Abstract
Ageing reduces endothelium-dependent vasodilatation in humans and animals, and in humans, exercise training reverses the ageing-associated reduction in endothelium-dependent vasodilatation. The purpose of this study was to determine the mechanism(s) by which 10–12 weeks of treadmill exercise enhances endothelium-dependent vasodilatation in muscles of differing fibre composition from young and old rats. Three- and 22-month-old male Fischer 344 rats were assigned to young sedentary, young exercise-trained, old sedentary, or old exercise-trained groups. Arterioles were isolated from the soleus and gastrocnemius muscles; luminal diameter changes were determined in response to the endothelium-dependent vasodilator acetylcholine (ACh, 10−9–10−4 mol l−1) alone and in the presence of the nitric oxide synthase (NOS) inhibitor l-NAME (10−5 mol l−1) or the combination of l-NAME and the cyclooxygenase inhibitor indomethacin (10−5 mol l−1). Training ameliorated the ageing-induced reduction in endothelium-dependent vasodilatation in soleus muscle arterioles. Treatment with l-NAME alone and in combination with indomethacin abolished differences in ACh vasodilatation occurring with ageing and training. Expression of endothelial NOS (eNOS) mRNA in soleus arterioles was unaltered by ageing, whereas eNOS protein was increased with age; training elevated both eNOS mRNA and protein. In gastrocnemius muscle arterioles, ageing did not alter maximal vasodilatation, but ageing and training increased maximal arteriolar diameter. These results demonstrate that ageing-induced reductions and training-induced enhancement of endothelial vasodilatation both occur through the nitric oxide signalling mechanism in highly oxidative skeletal muscle, but ageing and training do not appear to act on the same portion of the signalling cascade.
Maximal aerobic and exercise capacity is markedly reduced with advancing age (Ogawa et al. 1992; Fitzgerald et al. 1997). Although part of this decline results from a diminished elevation in cardiac output during exercise (Lakatta, 1995), alterations in the local control of skeletal muscle blood flow also contribute to this decline. For example, muscle blood flow is lower in older subjects, both when a small muscle mass is active and the limits of cardiac output are not approached (Irion et al. 1987; Haidet & Parsons, 1991; Haidet, 1992; Lawrenson et al. 2003, and when cardiac output is similar between young and old (Beere et al. 1999). The reduction in skeletal muscle blood flow appears to be related, at least in part, to an impaired endothelium-dependent vasodilatation. Several studies have shown an ageing-associated reduction in endothelial function in conduit and resistance arteries (Hongo et al. 1988; Tominaga et al. 1994; Delp et al. 1995; Gerhard et al. 1996; DeSouza et al. 2000; Woodman et al. 2002), including arterioles isolated from skeletal muscle (Muller-Delp et al. 2002a).
Aerobic exercise training has been reported to ameliorate age-associated reductions in both central and peripheral cardiovascular function. For example, cardiac output, stroke volume, and maximal aerobic capacity are increased as a result of exercise training (Ogawa et al. 1992). Furthermore, exercise training appears to reverse old age-associated reductions in endothelium-dependent vasodilatation in humans (DeSouza et al. 2000; Taddei et al. 2000). Although exercise training has been shown to improve the age-related decline in endothelium-dependent vasodilatation, the mechanism(s) by which ageing and exercise training alter endothelium-dependent function have not been determined. Therefore, the purpose of this study was to determine the mechanism(s) by which exercise training enhances endothelium-dependent vasodilatation in resistance arterioles from locomotory muscles of varying fibre composition in young and old rats. We hypothesized that exercise training would restore endothelium-dependent vasodilatation in skeletal muscle arterioles through enhancement of the nitric oxide (NO) signalling mechanisms.
Methods
This study was approved by the Texas A & M University Institutional Animal Care and Use Committee and conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Animals
Three-month- (n= 75) and 22-month-old (n= 63) male Fischer 344 rats were habituated to treadmill exercise, during which each rat walked on a motor-driven treadmill at 5 m min−1 (0 deg incline), 5 min day−1 for 3 days. Following habituation, young and old rats were randomly assigned to either sedentary (SED) control groups (young SED, n= 33 and old SED, n= 32) or exercise-trained (ET) groups (young ET, n= 42 and old ET, n= 31). Exercise-trained rats performed treadmill running at 15 m min−1 (15° incline), 5 days per week for 10–12 weeks. The length of time the rats ran on the treadmill was progressively increased, from 10 min day−1 to 60 min day−1, during the first 3 weeks. The rats continued to exercise 5 days per week for 60 min per day for the remainder of the 10–12 week training period.
Isolation and cannulation of skeletal muscle arterioles
Following the exercise-training period, the rats were anaesthetized with sodium pentobarbital (60 mg kg−1i.p.), killed by decapitation, and soleus–plantaris–gastrocnemius muscle groups were dissected free. First-order (1A) arterioles from the soleus muscle, composed primarily of high-oxidative fibres (Delp & Duan, 1996), and the superficial portion of the gastrocnemius muscle, composed primarily of low-oxidative glycolytic fibres (Delp & Duan, 1996), were isolated and prepared for in vitro videomicroscopy as previously described (McCurdy et al. 2000; Muller-Delp et al. 2002a,b).
Muscle oxidative enzyme activity
Sections of the soleus and gastrocnemius muscles from each animal were stored at −80°C for determination of citrate synthase activity, a measure of muscle oxidative capacity (Srere, 1969; Delp & Duan, 1996).
Evaluation of vasodilator responses
Once a steady level of spontaneous tone was achieved in vessel segments, vasodilator responses to the cumulative addition of the endothelium-mediated vasodilator acetylcholine (ACh, 10−9−10−4 mol l−1) and the nitric oxide donor sodium nitroprusside (SNP, 10−10−10−4 mol l−1) were determined as previously described (McCurdy et al. 2000; Muller-Delp et al. 2002a,2002b). At the end of the concentration–response determinations, the Mops buffer solution was replaced with Ca2+-free Mops buffer solution for 1 h to obtain the maximal passive diameter (McCurdy et al. 2000; Muller-Delp et al. 2002a,2002b).
