Abstract
Previous studies have outlined an important role for serotonin (5-HT) in the development of synaptic connectivity and function in the cerebral cortex. In this study, we have examined the effects of 5-HT on synaptic function in prefrontal cortex at a time of intense synapse formation and remodelling. Whole-cell recordings in slices derived from animals aged postnatal (P) days 16–20 showed that administration of 5-HT induced a robust increase in synaptic activity that was blocked by CNQX but not by bicuculline. This 5-HT-induced increase in glutamate-mediated synaptic activity was pharmacologically heterogeneous as it was differentially inhibited by the receptor subtype-selective antagonists SB-269970, MDL 100907 and GR 113808 and thus involved 5-HT7, 5-HT2A and 5-HT4 receptors. These results, obtained in juvenile cortex, contrast with those seen in adults where the increase in spontaneous excitatory postsynaptic currents (sEPSCs) was mediated solely by 5-HT2A receptors. In developing cortex, activation of 5-HT7, but not 5-HT2A or 5-HT4 receptors, elicited a robust inward current. However, the facilitation of synaptic activity mediated by all three of these receptors involved increases in both the amplitude and frequency of sEPSCs and was blocked by TTX. These results are best interpreted as indicating that all three receptor subtypes increase synaptic activity by exciting neuronal elements within the slice. No evidence was found for a postsynaptic facilitation of synaptic currents by 5-HT. Together, these results show that the repertoire of electrophysiologically active 5-HT receptors in prefrontal cortex is developmentally regulated, and that 5-HT7 and 5-HT4 receptors play a previously unsuspected role in regulating synaptic activity in this region.
The development of cortical function and connectivity is now widely recognized as resulting from the complex interplay between intrinsic and extrinsic factors (Levitt et al. 1997; Sur & Leamey, 2001). Among the latter, growing evidence implicates 5-HT (5-hydroxytryptamine; serotonin) and 5-HT-releasing fibres in regulating the refinement of synaptic connectivity during postnatal development. Thus, for example, 5-HT has been shown to play a key role in the development of barrel fields in somatosensory cortex, an action that is mediated by 5-HT1B receptors transiently expressed by thalamocortical afferents (Bennett-Clarke et al. 1994, 1995; Cases et al. 1996; Salichon et al. 2001). Similarly, 5-HT acting at 5-HT2C receptors transiently expressed in cat visual cortex has been shown to regulate synaptic plasticity in a spatially segregated pattern that may contribute to the formation of ocular dominance columns (Kojic et al. 1997; Kirkwood, 2000; Kojic et al. 2000). While these studies have been conducted in sensory cortices, it is likely that 5-HT may exert similar effects on other areas of cortex, most notably the prefrontal cortex, which are known to receive a robust serotonergic innervation during development., At the present time, very little is known about the specific mechanisms by which 5-HT regulates the development of cortical circuits. However, previous work in a variety of model systems has emphasized the importance of spontaneous and evoked synaptic activity in the formation and refinement of synaptic connections (see Zhang & Poo, 2001, for review). This suggests that one possible mechanism by which 5-HT could regulate the development of synaptic connectivity in the cerebral cortex may be by modulating the excitability of neurones and afferent fibres in the cortex. Here, we analyse the effects of 5-HT on synaptic activity in this region during the third postnatal week, a time of intensive synaptic refinement (Sur & Leamey, 2001). We find that 5-HT strongly regulates spontaneous synaptic activity in developing prefrontal cortex by activating 5-HT7, 5-HT4 and 5-HT2A receptors while only the latter receptor subtype accounted for the same response in adult. These results identify a previously unsuspected function for these 5-HT receptors subtypes in the developing prefrontal cortex and suggest a possible mechanism for 5-HT in fine-tuning the development of prefrontal cortex by controlling cellular and network excitability.
Methods
The procedures used for slice preparation were approved by the Wayne State University animal investigation committee. Briefly, male Sprague-Dawley rats aged (P) 15–19 or adults (>(P)35 days old, weighing 100–150 g) were anaesthetized with halothane (by inhalation) and killed by decapitation. The brain was quickly removed and cooled in ice-cold Ringer solution of the following composition (mm): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3 and 11 glucose, bubbled to saturation with 95% O2–5% CO2. The anterior pole of the brain was then isolated and affixed to a stage with cyanoacrylate glue. Coronal slices (300 μm thick) were cut using a vibratome (Lancer series 1000, Ted Pella, Irvine, CA, USA) and transferred to a holding chamber (Sakmann & Stuart, 1995) where they were allowed to recover for at least 1 h in Ringer solution. For recordings, one slice was transferred to a recording chamber of standard design (Sakmann & Stuart, 1995) while being perfused with normal Ringer solution bubbled to saturation with 95% O2–5% CO2.
Electrophysiological recordings
Whole-cell patch-clamp recordings were obtained from pyramidal neurones of layer V of the prelimbic or anterior cingulate subdivisions of the medial prefrontal cortex (Krettek & Price, 1977). Pyramidal cells were targeted using differential interference contrast (DIC) imaging on a fixed-stage upright microscope (Olympus, BX50WI). Electrical signals were recorded using an Axoclamp 2A, Axopatch 200 or Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) and recorded on-line with the use of a paper chart recorder (Gould 3400) or digitized and stored in an Intel-based PC. The recording pipettes were pulled from borosilicate glass (outer diameter, 1.2 mm) with the use of a Flaming-Brown horizontal puller to give resistance ranging from 3 to 6 MΩ when filled with an internal solution of the following composition (mm): 115 potassium gluconate, 20 KCl, 2 MgCl2, 4 Na2ATP, 10 sodium phosphocreatine, 0.3 GTP and 10 Hepes. The pH was adjusted to 7.3–7.4. An internal solution of the following composition was used in a limited number of experiments, as indicated: 77 caesium gluconate, 10 tetracaesium BAPTA, 5 TEA-Cl, 3 CaCl2, 10 Hepes, 4 ATP, 0.5 GTP, 5 QX-314 and 10 sodium phosphocreatine. In most experiments, series resistance was corrected by ∼60–70%. In the experiments involving recordings of miniature EPSCs (see below) series resistance was not compensated. Experiments in which access resistance increased by more than ∼30% were discarded.
