Abstract
An ex vivo, vagally innervated, lung preparation was used to address the hypothesis that vagal C-fibres comprise at least two distinct phenotypes. Histological and extracellular electrophysiological experiments revealed that vagal C-fibres innervating the pulmonary system are derived from cell bodies situated in two distinct vagal sensory ganglia. The jugular (superior) ganglion neurones project C-fibres to both the extrapulmonary airways (larynx, trachea and bronchus) and the lung parenchymal tissue. By contrast, C-fibres from nodose (inferior) neurones innervate primarily structures within the lungs. Histologically, nodose neurones projecting lung C-fibres were different from the jugular neurones in that they were significantly less likely to express neurokinins. The nerve terminals within the lungs of both nodose and jugular C-fibres responded with action potential discharge to capsaicin and bradykinin application, but only the nodose C-fibre population responded with action potential discharge to the P2X selective receptor agonist α,β-methylene-ATP. Whole cell patch clamp recording of capsaicin-sensitive nodose and jugular ganglion neurones retrogradely labelled from the lung tissue revealed that, like the nerve terminals, lung specific nodose C-fibre neurones express functional P2X receptors, whereas lung specific jugular C-fibres do not. The data support the hypothesis that both neural crest-derived neurones (jugular ganglia) and placode-derived neurones (nodose ganglia) project C-fibres in the vagus, and that these two C-fibre populations represent distinct phenotypes.
Sherrington (1906) categorized sensory nerves that interact with the outside environment as ‘surface receptors’. Those sensory receptors found deep in tissues that do not interact directly with the environment, but rather with ‘changes going on within the microcosm itself’, he termed ‘proprio-receptive’ from proprius (one's own) (Sherrington, 1906). He further subclassified surface receptors as those found on the outer surface of the body as ‘extero-receptors’ and those that interact with the outside environment at the body's internal surfaces such as the lining of the airways and alimentary tract as ‘intero-receptors’. Accordingly, intero-receptors and viscero-receptors are often considered synonymous. In some cases, however, visceral sensory nerves are not localized to the surface of internal organs but rather are situated deep within the tissue such that they respond mainly to stimuli within the ‘microcosm itself’.
The pulmonary system provides a good example of an organ that is innervated with sensory nerves situated both along the mucosal surface and deep within the parenchymal tissue. Bronchopulmonary afferent nerves comprise C-fibres and mechanosensitive A-fibres (Widdicombe, 2001). The vagus nerve provides sensory C-fibres that innervate the mucosa of the airways (classical intero-receptors), and also deeper structures within the lung parenchyma. The C-fibres in both compartments respond to noxious stimuli such as capsaicin, and when stimulated are thought to evoke classical defensive reflexes (Coleridge & Coleridge, 1984). These reflexes include apnoea, bradycardia, systemic hypotension, increases in parasympathetic tone with bronchoconstriction, and possibly cough. The bronchopulmonary C-fibres are therefore often referred to as bronchopulmonary nociceptors.
The idea that the C-fibres in the lung parenchyma are phenotypically different from those lining the internal surface of the airways was first raised by the seminal studies of the Coleridges and their colleagues (Coleridge et al. 1976; Coleridge & Coleridge, 1977, 1984). They noted that C-fibres could be divided into those that are most accessible to chemicals added to the pulmonary circulation (termed pulmonary C-fibres), and those most accessible to chemicals added to the systemic or bronchial circulation (termed bronchial C-fibres). Most of the pulmonary C-fibres are likely to be situated deep in the lung and may be synonymous with Paintal's juxtacapillary fibres (J-receptors) (Paintal, 1973). The bronchial C-fibres are thought to be those in the mucosal plexus of large airways.
The important question that has not been directly answered in the lungs or any other tissue is whether the intero-receptive C-fibres in the epithelial surface are phenotypically different from the proprio-receptive-like C-fibres located deep within the tissue. Pharmacological studies indicate that pulmonary and bronchial C-fibres may indeed represent distinct types of nerves. Although chemicals such as capsaicin stimulate both bronchial and pulmonary C-fibres, inflammatory mediators such as bradykinin, prostaglandins and adenosine, depending on the species and stimulus, seemed to preferentially stimulate one type over the other (Coleridge et al. 1976; Kaufman et al. 1980; Hong et al. 1998). It has been reasonably argued, however, that the difference in chemical sensitivities of the C-fibres studied in vivo may not represent differences in the nerves per se, but rather may reflect differences in access of the stimulus to the receptive field, or indirect effects of the chemicals on C-fibres in different locations within the organ (Lee & Pisarri, 2001; Undem & Carr, 2001).
In the present study, experiments were designed to directly address the hypothesis that vagal C-fibres innervating the lungs represent two distinct phenotypes. Our data indicate that two classes of vagal C-fibres can indeed be distinguished based on distribution within the lungs, chemical activation profile, and neuropeptide content. Moreover, based on the location of their respective cell bodies, we hypothesize that these two types of vagal nociceptive C-fibres have distinct embryonic origins.
Methods
All experiments were approved by the Johns Hopkins Animal Care and Use Committee. Male Hartley guinea-pigs (Hilltop Laboratory Animals, Inc., Scottsdale, PA, USA) weighing 100–300 g were used.
Retrograde tracing and histology
In a previous study we analysed the vagal ganglion neurones retrogradely labelled from a 1 μl injection of Fast Blue into the guinea-pig tracheal wall (Riccio et al. 1996a). In the present study we used nearly an identical technique to label neurones that project fibres deep into the lung. In this way a comparison of tracheal and pulmonary neurones can be made. Guinea-pigs were anaesthetized with intramuscular injection of ketamine (50 mg kg−1) and xylazine (2.5 mg kg−1). Animals were transdermally injected with 10 μl of 3% True Blue into the right and left lungs using a 50 μl Hamilton syringe (True Blue was used because Fast Blue became unavailable). In postmortem analysis we found that in more than half the animals, the dye had diffused into the large bronchi and vessels. To limit the dye diffusion within lung parenchyma, other animals were injected at two sites of the right lung with 1 μl of 3% True Blue containing 1% dimethyl sulfoxide with a 5 μl Hamilton syringe. Seven to 14 days after injection the animals were killed by an overdose of sodium pentobarbital (150 mg kg−1, intraperitoneally) and perfused via the ascending aorta with a rinsing solution containing procaine (100 mg ml−1) and heparin (10 000 IU l−1) in 0.1 m phosphate buffered saline (PBS; pH 7.4) followed by 4% paraformaldehyde (PFA). Intrathoracic organs and sensory vagal ganglia (jugular and nodose ganglia) were isolated and postfixed in 4% PFA for 2 h at 4°C and rinsed three times in PBS. The ganglia from only those animals in which we found the dye limited in lung parenchyma and not diffused to main bronchi, heart or oesophagus were included in the study. This represented four animals, two injected with 1 μl and two with 10 μl of dye. In the other animals the injection either missed the lung parenchyma, or was found to diffuse into large bronchi. Continuous serial cryostat sections (12 μm thick) of the vagal ganglion (nodose or jugular) were thaw-mounted on four different slides, such that the first slides had sections 1, 5, 9…, the second 2, 6, 10… and so on; alternate slides were used for the analysis. Slides were mounted and rinsed with water and PBS and incubated with goat serum (10%) diluted in PBS containing Tween 20 (0.5%) and bovine serum albumin (BSA, 1%) at room temperature for 1 h. Sections were then covered with primary antibodies diluted in PBS containing Triton X-100 (0.3%) and BSA (1%) (Triton–BSA–PBS) for 24 h at 4°C. After rinsing (3×) with Triton–BSA–PBS the sections were covered with secondary (antiprimary) antibodies diluted in Triton–BSA–PBS for 2 h at room temperature. The sections were then rinsed twice with PBS, once with saline buffered with phosphate to pH 8.6, then coversliped with antifade glycerol (antifade kit Fluoro-mount, Molecular Probes, Eugene, OR, USA).
