Abstract
We tested the hypothesis that a single allele deletion of neuronal nitric oxide synthase (nNOS) would impair the neural control of heart rate following physical training, and that this phenotype could be restored following targeted gene transfer of nNOS. Voluntary wheel-running (+EX) in heterozygous nNOS knockout mice (nNOS+/−, +EX; n = 52; peak performance 9.1 ± 1.8 km day−1) was undertaken and compared to wild-type mice (n = 38; 9.5 ± 0.8 km day−1). In anaesthetized wild-type mice, exercise increased phenylephrine-induced bradycardia by 67% (measured as heart rate change, in beats per minute, divided by the change in arterial blood pressure, in mmHg) or pulse interval response to phenylephrine by 52% (measured as interbeat interval change, in milliseconds, divided by the change in blood pressure). Heart rate changes or interbeat interval changes in response to right vagal nerve stimulation were also enhanced by exercise in wild-type atria (P < 0.05), whereas both in vivo and in vitro responses to exercise were absent in nNOS+/− mice. nNOS inhibition attenuated heart rate responses to vagal nerve stimulation in all atria (P < 0.05) and normalized the responses in wild-type, +EX with respect to wild-type with no exercise (−EX) atria. Atrial nNOS mRNA and protein were increased in wild-type, +EX compared to wild-type, −EX (P < 0.05), although exercise failed to have any effect in nNOS+/− atria. In vivo nNOS gene transfer using adenoviruses targeted to atrial ganglia enhanced choline acetyltransferase–nNOS co-localization (P < 0.05) and increased phenylephrine-induced bradycardia in vivo and heart rate responses to vagal nerve stimulation in vitro compared to gene transfer of enhanced green fluorescent protein (eGFP, P < 0.01). This difference was abolished by nNOS inhibition (P < 0.05). In conclusion, genomic regulation of NO bioavailability from nNOS in cardiac autonomic ganglia in response to training is dependent on both alleles of the gene. Although basal expression of nNOS is normal, polymorphisms of nNOS may interfere with neural regulation of heart rate following training. Targeted gene transfer of nNOS can restore this impairment.
Exercise has been implicated as an important factor in the up-regulation of both endothelial nitric oxide (NO) synthase (eNOS) (Sessa et al. 1994) and neuronal NOS (nNOS) (Mohan et al. 2000). The molecular mechanisms responsible for this in cardiac tissues are not established but may involve an interplay between haemodynamic elements (Ziegler et al. 1998) and oxidative stress (Drummond et al. 2000). The physiological significance of enhanced NO bioavailability may also be complex as NOS-containing cellular microdomains may be involved in site-specific and differential actions depending on the isoform relative to its target (Barouch et al. 2002; Paton et al. 2002). In general, NO derived from eNOS is important in establishing endothelium-dependent vasorelaxation (Huang et al. 1995) and myocardial perfusion (Gielen & Hambrecht, 2001), whereas NO derived from increased nNOS expression in intracardiac cholinergic ganglia enhances the release of acetylcholine (Herring & Paterson, 2001) and cardiac vagal responses to direct nerve stimulation following training (Danson & Paterson, 2003). Although the role of peripheral nNOS in cardiac responses to centrally mediated vagal activation remains to be demonstrated, this effect could be important in facilitating parasympathetic regulation of heart rate, which dramatically reduces mortality in cardiac disease (Cole et al. 1999; Li et al. 2004).
Polymorphisms of the nNOS gene are associated with a number of neurological diseases in humans (Levecque et al. 2003; Yu et al. 2003). However, the way in which these polymorphisms interfere with the regulation of gene expression during exercise, and whether they affect the cardiac vagal response to training are not known. Endothelial NOS (eNOS) gene polymorphisms accelerate coronary artery disease (Rossi et al. 2003), and an eNOS knockout mutation carried on one allele abrogates the eNOS training response in vascular endothelium (Kojda et al. 2001). Our aims were therefore: (1) to test whether the enhanced peripheral and central vagal control of heart rate were affected by a single nNOS allele knockout mutation; and (2) whether nNOS gene transfer could substitute for the enhanced intracardiac ganglionic nNOS expression seen following training where exercise alone was an ineffective promoter of increased vagal function.
Methods
Animals
Mice homozygous for targeted disruption of the nNOS gene (B6,129-nNOStm1plh, nNOS−/−) were purchased from Jackson Laboratories (Bar Harbour, ME, USA) and a colony was established by backcrossing the nNOS−/− on a C57BL/6 background. N4 littermate mice heterozygous for the nNOS gene (nNOS+/−, n = 98) were used as test animals whilst homozygous wild-type (WT, n = 76) were used as controls. The treatment of all animals was in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 and National Institutes of Health Guide for the Care and Use of Laboratory Animals and experiments were approved by the Physiology and Psychology local Ethics Review Committee, University of Oxford.
Procedures
Training protocol
All mice were housed singly in cages (+EX, n = 90; of which 52 were nNOS+/− and 38 were WT) and an equal number of each genotype were housed in cages without wheels (−EX, n = 84; of which 46 were nNOS+/− and 38 were WT). Voluntary running distances covered by +EX mice over a 24 h period for a training period of 5 weeks were measured as previously described (Danson & Paterson, 2003). Ventricular weights were measured post mortem and ventricle: body weight ratios were calculated as an index of training.
