Abstract
We have studied the interaction between extracellular K+ (K+o) and extracellular Na+ (Na+o) in human ether-à-go-go related gene (HERG)-encoded K+ channels expressed in Chinese hamster ovary (CHO-K1) cells, using the whole-cell voltage clamp technique. Prior studies indicate that Na+o potently inhibits HERG current (IC50 3 mm) by binding to an outer pore site, and also speeds recovery from inactivation. In this study, we sought to explore the relationship between the Na+o effect on recovery and Na+o inhibition of HERG current, and to determine whether inactivation gating plays a critical role in Na+o inhibition of HERG current. Na+o concentration–response relationships for current inhibition and speeding of recovery were different, with Na+o less potent at speeding recovery. Na+o inhibition of HERG current was relieved by physiological [K+]o, while Na+o speeded recovery from inactivation similarly in the absence or presence of physiological [K+]o. To examine the link between Na+o block and inactivation using an independent approach, we studied hyperpolarization-activated currents uncoupled from inactivation in the S4–S5 linker mutant D540K. Depolarization-activated D540K currents were inhibited by Na+o, while hyperpolarization-activated currents were augmented by Na+o. This result reveals a direct link between Na+o inhibition and a depolarization-induced conformational change, most likely inactivation. We attempted to simulate the disparate concentration–response relationships for the two effects of Na+o using a kinetic model that included Na+o site(s) affected by permeation and gating. While a model with only a single dynamic Na+o site was inadequate, a model with two distinct Na+o sites was sufficient to reproduce the data.
The human ether-à-go-go related gene (HERG) encodes voltage-gated K+ channel subunits. First identified in the hippocampus (Warmke & Ganetzky, 1994), the HERG channel also carries the cardiac delayed rectifier K+ current IKr (Sanguinetti et al. 1995), and it is in the heart that the role of HERG channels is best understood. IKr promotes rapid repolarization during phase III of the cardiac action potential. When IKr becomes suppressed, either by loss of function mutations in the HERG gene (Curran et al. 1995; Roden & Balser, 1999) or by untoward drug block (Monahan et al. 1990; Roy et al. 1996; Rampe et al. 1997), action potential prolongation results. On the surface electrocardiogram, this manifests as QT interval prolongation, or ‘long QT syndrome’. Both congenital and acquired (drug-induced) forms of long QT syndrome predispose patients to the potentially lethal arrhythmia torsades de pointes.
One characteristic feature of both IKr and heterologously expressed HERG current is exquisite sensitivity to extracellular [K+] ([K+]o). Modest increases in [K+]o dramatically increase these outward currents, a ‘paradoxical’ or ‘anti-Nernstian’ effect. This effect has clinical utility in that raising serum [K+] can help normalize abnormally prolonged QT intervals (Compton et al. 1996; Choy et al. 1997), but its biophysical mechanism has remained elusive for some time, and now appears to include novel cation interactions. While most voltage-gated K+ channels are blind to extracellular sodium (Na+o) under physiological conditions (Hille, 2001), Na+o potently inhibits outward HERG K+ currents, and this inhibition can be potently relieved by physiological [K+]o (Numaguchi et al. 2000a). We recently showed that Na+o inhibits HERG current by binding to an outer pore site, and that competition between Na+o and K+o for the outer pore is a plausible mechanism for the potent anti-Nernstian effect of K+o (Mullins et al. 2002).
IKr and HERG currents have an inwardly rectifying phenotype due to a fast inactivation mechanism with intrinsic voltage dependence. HERG inactivation resembles classical C-type inactivation (as defined by Hoshi et al. 1991) in its sensitivity to pore mutation and extracellular TEA (Smith et al. 1996), but also differs from classical C-type inactivation in several important ways (Wang et al. 1997). Both HERG inactivation (Wang et al. 1996, 1997) and classical C-type inactivation (Lopez-Barneo et al. 1993) are sensitive to the concentration of small external cations. In classical C-type inactivation, raising the concentration of external cations slows inactivation and speeds recovery, consistent with a simple destabilization of the inactivated state (Baukrowitz & Yellen, 1995). Wang et al. (1997) reported that for HERG, raising [K+]o to 98 mm slows both inactivation and recovery time constants, suggesting a more complex interaction. In that study, conducted in oocytes, [Na+]o was lowered as [K+]o was raised. In a later study focused on mechanisms of drug block, working in mammalian cells, we reported an effect of Na+o to favour steady-state inactivation, even as Na+o also hastened recovery from inactivation at hyperpolarized potentials (Numaguchi et al. 2000b). More recently, in the study demonstrating that Na+o interacts with the HERG channel at an outer pore site, we noted that several pore mutants with altered sensitivity to inhibition by Na+o also have impaired inactivation (Mullins et al. 2002). These findings all hint at an interaction between HERG inactivation gating and Na+o block.
In the present study, we have focused directly on the interaction between Na+o and the HERG inactivation mechanism. We have characterized the effect of Na+o to speed recovery from inactivation in detail, and directly compared the effect to hasten recovery with the effect of Na+o to inhibit current. Our results suggest the possibility of mechanistic separation between the two effects, with Na+o inhibition of HERG current occurring over a far lower concentration range. In a second series of experiments, we have utilized an independent means to determine whether inactivation plays an important role in Na+o inhibition of HERG currents. We show here that while Na+o cannot block hyperpolarization-activated currents uncoupled from inactivation in the S4–S5 linker mutant D540K (characterized in Sanguinetti & Xu, 1999; Mitcheson et al. 2000b), Na+o can block depolarization-activated currents in this mutant. We conclude that a depolarization-induced conformational change is important to Na+o inhibition, and that channel inactivation is the most likely candidate.
Methods
Plasmid cDNA constructs
Wild-type HERG cDNA was provided by Dr Mark Keating (University of Utah, Salt Lake City, UT, USA). DNA for the HERG point mutants D540A, D540K and S631A was provided by Dr Michael Sanguinetti (University of Utah). Wild-type and mutant constructs were subcloned into the vector pGFP-IRS (provided by David Johns, The Johns Hopkins University, Baltimore, MD, USA) for bicistronic expression of the channel protein and GFP reporter as previously described (Johns et al. 1997). The S624A and S624T mutations were generated by PCR using the overlap-extension technique (Tao & Lee, 1994) and subcloned back into our construct.
Cells
Chinese hamster ovary (CHO-K1) cells from the American Type Culture Collection were grown in Ham's F-12 medium with l-glutamine (Gibco Laboratories, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (Gibco Laboratories) and 1% penicillin–streptomycin (Gibco Laboratories) in a humidified, 5% CO2 incubator at 37°C.
Transfection
CHO-K1 cells were transfected using the lipofectamine (Gibco Laboratories) or FuGENE 6 (Roche Diagnostics Corp., Indianapolis, IN, USA) transfection reagents and method. Cells demonstrating green fluorescence were chosen for electrophysiological analysis.
Solutions
The standard intracellular (pipette) recording solution contained (mm): 110 KCl, 5 K2ATP, 5 K4BAPTA, 1 MgCl2, 10 Hepes, adjusted to pH 7.2 with KOH to yield a final intracellular [K+] of 145 mm.
The extracellular (bath) recording solution contained (mm): 146 XCl (X = sum of K+, Na+ and N-methyl-d-glucamine+ (NMDG)), 1 MgCl2, 2 CaCl2, 10 Hepes, 10 glucose, adjusted to pH 7.4 with HCl (low Na+ solutions) or NaOH (very high Na+ solutions). In limited experiments, Tris was used as the replacement ion instead of NMDG (data not shown). Although the results with Tris were more variable than results using NMDG, the overall effects of Na+o were similar with either replacement ion.
