Abstract
31P-magnetic resonance spectroscopy was used to study phosphocreatine (PCr) onset kinetics in exercising human gastrocnemius muscle under varied fractions of inspired O2 (FIO2). Five male subjects performed three identical work bouts (5 min duration; order randomised) at a submaximal workload while breathing 0.1, 0.21 or 1.0 FIO2. Either a single or double exponential model was fitted to the PCr kinetics. The phase I τ (0.1, 38.6 ± 7.5; 0.21, 34.5 ± 7.9; 1.0, 38.6 ± 9.2 s) and amplitude, A1 (0.1, 0.34 ± 0.03; 0.21, 0.28 ± 0.05; 1.0, 0.28 ± 0.03,% fall in PCr) were invariant (both P > 0.05) across FIO2 trials. The initial rate of change in PCr hydrolysis at exercise onset, calculated as A1/τ1 (%PCr reduction s−1), was the same across FIO2 trials. A PCr slow component (phase II) was present at an FIO2 of 0.1 and 0.21; however, breathing 1.0 FIO2 ablated the slow component. The onset of the slow component resulted in a greater (P ≤ 0.05) overall percentage fall in PCr (both phase I and II) as FIO2 decreased (0.43 ± 0.05, 0.34 ± 0.05, 0.28 ± 0.03) for 0.1, 0.21 and 1.0 FIO2, respectively. These data demonstrate that altering FIO2 does not affect the initial phase I PCr onset kinetics, which supports the notion that O2 driving pressure does not limit PCr kinetics at the onset of submaximal exercise. Thus, these data imply that the manner in which microvascular and intracellular PO2 regulates PCr hydrolysis in exercising muscle is not due to the initial kinetic fall in PCr at exercise onset.
Across the rest to work transition, [ATP] in working muscle is maintained by phosphocreatine (PCr) hydrolysis in a reaction catalysed by creatine kinase (CK). After a few seconds (if not immediately), both glycolysis (Connett et al. 1990; Howlett et al. 1999) and oxidative phosphorylation (Bangsbo, 2000; Behnke et al. 2003; Kindig et al. 2003) are activated. A direct proportionality between the kinetics of pulmonary V̇O2 and PCr hydrolysis has been demonstrated previously (Mahler, 1985; Meyer, 1988), and thus the time course of pulmonary V̇O2 onset kinetics has been related to changing [PCr] in muscle at the start of exercise (Marsh et al. 1993; Barstow et al. 1994; McCann et al. 1995; McCreary et al. 1996; Rossiter et al. 1999). In addition, the steady state level to which PCr falls is correlated positively to the steady state level of V̇O2 during exercise (Mahler, 1985; Meyer, 1988). These data suggest that PCr hydrolysis (specifically the increase in inorganic phosphate) is a potent regulator of oxidative phosphorylation (Meyer, 1988), and that PCr hydrolysis and regulation of oxidative phosphorylation are tightly coupled (Meyer & Foley, 1996).
Our laboratory has demonstrated previously that the tight coupling between the steady state levels of PCr depletion and V̇O2 can be dissociated by altering O2 availability (specifically, microvascular and intracellular PO2; Richardson et al. 1999) during exercise by breathing varied fractions of inspired oxygen (FIO2) (Haseler et al. 1998). Additionally, pulmonary V̇O2 kinetics have been modulated in an O2-dependent manner by altering the FIO2 (Hughes et al. 1968; Linnarsson et al. 1974; Engelen et al. 1996) although other investigations have reported invariant V̇O2 kinetics in the face of altered FIO2 (Linnarsson, 1974; Hughson & Kowalchuk, 1995). To date, whether PCr onset kinetics are O2 dependent has not been tested. Given the tight relationship between pulmonary V̇O2 and PCr hydrolysis, one possible scenario is that PCr kinetics will follow suit with V̇O2 kinetics (Hughes et al. 1968; Linnarsson et al. 1974; Engelen et al. 1996) and be slowed as FIO2 is reduced. Alternatively, despite the fact that V̇O2 kinetics can be slowed with hypoxia due to the necessity for O2 to serve as the terminal electron acceptor within the electron transport chain, PCr hydrolysis is catalysed by CK in a process largely dependent upon the metabolite signals arising from the contractile sites. Thus, regardless of O2 availability, PCr kinetics may remain unchanged due to the similar ATP demand of work.
