Abstract
This study investigated the function of FK506-binding protein (FKBP12.6) using adenoviral-mediated gene transfer to over-express FKBP12.6 (Ad-FKBP12.6) in adult rabbit ventricular cardiomyocytes. Infection with a β-galactosidase-expressing adenovirus (Ad-LacZ) was used as a control. Peak-systolic intracellular [Ca2+] (measured with Fura-2) was higher in the Ad-FKBP12.6 group compared to Ad-LacZ (1 Hz field stimulation at 37°C). The amplitude of caffeine-induced Ca2+ release was also greater, indicating a higher SR Ca2+ content in the Ad-FKBP12.6 group. Voltage clamp experiments indicated that FKBP12.6 over-expression did not change L-type Ca2+ current amplitude or Ca2+ efflux rates via the Na+ –Ca2+ exchanger. Ca2+ transients comparable to those after Ad-FKBP12.6 transfection could be obtained by enhancing SR Ca2+ content of Ad-LacZ infected cells with periods of high frequency stimulation. Line-scan confocal microscopy (Fluo-3 fluorescence) of intact cardiomyocytes stimulated at 0.5 Hz (20−21°C) revealed a higher degree of synchronicity of SR Ca2+ release and fewer non-responsive Ca2+ release sites in the Ad-FKBP12.6 group compared to control. Ca2+ spark morphology was measured in β-escin-permeabilized cardiomyocytes at a free [Ca2+]i of 150 nm. The average values of the spark parameters (amplitude, duration, width and frequency) were reduced in the Ad-FKBP12.6 group. Increasing [Ca2+]i to 400 nm caused coherent propagating Ca2+ waves in the Ad-FKBP12.6 group but only limited Ca2+ release events were recorded in the control group. These data indicate that FKBP12.6 over-expression enhances Ca2+ transient amplitude predominately by increasing SR Ca2+ content. Moreover, there is also evidence that FKBP12.6 can enhance the coupling between SR Ca2+ release sites independently of SR content.
FK506-binding protein 12.6 (FKBP12.6) is a member of a family of FKBP proteins expressed in a wide range of eukaryote cells including striated muscle cells (Marks, 1996). Previous work has suggested that FKBP12.6 modulates cardiac excitation–contraction (E–C) coupling by binding to the SR Ca2+ release channel (ryanodine receptor type 2, RyR2). Generally, three to four FKBP12.6 proteins bind to each RyR2 tetramer (Jeyakumar et al. 2001). The physiological pathways that modulate FKBP12.6 occupancy of RyR2 are currently under study. One possibility is that A-kinase-mediated phosphorylation of RyR2 dissociates FKBP12.6 and alters the properties of the channel (Marx et al. 2000). But recent work has failed to find a link between A-kinase-mediated phosphorylation and FKBP12.6 occupancy (Jiang et al. 2002; Stange et al. 2003). Changes in isolated RyR2 channel kinetics have been observed on addition of FKBP12.6 (Marx et al. 2001) or removal of FKBP12.6 by FK506 or rapamycin (Kaftan et al. 1996; Xiao et al. 1997). FKBP12.6 is also thought to mediate the coupled gating observed in RyR2 channel clusters (Marx et al. 2001). A prolongation of Ca2+ spark duration (Xiao et al. 1997), and increased spark frequency (McCall et al. 1996) were reported in response to FKBP sequestering drugs, but other studies have not shown any effects of FKBP12.6 added to isolated RyR2 (Timerman et al. 1996), or cardiac SR vesicles (Barg et al. 1997). Studies on FKBP12.6 knock-out mice indicate an increase in Ca2+ spark peak and width and slowed spark decay time. Larger Ca2+ transients accompanied these changes (Xin et al. 2002). Similarly, larger Ca2+ transients have also been noted on addition of FK506 or rapamycin to rat cardiomyocytes (McCall et al. 1996; Xiao et al. 1997). In apparent contradiction to these studies, over-expression of FKBP12.6 by up to six times the normal value increased twitch shortening in cultured adult rabbit cardiomyocytes (Prestle et al. 2001). In a recent study comparing mouse and rabbit cardiac myocytes, divergent effects of FK506 on Ca2+ transient amplitude were noted and attributed to species differences in intracellular Na+ levels (Su et al. 2003). The present study examines the effects of FKBP12.6 over-expression on Ca2+ transient, Ca2+ spark and Ca2+ wave characteristics in cultured adult rabbit cardiomyocytes.
Methods
Single ventricular cardiomyocyte isolation from the rabbit heart
New Zealand White rabbits (2–2.5 kg) were killed by administration of an intravenous injection of 500 IU heparin together with an overdose of sodium pentobarbitone (100 mg kg−1). Single rabbit ventricular cardiomyocytes were then isolated as previously described (Loughrey et al. 2002, 2003).
FK506-binding protein 12.6 over-expression within rabbit cardiomyocytes
Recombinant adenoviruses were generated by standard procedures using full-length cDNA of the human FKBP12.6 gene (Prestle et al. 2001). Adenoviral infection with a multiplicity of infection (MOI) of 100 was performed to produce two populations of adenovirus-transfected cardiomyocytes (i) over-expressing FKBP12.6 (Ad-FKBP12.6) and (ii) expressing β-galactosidase as control (Ad-LacZ). Infected cardiomyocytes were subsequently cultured in supplemented M199 medium (Sigma) for 48 h. Verification of transgene expression and virus transfection efficiency has been detailed elsewhere (Prestle et al. 2001). Previous measurements suggest that the level of FKBP12.6 over-expression was approximately 6 times normal values. Ad-LacZ (MOI-100) had no visible effects on the gross morphology of myocytes (compared to untransfected cells) including the density of transverse tubules (see Fig. 3 in Supplementary material, available online). Furthermore, preliminary measurements on intracellular Ca2+ or electrophysiological parameters failed to reveal any effects of infection with the Ad-LacZ virus. Therefore, differences observed between experimental groups (Ad-LacZ and Ad-FKBP12.6) arise from the effects FKBP12.6 over-expression, since infection with the control virus had no apparent effect on any of the parameters measured in this study.
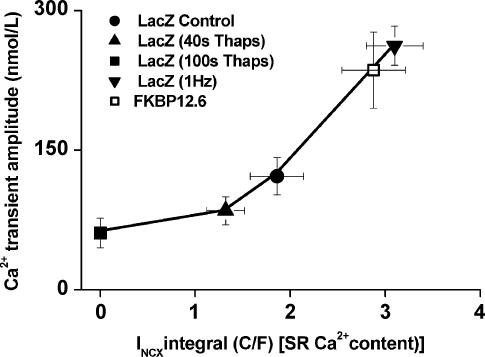
Figure 3. E–C coupling gain.
Relationship between INCX integral (an index of SR Ca2+ content) and Ca2+ transient amplitude for cardiomyocytes from Ad-LacZ (filled symbols) and Ad-FKBP12.6 (open symbol) groups. Control data (circle) were obtained in the steady state; lower SR loads were achieved by exposure to 5 μm thapsigargin for various times as indicated in the inset. Higher SR loads were achieved by 1–2 min periods of depolarizing pulses at 2 × the standard frequency.