Electrophysiology
HERG currents were recorded using the whole-cell patch clamp technique (Hamill et al. 1981). Cells were patch-clamped at room temperature (20−25°C) between 2 and 3 days post-transfection. Recordings utilized an Axopatch 200B patch clamp amplifier and a Digidata 1200 converter (Axon Instruments, Union City, CA, USA). Pipettes of resistance 2–8 MΩ were fabricated from borosilicate capillary glass (1.5 mm outer diameter, G150F-4, Warner Instruments, Inc., Hamden, CT, USA) using a Flaming/Brown puller (P-97; Sutter Instrument Co., Novato, CA, USA). Cell and pipette capacitances were nulled and series resistance was compensated (at least 80%) before recording. Data were acquired using pCLAMP6 programs (Axon Instruments). Both pCLAMP and Origin (OriginLab Corp., Northampton, MA, USA) programs were used in analysing and plotting data. For solution exchanges, our 0.5-ml bath was fully exchanged during a 2-min perfusion period prior to collecting data. Cells were equilibrated to new conditions by pulsing to +20 mV during this period. Cells were kept at the holding potential for 6 s between pulses during both the equilibration period and data collection. In some experiments, up to three traces from a single cell were used to generate an average trace used for display (figures) and data analysis. P values in the text and figure legends are from independent Student's t tests. In figures, error bars represent s.e.m., calculated from several cells. HERG D540K was the only channel that exhibited significant rundown. For other channels, no current rundown was observed for as long as 1 h. Na+o effects in these channels were completely reversible with our solution exchange setup (see Fig. 5B). Asterisks on the figures indicate those statistically significant results noted in the figure legends.
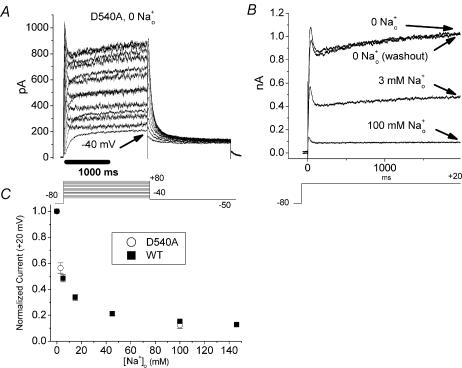
Figure 5. D540A phenotype and sensitivity to inhibition by Na+o.
A, representative family of D540A currents elicited by a series of depolarizing test pulses in 0 [Na+]o, 0 [K+]o. Note the fast activation and strong rectification of current and the sizable current induced by even a modest depolarizing step (−40 mV, arrows). B, representative experiment examining Na+o inhibition of D540A. Note return of current to original level after washout, which contrasts markedly with the result in D540K (Fig. 4). C, D540A inhibition placed in context of WT concentration–response curve for Na+o inhibition (same data as in Fig. 2A). Summarized results from several experiments like B (n = 3–4, open circles) suggest that D540A (open circles) is quite similar to WT HERG (filled squares) in its sensitivity to inhibition by Na+o.
Kinetic modelling
The simple five-state HERG kinetic model of Johnson et al. (1999a), adopted from Wang et al. (1997), has been used to successfully model several effects of extracellular gating modifiers on HERG (Johnson et al. 1999a,b; Wang et al. 2003); additionally, a similar model has been used to describe the blocking effects of Ba2+ on HERG (Weerapura et al. 2000). Here, the model of Johnson et al. (1999a) was used as a starting point. The simulations were carried out using ModelMaker software (Cherwell Scientific, Oxford, UK).
Results
Disparate effects of extracellular sodium on HERG current at depolarized versus hyperpolarized potentials
In the presence of physiological [K+]o, Na+o may have disparate effects on WT HERG current at depolarized versus hyperpolarized potentials. Specifically, removing all Na+o in the presence of physiological [K+]o results in increased current at depolarized potentials, but decreased peak tail current during a subsequent hyperpolarizing step (Numaguchi et al. 2000b). The increase in current at depolarized potentials upon removal of Na+o is fully consistent with the inhibition of outward HERG current by Na+o previously described (Numaguchi et al. 2000a; Mullins et al. 2002). The decrease in current at hyperpolarized potentials upon removal of Na+o results at least in part from slowing of kinetics of recovery from inactivation (Numaguchi et al. 2000b).
Extracellular sodium speeds recovery from inactivation in the absence of extracellular potassium
It is conceivable that the effect whereby removing all Na+o slows the kinetics of recovery from inactivation in physiological [K+]o (Numaguchi et al. 2000b) could be mediated indirectly. Very high (98 mm) [K+]o has been shown to slow the kinetics of recovery from inactivation for HERG channels expressed in oocytes (Wang et al. 1997). Na+o could in theory speed recovery kinetics by competing with K+o for a site that modulates recovery or by blocking the access of K+o to such a site. A purely indirect model of Na+o action predicts that recovery kinetics will be insensitive to Na+o in the absence of K+o. To study recovery kinetics, we applied a strong depolarizing pulse (+50 mV) to inactivate most channels, followed by stepoff to a more hyperpolarized test potential (−50 mV or −100 mV), causing channels to recover from inactivation. The early, increasing phase upon stepping to the test potential was fitted to an exponential function to generate time constants for recovery kinetics (arrows and dotted lines in Fig. 1A and B denote the region fit). Raising [Na+]o hastened recovery from inactivation in the absence of K+o (concentration–response, Fig. 1C), supporting a direct model of Na+o action.
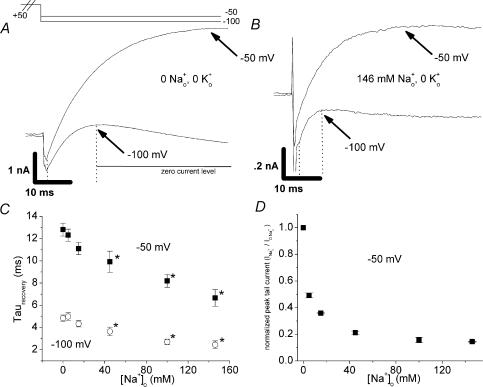
Figure 1. Effect of Na+o on HERG recovery from inactivation in the absence of K+o.
Membrane potential was stepped from −80 mV to +50 mV (2 s), followed by test potentials of −50 or −100 mV. A and B, representative currents at hyperpolarized test potentials for a paired experiment in which [Na+]o was changed from 0 (A) to 146 mm (B), with [K+]o held at 0. The currents in 146 mm Na+o were much smaller than those in 0 Na+o (note current scale is different while time scale is identical). Experiments as in A and B were carried out with several concentrations of Na+o. The early, increasing phase reflecting recovery from inactivation (ending at the point indicated by arrows) was fitted with a single exponential. Time constants at several [Na+]o are plotted in C (n = 4–5, paired observations). For test potentials of both −50 mV and −100 mV, recovery time constants were significantly smaller than baseline ([Na+]o= 0) for [Na+]o≥ 40 mm (P < 0.05). D, Na+o concentration–response relationship in the absence of K+o for peak tail current at −50 mV. Normalized peak tail current (INa/I0Na) is plotted on the y-axis. Note that the IC50 of 4.2 ± 1.2 mm is similar to that previously measured at +20 mV (Numaguchi et al. 2000a; Mullins et al. 2002) (see also Fig. 2A and inset).