At low work intensities (moderate work domain), pulmonary V̇O2 increases in a monoexponential fashion preceded by a short time delay. During work performed above the lactate threshold (i.e. heavy and severe exercise domains), a secondary rise in V̇O2 occurs around 2–3 min after exercise onset (Whipp & Wasserman, 1972; Gaesser & Poole, 1996). This secondary rise in pulmonary V̇O2, which has been termed the slow component, only occurs during a sustained lactic acidosis and represents an increased O2 cost for a constant work rate. A recent study demonstrated a slow component of PCr onset kinetics with similar magnitude and time course to the V̇O2 slow component (Rossiter et al. 2002). It has been well established that altering FIO2 can alter the absolute work rate at which the lactate threshold occurs (Welch, 1982; Hogan et al. 1983). Thus it is possible, during work performed at an intensity above the lactate threshold, that hyperoxia may shift the work intensity from the heavy to the moderate domain. If true, in this scenario one might reasonably expect an ablation of the V̇O2 slow component under hyperoxic conditions with a similar observation for the slow component of PCr onset kinetics.
To resolve these issues, we examined the effect of FIO2 on PCr onset kinetics, measured by 31P magnetic resonance spectroscopy (MRS), in human subjects performing plantar flexion exercise at a work intensity that elicited a ‘slow component’ during normoxia while breathing varied FIO2 (0.1, 0.21 and 1.00).
Methods
Subjects
Five healthy men, aged 21–42 years, volunteered to participate in this study and gave written informed consent. The Human Subjects Committee of the University of California, San Diego, approved the study in accordance with the Declaration of Helsinki. Subjects were all active, ranging from recreational to well-trained athletes. The subjects refrained from strenuous activity for 24 h prior to data collection.
Exercise protocol
Subjects were familiarized with plantar flexion exercise in the confines of a whole body MRI system. At this time, a work rate which elicited a PCr slow component while breathing room air was determined which corresponded with ∼60% of maximum work rate for each subject. Subjects performed constant load submaximal plantar flexion at this intensity (7.2 ± 0.6 W) at a frequency of 1 Hz (maintained with the aid of an electronic metronome) while lying supine in a superconducting 1.5 T magnet. At each FIO2 (0.1, 0.21 and 1.00), subjects performed a 5 min warm-up followed by 5 min of rest, and then 5 min of exercise followed by 5 min of recovery. Subjects were allowed 30 min of rest between each complete exercise bout. MRS data were acquired continuously for 2 min before exercise, and for the 5 min exercise bout. The order of the three treatments was randomized and blinded to the subjects to minimize any putative ordering effects.
Arterial saturation and arterial PO2
Throughout each exercise bout, subjects breathed through a low resistance two-way breathing valve (2700; Hans-Rudolph Inc., Kansas City, MO, USA). Arterial O2 saturation was monitored continuously throughout the experiment with a finger probe oximeter (Omni-Trak, In Vivo Research Inc., USA). During previous studies (Haseler et al. 1998; Haseler et al. 1999) a high correlation (r2= 0.9) between arterial O2 saturation calculated from end-tidal O2 gas measurement and that measured with this oximeter system was observed. These saturations were then used to calculate arterial PO2 using the Hill equation, assuming a normal O2 half-saturation pressure (P50).
31P MRS
MRS was performed using a clinical 1.5 T General Electric Signa system (5.4.2 version) operating at 25.86 MHz for 31P. 31P MRS data were acquired with a transmit/receive surface coil (diameters 20 and 10 cm, respectively) placed under the calf at its maximum diameter. The centring of the leg over the coil was confirmed by T1 weighted 1H localizing images obtained in the axial plane and the coil was repositioned if the major part of the gastrocnemius muscle was not within this range. Shimming on the proton signal from tissue water optimized magnetic field homogeneity and the 31P MRS signal was optimized by pre-scan transmitter gain adjustment so that ∼72% of the signal acquired was from tissue within 5 cm of the surface coil. A 500 μs hard pulse was used for signal excitation. The spectral width was 2500 Hz, and a single free induction decay (FID) was acquired every 4 s for 2 min prior to the onset of exercise and for the 5 min of exercise. As a result the data were expressed with a time resolution of 4 s.
Data analysis
Data were processed using SAGE/IDL software General Electric Medical Systems on a Silicon Graphics Indigo workstation. Each FID consisted of 1024 complex points and was processed with 5 Hz exponential line broadening prior to zero filling and Fourier transformation. All spectra were manually phased using zero and first order phase corrections. There were no phase variations between rest and exercise during the experiment. The levels of PCr determined from the intensity of that peak were normalized to 100% using the average value obtained from the last 40 s of rest acquired for each subject as a reference. Muscle intracellular pH was calculated from the chemical shift difference (δ) of the Pi peak relative to the PCr peak using the following equation:
(Taylor et al. 1983). Signal to noise ratios (∼30: 1) were sufficient to allow PCr levels to be determined with a temporal resolution of 4 s during the rest to exercise transition.