Synaptic events were filtered at 1 or 3 kHz and digitized at 10 kHz using a 12 bit A/D converter (Digidata 1200, Axon Instruments) under the control of pCLAMP 7.0 or 8.0 (Axon Instruments). Neurones were held at −70 mV and spontaneous activity was sampled for 1 s every 6 or 10 s for the duration of the entire experiment. The experiments aimed at determining the effect of bicuculline on the increase of sEPSCs induced by 5-HT were complicated by the tendency of bicuculline to induce rhythmic epileptiform discharge in the slice (usually less than one per minute). It was, however, possible to determine, between discharges, the frequency and amplitude of sEPSCs. In the experiments involving recordings of miniature EPSCs (mEPSCs), synaptic activity was continuously recorded for periods up to 20 min. For these experiments, slices were bathed in TTX (1–3 μm) and (−)bicuculline (30 μm). To increase the frequency of mEPSCs, some recordings were carried out in the presence of elevated [K+]o either by briefly applying a high K+ solution (50 mm KCl; 2–10 s every 30–60 s) from a broken pipette placed above the slice (puff application) or by directly perfusing the slice with Ringer solution containing 5 mm KCl. The marked increase in frequency of mEPSCs induced by puff applications showed some trial to trial variability and hence this method was used only for determining the effects of 5-HT on the amplitude of mEPSCs. In some experiments, synaptic currents were evoked every 15 s by electrical stimulation with a bipolar stimulating electrode placed in layer II–III about 150 μm away from the apical dendrite of the recorded neurones. For experiments aimed at isolating evoked EPSCs (eEPSCs), inhibitory synaptic potentials were minimized by bathing the slices in 1 μm bicuculline and by using an intracellular solution bringing ECl close to −70 mV (caesium based). This same solution was also used for evoking inhibitory postsynaptic currents (eIPSCs) in the presence of the glutamate receptor antagonists d-aminophosphono pentanoic acid(d-AP-5; 30 μm) and CNQX (30 μm). Exogenous glutamate was applied to some slices using a fast perfusion system (Warner Instruments) from a tube located above layer II–III. Analysis of synaptic events was carried out using the Mini Analysis Program (version 5.2; Synaptosoft, Leonia, NJ, USA). Parameters for the synaptic event detection algorithm were optimized for every experiment. The effects of 5-HT on the frequency and amplitude of spontaneous and miniature excitatory postsynaptic currents were plotted as inter-event interval histograms and as amplitude histograms and cumulative amplitude histograms, respectively. Statistical significance was assessed using the Kolmogorov-Smirnov (K-S) statistical method or Student's t test, as indicated (P < 0.05 was taken as indicating statistical significance). All drugs were bath applied dissolved in Ringer solution at a known concentration.
Chemicals
6-Cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), 5-hydroxytryptamine (5-HT), (−)bicuculline, d-AP-5, DOI and (−)cyanopindolol were purchased from Sigma-Aldrich. Tetrodotoxin (TTX) was purchased from Alomone Labs (Jerusalem, Israel). QX-314 was purchased from Calbiochem (San Diego, CA, USA). SB-269970 was a generous gift from SmithKline Beecham Pharmaceuticals (Harlow, UK) and GR 113808 was a generous gift of Glaxo (UK). MDL 100907 was a generous gift from Marion Merrel Dow (Strasbourg, France) and from Dr Kenner C. Rice (NIDDK, Bethesda, MD, USA).
Results
To address the effects of 5-HT in the developing medial prefrontal cortex, we obtained whole-cell recordings from pyramidal neurones of layer V of the medial subdivision of the prefrontal cortex. Pyramidal cells were identified based upon their morphology under DIC imaging and their distinctive electrophysiological properties (Connors & Gutnick, 1990). Administration of 5-HT (1–100 μm) to these cells during the third week of life induced a variable slow inward current (Fig. 1) that was accompanied by a marked increase in spontaneous synaptic activity (n > 100 cells). Previous studies have shown that synaptic activity plays a key role in the refinement of synaptic connections (Zhang & Poo, 2001). As such, these observations, at a time of intense synapse formation and remodelling (Sur & Leamey, 2001), suggest a role for 5-HT in regulating the development of prefrontal cortex. We therefore examined the mechanism underlying these effects of 5-HT, with special emphasis on the increase in synaptic activity. To simplify the analysis of this phenomenon, the vast majority of recordings reported upon in this report were obtained in the presence of 1 μm cyanopindolol to block 5-HT1A and 5-HT1B receptors.
Figure 1. Administration of 5-HT induces an inward current and an increase in the frequency and amplitude of spontaneous postsynaptic currents in layer V pyramidal neurones in the developing cortex.
Aa, in this recording from a P19 animal, bath administration of 5-HT (30 μm) induced an exceptionally large inward current and a large increase in synaptic activity. The increase in synaptic activity is depicted in more detail using an expanded time scale in the current traces at times 1 and 2. Ab, cumulative distribution of inter-event intervals for the cell illustrated in Aa. 5-HT significantly increased the frequency of synaptic currents (P < 0.05, K-S test). Ac, amplitude distribution of spontaneous synaptic currents during baseline acquisition (open bars) and in the presence of 5-HT (filled bars) for the same cell. Administration of 5-HT increased the frequency of synaptic currents at all amplitudes and shifted their distribution towards larger size classes. The noise distribution is shown as a dashed line. Ad, cumulative distribution of synaptic current amplitudes for the cell illustrated in A. 5-HT significantly shifted to the right the cumulative distribution synaptic current amplitudes (P < 0.05; K-S test). Holding potential, −70 mV; holding current, −5 pA. B, the synaptic events induced by 5-HT reversed at a membrane potential close to 0 mV. Current traces from a layer V pyramidal neurone recording using a caesium-based intracellular solution are shown during baseline acquisition and in the presence of 5-HT (30 μm). Traces are shown at holding potentials of −70, −40 and +40 mV. Note that no or very few synaptic events are readily detected as outward synaptic current in the presence of 5-HT at −40 mV. Inset, using the same caesium-based intracellular solution as for the recording depicted in B, GABAA receptor-mediated IPSCs were evoked with bipolar stimulating electrodes while holding a layer V pyramidal neurone at −80, −70 and −40 mV. These current traces (average of 5 consecutive traces) were obtained in the presence of CNQX (30 μm) and d-AP-5 (30 μm) and the IPSCs were completely blocked by bicuculline (30 μm; not shown). Note that GABAA receptor-mediated IPSCs are readily detected as outward currents at −40 mV.
Identification of the 5-HT receptor subtypes mediating the 5-HT-induced inward current
As a first step towards understanding the effects of 5-HT in developing prefrontal cortex, we determined which receptor subtype(s) mediated the slow inward current elicited by bath administration of 5-HT. Since activation of 5-HT2A receptors can depolarize pyramidal neurones in adult prefrontal cortex (Araneda & Andrade, 1991), we first examined the effects of the selective 5-HT2A receptor antagonist MDL 100907 (300 nm; (103 times its Kd for 5-HT2A receptors; Hagan et al. 2000). Surprisingly, bath administration of MDL 100907 only marginally inhibited the inward current elicited by 5-HT (∼30%n = 7; Fig. 2B, P < 0.05, Student's paired t test). A similar limited inhibition was obtained when slices were pretreated with MDL 100907 (300 nm, > 20 min) to ensure antagonist concentration equilibrium. Thus, administration of 5-HT (30 μm) induced an inward current averaging 58.2 ± 6.9 pA (mean ±s.e.m., n = 17; not shown) in control slices, while in slices pretreated with MDL 100907, it induced an inward current averaging 47.5 ± 5.7 pA (∼18% reduction; n = 6, not shown). The limited effectiveness of MDL 100907 indicates that one or more additional 5-HT receptor subtype(s) mediate(s) most of the inward current elicited by 5-HT in developing prefrontal cortex.
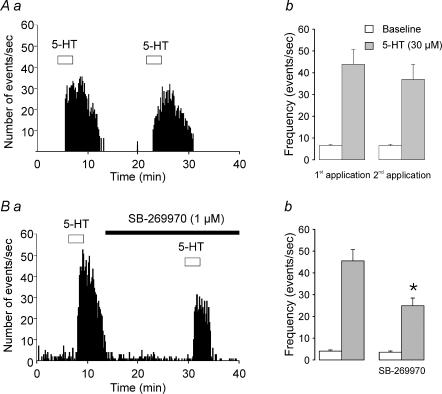
Figure 2. Predominant role of 5-HT7 receptor activation in the 5-HT-induced inward current in layer V pyramidal neurones.