Measurement of intracellular Ca2+ within intact cardiomyocytes
Cells were incubated with 1 μm Fura-2 AM for 10 min at 36–37°C, after which Fura-2 AM was removed and the cells incubated for a further 15 min. Cardiomyocytes were allowed to settle on a coverslip, placed on a custom-made microscope stage and superfused with a modified Krebs buffer with the following composition (mm): 140 NaCl, 4 KCl, 1 MgCl2, 5 Hepes, 11.1 glucose, 0.3 NaH2PO4.2H2O, 1.8 CaCl2 at 37°C. Cells were stimulated at 1 Hz with 2 ms duration voltage pulses delivered through parallel platinum wires (stimulation voltage was set to 1.5 times the threshold). The Fura-2 fluorescence was measured at 180 Hz using a spinning wheel spectrophotometer (Cairn Research Ltd UK). After 60–90 s stimulation at 1 Hz, SR Ca2+ load was estimated by rapidly switching the superfusing solution to one containing 20 mm caffeine for 7–8 s. After a 15 s wash to remove caffeine, electrical stimulation was restarted. This protocol was repeated several times on an individual cell. Fura-2 fluorescence measurements were converted to [Ca2+] using a calibration protocol previously outlined (McIntosh et al. 2000). Higher peak systolic and end-diastolic [Ca2+] were observed in cells field stimulated (1 Hz) and perfused with saline at 37°C when compared with voltage-clamped cells (0.5Hz) and perfused with saline at 20–21°C (Figs 1 and 2). The reason for this difference is unknown, but possibilities include systematic errors in calibration of the dye under these two different conditions.
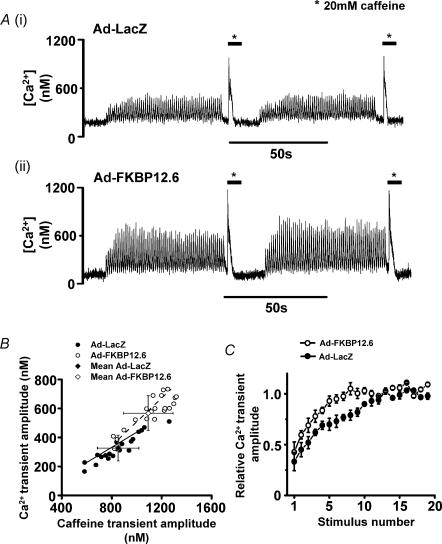
Figure 1. Ca2+ transients and caffeine-induced Ca2+ release.
A, intracellular [Ca2+] signals recorded 48 h after transfection with (i) Ad-LacZ (ii) Ad-FKBP12.6. B, a plot of caffeine-transient amplitude versus Ca2+ transient amplitude for individual cardiomyocytes from both Ad-LacZ (•) and Ad-FKBP12.6 (○) groups. Mean values (± s.d.) for both groups are shown by grey diamond symbols. The lines represent the best-fit linear correlation to the data: continuous line gradient (Ad-LacZ) = 0.50 ± 0.03(± s.d.); dashed line gradient (Ad-FKBP12.6) = 0.61 ± 0.07(± s.d.). C, time course of recovery of Ca2+ transient after a rest period; Ca2+ transients normalized to amplitude achieved in steady state; values are expressed as mean ± s.e.m., n = 20 in each group (○, Ad-FKBP12.6; •, Ad-LacZ).
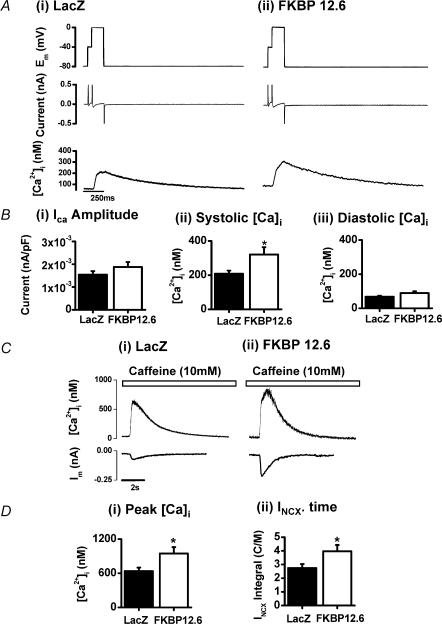
Figure 2. E–C coupling and Ca2+ fluxes.
Depolarization-induced Ca2+ transients recorded from Ad-LacZ- and Ad-FKBP12.6-transfected cardiomyocytes. A, records of membrane voltage (Em), membrane current and [Ca2+]i from single cardiomyocytes (average of 4 sequential signals). B, mean ± s.e.m. values of: (i) L-type Ca2+ current amplitude; (ii) peak systolic [Ca2+]i; (iii) end-diastolic [Ca2+]i. C, recordings of [Ca2+]i and membrane current recorded on rapid application of 10 mm caffeine (as indicated above the records) in Ad-LacZ-transfected (i) and Ad-FKBP12.6-transfected (ii) cardiomyocytes. D, mean ± s.e.m. values: (i) peak [Ca2+]i for Ad-LacZ (n = 21) and Ad-FKBP12.6 (n = 17) groups, P < 0.05); (ii) INCX × time integral, Ad-LacZ (n = 18) and Ad-FKBP12.6 (n = 13) groups (*P < 0.05).
Electrophysiological measurements in rabbit cardiomyocytes
Cardiomyocytes were superfused with a Hepes-based Krebs-Henseleit solution at 20–21°C in a chamber on an inverted microscope. Tetrodotoxin (TTX, 5 μm; Sigma) was included in the perfusate to suppress the inward Na+ current. Voltage clamp was achieved using the whole-cell ruptured patch technique using an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA, USA) operated in switch clamp mode. Pipette resistance was 7–10 MΩ. The pipette solution contained (mm): 20 KCl, 100 potassium aspartate, 20 tetraethylammonium chloride, 10 Hepes, 4.5 MgCl2 (calculated free Mg2+ ≈ 0.9 mm), 4 Na2ATP, 1 di-sodium creatine phosphate (free Na+ = 10 mm), 0.1 EGTA, pH 7.25 with KOH. No correction for liquid-junction potentials was applied, the small DC off-set was observed in Krebs-Henseleit solution was nulled prior to patching on to the cell.
E–C coupling protocol
Rabbit cardiomyocytes were held at −80 mV and the voltage stepped to −40 mV (50 ms) to inactivate the inward Na+ current, before stepping to 0 mV (150 ms) and then returning to −80 mV. This protocol was repeated every 2 s for 80 s to achieve steady-state Ca2+ transients. SR Ca2+ content and Na+ –Ca2+ exchanger (NCX) activity were then estimated by rapidly switching to 10 mm caffeine to cause SR Ca2+ release. In the continued presence of caffeine the SR is unable to re-accumulate Ca2+ and elimination of Ca2+ is mainly due to NCX (Fig. 3). The time course of the decay of [Ca2+] and INCX represent rates of extrusion of Ca2+ from the cell predominately via NCX (Diaz et al. 1997). These signals were fitted to exponential decays over > 80% of their amplitude. The magnitude of non-NCX Ca2+ removal mechanisms was estimated from the Ca2+ decay obtained by rapidly switching to 10 mm caffeine in the presence of 10 mm NiCl2 (Diaz et al. 1997).
E–C coupling studies at a range of SR Ca2+ loads
The relationship between SR Ca2+ content and Ca2+ transient amplitude was investigated by superfusing cardiomyocytes for set periods of time with thapsigargin (5 μm) in a manner similar to that described earlier (Seidler et al. 2003). This achieved a decrease in Ca2+ transient amplitude and SR Ca2+ content as a result of progressive SERCA2a inhibition (Bassani et al. 1993). Complete inhibition of the SR was achieved after 100 s perfusion with thapsigargin; rapidly switching to 10 mm caffeine did not generate a Ca2+ release or INCX. Shorter periods of perfusion of thapsigargin achieved intermediate caffeine responses representing intermediate SR Ca2+ contents. Separate groups of cells were exposed to 40 s and 100 s periods of perfusion with thapsigargin, and the Ca2+ transient amplitude was measured from the last four transients before caffeine application. Enhanced SR Ca2+ load above normal values was achieved by periods of higher frequency stimulation (1 Hz). Periods of 1 Hz stimulation (1–2 min) were used to increase SR content without affecting L-type Ca2+ channel activity or other sarcolemmal flux pathways. Measurements of L-type Ca2+ channel current amplitude and the calculation of the integral of this current were used to verify that none of the above protocols caused a significant change in either of these parameters (results not shown).