Double staining for neurofilament 160 kDa (NF)- and substance P (SP) immunoreactivity was performed using mouse anti-NF (2.5 μg ml−1, Chemicon, Temecula, CA, USA) and rat anti-SP (5 μg ml−1, Chemicon) as primary antibodies, and goat antirat Alexa Fluor 594-labelled antibody (20 μg ml−1, Molecular Probes) and goat antimouse Alexa Fluor 488-labelled secondary antibody (10 μg ml−1, Molecular Probes) used as secondary antibodies. Double staining for isolectin B4 and NF immunoreactivity was performed using mouse anti-NF (2.5 μg ml−1, Chemicon) and FITC-labelled isolectin B4 (7.5 μg ml−1, Sigma-Aldrich, St Louis, MO, USA) as primary antibodies, and antimouse Alexa Fluor 594-labelled antibodies (10 μg ml−1, Molecular Probes) as the secondary antibody.
Sections were examined under epifluorescence (Olympus DX60) using appropriate filter combinations for Fast Blue and True Blue (excitation filter 330–385 nm, barrier filter 400–420 nm), for Alexa Fluor 488 and FITC (excitation filter 450–480 nm, barrier filter 500–515 nm) and for Alexa Fluor 594 (excitation filter 510–550 nm, barrier filter 570–590 nm). Trachea, bronchi and lung parenchyma as well as heart, oesophagus and adjacent connective tissue were cut in 1–2 mm sections and evaluated for the presence and localization of the neuronal tracer.
We have previously reported that <5% of the large diameter NF-positive neurones in the nodose ganglia do not express appreciable substance P immunoreactivity (Riccio et al. 1996a; Hunter & Undem, 1999). However in the presence of viral or allergic pulmonary inflammation, substance P production is induced in this population of neurones (Carr et al. 2002; Myers et al. 2002). In two animals injected with the tracer dye we noted that more than 10% of the NF-positive neurones were also substance P positive. We hypothesize that this was reflecting ongoing lung inflammation and therefore data from these animals were not used in our studies.
Extracellular recording
Larynx, trachea and mainstem bronchus
The method for extracellular recordings from the vagal sensory neurones projecting to the guinea-pig trachea has been described in detail previously (Riccio et al. 1996b). Briefly, guinea-pigs were killed by CO2 inhalation and exsanguination. The trachea, larynx and right mainstem bronchus with intact right-side extrinsic vagal innervation (including right jugular and nodose ganglia) were dissected and the tissue was pinned in a small Sylgard-lined Perspex chamber filled with Krebs bicarbonate solution (KBS; composed of: NaCl, 118 mm; KCl, 5.4 mm; NaH2PO4, 1.0 mm; MgSO4, 1.2 mm; CaCl2, 1.9 mm; NaHCO3, 25.0 mm; dextrose, 11.1 mm; and gassed with 95% O2–5% CO2, pH 7.4, 25°C) containing indomethacin (3 μm). The chamber had two compartments, a larger tissue compartment in which the airways and vagus were pinned, and a second smaller recording compartment. A longitudinal cut was made along the ventral surface to open the larynx, trachea and bronchus and the airways were pinned mucosal side up. The rostral-most aspect of the vagus nerve containing the nodose and jugular ganglia was pulled through a small hole that separated the two compartments. The nodose and jugular ganglia were pinned to the floor of the recording compartment for extracellular recording of the nerve activity. The hole was then sealed with petroleum jelly to prevent diffusion of fluids between the compartments. The tissue and recording compartments were separately superfused with KBS (pH 7.4, 37°C, 4 ml min−1).
Lung preparation
A modification of the method described above was used for extracellular recording from the vagal sensory neurones projecting to the guinea-pig lungs. Guinea-pigs were killed by CO2 inhalation and exsanguination. The blood from the pulmonary circulation was mostly washed out by in situ perfusion with 20–30 ml of KBS, through a feeding needle inserted into the right heart chamber. The lungs were gently inflated with KBS (∼5–10 ml, 2–3 times) using the feeding needle inserted into the trachea through the larynx. The trachea and lungs with intact right-side extrinsic vagal innervation (including right jugular and nodose ganglia) were dissected (Fig. 1). The lungs and vagus were pinned in the tissue compartment and the nodose and jugular ganglia were pinned in the recording compartment. The hole between compartments was sealed with petroleum jelly. A piece of PE60 tubing was inserted into the pulmonary artery and connected to a Venoset Microdrip i.v. set for continuous perfusion with KBS (37°C, 4 ml min−1) of the pulmonary circulation. A piece of PE100 tubing was inserted into the trachea and connected to an infusion pump (model 944, Harvard Apparatus, Holliston, MA, USA) for continuous perfusion with KBS (37°C, 2 ml min−1) of the lungs. The lungs were punctured with a 27-gauge needle (1–2 mm deep, 2–8 per lobe) to allow perfusing KBS to exit the tissue. The tracheal perfusion pressure was recorded by a P23AA pressure transducer (Statham, Hata Rey, PR, USA) attached to the model TA240 chart recorder (Gould, Valley View, OH, USA). The resting tracheal perfusion pressure averaged approximately 5 cm of H2O. In addition to the lung perfusions, the tissue and recording chambers were separately superfused with KBS (pH 7.4, 37°C, 4 ml min−1). The pulmonary arterial perfusion of 4 ml min−1 was empirically chosen as that considered to be adequate to provide for tissue oxygenation (we were able to study the function of the nerve terminals for over 2 h without noticeable changes in function). The rate chosen also provided rapid and reproducible drug delivery (<2 ml min−1 provided poor drug delivery).
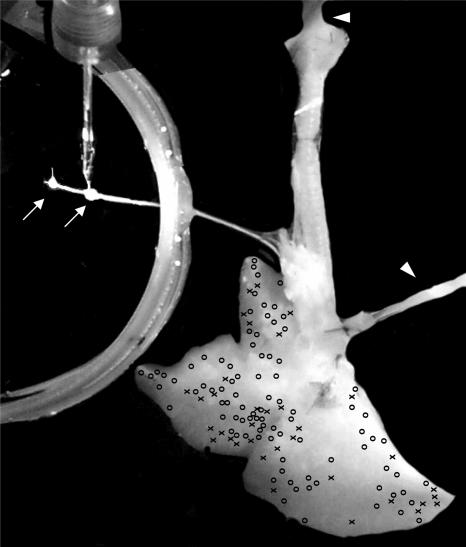
Figure 1. Photograph of the experimental preparation used to study the electrophysiology of jugular and nodose afferent nerves with receptive fields within the right lungs.