Evaluation of inhibitory effects of NG.-nitro-l-arginine methyl ester and indomethacin
To determine the role of nitric oxide and prostaglandins in ACh-induced vasodilatation, responses to ACh were evaluated after a 20-min incubation under one of the following conditions: (1) nitric oxide synthase (NOS) blocker NG-nitro-l-arginine methyl ester (l-NAME, 10−5 mol l−1), or (2) combination treatment of l-NAME (10−5 mol l−1) and the cyclooxygenase blocker indomethacin (10−5 mol l−1) (Koller & Kaley, 1990).
Evaluation of eNOS expression
RT-PCR.
Arterioles were snap frozen and stored at −80°C in 0.5 ml microcentrifuge tubes. Arterioles were pulverized in lysate buffer and total RNA was extracted with the RNAqueous filter system (Ambion). Five microlitres of total RNA was used to perform real-time PCR with TaqMan® probes designed with the use of Primer Express® from the published sequence for rat eNOS (forward primer: GAA CCT ACA GAG CAG CAA ATC CA; reverse primer: CAG TCC CTC CTG GCT TCC TCC A) and a TaqMan® oligonucleotide probe (probe: CGA GCC ACA ATC CTG GTC CGT CTT) labelled with a fluorescent reporter dye (VIC) and a quencher dye (TAMRA). Five microlitres of total RNA was also used to simultaneously amplify 18S ribosomal RNA. Reverse transcription and PCR were performed in 50 μl volumes using GeneAmp 96 well Optical Reaction plates. Each reaction well contained the following: 5 μl total RNA, 25 μl Universal PCR Master Mix, 1.25 μl (75 U) Multiscribe reverse transcriptase, 1.0 μl (300 nm) forward primer, 1.0 μl (300 nm) reverse primer, 1.0 μl (100 nm) labelled probe, and 15.75 μl DEPC-treated water. Reactions were performed in duplicate with all samples contained in the same reaction plate. Reverse transcription was carried out for 30 min at 48°C. PCR was initiated by a 10 min step at 95°C followed by 40 two-step cycles of 15 s at 95°C and then 1 min at 60°C. The fluorescence intensities of the dyes from each probe were measured by the ABI Prism 7700 Sequence Detection system at every temperature step and cycle during the reaction. The number of cycles required for the fluorescence signal from each tube to reach a fixed threshold is defined as the cycle threshold (CT). The fluorescence signals for 18S ribosomal RNA served as controls for differences in total RNA loading in the wells. Levels of the target sequence were quantified by calculating the difference between the CT for the target sequence and coamplified 18S ribosomal RNA (ΔCT). To insure that the efficiency of the amplification was similar for the target sequence and the 18S ribosomal RNA, a validation reaction was performed using serial dilutions of the same RNA sample. ΔCT values were plotted versus the log of the RNA concentrations in the serial dilutions. The slope of the line for this plot was less than 0.03, indicating that the efficiency of amplification reaction was similar for 18S and the target sequence, independent of the starting concentration of total RNA.
Immunoblot analysis of arteriolar protein.
Differences in eNOS protein expression in 1A arterioles from the soleus and superficial gastrocnemius muscles were assessed using immunoblot analysis. To provide adequate protein, multiple vessels were combined for analysis when necessary. Arterioles were placed in 25 μl of 2× sample buffer (Tris-Cl, pH 6.8, 0.126 m; glycerol 12.6%; 2-mercaptoethanol 1.44 m, bromophenol blue 0.004%; SDS 5%) and homogenized using a micropellet pestle homogenizer (Kimble). Arteriolar proteins and molecular weight standards (Invitrogen) were subjected to SDS–polyacrylamide gel electrophoresis (9–16.5% gradient gel) and transferred to nitrocellulose membrane. Membranes were cut and blocked for 2–4 h at 25°C in Tris-buffered saline containing 5% non-fat dry milk (Carnation) and 0.1% Tween 20, and incubated with primary eNOS (top portion of membrane) or GAPDH (bottom portion of membrane) antibodies overnight at 4°C. Primary antibody dilutions were as follows: eNOS, 1: 1250, and GAPDH, 1: 1000 in blocking buffer. After washing, membranes were incubated with the appropriate horseradish peroxidase-conjugated species-specific anti-IgG (1: 50,000–1: 100 000 depending on primary antibody) for two hours at 25°C. Peroxidase activity was detected using Super Signal Fempto (Pierce), which detects proteins in the femptogram range. Normalization for loading differences was accomplished using ratios of the densitometry signals for GAPDH versus eNOS protein.
Chemicals
Albumin was purchased from USB Chemicals and primary monoclonal antibodies for eNOS and GAPDH were obtained from Transduction Laboratories and Advanced Immunochemicals, respectively. All other chemicals were obtained from Sigma Chemical Co.
Statistical Analysis
To control for variations in vessel size, vasodilator responses were recorded as actual diameters and subsequently expressed as a percentage of maximal relaxation according to the following formula:
where Dm is the maximal diameter recorded at 60 cmH2O in calcium-free PSS, Ds is the steady-state diameter recorded after each dose of ACh, and Db is the initial baseline diameter recorded immediately prior to the first dose of ACh as previously described (McCurdy et al. 2000; Muller-Delp et al. 2002a,b). Concentration–diameter relations were evaluated by repeated measures ANOVA in order to detect differences within (dose) and between (experimental groups, muscle types) factors. Pairwise comparisons between specific levels were made through post hoc analysis (Scheffe's) when a significant main effect was found. A one-way ANOVA was used to determine differences among groups for citrate synthase activity, body weight, muscle weights, spontaneous tone, and maximal diameter. All values are presented as means ±s.e.m. Significance was defined as P= 0.05.
Results
Animals
The average age at the time of study was 6.0 ± 0.1 months (range, 4–7 months) for the young rats and 24.6 ± 0.1 months (range, 24–26 months) for the old rats. Body mass increased with age (young SED, 384 ± 7 g; old SED, 404 ± 7 g), and exercise training resulted in lower body mass in both young and old rats (young ET, 359 ± 4 g; old ET, 367 ± 6 g). Soleus muscle mass was not altered with ageing or exercise training (young SED, 0.169 ± 0.004 g; young ET, 0.174 ± 0.004 g; old SED, 0.156 ± 0.006 g; old ET, 0.168 ± 0.005 g). Gastrocnemius muscle mass was diminished by ageing (young SED, 1.92 ± 0.04 g; old SED, 1.52 ± 0.04 g), and by exercise training in the young rats (young ET, 1.81 ± 0.03 g). Exercise training did not alter gastrocnemius muscle mass in the old rats (old ET, 1.55 ± 0.04 g). Citrate synthase activity was higher in both muscles of young and old ET rats relative to the SED groups (Table 1), confirming the efficacy of the exercise training regimen.
