Abstract
The production of visually guided reaching movements relies on a large neural network. Based on indirect experimental evidence, it has been suggested that the superior colliculus, a subcortical centre known for its key role in controlling rapid orienting gaze shifts, also belongs to this network. The aim of the present study was to investigate the role of the cat superior colliculus (SC) in the control of visually guided reaching movements. To address this issue, we studied the effect of SC electrical stimulation on forelimb reaching movements in two cats trained to catch a piece of food. Electrical stimulation delivered just after the movement onset yielded a consistent perturbation of the movement trajectory of the forelimb extremity. This perturbation followed stimulation onset by 56 ± 11 ms on average, and consisted of a deviation of the spatial path and a deceleration of the movement. The forelimb perturbation was elicited in the absence of concomitant gaze or head displacement in 52% of the stimulation trials. Forelimb perturbations were followed by in-flight adjustments so that reaching movements reliably ended on the target. The present results constitute the first behavioural evidence for a contribution of the cat SC to the control of visually guided forelimb movements.
Programming and controlling visually guided reaching movements rely on a large neural network. Since the seminal work of Ungerleider & Mishkin (1982), which suggests the existence of two distinct visual streams in the cerebral cortex, a strong emphasis has been put on the critical role of the dorsal pathway in controlling reaching movements. The dorsal stream is known to transmit quickly visual information to the motor areas, matching the tight temporal constraints of visuo-motor control.
However, as proposed for lower mammals (Schneider, 1968; Trevarthen, 1968), and more recently for primates (Werner et al. 1997; Day & Brown, 2001), some subcortical structures, such as the superior colliculus (SC), may also be involved in the control of reaching movements. Indeed, it has been recently suggested that the SC, beyond its key role in controlling rapid orienting gaze shifts (see Guitton, 1999; Sparks, 1999 for review), also provides input signals to other motor systems such as the smooth pursuit (e.g. Missal et al. 2002). Another striking example of the possible SC contribution to a non-saccadic motor system came from the electrophysiological evidence of a tectospinal projection to C3–C4 propriospinal neurones in the cat (Illert et al. 1978). Furthermore, a spinal cord lesion made ventrally at the C2 level, and which interrupted the tectospinal pathway, was found to delay ‘mid-flight’ corrections of reaching movements (Alstermark et al. 1990). More recently, it has been shown in monkeys that some collicular cells discharge in relation to visually guided reaching movements of the forelimb (Werner et al. 1997; Stuphorn et al. 2000). However, the hypothesis that the SC may contribute to the control of reaching movements remains disputed because many of the ‘reaching cells’ recorded in the monkey were found in the mesencephalic reticular formation (Stuphorn et al. 1999). Moreover the conclusion of Alstermark et al. (1990) on the role of the tectospinal pathway in the control of the trajectory of reaching movements has recently been challenged in a study with more selective spinal cord lesions (Pettersson & Perfiliev, 2002). In addition, previous attempts to activate the limb motor system through electrical stimulation of the SC reported that the threshold to induce excitation on forelimb motoneurones was high in contrast to results previously observed with neck motoneurones (Anderson et al. 1972). Therefore, direct evidence for a causal relationship between transitory perturbation or inactivation of SC activity and deficits in reaching movements is still lacking.
To address this issue, we investigated the effects of SC electrical stimulation on visually guided reaching movements in two adult cats trained to catch a piece of food. Preliminary results have been reported previously in abstract form (Pélisson et al. 2002).
Methods
Experimental procedures
All experimental procedures were conducted in accordance with the guidelines of the French Ministry of Agriculture (87/848) and of the European Community (86/609/EEC), and animals were housed and cared for in accordance with these guidelines.