Cardiac gene transfer
Whilst experiments were commenced on some mice without gene transfer (WT, −EX, n = 6; WT, +EX, n = 6; nNOS+/−, −EX, n = 10; nNOS+/−, +EX, n = 10), other mice received percutaneous injections of adenovirus (5 × 109 to 5 × 1010 virus particles of Ad.eGFP (WT, −EX, n = 16; WT, +EX, n = 16; nNOS+/−, −EX, n = 18; nNOS+/−, +EX, n = 21) or Ad.nNOS (n = 71, same distribution of groups) in 100 µl phosphate-buffered saline (PBS)) directed to the right atrial wall, 5 days before the 5 week training period had elapsed, under halothane anaesthesia.
Replication-deficient adenoviral vectors encoding recombinant enhanced green fluorescent protein (Ad.eGFP) or neuronal NOS (Ad.nNOS) were generated and purified as previously described (Channon et al. 1996). This technique is described fully in Mohan et al. (2002).
Experimental protocols
In vitro measurement of direct vagal bradycardia
In order to test the effect of training on vagal bradycardia in WT and nNOS+/− mice, preparations of both right and left atria with intact right vagal innervation were isolated in vitro from mice from trained and untrained mice of both genotypes without gene transfer (WT, −EX, n = 6; WT, +EX, n = 6; nNOS+/−, −EX, n = 10; nNOS+/−, +EX, n = 10). Heart rate (HR; computed as spontaneous beating rate in beats per minute or pulse interval duration in milliseconds) responses to vagal nerve stimulation (VNS, 3 and 5 Hz; 10 V, 1 ms pulse interval) or bath-applied carbachol (CCh; Sigma, UK) in vitro (10−7 and 3 × 10−7 mol l−1) were measured. After control responses were repeated three times and a mean taken, atria from all groups were treated with the NOS inhibitor, vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine (l-VNIO (Amersham Biosciences, Little Chalfont, UK), 10−4 mol l−1, equilibration period 20 min) and the protocol was repeated.
In vivo measurement of phenylephrine-induced bradycardia
To test the effect of training on the vagal regulation of HR in vivo, the HR response to an i.v. bolus of phenylephrine was assessed in a group of trained and a group of untrained mice of each genotype following gene transfer of eGFP (WT, −EX, n = 12; WT, +EX, n = 6; nNOS+/−, −EX, n = 10; nNOS+/−, +EX, n = 10). In addition, to test the effect of cardiac gene transfer of nNOS on the vagal regulation of HR in vivo, Ad. eGFP treated tissues were compared with trained mice of each genotype following gene transfer of nNOS (WT, +EX, n = 6; nNOS+/−, +EX, n = 10). Spontaneously breathing mice were anaesthetized with continuous isoflurane (3%) via a nose cone and the abdominal aorta and vena cava were cannulated distal to the renal vessels after pedal reflexes were lost from the animals. Isoflurane was chosen as anaesthetic as it preserves cardiovascular reflexes relatively well. Temperature was measured by means of a rectal thermometer and maintained at 37 ± 0.5°C using an infrared lamp. HR was triggered from blood pressure pulses in the aorta. A bolus of propranolol (1 mg kg−1 i.v.) was given to block the sympathetic component of the baroreflex response, since different mechanisms may be involved in the regulation of sympathetic and parasympathetic limbs of the baroreflex. After a steady-state response to propranolol had been reached, a single bolus of phenylephrine (3 mg kg−1 i.v.) was then administered and the peak change in systolic blood pressure, HR and interbeat interval were recorded in order to test the vagally mediated cardioinhibitory limb of the baroreflex response. This was repeated a total of three times after mean arterial blood pressure (MAP) had returned to baseline level. After this, mice were humanely killed by cervical dislocation and atria were removed for in vitro experiments. The in vivo baroreceptor-mediated effects were expressed as bradycardia (ratio of change in HR to change in MAP; beats min−1 mmHg−1) or pulse interval response (ratio of change in interbeat (peak-to-peak) interval to change in MAP; ms mmHg−1).
In order to test the effects of gene transfer on vagal bradycardia in vitro, atria from completed in vivo experiments and some additional atria from humanely killed mice treated with Ad.eGFP (WT, −EX, n = 4; WT, +EX, n = 10; nNOS+/−, −EX, n = 8; nNOS+/−, +EX, n = 8) or Ad.nNOS (WT, −EX, n = 16; WT, +EX, n = 10; nNOS+/−, −EX, n = 18; nNOS+/−, +EX, n = 8) were isolated in vitro and HR responses to vagal/cholinergic activation were measured as has been described.