Note that tubing is secured in the trachea and pulmonary artery allowing for perfusion with oxygenated Krebs bicarbonate solution (37°C) (arrowheads). The arrows point to the jugular and nodose ganglia. An extracellular recording electrode is placed in the nodose ganglion. Excess tissue is left on the edges of the ganglia to assist in the pinning of the ganglia firmly to the Sylgard-lined dish. The photograph has been manipulated; the pins and the excess tissue around the ganglia were digitally removed, and the background was made black so that the outline of the two ganglia can be better visualized. The X's and O's represent the approximate location at which a punctate mechanical stimulus with a von Frey hair evoked a discharge of action potentials in a given jugular (O) or nodose (X) C-fibre. Note that several nodose and jugular C-fibres had receptive fields at the very margin of the lung lobes.
Extracellular electrophysiological recordings were performed using an aluminosilicate glass microelectrode (pulled with Flaming-Brown micropipette puller, Sutter Instrument Company, Novato, CA, USA) and filled with 3 m sodium chloride (electrode resistance ∼2 MΩ). The electrode was placed into an electrode holder connected directly to a headstage (A-M Systems, Everett, WA, USA). A return electrode of silver–silver chloride wire and an earthed silver–silver chloride pellet were placed in the perfusion fluid of the recording compartment. The recorded signal was amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut off, 0·3 kHz; high cut off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and a model TA240 chart recorder (Gould, Valley View, OH, USA). The data were stored and analysed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD, USA) and further processed using spreadsheet software (Microsoft Excel 98). The recording microelectrode was micromanipulated into different areas of either the jugular or the nodose ganglion (Fig. 1). A mechanosensitive receptive field was identified when the mechanical stimulus (von Frey hair, 1800–3000 mN) bluntly applied to mucosal surface of the airway or parenchymal surface of the lung lobe evoked a burst of action potentials. Once a mechanosensitive receptive field was identified, a brief (<1 ms) electrical stimulus was delivered by a small concentric electrode positioned over this discrete mechanosensitive region to determine the conduction velocity of the fibre. The voltage was increased until an action potential was evoked. Conduction velocity was calculated by dividing the distance along the nerve pathway by the time between the shock artifact and the action potential evoked by electrical stimulation of the mechanosensitive receptive field. In most (>90%) experiments one fibre per animal was recorded. Occasionally, two or three consecutive fibres were recorded in an individual preparation.
Various chemical stimulants were diluted from stock solutions and dissolved in KBS (37°C). In the airway preparation, the chemical stimulants were delivered directly to the mucosa over the mechanosensitive receptive field in a volume of 500 μl in ∼3 s using a transfer pipette. In the lung preparation, the chemical stimulants were infused into the lung at a rate of 50 μl s−1 through either the pulmonary artery or tracheal perfusion followed by at least 10 min washout with KBS. Most receptive fields could be accessed by the drug administered through either route, but there was often a quantitative difference in the response. The data presented refer to the larger response irrespective of route of delivery. The fibre was considered unresponsive to a particular stimulant when administration of an agonist through both routes failed to evoke a response, but a positive ‘control’ response could be obtained. Typically, capsaicin added at the end of the experiment provided a positive ‘control’ response (virtually all C-fibres were responsive to capsaicin). Distending the lungs by increasing the rate of tracheal perfusion such that the tracheal perfusion pressure was increased to >25 cmH2O failed to evoke responses in the C-fibres. By contrast this reproducibly activated mechanically sensitive A-fibre afferents in this preparation (data not shown). We have described similar preparation for the recording of vagal afferent nerve activity from the mouse lung (Kollarik et al. 2003).
Patch clamp recording
For patch clamp studies of lung labelled neurones, we used DiI (DiC18(3); Molecular Probes) as the retrograde tracer. The DiI solution (0.2%, 300 μl; dissolved in 10% of DMSO and 90% of normal saline) was injected into the tracheal lumen after a simple tracheostomy using a 27G needle under anaesthesia (50 mg kg−1 of ketamine and 2.5 mg kg−1 of xylazine; intraperitoneally) 7–9 days before isolation of the vagal sensory ganglia and dissociation of the neurones. When DiI was injected, the animals were positioned at 30 deg head-up to prevent the dye leakage to other tissues (especially through an oesophagus) except the airways and lungs. We found this procedure to label the large conducting airways as well as lung parenchymal tissue.
The nodose and jugular ganglia were rapidly (<10 min) removed from animals, cleared of adhering connective tissue, and then incubated in enzyme solution (10 mg collagenase type 1A (Sigma), 10 mg dispase II (Boehringer Mannheim, Mannheim, Germany), 10 ml Ca2+- and Mg2+-free Hanks' balanced salt solution) for 2 h at 37°C. Neurones were dissociated by trituration, washed by centrifugation (three times at 700 g for 45 s), suspended in L15 medium (Gibco BRL, Rockville, MD, USA) containing 10% fetal bovine serum (JRH Biosciences, Lenexa, KS, USA), then transferred onto circular 15 mm glass coverslips (Bellco Glass Inc., Vineland, NJ, USA) coated with poly d-lysine (0.1 mg ml−1, Sigma). The vagal sensory neurones adhering to coverslips were maintained in vitro at 37°C and used for recording approximately 12–24 h after plating.
The labelled cells were identified with a fluorescence microscope equipped with 560 nm excitation filter and 480 nm emission filter. All patch clamp recordings were done with labelled cells isolated from the jugular or nodose ganglion. Whole cell patch clamp recording was employed using an Axoclamp 200A amplifier and pCLAMP7 software (Axon Instruments, Union City, CA, USA). Patch electrodes (1.5–3 MΩ) were filled with a solution composed of (mm): 140 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 Hepes and 10 dextrose; titrated to pH 7.3 with KOH; 304 mosmol l−1. The membrane potentials of the cells were held at −60 mV. After forming a gigaohm seal and establishing whole-cell configuration, the membrane capacitance (Cm) and 60–80% of series resistance (Rs) were compensated using the ‘Membrane test’ application of pCLAMP7 software. Criteria for cell inclusion in the study were Rs < 10 MΩ and Rin > 100 MΩ. During the experiments, the cells were continuously superfused (2–4 ml min−1) by gravity with Locke's solution (34 ± 1°C); composition (mm): 136 NaCl, 5.6 KCl, 1.2 MgCl2, 2.2 CaCl2, 1.2 NaH2PO4, 14.3 NaHCO3 and 10 dextrose (pH 7.3–7.4). Capsaicin, ATP, or α,β-methylene-ATP diluted in Locke's solution was applied directly into a recording bath by switching the perfusion flow to the drug-containing Locke's solution. When more than one drug was studied the intervals between drug applications was at least 5 min for a cell to recover. The peak inward and the total inward current per unit membrane capacitance were measured.