Present data were gathered from two adult cats trained to stand immobile, while mildly restrained by a harness, in front of three parallel tubes placed horizontally at shoulder level with their open ends facing the animal. The internal tube diameter and intercentre distance were 30 mm; the middle tube was aligned with the cat’s right shoulder at a distance of about 100 mm from the average starting location of the paw. The cats were trained to reach for a piece of food presented, at random, in one of the three horizontal tubes. Before each trial, the three tubes were masked by a piece of cardboard; the trial started when the experimenter suddenly removed the cardboard.
When the training was completed, the animals underwent a single surgery whose detailed procedure has been already described (Guillaume & Pélisson, 2001). Briefly, the anaesthesia was induced and maintained by pentobarbital sodium (i.p. injection: 30 mg kg−1; i.v. perfusion: 1–3 mg kg−1 h−1). Cats were implanted with an eye coil to record gaze displacements. Then the skull was exposed, a craniotomy was made and a chamber was implanted to allow access to both SC with microelectrodes. A plastic post was set in the acrylic to restrain the head. A coil was also embedded into the headpiece to allow head movements to be recorded. After a recovery period of 10–15 days during which the wounds and the recording chamber were cleaned daily (use of aseptic agents) and the animals received i.m. injections of antibiotics (amoxicillin) for the first postoperative week, the awake animals were tested in the behavioural reaching task. At the end of the experiment, the animals were killed with an i.p. injection of 2.5 times the lethal dose of sodium pentobarbital.
SC stimulation
In this initial investigation, we did not systematically explore the entire SC map but rather focused on the contralateral (left) SC, and most SC sites encoded the lower right quadrant of visual field and of gaze motor space (see Table 1). Each experimental session started with the head restrained to allow us to lower a tungsten microelectrode in the SC. The dura matter was treated by a local anaesthetic agent (xylocaine) for 2 min before lowering the electrode through the dura by a conventional chronic electrode holder. Precisely positioning the electrode in any SC site required about 30–45 min (neuronal recording or electrical stimulation was carried out throughout the electrode penetration). The surface of the SC was first identified on the basis of the typical visual responses found when entering the superficial layers; then the electrode was lowered by 1.3–2.6 mm below the SC surface. After positioning the electrode in the SC deep layers, the animal’s head was unrestrained for the rest of the session (about 1 h). The site-specific gaze vector and its threshold (current intensity required to evoke a gaze shift with a probability of at least 0.75) were determined by applying a 300 ms train of 0.5 ms pulses delivered at 300 Hz. During reaching movements, electrical stimulation was applied at the same site but the train duration was reduced to 70–200 ms and stimulation intensity increased to about 1.3 × gaze threshold; other stimulation parameters remained unchanged. SC stimulation was triggered automatically near the onset of reaching movements, when the forelimb velocity reached about 180 mm s−1. During a given experimental session, stimulation trials were interleaved randomly with control trials, with a probability of 0.33. The whole session was performed with the lights on and was continuously video taped.
Table 1.