Immunohistochemical localization of nNOS in cholinergic ganglia
The remaining mice (nNOS+/−, +EX, n = 6) were treated with Ad.eGFP (n = 3) or Ad.nNOS (n = 3) as has been described, and were terminally anaesthetized (pentobarbitone i.p.) and then fixed by perfusion of the left ventricle with 4% paraformaldehyde–0.1% glutaraldehyde in 0.1 mol l−1 PBS (pH 7.1, 20 min). The atria were dissected free and treated for immunohistochemistry as previously described (Wang & Morris, 1996). After processing, the tissue was incubated in primary antisera against nNOS and choline acetyltransferase (ChAT) for 12 h. Tissue was then washed in 1% chicken egg albumin PBS solution and incubated in fluorescently labelled secondary antisera (rhodamine-conjugated anti-goat (1 : 200) and fluorescein-conjugated anti-sheep (1 : 200) antisera. Immunoreactivity was viewed using confocal microscopy (Leica).
Determination of nNOS mRNA levels
Atrial samples were taken after finishing in vitro experiments from tissue used for in vitro study only and expression of nNOS mRNA was measured using relative quantitative RT-PCR (RQ-RT-PCR) with QuantumRNA 18S rRNA internal standards from Ambion (WT, −EX, n = 5; WT, +EX, n = 5; nNOS+/−, −EX, n = 6; nNOS+/−, +EX, n = 6). Total RNA was purified from atria using TRI Reagent (Sigma), quantified spectrophotometrically and 200 µg reverse transcribed into cDNA using random DNA decamers and the Reverse-IT First Strand Synthesis kit (ABgene). These were then diluted 1/5 v/v with ultrapure water. RT-PCR was performed using 2 µl of each diluted cDNA with SuperTaq reagents (Enzyme Technologies) and the following primers: nNOS sense primer: 5′-AGCACCTTTGGCAATGGAGA-3′, nNOS antisense primer: 5′-ATCACAGGCTGCCTTGAAGA-3′ and universal 18S primers and competimers (Ambion). The expected amplicon sizes were 402 bp and 315 bp, respectively. Initial experiments were performed to ascertain the linear range of each PCR reaction and the optimal 18S primer: competimer ratio to bring the 18S amplicon to a similar level as the nNOS amplicon. This was to ensure that there was no unequal competition for reagents in the multiplex PCR due to the more abundant 18S rRNA target. Reaction products were separated by agarose gel electrophoresis. The optical density of each PCR fragment was established (Syngene Chemigenius Gel Documentation System) and the signal obtained for the nNOS amplicon divided by that for the 18S amplicon to give a corrected relative value for the nNOS product in each sample. Values were then compared between samples for an estimate of the relative changes in gene expression.
Determination of nNOS and eNOS protein levels
Atrial samples from WT, −EX (n = 5), WT, +EX (n = 5), nNOS+/−, −EX (n = 6) and nNOS+/−, +EX (n = 6), and the same number of samples of the thoracic aorta were also taken at random after completion of the in vivo/ in vitro experiments for measurement of the expression of nNOS and eNOS protein, respectively. Tissue was immersed in iced CelLytic MT lysis buffer (Sigma) containing a 1/40 v/v dilution of a proprietary mixture of mammalian protease inhibitors: 4-(2-aminoethyl)benzenesulphonyl fluoride, bestatin, pepstatinA, E-64, leupeptin and aprotinin (P8340, Sigma). Tissues were homogenized for 30 s in a Polytron homogenizer and homogenates centrifuged for 10 min at 10 000 g. Total protein levels were determined by the Bradford method (Bradford, 1976) and 200 µg protein loaded per well. Western blot analysis was performed as previously described (Danson & Paterson, 2003) using commercially available polyclonal antibodies to nNOS, eNOS and β-actin (BD Biosciences) and the Western Lightening detection system (Perkin Elmer Life Sciences). Protein levels were expressed as a ratio of the optical densities of the nNOS/eNOS bands and the β-actin band to control for any inaccuracies in the protein loading.
Results
Effect of nNOS allele deletion on voluntary exercise
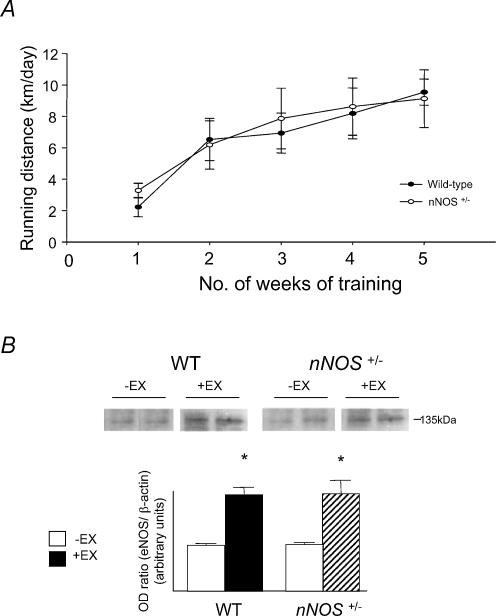
Average daily running distances of WT and nNOS+/− mice are shown in Fig. 1A. Both groups of animals demonstrated a similar running performance. Trained animals showed a significantly lower final body weight (BW) and higher ventricle/body weight ratio (Ve/BW) (WT, +EX: BW 35.4 ± 0.4 g, Ve/BW 5.5 ± 0.2 mg g−1; WT, −EX: BW 37.8 ± 0.6 g, Ve/BW 4.9 ± 0.2 mg g−1, P < 0.01, unpaired t test; nNOS+/−, +EX: BW 35.2 ± 0.5 g, Ve/BW 5.8 ± 0.3 mg g−1; nNOS+/−, −EX: BW 37.5 ± 0.9, Ve/BW 4.9 ± 0.2 mg g−1, P < 0.01, unpaired t test). There were no differences in the effects of exercise on BW or Ve/BW between genotypes.