Simultaneous field stimulation of intact cardiomyocytes with confocal imaging
Intact cardiomyocytes were loaded with Fluo-3 by incubation with 5 μm Fluo-3AM for 20 min. The incubation medium was removed by centrifugation; the cells were resuspended in modified Krebs solution and incubated for a further 20 min to ensure complete de-esterification of the dye. The cardiomyocytes were subsequently perfused with a modified Krebs solution (20–21°C) and field stimulated at 0.5 Hz. Confocal line-scan images were recorded using a Bio-Rad Radiance 2000 confocal system. Fluo-3 was excited at 488 nm (Kr laser) and measured >515 nm using epifluorescence optics of an inverted microscope with a × 60–1.2 NA water-immersion objective lens. Fluorescence was acquired in line-scan mode at 2 ms line−1; pixel dimension was 0.3 μm (512 pixels scan−1; zoom = 1.4). The scanning laser line was orientated parallel with the long axis of the cell and placed approximately equidistant between the outer edge of the cell and the nucleus/nuclei, to ensure the nuclear area was not included in the scan line. The exact timing of electrical stimulation was marked in the confocal image by activating a light-emitting diode mounted above the cell bath for 2 ms (i.e. the duration of one line-scan) 8 ms before electrical stimulation.
Ca2+ spark and wave measurements in permeabilized cardiomyocytes
Isolated rabbit cardiomyocytes were superfused with a mock intracellular solution and permeabilized using β-escin (Sigma) (see Fig. 1 in Supplementary material, available online). Fluo-3 or Fluo-5F (10 μm) in the perfusing solution was excited using a confocal microscope (see above). To enable this trace to be converted to [Ca2+] a series of calibration solutions were used at the end of each Ca2+ spark measurement period incorporating 10 mm EGTA as previously described (Loughrey et al. 2003) (see Fig. 1 in Supplementary material, available online). In all experiments concerning Ca2+ sparks, the [Ca2+] in the test solution was 145–160 nm. Ca2+ sparks recorded in Fluo-3-containing solutions were quantified using an automatic detection and measurement algorithm adapted from a previously published method (Cheng et al. 1999). All Ca2+ spark measurements were made within 3–4 min of cell permeabilization. This time was standardized to minimize loss of soluble proteins. Previous work on isolated SR vesicles indicates that FKBP loss after vigorous homogenization procedures is minimal (Timerman et al. 1996).
Statistics
Data was expressed as means ± s.e.m. For ion currents, intracellular [Ca2+] and Ca2+ spark parameters, comparisons were performed by using Students' unpaired t test; otherwise Students' paired t test was used and differences were considered significant when P < 0.05. ANOVA statistics with a Tukey's post hoc test were used in cases of multiple comparisons.
Results
Measurement of Ca2+ transients in intact ventricular cardiomyocytes using Fura-2
Figure 1A shows changes in intracellular [Ca2+] ([Ca2+]i) measured in field-stimulated (1 Hz) cardiomyocytes (36–37°C) after transfection with (i) the control virus Ad-LacZ or (ii) Ad-FKBP12.6. Peak systolic [Ca2+] was higher in cardiomyocytes over-expressing Ad-FKBP12.6 compared to the control (Ad-LacZ). After 60 s of continuous stimulation, SR Ca2+ load was assessed by rapid application of 20 mm caffeine for 7–8 s. As shown in Fig. 1A the caffeine-induced Ca2+ transient was larger in cardiomyocytes over-expressing Ad-FKBP12.6 compared to the control virus Ad-LacZ. The averaged data (Fig. 1B) confirms that both the amplitude of the Ca2+ transient and caffeine-induced release are significantly higher in FKBP12.6-over-expressing cardiomyoctes. Diastolic [Ca2+] was not significantly different between the two groups (Ad-FKBP12.6: 220 ± 55 nm, n = 25 versus Ad-LacZ: 250 ± 61 nm, n = 25). Mean Ca2+ transient amplitude and associated caffeine-induced Ca2+ release amplitude for individual cells from both experimental groups are plotted in Fig. 1B to illustrate the relationship between these two parameters. Both groups showed a generally positive correlation between Ca2+ transient amplitude and amplitude of caffeine-induced transients. As shown in Fig. 1B, there was significant overlap between the two groups of data indicating that myocytes from either group with comparable responses to caffeine generated Ca2+ transients of similar amplitudes. However, there was insufficient overlap to be able to determine whether both sets of data points were part of the same approximately hyperbolic relationship.
After a 15 s wash to remove caffeine, electrical stimulation was restarted and the time course of the restoration of the Ca2+ transient noted. As shown in Fig. 1C, a more rapid recovery of systolic [Ca2+] was observed in Ad-FKBP12.6-transfected cells compared to Ad-LacZ cells. The rate constant for the rise in Ca2+ transient amplitude was estimated for each cell. The mean time constant for the recovery of the Ca2+ transient after FKBP12.6 over-expression was significantly shorter (2.31 ± 0.15 s) in the Ad-FKBP12.6 group compared to the AD-LacZ group (4.5 ± 0.24 s, P < 0.05, n = 15 cells in each group).
[Ca2+]i measurements in voltage-clamped rabbit cardiomyocytes
The increase in Ca2+ transient amplitude and caffeine-induced Ca2+ release was also observed in voltage-clamped rabbit cardiomyocytes after Ad-FKBP12.6 transfection (Fig. 2A–D). As illustrated in Fig. 2A, ICa amplitude was monitored by incorporating a prepulse to –40 mV for 50 ms to inactivate the small amount of remaining inward INa (in the presence of 5 μm TTX). In addition to the measurements of ICa amplitude, the time integral of ICa was calculated and converted to a Ca2+ influx (normalized to cell capacitance). Neither the amplitude of ICa (Fig. 2Bi)) nor the integral of the current (result not shown) were different between the two experimental groups. This confirms the absence of an effect of FKBP12.6 over-expression on the ICa in these experiments.
SR Ca2+ content as assessed by rapid application of caffeine in rabbit cardiomyocytes
Application of caffeine caused a rapid increase of [Ca2+]i as a result of SR Ca2+ release. The subsequent reduction of [Ca2+] results from extrusion of Ca2+ across the sarcolemma mainly via NCX. The extrusion of Ca2+ via NCX generates a transient inward current, the amplitude and time course of which was monitored, together with the Ca2+ transient (Fig. 2C). As shown in Fig. 2C (mean values shown in Fig. 2D), the peak of the caffeine-induced Ca2+ release was significantly larger in Ad-FKBP12.6-transfected cardiomyocytes, suggesting an increased SR Ca2+ content. Assuming the caffeine-induced transient inward current is entirely due to the activity of NCX, the time integral of the current can be used as a measure of the amount of Ca2+ extruded by NCX during a caffeine application (an indicator of the SR Ca2+ content). As shown in Fig. 2Dii, the mean integral of the NCX-mediated inward current (INCX) in the Ad-FKBP12.6-transfected group was significantly higher than the control (LacZ) transfected group (normalized to cell capacitance), supporting the conclusion that SR Ca2+ content was significantly higher in FKBP12.6-over-expressing cardiomyocytes.
Sarcolemmal Ca2+ efflux rates in rabbit cardiomyocytes
Sarcolemmal flux rates can be estimated from the time course of the inward current decay and the corresponding decrease in [Ca2+] after rapid application of 10 mm caffeine. As shown in Fig. 2Ci and ii, both INCX and [Ca2+] decayed with a similar time course in Ad-FKBP12.6-transfected cardiomyocytes. These decays were fitted to a single exponential function and mean rate constants were calculated. The rate constants for Ca2+ extrusion were not significantly different in the Ad-LacZ versus Ad-FKBP12.6 groups (INCX, 0.65 ± 0.05 s−1 versus 0.78 ± 0.09 s−1; [Ca2+], 0.37 ± 0.02 s−1 versus 0.45 ± 0.05 s−1, n = 8), suggesting that the rate of extrusion of Ca2+ via NCX was not affected by FKBP12.6 over-expression.