Table 1.
Arteriolar characteristics and oxidative capacity of the soleus and the superficial portion of gastrocnemius muscles
| Soleus Muscle | Gastrocnemius Muscle | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Young SED | Young ET | Old SED | Old ET | Young SED | Young ET | Old SED | Old ET | ||
| Tone (%) | |||||||||
| Pre-Acetylcholine | 47 ± 3 | 55 ± 5 | 51 ± 4 | 48 ± 4 | 31 ± 2 | 39 ± 3† | 35 ± 4 | 39 ± 3 | |
| n = 14 | n = 11 | n = 11 | n = 13 | n = 13 | n = 13 | n = 13 | n = 11 | ||
| Pre-l-NAME | 60 ± 4 | 65 ± 5 | 55 ± 6 | 48 ± 7 | 38 ± 6 | 35 ± 6 | 38 ± 7 | 45 ± 5 | |
| n = 7 | n = 6 | n = 8 | n = 8 | n = 5 | n = 7 | n = 8 | n = 9 | ||
| Post-l-NAME | 71 ± 5 | 73 ± 2# | 60 ± 6 | 64 ± 5 | 42 ± 3 | 53 ± 4# | 44 ± 3 | 53 ± 5 | |
| n = 7 | n = 6 | n = 8 | n = 8 | n = 5 | n = 7 | n = 8 | n = 9 | ||
| Pre-l-NAME | |||||||||
| + Indomethacin | 52 ± 3 | 65 ± 3 | 58 ± 6 | 51 ± 6 | 27 ± 10 | 34 ± 11 | 44 ± 5 | 49 ± 7 | |
| n = 5 | n = 7 | n = 10 | n = 9 | n = 5 | n = 5 | n = 9 | n = 8 | ||
| Post-l-NAME | |||||||||
| + Indomethacin | 62 ± 10 | 73 ± 5# | 69 ± 7 | 68 ± 4# | 38 ± 8 | 56 ± 8# | 60 ± 5# | 60 ± 6# | |
| n = 5 | n = 7 | n = 10 | n = 8 | n = 5 | n = 5 | n = 9 | n = 8 | ||
| Citrate Synthase Activity | |||||||||
| (μmol min−1 g−1 wet wt.) | 17 ± 0.4 | 21 ± 0.7* | 12 ± 0.5* | 15 ± 0.8† | 16 ± 1.1 | 21 ± 0.9* | 14 ± 1.2 | 25 ± 1.5*† | |
| n = 33 | n = 42 | n = 26 | n = 31 | n = 27 | n = 34 | n = 20 | n = 31 | ||
Values are means ±s.e.m.
Indicates group mean significantly different from young SED group (P = 0.05).
Indicates group mean significantly different from old SED group (P = 0.05).
Indicates significance difference from respective pretreatment level of spontaneous tone (P = 0.05).
Characteristics of isolated vessels
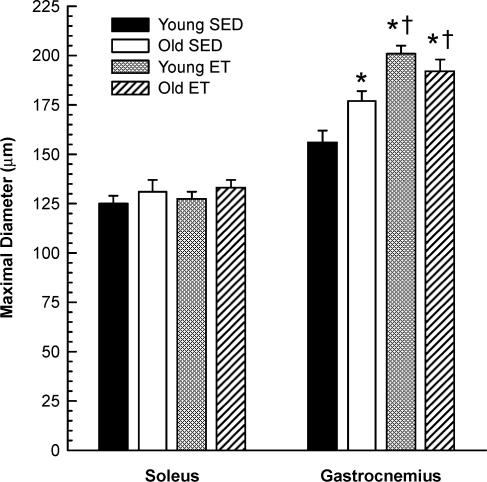
Maximal intraluminal diameter of soleus muscle arterioles was not different among groups, whereas maximal diameter of arterioles from the superficial portion of the gastrocnemius muscle was greater in the old SED group relative to young SED group and in the ET groups relative to the SED groups (Fig. 1). Maximal arteriolar diameters from the gastrocnemius muscle were greater than those from the soleus muscle for all groups.
Figure 1. Maximal diameter of arterioles from the soleus (young SED, n = 22; old SED, n = 23; young ET, n = 20; old ET, n = 25) and gastrocnemius (young SED, n = 23; old SED, n = 31; young ET, n = 25; old ET, n = 26) muscles.
Values are means ±s.e.m.*Indicates significant difference from young SED group (P = 0.05). †Indicates significant difference from old SED group (P = 0.05). All soleus muscle arteriolar maximal diameters are significantly less than that of gastrocnemius muscle arterioles (P = 0.05).
The level of initial spontaneous tone was similar for all groups except for gastrocnemius muscle arterioles from young SED and young ET rats (Table 1). Treatment with l-NAME significantly increased tone in soleus muscle arterioles from young ET rats, but also tended to increase tone in old ET rats (P < 0.07). Likewise, treatment with l-NAME significantly increased tone in gastrocnemius muscle arterioles of young ET rats. The combined treatment of l-NAME and indomethacin increased tone in soleus muscle arterioles from young and old ET rats. In gastrocnemius muscle arterioles, the combined treatment increased tone in young and old ET groups, as well as in old SED animals.
