Abstract
Post-ischaemic reperfusion may precipitate cardiomyocyte death upon correction of intracellular acidosis due in part to mitochondrial permeability transition. We investigated whether glycine, an amino acid with poorly understood cytoprotective properties, may interfere with this mechanism. In cardiomyocyte cultures, addition of glycine during re-energization following 1 h of simulated ischaemia (NaCN/2-deoxyglucose, pH 6.4) completely prevented necrotic cell death associated with pH normalization. Glycine also protected against cell death associated with pH normalization in reoxygenated rat hearts. Glycine prevented cyclosporin-sensitive swelling and calcein release associated with re-energization in rat heart mitochondria submitted to simulated ischaemia or to Ca2+ stress under normoxia. NMR spectroscopy revealed a marked glycine depletion in re-energized cardiomyocytes that was reversed by exposure to 3 mm glycine. These results suggest that intracellular glycine exerts a previously unrecognized inhibition on mitochondrial permeability transition in cardiac myocytes, and that intracellular glycine depletion during myocardial hypoxia/reoxygenation makes the cell more vulnerable to necrotic death.
Mitochondria are increasingly recognized as central regulators of cell death and survival in cardiac myocytes exposed to ischaemia–reperfusion (Duchen, 1999). A prominent player in the mitochondrial death pathway is the mitochondrial permeability transition pore, a largely unselective megachannel connecting the mitochondrial matrix with the cytosol and allowing the passage of molecules up to 1.5 kDa. During reperfusion, mitochondrial permeability transition (MPT) may be triggered by elevated Ca2+ concentrations, radical oxygen species and normalization of intracellular pH, and it is followed by the collapse of mitochondrial membrane potential, interruption of ATP synthesis, release of pro-apoptotic molecules into the cytosol (Petronilli et al. 2001; Di Lisa et al. 2001) and profound ionic disturbances that may result in necrotic or apoptotic death (Lemasters et al. 1998; Kim et al. 2003). It has been recently proposed that inhibition of MPT may have a strong protective effect by preventing the development of structural cell fragility (Hausenloy et al. 2003; Javadov et al. 2003).
The amino acid glycine (Gly) has been shown to exert a protective effect against cell death after diverse insults including ischaemia–reperfusion injury in different cell types other than cardiomyocytes, but the mechanism of this effect remains elusive (Nishimura et al. 1998; Schemmer et al. 1999; Nishimura & Lemasters, 2001; Dong et al. 2001; Zhang et al. 2003). In this study we investigated the effect of Gly on MPT and reoxygenation-induced cell death in cultured HL-1 cardiac myocytes, intact rat myocardium and rat heart mitochondria. The results show that Gly, an endogenous amino acid, may play a cardioprotective effect in reoxygenated cardiomyocytes by preventing MPT associated with pH normalization.
Methods
This study was performed on cultured HL-1 cardiac myocytes, Langendorf-perfused hearts from Sprague-Dawley rats, and isolated mitochondria. The experimental procedures conformed with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication no. 85–23, revised 1996), and were approved by the Research Commission on Ethics of the Hospital Vall d'Hebron.
Studies in HL-1 cardiomyocytes
HL-1 cardiac myocytes were plated at 20 000 cells cm−2 density in glass-bottomed culture dishes until a 70–80% confluence was achieved, as previously described (Ruiz-Meana et al. 2003).
Simulated ischaemia–reoxygenation
Cell pellets were submitted to 1 h simulated ischaemia (SI) by suspending them in a buffer containing (mm): NaCl 140, KCl 3.6, CaCl2 2, MgSO4 1.2, Hepes 20, NaCN 2, 2-deoxyglucose 20, at pH 6.4, 37°C. Reoxygenation was performed by centrifugation and resuspension of the pellet in a control normoxic buffer at pH 7.4, with 5 mm glucose.
Experimental interventions
The effect of modifying the reoxygenation buffer by lowering its pH (up to 6.4) or by adding Gly (3 mm) was investigated. The role of Ca2+ was studied by sequestration of either intra- or extracellular Ca2+ (addition of 30 μm BAPTA or removal of CaCl2 in the presence of 2 mm EGTA).
Cell volume and viability
Changes in cell volume were determined by analysis of forward scatter (FACscalibur flow cytometer, Becton Dickinson, USA) and cell viability by exclusion of propidium iodide (PI). Double staining with annexinV–fluorescein isothiocyanate (FITC)–PI was used to quantify the contribution of apoptosis (annexin–FITC+–PI− cells) to cell death.
Intracellular pH and Na+
Changes in cytosolic [H+] and [Na+] during SI–reoxygenation were monitored by ratio-fluorescence imaging in HL-1 cells loaded, respectively, with 3 μm BCECF-AM or 5 μm SBFI (Molecular Probes, USA) (Ruiz-Meana et al. 2003).
Studies in isolated rat hearts
Heart preparation
After an intraperitoneal injection of sodium pentobarbital (100 mg kg−1), the hearts from 18 Sprague-Dawley rats (280–350 g) were rapidly removed and perfused with an oxygenated Krebs solution at 37°C, as previously described (Rodriguez-Sinovas et al. 2003).