Figure 1. Running distances and aortic eNOS protein levels in mice.
A, daily running distances for wild-type (WT) and nNOS+/− mice. B, Western Blot analysis showed elevated aortic eNOS protein levels in vessels from WT, +EX compared to WT, −EX and in vessels from nNOS+/−, +EX compared to nNOS+/−, −EX (*P < 0.01, unpaired t test).
Exercise training also increased the expression of eNOS protein in the aortas of both WT and nNOS+/− mice suggesting that the nNOS allele mutation had no effect on the exercise-related induction of eNOS gene expression (see Fig. 1B).
Effect of exercise on atrial nNOS mRNA and protein levels
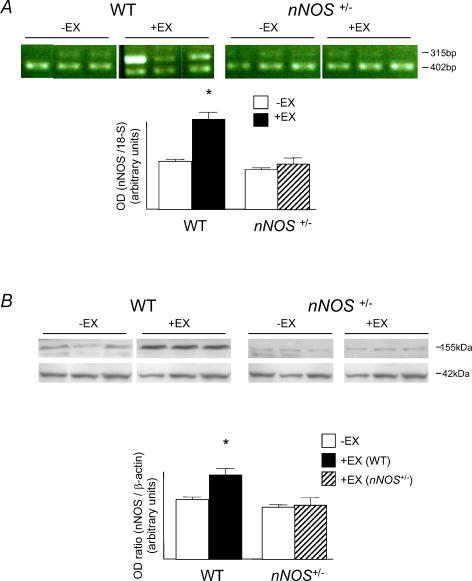
RT-PCR showed that although nNOS mRNA levels were unchanged in WT, −EX compared to nNOS+/−, −EX groups, training significantly enhanced levels in the WT group (P < 0.05, unpaired t test) but failed to have any effect in the nNOS+/− group (see Fig. 2). Western blot appeared to reveal bands at both 155 kDa and 120 kDa in some tissues although we decided not to include the 120 kDa bands in the analysis of nNOS expression since these may represent cross-reactivity of the nNOS antibody with eNOS protein, and were only picked up as faint bands in some samples. Importantly, the 155 kDa bands revealed the same effect of exercise on nNOS protein as was demonstrated by RQ-RT-PCR (P < 0.05, unpaired t test).
Figure 2. nNOS mRNA and protein levels in atria.
A, PCR-amplified nNOS mRNA expression revealed that nNOS mRNA was increased in WT, +EX atria with respect to WT, −EX (*P < 0.05, unpaired t test), but this effect was not evident in nNOS+/− atria. B, Western Blot analysis showed elevated nNOS protein levels in WT, +EX atria compared to WT, −EX (*P < 0.05, unpaired t test), but no effect in nNOS+/− atria.
Effect of exercise on phenylephrine-induced bradycardia and vagal bradycardia
In WT mice, phenylephrine-induced bradycardia (in beats min−1) and pulse interval response (in ms) were significantly augmented by exercise training (see Table 1; from 2.1 ± 0.1 to 3.2 ± 0.3 ms mmHg−1, P < 0.05, unpaired t test). However, this effect was absent in the nNOS+/− mice (from 2.2 ± 0.1 to 2.7 ± 0.2 ms mmHg−1, despite there being no difference between animals without training.
Table 1.
Heart rate (HR) and mean arterial blood pressure (MAP) changes in response to phenylephrine in anaesthetized mice
| Wild-type | nNOS+/− | |||||
|---|---|---|---|---|---|---|
| −EX | +EX | −EX | +EX | |||
| Ad.eGFP | Ad.eGFP | Ad.nNOS | Ad.eGFP | Ad.eGFP | Ad.nNOS | |
| Resting HR (beats min−1) | 686 ± 12 | 641 ± 17* | 650 ± 16 | 712 ± 11 | 707 ± 12 | 650 ± 16** |
| ΔHR (PRL) (beats min−1) | 46 ± 3 | 33 ± 5 | 35 ± 4 | 51 ± 3 | 35 ± 6* | 40 ± 2 |
| ΔMAP (Phe) (mmHg) | 61 ± 3 | 53 ± 2 | 40 ± 1 | 58 ± 3 | 47 ± 4 | 53 ± 3 |
| ΔHR (Phe) (beats min−1) | 167 ± 7 | 263 ± 15 | 268 ± 14 | 174 ± 18 | 176 ± 17 | 260 ± 9 |
| ΔHR/ΔMAP (beats min−1 mmHg−1) | 2.7 ± 0.1 | 4.5 ± 0.3* | 6.7 ± 0.5** | 3.0 ± 0.3 | 3.8 ± 0.3 | 5.0 ± 0.4** |
| ΔRR interval/ΔMAP (ms mmHg−1) | 2.1 ± 0.1 | 3.2 ± 0.3* | 4.5 ± 0.5** | 2.2 ± 0.1 | 2.7 ± 0.2 | 3.3 ± 0.3** |
Cardiac parameters measured via aortic catheter in isoflurane-anaesthetized mice. Phenylephrine (Phe)-induced bradycardia (HR change (beats min−1) or interbeat interval (RR) change (ms)/aortic blood pressure change (mmHg)) was significantly elevated in response to exercise training in wild-type (WT) mice, whereas resting heart rate before β-adrenoceptor blockade (with propranolol PRL) was significantly reduced
P < 0.05, unpaired t test). These effects were absent in mice lacking one allele of neuronal nitric oxide synthase (nNOS+/−). However, gene transfer of nNOS to the atria significantly enhanced phenylephrine-induced bradycardia in WT and nNOS+/− mice
P < 0.01, unpaired t test).