Summary of the 11 experimental sessions
| Stimulation sites | Gaze parameters | Control forelimb movements | Perturbed forelimb movements | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session | Cat | Stereotax. (A-L) (mm) | Depth (re SC surf.) (mm) | Gaze thresh. (μA) | Gaze H (deg) | Gaze V (deg) | Duration (ms) | Peak velocity (mm s−1) | No. of trials | Duration (ms) | Peak velocity (mm s−1) | Perturb. lat. (ms) | Perturb. ampl. (mm) | No. of trials |
| 1 | Y | 2.6–2.4 | 2.2 | 10 | 37.5 | −32 | 271 | 872 | 38 | 415 | 687 | 46 | 31.2 | 32 |
| 2 | Y | 1.8–2.1 | 2.0 | 8 | 41 | −7 | 307 | 765 | 31 | 471 | 463 | 61 | 24.4 | 21 |
| 3 | Y | 2.7–2.1 | 2.2 | 8 | 28 | −3 | 289 | 929 | 29 | 395 | 505 | 52 | 23.2 | 26 |
| 4 | Y | 3.0–2.5 | 2.2 | 15 | 20 | −0.2 | 288 | 857 | 34 | 376 | 549 | 57 | 22.7 | 25 |
| 5 | Y | 1.3–2.9 | 2.0 | — | — | — | 306 | 681 | 21 | 463 | 441 | 71 | 23.2 | 6 |
| 6 | Y | 2.6–2.4 | 2.0 | 15 | 29 | −2 | 253 | 791 | 40 | 356 | 722 | 38 | 35.0 | 35 |
| 7 | Y | 1.1–2.3 | 2.6 | 40 | 15 | −8 | 242 | 1018 | 16 | 373 | 724 | 61 | 28.5 | 4 |
| 8 | Y | 2.7–2.4 | 2.0 | 18 | 34 | −11 | 295 | 807 | 43 | 373 | 533 | 43 | 18.8 | 16 |
| 9 | Y | 2.7–2.4 | 2.0 | 18 | 34 | −11 | 283 | 1001 | 16 | 397 | 433 | 72 | 28.3 | 15 |
| 10 | Y | 2.7–2.4 | 2.0 | 18 | 34 | −11 | 308 | 1005 | 39 | 368 | 572 | 58 | 28.4 | 8 |
| Mean | — | — | — | 16.6 | 30.3 | 9.3 | 284 | 873 | — | 399 | 563 | 56 | 26.4 | — |
| (s.d.) | (10) | (8.3) | (9.4) | (23) | (114) | (40) | (112) | (11) | (4.8) | — | ||||
| 11 | Z | 3.9–3.4 | 1.3 | 15 | 61 | − 27 | 469 | 569 | 7 | 663 | 259 | 73 | 40.4 | 9 |
Mean (s.d.) indicates the average of all sessions performed in cat Y (n= 10). In sessions Y8, Y9 and Y10, the electrode was permanently implanted in the SC, so the stereotaxic coordinates and the elicited gaze parameters (threshold and H and V. displacements) are identical. Note also that the elicited gaze parameters are not available for session Y5.
Data acquisition and analysis
Vertical and horizontal gaze and head position signals were linearized and calibrated on-line, sampled at 500 Hz, digitally filtered (FIR filter, 70 Hz cut-off frequency) and stored on a PC for offline analysis. The position of the right forelimb extremity was measured by a method previously described (Urquizar & Pélisson, 1992). In brief, the 3D coordinates of a pair of infra-red LEDs fixed on the cat’s wrist were monitored by two orthogonally mounted 2-D sensors (Hamamatsu, spatial and temporal resolution = 0.1 mm and 324 Hz) and stored on a hard disk.
Signal processing and measurements were performed off-line by computer programs developed in the laboratory by M. Thevenet, Y. Paulignan & C. Prablanc (© UCBL-CNRS-INSERM). Position signals from the forelimb were calibrated and expressed as x (depth), y (azimuth) and z (elevation) coordinates and vectorial velocity was calculated. The onset of reaching movements was determined by using a vectorial velocity criterion of 50 mm s−1 and movement offset was defined as the moment the forelimb extremity entered the target tube, i.e. when x wrist‘s position equalled the x coordinate of the tube edge. When a perturbation of reaching movements was elicited by SC stimulation, we also determined the perturbation onset, defined as the time when the vectorial velocity profile reversed, and the onset of voluntary correction, defined as the next velocity reversal, when the limb re-accelerated after the stimulation train (see Fig. 2). Finally, the effect of electrical stimulation on reaching movements was quantified by computing a ‘perturbation vector’. This vector was measured by computing the maximum difference between the normalized trajectory of each perturbed movement and the average normalized trajectory of control movements performed towards the same target.