Relationship between SR Ca2+ content and Ca2+ transient amplitude in rabbit cardiomyocytes
To determine the relationship between SR Ca2+ content and Ca2+ transient amplitude, measurements were made using thapsigargin to progressively decrease SR Ca2+ content and short periods of increased stimulation rate (1 Hz) to increase SR Ca2+ content. As shown in Fig. 3, the plot of the INCX integral and Ca2+ transient amplitude for the Ad-LacZ group generated an approximately hyperbolic relationship. The data from the Ad-FKBP12.6 group lay on the relationship described for the Ad-LacZ group. Analysis of ICa indicated that there were no significant changes in the amplitude or time course of this current in any of the data-sets (data not shown). Therefore, the hyperbolic relationship described by the Ad-LacZ data represents the relationship between SR Ca2+ content and the ability of ICa to trigger Ca2+ release from the SR, i.e. E–C coupling ‘gain’ (Bassani et al. 1995). Thus, within the limits of the measurements, the effects of FKBP12.6 over-expression on E–C coupling could be explained by an increase in SR Ca2+ content alone, with no evidence for alteration of E–C coupling ‘gain’. This latter effect would be evident as an Ad-FKBP12.6 data-set that did not lie on the hyperbolic relationship described by the Ad-LacZ data.
Line-scan imaging of Ca2+ transients in cardiomyocytes
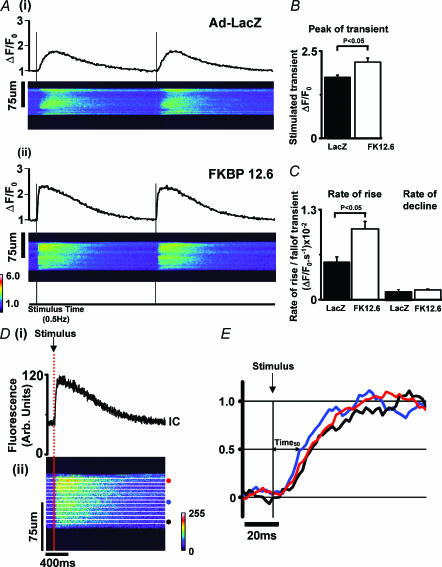
In separate experiments, intact cardiomyocytes were loaded with Fluo-3 and subsequently field stimulated at 0.5 Hz (20–21°C). Fluo-3 fluorescence was monitored with line-scan confocal imaging, and the scan line was arranged parallel to the long axis of the cardiomyocyte. Figure 4A shows the average cellular fluorescence and the corresponding line-scan image from cardiomyocytes transfected with the control virus Ad-LacZ (Fig. 4Ai) and Ad-FKBP12.6 (Fig. 4Aii). The average of six sequential Ca2+ transients from each cardiomyocyte was used to assess the kinetics of the transient. As shown in Fig. 4B, the mean peak (F/F0) of the averaged transient was significantly greater in the Ad-FKBP12.6 group by ∼125%. The rate of rise (ΔF/F0 s−1) of the Ca2+ transient at a standard [Ca2+] (F/F0 = 1.4, ∼300 nm) is greater by ∼190% in the cardiomyocytes over-expressing FKBP12.6 than control cells (Fig. 4C). In contrast, there is no significant difference in the rate of decline of [Ca2+] in the two groups.
Figure 4. Confocal imaging of Ca2+ transients.
A, confocal line-scan images and the corresponding mean cellular fluorescence recorded in Fluo-3 loaded cardiac myocytes after (i) Ad-LacZ infection and (ii) Ad-FKBP12.6 infection (stimulated at 0.5 Hz at 20–21°C). B, mean (± s.e.m.) Ca2+ transient peak (F/Fo values) in the two experimental groups (n = 9 cells in each group). C, mean rate of rise and rate of decline of the Ca2+ transient at a set [Ca2+] (F/Fo = 1.4). D, synchronicity analysis, confocal line-scan image was split into 12 × 20 pixel sections as indicated in the line scan image. The mean fluorescence signal in each 20-pixel section was used to measure the time from stimulus to 50% of the transient amplitude (Time50). An example of 3 selected regions indicated by circles in Dii is shown as 3 separate transients in Fig. 4E.
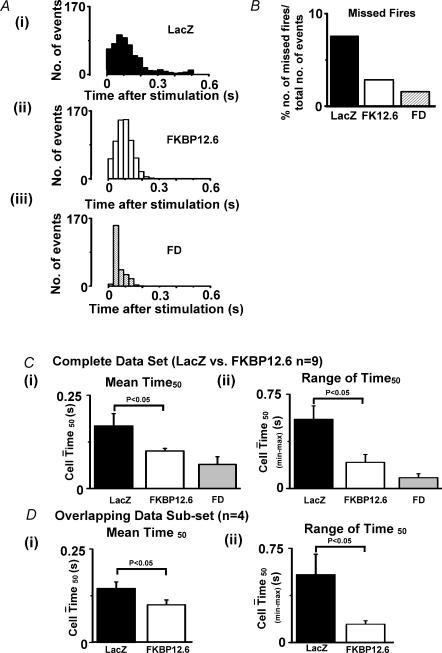
Quantitative assessment of the degree of synchrony of Ca2+ release during stimulated Ca2+ transients using confocal line-scan imaging
On examining the confocal images in Fig. 4A, the cardiomyocytes over-expressing FKBP12.6 appeared to have a more uniform release pattern compared to Ad-LacZ-transfected cardiomyocytes. Some sites along the length of the Ad-LacZ cardiomyocyte did not respond to stimulation at every stimulus, others sites responded after a delay. This observation was quantified in the following manner: (1) the central intracellular area was divided into 12 × 20 pixel bands as illustrated in Fig. 4Dii; (2) the average fluorescence within each 20 pixel band was plotted as 12 individual Ca2+ transients (an example of 3 transients are shown in Fig. 4E); (3) the time from stimulation to 50% of the peak of each transient (Time50) was calculated for each of the 12 bands (see Fig. 4E). These measurements were made on six serial steady-state Ca2+ transients from each cardiomyocyte using an analysis program (written in Labtalk, Origin v6.1). The ranges of Time50 values measured in a number of cardiomyocytes from the two experimental groups were displayed as histograms (Fig. 5A) for: (i) Ad-LacZ cells; (ii) Ad-FKBP12.6; (iii) and freshly dissociated cardiomyocytes (FD), i.e. cells used within 3–4 h of animal kill. Only the Time50 values less then 0.5 s are shown; values greater than 0.5 s are considered ‘missed-fires’ and quantified separately (Fig. 5B). The distribution of Time50 values for the cells infected with Ad-FKBP12.6 had a much narrower profile than the control Ad-LacZ cells. As indicated in Fig. 5B, the corresponding number of missed-fires was lower in Ad-FKBP12.6-infected cells (2.73%) than Ad-LacZ cells (7.5%). In comparison, cardiomyocytes that were freshly dissociated (FD), showed the lowest number of missed-fires (1.43%) of all three groups. In none of the cells studied were missed-fires caused by a permanent absence of release; all sites fired at least once during the period examined. The mean Time50 value (± s.e.m.) and the mean of the range of Time50 values observed in each cell are shown in Fig. 5C. These data indicate that both of these parameters were reduced by FKBP12.6 over-expression, but the values still remained higher than that of the FD group.
Figure 5. Synchronicity of E–C coupling.
A, distribution histograms of Time50 values measured from Ad-LacZ group (n = 9 cells), Ad-FKBP12.6 group (n = 9 cells) and freshly dissociated (FD) cells (n = 4 cells). B, percentage of sites not generating a Ca2+ transient (Time50 > 0.5 s) in the three experimental groups. Ci, mean Time50 and Cii mean range of Time50 values for Ad-Lacz (n = 9 cells), Ad-FKBP12.6 (n = 9 cells) and freshly dissociated cardiomyocytes (FD, n = 4 cells). Di mean Time50 and Dii mean range of Time50 values for a subset of the Ad-LacZ and Ad-FKBP12.6 groups with similar Ca2+ -transient amplitudes.