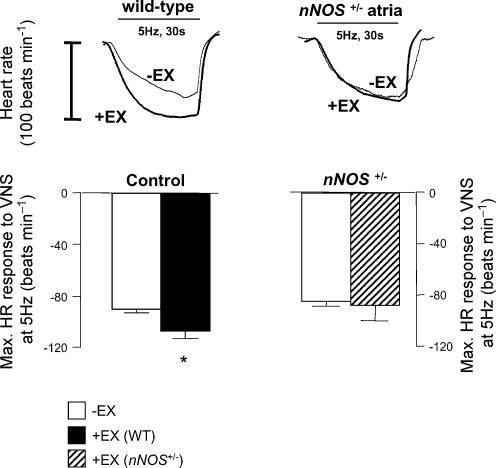
Similarly, HR responses to VNS were significantly enhanced in +EX atria from WT mice in vitro compared to −EX at both 3 Hz (see Table 2, P < 0.01, unpaired t test) and 5 Hz stimulation frequencies (see Fig. 3, P < 0.01, unpaired t test). However, HR responses were not different in nNOS+/−, +EX atria compared to nNOS+/−, −EX at either 3 Hz (see Table 2, P < 0.01, unpaired t test) or 5 Hz (see Fig. 3), despite there being no impairment in nNOS+/−, −EX responses compared to WT, −EX responses. Statistical differences between pulse rate responses to VNS were also apparent between the same comparisons made when data were expressed as pulse interval change.
Table 2.
Effects of nNOS inhibition on HR responses (A, beats min−1) or interbeat interval changes (B, ms) to vagal nerve stimulation in isolated atria in vitro
| A. | Wild-type | nNOS+/− | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −EX | +EX | −EX | +EX | |||||||||
| Con | Ad.eGFP | Ad.nNOS | Con | Ad.eGFP | Ad.nNOS | Con | Ad.eGFP | Ad.nNOS | Con | Ad.eGFP | Ad.nNOS | |
| Control | ||||||||||||
| 3 Hz | 50 | 44 | 123 | 99 | 76 | 126 | 58 | 60 | 139 | 48 | 55 | 137 |
| ± 5 | ± 4 | ± 12* | ± 11** | ± 3** | ± 8* | ± 8 | ± 10 | ± 36* | ± 8 | ± 3 | ± 10* | |
| 5 Hz | 89 | 82 | 159 | 112 | 125 | 192 | 86 | 92 | 153 | 85 | 92 | 202 |
| ± 6 | ± 5 | ± 20* | ± 11** | ± 8** | ± 13* | ± 7 | ± 10 | ± 35* | ± 16 | ± 3 | ± 12* | |
| l-VNIO | ||||||||||||
| 3 Hz | 44 | 31 | 59 | 53 | 51 | 59 | 51 | 40 | 40 | 39 | 50 | 57 |
| ± 5** | ± 8** | ± 9** | ± 17** | ± 4** | ± 8** | ± 7** | ± 5** | ± 10** | ± 7** | ± 6** | ± 5** | |
| 5 Hz | 66 | 53 | 78 | 67 | 81 | 86 | 78 | 84 | 75 | 70 | 76 | 91 |
| ± 12** | ± 17** | ± 17** | ± 10** | ± 4** | ± 7** | ± 4** | ± 11** | ± 23** | ± 13** | ± 5** | ± 7** | |
| l-Arg | ||||||||||||
| 3 Hz | 48 | 62 | 107 | 96 | 82 | 98 | 55 | 59 | 108 | 53 | 58 | 96 |
| ± 3 | ± 11 | ± 5* | ± 13** | ± 11** | ± 6* | ± 4 | ± 6 | ± 24* | ± 10** | ± 5 | ± 17* | |
| 5 Hz | 83 | 103 | 140 | 103 | 107 | 132 | 83 | 98 | 116 | 80 | 89 | 147 |
| ± 5 | ± 10 | ± 40* | ± 8** | ± 11** | ± 11* | ± 7 | ± 8 | ± 20* | ± 12** | ± 4 | ± 22* | |
| Baseline HR (beats min−1) | ||||||||||||
| 376 | 371 | 378 | 350 | 349 | 367 | 383 | 398 | 388 | 354 | 379 | 347 | |
| ± 25 | ± 18 | ± 29 | ± 19 | ± 33 | ± 30 | ± 20 | ± 29 | ± 25 | ± 23 | ± 34 | ± 32 | |
| B. | Wild-type | nNOS+/− | ||||||||||
| −EX | +EX | −EX | +EX | |||||||||
| Con | Ad.eGFP | Ad.nNOS | Con | Ad.eGFP | Ad.nNOS | Con | Ad.eGFP | Ad.nNOS | Con | Ad.eGFP | Ad.nNOS | |
| Control | ||||||||||||
| 3 Hz | 34 | 31 | 87 | 69 | 49 | 99 | 28 | 29 | 89 | 27 | 32 | 108 |
| ± 2 | ± 1 | ± 5* | ± 7** | ± 3** | ± 5* | ± 3 | ± 4 | ± 16* | ± 4 | ± 2 | ± 5* | |
| 5 Hz | 59 | 54 | 127 | 82 | 97 | 210 | 45 | 49 | 104 | 54 | 60 | 226 |
| ± 2 | ± 2 | ± 9* | ± 7** | ± 5** | ± 8* | ± 3 | ± 4 | ± 15* | ± 9 | ± 2 | ± 6* | |
| l-VNIO | ||||||||||||
| 3 Hz | 31 | 24 | 39 | 32 | 31 | 36 | 24 | 18 | 18 | 21 | 28 | 33 |
| ± 12 | ± 13 | ± 13† | ± 10† | ± 3† | ± 5† | ± 3 | ± 2† | ± 4† | ± 4† | ± 3† | ± 3† | |