Data analysis
Histology data are presented as the mean ±s.e.m. Extracellular recording data are presented as the mean ±s.e.m. of the total number of action potentials and the peak frequency (Hz). The peak frequency was taken as the maximum number of impulses observed in any 1 s period. Conduction velocities are also presented as the mean ±s.e.m. Student's paired and non-paired t tests were used as appropriate to quantitatively compare the responses.
Drugs and chemicals
The following chemicals were dissolved in the distilled water (stock concentrations in brackets): α,β-methylene-ATP (10 mm) and bradykinin (1 mm). Indomethacin (30 mm) and capsaicin (10 mm) were dissolved in ethanol. The stock solutions were stored at −20°C (indomethacin stock was stored at 4°C) and the drugs were diluted to their final concentrations on the day of use. Adenosine 5′-triphosphate (ATP) was diluted to its final concentration at the day of use. All drugs were purchased from Sigma-Aldrich (St Louis, MO, USA) except for bradykinin (Peninsula Laboratories, Belmont, CA, USA).
Results
Histology
Neurones in the vagal sensory ganglia retrogradely labelled from the lung were compared to those labelled specifically from the trachea (Fig. 2). All labelled neurones were counted, but special emphasis was placed on the neurofilament (NF)-negative neurones, as we and others have found that they project unmylinated C-fibres (Lawson & Waddell, 1991; Riccio et al. 1996a; Hunter et al. 2000).
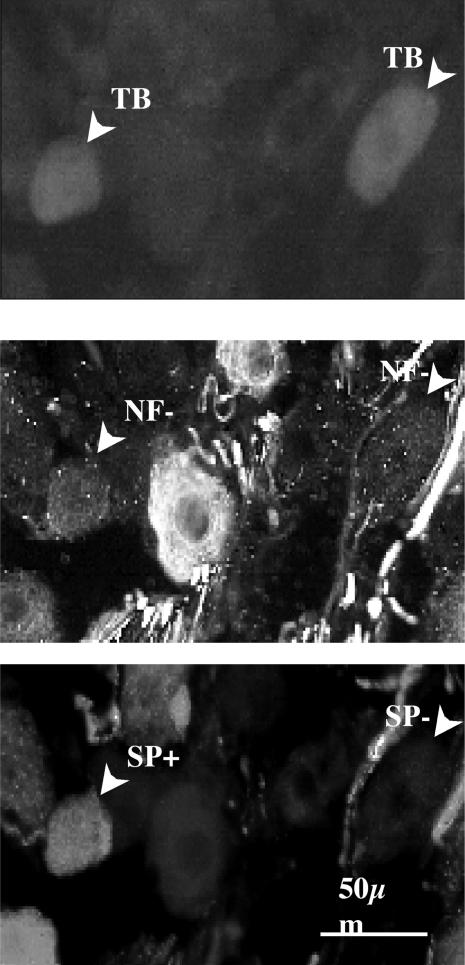
Figure 2. Photomicrographs showing examples of substance P-positive and substance P-negative, True Blue labelled NF-negative neurones in the nodose ganglion.
Top, photomicrograph of two right nodose ganglion cell bodies retrogradely labelled by True Blue injected into the right lung lobe. Middle, the True Blue labelled neurones were both NF-negative, indicative of C-fibre neurones. Bottom, immunoreactivity results showing that one of the lung specific neurones was substance P-positive and the other substance P-negative. The data from these experiments is quantified in Table 1.
Tracheal injections
In three previously published studies we evaluated neurones in the vagal sensory ganglia that were retrogradely labelled with dye injected into the trachea (Riccio et al. 1996a; Hunter & Undem, 1999; Myers et al. 2002). The conclusions drawn from these studies regarding C-fibre neurones innervating the trachea were the same in each case; the data from our original study (Riccio et al. 1996a) is presented here for purposes of comparison (Table 1). Neurones in both the nodose (inferior) and jugular (superior) vagal ganglia were labelled from the trachea. Nearly all of the nodose neurones projecting fibres to the trachea were neurofilament (NF)-positive, large diameter (mean ≈40 μm) and were devoid of substance P and CGRP immunoreactivity. By contrast approximately 50% of the neurones in the jugular ganglia that project fibres to the trachea were NF-negative, with small diameters (15–25 μm). All NF-negative neurones in the jugular ganglia labelled from the trachea were positive for substance P immunoreactivity. In other words, nearly all of the C-fibres innervating the trachea are substance P containing nerves derived from cell bodies located in the jugular ganglia. In the present study we found that only 14 of the 727 (<2%) nodose neurones labelled from the trachea were NF-negative. All 14 of these neurones were immunoreactive for substance P.
Table 1.
Innervation of trachea and lungs by NF-negative (C-fibre) neurones in jugular and nodose vagal ganglia
| NF-negative neurones (%) | % of NF-negative neurones positive for substance P | |||
|---|---|---|---|---|
| Tracer injection site | Nodose | Jugular | Nodose | Jugular |
| Tracheal | 7/448 (1.5%) | 290/571 (51%) | > 99% | > 99% |
| Lung | 262/407 (64%) | 171/217 (79%) | 55 ± 8% | 89 ± 4%* |
Neuronal tracer was injected directly into the tracheal or lung parenchymal tissue. At 7–14 days after the injection of the retrograde tracing dye, the total number of dye-labelled NF-negative (C-fibre) neurones was quantified in the nodose and jugular ganglia. The data were obtained from 6 and 4 ganglia in the trachea and lung injection studies, respectively. An asterisk denotes a significant difference between jugular and nodose values. The trachea data was obtained from our previous study (Riccio et al. 1996b) and presented here for comparison.
Lung parenchymal injections.
Either 10 or 1 μl of True Blue dye was injected transcutaneously into a lobe of the lungs. As discussed in Methods, ganglia were obtained and studied only from those animals in which the dye was noted upon postmortem analysis to be delimited to a localized region of the lung lobe in which it was injected, with little dye seen in the larger bronchi, and no dye seen in extrapulmonary tissues. An average of 82 ± 14 nodose neurones per ganglion took up the lung-injected tracer (n= 4). Among these nodose neurones an average of 64% were NF-negative neurones. An average of 66 ± 13 jugular neurones per ganglion took up the lung injected tracer dye (n= 4). Among these jugular neurones, nearly 80% were NF-negative neurones (Table 1). Thus, by contrast to the extrapulmonary tissue, both jugular and nodose neurones appear to project C-fibres to the intrapulmonary compartment.
We have previously reported that > 99% of the jugular NF-negative neurones labelled from the trachea are substance P immunoreactive (Riccio et al. 1996a). Similarly, we found that the vast majority (89 ± 4%) of jugular NF-negative neurones labelled from the lung parenchymal injections were positive for substance P immunoreactivity. By contrast, only about half (55 ± 8%) of the intrapulmonary NF-negative nodose neurones were substance P-positive (P < 0.05, compared to paired jugular values) (see Fig. 2, Table 1). Among the NF-positive population labelled from the lung parenchymal injections in both ganglia, only 4 ± 2% of neurones were substance P-positive.