Kinetic analysis
Curve fitting (Kaleidagraph 3.5, Synergy software, Reading PA, USA) was performed on the PCr data using a monoexponential function:
and a double-exponential function
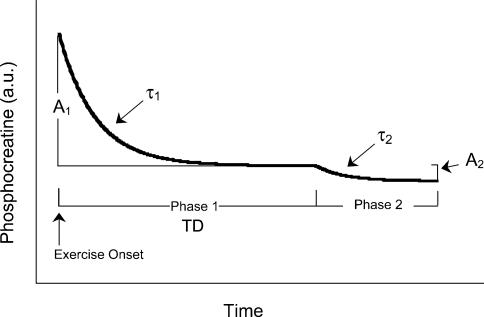
where PCr(t), PCrb and ΔPCrss are PCr at time t, baseline (pre-exercise) and the fall in PCr from resting baseline to steady-state exercising values, respectively. For the more complex double-exponential model, ΔPCrprimary and ΔPCrsecondary designate the asymptotic value to which that component of the ΔPCr is projecting. Figure 1 is a schematic diagram illustrating the kinetic analysis described above and referred to in the results. The τ1 and τ2 are the respective time constants and TD is the independent time delay prior to the onset of the secondary phase (slow component). Goodness of fit was assessed via visual inspection and analysis of the residual fit to the model as well as the coefficient of determination and Chi square values. In addition, time to 50% of the overall fall (T50) was calculated, independent of modelling procedures.
Figure 1. A schematic diagram of the model and kinetic parameters used to fit the data as described in Methods and Results.
A1, amplitude of the initial phase I fall in PCr; τ1, time constant for the phase I fall in PCr; A2, amplitude of the phase II fall in PCr (slow component); τ2, time constant of the phase II kinetics; TD, time delay prior to slow component onset.
Statistical analysis
Data were tested with repeated measures ANOVA (Tukey's post hoc test) using a commercially available software package (InStat, GraphPad Software Inc., San Diego, CA, USA). Data were considered significantly different when P≤ 0.05. Data are presented as means ± s.e.m.
Results
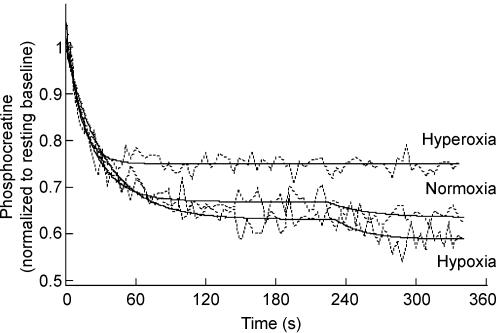
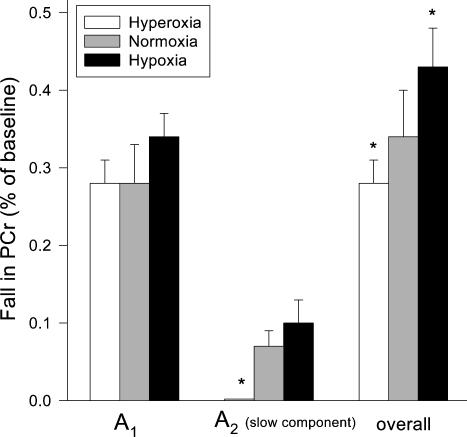
Normalized PCr data at rest and across the three exercise bouts for a representative subject (raw data (4 s time resolution) with model fit for each trial) are shown in Fig. 2. The PCr phase I amplitude (A1), expressed as a fraction of resting PCr levels, was not different (0.1, 0.34 ± 0.03; 0.21, 0.28 ± 0.05; 1.0, 0.28 ± 0.03; P > 0.05) across the range of FIO2 values employed in this study (Fig. 3). A PCr slow component was present at an FIO2 of 0.1 and 0.21; however, at 1.0 FIO2 there was no discernible slow component (Figs 2 and 3). This was confirmed statistically with significantly greater (P < 0.05) coefficient of determination and Chi square values for the 2- versus 1-component model at FIO2 of 0.1 and 0.21, yet unchanged (both P > 0.05) values at FIO2 = 1.0. The onset of the slow component (i.e. TD) at 0.1 and 0.21 FIO2 occurred 181 ± 16 and 188 ± 27 s into the exercise bout, respectively. The overall percentage fall in PCr (including phase II ‘slow component’) increased significantly as FIO2 decreased (0.1, 0.43 ± 0.05; 0.21, 0.34 ± 0.05; 1.0, 0.28 ± 0.03; Fig. 3).