Experimental protocol
After 40 min of equilibration, hearts were submitted to 2 h of hypoxia at pH 6.4 followed by 1 h of reoxygenation. For hypoxic perfusion, a modified Krebs buffer (mm: NaCl 139.5, KCl 4.7, MgSO4 1.2, CaCl2 2.5, NaHCO3 3.5, KH2PO4 1.2, and sucrose 11) was bubbled with 95% N2–5% CO2. Reoxygenation was performed by switching the hypoxic Krebs solution to a glucose-containing oxygenated (95% O2–5% CO2) Krebs solution. Hearts were allocated to one of three groups: (1) control reoxygenation (n = 6) at pH 7.4; (2) acidic reoxygenation (n = 5) at pH 6.4; (3) reoxygenation in the presence of 10 mm Gly (n = 5). Two additional hearts were used to calculate cumulative concentration–response curves of developed tension and transmembrane action potential to Gly (from 10−4 to 10−1m, each concentration given during 15 min) under normoxia. Hearts were paced from the base (2.5 ms, 4 V pulses at 2.5 Hz) and transmembrane action potentials were recorded from the apex, as described (Rodriguez-Sinovas et al. 2003).
Enzyme release
Lactate dehydrogenase (LDH) release during reoxygenation was measured in samples taken from the effluent as previously described (Rodriguez-Sinovas et al. 2003).
Studies in isolated mitochondria
Isolation of mitochondria
Rat heart mitochondria were isolated by differential centrifugation, according to the method described by Holmuhamedov et al. (1998). Protein was adjusted to 1 mg ml−1.
Experimental interventions
Mitochondria were submitted to either (1) simulated ischaemia (SI)–reoxygenation or (2) 15 min Ca2+ overload. To simulate ischaemia, the mitochondrial pellet was suspended in a metabolic inhibition buffer (mm: KCl 150, NaCl 7, Hepes 6, KH2PO4 0.5, sucrose 50, MgCl2 1, ADP 0.1, CaCl2 0.01 and NaCN 2), at pH 6.4, for 1 h at 37°C. Reoxygenation was performed by switching to a NaCN-free solution containing (mm): KCl 150, NaCl 7, Hepes 6, KH2PO4 0.5, sucrose 50, MgCl2 1, ADP 0.1, CaCl2 0.05, ATP 0.3, succinate 0.3, ascorbic acid 2.5, at pH 7.2. The effects of acid pH (6.4), Gly (3 mm) or cyclosporin A (CsA, 1 μm), as well as substrates (5 mm succinate, 5 mm pyruvate or 5 mml-alanine) on MPT were assessed during the first 30 min of reoxygenation. Also, the contribution of Ca2+ to the MPT was tested by adding 2 mm EGTA or by increasing the extracellular Ca2+ to 200 μm in the reoxygenation buffer. In the control group, mitochondria were submitted to the same experimental manipulations in a NaCN-free, low Ca2+ (0.1 μm) buffer containing (mm): KCl 150, NaCl 7, Hepes 6, KH2PO4 0.5, sucrose 50, MgCl2 1, ADP 0.1, ATP 0.3, succinate 5, ascorbic acid 2.5, at pH 7.2. The inhibitory effect of acid pH and Gly on MPT was also assessed in normoxic mitochondria submitted to 200 μm CaCl2 for 15 min.
Swelling assay
Changes in mitochondrial volume were assayed spectrophotometrically in mitochondrial suspensions at 25°C using either 96-well-plates (Garlid et al. 1996) or 500 μl cuvettes. Light absorbance was determined at 520 nm under basal conditions, after 60 min of SI, and at the end of reoxygenation. Changes in light absorbance were normalized with respect to basal values. In normoxic Ca2+-stressed mitochondria light absorbance was monitored throughout time following addition of 200 μm Ca2+ to the mitochondrial suspension. MPT was identified as a CsA-sensitive abrupt decrease in light absorbance, reflecting passive matrix swelling.
Assessment of calcein release
Calcein release was monitored in mitochondria using a 96-well fluorometry assay. The mitochondrial pellet was loaded with 1 μm calcein-AM at 37°C (Molecular Probes, USA), washed and submitted to 200 μm CaCl2. At the end of the experiment, mitochondria were centrifuged and calcein fluorescence was determined (Excitation Wavelength: 485 nm, Emission Wavelength: 538 nm) in the pellet (intramitochondrial compartment) and in the supernatant (extramitochondrial compartment). MPT was identified as a CsA-sensitive loss of calcein from the intramitochondrial compartment. This effect was invariably associated with an increase in the calcein at the extramitochondrial compartment.
NMR spectroscopy
Cell pellets ((3–5) × 106 cells per sample) were extracted with 1.2 ml of chloroform–methanol (1: 2) at room temperature for 1 h. After addition of 0.5 ml chloroform and 0.5 ml of water, two phases were separated by centrifugation (1500 g, 5 min). The upper phase (methanol–water) was separated and dried under N2. Dried extracts were dissolved with 400 μl of D2O containing 1 mm 3-(trimethylsilyl) propionic acid (TSP) as chemical shift and concentration reference. Spectroscopy was performed at 27° C with a 400 MHz Bruker ARX spectrometer. Peak areas were measured by integration in fully relaxed spectra.
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS for Windows 8.0). Comparisons between two independent groups were performed by Student's t test. The effect of SI–reoxygenation with or without Gly supplementation on intracellular Gly content was assessed by means of a t test for paired samples. Evaluation of unhomogeneity between multiple groups was performed by analysis of variance (ANOVA). Curves of LDH release were compared by repeated measures ANOVA. Data are expressed as the mean ±s.e.m. The significance level was set at 0.05.