Aa, current trace from a layer V pyramidal neurone illustrating the inward current induced by bath administration of 5-HT (30 μm). This effect is inhibited by the selective 5-HT7 receptor antagonist SB-269970 (1 μm). The three open circles represent a period of 17 min. Holding potential, −70 mV; holding current, −15 pA. Ab, summary plot depicting the amplitude of the 5-HT-induced inward current before and during bath administration of SB-269970 (1 μm; n = 7; P < 0.01, Student's paired t test). Ba, current trace from a layer V pyramidal neurone illustrating the inward current induced by bath administration of 5-HT (30 μm) before and during bath administration of the selective 5-HT2A receptor antagonist MDL 100907 (300 nm). The three open circles represent a period of 15 min. Bb, summary plot depicting the amplitude of the 5-HT-induced inward current before and during bath administration of MDL 100907 (300 nm to 1 μm; n = 7; P < 0.05; Student's paired t test).
Serotonin receptors of the 5-HT7 subtype have been shown to mediate membrane depolarizations elsewhere in the brain (Chapin & Andrade, 2001). Therefore we next tested for the possible involvement of this receptor subtype. Consistent with a role for 5-HT7 receptors in mediating the 5-HT-induced inward current in developing prefrontal cortex, the ability of 5-HT to induce an inward current was greatly inhibited (72.2 ± 0.1% inhibition; P < 0.01, Student's paired t test, Fig. 2A) by bath administration of the selective 5-HT7 receptor antagonist SB-269970 (n = 7, 1 μm, ∼103 times its Kd for 5-HT7 receptors; Kehne et al. 1996). Essentially identical results were obtained when slices were pretreated with SB-269970 (1 μm, > 20 min, 11.5 ± 3.1 pA ∼80% reduction; n = 27). In a subset of these slices, the small residual current induced by 5-HT was significantly reduced by MDL 100907 (300 nm; n = 9; P < 0.05; Student's t test; not shown), thus confirming a small, but significant, contribution by 5-HT2A receptors to the inward current. Co-administration of SB-299970 and MDL 100907 completely blocked the ability of 5-HT to induce an inward current in 4 out of 9 cells tested. In the remaining 5 cells, 5-HT still elicited a miniscule current (7.6 ± 1.1 pA, n = 5 cells). These results suggest that 5-HT7, and to a much lesser extent 5-HT2A receptors, mediate the inward current elicited by 5-HT in developing prefrontal cortex. However, the possible involvement of yet another receptor present in a limited number of cells cannot be ruled out.
Administration of 5-HT induces a marked increase in both the frequency and amplitude of sEPSCs that is blocked by TTX
In addition to eliciting an inward current, administration of 5-HT elicited a marked increase in spontaneous synaptic activity (Figs 1A, 3 and 4). In the current sample, administration of 30 μm 5-HT increased the frequency of spontaneous synaptic activity from a baseline of 3.7 ± 0.4 events s−1 to 42.7 ± 3.4 events s−1 (n = 27). The 5-HT-induced increase in synaptic activity displayed only modest desensitization when assessed using consecutive applications of 5-HT (see below and Fig. 4) or continuous application of this agonist (15 min, 30 μm, n = 3 cells, Fig. 3A). This made it possible to quantitatively analyse changes in the amplitude and frequency of synaptic events in response to 5-HT.
Figure 3. The 5-HT-induced increase in synaptic activity reflects action potential-dependent release of glutamate.
Aa, histogram depicting the frequency of synaptic currents as a function of time. Prolonged administration of 5-HT induces a persistent increase in the frequency of synaptic potentials. Ab, sample current traces for the experiment illustrated in Aa. Ba, administration of TTX (1 μm) induces a rapid and complete suppression of the synaptic activity induced by 5-HT. Bb, sample current traces for the experiment depicted in Ba. Ca, administration of the AMPA/kainate antagonist CNQX (30 μm) blocks the 5-HT-induced increase in synaptic activity, and thus identifies the synaptic currents as sEPSCs. Cb, sample current traces for the experiment depicted in Ca.
Figure 4. Activation of 5-HT7 receptors mediates, in part, the 5-HT-induced increase in sEPSCs in layer V pyramidal neurones of developing prefrontal cortex.
Aa, histogram depicting the increase in sEPSC frequency induced by two successive applications of 5-HT (30 μm). Ab, plot comparing the response elicited by 5-HT (30 μm; n = 4) on consecutive administrations. Although the second 5-HT administration tended to elicit somewhat smaller responses, this difference did not reach statistical significance (P = 0.2, Student's paired t test). Ba, histogram illustrating the antagonism of the 5-HT-induced increase in sEPSCs by SB-269970 (1 μm). Bb, plot summarizing the effect of SB-269970 (1 μm). SB-269970 significantly inhibited the effect of 5-HT on sEPSCs (n = 6; P < 0.01, Student's paired t test).
As illustrated in Fig. 1A, administration of 30 μm 5-HT induced a robust increase in the frequency of synaptic currents (plotted as a decrease in inter-event intervals) and also an increase in their amplitude (22 out of 27 cells, P < 0.05, K-S test). The simplest interpretation for these observations is that 5-HT excites cellular elements within the slice that synapse onto the recorded pyramidal cells. The firing of these cellular elements would increase the proportion of multiquantal, action potential-dependent, release events thus increasing the frequency of synaptic events and skewing the amplitude distribution of synaptic currents towards larger size classes (Figs 1 and 2). If this interpretation were correct, this effect should be sensitive to blockade of action potential propagation. Consistent with this idea, administration of the sodium channel blocker tetrodotoxin (TTX, 1 μm) completely suppressed the ability of 5-HT to increase spontaneous synaptic activity (n = 5 cells, Fig. 3B). TTX also significantly reduced sEPSC frequency and amplitude under control conditions (P < 0.05; K-S test; n = 4; not shown).
Under our recording conditions, the observed increase in synaptic activity elicited by 5-HT could, in principle, result from an increase in glutamatergic or GABAergic synaptic activity. We sought to discriminate between these possibilities, first, by looking at the reversal potential of the synaptic events induced by 5-HT. By holding the cell at −40 mV, where changes in chloride permeability would appear as outward currents, we could estimate the fraction of chloride-mediated (presumably GABAergic) synaptic events contributing to the increase in synaptic activity elicited by 5-HT. Overall, we found that less than 10% of the total number of synaptic events elicited by serotonin (range 0–15%) were outward at −40 mV (not shown; n = 8). Similar experiments were carried out using a caesium-based intracellular solution that exhibits a slightly more negative reversal potential for chloride ions (∼−70 mV). Under these conditions, 5-HT again induced a large increase in synaptic activity when holding at ECl and, as seen with the potassium-based intracellular solution, very few outward synaptic currents could be detected at −40 mV (Fig. 1C; n = 6). This failure was unlikely to reflect an inability to record GABAergic synaptic currents since evoked outward GABAA receptor-mediated inhibitory postsynaptic currents (eIPSCs) could be readily detected under these same conditions (Fig. 1C, inset). As expected for glutamate receptor-mediated currents, the overwhelming majority of synaptic events induced by 5-HT reversed at around 0 mV and were detected as outward current at +40 mV (Fig. 1C; n = 6). These observations suggest that the vast majority of synaptic currents induced by 5-HT were glutamatergic in nature, while a minor fraction represented GABAergic events.