In 0 [K+]o, tail currents were reduced when [Na+]o was raised despite the faster recovery kinetics (compare paired experiment in Fig. 1A and B; arrows indicate end of rising phase fit to an exponential function; scale changes for current magnitude but not for time). This reduction of tail current stands in sharp contrast to the result that initially motivated the study of Na+o effects on recovery kinetics: that peak tail currents were actually increased by Na+o in the presence of 5.4 mm[K+]o (Numaguchi et al. 2000b). This difference suggests the possibility that the effects of Na+o to inhibit current and speed recovery kinetics may differ in their susceptibility to relief by K+o. A Na+o concentration–response relationship in the absence of K+o for peak tail current (−50 mV) is shown in Fig. 1D, and is similar to the concentration–response relationships previously reported at +20 mV (Numaguchi et al. 2000a; Mullins et al. 2002), with an IC50 of 4.2 ± 1.2 mm (also see Fig. 2A and inset). The data in Fig. 1, then, show that although Na+o can speed recovery from inactivation in the absence of K+o (Fig. 1A–C), this effect does not appreciably decrease the potency of Na+o for inhibiting current during a step to −50 mV following a depolarization (Fig. 1D).
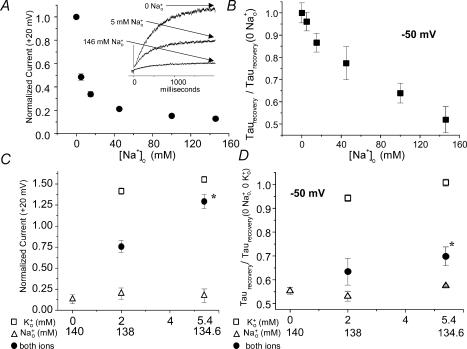
Figure 2. Comparison of concentration–response and mole-fraction relationships for the two effects of Na+o.
A and B, Na+o concentration–response relationships measured in the absence of K+o. Note data in A and B were collected from the same cells. A, concentration–response relationship for effect of Na+o to inhibit HERG current at depolarized potential (2 s step to +20 from −80 mV, current at end of step (arrows, inset) normalized to value in 0 Na+o, n = 4–7); similar design to experiments in Numaguchi et al. (2000a). IC50= 4.1 ± 1.1 mm. B, concentration–response relationship for effect of Na+o to speed HERG recovery from inactivation at −50 mV (same data as shown in Fig. 1C, n = 4–5). Note that while 4.1 ± 1.1 mm[Na+]o caused a half-maximal inhibitory effect (A), much greater [Na+]o was necessary for a half-maximal effect in speeding recovery kinetics (B). Because it is unclear from the concentration–response data for speeding of recovery whether the effect has saturated at the highest [Na+]o tested, the calculated [Na+]o giving a half-maximal effect depended greatly on the equation used to fit the curve. We fitted the [Na+]o concentration–response relationships for recovery time constants using the modified Hill equation:
yielding an EC50 of 176 ± 15 mm and a Hill coefficient of 0.85 ± 0.07, and also to the same equation with an offset term y′:
yielding an EC50 of 55 ± 22 mm, Hill coefficient of 0.54 ± 0.19, and y′= 0.2. C and D, mole-fraction experiments comparing the two effects of Na+o. In each experiment, currents were recorded first in 0 [K+]o, 0 [Na+]o and all data from a given cell were normalized to this measurement. Note that the x-axes for Na+o and K+o have opposite polarity. Open symbols indicate single ion concentration–response data (triangles for Na+o, squares for K+o). The filled circles show the calculated result when Na+o and K+o were mixed in the proportions indicated on the abscissa. n = 4–5 for each point. Physiological [K+]o antagonized both effects of Na+o in a non-additive manner; K+o relieved the effect of Na+o on current magnitude (C) more effectively than it relieved the effect on τrecovery (D). For both measurements, the normalized value in 5.4 mm[K+]o, 134.6 mm[Na+]o was significantly greater than the value in 0 [K+]o, 134.6 mm[Na+]o (P < 0.05).
Two effects of extracellular sodium: different concentration–response and mole-fraction relationships
Na+o potently inhibits outward HERG current at depolarized potentials (Numaguchi et al. 2000a) and also speeds recovery from inactivation, even in the absence of K+o (Fig. 1). As a preliminary means of assessing whether these effects were likely to be mediated by distinct binding sites, we compared concentration–response relationships for the two effects of Na+o, measured in the absence of K+o (Fig. 2A and B; Fig. 2B contains normalized data replotted from Fig. 1C). Whereas current at +20 mV was inhibited with an IC50 of 4.1 ± 1.1 mm (Fig. 2A, note raw traces in inset), much greater [Na+]o was required to elicit a half-maximal speeding of recovery kinetics at −50 mV, and it is not clear that the recovery effect is saturated at the highest [Na+]o (see Fig. 2B and legend for summary of curve fitting). We also performed mole fraction experiments to compare the ability of K+o to antagonize the two effects of Na+o (Fig. 2C and D, [K+]o and [Na+]o plotted on oppositely directed x-axes). Physiological [K+]o antagonized both effects in a non-additive manner (filled circles, indicating experiments in which Na+o and K+o were mixed in the proportions indicated on the x-axis). That is, for both parameters studied, the effect of K+o to oppose Na+o as measured in the mole fraction experiments was consistently greater than the sum of the individual K+o and Na+o effects measured in the absence of the other external cation. This non-additivity suggests that K+o and Na+o interact functionally (as opposed to exerting opposite but functionally independent effects on the channel).
Interestingly, K+o relieved the current inhibition (Fig. 2C) much more potently than it relieved the speeding of recovery kinetics (Fig. 2D). This difference is consistent with the observation that Na+o increases peak tail current in the presence of K+o (Numaguchi et al. 2000b), but begs the question of how such a difference could arise mechanistically.
Effect of extracellular sodium on recovery in mutants with impaired sensitivity to Na+o inhibition
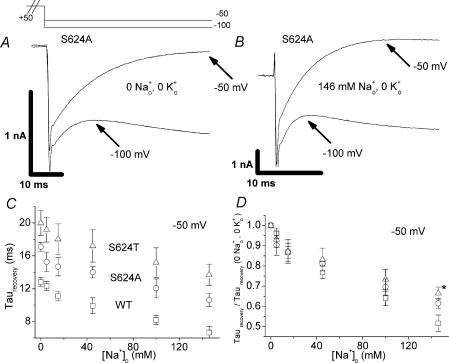
In a previous study of inhibition of HERG currents by Na+o, we identified two HERG point mutants (S624A and S624T) with intact K+ selectivity and impaired sensitivity to inhibition by Na+o; S624A and S624T channels also demonstrate impaired inactivation at potentials of −20 mV and above (Mullins et al. 2002). To gain additional insight into the relationship between Na+o inhibition of current and Na+o hastening of recovery, we compared the Na+o sensitivity of the S624A (raw traces, Fig. 3A and B), S624T and WT channels with respect to kinetics of recovery from inactivation. While the baseline (0 [Na+]o, 0 [K+]o) kinetics of recovery were significantly slower in the mutant channels (Fig. 3A), Na+o was able to speed recovery for both S624A and S624T (Fig. 3C). When data were normalized to the baseline τrecovery at −50 mV, recovery from inactivation in the mutant channels appeared to have Na+o sensitivity comparable to wild-type (Fig. 3D, see legend). Hence, although the mutant channels are less vulnerable to Na+o inhibition (Mullins et al. 2002), the mutations did not dramatically alter the effect of Na+o on recovery from inactivation (Fig. 3D), again suggesting possible mechanistic separation of these two Na+o effects. Na+o block of HERG current seems dependent on a fully intact inactivation mechanism, while Na+o effects on recovery from inactivation, ironically, seem less sensitive to the integrity of the inactivation mechanism.