Results
Cell death during SI–reoxygenation
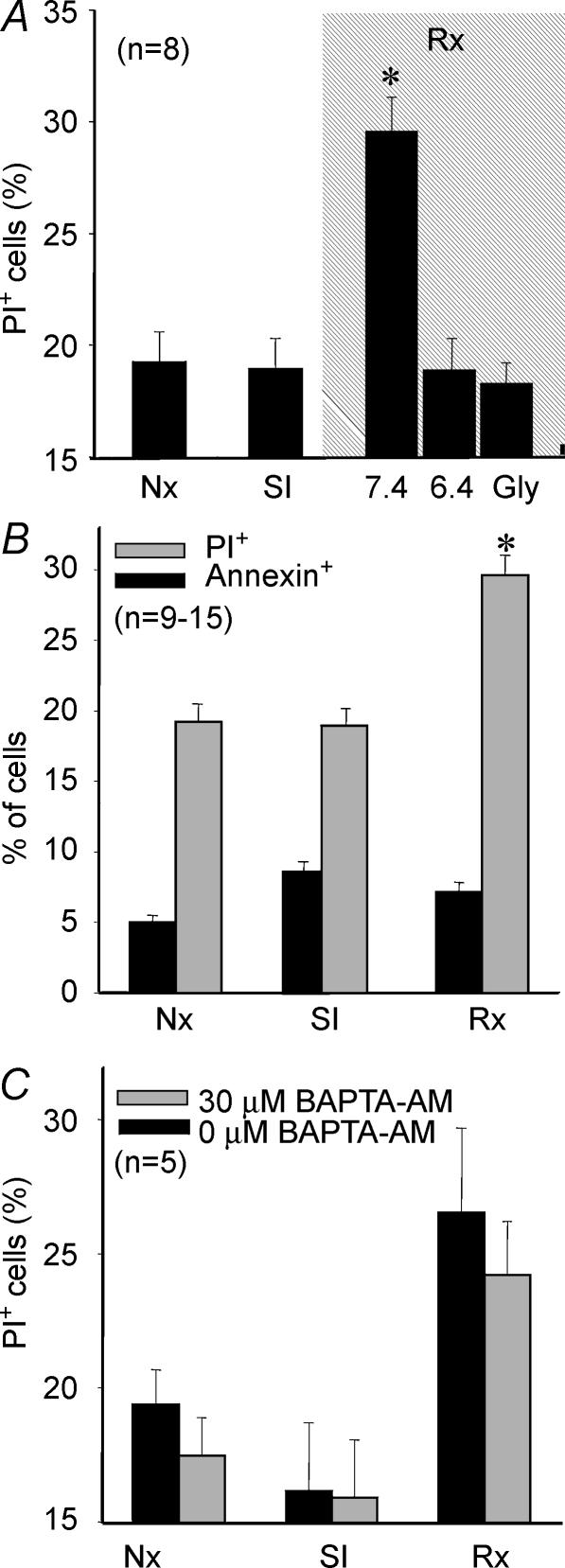
SI did not result in detectable cell death (Fig. 1A). However, reoxygenation after 1 h of SI was associated with a marked increase in the number of PI+ cardiomyocytes, reflecting sarcolemmal disruption (Fig. 1A). Sarcolemmal rupture occurred early upon reoxygenation, was fully observable at 30 min, and did not further increase when the duration of reoxygenation was prolonged to 2 h (data not shown). Apoptosis seemed not to contribute to cell death: the proportion of annexin+–PI− cells after 30 min of reoxygenation was low and remained unchanged after 2 h of reoxygenation (data not shown). Furthermore, this proportion was not significantly modified by either SI or reoxygenation (Fig. 1B).
Figure 1.
Cell death in cardiomyocytes submitted to 1 h SI–30 min reoxygenation
A, necrotic cell death (PI+) when reoxygenation was performed at pH 7.4, at pH 6.4 or at pH 7.4 in the presence of 3 mm Gly. Cell death was associated with normalization of pH upon reoxygenation. Addition of Gly to the reoxygenation buffer prevented cell death associated with pH normalization. B, contribution of apoptosis (annexin+–PI− cells) to total cell death was small and was not significantly increased by either SI or reoxygenation. C, sequestration of intracellular Ca2+ with BAPTA throughout the reoxygenation period did not afford any significant protection against cell death. P < 0.05 versus normoxic cells.
Cell death associated with reoxygenation was closely related to pH normalization and could be abolished when pH remained at 6.4 during reoxygenation (Fig. 1A). Addition of 3 mm Gly to the reoxygenation buffer completely prevented pH-dependent cell death (Fig. 1A). Chelation of Ca2+ from the extracellular medium with EGTA had no effect on reoxygenation-induced cell death (15.1 ± 0.4% and 16.3 ± 0.26% in control and EGTA-treated cells, respectively, n.s.). Similarly, intracellular Ca2+ sequestration with BAPTA, did not reduce cell death during reoxygenation (Fig. 1C).