We next sought to confirm the identity of the synaptic events induced by 5-HT using a pharmacological analysis. As illustrated in Fig. 3C, bath administration of the AMPA/kainate receptor antagonist CNQX (30 μm) effectively reversed the increase in spontaneous synaptic activity if applied during the continued application of 5-HT (P < 0.01; n = 3, Fig. 3C). Similarly, CNQX blocked the ability of 5-HT to increase spontaneous synaptic activity when determined by two sequential administrations of 5-HT (5-HT alone, 35.5 ± 7.1 Hz; 5-HT in the presence of CNQX, 1.4 ± 0.6 Hz; n = 6 cells, P < 0.01 Student's paired t test; not shown). These results support the conclusion that most of the synaptic events elicited by serotonin represent glutamatergic sEPSCs. CNQX also significantly reduced the baseline value of sEPSCs frequency (2.6 ± 0.6 to 0.1 ± 0.07 Hz; n = 6, not shown).
In a second experiment, we determined whether administration of a saturating concentration of the GABAA receptor antagonist bicuculline (30 μm) could reduce the increase in spontaneous synaptic events induced by sequential administration of 5-HT. In these experiments, 5-HT increased the frequency of sEPSCs to 34.8 ± 9.9 Hz. A second administration of 5-HT to these same cells in the presence of bicuculline increased synaptic activity to 27.6 ± 7.3 Hz (n = 7; P = 0.08, Student's paired t test; not shown). A comparable small decrease in the response to 5-HT on applications was also observed in the absence of bicuculline and reflects a small, but non-significant, desensitization of the response (see below). Taken together, these results indicate that 5-HT increased spontaneous synaptic activity in developing prefrontal cortex primarily by increasing the frequency and amplitude of glutamate-mediated sEPSCs.
5-HT7 receptors contribute to the increase in synaptic activity induced by administration of 5-HT
Since cortical pyramidal cells, including those of layer V, are extensively interconnected (Feldmeyer & Sakmann, 2000; Schubert et al. 2001), a potential explanation for the increase in synaptic activity induced by administration of 5-HT observed during the P15–P19 developmental period could be that it reflects the excitation of intracortical circuits. Administration of 5-HT induced a robust inward current which can be expected to depolarize and excite pyramidal cells. Such an effect could in principle account for the increase in synaptic activity. If that were the case, and since 5-HT7 receptors are overwhelmingly responsible for the 5-HT-induced inward current, it could be expected that these receptors would also be responsible for the increase in synaptic activity. Unexpectedly, administration of SB-269970 (1 μm) only partially inhibited the 5-HT-induced increase in sEPSCs (45% inhibition, n = 6, P < 0.05; Student's paired t test, Fig. 4). This partial blockade did not simply reflect incomplete equilibrium of the antagonist in the slice, since 5-HT still elicited significant increases in both the frequency and amplitude of sEPSCs in slices preincubated with the antagonist (> 20 min, 1–3 μm, n = 27, not shown). Thus, the activation of 5-HT7 receptors mediates the vast majority of the inward current elicited by 5-HT in layer V, but is responsible for only about half of the increase in synaptic activity. As such, these results indicate that, while 5-HT7 receptors are important contributors to the 5-HT-induced increase in sEPSCs, they do not fully account for it.
5-HT2A and 5-HT4 receptors contribute to the increase in spontaneous synaptic activity elicited by 5-HT in the developing prefrontal cortex
5-HT2A receptors are widely expressed in prefrontal cortex, and have been shown to account for the increase in sEPSCs induced by administration of 5-HT in this region in adult animals (Aghajanian & Marek, 1997). This raised the possibility that 5-HT2A receptors, while having only very modest effect on holding current/membrane potential in layer V pyramidal neurones, could mediate the non-5-HT7 receptor-mediated effect of 5-HT on sEPSCs in developing prefrontal cortex. To test this idea, we examined the ability of the selective 5-HT2A receptor antagonist MDL 100907 to inhibit the 5-HT-induced increase in sEPSCs. Consistent with a role for 5-HT2A receptors, administration of MDL 100907 (300 nm to 1 μm) reduced the effect of 5-HT on the frequency of sEPSCs (5-HT: 39.9 ± 3.9 events s−1 and 5-HT in MDL 100907: 22.1 ± 2.9 events s−1; P < 0.05, Student's paired t test, not shown, n = 7). In contrast to this effect on spontaneous synaptic activity, activation of 5-HT2A receptors with the selective 5-HT2 partial agonist DOI (up to 100 μm) had no detectable effect of the amplitude of evoked EPSCs (n = 7; not shown).
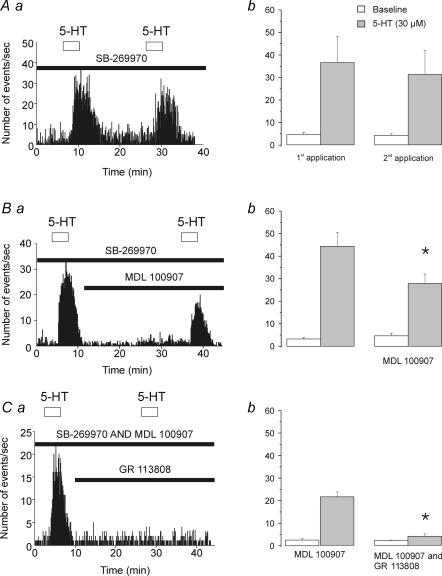
If the activation of 5-HT2A receptors was entirely responsible for the residual, non-5-HT7 receptor-mediated increase in synaptic activity induced by 5-HT, administration of MDL 100907 should completely block the 5-HT-induced increase in synaptic activity during the continuous blockade of 5-HT7 receptors. To test this idea, we incubated slices for a prolonged period of time in the 5-HT7 receptor antagonist SB-269970 (1 μm). Under these conditions, administration of 5-HT (30 μm) induced a robust increase in synaptic activity which showed modest desensitization (Fig. 5A; n = 4; P = 0.36, Student's paired t test) and was blocked by administration of CNQX (5-HT, 35 ± 7.07 events s−1; 5-HT after CNQX, 1.52 ± 0.63 events s−1; P < 0.01, Student's paired t test, not shown; n = 4). Administration of MDL 100907 (300 nm) significantly reduced this 5-HT-induced increase in synaptic activity (38% reduction; Fig. 5B; P < 0.01, Student's t test; n = 8) but again did not completely block it. These results indicate that, while 5-HT2A receptors and 5-HT7 receptors both contributed to the 5-HT-induced increase in synaptic activity, another 5-HT receptor subtype is also involved.
Figure 5. Activation of 5-HT2A and 5-HT4 receptors also contribute to the 5-HT-induced increase in sEPSCs recorded from layer V pyramidal neurones.