Figure 2. Raw data and the model fit for PCr hydrolysis at exercise onset in a representative subject under each FIO2 (hypoxia 0.1, normoxia 0.21, hyperoxia 1.0).
Note that the slow component of PCr hydrolysis, manifest in the hypoxic and normoxic trials, is ablated in the hyperoxic condition.
Figure 3. The effect of FIO2 on the phase I, phase II and overall PCr amplitudes.
The amplitude of the initial phase I fall of PCr (A1), expressed as a fraction of resting PCr, was not different as FIO2 was increased. A slow component of PCr was present at FIO2 of 0.1 and 0.21 (A2). The overall fall in PCr, both A1 and A2, increased significantly as FIO2 was decreased.
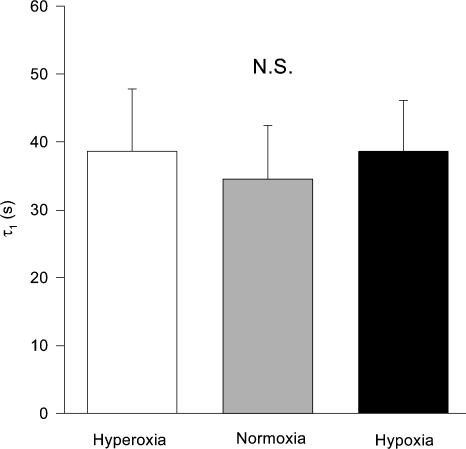
Figure 4 demonstrates that the PCr A1 time constants (i.e. τ1) were invariant (P > 0.05) across the varied FIO2 trials (0.1, 38.6 ± 7.5; 0.21, 34.5 ± 7.9; 1.0 38.6 ± 9.2 s). In addition, the initial rate of change in PCr hydrolysis at exercise onset, calculated as A1/τ1 (%PCr reduction s−1), was the same across FIO2 trials (Table 1). However, if the ‘speed of the PCr fall at exercise onset’ is simply assessed as time to 50% of the overall PCr fall (i.e. independent of modelling procedures), the T50 was significantly slowed as FIO2 was reduced from 1.0 to 0.1 (Table 1).
Figure 4. The time constants, τ1, of the initial phase I kinetics of PCr hydrolysis.
The initial phase I kinetics of PCr breakdown at the onset of exercise were invariant (P > 0.05) across the varied FIO2 trials.
Table 1.
A summary of data acquired under eachFIO2condition. The intracellular pH was determined for the last 40 s of exercise
| Hyperoxia | Normoxia | Hypoxia | |
|---|---|---|---|
| T50 (s) | 16.6 ± 3.7* | 26.6 ± 4.9 | 38.6 ± 8.0* |
| A1/τ1 (s) | 0.01 ± 0.003 | 0.01 ± 0.003 | 0.01 ± 0.002 |
| Intracellular pH | 7.09 ± 0.01* | 7.07 ± 0.01 | 7.01 ± 0.02* |
| SaO2 | 100 ± 1.0 | 98.2 ± 0.3 | 70.1 ± 3.6* |
| Arterial PO2 | 650 ± 7* | 120 ± 4 | 42 ± 10* |
Significantly different from Normoxia, P < 0.05.
Intracellular pH, calculated from the 31P MRS spectra obtained during the last 40 s of exercise, was significantly greater during the hyperoxic condition and lower during the hypoxic condition, than during normoxic breathing (Table 1). The arterial O2 saturations reported in Table 1 were significantly reduced with hypoxic breathing compared to normoxia with only a minimal increase with hyperoxia. The arterial PO2 calculated from the arterial saturations increased with increasing FIO2 (Table 1).
Discussion
To our knowledge, this is the first study to investigate the effect of FIO2 on PCr onset kinetics in humans performing dynamic submaximal plantar flexion exercise. In the present study, we altered FIO2 in order to evoke large changes in arterial PO2. Thus, reducing FIO2 from 1.0 to 0.21–0.1 resulted in a reduced microvascular PO2 and ultimately less diffusive O2 flux to the myocyte at a given workload regardless of O2 delivery (Richardson et al. 1999). Two key and novel findings in this current study are that (1) altered FIO2 did not alter phase I PCr on-kinetics and (2) a hyperoxic FIO2 ablated the PCr slow component manifest during normoxic and hypoxic exercise. The data demonstrate that the initial phase of the fall in PCr does not depend critically upon the O2 driving gradient from the microvasculature to the mitochondria, at least across the range evoked by breathing gases across the FIO2 range from 0.1 to 1.0. The ablation of the PCr slow component with an FIO2 of 1.0 suggests that PO2 (both microvascular and intracellular) is important in the regulation of PCr hydrolysis after the initial phase I kinetics. As a whole, these data suggest that the mechanism by which altering FIO2 impacts upon the cellular metabolic state in exercising muscle is not related to the speed of the initial PCr fall, but rather to events occurring thereafter.