Isolectin B4 (IB4) has been used as a marker of distinct phenotypes of C-fibre neurones in the somatosensory system (Plenderleith & Snow, 1993). We found that IB4 immunoreactivity did not differentiate among the nodose and jugular pulmonary C-fibre population. The IB4 staining was found in 114/117 NF-negative nodose neurones, and 89/95 NF-negative jugular neurones labelled from the lung. The NF-positive populations of labelled neurones, by contrast, were mostly IB4-negative in both ganglia. We found that among the 25 NF-positive jugular neurones labelled from the lungs none were positive for IB4 staining, and only 30 of 93 NF-positive nodose neurones were IB4-postive.
Electrophysiology
Conduction velocities
We evaluated the ganglionic location (nodose or jugular) of C-fibres with receptive fields in the extrapulmonary airways and within the lungs (Table 2). Based on our previous analysis of the vagal compound action potential, C-fibres were considered those with conduction velocities of <1 m s−1 (Canning & Undem, 1993; Riccio et al. 1996a). These fibres uniformly responded to capsaicin.
Table 2.
Ganglionic location of neurones projecting C-fibres to larynx, trachea, main bronchus and the lungs; proportion of fibres with conduction velocities < 1 m s−1
| Nodose ganglion | Jugular ganglion | |
|---|---|---|
| Larynx | 6/42 (14%) | 52/69 (75%) |
| Trachea | 6/137 (4%) | 69/149 (46%) |
| Main bronchus | 1/39 (3%) | 28/85 (29%) |
| Within lungs | 47/92 (51%) | 31/36 (86%) |
Conduction velocities of nerve fibres with receptive fields in the various tissues were determined using extracellular recording technique as described in methods. The cut-off of 1 m s−1 is based on analysis of the compound action potential as well as chemical sensitivity to capsaicin. The number in the denominator was the number of fibres analysed. The conduction velocities of those fibres that were faster than 1 m s−1 are given in the text.
Extrapulmonary fibres
We evaluated the C-fibre distribution among nodose and jugular ganglion fibres with receptive fields in the extrapulmonary airways (larynx, trachea and mainstem bronchus). Within each of these compartments a substantial percentage of the jugular ganglion fibres were C-fibres. By contrast, very few (<10%) of the nodose fibres projecting to the extrapulmonary airways were C-fibres. The conduction velocities of C-fibres in the larynx, trachea and main bronchus were not different from one another and averaged 0.7 ± 0.01 m s−1 (n= 149). The average conduction velocity of the few nodose C-fibres innervating the extrapulmonary structures was 0.9 ± 0.07 m s−1 (n= 13). The conduction velocity of Aδ fibres within the extrapulmonary compartments were similar between jugular and nodose fibres and averaged 6.2 ± 0.3 m s−1 (n= 130) and 5.3 ± 0.2 m s−1 (n= 185), respectively.
Intrapulmonary fibres
We recorded from 36 jugular afferent fibres and from 92 nodose afferent fibres with defined receptive fields within the lungs. By contrast to the extrapulmonary airways, a substantial percentage of the C-fibres within the lungs were derived from neurones situated within the nodose ganglia (Table 2). The conduction velocity of jugular and nodose C-fibres with receptive fields in the lungs averaged 0.6 ± 0.02 m s−1 (n= 31) and 0.9 ± 0.1 m s−1 (n= 47), respectively. The conduction velocity of the few (5/36) jugular A-fibres in the lungs was not significantly different than those in the extrapulmonary airways (3 ± 0.5 m s−1). Interestingly, nodose A-fibres projecting to the lungs were about 3 times faster than the nodose A-fibres in the extrapulmonary airways, averaging 15.0 ± 1.0 m s−1 (n= 23). Although not shown, intrapulmonary nodose A-fibres responded to lung distention with characteristics consistent with SAR and RAR fibres.
These data are consistent with our histological studies revealing that jugular C-fibre innervate intra- and extra-pulmonary tissues, whereas the nodose C-fibres are found nearly exclusively in the intrapulmonary compartment.
Receptive fields
The ‘lung surface’ receptive fields of the fibres within the lung, as determined by punctate mechanical stimulation with a von Frey fibre, is illustrated in Fig. 1. With only three exceptions each C-fibre studied had a single discrete receptive field, such that moving the von Frey fibre more than a few millimeters from the centre of the field evoked no response. Three fibres had two distinct receptive fields, and in one case the two distinct receptive fields were in different lobes. When the surface receptive field is over the central region of the lung it is not possible to conclude whether the mechanosensor is within an underlying conducting airway or large blood vessel, or in the parenchymal tissue closer to the surface. When the surface receptive field is within 2 mm of the edge of the lung lobe, however, it can be concluded with caution that the mechanosensor is outside the region of conducting airways. We found that 13% and 19% of the jugular and nodose C-fibres evaluated had their receptive fields at edges of the lungs, respectively.
Pharmacology
We evaluated the response of jugular and nodose C-fibres with receptive fields within the lung to frequently studied chemical activators of vagal C-fibre afferents (Table 3 and Fig. 3).
Table 3.
Pharmacology of nodose and jugular C-fibres with receptive fields in the lungs
| Nodose C-fibres | Jugular C-fibres | |||
|---|---|---|---|---|
| % responding | Peak f (Hz) | % responding | Peak f (Hz) | |
| Capsaicin | 15/15 (100%) | 12 ± 3 | 20/20 (100%) | 14 ± 3 |
| Bradykinin | 11/11 (100%) | 20 ± 4 | 11/13 (84%) | 4 ± 0.5* |
| ATP | 22/24 (92%) | 12 ± 2 | 6/29 (21%) | 6 ± 2* |
| α,β-Methylene-ATP | 21/21 (100%) | 16 ± 2 | 0/7 (0%) | —* |
After a receptive field was identified and the conduction velocity determined to be < 1 m s−1, 1 ml of capsaicin (0.3 μm), bradykinin (1 μm), ATP (30 μm) or α,β-methylene-ATP (30 μm) was infused via the trachea or pulmonary artery. The largest response was recorded regardless of the route of administration. If a chemical failed to evoke a response it was considered negative only if capsaicin or bradykinin evoked a response at the end of the experiment. If no chemical activated the fibre (about 2/50 fibres), the fibre was not used for analysis as there is no way of assuring adequate delivery of the chemical to the receptive site. The action potential discharge was quantified and segregated into 1 s bins. The peak frequency in hertz (Hz) was taken as the largest number of action potential per any 1 s bin. An asterisk denotes a significant difference (P < 0.05) in the response between jugular and nodose fibre populations. See Fig. 3 for examples of extracellular recordings.
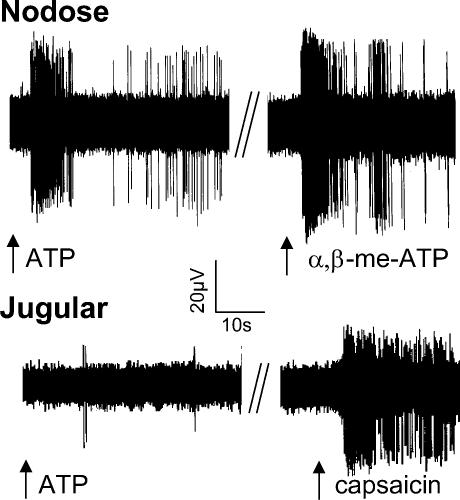
Figure 3. Representative extracellular recordings of action potential discharge from nodose and jugular C-fibres with receptive fields within the right lung.