These experiments were all conducted in the presence of SB-269970 (1 μm) to block 5-HT7 receptors. Aa, histogram depicting the increase in sEPSC frequency induced by two successive applications of 5-HT (30 μm). Ab, plot comparing the response to successive applications of 5-HT in the presence of SB-269970 (1 μm, n = 4, P = 0.36). Ba, histogram illustrating the effect of the 5-HT2A receptor antagonist MDL 100907 (300 nm) on the 5-HT-induced increase in sEPSC frequency. Bb, plot summarizing the effect of MDL 100907 (1 μm; n = 8). MDL 100907 partially inhibited the effect of 5-HT (P < 0.01; Student's paired t test). Ca, histogram illustrating the effect of GR 113808 (1 μm) co-administered with MDL 100907 (300 nm) on the increase in synaptic activity elicited by 5-HT. Cb, plot summarizing the effect of GR 113808 + MDL 100907 (300 nm; n = 4). Co-administration of these antagonists almost completely suppressed the effect of 5-HT (P < 0.01; Student's paired t test).
Previous studies have documented the presence of 5-HT4 receptors in the rat prefrontal cortex (Waeber et al. 1996). Since 5-HT4 receptors increase neuronal excitability in other brain regions (Andrade & Chaput, 1991; Torres et al. 1994; Ansanay et al. 1995), we reasoned that this receptor subtype might additionally contribute to the increase in synaptic activity induced by 5-HT observed here. Consistent with this possibility, we found that bath administration of the selective 5-HT4 antagonist GR 113808 (1 μm) essentially blocked the residual increase in synaptic activity induced by 5-HT in the presence of SB-269970 and MDL 100907 (Fig. 5C; n = 4, P < 0.01; Student's paired t test). Together, these results indicate that the effect of 5-HT on sEPSCs in developing prefrontal cortex is mediated by 5-HT receptors of the 5-HT7, 5-HT2A and 5-HT4 subtypes.
Selective activation of 5-HT4 and 5-HT2A receptors increases the amplitude of sEPSCs
As outlined above, the activation of 5-HT7 receptors most probably induces an increase in sEPSCs by exciting pyramidal cells in layer V (and/or other layers) that are presynaptic to the recorded neurone. However, the lack of robust inward current elicited by 5-HT in the presence of SB-269970 suggest that neither 5-HT2A nor 5-HT4 receptors induce a depolarization/inward currents of sufficient magnitude to excite pyramidal cells of layer V during the developmental period studied. This suggests that these 5-HT receptors either activate glutamate-releasing elements within the slice other than layer V pyramidal neurones, or increase sEPSCs (frequency and/or amplitude) through a postsynaptic mechanism. To distinguish between these possibilities, we next examined the mechanisms by which activation of 5-HT2A and 5-HT4 receptors increased synaptic activity.
Experiments aimed at identifying the mechanisms whereby 5-HT2A and 5-HT4 increase synaptic activity were complicated by the paucity of selective agonists displaying full intrinsic activity for these receptors. Therefore, for these experiments, we chose to selectively activate 5-HT4 or 5-HT2A receptors using 5-HT as an agonist, while masking 5-HT7 receptors with SB-269970 and either 5-HT2A or 5-HT4 receptors with MDL 100907 and GR 113808, respectively. As illustrated in Fig. 6A, selective activation of 5-HT4 receptors under these conditions induced a robust increase in sEPSC frequency as well as a shift in the sEPSC amplitude distribution towards larger size classes (n = 8; Fig. 6). A qualitatively similar effect was observed following activation of 5-HT2A receptors in isolation (5-HT in the presence of SB-269970 and GR 113808; Fig. 6B; n = 3). These results, in conjunction with the observation that TTX and CNQX completely suppress the effect of 5-HT on sEPSCs (see above), suggest that both 5-HT2A and 5-HT4, like 5-HT7 receptors, increased spontaneous synaptic activity by activating glutamate-releasing elements presynaptic to the recorded neurone.
Figure 6. Selective activation of 5-HT4 or 5-HT2A receptors increases the frequency and amplitude of sEPSCs.
A, effect of 5-HT in the presence of 1 μm MDL 100907 and 1 μm SB-269970 (selective activation of 5-HT4 receptors). Aa, current trace from a layer V pyramidal neurone showing the effect of bath administration of 30 μm 5-HT. Holding potential, −70 mV; holding current, −25 pA. Ab, administration of 5-HT significantly shifts to the left the cumulative distribution of inter-event intervals indicating that 5-HT significantly increased the frequency of sEPSCs (P < 0.05, K-S test). Ac, amplitude distribution of sEPSCs during baseline acquisition (open bars) and in the presence of 5-HT (filled bars). 5-HT shifts the amplitude distribution of synaptic currents towards larger size classes. The noise distribution is shown as a dashed line. Ad, the cumulative distribution of sEPSC amplitudes (P < 0.05; K-S test) indicates that 5-HT increased the amplitude of sEPSCs. B, effect of 5-HT in the presence of 1 μm GR 113808 and 1 μm SB-269970 (selective activation of 5-HT2A receptors). Ba, current trace from a layer V pyramidal neurone showing the effect of bath administration of 30 μm 5-HT. Bb, administration of 5-HT significantly shifted to the left the cumulative distribution of inter-events intervals (P < 0.05, K-S test) indicating that 5-HT significantly increased the frequency of sEPSCs. Bc, amplitude distribution of sEPSCs during baseline (open bars) and in the presence of 5-HT (filled bars). 5-HT shifted the amplitude distribution of synaptic currents towards larger size classes. The noise distribution is shown as a dashed line. Bd, the cumulative distribution of sEPSC amplitudes shows that 5-HT increases the amplitude of sEPSCs (P < 0.05; K-S test).
The effects of 5-HT4 and 5-HT2A receptor activation on sEPSCs do not involve changes in the number or function of postsynaptic AMPA receptors
While the results above point to a mechanism involving action potential-dependent glutamate release, they cannot exclude an additional contribution by postsynaptic mechanisms. Specifically, part of the 5-HT response could have resulted from an additional increase in the sensitivity to synaptically released glutamate. To address this possibility, we next examined the effects of 5-HT on miniature EPSCs (mEPSCs) recorded in the presence of TTX. Since bath administration of TTX (1 μm) reduced the frequency (and amplitude) of sEPSCs (P < 0.05; K-S test; n = 4; not shown), we conducted these experiments in elevated extracellular potassium to increase the frequency of mEPSCs (see Methods). Administration of 5-HT (30 μm; 5 min) failed to alter the amplitude of mEPSCs in 6 out of the 9 cells tested (K-S test; P > 0.05; Fig. 7) while in the remaining 3 cells, it induced a small decrease in mEPSC amplitude (K-S test; P < 0.05; not shown). In addition, in recordings carried out in the presence of continuous 5 mm extracellular potassium, administration of 5-HT failed to alter mEPSC frequency in 3 out of 5 cells, while it slightly decreased it in the remaining 2 cells (K-S test; P < 0.05, not shown). Together, these results show that administration of 5-HT did not increase the frequency or the amplitude of mEPSCs and therefore indicate that neither an increase in the probability of release nor an increase in the number of functional (AMPA receptor-containing) synapses are likely to contribute significantly to the effect of 5-HT on sEPSCs.