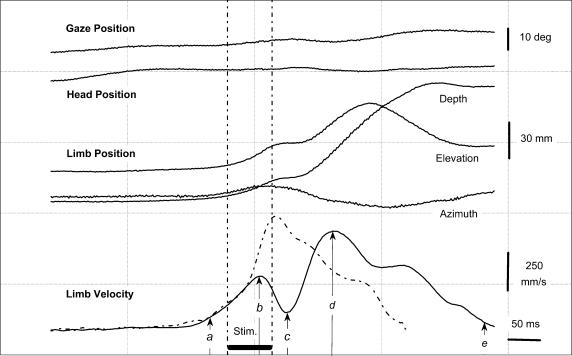
Figure 2. Time course of a perturbed forelimb movement (session Y1, centre target).
Continuous lines plot, relative to the time of target presentation in a perturbed trial, the horizontal position of gaze and head, the 3 coordinates of the forelimb position and its vectorial velocity. The velocity profile of a control forelimb movement (dashed line) is plotted for comparison with its onset time-aligned with that of the perturbed movement. Labels a–e indicate the analysed temporal parameters: onset (a), peak velocity (d) and end (e) of reaching, the onset of the perturbation (b) and of the compensation (c). Horizontal bar (‘Stim’) indicates the electrical microstimulation applied to the left SC (300 Hz, 70 ms, 14μA).
Results
Ten experimental sessions were performed in cat Y and one in cat Z. As shown in Table 1, although cat Z performed slower reaching movements, the effects of SC stimulation were comparable in the two cats and therefore for the sake of clarity, only data collected in cat Y are presented. The possibility of eliciting low-threshold gaze displacements was checked before all but one experimental session. In cat Y, the threshold for evoking gaze displacements ranged from 8 to 40 μA (mean ±s.d.: 16.6 ± 10.4 μA, n= 9); their amplitude varied from 18 to 53 deg (mean ±s.d.: 34.0 ± 10.6 deg., n= 9). All stimulation sites proved to be effective for inducing perturbations of reaching movements, using a stimulation current intensity which was raised to 1.3 × gaze threshold (see Methods).
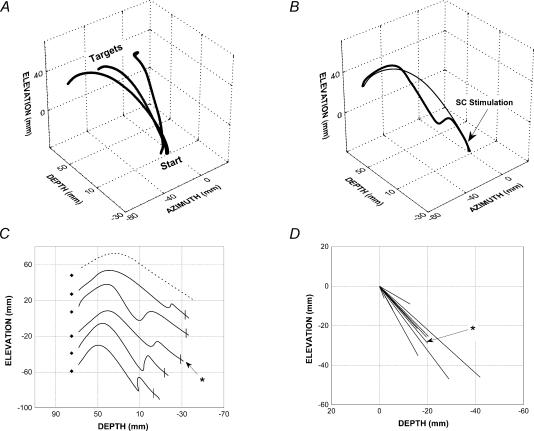
Figure 1A shows an example of averaged control reaching movements. In order to reach a given target, the cats had both to raise (z-axis) and extend (x-axis) the forelimb and, in addition, for the two eccentric targets, a displacement along the y-axis was required.
Figure 1. Trajectories of selected forelimb reaching movements (session Y1).
A, 3-D averaged trajectories of unperturbed movements directed toward the 3 targets. After synchronization on movement onset, the trajectory of 7, 10 and 4 individual responses to the left, centre and right targets, respectively, were pooled. B–D, forelimb responses to the left target recorded in session Y1. B, 3-D trajectories of an individual perturbed response (thick line, stimulation parameters: 300 Hz, 100 ms, 12 μA) and of the average of control responses (thin line). C, lateral view of different perturbed responses (continuous line) and of a typical control response (dashed line). Target centre positions are shown by diamonds. The perturbed response shown in B is indicated by the asterisk. D, lateral view of perturbation vectors. The vector of the perturbed movement shown in B and C is indicated by the asterisk. Note the different scales as compared to C.