| 5 Hz | 44 | 36 | 51 | 42 | 53 | 57 | 40 | 44 | 38 | 42 | 47 | 59 |
| ± 15 | ± 17 | ± 17† | ± 6† | ± 3† | ± 5† | ± 1† | ± 4 | ± 10† | ± 7† | ± 3† | ± 4† | |
| l-Arg | ||||||||||||
| 3 Hz | 32 | 41 | 73 | 66 | 54 | 68 | 26 | 28 | 61 | 30 | 34 | 64 |
| ± 1 | ± 4 | ± 2* | ± 8** | ± 7** | ± 4* | ± 1 | ± 2 | ± 10* | ± 5† | ± 3 | ± 9* | |
| 5 Hz | 55 | 70 | 104 | 73 | 77 | 105 | 43 | 54 | 68 | 50 | 57 | 121 |
| ± 2 | ± 4 | ± 2* | ± 5** | ± 7** | ± 7* | ± 3 | ± 3 | ± 8* | ± 6† | ± 2 | ± 12* | |
In vitro atrial beating rate responses to vagal nerve stimulation (VNS) at 3 and 5 Hz stimulation frequencies. Part A shows rate responses calculated as beats per minute change and part B shows responses expressed as change in contraction interval (ms). Control responses were significantly increased in trained (+EX) compared to untrained (−EX) in wild-type tissue
P < 0.05, unpaired t test), although this effect was not present in atria from mice lacking one allele of neuronal nitric oxide synthase (nNOS+/−). Gene transfer using adenoviruses containing nNOS (Ad.nNOS) increased responses to VNS in all atria compared to gene transfer using adenoviruses containing enhanced green fluorescent protein (Ad.eGFP) and control (Con, no gene transfer) atria
P < 0.01, unpaired t test). Selective nNOS inhibition with vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine (l-VNIO, 100 µmol l−1) attenuated the response to nerve stimulation in all atria, and abolished the effects of training and Ad.nNOS treatment. This effect was reversed in all cases with l-arginine l-Arg, 1 mmol l−1,
P < 0.05, repeated measures ANOVA.
Figure 3. Heart rate responses to vagal stimulation in vitro.
The decrease in heart rate associated with vagal nerve stimulation (5 Hz) in isolated atria in vitro was enhanced in trained (+EX) wild-type atria with respect to untrained (−EX; *P < 0.05, unpaired t test). This effect was not evident in nNOS+/− atria.
Responses to CCh were not affected by exercise training or allele ablation (data not shown), although this is not surprising as we have previously shown that nNOS gene knockout (Choate et al. 2001), nNOS gene transfer (Mohan et al. 2002) or exercise training (Danson & Paterson, 2003) have no independent effect on the IC50 dose of CCh.
The intrinsic rates of atria in vitro did not reveal any statistical differences between groups, although there was a trend for training to reduce intrinsic sinus rate in both WT (from 376 ± 25 to 350 ± 19 beats min−1) and nNOS+/− atria (from 383 ± 20 to 354 ± 23 beats min−1) (see Table 2).
Effect of nNOS inhibition on vagal HR responses in vitro
Selective nNOS inhibition attenuated HR responses to VNS in WT, +EX and nNOS+/−, +EX atria at 3 Hz (see Table 2), an effect reversed by excess l-arginine (P < 0.05, ANOVA with repeated measures). Furthermore, l-VNIO normalized the enhanced vagal responsiveness in WT, +EX atria with respect to nNOS+/−, +EX atria, suggesting that the enhanced vagal responsiveness in WT versus nNOS+/− atria is nNOS dependent.