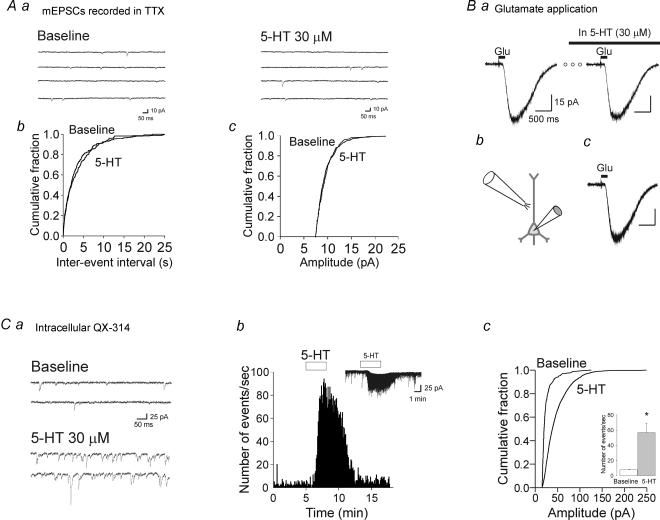
Figure 7. The increase in synaptic activity induced by 5-HT does not involve a postsynaptic facilitation of glutamate-mediated synaptic currents.
A, administration of 5-HT does not increase the frequency or the amplitude of mEPSCs. Aa, current traces recorded from a layer V pyramidal neurones recorded in the presence of TTX (1 μm) and (−)bicuculline (30 μm), before and during bath administration of 5-HT (30 μm). Ab, cumulative probability plot for mEPSC inter-event intervals recorded from the cell in Aa before and during the application of 30 μm 5-HT (P > 0.05; K-S test). Ac, cumulative probability plot for mEPSC amplitudes recorded from the same cell, before and during the application of 30 μm 5-HT (P > 0.05; K-S test). B, administration of 5-HT does not alter the amplitude of the inward current induced by exogenous glutamate. Ba, current traces (averaged from 10 consecutive traces) recorded from a layer V pyramidal neurone showing the rapid inward current induced by a 200 ms long application of glutamate (1 μm; from a perfusion tube located above the apical dendrite of the recorded neurone, as depicted in Bb) before, and during, bath administration of 30 μm 5-HT. Recordings were carried out in the continuous presence of TTX. Bc, the traces in Ba are shown here superimposed. Holding potential, −70 mV; holding current, −10 pA. C, intracellular administration of the sodium channel blocker QX-314 did not prevent the increase in frequency and amplitude of sEPSCs induced by bath administration of 5-HT. Ca, current traces of a layer V pyramidal neurone recorded with an intracellular solution containing QX-314 (5 mm) before and after bath administration of 30 μm 5-HT. Holding potential, −70 mV; holding current, +20 pA. Cb, histogram depicting the increase in sEPSC frequency elicited by 5-HT under these conditions. Inset, current trace for this neurone depicted using a slower time scale. Cc, cumulative distribution of sEPSC amplitudes for this same cell. 5-HT significantly increased the amplitude of sEPSCs (P < 0.05, K-S test). Inset, plot depicting the average increase in sEPSC frequency induced by 5-HT in whole-cell recordings with intracellular QX-314 (n = 11, P < 0.01).
To further test for changes in the function of postsynaptic AMPA receptor function, we determined the effect of 5-HT on the amplitude of inward current induced by brief application of exogenous glutamate. In the presence of TTX, brief application of glutamate (100–250 ms, 1/15 s) from a tube located over layer II–III close to the apical dendrite of the recorded pyramidal neurone, induced rapid inward currents (Fig. 7B). Consistent with the results obtained when examining mEPSCs, the amplitude of these glutamate-induced inward current was not significantly altered by bath administration of 5-HT (30 μm; 8% reduction; P = 0.43; n = 6; Fig. 7B). These results further supported the idea that the effects of 5-HT on sEPSCs are not caused by an altered function/number of postsynaptic AMPA receptors.
Effect of QX-314 on the 5-HT-induced increase in sEPSCs
Finally, it is possible that 5-HT could increase sEPSCs recorded at the soma by modulating the electrical properties of the dendritic tree. In particular, sodium channels have been reported to enhance excitatory postsynaptic potentials (EPSPs) generated in the distal dendrites of layer V pyramidal neurones of the prefrontal cortex (Gonzalez-Burgos & Barrionuevo, 2001), and Aghajanian & Marek (1997) have suggested that 5-HT may take advantage of such a mechanism to amplify sEPSPs originating in distal dendrites. Such an amplification mechanism should, in principle, be prevented by voltage clamping the membrane potential, as done in the current experiments. However, it is possible that this mechanism remained operational in the distal dendrites of the recorded cell where control of membrane voltage may not be optimal. One key prediction of this mechanism is that inactivating or blocking sodium channels in the recorded cell should prevent it. As outlined above (Fig. 1C), the ability of 5-HT to induce an increase in sEPSCs was not prevented by holding the cell at +40 mV, conditions that could be expected to isolate AMPA synaptic currents from the influence of voltage-dependent sodium (and other) channels. Nevertheless, to test this idea further we examined the effect of 5-HT (30 μm in SB-269970) while blocking sodium channels selectively in the neurone under study using the cell-impermeant sodium channel blocker QX-314 (5 μm; dissolved in the intracellular recording solution). Under these conditions, action potential firing was completely suppressed (not shown), yet administration of 5-HT (> 10 min after break-in) still induced robust increases in both the frequency and amplitude of sEPSCs, at least comparable to those seen in controls (Fig. 7C; n = 11 cells). These results, together with the general lack of effect of 5-HT on the amplitude of mEPSCs and exogenous glutamate, do not support the idea that 5-HT facilitates synaptic activity in developing prefrontal cortex by increasing the postsynaptic effects of synaptically released glutamate.
Activation of 5-HT2A receptors is solely responsible for the 5-HT-induced increase in synaptic activity in the adult rat prefrontal cortex
The results outlined above indicated that the activation of 5-HT7, 5-HT2A and 5-HT4 receptors accounts for the effect of 5-HT on spontaneous synaptic transmission in developing rat prefrontal cortex. This observation contrasts with previous reports indicating that, in adult rat prefrontal cortex, the effects of 5-HT on sEPSCs is mediated solely by the activation of 5-HT2A receptors (Aghajanian & Marek, 1997; Marek & Aghajanian, 1999). Therefore, to rule out potential methodological differences, we re-examined the contribution of 5-HT2A receptors to the increase in synaptic activity elicited by 5-HT in adult prefrontal cortex. Application of 5-HT (30 μm) to cortical slices derived from adult rats induced a marked, generally non-desensitizing, increase in the frequency and amplitude of sEPSCs in layer V pyramidal neurones (Fig. 8). As in developing cortex, this increase resulted from an increase in sEPSC frequency as well as a shift in the sEPSC amplitude distribution towards larger size classes (Fig. 8B). However, in contrast to what was observed in the developing prefrontal cortex, this increase in synaptic activity was entirely blocked by bath administration of the 5-HT2A antagonist MDL 100907 (300 nm, n = 3; Fig. 8C).
Figure 8. The 5-HT-induced increase in sEPSC frequency and amplitude in the adult rat prefrontal cortex is entirely dependent on the activation of 5-HT2A receptors.