Figure 3. Comparison of ability of Na+o to speed recovery kinetics in WT and mutant HERG channels.
As in Fig. 1, experiments were carried out in the absence of K+o and membrane potential was stepped from −80 mV to +50 mV (2 s), followed by test potentials of −50 or −100 mV. A and B, representative S624A currents at hyperpolarized test potentials for a paired experiment in which [Na+]o was changed from 0 (A) to 146 mm (B). Experiments as in A and B were carried out with several concentrations of Na+o for the S624A and S624T mutant channels. The early, increasing phase reflecting recovery from inactivation (ending at the point indicated by arrows) was fitted with a single exponential. Time constants at several [Na+]o for WT (squares), S624A (circles), and S624T (triangles) channels are plotted in C (n = 4–5, paired observations). Data collected at a test potential of −50 mV are shown with open symbols. The WT data are the same data shown in Fig. 1C. WT HERG had significantly faster kinetics of recovery from inactivation as compared to the mutant channels both at baseline (0 [Na+]o) and in each [Na+]o tested (P < 0.05). High [Na+]o significantly speeded recovery kinetics in both mutant channels (0 versus 146 mm Na+o, P < 0.05). D shows recovery time constants at −50 mV for each channel (open symbols, C) normalized to the value in 0 [Na+]o, 0 [K+]o. There was no significant difference in normalized data for WT versus mutant channels (P > 0.05 for each [Na+]o tested), with the exception of S624T in 146 mm[Na+]o (P < 0.05).
Extracellular sodium inhibits depolarization-activated currents but does not inhibit hyperpolarization-activated currents in the S4–S5 linker mutant HERG D540K
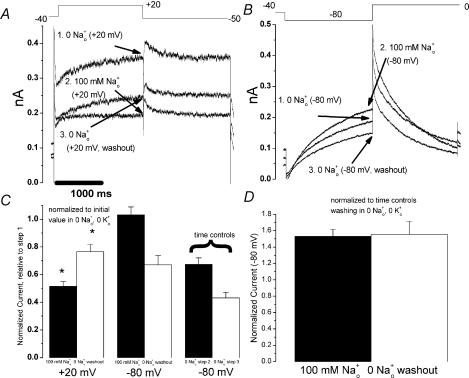
While channels with pore mutations and destabilized inactivation exhibit impaired Na+o block (Mullins et al. 2002), this effect could result primarily from an alteration of the Na+o binding site in the pore. Still, the effect of Na+o to speed recovery from inactivation in the absence of K+o (Fig. 1) suggests an interaction between Na+o and the HERG inactivated state. As an additional means of exploring the relationship between HERG inactivation and Na+o block, we studied the S4–S5 linker mutation D540K. Because of a repulsive interaction of residue 540 with the S6 residue R665 (Tristani-Firouzi et al. 2002), D540K channels can open at hyperpolarized potentials where inactivation is expected to be negligible (Sanguinetti & Xu, 1999). Therefore the hypothesis that inactivation facilitates Na+o inhibition predicts that depolarization-activated D540K currents will be more potently inhibited by Na+o than hyperpolarization-activated currents. The single-channel conductance and selectivity properties of the hyperpolarization-activated state of D540K HERG closely resemble those of WT HERG activated by depolarization (Mitcheson et al. 2000b). D540K currents in CHO cells (Fig. 4) were morphologically similar to those reported in oocytes (Sanguinetti & Xu, 1999). In 0 [Na+]o, 0 [K+]o, significant outward current was present at baseline at all holding potentials. Baseline current was minimal at −40 mV, so cells were held at that potential. Channels were further activated by either a depolarizing pulse or a hyperpolarizing pulse (clamp protocols are shown above Fig. 4A and B). Consistent with results in oocytes (Sanguinetti & Xu, 1999), depolarization-induced D540K currents (Fig. 4A) demonstrated inward rectification due to WT-like rapid inactivation (data not shown), but activated more rapidly than WT (Fig. 2A), or than hyperpolarization-induced currents (Fig. 4B).
Figure 4. Na+o effects on depolarization- and hyperpolarization-activated currents in HERG D540K.
A, representative currents from an experiment examining Na+o effects on depolarization-activated D540K current. From a holding potential of −40 mV, cells were stepped to +20 mV for 2 s, followed by a 2 s step to −50 mV. Current was recorded in three consecutive solutions, as shown. B, representative currents from an experiment examining Na+o effects on hyperpolarization-activated D540K current. From a holding potential of −40 mV, cells were stepped to −80 mV for 2 s, followed by a 2 s step to 0 mV. Current was recorded in three consecutive solutions, as shown. C, summarized data from several experiments as in A (n = 10) and B (n = 8). Current at the end of the first 2 s voltage step (arrows, A and B) was normalized to the initial value (‘step 1’) in 0 [Na+]o, 0 [K+]o. While significant inhibition of depolarization-activated currents (+20 mV) by 100 mm[Na+]o was observed (I100mM/I0mM = 0.52 ± 0.03) and much of the inhibitory effect could be washed out (Iwashout/I0mM= 0.77 ± 0.05, P < 0.01 versus I100mM/I0mM), no significant inhibition of hyperpolarization-activated current was observed (−80 mV, I100mM= 1.03 ± 0.06). C also includes summarized data from time control experiments (n = 4) in which solution containing 0 [Na+]o was washed in twice (step 2, step 3). These time control data were used to calculate the ‘doubly normalized’ data in D, which suggest that 100 mm[Na+]o actually increases hyperpolarization-activated currents measured at −80 mV (I100mM/Itimecontrol = 1.53 ± 0.08, P < 0.01 versus 1).
Unlike WT HERG currents and other mutant currents, both depolarization-induced and hyperpolarization-induced D540K currents progressively decreased (‘ran down’) over the course of our experiments. Taking rundown into account, we designed the following three-step experimental protocol (Fig. 4A and B). In step 1, current was measured in 0 [Na+]o to establish a baseline. Next, in step 2, bath solution containing 100 mm[Na+]o was washed in, and currents were again recorded. Finally, in step 3, 0 [Na+]o solution was washed back in and current was again recorded to estimate the extent of rundown. For hyperpolarization-activated currents (Fig. 4B), current after wash-in was further assessed by parallel time control experiments in which fresh 0 Na+o solution was washed in at step 2 rather than 100 mm[Na+]o (see Fig. 4C, right). 100 mm[Na+]o inhibited D540K currents measured at +20 mV (Fig. 4A and C, I100mM/I0mM= 0.52 ± 0.03, n = 10), and much of the inhibitory effect could be washed out (Iwashout/I0mM = 0.77 ± 0.05, n = 10, P < 0.01 versus I100mM/I0mM) with fresh 0 Na+o solution. In contrast, 100 mm[Na+]o did not inhibit, and even increased, the current at −80 mV (Fig. 4B, C and D, I100mM/Itimecontrol= 1.53, P < 0.01versus 1, n = 8). The inhibition of depolarization-activated D540K currents by Na+o (Fig. 4A) and the time course of hyperpolarization-activated current rundown in 0 [Na+]o (Fig. 4C) provide essential context for interpreting the result that hyperpolarization-activated current is not inhibited by Na+o (Fig. 4B). Our observations that depolarization-activated D540K current was inhibited by Na+o and hyperpolarization-activated D540K current was increased by Na+o are consistent with a positive interaction between inactivation and Na+o inhibition.