V̇O2 and PCr hydrolysis
Recently, Rossiter et al. (2002) simultaneously acquired both muscle PCr kinetics and pulmonary V̇O2 kinetics across the rest to work transition in humans performing both moderate and heavy intensity knee extensor exercise. Cogent to the current investigation, two key findings were observed. First, the time course of the fall in PCr matched that for the rise in V̇O2 at exercise onset as previously described (Barstow et al. 1994; McCreary et al. 1996). Second, the onset of the V̇O2 slow component was in close temporal proximity with a slow component of PCr hydrolysis. These data support the tenet that the slow component originates within the exercising muscle (Poole, 1994; Gaesser & Poole, 1996). Also, these findings set the stage to discuss the mechanisms for the PCr slow component acquired in the current study in the context of data reported previously for pulmonary V̇O2.
Effect of FIO2 on metabolism
It has long been accepted that altering FIO2 can result in a change in the absolute work rate at which the lactate threshold is reached during a ramped increase in work. Welch (1982) demonstrated that, during cycle ergometry, lowering the FIO2 shifted the lactate threshold to the left (i.e. a lower absolute work rate and V̇O2 %) whereas breathing hyperoxic gas shifted the lactate threshold to the right (Welch, 1982). The interpretation of these findings are confounded by the likelihood that blood flow to the working muscle was elevated during hypoxia and possibly reduced during hyperoxia to counteract the reduced haemoglobin saturation such that oxygen delivery (Q̇O2) remained unchanged (Rowell et al. 1986; Hogan et al. 1988). However, microvascular PO2 would be elevated at each progressively greater FIO2, which would increase the potential for O2 flux from the capillary to the mitochondrion during the exercise bout.
At the transition to moderate intensity exercise, the speed of the pulmonary V̇O2 response does not depend critically on O2 availability in normal healthy subjects whereas above the lactate threshold, the V̇O2 kinetics may be dependent, in part, on the O2 concentration (Hughson & Morrissey, 1982; Gerbino et al. 1996; Tschakovsky & Hughson, 1999). To date, the specific PO2 within the microcirculation that may alter the initial metabolic response from rest to high-intensity exercise is not known. Wilson & Rumsey (1988) demonstrated that a PO2 of 30 Torr requires compensatory changes in the phosphorylation and redox potential to maintain mitochondrial respiration in cell suspensions but the changes are minor until the PO2 drops to around 10 Torr. This finding has been corroborated during small muscle mass exercise in humans, in which manipulations in FIO2 induced significant alterations in steady-state PCr levels and intracellular PO2 (Haseler et al. 1998; Richardson et al. 1999).
Furthermore, and possibly more pertinent to the current study, previous investigations have demonstrated that alterations in FIO2 may alter the kinetics of V̇O2 in an O2-dependent manner (Linnarsson et al. 1974; Engelen et al. 1996; MacDonald et al. 1997). However, other investigations have observed no changes in V̇O2 kinetics with altered FIO2 (Linnarsson, 1974; Hughson & Kowalchuk, 1995). In a series of investigations by Grassi and colleagues using the isolated dog hindlimb preparation, acute manipulations in Q̇O2 and/or availability of O2 did not affect V̇O2 on-kinetics during moderate intensity exercise (Grassi et al. 1998a, b), but increasing O2 delivery did speed V̇O2 kinetics in response to maximal intensity exercise (Grassi et al. 2000). The data presented here suggest that altering the FIO2 does not affect the primary amplitude of PCr kinetics in humans, at least during small muscle mass exercise, which implies that the metabolic perturbation seen with reduced O2 availability during steady state exercise is not due to altered primary (initial) PCr kinetics (Haseler et al. 1998).