Subsets of the data were constructed on the basis of Ca2+ transient amplitude; the four largest Ca2+ transients in the Ad-LacZ group were compared with the four smallest Ca2+ transients in the Ad-FKBP12.6. The mean Ca2+ transient peak in these two subgroups was not significantly different (F/F0 = 1.95 ± 0.06 versus 1.93 ± 0.06, Ad-LacZ versus Ad-FKBP12.6), yet, as shown in Fig. 5D, the mean Time50 and range of Time50 values were still significantly lower in the Ad-FKBP12.6 group (mean Time50 is 31% lower than Ad-LacZ; the range of Time50 values is 73% lower than the Ad-LacZ group). Comparable Ca2+ transient amplitudes in the two experimental groups occurred at similar SR Ca2+ contents, as indicated by caffeine-induced release data (see Fig. 1B). Therefore, only a part of the increased synchrony observed on FKBP12.6 over-expression is a consequence of increased SR Ca2+ content. The remaining component may be the result of a direct effect of FKBP12.6 over-expression on the functioning of the RyR2 cluster.
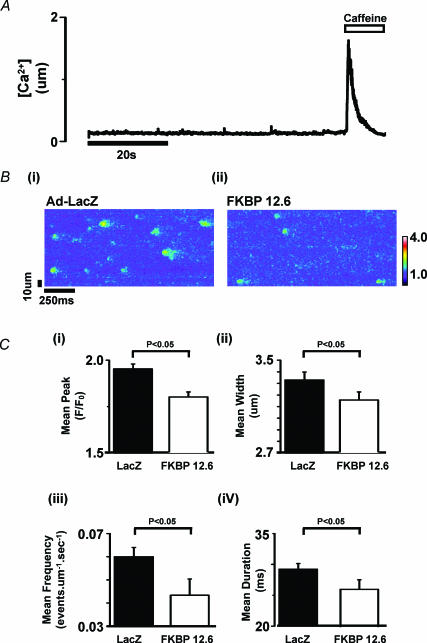
Ca2+ sparks and caffeine-induced Ca2+ release in permeabilized cardiomyocytes over-expressing FKBP12.6
The above results suggest that FKBP12.6 modulates Ca2+ release from cardiac SR. The release of Ca2+ from the SR is via clusters of RyR2 units. The activity of individual RyR2 clusters generates localized Ca2+ release events termed Ca2+ sparks. The frequency, amplitude and time course of Ca2+ sparks contributes to the spontaneous Ca2+ leak from the SR. An investigation of the direct effects of FKBP12.6 over-expression on Ca2+ sparks in intact rabbit cardiomyocytes is complicated by the rapid sarcolemmal extrusion of intracellular Ca2+ and loss of SR Ca2+ during the quiescent periods required for Ca2+ spark recording. For this reason, sarcolemmal fluxes were functionally bypassed by the acute permeabilization of the sarcolemma with β-escin. Under these circumstances, single cardiomyocytes can be superfused with a standardized [Ca2+] and pH in the presence of ATP and creatine phosphate. Ca2+ spark activity was monitored by the inclusion of 10 μm Fluo-3 in the perfusing solution. Examples of line-scan data and the procedure used to calibrate the Ca2+ signal are included in the online Supplementary material (Fig. 1). A typical protocol is shown in Fig. 6A. SR Ca2+ content was assessed at the end of spark recording by rapid application of 10 mm caffeine; the mean values are shown in Fig. 7. To quantify Ca2+ spark activity (Fig. 6Bi and ii) the first 15 s of each line-scan image was analysed using an automated spark detection program adapted from a previously published method for use in permeabilized cardiomyocytes (Cheng et al. 1999). The results collated from a number of cardiomyocytes are shown in Fig. 6C. It can be seen from this summary that the mean values of peak, width, duration and frequency of the Ca2+ sparks are all significantly less in cardiomyocytes over-expressing FKBP12.6 compared to Ad-LacZ; Ca2+ spark peak F/F0 (1.80 ± 0.03 versus 1.96 ± 0.02), width (3.15 ± 0.07 versus 3.33 ± 0.07 μm); duration (26.09 ± 1.5 versus 29.21 ± 1.0 ms) and frequency (0.04 ± 0.007 versus 0.06 ± 0.004 events μm−1 s−1). The spark peak, duration and width distribution histograms are shown separately (see Fig. 2 in Supplementary material, available online).
Figure 6. Ca2+ sparks and caffeine-induced Ca2+ release in permeabilized cardiomyocytes transfected with Ad-LacZ (control) and Ad-FK12.6.
A, Fluo-3 fluorescence signal from 6 μm section of a confocal line-scan image of a permeabilized myocyte to illustrate the experimental protocol. Initial perfusion with 150 mm Ca2+ (0.05 mm EGTA) was followed by rapid application of 10 mm caffeine. B, line-scan epifluorescence image from single permeabilized cardiomyocytes after transfection with Ad-LacZ and Ad-FKBP12.6 perfused with 150 nm Ca2+ C, mean ± s.e.m. values for: (i) peak F/Fo; (ii) spark width (full width half maximal) (iii) spark frequency; (iv) spark duration (full width half maximal). Ad-LacZ group, n = 9 cells; Ad-FKBP12.6, n = 18 cells.
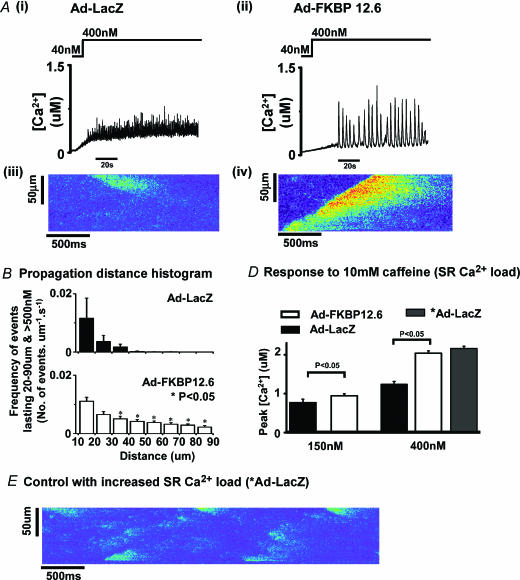
Figure 7. Ca2+ wave characteristics.
Ai and ii, examples of Ca2+ waves recorded in 20 pixel (6 μm) regions of permeabilized cardiomyocytes after increasing the [Ca2+] in the perfusion solution to from 40 to ∼400 nm. Aiii and iv, line-scan images of Ca2+ release events recorded in permeabilized cardiomyocytes from Ad-LacZ and Ad-FKBP12.6 groups. B, distribution histograms of the distance propagated by Ca2+ release events recorded from Ad-LacZ (n = 4 cells) and Ad-FKBP12.6 groups (n = 6 cells). C, mean peak value of the [Ca2+] achieved on rapid application of caffeine in permeabilized cardiomyocytes equilibrated in 150 or 400 nm Ca2+. D, an example of a line-scan image of Ca2+ release events recorded in permeabilized cardiomyocytes (Ad-LacZ) after 1 min pretreatment with high [Ca2+] (∼1 μm) to increase SR Ca2+ content (see C).