Although Na+o inhibited depolarization-induced D540K current, the reduced potency of Na+o in D540K compared to WT prompted additional experiments. One hundred mm[Na+]o is sufficient to inhibit WT HERG current at +20 mV almost completely (Fig. 2A), but is less effective at inhibiting D540K current at +20 mV (Fig. 4A). In addition to inducing channel opening in response to hyperpolarization, the D540K mutation markedly speeds activation in response to depolarization
(compare Figs 2A and 4A). Another mutant, D540A, does not open in response to hyperpolarization, but exhibits depolarization-activated current which has the same speeded activation gating as D540K (Sanguinetti & Xu, 1999). Therefore, studying the Na+o sensitivity of D540A channels provides a means of controlling for the possibility that the change in activation kinetics alone alters Na+o inhibition, thereby further informing the interpretation of results from D540K. Figure 5A shows a family of D540A currents elicited by a series of depolarizing steps from −80 mV. Not only is activation faster than WT HERG, but significant activation occurs with very mild depolarization (Fig. 5A, arrow). This result implies that some of the D540K current present at a holding potential of −40 mV (Fig. 4) may be depolarization- rather than hyperpolarization-activated. Indeed, a decrease in D540K current at the holding potential is observed when Na+o is washed in (Fig. 4A). D540A current was inhibited by Na+o with a potency indistinguishable from WT (Fig. 5B and C), suggesting that the kinetics of activation gating do not affect Na+o inhibition and that mutation of D540 does not impart an obligatory change to the Na+o binding site.
The results with D540A, then, do not illuminate the decreased potency of Na+o for inhibition of depolarization-activated D540K currents. One possibility which cannot be ruled out is that some ‘hyperpolarization-activated’ D540K current may remain even at depolarized potentials, and that Na+o may even augment that current such that the overall observed result is a weighted sum of current from the ‘depolarization-activated’ state (inhibited by Na+o, Fig. 4A) and current from the ‘hyperpolarization-activated’ state (potentiated by Na+o, Fig. 4B), yielding an apparent decrease in the inhibitory potency of Na+o.
Continuous potassium efflux at the holding potential attenuates current inhibition by extracellular sodium
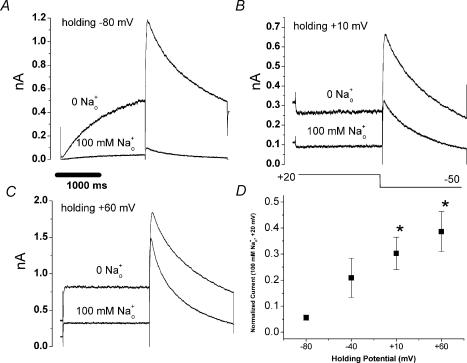
An alternative interpretation of the reduced inhibitory potency of Na+o in D540K is motivated by the recent definition of an outer pore binding site for Na+o (Mullins et al. 2002). This interpretation is that continuous K+ efflux clears Na+ from its binding site in the pore and/or continuously occupies the Na+o binding site such that Na+o has no opportunity to reach its inhibitory site. In this scenario, an ‘inverted’ version of Armstrong's classic model of antagonism of internal TEA block by permeating K+ (Armstrong, 1971), the high inhibitory potency of Na+o in wild-type channels would depend in part on the absence of K+ efflux at the holding potential. On this basis, we tested the idea that continuous K+ efflux could attenuate Na+o inhibition of WT HERG channels as a means of providing further context to data from D540K. Our experimental design was to vary the holding potential and measure the inhibitory potency of Na+o during subsequent steps to +20 mV (Fig. 6). Inhibition by 100 mm[Na+]o at +20 mV was significantly less potent when channels were held for sustained periods at current-generating potentials (+10 mV, +60 mV) compared to the standard holding potential of −80 mV (Fig. 6D, P < 0.05 in both cases).
Figure 6. Effect of continuous K+ efflux on Na+o inhibition of WT HERG channels.
Cells were clamped at a variable holding potential and subsequently stepped to +20 mV for 2 s, followed by a 2 s step to −50 mV. In each experiment, currents were recorded first in 0 [Na+]o, 0 [K+]o and subsequently in 100 mm[Na+]o, 0 [K+]o. A, B and C, representative records at holding potentials of −80 mV, +10 mV and +60 mV, respectively. D, summary data from several cells (n = 3–5), all normalized to the initial value in 0 [Na+]o, 0 [K+]o. Normalized current at holding potentials of +10 and +60 mV was significantly greater than current with a holding potential of −80 mV (P < 0.05), whereas normalized current when holding at −40 mV was not significantly different (P = 0.18).
Together with the data from D540A channels (Fig. 5), the data in which the holding potential was varied in WT channels (Fig. 6) support the idea that Na+o inhibition of depolarization-activated D540K current (Fig. 4A) occurs by the same mechanism as Na+o inhibition of WT HERG channels. The apparent decrease in Na+o inhibition of depolarized D540K current is probably related to the fact that there is D540K current at all holding potentials, even −40 mV. Far fewer WT than D540K channels open at −40 mV, so the absence of a significant effect of holding at −40 mV (Fig. 6D) in WT channels is not surprising. There is no strong effect of test potential on Na+o inhibition (Mullins et al. 2002), which suggests it is unlikely that the reduced inhibitory potency of Na+o at more depolarized holding potentials (+10 mV, +60 mV, Fig. 6) results primarily from a strong, predominant field effect. However, we cannot completely rule out the possibility that, in both D540K and WT HERG, reduced inhibition by Na+o at more depolarized holding potentials might result not from K+ efflux but instead from a previously unidentified channel state with low affinity for Na+o.
Discussion
The major findings of this study are: (i) speeding of HERG recovery from inactivation by Na+o (EC50 > 50 mm) occurs with lower potency than the inhibition of HERG current by Na+o (IC50= 4.1 ± 1.1 mm), (ii) the effect on recovery is largely [K+]o independent while the inhibitory effect is potently relieved by physiological [K+]o, (iii) hyperpolarization-induced currents uncoupled from inactivation (D540K) are not inhibited by Na+o, and (iv) more positive holding potentials attenuate Na+o inhibition, probably via continuous K+ efflux.
Interpretation of speeding of recovery from inactivation by extracellular sodium
As an aside, it is theoretically possible that a very dramatic change in deactivation kinetics could alter apparent kinetics of recovery from inactivation in HERG channels. However, we can be certain that speeding deactivation is not the primary effect of Na+o. A simple speeding of deactivation would be expected to decrease peak tail current magnitude, whereas Na+o increases peak tail current magnitude in the presence of 5.4 mm[K+]o (Numaguchi et al. 2000b). Even so, one could conceive of the possibility that a modest speeding of deactivation could be superimposed on a speeding of recovery and thereby distort the Na+o concentration–response relationships for recovery kinetics (Fig. 1C). In fact, there is a test case of this scenario using methods identical to ours (Johnson et al. 1999a). In that study, the authors describe a specific effect of Ca2+o on activation gating of HERG channels. Raising [Ca2+]o greatly speeds HERG channel deactivation (an effect clearly visible in current records), but there is no effect of Ca2+o on rate of recovery as measured by a protocol identical to the one used here. By contrast, studies with Cd2+o have shown that a specific effect to speed HERG recovery can speed apparent deactivation (Johnson et al. 1999b).