A, current traces depicting the effect of 30 μm 5-HT on a layer V pyramidal neurone. Traces 1 and 2 depict current traces plotted using an expanded time. Ba, 5-HT significantly shifted to the left the cumulative distribution of inter-event intervals (P < 0.05, K-S test) indicating that 5-HT significantly increased the frequency of sEPSCs. Bb, amplitude distribution of sEPSCs during baseline acquisition (open bars) and in the presence of 5-HT (filled bars). 5-HT increased the frequency of sEPSCs at all amplitudes and shifted the distribution towards larger size classes. The noise distribution is shown as a dashed line. Bc, the cumulative distribution for sEPSC amplitudes is significantly shifted to the right by 5-HT (P < 0.05; K-S test. Holding potential, −70 mV. Ca, the increase in sEPSC frequency induced by bath administration of 5-HT is blocked by the selective 5-HT2A antagonist MDL 100907 (300 nm). Cb, plot comparing the effect of two consecutive applications of 5-HT on sEPSC frequency. The increase in frequency of sEPSCs induced by two successive applications of 5-HT did not show significant desensitization (n = 5; Student's paired t test). Cc, plot summarizing the effect of MDL 100907 (300 nm; n = 3). MDL 100907 completely blocked the effect of 5-HT on sEPSCs (P < 0.01; Student's paired t test).
Discussion
In this study, we have examined the effects of 5-HT on spontaneous synaptic activity in developing (P15–P19) prefrontal cortex using whole-cell recordings in brain slices. Administration of 5-HT induced a robust increase in spontaneous synaptic activity, as revealed by somatic recordings from layer V pyramidal neurones. Surprisingly, in light of the situation that prevails in adult prefrontal cortex, we find that this effect is mediated by the activation of receptors of the 5-HT7, 5-HT2A and 5-HT4 subtypes. These results outline marked developmental differences in the regulation of synaptic activity by 5-HT in prefrontal cortex and raise the possibility that 5-HT7 and 5-HT4 receptors may play a previously unsuspected role in the development of this cortical region.
Administration of 5-HT induced a marked increase in the frequency and amplitude of sEPSCs in layer V pyramidal neurones of the developing rat prefrontal cortex. This response could be recorded at potentials well depolarized from ECl, was blocked by bath application of the AMPA receptor antagonist CNQX, and was insensitive to the GABAA receptor antagonist bicuculline. These results indicate that, under our recording conditions, the synaptic events elicited by 5-HT reflect predominantly the release of glutamate from excitatory afferents impinging upon the recorded neurone. Based upon the sensitivity of this response to SB-269970, we conclude that a large fraction of this increase was attributable to the activation of 5-HT7 receptors. The remaining response could be attributed to the activation of 5-HT4 and 5-HT2A receptors. Simultaneous blockade of all three receptor subtypes essentially abolished the response to 5-HT suggesting that their coactivation probably accounts for most, if not all, of the effect of 5-HT on spontaneous synaptic activity in prefrontal cortex. These results contrast with the situation that prevails in the adult prefrontal cortex where the ability of 5-HT to increase spontaneous activity is entirely dependent on the activation of 5-HT2A receptors (Aghajanian & Marek, 1997; Marek & Aghajanian, 1999; as well as the present work).
A key issue regarding the actions of 5-HT on synaptic activity in the developing prefrontal cortex concerns its cellular basis. In the case of the 5-HT7 receptor-mediated increase in synaptic activity, we found that activation of 5-HT7 receptors induces an inward current that can depolarize and excite layer V pyramidal neurones. Since cortical pyramidal neurones of layer V make multiple (range 4–8) and reciprocal synaptic contacts (Markram et al. 1997b; Feldmeyer & Sakmann, 2000), the 5-HT7 receptor-mediated increase in both the amplitude and frequency of sEPSCs therefore most likely reflects the excitation, and concurrent action potential firing, of interconnected pyramidal cells.
A similar interpretation, however, cannot be readily extended to the 5-HT4 and 5-HT2A receptor-mediated increases in synaptic activity. In our hands, activation of 5-HT2A or 5-HT4 receptors, either individually or together, induce robust increases in synaptic activity while having a minimal effect on membrane potential/holding current (present work and authors' unpublished observations). These observations prompted us to look for alternative mechanisms that could account for the increase in synaptic activity induced by the activation of these two receptor subtypes. In principle, an increase in sEPSCs could be caused, at least in part, by a direct facilitation of the function of postsynaptic AMPA receptors. However, we found that while the activation of 5-HT2A and/or 5-HT4 receptors increased sEPSC frequency and amplitude, it had no effect on the amplitude of mEPSCs, nor on the amplitude of the inward current induced by direct glutamate application. In addition, we found no evidence for a postsynaptic facilitation of sEPSCs mediated through voltage-dependent sodium (or other) channels. Together, these results are best interpreted as indicating that 5-HT2A and 5-HT4 receptors increase synaptic activity predominantly, if not exclusively, by increasing glutamate release from presynaptic terminals impinging on the recorded neurone. This situation is broadly analogous to that encountered in adult prefrontal cortex where 5-HT2A receptor activation leads to a strong increase in synaptic activity in the absence of reliable excitation of pyramidal neurones (Aghajanian & Marek, 1997).
It is possible to imagine several mechanisms that could account for an increase in glutamate release. If 5-HT2A and/or 5-HT4 receptors were located on presynaptic terminals, they could increase synaptic activity simply by facilitating ongoing action potential-mediated glutamate release. However, changes in release probability are thought to be accompanied by changes in mEPSC frequency (Malenka & Nicoll, 1999), yet in the current study, 5-HT failed to increase the frequency of mEPSCs. Furthermore, previous work has shown that 5-HT2A receptors are located predominantly postsynaptically in adult prefrontal cortex (Jakab & Goldman-Rakic, 1998; Cornea-Hebert et al. 1999; Miner et al. 2003) and a similar distribution likely occurs in developing cortex. Finally, in the current study, the selective 5-HT2 agonist DOI failed to increase the amplitude of evoked EPSCs. Together, these results make it unlikely that the increase in sEPSC frequency induced by 5-HT2A or 5-HT4 receptors reflects an increase in the probability of release at glutamate-secreting synapses.
Another possibility is that 5-HT2A and 5-HT4 receptors may excite presynaptic elements impinging onto the recorded neurone. The simplest scenario in this regard is that 5-HT2A and/or 5-HT4 receptors may excite specific subpopulations of pyramidal cells in developing prefrontal cortex. Admittedly, we have not encountered such cells in our recordings in layer V. However, such failure is perhaps not unexpected. The increase in synaptic activity induced by the activation of 5-HT2A and 5-HT4 receptors is generally less than 40 Hz, and hence can be accounted for by the excitation of a very small number of neurones, conceivably as few as one or two. Given the cell packing density of cortex and the pattern of synaptic connectivity between pyramidal cells (Markram et al. 1997a; Reyes & Sakmann, 1999; Feldmeyer & Sakmann, 2000), it can be expected that several hundred pyramidal cells scattered over the cortex may connect with the cell being recorded. Therefore, 5-HT would need to excite only a vanishingly small fraction of the cells in the slice to account for the observed increase in synaptic activity. Such cells are unlikely to be sampled in single-cell studies such as this one. Previous imaging studies sampling large numbers of neurones in developing visual cortex, however, have documented the presence of spontaneously active pyramidal cells (Mao et al. 2001). Similar cells probably exist in prefrontal cortex slices since administration of TTX reduced basal spontaneous synaptic activity (both frequency and amplitude). Even a modest depolarization of such cells would lead to marked increases in action potential discharge frequency and to the appearance of large, presumably multiquantal, synaptic events in connected cells. As such, these cells may represent the cellular substrate for the increase in synaptic activity elicited by 5-HT. Alternatively, it is also possible that a cell subpopulation, other than the canonical layer V cells studied here, although synapsing onto them, may exhibit much more robust depolarizing responses to 5-HT. As a last possible scenario, it has been proposed that pyramidal cells in adult prefrontal cortex may release a retrograde messenger capable of exciting isolated thalamocortical axons present in the slice in response to 5-HT2A receptor activation (Lambe & Aghajanian, 2001; Marek et al. 2001). Such a mechanism could also potentially explain the effects of 5-HT2A (and 5-HT4) receptor activation in the current study, although we favour the simpler hypotheses outlined above.