Mechanisms of ACh-induced vasodilatation in soleus muscle
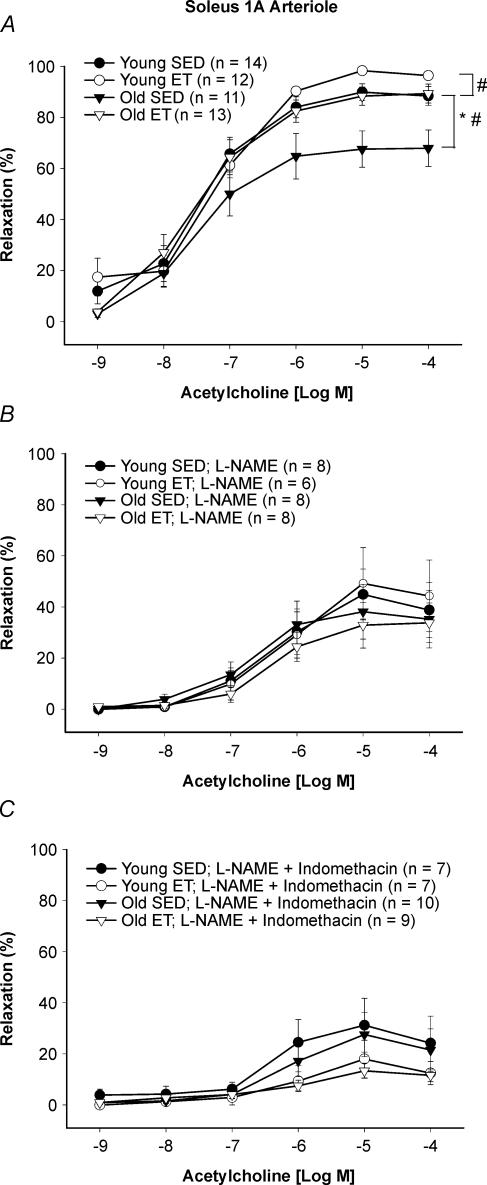
Vasodilator responses to ACh were diminished in soleus muscle arterioles from old SED rats relative to young SED rats (Fig. 2A). Exercise training restored ACh-induced vasodilatation in soleus muscle arterioles from old rats to a level that was equivalent to that in young SED rats; training further enhanced maximal ACh-induced vasodilatation in arterioles from young rats (Fig. 2A).
Figure 2. Concentration–response relation of soleus muscle arterioles from young and old, SED and ET rats to acetylcholine (ACh).
A, age significantly reduced vasodilator responses to ACh in soleus muscle arterioles. Exercise training restored vasodilator responses to ACh in soleus muscle arterioles from old rats and enhanced vasodilator responses to ACh in soleus muscle arterioles from young rats. B, l-NAME inhibited the vasodilator response to ACh in arterioles from all groups, and abolished the ageing and training-induced differences in ACh vasodilatation. C, combination of l-NAME and indomethacin inhibited the vasodilator response to ACh in arterioles from all groups, and eliminated the ageing and training-induced differences in ACh vasodilatation. Values are means ±s.e.m.*Indicates young significantly different from old (P = 0.05). #Indicates ET rats significantly different from respective SED rats (P = 0.05).
l-NAME reduced ACh-induced vasodilatation in soleus muscle arterioles from all groups (Fig. 2B). NOS inhibition abolished both ageing and training differences among groups (Fig. 2B). The combined l-NAME and indomethacin treatment further reduced ACh-induced vasodilatation in soleus muscle arterioles from all groups (Fig. 2C). These attenuated vasodilator responses were not different among groups.
Mechanisms of ACh vasodilatation in gastrocnemius muscle
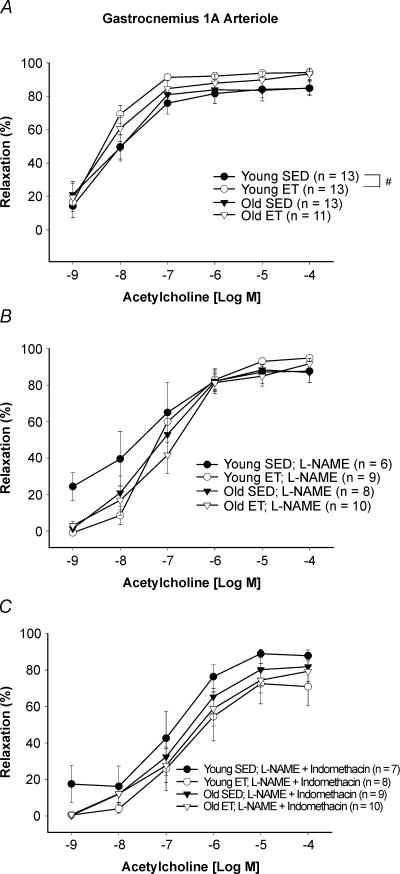
In gastrocnemius muscle, arteriolar vasodilatation to ACh was not different between old SED animals and young SED animals (Fig. 3A). Exercise training enhanced maximal ACh-induced vasodilatation in the young rats, but had no effect on vasodilator responses in gastrocnemius muscle arterioles from old animals, despite a larger increase in gastrocnemius muscle oxidative capacity in old rats (Table 1).
Figure 3. Concentration–response relation of gastrocnemius muscle arterioles from young and old SED and ET rats to acetylcholine (ACh).
A, vasodilator responses to ACh in gastrocnemius muscles were not affected by age. Exercise training enhanced vasodilatation to ACh in gastrocnemius muscle arterioles from young rats but not old rats. B, l-NAME did not inhibit maximal vasodilator responsiveness to ACh in any group, but reduced sensitivity (IC50) to ACh in the old SED rats and in the young and old ET rats. C, combination of l-NAME and indomethacin reduced maximal vasodilatation to ACh in young ET rats, while sensitivity (IC50) to ACh was reduced in all groups. Values are means ±s.e.m.#Indicates young ET significantly different from young SED (P = 0.05).
Treatment with l-NAME had no effect on maximal ACh vasodilatation (10−6–10−4 mol l−1) in both young and old, SED and ET rats (Fig. 3B). However, NOS inhibition reduced arteriolar sensitivity (IC50) to ACh in all groups except the young SED (Fig. 3B). The combined treatment of l-NAME and indomethacin reduced arteriolar sensitivity (IC50) to ACh in all groups, but only reduced vasodilatation at the highest doses of ACh in young ET rats (Fig. 3C). There were no differences in the maximal response or sensitivity to ACh among young and old, SED and ET groups in the presence of l-NAME plus indomethacin.