Possible significance of extracellular sodium speeding of recovery from inactivation
HERG current early in the cardiac action potential is limited due to rapid, voltage-dependent inactivation. As other currents (such as IKs) begin to initiate repolarization, inactivated HERG channels rush back from the inactivated state into the open state (recovery), resulting in peak current targeted to phase III, which accelerates repolarization. That Na+o would act to speed recovery from inactivation (Fig. 1) and that this effect would persist in physiological [K+]o (Fig. 2D), then, are consistent with the biological function of the HERG K+ channel as inferred from the long QT phenotype and response of HERG K+ channels to action potential waveforms (Miller, 1996; Smith et al. 1996; Vandenberg et al. 2001). That is, whatever the biophysical mechanism, Na+o probably aids HERG channels in carrying out their physiological function by speeding recovery.
Interestingly, Na+o actually appears to favour onset of inactivation at depolarized potentials as studied by standard steady-state and kinetic measurements (Numaguchi et al. 2000b). The feature of apparently increasing rates of both inactivation and recovery due to Na+o parallels the observation by Wang et al. (1997) that raising [K+]o to 98 mm slows both inactivation and recovery time constants. In that study, conducted in oocytes, [Na+]o was lowered as [K+]o was raised. Working in mammalian cells, we find that changing [K+]o in the absence of Na+o does not yield large effects on recovery in the physiological range (Fig. 2D). As noted by Wang et al., the [K+]o effect on classical C-type inactivation is to exert opposite effects on rates of inactivation and recovery (Baukrowitz and Yellen, 1995; Wang et al. 1997). That Na+o appears to speed both development of and recovery from inactivation, then, is another feature suggestive of fundamental differences between external cation interactions with classical C-type inactivation and HERG inactivation. The dual speeding is also potentially consistent with Na+o acting as a ‘catalyst’ to amplify the efficiency of HERG's intrinsic gating mechanism independent of transition direction. This possibility is considered in more detail below (Fig. 7).
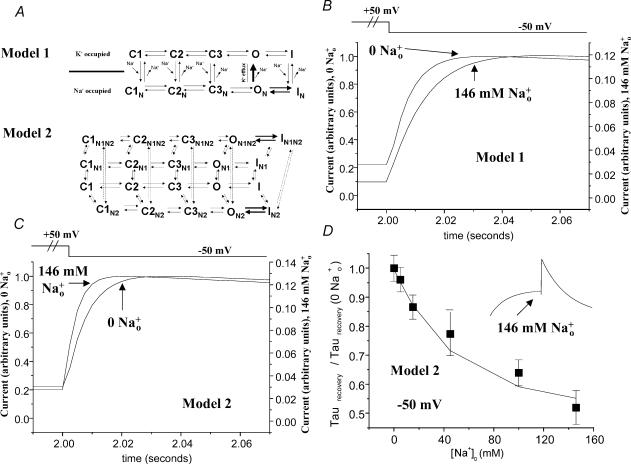
Figure 7. Evaluation of ability of kinetic models to simulate effects of Na+o.
A, kinetic schemes evaluated. The baseline 5-state model was that of Johnson et al. (1999a), adopted from Wang et al. (1997). Rate constants were implemented in ModelMaker software according to equations of form α = (kT/h)exp[ΔGα+ (zδeEm)/kT]. For ON and IN states (Model 1) or ON2, ON1N2, IN2, and IN1N2 states (Model 2), a shift in ΔG of 1 unit (lowering the energy barrier) was applied for both inactivation and recovery transitions. In Model 1 as evaluated in B, Na+ on-rates for closed and inactivated states were [Na+]o (m) × 106 s−1, and Na+ off-rates for closed and inactivated states were 1 s−1. The Na+ off-rate for the ON state was 10 s−1. Only the O state conducts current in Model 1. In Model 2 as evaluated in C and D, Na+ on-rates for the N1 and N2 states were [Na+]o (m) × 106 s−1. Na+ off-rates were 3000 and 140 000 for the N1 and N2 states, corresponding to site affinities of 3 mm and 140 mm, respectively. Both the O and ON2 states conduct current in Model 2. B, effect of Na+o on recovery from inactivation for Model 1. A 2 s depolarization to +50 mV was followed by a 2 s hyperpolarization to −50 mV, allowing for evaluation of kinetics of recovery from inactivation. Simulated currents are scaled to maximum tail current to allow direct comparison of recovery kinetics (note different y-axes for the two traces). To achieve current inhibition at levels approximating those of experimental records, the Na+ off-rate for the ON state had to be limited. This resulted in apparent slowing of recovery from inactivation by Na+o, an effect opposite that seen experimentally. C, effect of Na+o on recovery from inactivation for Model 2. Note Na+o speeds apparent recovery from inactivation, as observed in experimental records (compare to Fig. 1). D, concentration–response curve for speeding of recovery from inactivation by Na+o as generated by Model 2. Experimental data are replotted from Fig. 2B. Model data are indicated by broken line. Inset shows simulated current elicited by sequential 2 s steps to +20 and −50 mV with 146 mm Na+o, illustrating that the characteristic morphology of HERG currents is preserved with this model, even in high [Na+]o.
Extracelluar sodium effects on HERG (current inhibition and speeding of recovery) do not occur via a simple common mechanism
Three features suggest that Na+o inhibition and Na+o speeding of recovery from inactivation do not occur via a simple common mechanism such as binding to a single site unaffected by gating. First, the concentration–response relationships for the two effects differ dramatically (Fig. 2A and B), and the difference cannot be explained by a simple field effect. Not only do we see no direct evidence for a strong, predominant field effect with respect to current inhibition (Mullins et al. 2002), but the less potent effect (speeding recovery) is measured at the more hyperpolarized potential, the opposite of the expectation for a positively charged external cation experiencing the transmembrane field. Second, the ability of K+o to antagonize the two effects differs dramatically (Fig. 2C and D). Third, the P-loop mutants S624A and S624T are markedly affected in their sensitivity to Na+o inhibition (Mullins et al. 2002), but are only marginally less sensitive to speeding of recovery by Na+o (Fig. 3D). Interestingly, ‘baseline’ steady-state inactivation (0 [Na+]o, 0 [K+]o) is impaired (Mullins et al. 2002) and kinetics of recovery are slowed (Fig. 3C) in both S624A and S624T mutant channels. Like the effect of Na+o on the two gating processes in WT HERG, this result is not easily explained by simple stabilization of the inactivated or open state.
A depolarization-induced conformational change is necessary for current inhibition by extracellular sodium
Although Na+o inhibition of HERG current occurs via a pore-occluding rather than a remote allosteric mechanism (Mullins et al. 2002), that result did not rule out the possibility that a conformational change like channel inactivation might play a critical role in Na+o inhibition.
The experiments with D540K (Fig. 4) were designed to test the hypothesis that inactivation is important to Na+o inhibition. The D540K channel serves as its own internal control. Because depolarization-induced D540K currents are inhibited by Na+o, we know that the receptor mediating Na+o inhibition can be occupied in this mutant channel. The augmentation of hyperpolarization-induced D540K currents by Na+o suggests a fundamentally different interaction with the channel and, at a minimum, supports the idea that a depolarization-induced conformational change is important to Na+o inhibition. The single-channel conductance and selectivity properties of the hyperpolarization-activated state of D540K HERG closely resemble those of WT HERG activated by depolarization (Mitcheson et al. 2000b). If we assume the hyperpolarization-induced open state is identical to the depolarization-induced open state, then the leading candidate for a depolarization-induced conformational change favouring Na+o inhibition is channel inactivation. Therefore, while the opposing effects of Na+o and K+o on outward HERG current magnitude can be explained by a model in which the two cations simply compete for the channel pore (Mullins et al. 2002), it remains possible that gating-mediated mechanisms are also important. The observation that K+o hinders HERG inactivation (Wang et al. 1997; Yang et al. 1997), together with the apparent importance of inactivation to current inhibition by Na+o noted here, suggests that an indirect effect mediated by inactivation gating could contribute to the potent ability of K+o to antagonize inhibition by Na+o.