Kinetics of initial PCr fall
At the onset of exercise, the rate of PCr hydrolysis increases to meet the increase in ATP demand. PCr hydrolysis and oxidative phosphorylation are tightly coupled such that PCr falls with the same time course and in proportion to pulmonary V̇O2 (Mahler, 1985; Rossiter et al. 1999). The regulation of this kinetic response has been the subject of some controversy with investigations focusing on two hypotheses: (1) the oxidative enzyme inertia hypothesis (Cerretelli et al. 1980; Grassi et al. 1998a; Grassi et al. 1998b), where the delayed rise in V̇O2 reflects a metabolic inertia determined solely by levels of cellular metabolic controllers (phosphorylation potential and redox state) and/or mitochondrial enzyme activation, and (2) the O2 delivery hypothesis, where the speed of the rise in V̇O2 is related to the rate of adjustment of O2 delivery to the exercising muscles (Gerbino et al. 1996; Hughson et al. 1996; MacDonald et al. 1997). A complex interaction between the cellular metabolic state, enzyme activation state, and O2 availability is likely to be responsible for determining the rate of change in oxidative phosphorylation at the onset of exercise (Tschakovsky & Hughson, 1999).
In the current investigation, O2 availability was manipulated by having the subjects exercise while breathing a range of FIO2. As discussed in the previous section, the effect of varying FIO2 on convective O2 delivery is dampened by an alteration in muscle blood flow (Rowell et al. 1986). The most significant effect of hyperoxia is to raise blood PO2, as Hb saturation is already close to its ceiling in normoxia (and muscle blood flow tends to fall in hyperoxia). Hence, it is suggested that in hyperoxia the driving gradient from blood to muscle was enhanced resulting in an elevated intracellular PO2; conversely, hypoxia may result in elevated blood flow, but a reduced O2 driving gradient (Richardson et al. 1999). The diffusive component of O2 transport would be enhanced with increasing FIO2 due to the increasing microvascular and intracellular PO2. The time constant of the initial fall in PCr was unchanged with increasing FIO2 (Fig. 4), as was the initial rate of change in PCr hydrolysis (Table 1) consistent with data reported by Kindig et al. (2003) in frog single skeletal muscle fibres. The present data suggest that O2 availability (diffusive component with altered FIO2) is not a key determinant of the initial PCr hydrolysis response at the onset of exercise in humans. However, what this investigation fails to address is whether V̇O2 kinetics were altered during the plantar flexion exercise with changing FIO2. One postulate would be that the V̇O2 kinetics would not be changed in the face of invariant PCr kinetics and thus the PCr and V̇O2 kinetic relationship is maintained as previously described (Mahler, 1985; Rossiter et al. 1999). However, while oxidative phosphorylation is ultimately dependent upon O2 as the terminal electron acceptor within the electron transport chain, the CK-catalysed breakdown of PCr is primarily dependent upon the metabolic signals arising from the contractile sites. Thus, the 0.1 FIO2 condition represents a unique situation in which PCr kinetics may be dissociated from V̇O2 kinetics.
PCr hydrolysis and the slow component
It has been well documented that a V̇O2 slow component occurs solely during work performed above the lactate threshold (Gaesser & Poole, 1996). This apparent reduction in muscle ‘efficiency’ does not occur immediately, but rather some 2–3 min after the onset of aerobic work. This additional V̇O2 gain slows the overall speed of the V̇O2 kinetic response (Whipp & Wasserman, 1972; Linnarsson, 1974). Mechanisms responsible for the additional O2 cost associated with the V̇O2 slow component have yet to be resolved (Gaesser & Poole, 1996), although recent research suggests that it may be related to muscle fibre or muscle fibre type recruitment consequent to the sustained lactic acidemia (Shinohara & Moritani, 1992; Poole, 1994; Ozyener et al. 2001; Burnley et al. 2002).
Previous investigations have been able to manipulate the slow component to a certain extent. Originally, it was demonstrated that exercise training shifts the lactate threshold to an overall greater absolute workload, such that performing work, which would have evoked a slow component prior to training, will no longer do so (Gaesser & Poole, 1996). Furthermore, Kindig et al. (2001) demonstrated an earlier onset of the slow component, yet unaltered amplitude, after nitric oxide synthase inhibition, which was thought to be due to an early onset of fatigue induced by reduced O2 delivery. Burnley et al. (2000) have observed a significantly reduced slow component amplitude following an initial ‘priming’ bout of exercise. Additionally, one investigation by MacDonald et al. (1997) demonstrated that hyperoxia (FIO2= 0.70) shortened the V̇O2 mean response time and reduced the amplitude of the slow component. One shortcoming of that investigation was the inability to utilize 100% O2 (i.e. the inability to perform the Haldane transformation) and thereby further increase the O2 driving gradient from the microvasculature to the myocyte. In the current investigation, an FIO2 of 1.00 was employed and this manipulation ablated the slow component of PCr hydrolysis. Given that the slow component is only evidenced at work performed above the lactate threshold, the most logical explanation for this would be that hyperoxia shifted the lactate threshold such that during hyperoxic breathing, the work intensity was actually in the moderate rather than the heavy domain. Additionally, and along a similar line to that of MacDonald et al. (1997), the greater O2 driving gradient may have reduced the fatigue incurred, thus slowing the recruitment of additional fibres thought to be integral to the mechanism of the slow component.