eNOS expression
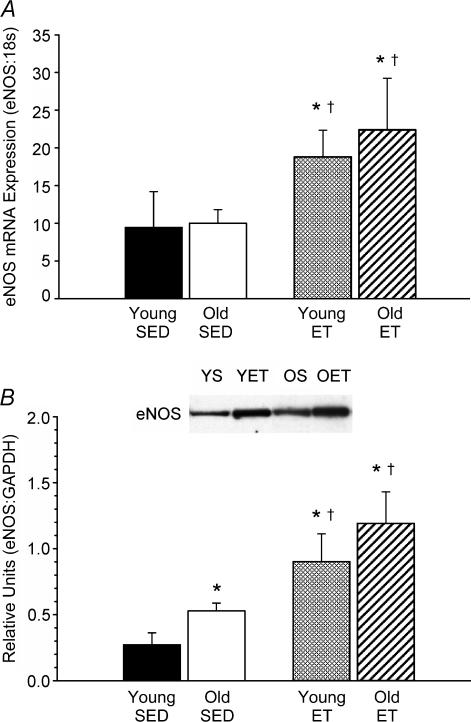
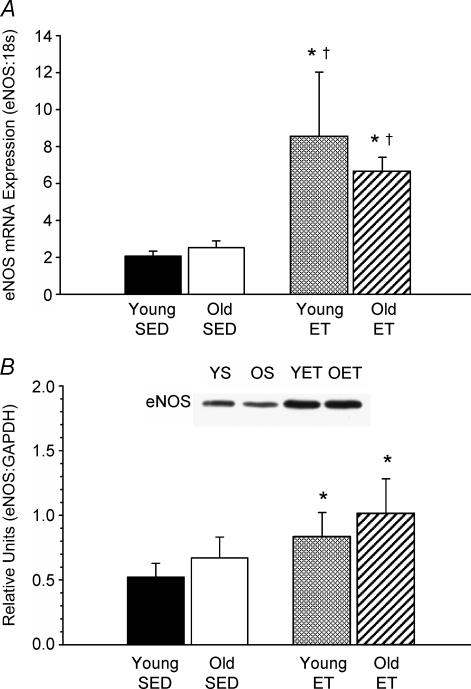
In soleus muscle arterioles, ageing had no effect on eNOS mRNA expression (Fig. 4A), but resulted in a greater eNOS protein content (Fig. 4B). Exercise training resulted in elevations in both eNOS mRNA and protein expression in soleus muscle arterioles from young and old rats. In gastrocnemius muscle arterioles, ageing did not alter eNOS mRNA (Fig. 5A) or protein levels (Fig. 5B). Exercise training elevated eNOS mRNA expression in gastrocnemius muscle arterioles from young and old rats, but eNOS protein concentration was only elevated in the young rats with training. Representative protein immunoslot show in Fig. 6.
Figure 4. eNOS mRNA (A) and protein (B) expression in soleus muscle 1A arterioles of young and old, SED and ET rats.
Values are means ±s.e.m.*Indicates significant difference from young SED group (P = 0.05). †Indicates significant difference from old SED group (P = 0.05).
Figure 5. eNOS mRNA (A) and protein (B) expression in 1A arterioles from gastrocnemius muscle of young and old, SED and ET rats.
Values are means ±s.e.m.*Indicates significant difference from young SED group (P = 0.05). †Indicates significant difference from old SED group (P = 0.05).
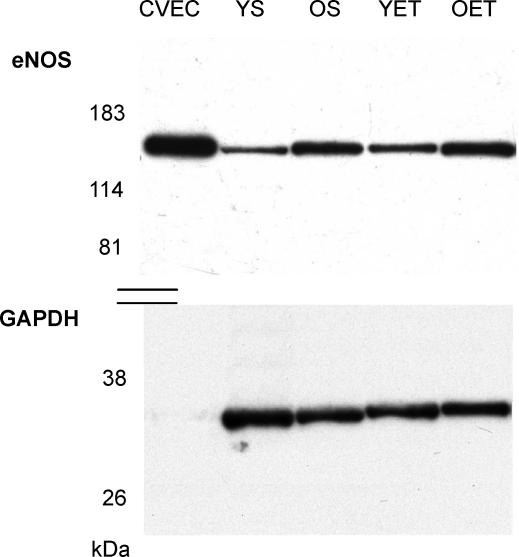
Figure 6.
Sample immunoblot showing eNOS and GAPDH proteins from cultured coronary venular endothelial cells (CVEC, an internal control sample) and soleus muscle arterioles of young sedentary (YS), old sedentary (OS), young exercise trained (YET), and old exercise trained (OET) rats
Vasodilator responses to SNP
Responses to the exogenous nitric oxide donor, SNP, were not altered by age or training status in arterioles from either the soleus or gastrocnemius muscles (data not shown).
Discussion
The purpose of this study was to determine the effects of ageing and exercise training on endothelium-dependent vasodilator responses of skeletal muscle resistance arterioles. The results of this study provide new insight into the effects of ageing and exercise training on the vasodilatory responses of the resistance vasculature within skeletal muscles of varying fibre composition. First, the results confirm that endothelium-dependent vasodilator responses to ACh are diminished with age in arterioles from the soleus muscle (Fig. 2A), a highly oxidative muscle, but not in arterioles from the superficial portion of gastrocnemius muscle (Fig. 3A), a low-oxidative glycolytic muscle (Muller-Delp et al. 2002a). Second, the present findings demonstrate that the mechanism of the reduction in ACh-mediated vasodilatation with senescence in soleus muscle arterioles is through an endothelial NO pathway, although this decrement is not the result of lower arteriolar eNOS protein content (Fig. 4B). And finally, the results demonstrate that exercise training restores endothelium-dependent vasodilatation in soleus muscle arterioles from old rats to levels occurring in young sedentary rats. However, training in young rats enhances endothelium-dependent vasodilatation in soleus and gastrocnemius muscles beyond that of the old trained animals. Although both ageing- and training-induced alterations of endothelium-dependent function occur through an NO-dependent pathway, diverse mechanisms of NO-mediated vasodilatation are affected by ageing and training. To our knowledge, this is the first study to demonstrate that the ageing-induced impairment of ACh-mediated vasodilatation is not associated with reduced eNOS mRNA and protein expression in resistance arteries, whereas the training-induced enhancement of ACh-mediated vasodilatation is accompanied by an increase in eNOS mRNA and protein (Figs 4 and 5).
Ageing and endothelium-dependent vasodilatation
Previous studies have shown old age-associated reductions in endothelium-dependent vasodilatation to ACh in rats (Tominaga et al. 1994; Delp et al. 1995; Muller-Delp et al. 2002a; Woodman et al. 2002) and humans (Egashira et al. 1993; Taddei et al. 1995,2000; Gerhard et al. 1996; DeSouza et al. 2000). In humans, vascular responsiveness to ACh administered in the forearm is reduced in aged men (Taddei et al. 1995,2000; Gerhard et al. 1996; DeSouza et al. 2000) and women (Taddei et al. 1995; Gerhard et al. 1996), whereas vasodilatation to nitroprusside, an endothelium-independent vasodilator, is unchanged (Taddei et al. 1995; Gerhard et al. 1996; DeSouza et al. 2000). The results of the current study and previous work with rats (Muller-Delp et al. 2002a) show similar old-age-associated reductions in vasodilator responsiveness to ACh in soleus muscle arterioles with no change in the smooth muscle response to the NO donor nitroprusside. Results from the present study demonstrate that differences in ACh-induced vasodilatation between soleus muscle arterioles of young and old rats were abolished during NOS blockade, and that the addition of the cyclooxygenase inhibitor indomethacin did not reveal any further ageing effect, indicating that ageing diminishes the NO-dependent component of ACh-mediated vasodilatation in soleus muscle arterioles.