Effect of holding potential on inhbition by extracellular sodium suggests an important role for potassium efflux
The low inhibitory potency of Na+o for depolarization-activated D540K currents despite strong rectification indicative of intact inactivation prompted an experiment revealing that relatively depolarized holding potentials (like those used for D540K, Fig. 4) can attenuate Na+o inhibition in WT HERG (Fig. 6). Given that Na+o inhibits HERG current by occupying an outer pore site (Mullins et al. 2002), the reduction in Na+o inhibition at more depolarized holding potentials most likely results from continuous K+ efflux at the holding potential. Again, this model is a variation of Armstrong's classic model of antagonism of internal TEA block by permeating K+ (Armstrong, 1971). If permeating K+ were the sole determinant of block by Na+o, then inhibition of HERG current by a given [Na+]o would be expected to be greater at +60 mV than at +10 mV. That current inhibition by Na+o is not significantly greater when holding at +60 mV than when holding at +10 mV (Fig. 6B–D) most likely reflects a small effect of the transmembrane field on Na+o binding in the pore, and is consistent with previous experiments in which the test potential was varied (Mullins et al. 2002).
Towards a comprehensive model of sodium and potassium interactions with HERG
The mechanistic relationship between Na+o inhibition and Na+o speeding of recovery from inactivation is still uncertain. It is likely that Na+o inhibition of HERG current involves interactions with, at a minimum, the closed (Fig. 6) (Mullins et al. 2002) and inactivated (Fig. 4) channel states. The apparent state dependence of Na+o interactions with HERG may reflect disparate Na+o affinities of channel conformational states and/or indirect effects mediated by the influence of conformational states on permeating K+ (see discussion above and in Mullins et al. 2002). Terlau et al. (1999) have developed in detail a model that incorporates K+ permeation to explain the state dependence of κ-conotoxin PVIIA block of Shaker channels.
We considered the possibility that the placement of Na+o in the HERG pore (Mullins et al. 2002) might provide the complexity necessary for a single site to account for the data supporting mechanistic separation of the two effects (Na+o inhibition and Na+o speeding recovery from inactivation). Specifically, we investigated whether a simple established kinetic model (Wang et al. 1997; Johnson et al. 1999a) could be expanded and modified via an implicit effect of K+ permeation to simulate the two effects of Na+o with a single site. The relevant scheme is shown in Fig. 7A, Model 1. The five-state model of Johnson et al. (1999a) is shown at the top and conceived of as ‘K+-occupied’. A parallel set of states (shown below) is conceived of as ‘Na+-occupied’; the ‘Na+-occupied’ states are inaccessible in the absence of Na+o. Only open, K+-occupied channels conduct current. Na+ has identical on and off rates for closed and inactivated states. Continuous K+ permeation through open channels is conceived of as preventing Na+ binding; that is, there is no on-rate for Na+ binding to the open state in Model 1. Na+ is conceived of as a ‘catalyst’ for inactivation gating transitions independent of direction (that is, both O→I and I→O transitions are speeded when Na+ is bound). Practically, this was accomplished by lowering the membrane potential-independent portion of the free energy barrier for gating transitions (see Fig. 7 legend). In theory, the combination of rapid equilibrium between open and inactivated Na+-bound states and rapid ‘release’ of Na+ from the outer pore of open channels would manifest as fast recovery from inactivation in current records, as observed experimentally. In practice, we found that the rate for the ON→O transition had to be limited to maintain the level of current inhibition seen in experimental records; that is, channels spent enough time in the ON state that a fast off-rate for Na+ effectively eliminated current inhibition. An off-rate sufficiently low to permit potent current inhibition resulted in Na+o actually slowing apparent recovery from inactivation (Fig. 7B), an effect opposite to that observed experimentally. Thus, we do not believe this particular model is tenable.
We next investigated whether a two-site model would be able to approximate the disparate dose–response relationships for the two effects of Na+o. The scheme we implemented is shown as Model 2 in Fig. 7A. Beginning from the same baseline model (Johnson et al. 1999a), we added two sites for Na+o, denoted N1 and N2. The Na+o-bound states are again strictly inaccessible in the absence of Na+o. The N1 site is envisioned as the pore site where Na+o inhibits current (Mullins et al. 2002). Occupancy of the N2 site did not block current, but speeded inactivation gating transitions bidirectionally, with the effect implemented as described for Model 1. In Model 2, then, current passes through channels in both the O and ON2 states. It was not necessary to assign state dependence to site affinities in Model 2. Further, because symmetrically lowering the energy barrier for inactivation gating transitions increases the rates of inactivation and recovery by identical factors, the model satisfies microscopic reversibility. Figure 7C shows a simulated trace from Model 2, illustrating the appropriate directional change in recovery kinetics in high [Na+]o (protocol identical to Fig. 7B). Concentration–response curves for the two effects were adequately fitted by assigning an affinity of 3 mm to the N1 site and an affinity of 140 mm to the N2 site. Figure 7D shows concentration–response for the recovery effect. The inset in Fig. 7D illustrates the ability of Model 2 to generate a morphologically appropriate HERG current trace in the presence of high [Na+]o (protocol as in Fig. 6A). That Model 2 is able to simulate several features of our experimental data should be taken not as evidence that its mechanisms of [Na+]o action are the correct ones, but instead as evidence that these mechanisms remain within the set of tenable models.
Implications for HERG inactivation
Motivated by analogy with classical C-type inactivation, the final conformational change in HERG inactivation has most often been envisioned as a simple collapse of the pore. Recent studies of the HERG extracellular S5–P linker suggest a more complex picture. The S5–P linker of the HERG channel is longer than the S5–P linkers of channels for which crystal structures are available. This difference has inspired recent mutagenesis, structural and electrophysiological studies that suggest an intimate association between the S5–P linker and the HERG pore. Specifically, mutations in the linker produce a common mutant phenotype in which inactivation is disrupted, K+ selectivity is reduced, and voltage dependence of activation is shifted. Further, the severity of the mutant phenotype follows an alpha-helical periodicity (Liu et al. 2002). Circular dichroism and NMR measurements of a peptide corresponding to the S5–P linker have now verified an alpha-helical structure, and the linker peptide has been shown to have a specific effect on HERG channels heterologously expressed in CHO cells (Torres et al. 2003).
The effect of the linker peptide is complex and voltage dependent, with binding apparently favoured at negative potentials. Peptide application augments current at positive potentials (attributed to a disruption of inactivation), decreases current at more negative potentials, and reduces the K+ selectivity of the channels as inferred from changes in reversal potential. Intriguingly, the effects of the linker peptide appear to be largely dependent on the presence of Na+o.