The arrow denotes the point at which 1 ml of ATP (30 μm), α,β-methylene-ATP (30 μm), or capsaicin (0.3 μm) is infused into the trachea or pulmonary artery. The top tracing is from a nodose C-fibre (conduction velocity = 0.7 m s−1) with a receptive field in the middle lobe of the right lung. The bottom tracing is from a jugular C-fibre (conduction velocity = 0.6 m s−1) with its receptive field in the middle lobe of the right lung. Note that the jugular C-fibre is unresponsive to ATP, but is stimulated by capsaicin. By contrast, the nodose C-fibre responds vigorously to ATP. This ATP effect is mimicked by the P2X receptor selective agonist αβ-methylene-ATP. A quantitative summary of this data is presented in Table 3.
Intrapulmonary Jugular C-fibres
The jugular C-fibres projecting to the lungs responded to chemical stimulants in a fashion similar to those that project to the trachea/bronchus. Capsaicin (1 ml of 0.3 μm solution) stimulated every jugular C-fibre with its receptive field in the lungs (n= 20). The peak discharge frequency was 14 ± 3 Hz. The total number of action potentials evoked averaged 189 ± 63. Likewise, bradykinin (1 ml of 1 μm solution) activated the vast majority of jugular C-fibres projecting to the lungs. The peak discharge frequency and total number of action potentials averaged 4 ± 0.5 Hz and 56 ± 25, respectively (n= 11).
Very few (6 of 29) jugular C-fibres projecting to the lungs responded to ATP (1 ml of 30 μm) solution. The total number of action potentials in the six fibres that responded to ATP was only 42 ± 12. In two fibres that were unresponsive at the 30 μm concentration of ATP increasing the concentration to 100 μm did not uncover a response. No jugular C-fibre responded to the P2X receptor-selective agonist α,β-methylene-ATP (30 μm). Two of the fibres that responded to ATP failed to respond to the selective P2X receptor agonist, suggesting that ATP may have indirectly led to activation of these fibres independently of direct action on P2X receptors.
Intrapulmonary nodose C-fibres
As with the jugular C-fibres, all intrapulmonary nodose C-fibres studied responded to capsaicin (n= 15). The response to capsaicin was quantitatively similar to that observed with jugular C-fibres. The average peak frequency and total number of action potentials was 12 ± 3 Hz and 141 ± 52, respectively. Bradykinin likewise stimulated all nodose C-fibres studied (n= 11). The response of nodose C-fibres to bradykinin was significantly greater (P < 0.05) than that observed with jugular C-fibres. The peak frequency (20 ± 4 Hz) was nearly 5-fold greater then the average response of jugular C-fibres, and the total number of action potentials (290 ± 82) was also 5-fold greater (P < 0.01).
By contrast to the jugular C-fibres, nearly all (22/24) nodose C-fibres responded vigorously to ATP application (1 ml, 30 μm; Fig. 3). The peak frequency of discharge was 12 ± 2 Hz and the total number of action potentials averaged 100 ± 14 (n= 22). The effect of the P2X selective agonist α,β-methylene-ATP (30 μm) was studied in an additional 21 nodose C-fibres in the lungs. All 21 fibres responded; the average peak frequency of discharge was 16 ± 2 Hz and the total number of action potentials was 173 ± 31 (Fig. 3).
Patch clamp studies
The response to the P2X agonist reflects an obvious qualitative difference between jugular and nodose bronchopulmonary C-fibres in the guinea-pig. We used whole cell patch clamp technique to specifically address the hypothesis that this difference is due to a distinction in the neuronal phenotype among these two populations of C-fibres rather than merely reflecting pharmacokinetic differences or differences in potential indirect influences of the purines on the nerves.
Neurones dissociated from the nodose and jugular ganglia that were retrogradely labelled from the lungs were studied. We focused only on those neurones that projected C-fibres as denoted by their positive response to capsaicin applied at the end of the experiment. Consistent with the responses at the nerve endings, 16 of 18 labelled capsaicin-sensitive nodose neurones responded to ATP (1–100 μm, extrapolated EC50= 7 μm) (Fig. 4). The maximum response averaged 89 ± 17 pA pF−1 and was obtained with either 30 μm or 100 μm. A detailed kinetic analysis of the response was not undertaken, but in general the ATP-induced current was either rapidly inactivating as seen in Fig. 4Aa, or somewhat more slowly inactivating as seen in Fig. 4Ab. In 5 of 5 nodose neurones, the P2X selective agonist α,β-methylene-ATP (10 μm) evoked a rapid inward current (20 ± 2 pA pF−1) similar to that observed with ATP (Fig. 4). Consistent with the lack of effect of P2X responses in jugular C-fibre endings in the lungs, only 1 of 12 capsaicin-sensitive lung-specific jugular neurones responded to ATP (100 μm), and this response was slow in onset and of trivial magnitude (<1 pA pF−1).
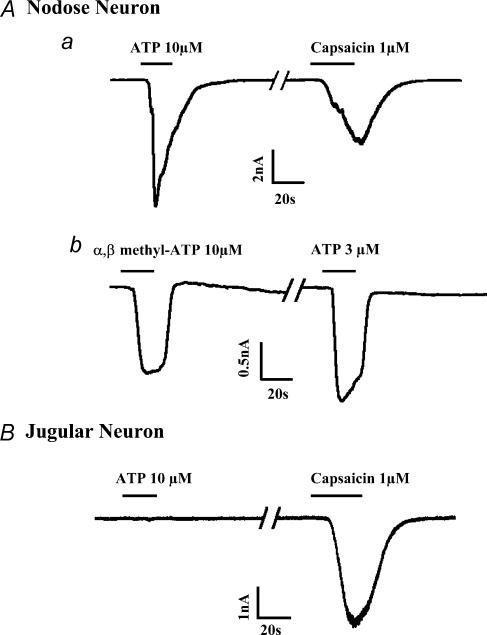
Figure 4. Representative inward ionic currents obtained with whole cell patch recordings of nodose (A) or jugular (B) neurones retrogradely labelled from the lung.
A, all nodose neurones responded to ATP (e.g. a and b) or the P2X receptor selective agonist, α,β-methylene-ATP (e.g. b) with a rapid inward current. The ATP current was either rapidly inactivating (e.g. neurone a) or more slowly inactivating (e.g. neurone b). B, by contrast to nodose neurones, retrogradely labelled, capsaicin-sensitive jugular neurones did not respond to ATP. See text for the quantification of the data from these experiments.
Discussion
The results of the present study support the hypothesis that vagal afferent C-fibres in the guinea-pig pulmonary system comprise at least two distinct phenotypes. Based on the location of the cell bodies (nodose or jugular ganglia) the two types of vagal C-fibres are likely to have different embryonic origins. In addition, distinctions in the C-fibre phenotypes are evident at the level of the expression of ionotropic membrane receptors (e.g. P2X receptors), neuropeptide content, and distribution within the pulmonary system.