As illustrated in Fig. 1B and C, stimulation of the contralateral SC induced a marked perturbation of reaching movements. The effect of SC stimulation on one individual reaching movement is illustrated in Fig. 1B (thick line) and compared with the averaged control movements (thin line) directed to the same, left, target. In this particular example, SC stimulation (arrow) resulted in a downward deviation of the forelimb trajectory. Since in this session the induced perturbation was restricted to the sagittal (x–z) plane, this movement is represented in a lateral 2-D view, along with the trajectories of four other perturbed movements and that of a control movement (dotted line) for comparison (Fig. 1C). This plot indicates that, for a given stimulation site, SC stimulation resulted in a rather consistent deviation of reaching movements. A marked velocity reduction occurred concomitantly with this deviation (see below for details). The perturbation vectors (see Methods) of reaching movements for this stimulation site are shown in Fig. 1D (n= 10) and confirm that SC stimulation produced a fairly reproducible perturbation. For this site, perturbation vectors were directed downward and backward, signalling, respectively, a downward deviation of the forelimb trajectory and a deceleration of the movement relative to the average control movement. This is representative of the perturbation vectors found in most experiments (coordinates of vector averaged across the 10 experiments: x=−11.6 mm, y=−2.8 mm, z=−18.9 mm). In addition a significant lateral deviation of the perturbation vector was also observed for some experiments (subtending an angle of more than 45 deg relative to straight ahead in 4 out of 10 experiments). Recall that the SC space sampled in this study was restricted (see Methods), which prevents us from correlating the direction or amplitude of the perturbation vectors to the position of the stimulated SC sites. Table 1 shows, for both cats and for all experimental sessions, the mean amplitude of the perturbation vectors.
Figure 2 illustrates the time course of typical reaching movements. The velocity profile of control reaching movements (dashed line) was asymmetrical, with an acceleration phase shorter than the deceleration phase. Over all recording sessions, control reaching movements had a mean duration of 284 ms (s.d.: 23 ms) and a peak velocity of 873 mm s−1 (s.d.: 114 mm s−1, n= 10). The effect of SC stimulation was evident when the velocity profile of the control movement is compared with that of a perturbed movement (continuous lines). Indeed, a clear reversal of the velocity profile can be seen, corresponding to a deceleration effect of the collicular stimulation. In this example, the perturbation had a latency of 52 ms; on average, the latency of all perturbations elicited from this stimulation site was 46.4 ± 9.5 ms (n= 32). When data from all sites explored in this cat were pooled, the mean latency of perturbations was 55.9 ± 11.1 ms (n= 10). The velocity decrease produced by SC stimulation was then followed by a re-acceleration, which corresponds to a movement correction phase during which the limb was redirected toward the target. Another general finding was that, although the stimulus intensity used was on average 30% larger than the gaze threshold determined prior to the reaching task (see Methods), forelimb perturbation were not accompanied by significant gaze shift or head movement. In 52% of the stimulation trials, as shown in Fig. 2, no detectable gaze or head displacement was found. In the remaining trials, the elicited gaze displacements had an amplitude equal, on average, to 23% of the gaze displacement vector evoked using the threshold intensity determined prior to the reaching task.
Because of its short latency and of its backward component, the induced forelimb perturbation led to a decrease in the peak velocity of reaching movements (563 ± 112 mm s−1) when compared with controls (873 ± 114 mm s−1; paired t test: t(9) = 7.0, P < 0.001). Electrically induced perturbations never resulted in a complete movement disruption and were systematically followed by a mid-flight correction of the reaching movement trajectory. Consequently, all reaching movements ended reliably within the appropriate tube (see the endpoint of the movements illustrated in Fig. 1C). The mean latency of the movement correction, estimated with respect to the stimulation offset, was 28.0 ± 35 ms (mean ±s.d., n= 10). Because of the perturbation itself and the consecutive correction, “perturbed” reaching movements had a longer duration (mean ±s.d.: 399 ± 40 ms, n= 10) than controls (284 ± 23 ms; paired t test, t(9) = 10.5, P < 0.001).