Changes in cell volume, intracellular pH and Na+ during SI–reoxygenation
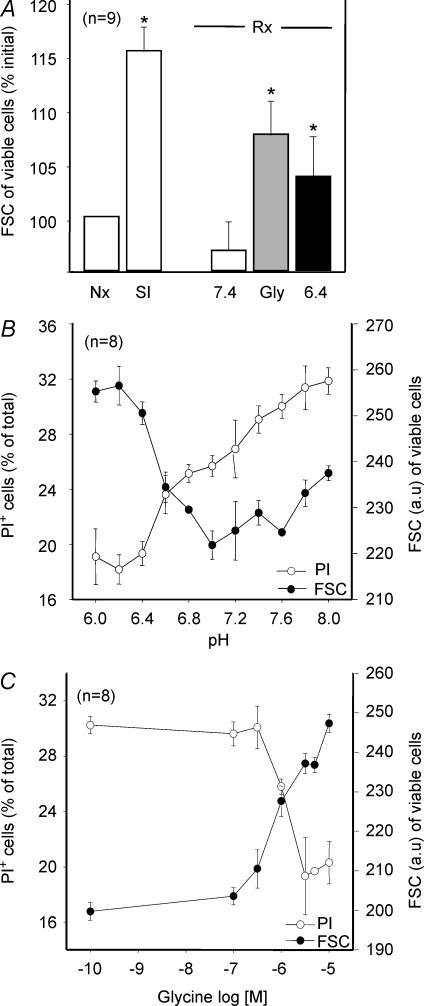
SI induced a significant volume increase (cell swelling), followed by cell size correction upon restoration of energy and normalization of pH. Interestingly, the protective effect of acid pH and Gly on cell death was closely associated with an impaired capacity of cells to recover their volume (Fig. 2A). In both interventions, dose–effect studies demonstrated a narrow inverse relationship between the degree of protection against reoxygenation-induced cell death and the magnitude of correction of cell volume (Fig. 2B and C).
Figure 2.
Effect of SI and reoxygenation on cardiomyocyte volume and death (PI+ cells) under different conditions
A, cells submitted to SI experienced a significant increase in their volume, which was corrected upon reoxygenation at pH 7.4. Cell volume did not recover when Gly was present during reoxygenation or when reoxygenation was performed at pH 6.4. P < 0.05 versus normoxic cells. B, the degree of protection against death afforded by low pH closely correlated with the magnitude of the effect on cell volume. C, the degree of protection afforded by Gly was also closely correlated with its effect on cell volume.
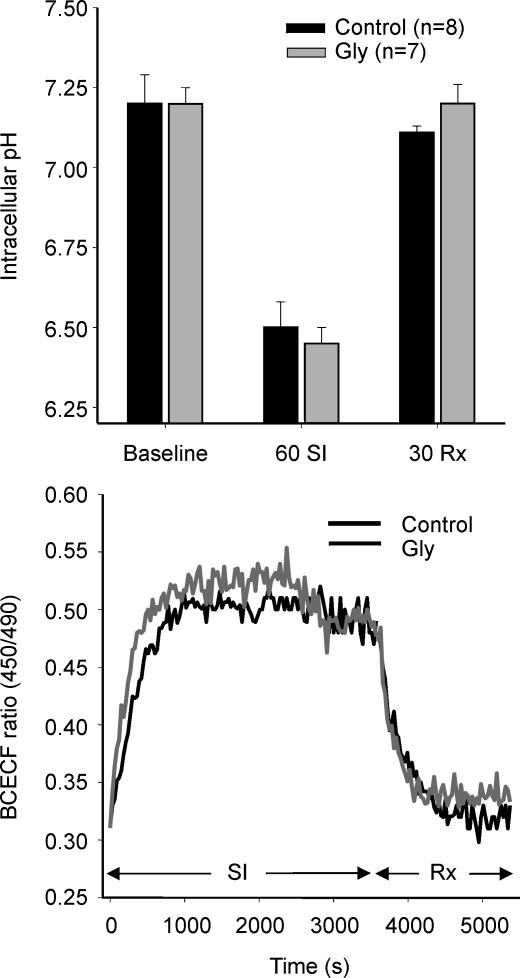
Intracellular pH decreased rapidly during SI, reaching 6.4 ± 0.04 within 15 min, and recovered to 7.2 in the first 10 min of reoxygenation. Addition of Gly to the reoxygenation buffer did not produce any delay in pH recovery (Fig. 3). Intracellular Na+ concentration steadily rose during the whole SI period (from 1 ± 0 to 1.35 ± 0.05 a.u. of SBFI ratio fluorescence), remained stable during the 30 min reoxygenation (1.33 ± 0.1 a.u), and was not influenced by the presence of Gly in the reoxygenation buffer (1.32 ± 0.16 a.u., n.s.).
Figure 3.