Surprisingly, the key roles played by 5-HT7 and 5-HT4 receptors in the developing prefrontal cortex appears to have gone undetected in earlier studies (Zhou & Hablitz, 1999; Lambe & Aghajanian, 2001). This is perhaps attributable to the superficial similarity in the responses to 5-HT in the developing and adult cortices, which would lead to the assumption that the effect in developing prefrontal cortex also reflects 5-HT2A receptor activation. This situation was most likely compounded by the very high affinity of several widely used 5-HT2 receptor antagonists for 5-HT7 receptors. Zhou & Hablitz (1999) have reported that 5-HT also increases inhibitory synaptic activity (sIPSCs) in the anterior cingulate cortex by acting on 5-HT2A receptors. In the current study, CNQX completely blocked the 5-HT-induced increase in synaptic activity while bicuculline had little effect. Although our recording conditions were not optimal to detect robust effects on spontaneous GABAergic synaptic transmission (the driving force for chloride ions in our recordings was approximately half of that used by Zhou and Hablitz) we found that very few synaptic events induced by 5-HT reversed polarity near ECl. One possible explanation for this discrepancy is that these authors report a robust effect on sIPSCs only when using a concentration of 5-HT (100 μm) higher than that used here. Future studies will be needed to determine if 5-HT4 and 5-HT7 receptors affect GABAergic synaptic activity in the developing prefrontal cortex.
Functional implications
The emergence of normal adult cortical function is dependent on the appropriate development and refinement of intra- and extracortical synaptic connections during development. Although synapse formation can occur in relative isolation (Bonhoeffer & Yuste, 2002), extrinsic cues have been shown to regulate both synaptic development and circuitry formation. One potential mechanism by which extrinsic serotonergic inputs may regulate the development of cortical connectivity is by regulating the excitability of different cellular elements within the cortex. Thalamic afferents enter the cortex during early development, while reciprocal corticothalamic projection develops later, between P6 and P8 in the cortex (Van Eden, 1986). Interestingly, serotonergic fibres arrive in the cerebral cortex during the prenatal period and this innervation expands in concert with the development of the cortical cell layers (Vu & Tork, 1992; Dori et al. 1996). These developmental processes are accompanied by a dynamic pattern of 5-HT receptor subtype expression. In this study, we have shown that 5-HT exerts profound effects on synaptic activity at a critical time for the formation of synaptic connections in prefrontal cortex. Given the importance of electrical activity for synapse formation and refinement, it is tempting to speculate that 5-HT7, 5-HT2A and 5-HT4 receptors, by regulating synaptic activity between discrete cell populations in developing prefrontal cortex, may play defining roles in the development of synaptic networks in this area.
Acknowledgments
The present study was supported by National Institutes of Health grant MH43985 (R.A.). J.-C.B. is in receipt of a postdoctoral fellowship from the Canadian Institutes for Health Research (CIHR).
References
- Aghajanian GK, Marek GJ. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Andrade R, Chaput Y. 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther. 1991;257:930–937. [PubMed] [Google Scholar]
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L. cAMP-dependent, long-lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci U S A. 1995;92:6635–6639. doi: 10.1073/pnas.92.14.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Lane RD, Rhoades RW. Fenfluramine depletes serotonin from the developing cortex and alters thalamocortical organization. Brain Res. 1995;702:255–260. doi: 10.1016/0006-8993(95)00867-5. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat's somatosensory cortex. J Neurosci. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhoeffer T, Yuste R. Spine motility. Phenomenology, mechanisms, and function. Neuron. 2002;35:1019–1027. doi: 10.1016/s0896-6273(02)00906-6. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT7 receptor-mediate depolarization the anterodorsal thalamus: I. Pharmacological characterization. J Pharmacol Exp Ther. 2001;297:395–402. [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Dori I, Dinopoulos A, Blue ME, Parnavelas JG. Regional differences in the ontogeny of the serotonergic projection to the cerebral cortex. Exp Neurol. 1996;138:1–14. doi: 10.1006/exnr.1996.0041. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Sakmann B. Synaptic efficacy and reliability of excitatory connections between the principal neurones of the input (layer 4) and output layer (layer 5) of the neocortex. J Physiol. 2000;525:31–39. doi: 10.1111/j.1469-7793.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Barrionuevo G. Voltage-gated sodium channels shape subthreshold EPSPs in layer 5 pyramidal neurons from rat prefrontal cortex. J Neurophysiol. 2001;86:1671–1684. doi: 10.1152/jn.2001.86.4.1671. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci U S A. 1998;95:735–740. doi: 10.1073/pnas.95.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci U S A. 2000;97:1951–1952. doi: 10.1073/pnas.070044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci U S A. 2000;97:1841–1844. doi: 10.1073/pnas.97.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–192. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997;20:269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mao BQ, Hamzei-Sichani F, Aronov D, Froemke RC, Yuste R. Dynamics of spontaneous activity in neocortical slices. Neuron. 2001;32:883–898. doi: 10.1016/s0896-6273(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J Physiol. 1997a;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997b;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Ultrastructural localization of serotonin (2A) receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Stuart G. Patch-pipette recordings from the soma, dendrites, and axon of neurons in brain slices. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum Press; 1995. pp. 199–211. [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M, De Maeyer E, Murphy DL, Mossner R, Lesch KP, Hen R, Seif I. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase A and 5-HT transporter knock-out mice. J Neurosci. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Zilles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci. 2001;21:3580–3592. doi: 10.1523/JNEUROSCI.21-10-03580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- Torres GE, Holt IL, Andrade R. Antagonists of 5-HT4 receptor-mediated responses in adult hippocampal neurons. J Pharmacol Exp Ther. 1994;271:255–261. [PubMed] [Google Scholar]
- Van Eden CG. Development of connections between the mediodorsal nucleus of the thalamus and the prefrontal cortex in the rat. J Comp Neurol. 1986;244:349–359. doi: 10.1002/cne.902440307. [DOI] [PubMed] [Google Scholar]
- Vu DH, Tork I. Differential development of the dual serotoninergic fiber system in the cerebral cortex of the cat. J Comp Neurol. 1992;317:156–174. doi: 10.1002/cne.903170205. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rat brain. Behav Brain Res. 1996;73:259–262. doi: 10.1016/0166-4328(96)00108-8. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(Suppl.):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]