It is well recognized that two separate sensory ganglia are situated along the cranial nerves VII, IX and X (vagus). Embryologically, the neurones in the distal ganglia in each case are placode-derived, whereas the neurones in the proximal ganglia are derived from the neural crest (Baker & Bronner-Fraser, 2001). With respect to the vagus nerve the two sensory ganglia are referred to as nodose ganglion (epibranchial placode-derived) and jugular ganglion (neural crest-derived). In guinea-pigs as well as in larger mammals, these two ganglia are readily distinguishable with the naked eye, whereas in rodents such as mice and rats, they may merge into a less defined jugular–nodose (and in some cases petrosal) complex. Neurones within the nodose and jugular ganglia express different growth factor receptors and depend on different neurotrophins for development and survival (Zhuo et al. 1997; Baker & Bronner-Fraser, 2000, 2001; Begbie et al. 2002; Ernfors et al. 1992). It may not be surprising therefore, that even though neurones in both ganglia project C-fibres in the vagus nerve, their phenotypes are distinct.
Our results support the hypothesis that nodose neurones project C-fibres to intrapulmonary aspects of the respiratory system, whereas the jugular ganglion projects C-fibres to both the peripheral lung and the extrapulmonary airways. This hypothesis is based on retrograde tracing and electrophysiological analysis. Previous studies from our laboratory (Riccio et al. 1996a; Hunter & Undem, 1999), and additional data presented here, show that the trachea and mainstem bronchus receives vagal C-fibres nearly exclusively from neurones situated in the jugular ganglia. The nodose ganglia by contrast, project mainly myelinated Aδ fibres to the trachea and mainstem bronchi, with the endings situated beneath the epithelium (Hunter & Undem, 1999). We have previously noted that nodose Aδ fibres in the guinea-pig trachea and main bronchus are unresponsive to capsaicin and bradykinin, but are exquisitely sensitive to punctate mechanical stimulation (Riccio et al. 1996a; Kajekar et al. 1999; McAlexander et al. 1999).
The sensory C-fibre innervation of the intrapulmonary system is different from that observed in the extrapulmonary airways. When the tracing dye was presented to the intrapulmonary tissue, numerous NF-negative (C-fibre) neurones were labelled in both the nodose and jugular ganglia. In fact, more than 60% of the total number of NF-negative neurones labelled from the lungs were situated in the nodose ganglia. Our electrophysiological experiments independently support the conclusion that, unlike the trachea and mainstem bronchi, the capsaicin-sensitive C-fibres with terminals within the lungs are derived from both the nodose (placode-derived) and jugular ganglion (neural crest-derived) neurones.
In other species, C-fibres in different compartments of the intrapulmonary system have been discerned by taking advantage of the two circulations in the lung. When a chemical C-fibre stimulant such as capsaicin is added to the pulmonary circulation, it will first contact the receptive fields in the peripheral lung, whereas when the stimulus is added to the bronchial circulation, the receptive fields in the conducting airway will first be reached. This type of analysis has led to the concept of ‘pulmonary’ and ‘bronchial’ C-fibres, respectively (Coleridge & Coleridge, 1977). A review of the history and basis for this nomenclature can be found in (Coleridge & Coleridge, 1984). One is tempted to speculate that the jugular neurones project ‘surface or intero-receptive’ C-fibres to the mucosa of the conducting airways (i.e. bronchial C-fibres), whereas the nodose projects fibres only to more peripheral aspects of the lung (i.e. pulmonary C-fibres). The resolution of the techniques used in our ex vivo preparation can provide only some general comments on this issue. When a punctuate mechanical receptive field is located in the central portion of the lung it is not possible to know if the nerve is in the underlying conducting airways or in the overlying parenchymal tissue. When the receptive field is at the very margins of the lung lobe, however, it is clear that it is outside the region of the conducting airways. Our finding that about 10–20% of the fibres studied, irrespective of ganglionic origin, were found within the lung margins indicates that both types of C-fibres can be found outside the conducting airways. From data presented in Table 2, we can conclude that nodose C-fibres do not appreciably innervate the trachea and mainstem bronchus. By extension, one might conclude that they do not innervate the conducting airways within the lungs. If this assumption is valid, the nodose fibres correspond to the pulmonary C-fibres whereas the jugular C-fibres include all bronchial C-fibres and some pulmonary C-fibres.
The nodose and jugular C-fibres are not only different in their relative projections to the bronchopulmonary system, they also differ in their neuropeptide content and response to pharmacological agents. We found that significantly fewer NF-negative pulmonary neurones in the nodose ganglion express substance P immunoreactivity when compared to their jugular ganglion counterparts. These data are consistent with that of Kummer et al. (1992) who noted in their histochemical analysis of pulmonary afferent nerves, that the substance P containing vagal sensory neurones innervating the pulmonary system were located in the jugular ganglia, but not nodose ganglia (Kummer et al. 1992). Although nodose neurones were retrogradely labelled from the lungs, they were found to be uniformly substance P-negative. Similarly, jugular but not nodose lung-specific neurones were found to express neuropeptide calcitonin gene related peptide (CGRP) in rat (Springall, 1987). This would suggest that the neural crest-derived C-fibres innervating the pulmonary system are more likely to express neuropeptides such as substance P and CGRP than those derived from the placodes.
Target derived signals are likely to be critical in determining the expression of neuropeptide genes. For example, although nodose neurones project primarily myelinated A-fibres to the trachea, among the >700 nodose neurones traced from the trachea, we had occasion to find a small number of NF-negative neurones. These neurones were each substance P-positive. Moreover, upon allergic inflammation or viral infection many airway-labelled nodose NF-positive neurones (A-fibre) are induced to express the RNA for preprotachykinin A, and substance P immunoreactivity (Fischer et al. 1996; Myers et al. 2002). Considered together, the data support the hypothesis that nodose C-fibres terminate in a compartment of the lungs different from the jugular C-fibres, with the latter having access to signals involved in the PPT RNA expression. One signal that may be relevant in this regard is nerve growth factor (NGF). Jugular C-fibres in the extrapulmonary airways are situated within the epithelium (Hunter & Undem, 1999), and NGF is synthesized by airway epithelial cells (Renz, 2001; Olgart Hoglund et al. 2002). In addition, we noted that injecting NGF to the airway increases the percentage of vagal sensory neurones that express substance P immunoreactivity (Hunter et al. 2000).
Tachykinin-positive and -negative C-fibre neurones have also been observed in the somatosensory system. In DRG neurones, the isolectin B4 (IB4) has proven to be a useful marker that differentiated between these two C-fibre phenotypes, with IB4 staining primarily the substance P-negative population (Silverman & Kruger, 1990). In the present study we found that IB4 rather indiscriminately stained both jugular and nodose C-fibre neurones. There was selectivity in IB4 staining in that none of the larger diameter NF-positive neurones in the jugular ganglia were stained with IB4, and only a fraction of the NF-positive neurones in the nodose ganglia were stained with the IB4.