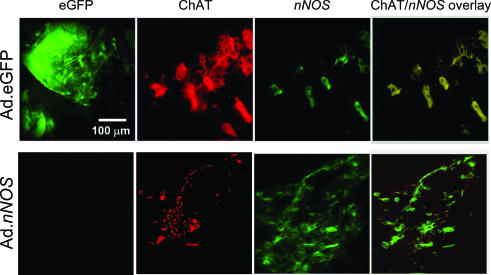
Immunolocalization of eGFP and nNOS following adenoviral gene transfer nNOS staining in cholinergic ganglia was increased in Ad.nNOS-treated atria compared with Ad.eGFP-treated atria. In addition, eGFP fluorescence was only evident in atria from Ad.eGFP-treated mice (see Fig. 4). nNOS localization in cholinergic ganglia, shown by co-localization of nNOS and ChAT, was also increased in atria from Ad.nNOS-treated mice.
Figure 4. Immunohistochemical localization of nNOS in cholinergic ganglia.
Confocal micrograph illustrates enhanced green fluorescence (far left, green panels) immunoreactivity against choline acetyltransferase (ChAT, middle, red panels) and nNOS (far right, green panels) in atria from nNOS+/−, +EX mice pretreated with adenoviruses containing either eGFP (top row) or nNOS genes (bottom row). The panels show the presence of enhanced green fluorescence located with ChAT-positive cholinergic ganglia in an Ad.eGFP specimen with some neurones coexpressing nNOS. In the Ad.nNOS specimen, enhanced green fluorescence is not present, and coexpression of nNOS in cholinergic neurones is increased with respect to the Ad.eGFP group.
Effect of gene transfer on total atrial nNOS protein levels
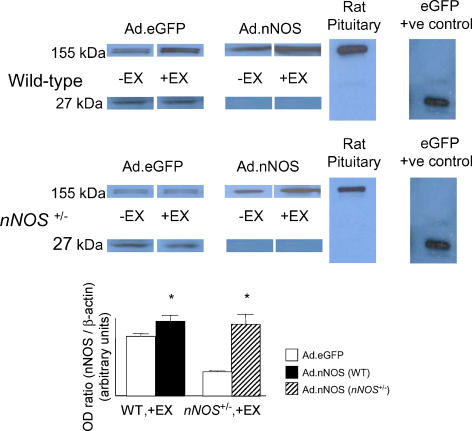
Western blot analysis showed that our percutaneous adenoviral gene transfer method was effective at increasing total eGFP or nNOS protein expression in atria (see Fig. 5, P < 0.001).
Figure 5. nNOS and eGFP protein levels in adenovirus-transfected atria.
Western Blot analysis showed expression of nNOS (155 kDa) and eGFP (27 kDa) in wild-type, −EX and +EX; and nNOS+/−, −EX and +EX atria treated with Ad.nNOS or Ad.eGFP adenovirus. Ad.eGFP treatment was successful in causing atria to express eGFP protein. Ad.nNOS significantly increased atrial expression of nNOS protein in all groups (*P < 0.001, unpaired t test).
Effect of gene transfer on phenylephrine-induced bradycardia and vagal bradycardia
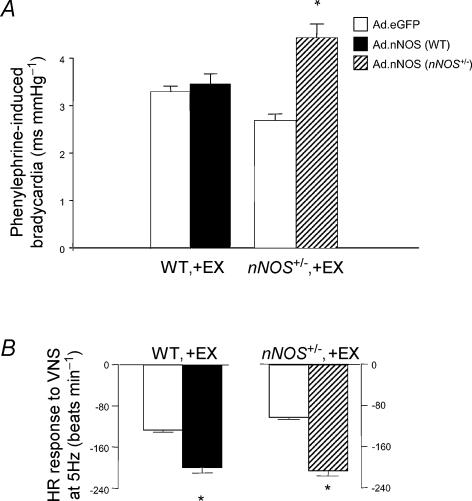
Ad.nNOS treatment increased phenylephrine-induced bradycardia in WT and nNOS+/− mice compared to Ad.eGFP. This effect was statistically significant in both nNOS+/−, −EX and +EX mice, but only WT, −EX mice (see Fig. 6A). The absence of any effect of nNOS gene transfer in WT, +EX groups may be related to the fact this group demonstrated enhanced parasympathetic activity and nNOS expression compared with all other groups in response to exercise training.
Figure 6. Effects of nNOS gene transfer on in vivo phenylephrine-induced bradycardia and in vitro vagal-induced bradycardia.
A, phenylephrine-induced bradycardia, expressed as change in interpulse interval (ms) per change in mean arterial blood pressure (mmHg), in response to pressor challenge was increased in anaesthetized nNOS+/−, +EX mice treated with Ad.nNOS compared to treatment with Ad.eGFP adenovirus (*P < 0.05, unpaired t test). Ad.nNOS treatment failed to have a significant effect on phenylephrine-induced bradycardia in WT, +EX mice, although Ad.nNOS increased bradycardia in WT, −EX mice by 64% compared to Ad.eGFP (P < 0.05, unpaired t test; data not shown). B, bradycardia to vagal nerve stimulation (5 Hz) in isolated atria in vitro was enhanced in nNOS+/−, +EX mice treated with Ad.nNOS compared to treatment with Ad.eGFP adenovirus (*P < 0.01, unpaired t test).