Changes of intracellular pH in cardiomyocytes submitted to 1 h SI–30 min reoxygenation (Rx)
A, intracellular pH immediately before SI (baseline), after 1 h SI and at the end of reoxygenation was not modified by the addition of 3 mm Gly during the reoxygenation period. B, original measurements of intracellular H+ concentration (a.u. of BCECF ratio fluorescence) in 2 representative experiments. Addition of Gly did not modify the time course of pH recovery during reoxygenation
Effect of Gly on isolated rat hearts
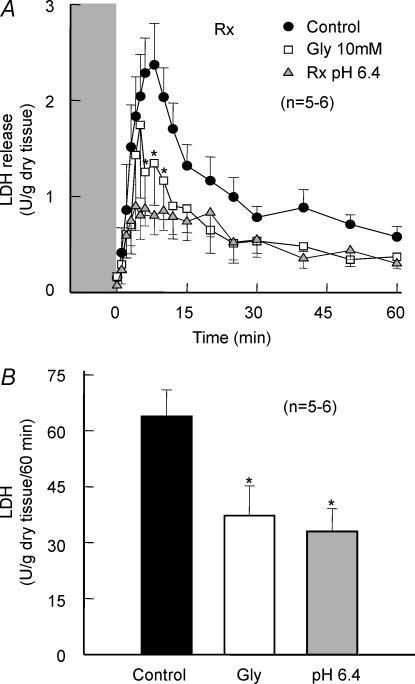
Reoxygenation induced an important LDH release (Fig. 4A). In hearts submitted to reoxygenation at pH 6.4, total LDH release was reduced by approximately 50% (33.1 ± 6.0 U (g dry tissue)−1 (60 min)−1). Addition of 10 mm Gly during reoxygenation had a similar effect (LDH release was 37.4 ± 7.8 U (g dry tissue)−1 (60 min)−1, Fig. 4B). Normoxic perfusion with a Gly-containing Krebs solution, from 10−4 to 10−1m, did not induce any change in transmembrane action potential characteristics, conduction velocity or contractile function (data not shown).
Figure 4.
Reoxygenation injury in isolated rat hearts
A, reoxygenation-induced LDH release in control and Gly-treated hearts, and in hearts reoxygenated at pH 6.4. B, total LDH release during reoxygenation.
Mitochondrial swelling during reoxygenation
In isolated mitochondria, reoxygenation at pH 7.2 after 1 h SI at pH 6.4 was associated with an increase in mitochondrial volume, as measured by a fall in light absorbance. Reoxygenation at pH 6.4 abolished mitochondrial swelling. Reoxygenation-induced swelling can be attributed to MPT since it was blocked by 1 μm CsA and could be precipitated by increasing Ca2+ to 200 μm during reoxygenation (Fig. 5A). MPT was not dependent on substrate availability, since the increase of succinate concentration from 0.3 to 5 mm, or addition of 5 mm pyruvate did not reduce mitochondrial swelling (Fig. 5A). Addition of 3 mm Gly to the reoxygenation buffer completely prevented mitochondrial swelling despite pH normalization (Fig. 5B). This protective effect against MPT could not be mimicked by 5 mml-alanine or by sequestration of extracellular Ca2+ with 2 mm EGTA (Fig. 5B). To further assess the potential inhibitory effect of Gly on MPT, normoxic mitochondria were stressed with 200 μm CaCl2, a manoeuvre known to precipitate mitochondrial permeabilization. The minimum concentration of Gly found to be protective against SI–reoxygenation (3 mm) also reduced mitochondrial swelling induced by high Ca2+ in a similar manner as acidic pH (Fig. 5C and D).
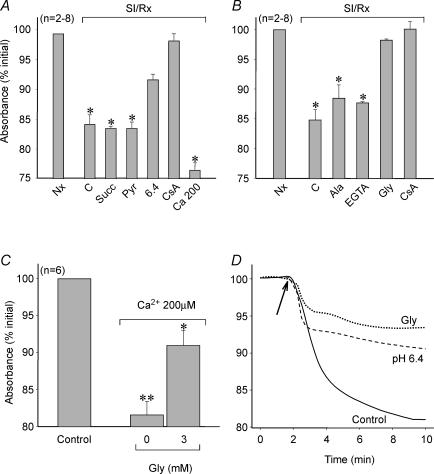
Figure 5.
Effect of pH and Gly on mitochondrial swelling
Changes in light absorbance at 520 nm in suspensions of rat heart mitochondria submitted to different experimental protocols. A, 30 min of reoxygenation (Rx) after 1 h of SI under the following conditions: at pH 7.2 (C), with 2 different respiratory substrates (5 mm succinate or 5 mm pyruvate), at pH 6.4, with 1 μm CsA or with 200 μm CaCl2. Absorbance is expressed as the percentage value with respect to normoxic mitochondria (Nx). Both acidic reoxygenation (6.4) and CsA prevented the reoxygenation-induced fall in light absorbance, indicative of MPT. *P < 0.05 versus Nx. B, 30 min of reoxygenation (Rx) after 1 h of SI under the following conditions: at pH 7.2 (C), with 5 mm l-alanine, with 2 mm EGTA, with 3 mm Gly and with 1 μm CsA. Absorbance is expressed as the percentage value with respect to normoxic mitochondria (Nx). Addition of Gly to the reoxygenation buffer was able to completely prevent reoxygenation-induced mitochondrial swelling, as did CsA. *P < 0.05 versus Nx. C, exposure of normoxic mitochondria to 15 min of 200 μm CaCl2 to promote MPT, with or without 3 mm Gly. Control mitochondria were not submitted to high Ca2+ concentration. Addition of 3 mm Gly significantly reduced swelling in Ca2+-stressed mitochondria. *P < 0.05 versus Control, **P < 0.05 versus 3 mm Gly. D, changes in light absorbance throughout time in fully energized mitochondria submitted to 200 μm CaCl2 to promote MPT. The arrows indicate the time at which Ca2+ was added. Addition of 3 mm Gly during Ca2+ overload was able to prevent mitochondrial swelling more efectively than acid pH.
Calcein release in isolated mitochondria
Addition of 200 μm CaCl2 to calcein-loaded mitochondria induced a rapid and abrupt release of the fluorochrom into the extramitochondrial space (Fig. 6). When Gly was added at the concentrations used to prevent mitochondrial swelling (3 mm or higher), Ca2+-induced calcein release was completely inhibited (Fig. 6). Release of calcein clearly reflected MPT since it could be blocked by CsA (0.1–1 μm).