Effects of higher [Ca2+] on permeabilized cardiomyocytes overexpressing FKBP12.6
Perfusing with a weakly buffered solution containing ∼400 nm Ca2+ has previously been shown to cause spontaneous Ca2+ release and propagated Ca2+ waves in freshly dissociated permeabilized cardiomyocytes (Loughrey et al. 2002). Figure 7Ai shows signals from a 20-pixel intracellular region of a permeabilized cardiomyocyte infected with Ad-LacZ and Fig. 7Aiii a sample of a line-scan image. Note that in these cells, SR Ca2+ release consists of small amplitude events that did not propagate significant distances (Fig. 7Aiii). However, as illustrated in Fig. 7Aii and iv, the same protocol applied to Ad-FKBP12.6-transfected cardiomyocytes causes coherent large amplitude Ca2+ waves that frequently propagate the entire length of the cardiomyocyte. To quantify the distances propagated by Ca2+ release events, the line-scan images were adjusted to display Ca2+ release events over 500 nm, and the distances propagated by these release events were measured in each cell. The distribution histogram of the release events is shown in Fig. 7B. It is clear from this graph that there were very few release events that propagated further than 40 μm in Ad-LacZ-transfected cardiomyocytes, while a significant number of events propagated up to 90 μm in the Ad-FKBP transfection group. Correspondingly fewer events of distance <10 μm occurred in the FKBP12.6 over-expressing cardiomyocytes compared to the control group (results not shown). Finally, the average peak value of the Ca2+ transient induced by rapid application of 10 mm caffeine (an indication of SR Ca2+ load) is shown in Fig. 7D for the two bathing [Ca2+] used in this study, i.e. ∼150 nm and ∼400 nm. Under both conditions (150 nm and 400 nm) the caffeine-induced Ca2+ release was significantly larger in the Ad-FKBP12.6 group. The presence of Ca2+ waves in FKBP12.6-over-expressing cells could simply be the result of an enhanced SR Ca2+ content in this group. In an attempt to test this hypothesis, the SR Ca2+ load in a group of Ad-LacZ myocytes was temporarily enhanced by short-term (1 min) perfusion with ∼1 μm Ca2+. As shown in Fig. 7E, reperfusion with ∼400 nm Ca2+ generated numerous limited Ca2+ release events but no propagated waves. Rapid caffeine application after 1 min perfusion with 400 nm Ca2+ was used to assess the SR content. As shown in Fig. 7D, the mean peak of the caffeine-induced release after this procedure (*Ad-LacZ) was comparable to that observed after Ad-FKBP12.6 transfection. These results support the conclusion that, independently of the increased SR content, FKBP12.6 over-expression enhances the ability for SR Ca2+ release to propagate along the length of the cultured cardiomyocyte.
Discussion
This study examines the effects of FKBP12.6 over-expression in cardiomyocytes cultured for 48 h. Data from Fura-2-loaded cardiomyocytes indicated that FKBP12.6 over-expression increased the amplitude of the Ca2+ transient and SR Ca2+ content. As shown in Fig. 1, the Ad-FKBP12.6 group produced similar Ca2+ transients for comparable caffeine-induced release, suggesting that there were no major changes in the relationship between SR Ca2+ content and Ca2+ transients in FKBP12.6-over-expressing cells. Measurements on voltage-clamped cardiomyocytes enabled the quantification of Ca2+ influx and efflux. The amplitude and time course of the L-type Ca2+ transient and the rate of extrusion of Ca2+ via NCX was unaffected by FKBP12.6 over-expression. These results support the conclusion that the increased Ca2+ transient amplitude observed during FKBP12.6 over-expression is due to effects on the SR function alone.
The term ‘E–C coupling gain’ represents the effectiveness with which the L-type Ca2+ channel releases Ca2+ from the SR. Previous reports have indicated increased E–C coupling gain on removal of FKBP12.6 (McCall et al. 1996; Su et al. 2003). In a recent study on FKBP12.6 knock-out mice (Xin et al. 2001), the absence of FKBP12.6 was associated with larger Ca2+ transients in cardiomyocytes. On the basis of these studies, over-expression of FKBP12.6 (this study) should decrease E–C coupling gain and therefore decrease the Ca2+ transient amplitude anticipated at a SR Ca2+ load. But the measurements from both stimulated and voltage-clamped myocytes failed to show any significant change in the overall ‘gain’ of the SR Ca2+ release mechanism (Figs 1 and 3).
Effects of FKBP12.6 over-expression on synchronicity of E–C coupling
As shown in Fig. 4, marked asynchrony of Ca2+ release was evident in cardiomyocytes transfected with the control virus Ad-LacZ. Similar asynchrony was observed in myocytes cultured for the same period of time (48 h) without virus infection (results not shown). Asynchronous release has been observed in atrial myocytes and attributed to decreased transverse (T)-tubular system density (Lipp et al. 1996; Kirk et al. 2003). Reduction of T-tubule density occurs progressively during quiescent culture of ventricular myocytes (Mitcheson et al. 1998). Measurements using the membrane dye Di-8-ANEPPS showed a decrease in T-tubular system staining in cells, from ∼14% in freshly dissociated cells to ∼8–9% in Ad-LacZ-transfected cells (see Fig. 3 in Supplementary material, available online). But the T-tubule density was not significantly higher in the FKBP12.6 over-expression group (compared to Ad-LacZ) and therefore it is unlikely that the affects of FKBP12.6 over-expression on E–C coupling are mediated by changes in T-tubular structure. These measurements of T-tubular density were supported by independent measurements of cell capacitance on separate groups of cells; mean values for cells in the Ad-LacZ group were not significantly different from the Ad-FKBP12.6 group (86 ± 5.1 μF, n = 35 versus 82 ± 3.5 μF, n = 31). However, the mean capacitance values of both groups were significantly lower than that of freshly dissociated cells (105 ± 4.3 μF, n = 43, P < 0.05).
In cardiomyocytes over-expressing FKBP12.6, larger Ca2+ transients were accompanied by more synchronous Ca2+ release. The higher degree of synchrony is consistent with the more rapid rate of rise of the Ca2+ transient (Fig. 4B). These results show for the first time that E–C coupling synchrony can be increased by FKBP12.6 over-expression. However, in determining the mechanism it is difficult to distinguish between two options: (i) an increased synchrony as a result of a higher SR Ca2+ content or (ii) a direct effect on the coupling L-type Ca2+ channel activity and SR Ca2+ release. Evidence for the latter comes from comparing Ca2+ transients of similar amplitudes (and therefore similar SR Ca2+ content) as shown in Fig. 5B. In this subset, the Ad-FKBP12.6 group still retained a lower mean Time50 and range of Time50 values than the control group. This latter result indicates that enhanced SR Ca2+ content alone cannot account for the increased synchrony of release and supports the hypothesis that the coupling of L-type Ca2+ channel activity to SR Ca2+ release is enhanced after FKBP12.6 over-expression.