Summary
These data demonstrate that altering FIO2 does not alter the initial phase I PCr onset kinetics, which supports the notion that O2 driving pressure does not limit energetics at the onset of submaximal exercise. However, the ablation of the slow component with hyperoxic breathing suggests an important role for microvascular and intracellular PO2 in the regulation of PCr hydrolysis in exercising muscle after the initial phase I of PCr kinetics which modulates the level of metabolic perturbation induced by varying FIO2.
Acknowledgments
The authors thank the subjects for their time in volunteering for this research. This research was supported by grants from NIH NHLBI HL-17731, NIH NIAMSD AR-40155 and AR-48461, a Grant in Aid from the American Heart Association (AHA 9960064Y), National Center for Regional Resources (RR14785) and TRDRP 10KT-0335. C. A. K. was a Parker B. Francis Fellow.
References
- Bangsbo J. Muscle oxygen uptake in humans at onset of and during intense exercise. Acta Physiol Scand. 2000;168:457–464. doi: 10.1046/j.1365-201x.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen uptake kinetics during moderate exercise. J Appl Physiol. 1994;77:1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI, Poole DC. Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol. 2003;549:597–605. doi: 10.1113/jphysiol.2002.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnley M, Doust JH, Ball D, Jones AM. Effects of prior heavy exercise on VO2 kinetics during heavy exercise are related to changes in muscle activity. J Appl Physiol. 2002;93:167–174. doi: 10.1152/japplphysiol.01217.2001. [DOI] [PubMed] [Google Scholar]
- Burnley M, Jones AM, Carter H, Doust JH. Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol. 2000;89:1387–1396. doi: 10.1152/jappl.2000.89.4.1387. [DOI] [PubMed] [Google Scholar]
- Cerretelli P, Rennie DW, Pendergast DR. Kinetics of metabolic transients during exercise. In: Cerrretelli P, Whipp BJ, editors. Exercise Bioenergetics and Gas Exchange. Amsterdam: Elsevier; 1980. pp. 187–209. [Google Scholar]
- Connett RJ, Honig CR, Gayeski TEJ, Brooks GA. Defining hypoxia: a systems view of VO2, glycolysis, energetics, and intracellular PO2. J Appl Physiol. 1990;68:833–842. doi: 10.1152/jappl.1990.68.3.833. [DOI] [PubMed] [Google Scholar]
- Engelen M, Porszasz J, Riley M, Wasserman K, Maehara K, Barstow TJ. Effects of hypoxic hypoxia on O2 uptake and heart rate kinetics during heavy exercise. J Appl Physiol. 1996;81:2500–2508. doi: 10.1152/jappl.1996.81.6.2500. [DOI] [PubMed] [Google Scholar]
- Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exerc Sport Sci Rev. 1996;24:35–71. [PubMed] [Google Scholar]
- Gerbino A, Ward SA, Whipp BJ. Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J Appl Physiol. 1996;80:99–107. doi: 10.1152/jappl.1996.80.1.99. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998a;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect VO2 on-kinetics in isolated insitu canine muscle. J Appl Physiol. 1998b;85:1404–1412. doi: 10.1152/jappl.1998.85.4.1404. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden LB. Role of convective O2 delivery in determining VO2 on-kinetics in canine muscle contracting at peak VO2. J Appl Physiol. 2000;89:1293–1301. doi: 10.1152/jappl.2000.89.4.1293. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Hogan MC, Richardson RS. Skeletal muscle phosphocreatine recovery in exercise trained humans is dependent on O2 availability. J Appl Physiol. 1999;86:2013–2018. doi: 10.1152/jappl.1999.86.6.2013. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol. 1998;85:1457–1463. doi: 10.1152/jappl.1998.85.4.1457. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Cox RH, Welch HG. Lactate accumulation during incremental exercise with varied inspired oxygen fractions. J Appl Physiol. 1983;55:1134–1140. doi: 10.1152/jappl.1983.55.4.1134. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Roca J, Wagner PD, West JB. Limitation of maximal O2 uptake and performance by acute hypoxia in dog skeletal muscle in situ. J Appl Physiol. 1988;65:815–821. doi: 10.1152/jappl.1988.65.2.815. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Heigenhauser GJ, Hultman E, Hollidge-Horvat MG, Spriet LL. Effects of dichloroacetate infusion on human skeletal muscle metabolism at the onset of exercise. Am J Physiol. 1999;277:E18–E25. doi: 10.1152/ajpendo.1999.277.1.E18. [DOI] [PubMed] [Google Scholar]