Despite the fact that old age is associated with endothelial dysfunction of the NO-dependent pathway in soleus muscle arterioles, results from the present study demonstrate that this is not due to a decrease in eNOS protein content (Figs 4B and 6). Although the present investigation is the first to report the effects of old age on skeletal muscle arteriolar eNOS protein levels, other studies have shown mixed results with eNOS protein in other arterial segments. Similar to the present study, several studies have shown impaired ACh-mediated vasodilatation and greater eNOS protein content in the aorta of old rats relative to that in young and middle aged rats (Cernadas et al. 1998; van der Loo et al. 2000). In contrast, Woodman et al. (2002) reported that a lower ACh-mediated vasodilatation of soleus muscle feed arteries was associated with lower levels of eNOS protein in old rats. The reason for the discrepancy in eNOS protein expression among studies is unclear, but may be related to the differential metabolic milieu and mechanical influences to which conduit arteries, feed arteries and muscle arterioles are exposed with old age.
It has been previously proposed that the stimulus for the ageing-induced decrement in endothelial NO-dependent vasodilatation in skeletal muscle arterioles is physical inactivity and deconditioning (Muller-Delp et al. 2002a). Several lines of evidence support this hypothesis. First, imposed muscular inactivity in rats via hindlimb unloading diminishes ACh-induced vasodilatation in skeletal muscle arterioles through a NOS mechanism; and second, this decrement in endothelium-dependent vasodilatation only occurs in arterioles from muscles composed of high-oxidative fibres, such as the soleus muscle, and not the superficial gastrocnemius muscle composed of low-oxidative, glycolytic fibres (Delp et al. 2000; Schrage et al. 2000; Woodman et al. 2001). Vascular ‘deconditioning’ would primarily be expected to occur in highly oxidative muscles, since this muscle type is recruited during low to moderate intensity activities and is the muscle group principally affected by decrements in activity (Armstrong & Laughlin, 1985; Delp & Duan, 1996; Baldwin & Haddad, 2001). In contrast, low-oxidative glycolytic muscles are recruited during high intensity activity (Armstrong & Laughlin, 1985; Delp & Duan, 1996) and therefore in normal sedentary organisms, muscles of this type are largely unaffected by physical inactivity (Armstrong & Laughlin, 1985; Delp & Duan, 1996; Baldwin et al. 2001). Indeed, the old-age-related decrement in soleus muscle citrate synthase activity in the present study (Table 1), but not in gastrocnemius muscle, indicates muscular deconditioning occurs primarily in the highly oxidative muscle where endothelium-dependent vasodilator dysfunction likewise occurs. There are, however, important distinctions between ageing-induced and deconditioning-induced decrements in endothelium-dependent vasodilatation in skeletal muscle arterioles. Deconditioning-induced decrements in ACh-mediated vasodilatation in soleus muscle arterioles are accompanied by decreases in eNOS mRNA and protein expression (Schrage et al. 2000; Woodman et al. 2001). In contrast, the ageing-induced decrease in ACh-mediated vasodilatation is accompanied by no change in eNOS mRNA expression and an increase in eNOS protein concentration (Figs 4 and 6). These data suggest that eNOS protein levels are a target for inactivity-related changes in the NO signalling mechanism of endothelium-dependent vasodilatation, whereas ageing alters another component of the eNOS signalling pathway.
It has been proposed that the greater eNOS protein content and activity in arteries from old animals is a compensatory mechanism to counter a reduction in NO bioavailability due to greater oxidative stress associated with old age (van der Loo et al. 2000). A reduction in NO bioavailability and, correspondingly, NO-dependent vasodilatation may be related to enhanced superoxide production and a concomitant reduction in arterial antioxidant capacity (Demaree et al. 1999; van der Loo et al. 2000; Woodman et al. 2002). Alternatively, a diminished availability of substrate and cofactor for NO synthesis could impair endothelium-dependent vasodilatation of arterioles with old age (Berkowitz et al. 2003). Such possibilities merit further investigation in the skeletal muscle microcirculation
Exercise training and endothelium-dependent vasodilatation
Studies in humans have shown that as little as 3 months of endurance training results in increased leg blood flow and vasodilator capacity in aged men (Martin et al. 1990; Beere et al. 1999) and women (Martin et al. 1990). Aged endurance-trained athletes likewise demonstrate greater ACh-induced vasodilatation in the forearm when compared to age-matched sedentary men (DeSouza et al. 2000; Taddei et al. 2000). The physically active individuals appear to have attenuated the age-associated decreases in endothelium-dependent vasodilatation via a NOS-dependent mechanism (Taddei et al. 2000). Unfortunately, due to the inherent limitations of the cross-sectional study design and the inclusion of smokers in the subject population, it is difficult to definitively conclude from this latter study that exercise training increases NO-dependent vasodilatation.