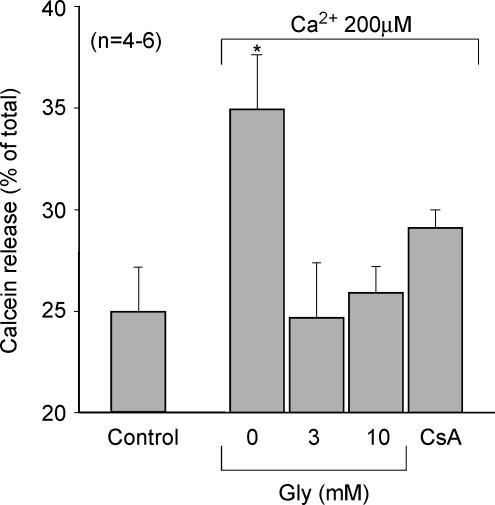
Figure 6.
Effect of Gly on mitochondrial calcein release
Calcein release from rat heart mitochondria submitted to 15 min of 200 μm CaCl2 to promote MPT, in the presence of Gly at 2 different concentrations, in its absence, and when MPT was blocked with 0.1 μm CsA. Control group corresponds to mitochondria not submitted to Ca2+ overload. Gly 3 mm and 10 mm prevented calcein release, as did CsA. P < 0.05 versus control.
Intracellular Gly content
NMR spectroscopy revealed that SI–reoxygenation induced a significant reduction of intracellular Gly content. In contrast, a marked increase in intracellular Gly concentration was observed after 30 min reoxygenation when reoxygenation buffer contained 3 mm Gly (Fig. 7).
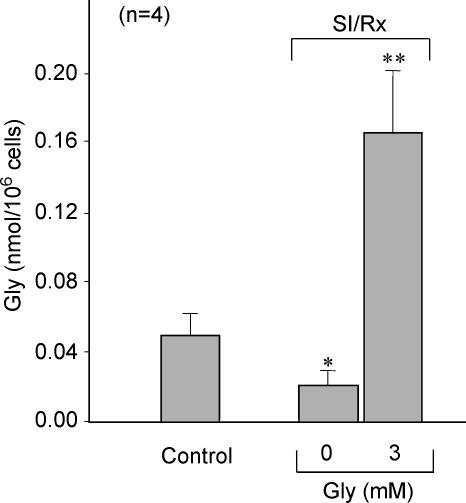
Figure 7.
Gly content during SI–reoxygenation (Rx)
Intracellular Gly content measured by NMR in cardiomyocytes submitted to 1 h SI–30 min reoxygenation in the presence (Gly+) or absence (Gly−) of Gly in the reoxygenation buffer. Control group corresponds to cells maintained under Gly-free normoxic conditions. SI–reoxygenation resulted in a reduction of intracellular Gly content while addition of 3 mm Gly to the reoxygenation buffer induced a significant increase in the intracellular Gly content. P < 0.05 versus normoxic cells, **P < 0.01 versus normoxic cells.
Discussion
The present study shows that the amino acid Gly has a powerful and previously unrecognized inhibitory effect on MPT, similar to that of low pH, in rat heart mitochondria. We also demonstrate for the first time that addition of 3–10 mm Gly during reoxygenation prevents acute necrotic cell death associated with pH normalization in cultured cardiac myocytes and LDH release in isolated rat hearts. Moreover, exposure to Gly during reoxygenation not only reverses cellular Gly depletion observed in cells submitted to SI–reoxygenation, but markedly raises intracellular Gly content up to the range proven to inhibit MPT in isolated mitochondria. Altogether, these results indicate that Gly protects cardiomyocytes from reoxygenation-induced cell death by preventing MPT associated with pH normalization.
MPT contributes to cardiomyocyte death induced by normalization of pH during reoxygenation
Disruption of the mitochondrial inner membrane by MPT triggers cell death by different pathways, including the release of cytochrome c and other proapoptotic factors that activate caspases and initiate apoptosis (Zamzami & Kroemer, 2001; De Giorgi et al. 2002). Less known, but increasingly recognized, is the contribution of MPT to acute necrotic cell death through impaired ATP synthesis and alterations in cation, in particular of Ca2+, homeostasis (Crompton, 1999; Kim et al. 2003).
Growing evidence indicates that MPT may contribute to myocardial reperfusion or reoxygenation injury. During prolonged ischaemia, the rapid and profound fall in intracellular pH exerts a powerful inhibitory effect on MPT by antagonizing Ca2+ binding to the adenine nucleotide translocase (ANT; Halestrap, 1991). Upon reperfusion, the protective inhibitory effect of low pH is abrogated by the rapid correction of intracellular acidosis, while exacerbated oxidative stress and persistent abnormalities of Ca2+ handling may stimulate MPT. Experimental evidence of MPT during reperfusion has been provided by fluorescence microscopy of cardiomyocytes re-energized after SI showing CsA-sensitive mitochondrial depolarization (Duchen et al. 1993) and calcein loading (Petronilli et al. 1999). In reperfused myocardium, MPT has been demonstrated by the loss of mitochondrial NAD+ (Di Lisa et al. 2001) and mitochondrial entrapment of [3H]deoxyglucose 6-phosphate (Javadov et al. 2000), with a time course that reflects that of pH normalization.