Effect of FKBP12.6 over-expression of Ca2+ sparks in permeabilized cardiomyocytes
Measurement of Ca2+ spark activity in the steady state is problematic in intact rabbit cardiomyocytes because [Ca2+]i and SR Ca2+ content rapidly decreases on cessation of stimulation due to net sarcolemmal efflux. Furthermore, Ca2+ spark parameters are very sensitive to [Ca2+]i (Cheng et al. 1996; Lukyanenko & Györke, 1999), therefore interpretation of results is difficult without knowledge and/or control of the [Ca2+]i in cells from different experimental groups. For these reasons, Ca2+ spark activity was monitored in permeabilized cardiomyocytes at a precisely measured bathing [Ca2+]. Previous work has established that Ca2+ spark characteristics (amplitude, time course and frequency) in permeabilized cells are indistinguishable from those observed in intact cells (Lukyanenko & Györke, 1999) and regulated by known modulators of RyR2 activity (Ca2+ –calmodulin and cyclic-ADPribose) (Lukyanenko & Györke, 1999) but with the added advantage that cytoplasmic conditions can be standardized. As shown in Fig. 6, Ca2+ sparks occurred less frequently and were smaller in amplitude, width and duration in FKBP12.6 over-expressing cardiomyocytes. These results are consistent with previous measurements of Ca2+ sparks on removal of FKBP12.6, since increases in Ca2+ spark frequency (McCall et al. 1996), amplitude (Xin et al. 2002), width and duration (Xiao et al. 1997; Xin et al. 2002) were noted. These reports plus others on isolated RyR2 channels suggest that association of FKBP12.6 with RyR2 would shorten the average duration of a Ca2+ spark by decreasing the channel open time (Xiao et al. 1997) and enhancing the degree of coupled gating (Marx et al. 2001). The overall effects of FKBP12.6 over-expression on Ca2+ spark activity is consistent with the reduction of diastolic SR Ca2+ leak indicated by the more rapid restoration of the steady-state Ca2+ transient amplitude after rest (Fig. 1B). Previous work has suggested that occupancy of RyR2 with FKBP12.6 is close to maximal (4 FKBP12.6: 1 RyR2 tetramer) under basal conditions (Marx et al. 2000). Thus, over-expression of FKBP12.6 would not be expected to boost FKBP12.6: RyR2 stoichiometry significantly. However, two lines of evidence suggest that FKBP12.6 binding to RyR2 is considerably lower than normal in 2-day-cultured myocytes: (i) previous work indicates that rapamycin has no effect on RyR2-mediated Ca2+ leak in 2-day-cultured myocytes, but rapamycin sensitivity is regained after FKBP12.6 over-expression (Prestle et al. 2001); (ii) Ca2+ spark characteristics after 2 days in culture are significantly different from those observed in freshly dissociated myocytes. Ca2+ sparks measured in freshly dissociated myocytes (n = 20 cells) were significantly smaller in amplitude (ΔF/F0 = 1.72 ± 0.02), narrower in width (2.90 ± 0.03 μm), shorter in duration (24.1 ± 0.97 ms) compared to Ad-LacZ-transfected cardiomyocytes. Therefore a possible explanation of the results is that 2-day-cultured cardiomyocytes have a lower than normal FKBP12.6: RyR2 stoichiometry which dramatically increases diastolic Ca2+ leak. Over-expression of FKBP12.6 in these cells returns the FKBP12.6: RyR2 stoichiometry towards normal, reducing diastolic SR Ca2+ leak, increasing SR Ca2+ content and thereby increasing Ca2+ transient amplitude. However, there is a change in the expression of many proteins and myocyte structure during culture, and therefore it would be anticipated that E–C coupling characteristics are distinct from freshly dissociated cells. The extent to which leak via RyR during diastole modulates SR Ca2+ content is under debate. Some studies have shown that under normal conditions, leak through RyR2 during diastole is small relative to other Ca2+ flux mechanisms (Ginsburg et al. 1998). But other studies indicate that Ca2+ leak during diastole can influence SR Ca2+ content (Lukyanenko et al. 2002). If diastolic leak is low, FKBP12.6 over-expression may not dramatically affect SR Ca2+ content. Instead the effects of FKBP12.6 on Ca2+ release during systole may be the predominant influence.
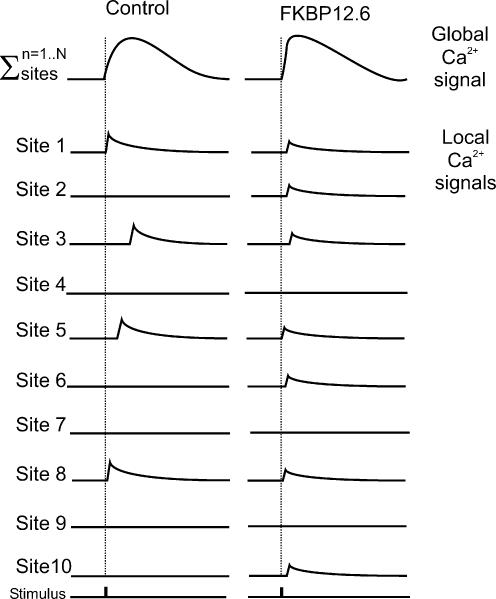
Local gain versus global gain hypothesis
The Ca2+ transient in cardiac muscle is considered to be due to the temporal and spatial summation of Ca2+ sparks triggered by the L-type Ca2+ channel (Cheng et al. 1993). Therefore, if ICa is constant, then changes in the Ca2+ transient amplitude should be reflected in the amplitude of the spontaneously occurring Ca2+ spark. On this basis, the two main experimental observations reported in this study are initially difficult to reconcile, i.e. (i) the enhanced Ca2+ transient amplitude and (ii) decreased Ca2+ spark amplitude. However, if (as observed) FKBP12.6 over-expression causes increased synchronicity and fewer missed-fires of Ca2+ release events, the two effects described could be reconciled. As shown diagrammatically in Fig. 8 the total number and synchrony of release events in each systole is increased after FKBP12.6 over-expression, so although the events are individually smaller the increase in the number of release events results in a comparable global transient. This hypothesis may explain the previously published work suggesting FKBP12.6 decreases E–C coupling gain (McCall et al. 1996). If increased FKBP12.6-RyR binding occurred in cardiac cells that already have highly synchronous activation, there is little scope to increase the number and synchronicity of sites. Under these circumstances the predominant effect is the decreased spark size, leading to a smaller than expected Ca2+ transient (i.e. decreased E–C coupling gain).
Figure 8. Ca2+ transient arising from the temporal and spatial summation of Ca2+ sparks.
Top traces represent the global Ca2+ transient from two different cells. The individual sites represent 10 possible release sites within each cell. The summation of the events at all 10 sites generates the global transient. Two situations are considered: (i) the control situation (Ad-LacZ group) where few sites fire with variable delay but with large individual release events; (ii) the situation after FKBP12.6 over-expression, where the majority of sites fire, with a short delay and small individual release events.
Effects of FKBP12.6 over-expression on spontaneous SR Ca2+ release
Previous work has shown that increasing cytosolic [Ca2+] above 200 nm in permeabilized cardiomyocytes initiates spontaneous SR Ca2+ release and propagated Ca2+ waves similar to that observed in Ca2+ -overloaded intact cardiomyocytes (Lukyanenko & Györke, 1999; Loughrey et al. 2002). However, as shown in Fig. 7, spontaneous Ca2+ release in permeabilized cardiomyocytes after 2 days of quiescent culture consisted of small amplitude events that propagated only limited distances within the cytosol. In contrast, when cardiomyocytes over-expressing FKBP12.6 were perfused with identical solutions, coherent Ca2+ waves that propagated along the majority of the length of the myocyte occurred in a similar fashion to those observed in freshly dissociated cells. Measurements of caffeine-induced Ca2+ release suggested that SR Ca2+ content was higher than in the Ad-LacZ-transfected cardiomyocytes when perfused with 400 nm Ca2+. The frequent low-amplitude Ca2+ release events observed in Ad-LacZ-transfected cardiomyocytes would suggest a large diastolic Ca2+ leak and explain the reduced ability of the SR to retain Ca2+. FKBP12.6 over-expression not only reduced the number of release events, allowing the SR Ca2+ content to increase to a higher steady state, but also permitted the generation of large amplitude propagated Ca2+ waves. Low amplitude Ca2+ release may not generate a sufficiently large cytosolic Ca2+ signal at the adjacent release site to allow propagation (Cheng et al. 1996; Subramanian et al. 2000; Smith & O'Neill, 2002). This appears to be the case in the Ad-LacZ group; the higher SR Ca2+ content in FKBP12.6 over-expressing cells supports sufficiently large SR Ca2+ release to allow propagation. However, the effect is not simply related to SR Ca2+ content since manoeuvres that increase SR Ca2+ content to comparable levels to the Ad-FKBP12.6 group did not restore Ca2+ waves (Fig. 7D). Thus FKBP12.6 over-expression must also alter the characteristics of the RyR2 cluster and its sensitivity to cytoplasmic Ca2+.