Similar to that occurring in humans, exercise training in young animals has been shown to elicit greater endothelium-dependent vasodilatation in conduit arteries (Delp et al. 1993; Delp & Laughlin, 1997) and skeletal muscle arterioles (Sun et al. 1994) through a NOS mechanism. Therefore, we sought to determine whether and through what mechanism(s) exercise training reverses ageing-related declines in endothelium-mediated vasodilatation in skeletal muscle arterioles. As in humans, the results demonstrate that exercise training restores ACh-induced vasodilatation in old rat soleus muscle arterioles to levels equivalent to that of young sedentary rats. The data additionally demonstrate that differences in maximal vasodilatation between old sedentary and trained rats, as well as between young sedentary and trained animals, were abolished in the presence of l-NAME and in the presence of l-NAME and indomethacin (Figs 2 and 3), indicating that the training-associated enhancement in ACh-mediated vasodilatation occurred through a NOS signalling mechanism. This assertion is further supported by the observation that in each instance where endothelium-dependent vasodilatation was greater in the trained versus sedentary arterioles, i.e. in young soleus, old soleus and young gastrocnemius muscle arterioles, there was a concomitant increase in eNOS protein expression (Figs 4B and 5B). These observations are consistent with previous reports that exercise training in young adult animals is associated with greater endothelium-dependent vasodilatation and eNOS mRNA and protein expression in the aorta and coronary resistance arteries (Delp et al. 1993; Muller et al. 1994; Sessa et al. 1994; Delp & Laughlin, 1997; Woodman et al. 1997; Kojda et al. 2001). In addition, the data collectively demonstrate that vascular eNOS protein expression is sensitive to alterations in physical activity, both increases (Delp et al. 1993; Muller et al. 1994; Sessa et al. 1994; Delp & Laughlin, 1997; Woodman et al. 1997; Kojda et al. 2001) and decreases (Schrage et al. 2000; Woodman et al. 2001), and appears to be a key component in adaptive responses of the vascular endothelium to exercise training and deconditioning. Although ageing-induced deficits in endothelium-dependent vasodilatation in skeletal muscle arterioles are not the result of reductions in vascular eNOS protein, increases in eNOS protein stimulated by chronic exercise appear to be part of an adaptive compensatory response to the old-age-associated deficit in a regulatory component of endothelial NO vasodilatation.
Ageing, training and vascular remodeling
Unlike vasodilator responses in soleus muscle arterioles, old age did not impair ACh-induced vasodilatation in gastrocnemius muscle arterioles (Fig. 3), nor did exercise training enhance vasodilator responses in old rats, despite the training-associated elevation in gastrocnemius muscle oxidative capacity (Table 1). However, NOS inhibition with l-NAME unmasked subtle ageing and training differences in the mechanism of how endothelium-dependent vasodilatation is achieved. In gastrocnemius muscle arterioles, NOS inhibition had no effect on ACh-induced vasodilatation in young sedentary animals, but decreased the arteriolar sensitivity (IC50) to ACh with old age and with training. Therefore, these data indicate a greater dependence on NO signalling in gastrocnemius muscle arterioles of old sedentary rats, as well as young and old trained rats.
One possibility for the lack of difference in maximal endothelium-dependent vasodilatation of gastrocnemius arterioles with ageing and with training in old rats is the structural remodelling of the vessels, i.e. the increase in maximal diameter (Fig. 1). One of the two primary mechanical forces that act upon the vasculature to induce structural adaptations is the shear stress that arises from the blood acting on endothelial cells (Kamiya & Togawa, 1980; Langille, 1993). Increases in vessel diameter are indicative of chronic increases in blood flow and, correspondingly, wall shear stress; the structural increase in maximal diameter serves to maintain shear stress within a given range despite sustained elevations in blood flow through the vessel (Kamiya & Togawa, 1980; Langille, 1993). An increase in maximal diameter of gastrocnemius muscle arterioles being related to an increase in blood flow with ageing seems contrary to published work demonstrating blood flow remains unchanged or may be lower with old age (Delp et al. 1998; Muller-Delp et al. 2002a). As discussed previously (Muller-Delp et al. 2002a), the lack of an ageing effect on maximal acetylcholine-induced vasodilatation and the larger maximal diameter of gastrocnemius muscle arterioles in old rats could be related to diminished physical activity, lower gastrocnemius muscle blood flow, and arteriolar rarefaction. Therefore, despite a putative decrease in total muscle blood flow that would be predicted from muscle atrophy and inactivity, a reduction in arteriolar density could increase blood flow and shear stress in the remaining arterioles and provide a stimulus for an adaptive increase in maximal diameter (Fig. 1) (Kamiya & Togawa, 1980; Langille, 1993) and greater dependence on NO signalling in acetylcholine-induced vasodilatation (Fig. 3B) (Nadaud et al. 1996) of gastrocnemius muscle arterioles from aged rats.
The larger maximal diameter of gastrocnemius muscle arterioles (Fig. 1) associated with exercise training, and the increased dependence on NO signalling (Fig. 3B) and eNOS expression (Fig. 5) in young and old rats are likely to be the result of the repetitive muscle hyperaemia and consequent elevations in shear stress that occurs with the exercise bouts throughout the 10–12 week training period. Of interest then are the seemingly disparate adaptations to training that occur between the soleus (greater maximal endothelium-dependent vasodilatation) and gastrocnemius (greater maximal diameter) muscle arterioles. We speculate that these differences may be due to the magnitude of the training stimulus. For example, blood flow to the postural soleus muscle is high at rest relative to that in the gastrocnemius muscle (Laughlin & Armstrong, 1983; Delp et al. 1998). During prolonged bouts of exercise, perfusion increases to both the soleus and gastrocnemius muscles, but the greatest relative increase occurs within the gastrocnemius muscle (Laughlin & Armstrong, 1983). Thus, the chronic and repetitive elevations of shear stress in soleus muscle arterioles may induce increases in maximal endothelium-dependent vasodilatation. In contrast, a potentially greater chronic and repetitive elevation of shear stress occurs in gastrocnemius muscle arterioles that may initially elicit increases in maximal NO-dependent vasodilatation, but then progresses further to induce a structural remodelling which increases maximal diameter and returns shear stress to levels occurring in sedentary animals. A time-course study which includes measures of vascular dimensions and muscle blood flow will be necessary to confirm this hypothesis.
Significance and conclusions
The onset of impaired endothelial vasodilator function with advancing age has been proposed to account, at least in part, for the increased incidence of cardiovascular disorders among the elderly, including atherosclerosis and hypertension (Matz & Andriantsitohaina, 2003). Therefore, various strategies to maintain endothelium-dependent vasodilatation have been considered to minimize long-term cardiovascular risk with old age. These include pharmacological intervention, hormone therapy and physical activity (Matz, 2003). The present study demonstrates that an exercise intervention during old age can restore deficits in endothelium-dependent vasodilator responsiveness in skeletal muscle arterioles (Fig. 2), as well as increase dependence on NO signalling (Fig. 3B) in all muscle types studied. Given the antiatherosclerotic properties of endothelial NO, including inhibition of platelet aggregation and smooth muscle proliferation (Mooradian et al. 1995; Tsao et al. 1995), these results support the use of exercise as a strategy in the management of cardiovascular risk factors.