In the present study, acidic reoxygenation markedly attenuated cell death in cultured HL-1 myocytes and in rat hearts. The protective effect of low pH was independent of the inhibitory effect of acidosis on contractility (since HL-1 cells have only a rudimentary contractile machinery and do not hypercontract upon reoxygenation), was not associated with attenuated cytosolic Na+ overload, and was not modified by elimination of extra or intracellular Ca2+. The protective effect of acidic pH cannot be thus explained by attenuation of Ca2+ overload or hypercontracture, but it rather appears to be related to its inhibitory effect on MPT, as observed in isolated mitochondria.
Gly prevents reoxygenation injury by interfering with MPT
The direct protective effect of Gly against necrotic and apoptotic cell death induced by different insults, including ischaemia–reperfusion, and a variety of other pathological conditions – i.e. endotoxaemic shock, alcoholic hepatitis, hepatic fibrosis, arthritis, tumour and drug toxicity – has been characterized in detail (Zhong et al. 1996; Nishimura et al. 1998; Nishimura & Lemasters, 2001; Dong et al. 2001; Zhang et al. 2003). Gly delays sarcolemmal rupture induced by hypoxia, which was initially explained by attenuated Na+ accumulation obscurely related to stimulation of the Gly-gated Cl− channels, originally described in the postsynaptic membranes of the spinal cord (Carini et al. 2000). Other authors suggested that the protective effect of Gly was independent of receptor stimulation but rather related to the closure of non-specific leaks in plasma membranes induced by hypoxia (Dong et al. 1998; Frank et al. 2000; Nishimura & Lemasters, 2001). Confocal microscopy analysis of membrane permeabilization to fluorescent molecules of different sizes demonstrated that Gly delays membrane permeabilization during hypoxia, which could reflect the opening of large anionic channels (‘death pores’) eventually leading to massive cell swelling and sarcolemmal rupture (Nishimura & Lemasters, 2001). Previous studies were coincident in interpreting that Gly prevented plasma membrane failure, the final event in the pathway to necrotic cell death (Frank et al. 2000).
In the present study Gly completely prevented cell death associated with normalization of pH during reoxygenation in cardiomyocyte cultures, and mimicked the protective effect of acidic reoxygenation on LDH release in isolated rat hearts. Since acute cell death observed during reoxygenation in our model was ascribable to MPT, we hypothesized that Gly could act at the mitochondrial level, interfering with MPT. The experiments in isolated mitochondria demonstrated that Gly indeed prevents CsA-sensitive mitochondrial swelling and calcein release induced by re-energization after a transient SI or by exposure to high Ca2+. The protective effect of Gly is not related to its utilization as a respiratory substrate or as a source of NAD(P)H, since it was not mimicked by other substrates (succinate or pyruvate) and was present in fully energized mitochondria submitted to Ca2+ overload.
The inhibitory effect of Gly on MPT was equivalent to that of low pH (6.4) and was observed at high concentrations (3 mm). Intracellular Gly concentration, measured by NMR spectroscopy, was reduced in HL-1 cells exposed to SI–reoxygenation, as compared to normoxic cells. Exposure to 3 mm Gly during reoxygenation raised intracellular Gly concentration up to the range proven to inhibit MPT. Measured intracellular content corresponds to an estimated Gly concentration of approximately 7 mm, compared to approximately 1 mm in normoxic cells and 0.6 mm in cells reoxygenated without Gly.
Hypothetical role of Gly depletion in reoxygenation
The mechanism of Gly depletion in cardiomyocytes submitted to SI–reoxygenation was not investigated in the present study. However, amino acid and Cl− release play an important role in regulating volume decrease in swollen cells (Rasmusson et al. 1993; Song et al. 1998). It should be thus expected that the rapid reduction in cell volume occurring during reoxygenation and normalization of intracellular pH results in Gly efflux. Although the contribution of Gly efflux to volume decrease has not been established, its abolition by exposure to increased Gly concentration in the extracellular medium should reduce it and hamper cell volume correction. In this study, addition of Gly to the reoxygenation buffer attenuated volume decrease, and this effect was closely correlated with the magnitude of the protection afforded against cell death.
A hypothesis that integrates the results obtained in the present study is outlined in Fig. 8. Rapid pH normalization during reoxygenation favours MPT and cell death. Intracellular Gly acts as an inhibitor of MPT. However, volume regulatory decrease occurring upon normalization of extracellular pH involves cellular Gly loss, which makes the cell more vulnerable to MPT, an effect that can be reversed by increasing extracellular Gly concentration.
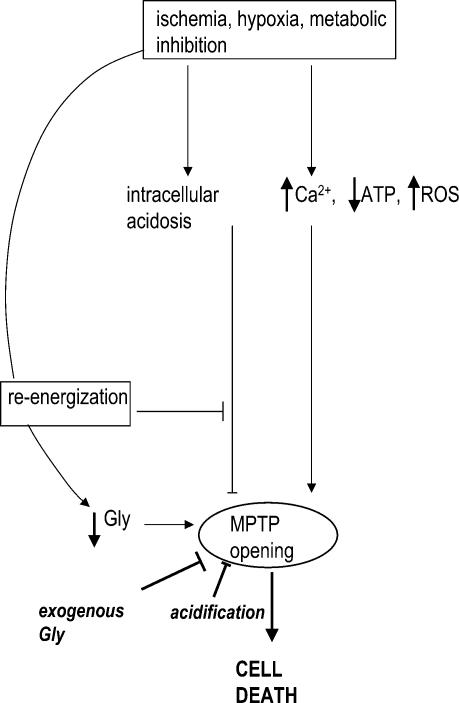
Figure 8.