Discussion
The present study showed that SC stimulation applied at the onset of reaching movements yielded a consistent perturbation of the forelimb trajectory. This perturbation consisted of a change in movement direction associated with an abrupt decrease in movement velocity that occurred 50–60 ms after stimulation onset. Reaching movement perturbations could be elicited from all SC sites investigated and were, in about 50% of the trials, not accompanied by a gaze or head displacement. The induced perturbations were systematically followed by a rapid in-flight correction of reaching movements.
Because the perturbations of reaching movements elicited by SC stimulation were not accompanied by significant gaze or head displacements, we can rule out that the effect of collicular stimulation on forelimb movements was an indirect consequence of shifting gaze direction. In addition, the low intensity of electrical stimulation required to perturb reaching movements, applied at sites where gaze displacements could be elicited with longer trains, supports the hypothesis that these effects were mediated by efferent collicular neurones. The finding that no gaze displacement, or only small amplitude gaze shifts, was elicited during reaching movements may appear somewhat puzzling since we used a supra-threshold stimulation current. However, it is likely that, during the reaching task, the animals attentively fixated the target; this is known to decrease the probability and amplitude of elicited gaze shifts (Paréet al. 1994). In addition, it is worth noting that the stimulation train we used to perturb reaching movements was shorter than that used to determine gaze displacements threshold. Altogether, these observations support the hypothesis that the perturbations of reaching movements described in the present study result directly from the activation of SC neurones by electrical stimulation. The stimulation sites tested in this study were confined to a rather restricted area in the SC deep layers (see Methods). Thus, further mapping experiments are necessary to determine whether the limb perturbations reported in the present study are in spatial register with the visual and gaze shift maps found in the SC deep layers. Nonetheless, the present data provide the first functional evidence that the cat SC directly contributes to the control of visually guided reaching movements.
This conclusion is supported by anatomical data in cats showing that direct collicular projections to the spinal cord (i) are particularly dense (Nudo & Masterton, 1989; Olivier et al. 1991) and (ii) establish very strong connections with C3–C4 propriospinal neurones involved in the control of reaching movements (Illert et al. 1978).
As mentioned in the Introduction, the rapid processing of visuo-motor signals necessary to execute goal-directed limb movements has led to the suggestion that fast subcortical loops may be involved in their control. The SC contribution to the control of such movements was first suggested by the finding that, in the cat, the latency of limb trajectory corrections in response to a target jump was prolonged after a lesion of the spinal cord at a C2 level involving the tectospinal pathway (Alstermark et al. 1990). Besides the fact that this conclusion has been recently challenged (Pettersson & Perfiliev, 2002), the results of such lesion-based studies are always difficult to interpret because the section of the tectospinal pathway was performed in the spinal cord and is likely to involve other descending fibres. In addition, in these experiments, the animals were tested several days (10–15 days) after the surgery. The present approach allows us to circumvent these limitations and to conclude that the SC is involved in forelimb movement control.
It is also worth noting that the short latency of limb perturbation following SC stimulation (50–60 ms) is compatible with the previously suggested involvement of the SC in the control of mid-flight corrections of reaching movements (Alstermark et al. 1990). Another, non-exclusive role of the SC in the control of visually guided forelimb movements would be to coordinate forelimb and gaze orienting movements during a reaching task. Further studies are necessary to distinguish between these different hypotheses.
Another interesting finding of the present study is the short-latency corrections that systematically followed perturbations of visually guided reaching movements. These corrections suggest that the electrical stimulation did not completely reset the limb-related neuronal activity in the SC. In addition, because even a high intensity SC stimulation (up to 6 times threshold; see, e.g. Guillaume & Pélisson, 2001) never evoked limb movements, the possibility that SC stimulation can elicit descending commands for movement initiation is unlikely. Instead, it may be hypothesized that the transient increase of neuronal activity resulting from collicular stimulation momentarily modifies the saliency of target-related signals encoded by the SC.