Previous studies in intact dogs have noted that the pharmacological activation of pulmonary C-fibres could be differentiated from the bronchial C-fibre population (Coleridge & Coleridge, 1984). For example the C-fibres most accessible by the bronchial circulation were more sensitive to bradykinin than those most accessible by the pulmonary circulation (Kaufman et al. 1980). We noted in our ex vivo preparation that the 100% of nodose and jugular C-fibres regardless of their termination sites responded in a quantitative similar fashion to capsaicin. Bradykinin, by contrast, resulted in a more vigorous responses (∼20Hz) in the nodose C-fibre population as compared to jugular C-fibres (∼4Hz). The response of jugular C-fibres in the lungs was similar to what we have found with jugular C-fibres in the trachea (Kajekar et al. 1999), indicating the difference noted is not simply due to location of the jugular C-fibre receptive field. Nevertheless, in these ex vivo studies, as well as in in vivo studies, it is not clear whether this quantitative difference represents a phenotypic difference in the two types of C-fibres, or whether this represents pharmacokinetic differences (drug metabolism or access rate differences), or is merely a reflection of indirect effects of bradykinin. Our studies with the purinergic receptor agonists more directly address this issue.
ATP, and the P2X receptor-selective agonist α,β-methylene-ATP in particular (Khakh et al. 2001), showed a clear demarcation in the responsiveness of the nodose versus jugular bronchopulmonary C-fibres. The jugular C-fibres were categorically unresponsive to the selective P2X receptor agonist, whereas the nodose C-fibres were uniformly responsive. Patch clamp recording of whole cell currents in dissociated neurones labelled from the lungs were in agreement with the data obtained from the C-fibre terminals in the lungs. When capsaicin-sensitive nodose neurones labelled from the lungs were isolated and studied using whole cell patch clamp techniques, ATP and α,β-methylene-ATP caused a rapid and large inward current indicative of activation of the ionotropic P2X receptor. Although a detailed pharmacological analysis was not undertaken in the present study, the response to α,β-methylene-ATP, and the response kinetics are consistent with the conclusion based on studies of mouse nodose neurones, that the P2X receptor involved includes the P2X3 and P2X2/3 subtypes (Zhong et al. 2001). By contrast, retrogradely labelled capsaicin-sensitive jugular neurones were unresponsive to the P2X agonist. It would seem likely therefore that the explanation for why nodose, but not jugular, C-fibres in the lung respond to P2X receptor agonists is because of a difference in their phenotype. That is, nodose neurones projecting C-fibres to the lungs express functional P2X receptors, whereas jugular neurones do not. The response of guinea-pig vagal C-fibres in the lungs to P2X receptor activation is consistent with the findings that P2X receptor agonists stimulate vagal C-fibres in the canine lung (Pelleg & Hurt, 1996).
The results demonstrate that capsaicin-sensitive vagal C-fibres comprise at least two distinct phenotypes. The two types of vagal C-fibres innervating the guinea-pig bronchopulmonary system can be distinguished based on the ganglionic location of their cell bodies, neuropeptide content, and expression of ionotropic receptors in their terminal membranes. It would seem likely that based on their differential development and location within the tissue, these two types of C-fibres have been adapted to subserve different functions within the organism.
Acknowledgments
The authors acknowledge Ms Sonya Meeker for her outstanding technical assistance, and the NIH, Bethesda, MD for financial support.
References
- Baker CV, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–511. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Undem BJ. Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea-pig trachealis. J Physiol. 1993;471:25–40. doi: 10.1113/jphysiol.1993.sp019889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med. 2002;165:1071–1075. doi: 10.1164/ajrccm.165.8.2108065. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Impulse activity in afferent vagal C-fibres with endings in the intrapulmonary airways of dogs. Respir Physiol. 1977;29:125–142. doi: 10.1016/0034-5687(77)90086-x. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC, Ginzel KH, Baker DG, Banzett RB, Morrison MA. Stimulation of ‘irritant’ receptors and afferent C-fibres in the lungs by prostaglandins. Nature. 1976;264:451–453. doi: 10.1038/264451a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JL, Ho CY, Kwong K, Lee LY. Activation of pulmonary C fibres by adenosine in anaesthetized rats: role of adenosine A1 receptors. J Physiol. 1998;508:109–118. doi: 10.1111/j.1469-7793.1998.109br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med. 2000;161:1985–1990. doi: 10.1164/ajrccm.161.6.9908051. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- Kajekar R, Proud D, Myers AC, Meeker SN, Undem BJ. Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther. 1999;289:682–687. [PubMed] [Google Scholar]
- Kaufman MP, Coleridge HM, Coleridge JC, Baker DG. Bradykinin stimulates afferent vagal C-fibers in intrapulmonary airways of dogs. J Appl Physiol. 1980;48:511–517. doi: 10.1152/jappl.1980.48.3.511. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- McAlexander MA, Myers AC, Undem BJ. Adaptation of guinea-pig vagal airway afferent neurones to mechanical stimulation. J Physiol. 1999;521:239–247. doi: 10.1111/j.1469-7793.1999.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol. 2002;282:L775–L781. doi: 10.1152/ajplung.00353.2001. [DOI] [PubMed] [Google Scholar]
- Olgart Hoglund C, de Blay F, Oster JP, Duvernelle C, Kassel O, Pauli G, Frossard N. Nerve growth factor levels and localisation in human asthmatic bronchi. Eur Respir J. 2002;20:1110–1116. doi: 10.1183/09031936.02.00205402. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Vagal sensory receptors and their reflex effects. Physiol Rev. 1973;53:159–227. doi: 10.1152/physrev.1973.53.1.159. [DOI] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM. Mechanism of action of ATP on canine pulmonary vagal C fibre nerve terminals. J Physiol. 1996;490:265–275. doi: 10.1113/jphysiol.1996.sp021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenderleith MB, Snow PJ. The plant lectin Bandeiraea simplicifolia I–B4 identifies a subpopulation of small diameter primary sensory neurones which innervate the skin in the rat. Neurosci Lett. 1993;159:17–20. doi: 10.1016/0304-3940(93)90787-l. [DOI] [PubMed] [Google Scholar]
- Renz H. Neurotrophins in bronchial asthma. Respir Res. 2001;2:265–268. doi: 10.1186/rr66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996a;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. J Physiol. 1996b;491:499–509. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington SC. The Integrative Action of the Nervous System. New Haven: Yale University Press; 1906. [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Springall DR, Cadieux A, Oliveira H, Su H, Royston D, Polak JM. Retrograde tracing shows that CGRP-immunoereactive nerves of rat trachea and lung originate from vagal and dorsal root ganglia. J Auton Nerv Syst. 1987;20:155–166. doi: 10.1016/0165-1838(87)90113-5. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Carr MJ. Pharmacology of airway afferent nerve activity. Respir Res. 2001;2:234–244. doi: 10.1186/rr62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. Airway receptors. Respir Physiol. 2001;125:3–15. doi: 10.1016/s0034-5687(00)00201-2. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Bardini M, Ford AP, Cockayne DA, Burnstock G. Changes in P2X receptor responses of sensory neurons from P2X3-deficient mice. Eur J Neurosci. 2001;14:1784–1792. doi: 10.1046/j.0953-816x.2001.01805.x. [DOI] [PubMed] [Google Scholar]
- Zhuo H, Ichikawa H, Helke CJ. Neurochemistry of the nodose ganglion. Prog Neurobiol. 1997;52:79–107. doi: 10.1016/s0301-0082(97)00003-8. [DOI] [PubMed] [Google Scholar]