- Hughes RL, Clode M, Edwards RHT, Goodwin TJ, Jones NL. Effect of inspired O2 on cardiopulmonary and metabolic responses to exercise in man. J Appl Physiol. 1968;24:336–347. doi: 10.1152/jappl.1968.24.3.336. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Kowalchuk JM. Kinetics of oxygen uptake for submaximal exercise in hyperoxia, normoxia, and hypoxia. Can J Appl Physiol. 1995;20:198–210. doi: 10.1139/h95-014. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Morrissey M. Delayed kinetics of respiratory gas exchange in the transition from prior exercise. J Appl Physiol. 1982;52:921–929. doi: 10.1152/jappl.1982.52.4.921. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Shoemaker JK, Tschakovsky ME, Kowalchuk JM. Dependence of muscle VO2 on blood flow dynamics at onset of forearm exercise. J Appl Physiol. 1996;81:1619–1626. doi: 10.1152/jappl.1996.81.4.1619. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Hogan MC. Effect of extracellular PO2 on the fall in intracellular PO2 in contracting single myocytes. J Appl Physiol. 2003;94:1964–1970. doi: 10.1152/japplphysiol.00893.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of L-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol. 2001;91:891–896. doi: 10.1152/jappl.2001.91.2.891. [DOI] [PubMed] [Google Scholar]
- Linnarsson D. Dynamics of pulmonary gas exchange and heart rate changes at start and end of exercise. Acta Physiol Scand Suppl. 1974;415:1–68. [PubMed] [Google Scholar]
- Linnarsson D, Karlsson J, Fagraeus L, Saltin B. Muscle metabolites and oxygen deficit with exercise in hypoxia and hyperoxia. J Appl Physiol. 1974;36:399–402. doi: 10.1152/jappl.1974.36.4.399. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Pedersen PK, Hughson RL. Acceleration of VO2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. J Gen Physiol. 1985;86:135–165. doi: 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh GD, Paterson DH, Potwarka JJ, Thompson RT. Transient changes in muscle high-energy phosphates during moderate exercise. J Appl Physiol. 1993;75:648–656. doi: 10.1152/jappl.1993.75.2.648. [DOI] [PubMed] [Google Scholar]
- McCann DJ, Mole PA, Caton JR. Phosphocreatine kinetics in humans during exercise and recovery. Med Sports Exerc. 1995;27:378–387. [PubMed] [Google Scholar]
- McCreary CR, Chilibeck PD, Marsh GD, Paterson DH, Cunningham DA, Thompson RT. Kinetics of pulmonary oxygen uptake and muscle phosphates during moderate intensity calf exercise. J Appl Physiol. 1996;81:1331–1338. doi: 10.1152/jappl.1996.81.3.1331. [DOI] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988;254:C548–C553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Foley JM. Cellular processes integrating the metabolic response to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. Bethesda: American Physiological Society; 1996. pp. 841–869. [Google Scholar]
- Ozyener F, Rossiter HB, Ward SA, Whipp BJ. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol. 2001;533:891–902. doi: 10.1111/j.1469-7793.2001.t01-1-00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC. Role of exercising muscle in slow component of VO2. Med Sci Sport Exerc. 1994;26:1335–1340. [PubMed] [Google Scholar]
- Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O2 consumption in trained human skeletal muscle. J Appl Physiol. 1999;87:325–331. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Doyle VL, Howe FA, Griffiths JR, Whipp BJ. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol. 1999;518:921–932. doi: 10.1111/j.1469-7793.1999.0921p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Kowalchuk JM, Griffiths JR, Whipp BJ. Dynamics of intramuscular 31P-MRS Pi peak splitting and the slow components of PCr and O2 uptake during exercise. J Appl Physiol. 2002;93:2059–2069. doi: 10.1152/japplphysiol.00446.2002. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypopoxemia. Am J Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Moritani T. Increase in neuromuscular activity and oxygen uptake during heavy exercise. Ann Physiol Anthropol. 1992;11:257–262. [PubMed] [Google Scholar]
- Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- Welch HG. Hyperoxia and human performance: a brief review. Med Sci Sport Exerc. 1982;14:253–262. doi: 10.1249/00005768-198204000-00001. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant load work. J Appl Physiol. 1972;33:351–356. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Rumsey WL. Factors modulating the oxygen dependence of mitochondrial oxidative phosphorylation. Adv Exp Med Biol. 1988;222:121–131. doi: 10.1007/978-1-4615-9510-6_14. [DOI] [PubMed] [Google Scholar]