Spontaneous Ca2+ waves are thought to be beneficial to the cell under conditions of Ca2+ overload since the transient increases of [Ca2+]i are an efficient method of stimulating Ca2+ efflux from the cardiomyocyte while preventing the development of a sustained contraction (Diaz et al. 1997; Kort & Lakatta, 1988; Loughrey et al. 2002). However, spontaneous Ca2+ waves also generate potentially arrhythmic depolarizing currents and are thought to be a common cause of triggered arrhythmias. Recent work on myocardium from failing human hearts and animal models of heart failure have shown a reduction in FKBP12.6 binding to RyR2 (Marx et al. 2000). This change causes increased diastolic SR Ca2+ leak (Yano et al. 2000) and therefore reduced twitch amplitude (Marx et al. 2000). The results presented in this study support the idea that increasing FKBP12.6: RyR2 stoichiometry will, under some circumstances, increase Ca2+ transient amplitude and may be a possible therapeutic approach in heart failure (Yano et al. 2003). However, the data also indicate the possible detrimental consequences of this approach. If the mean cytosolic Ca2+ is above a crucial level as a result of other pathological changes, increasing FKBP12.6: RyR2 stoichiometry may increase SR Ca2+ content and alter RyR2 function in such a way as to increase the amplitude of spontaneous Ca2+ release from the SR and consequent arrhythmic depolarizing currents.
Acknowledgments
Expert technical assistance from Aileen Rankin and Anne Ward is gratefully acknowledged. This study was supported by grants from the Wellcome Trust, British Heart Foundation (BHF) the Deutsche Forschungsgemeinshaft (DFG) and German National Genome Research Network (NGFN). An Engineering and Physical Sciences Research Council studentship (EPSRC) and the Faculty of Veterinary Medicine, Glasgow University, supported C.L. and a BHF PhD scholarship supported S.L.W.M. The authors wish to thank Mhorven Laurie for providing data on cultured cardiomyocytes structure.
Supplementary material
The online version of this paper can be found at:
DOI: 10.1113/jphysiol.2003.057166 and contains supplementary material entitled:
Expanded Materials and Methods: Calibration of the Fura-2 fluorescence signal in intact myocytes. They can also be accessed at http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp202/tjp202sm.htm
References
- Barg S, Copello JA, Fleischer S. Different interactions of cardiac and skeletal muscle ryanodine receptors with FK-506 binding protein isoforms. Am J Physiol. 1997;272:C1726–C1733. doi: 10.1152/ajpcell.1997.272.5.C1726. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol. 1993;265:C533–C540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–C1329. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J. 1999;76:606–617. doi: 10.1016/S0006-3495(99)77229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz ME, Trafford AWO, Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol 501. 1997;1:3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg KS, Weber CR, Bers DM. Control of maximum sarcoplasmic reticulum Ca load in intact ferret ventricular myocytes. Effects of thapsigargin and isoproterenol. J Generalphysiol. 1998;111:491–504. doi: 10.1085/jgp.111.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar LH, Ballester L, Cheng DS, McIntyre JO, Chang P, Olivey HE, Rollins-Smith L, Barnett JV, Murray K, Xin HB, Fleischer S. FKBP binding characteristics of cardiac microsomes from diverse vertebrates. Biochem Biophys Res Commun. 2001;281:979–986. doi: 10.1006/bbrc.2001.4444. [DOI] [PubMed] [Google Scholar]
- Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circulation Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- Kaftan E, Marks AR, Ehrlich BE. Effects of rapamycin on ryanodine receptor/Ca2+ -release channels from cardiac muscle. Circulation Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- Kirk MM, Izu LT, Chen-Izu Y, McCulle SL, Wier WG, Balke CW, Shorofsky SR. Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes. J Physiol. 2003;547:441–451. doi: 10.1113/jphysiol.2002.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort AA, Lakatta EG. Spontaneous sarcoplasmic reticulum calcium release in rat and rabbit cardiac muscle: Relation to transcient and rested-state twitch tension. Circulation Res. 1988;63:969–979. doi: 10.1161/01.res.63.5.969. [DOI] [PubMed] [Google Scholar]
- Lipp P, Huser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol. 1996;497:589–597. doi: 10.1113/jphysiol.1996.sp021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrey CM, MacEachern KE, Cooper JM, Smith GL. Measurement of the dissociation constant of Fluo-3 for Ca2+ in isolated rabbit cardiomyocytes using Ca2+ wave characteristics. Cell Calcium. 2003;34:1–9. doi: 10.1016/s0143-4160(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Loughrey CM, MacEachern KE, Neary P, Smith GL. The relationship between intracellular [Ca2+] and Ca2+ wave characteristics in permeabilised cardiomyocytes from the rabbit. J Physiol. 2002;543:859–870. doi: 10.1113/jphysiol.2002.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J Physiol. 1999;521:575–585. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner S, Györke I. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+ -sensitive leak in rat ventricular myocytes. Biophys J. 2002;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall E, Li L, Shannon TR, Blatter LA, Bers DM. Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circulation Res. 1996;79:1110–1121. doi: 10.1161/01.res.79.6.1110. [DOI] [PubMed] [Google Scholar]
- McIntosh MA, Cobbe SM, Smith GL. Heterogeneous changes in action potential duration and intracellular Ca2+ in left ventricular myocytes sub-types from rabbits with heart failure. Cardiovascular Res. 2000;45:379–409. doi: 10.1016/s0008-6363(99)00360-0. [DOI] [PubMed] [Google Scholar]
- Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circulation Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKB12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: Future applications, culture methods, morphological and electrophysiological properties. Cardiovascular Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- Prestle J, Janssen PML, Janssen AP, Zeitz O, Lehnart SE, Bruce L, Smith GL, Hasenfuss G. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated ca2+ leak from the sarcoplasmic reticulum and increases contractility. Circulation Res. 2001;88:188–194. doi: 10.1161/01.res.88.2.188. [DOI] [PubMed] [Google Scholar]
- Seidler T, Miller SLW, Loughrey CM, Kania A, Burow A, Kettlewell S, Teucher N, Wagner S, Kögler H, Meyers MB, Hasenfuss G, Smith GL. The effects of adenoviral-mediated sorcin over-expression on excitation-contraction coupling in isolated rabbit cardiomyocytes. Circulation Res. 2003;93:132–139. doi: 10.1161/01.RES.0000081596.90205.E2. [DOI] [PubMed] [Google Scholar]
- Smith GL, O'Neill SC. A comparison of the effects of ATP and tetracaine on spontaneous Ca2+ release from rat permeabilised cardiac myocytes. J Physiol. 2002;534:37–47. doi: 10.1111/j.1469-7793.2001.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- Su Z, Sugishita K, Li F, Ritter M, Barry WH. Effects of FK506 on [Ca2+]i differ in mouse and rabbit ventricular myocytes. J Pharmacol Exp Ther. 2003;304:334–341. doi: 10.1124/jpet.102.041210. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Viatchenko-Karpinski S, Lukyanenko V, Györke S. Underlying mechanisms of symmetric calcium wave propagation in rat ventricular myocytes. Biophys J. 2000;80:1–11. doi: 10.1016/S0006-3495(01)75991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht GJ, Fleischer S. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- Xiao R-P, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. J Physiol. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- Xin HB, Senbonmatsu T, Wang Y-X, Copello J, Ji G-J, Collier ML, Deng K-Y, Jeyakumar L, Sutherland M, Magnuson M, Inagami T, Fleischer S. Sex-specific cardiac hypertrophy in mail mice lacking FK506 binding protein 12.6. Biophys J. 2001;80:579a. [Google Scholar]
- Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okauda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Kohno M, Matsuzaki M. FKBP12.6-mediated stabilization of calcium-release channel (Ryanodine Receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, Kobayashi S, Hisamatsu Y, Yamamoto T, Noguchi N, Takasawa S, Okamoto H, Matsuzaki M. Altered stoichiometry of fkbp12.6 versus ryanodine receptor as a cause of abnormal Ca2+ leak through ryandone receptor in heart failure. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]