Proposed role of Gly during reperfusion injury
Ischaemia and ischaemia-related conditions are associated with cytosolic derangements that favour MPT, but also with acidosis that prevents it. Reoxygenation rapidly corrects intracellular acidosis, thus allowing MPT, which is facilitated by depletion of intracellular Gly (probably through volume regulatory decrease). Treatment with Gly at the time of reoxygenation inhibits MPT despite correction of acidosis, and prevents cell death.
The contribution of Gly to cardiomyocyte survival after an ischaemic insult represents a promising therapeutic approach to preventing cell death in reperfused myocardium. Studies in volunteers have shown that plasmatic Gly concentrations higher than those found to be protective in this study can be rapidly achieved by intravenous infusion, and are well tolerated (Nilsson et al. 1996).
Acknowledgments
This work was partially supported by grants from the Spanish Ministry of Science & Technology, CICYT-SAF/2002-00759, and Fondo de Investigación Sanitaria 01/3135. A.R.-S. has a grant from the Ministerio de Sanidad y Consumo (99/3142).
References
- Carini R, De Cesaris MG, Splendore R, Bagnati M, Bellomo G, Albano E. Alterations of Na+ homeostasis in hepatocyte reoxygenation injury. Biochim Biophys Acta. 2000;1500:297–305. doi: 10.1016/s0925-4439(99)00114-3. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ, Ichas F. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Dong Z, Patel Y, Saikumar P, Weinberg JM, Venkatachalam MA. Development of porous defects in plasma membranes of adenosine triphosphate-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Laboratory Invest. 1998;78:657–668. [PubMed] [Google Scholar]
- Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y. Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol. 2001;158:1021–1028. doi: 10.1016/S0002-9440(10)64049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, McGuinness O, Brown LA, Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32:58–66. doi: 10.1016/s0168-8278(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at pH values below 7. Implications for the protective effect of low pH against chemical and hypoxic cell damage. Biochem J. 1991;278:715–719. doi: 10.1042/bj2780715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res. 2003;60:617–625. doi: 10.1016/j.cardiores.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol. 1998;275:H1567–H1576. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549:513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadov SA, Lim KH, Kerr PM, Suleiman MS, Angelini GD, Halestrap AP. Protection of hearts from reperfusion injury by propofol is associated with inhibition of the mitochondrial permeability transition. Cardiovasc Res. 2000;45:360–369. doi: 10.1016/s0008-6363(99)00365-x. [DOI] [PubMed] [Google Scholar]
- Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Randmaa I, Hahn RG. Haemodynamic effects of irrigating fluids studied by Doppler ultrasonography in volunteers. Br J Urol. 1996;77:541–546. doi: 10.1046/j.1464-410x.1996.94411.x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Lemasters JJ. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ. 2001;8:850–858. doi: 10.1038/sj.cdd.4400877. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Romer LH, Lemasters JJ. Mitochondrial dysfunction and cytoskeletal disruption during chemical hypoxia to cultured rat hepatic sinusoidal endothelial cells: the pH paradox and cytoprotection by glucose, acidotic pH, and glycine. Hepatology. 1998;27:1039–1049. doi: 10.1002/hep.510270420. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Rasmusson RL, Davis DG, Lieberman M. Amino acid loss during volume regulatory decrease in cultured chick heart cells. Am J Physiol. 1993;264:C136–C145. doi: 10.1152/ajpcell.1993.264.1.C136. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A, Garcia-Dorado D, Padilla F, Inserte J, Barrabes JA, Ruiz-Meana M, Agullo L, Soler-Soler J. Pre-treatment with the Na+/H+ exchange inhibitor cariporide delays cell-to-cell electrical uncoupling during myocardial ischemia. Cardiovasc Res. 2003;58:109–117. doi: 10.1016/s0008-6363(02)00840-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Garcia-Dorado D, Pina P, Inserte J, Agullo L, Soler-Soler J. Cariporide preserves mitochondrial proton gradient and delays ATP depletion in cardiomyocytes during ischemic conditions. Am J Physiol Heart Circ Physiol. 2003;285:H999–H1006. doi: 10.1152/ajpheart.00035.2003. [DOI] [PubMed] [Google Scholar]
- Schemmer P, Bradford BU, Rose ML, Bunzendahl H, Raleigh JA, Lemasters JJ, Thurman RG. Intravenous glycine improves survival in rat liver transplantation. Am J Physiol. 1999;276:G924–G932. doi: 10.1152/ajpgi.1999.276.4.G924. [DOI] [PubMed] [Google Scholar]
- Song D, O'Regan MH, Phillis JW. Amino acid release during volume regulation by cardiac cells: cellular mechanisms. Eur J Pharmacol. 1998;341:273–280. doi: 10.1016/s0014-2999(97)01440-4. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- Zhang K, Weinberg JM, Venkatachalam MA, Dong Z. Glycine protection of PC-12 cells against injury by ATP-depletion. Neurochem Res. 2003;28:893–901. doi: 10.1023/a:1023275426637. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Jones S, Thurman RG. Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol. 1996;270:G332–G338. doi: 10.1152/ajpgi.1996.270.2.G332. [DOI] [PubMed] [Google Scholar]