The recent work of Torres et al. (2003), then, raises the possibility that the speeding of recovery from inactivation by Na+o in WT HERG channels that we have described here may result from Na+o facilitation of an S5–P linker interaction with the pore. It is conceivable that Na+o could bind either within or outside the pore to promote such a linker–pore interaction. We have considered the hypothesis that the effects of Na+o to inhibit current and speed recovery could occur at two distinct sites (as suggested by Model 2, Fig. 7). The identification of a conformational change outside the pore region that may be associated with inactivation gating (Smith & Yellen, 2002) increases the plausibility of the idea that Na+o could speed recovery via a site outside the pore. In theory, such a scenario might also help explain the relative inability of K+o to antagonize the speeding of recovery by Na+o (Fig. 2C and D). However, we cannot rule out the possibility that Na+o might act dynamically within the pore via an undefined mechanism to produce its effects on both current magnitude and recovery kinetics.
Acknowledgments
We thank Dr Michael Sanguinetti (University of Utah) for the gift of D540K and D540A mutant constructs. We thank Drs Lou DeFelice, Dan Roden, Dave Lovinger and Christina Petersen for their helpful comments on the manuscript. This project was completed in partial fulfilment of the requirements for the Ph.D. degree in Pharmacology at Vanderbilt University School of Medicine (F.M.), and supported by a Medical Student Research Fellowship from the Pharmaceutical Research and Manufacturers of America Foundation (F.M.). The work was also funded by the Medical Scientist Training Program, grant no. T32 G07347-23, awarded by the National Institute of General Medical Sciences through the National Institutes of Health (F.M.) and by a program project grant from the National Institutes of Health (J.R.B.) (P01 HL46681).
References
- Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J General Physiol. 1971;58:413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Choy AM, Lang CC, Chomsky DM, Rayos GH, Wilson JR, Roden DM. Normalization of acquired QT prolongation in humans by intravenous potassium. Circulation. 1997;96:2149–2154. doi: 10.1161/01.cir.96.7.2149. [DOI] [PubMed] [Google Scholar]
- Compton SJ, Lux RL, Ramsey MR, Strelich KR, Sanguinetti MC, Green LS, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation. 1996;94:1018–1022. doi: 10.1161/01.cir.94.5.1018. [DOI] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille . Ion Channels of Excitable Membranes. Sunderland MA USA: Sinauer; 2001. [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Johns DC, Nuss HB, Marban E. Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant-negative Kv4.2 constructs. J Biol Chem. 1997;272:31598–31603. doi: 10.1074/jbc.272.50.31598. [DOI] [PubMed] [Google Scholar]
- Johnson JP, Jr, Balser JR, Bennett PB. Enhancement of HERG K+ currents by Cd2+ destabilization of the inactivated state. Biophys J. 1999b;77:2534–2541. doi: 10.1016/s0006-3495(99)77088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JP, Jr, Mullins FM, Bennett PB. Human ether-a-go-go-related gene K+ channel gating probed with extracellular Ca2+. Evidence for two distinct voltage sensors. J General Physiol. 1999a;113:565–580. doi: 10.1085/jgp.113.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang M, Jiang M, Tseng GN. Structural and functional role of the extracellular s5-p linker in the HERG potassium channel. J General Physiol. 2002;120:723–737. doi: 10.1085/jgp.20028687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- Miller C. The inconstancy of the human heart. Nature. 1996;379:767–768. doi: 10.1038/379767a0. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Chen J, Sanguinetti MC. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J General Physiol. 2000b;115:229–240. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR., Jr Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–2790. [PubMed] [Google Scholar]
- Mullins FM, Stepanovic SZ, Desai RR, George AL, Jr, Balser JR. Extracellular sodium interacts with the HERG channel at an outer pore site. J General Physiol. 2002;120:517–537. doi: 10.1085/jgp.20028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaguchi H, Johnson JP, Jr, Petersen CI, Balser JR. A sensitive mechanism for cation modulation of potassium current. Nat Neurosci. 2000a;3:429–430. doi: 10.1038/74793. [DOI] [PubMed] [Google Scholar]
- Numaguchi H, Mullins FM, Johnson JP, Jr, Johns DC, Po SS, Yang IC, et al. Probing the interaction between inactivation gating and Dd-sotalol block of HERG. Circ Res. 2000b;87:1012–1018. doi: 10.1161/01.res.87.11.1012. [DOI] [PubMed] [Google Scholar]
- Rampe D, Roy ML, Dennis A, Brown AM. A mechanism for the proarrhythmic effects of cisapride (Propulsid): high affinity blockade of the human cardiac potassium channel HERG. FEBS Lett. 1997;417:28–32. doi: 10.1016/s0014-5793(97)01249-0. [DOI] [PubMed] [Google Scholar]
- Roden DM, Balser JR. A plethora of mechanisms in the HERG-related long QT syndrome. Genetics meets electrophysiology. Cardiovasc Res. 1999;44:242–246. doi: 10.1016/s0008-6363(99)00224-2. [DOI] [PubMed] [Google Scholar]
- Roy M, Dumaine R, Brown AM. HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation. 1996;94:817–823. doi: 10.1161/01.cir.94.4.817. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Xu QP. Mutations of the S4–S5 linker alter activation properties of HERG potassium channels expressed in Xenopus oocytes. J Physiol. 1999;514:667–675. doi: 10.1111/j.1469-7793.1999.667ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Smith PL, Yellen G. Fast and slow voltage sensor movements in HERG potassium channels. J General Physiol. 2002;119:275–293. doi: 10.1085/jgp.20028534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao BY, Lee KCP. Mutagenesis by PCR. In: Griffin HG, Griffin AM, editors. PCR Technology: Current Innovations. London: CRC Press; 1994. pp. 69–84. [Google Scholar]
- Terlau H, Boccaccio A, Olivera BM, Conti F. The block of Shaker K+ channels by kappa-conotoxin PVIIA is state dependent. J General Physiol. 1999;114:125–140. doi: 10.1085/jgp.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres AM, Bansal PS, Sunde M, Clarke CE, Bursill JA, Smith DJ, et al. Structure of the HERG K+ channel S5P extracellular linker: role of an amphipathic alpha-helix in C-type inactivation. J Biol Chem. 2003;278:42136–42148. doi: 10.1074/jbc.M212824200. [DOI] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Chen J, Sanguinetti MC. Interactions between the S4–S5 linker and the S6 transmembrane domain modulate gating of HERG K+ channels. J Biol Chem. 2002;277:18994–19000. doi: 10.1074/jbc.M200410200. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. doi: 10.1016/s0165-6147(00)01662-x. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J Physiol. 1997;502:45–60. doi: 10.1111/j.1469-7793.1997.045bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Morales MJ, Liu S, Strauss HC, Rasmusson RL. Time, voltage and ionic concentration dependence of rectification of h-erg expressed in Xenopus oocytes. FEBS Lett. 1996;389:167–173. doi: 10.1016/0014-5793(96)00570-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Salata JJ, Bennett PB. Saxitoxin is a gating modifier of HERG K+ channels. J General Physiol. 1997;121:583–598. doi: 10.1085/jgp.200308812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapura M, Nattel S, Courtemanche M, Doern D, Ethier N, Hebert T. State-dependent barium block of wild-type and inactivation-deficient HERG channels in Xenopus oocytes. J Physiol. 2000;526:265–278. doi: 10.1111/j.1469-7793.2000.t01-1-00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Snyders DJ, Roden DM. Rapid inactivation determines the rectification and [K+]o dependence of the rapid component of the delayed rectifier K+ current in cardiac cells. Circ Res. 1997;80:782–789. doi: 10.1161/01.res.80.6.782. [DOI] [PubMed] [Google Scholar]