In summary, the results of this study confirm previous findings (Muller-Delp et al. 2002a) that ageing impairs endothelium-dependent vasodilatation to ACh in arterioles from highly oxidative muscle (Fig. 2), but not in arterioles from low-oxidative glycolytic muscle (Fig. 3). The present findings further demonstrate that the diminished vasodilatation of soleus muscle arterioles occurs through an impairment of NO signalling (Fig. 2), but this is not the result of reduced arteriolar eNOS protein content (Fig. 4B). This observation suggests that the ageing-induced impairment of endothelium-dependent vasodilatation is due to a primary ageing process and not simply the result of physical inactivity and deconditioning. Exercise training ameliorated the age-associated reduction of the ACh-mediated vasodilatation in soleus muscle arterioles, and this adaptive response was also mediated through the NO signalling pathway. However, unlike the old-age-associated vasodilator dysfunction, the training-induced enhancement of endothelium-dependent vasodilatation was associated with an increase in eNOS protein expression. Thus, old age and exercise training both affect NO signalling of endothelium-dependent vasodilatation, but they do not appear to act on the same portion of the NOS signalling cascade.
Acknowledgments
This work was supported by American Heart Association, Texas Affiliate, Grant 98BG-801, National Institute on Ageing Grant R21AG19248-01, and National Aeronautics and Space Administration grant NAG2-1340. The authors gratefully acknowledge Katherine A. Kelly for her technical contributions to this work.
References
- Armstrong RB, Laughlin MH. Metabolic indicators of fibre recruitment in mammalian muscles during locomotion. J Exp Biol. 1985;115:201–213. doi: 10.1242/jeb.115.1.201. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- Berkowitz DE, White R, Dechun L, Khalid MM, Cernetich A, Kim S, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- Cernadas MR, Sánchez de Miguel L, García-Durán M, González-Fernández F, Millás I, Montón M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- Delp MD, Brown M, Laughlin MH, Hasser EM. Rat aortic vasoreactivity is altered by old age and hindlimb unloading. J Appl Physiol. 1995;78:2079–2086. doi: 10.1152/jappl.1995.78.6.2079. [DOI] [PubMed] [Google Scholar]
- Delp MD, Colleran PN, Wilkerson MK, McCurdy MR, Muller-Delp J. Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am J Physiol Heart Circ Physiol. 2000;278:H1866–H1873. doi: 10.1152/ajpheart.2000.278.6.H1866. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol. 1998;85:1813–1822. doi: 10.1152/jappl.1998.85.5.1813. [DOI] [PubMed] [Google Scholar]
- Delp MD, Laughlin MH. Time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exerc. 1997;29:1454–1461. doi: 10.1097/00005768-199711000-00011. [DOI] [PubMed] [Google Scholar]
- Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vasoreactivity of rat abdominal aorta. J Appl Physiol. 1993;75:1354–1363. doi: 10.1152/jappl.1993.75.3.1354. [DOI] [PubMed] [Google Scholar]
- Demaree SR, Lawler JM, Linehan J, Delp MD. Ageing alters aortic antioxidant enzyme activities in Fischer-344 rats. Acta Physiol Scand. 1999;16:203–208. doi: 10.1046/j.1365-201x.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Roddy M-A, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- Haidet GC. Effect of age on cardiovascular responses to static muscular contraction in beagles. J Appl Physiol. 1992;73:2320–2327. doi: 10.1152/jappl.1992.73.6.2320. [DOI] [PubMed] [Google Scholar]
- Haidet GC, Parsons D. Reduced exercise capacity in senescent beagles: an evaluation of the periphery. Am J Physiol Heart Circ Physiol. 1991;260:H173–H182. doi: 10.1152/ajpheart.1991.260.1.H173. [DOI] [PubMed] [Google Scholar]
- Hongo K, Nakagomi T, Kassell NF, Sasaki T, Lehman M, Vollmer DG, et al. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988;19:892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol. 1987;42:660–665. doi: 10.1093/geronj/42.6.660. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–2844. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990;6:529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Cardiovascular system. In: Masoro EJ, editor. Handbook of Physiology. New York: Oxford University Press; 1995. pp. 413–474. section 11, Aging. [Google Scholar]
- Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21(Suppl.):S11–S17. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am J Physiol Heart Circ Physiol. 1983;244:H814–H824. doi: 10.1152/ajpheart.1983.244.6.H814. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: the effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Martin WHI, Kohrt WM, Malley MT, Korte E, Stoltz S. Exercise training enhances leg vasodilatory capacity of 65-yr-old men and women. J Appl Physiol. 1990;69:1804–1809. doi: 10.1152/jappl.1990.69.5.1804. [DOI] [PubMed] [Google Scholar]
- Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003;20:527–550. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol. 2000;89:398–405. doi: 10.1152/jappl.2000.89.1.398. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation. 1994;89:2308–2314. doi: 10.1161/01.cir.89.5.2308. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002a;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002b;282:H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Nadaud S, Philippe M, Arnal JF, Michel JB, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857–863. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol. 2000;89:1483–1490. doi: 10.1152/jappl.2000.89.4.1483. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circulation Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Meth Enzymol. 1969;13:3–5. [Google Scholar]
- Sun D, Koller A, Kaley G. Short-term daily exercise activity enhances endothelial NO synthesis in skeletal muscle arterioles of rats. J Appl Physiol. 1994;76:2241–2247. doi: 10.1152/jappl.1994.76.5.2241. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Fujii K, Abe I, Takata Y, Kobayashi K, Fujishima M. Hypertension and ageing impair acetylcholine-induced vasodilation in rats. J Hypertension. 1994;12:259–268. [PubMed] [Google Scholar]
- Tsao PS, Lewis NP, Alpert S, Cooke JP. Exposure to shear stress alters endothelial adhesiveness. Role of nitric oxide. Circulation. 1995;92:3513–3519. doi: 10.1161/01.cir.92.12.3513. [DOI] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1743. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Muller JM, Laughlin MH, Price EM. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am J Physiol Heart Circ Physiol. 1997;273:H2575–H2579. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Schrage WG, Rush JWE, Ray CA, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases endothelium-dependent dilation and eNOS expression in soleus not gastrocnemius. J Appl Physiol. 2001;91:1091–1098. doi: 10.1152/jappl.2001.91.3.1091. [DOI] [PubMed] [Google Scholar]