Acknowledgments
We acknowledge Marie-Line Loyalle for taking care of the animals. This research was supported by Institut National de la Santé et de la Recherche Médicale U534 and by a CFB-INSERM agreement.
References
- Alstermark B, Gorska T, Lundberg A, Pettersson LG. Integration in descending motor pathways controlling the forelimb in the cat. 16. Visually guided switching of target-reaching. Exp Brain Res. 1990;80:1–11. doi: 10.1007/BF00228841. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Yoshida M, Wilson VJ. Tectal and tegmental influences on cat forelimb and hindlimb motoneurones. J Neurophysiol. 1972;35:462–470. doi: 10.1152/jn.1972.35.4.462. [DOI] [PubMed] [Google Scholar]
- Day BL, Brown P. Evidence for subcortical involvement in the visual control of human reaching. Brain. 2001;124:1832–1840. doi: 10.1093/brain/124.9.1832. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pélisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. I. Effect of the locus and of the parameters of stimulation. Eur J Neurosci. 2001;14:1331–1344. doi: 10.1046/j.0953-816x.2001.01744.x. [DOI] [PubMed] [Google Scholar]
- Guitton D. Gaze shifts in three-dimensional space: a closer look at the superior colliculus. J Comp Neurol. 1999;413:77–82. doi: 10.1002/(sici)1096-9861(19991011)413:1<77::aid-cne5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Illert M, Lundeberg A, Padel Y, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3–C4 propriospinal neurones. Exp Brain Res. 1978;33:101–130. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- Missal M, Coimbra A, Lefèvre P, Olivier E. Further evidence that separate circuits drive slow eye movements and saccades through a shared efferent collicular pathway. Exp Brain Res. 2002;147:344–352. doi: 10.1007/s00221-002-1274-7. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB. Descending pathways to the spinal cord. II. Quantitative study of the tectospinal tract in 23 mammals. J Comp Neurol. 1989;286:96–119. doi: 10.1002/cne.902860107. [DOI] [PubMed] [Google Scholar]
- Olivier E, Chat M, Grantyn A. Rostrocaudal and lateromedial density distributions of superior colliculus neurons projecting in the predorsal bundle and to the spinal cord: a retrograde HRP study in the cat. Exp Brain Res. 1991;87:268–282. doi: 10.1007/BF00231844. [DOI] [PubMed] [Google Scholar]
- Paré M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 1994;101:123–139. doi: 10.1007/BF00243222. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Courjon JH, Olivier E. Perturbation of visually-guided reaching movements by electrical microstimulation of the superior colliculus in the cat. Soc Neurosci Abs. 2002:170.6. [Google Scholar]
- Pettersson L-G, Perfiliev S. Descending pathways controlling visually-guided updating of reaching in cats. Eur J Neurosci. 2002;16:1349–1360. doi: 10.1046/j.1460-9568.2002.02203.x. [DOI] [PubMed] [Google Scholar]
- Schneider G. Two visual systems. Science. 1968;163:895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opinion Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Bauswein E, Hoffmann KP. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J Neurophysiol. 2000;83:1283–1299. doi: 10.1152/jn.2000.83.3.1283. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Hoffmann KP, Miller LE. Correlation of primate superior colliculus and reticular formation discharge with proximal limb muscle activity. J Neurophysiol. 1999;81:1978–1982. doi: 10.1152/jn.1999.81.4.1978. [DOI] [PubMed] [Google Scholar]
- Trevarthen CB. Two mechanisms of vision in primates. Psychologische Forsch. 1968;31:299–337. doi: 10.1007/BF00422717. [DOI] [PubMed] [Google Scholar]
- Ungerleider L, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Motor Behavior. Cambridge, MA, USA.: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Urquizar C, Pélisson D. A non-contact system for 2-dimensional trajectory recording. J Neurosci Meth. 1992;43:77–82. doi: 10.1016/0165-0270(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]