The effect of nNOS gene transfer on vagal function in vitro mirrored that seen in vivo (see Fig. 6B). Vagal bradycardia was enhanced in all Ad.nNOS groups compared to Ad.eGFP except in the nNOS+/−, −EX group which displayed high variance in responsiveness to vagal stimulation following nNOS gene transfer, most likely reflecting variability in the transfection efficiency in this group. In addition, the effects of nNOS gene transfer could be blocked by selective nNOS inhibition in vitro (P < 0.05, ANOVA with repeated measures, see Table 2).
Discussion
Regular aerobic exercise training induces numerous beneficial structural and regulatory adaptations in the cardiovascular system. Neurally mediated adjustments in the heart underlie an important part of this milieu of changes and lead to both sympathoinhibition and enhanced vagal activity (Buch et al. 2002). Although sympathoinhibition can largely be attributed to a reduction in circulating catecholamines, desensitization and down-regulation of cardiac β-adrenergic receptors and increased central blood volume load on central cardiopulmonary baroreceptors (Shi et al. 1996), the mechanisms leading to enhanced vagal responsiveness are less well defined. Wheel-running activity in mice can significantly enhance the action of the parasympathetic nervous system on the heart (i.e. the decrease in beats per minute or increase in interbeat interval in milliseconds) by direct electrical stimulation of the vagus nerve in vitro, an effect that is blocked by NOS inhibition (Danson & Paterson, 2003). Given that this response is seen through vagal activation at the level of the vagal trunk suggests that an important response to training is peripheral neuronal modulation of cardiac excitability by NO. Here we provide direct evidence that this effect can be seen through central activation of the vagus, and that enhanced vagal responsiveness in vivo and in vitro brought about by physical training requires the presence of two intact alleles of nNOS. Furthermore, dysfunctional regulation of the nNOS gene in heterozygous mice can be overcome by nNOS gene transfer to intracardiac ganglia which restores vagal responsiveness and the autonomic phenotype, seen after exercise training.
Although the main limitation of our adenoviral atrial gene transfer technique over exercise training continues to be the lack of specificity in cell transfection and marginally more variable protein expression, our data are consistent with the responses seen following exercise training. In addition we show that nNOS gene transfer increases baroreflex-mediated parasympathetic bradycardia or pulse interval response in nNOS+/− mice. This appears to be mediated predominantly by increased NO bioavailability at intracardiac cholinergic ganglia, since bradycardia in response to VNS in vitro is enhanced in an NO-dependent manner. Furthermore, the margin of increase in vagal responsiveness resulting from nNOS gene transfer is greater in our in vitro preparations than in vivo. This is particularly striking in certain groups, e.g. in the WT, +EX group (see Fig. 6), suggesting that the central nervous system may, under certain circumstances, actually limit peripheral vagal facilitation by NO. This idea would be consistent with data showing NO-dependent inhibition of baroreflex vagal outflow in the nucleus tractus solitarii (Paton et al. 2001). Another possibility is that NO acts in a biphasic concentration-dependent manner in modulating cardiac parasympathetic signalling, and high levels of neuronal NO (from augmented nNOS activity resulting from the combined effects of training and gene transfer in WT mice) may begin to inhibit vagal control of heart rate.
It has been suggested that both eNOS (within endothelial cells and cardiomyocytes) and nNOS protein (from intracardiac neurones) may play important roles in establishing the peripheral neuronal response to exercise as the expression of both isoforms increases following training (Sessa et al. 1994; Mohan et al. 2000). However, the putatively selective nNOS inhibitor l-VNIO can normalize the enhanced vagal response in vitro suggesting that NO bioavailability at the level of ganglia is important in bringing about the vagal response to training (Danson & Paterson, 2003). In addition, although eNOS gene knockout results in mild bradycardia in vivo (Kojda et al. 1999), these effects are thought to result entirely from hypertension and baroreflex cardioinhibition since this effect has not been consistently demonstrated in normotensive animals or in isolated hearts. Furthermore, non-selective NOS inhibition has no effect on the heart rate response to carbachol (Sears et al. 1998), suggesting that there is little or no action of NO on heart rate at sinoatrial node myocytes. When eNOS is overexpressed in cardiomyocytes (40–90 times normal rate) – albeit without any direct evidence of changes in expression in sinoatrial node myocytes – there is also no effect on heart rate (Brunner et al. 2001). Conversely, increases in nNOS mRNA or protein in WT relative to nNOS+/− mice were associated with significant increases in vagal bradycardia in vivo and in vitro. Although we cannot conclusively rule out that nNOS expression may have been influenced over the course of the in vitro protocol, relative changes in protein and mRNA levels from this preparation were similar to those reported from freshly isolated tissue. We believe that the major source of this nNOS is from cholinergic ganglia, and evidence suggests that NO derived from nNOS can modulate neurotransmission at the pre-postganglionic synapse (Markos et al. 2002), and presynaptically at the postganglionic neuroeffector junction (Mohan et al. 2004). In contrast, there is no compelling evidence that nNOS is expressed at significant levels in sinoatrial node myocytes; however, it is now widely recognized that nNOS derived from ventricular myocytes may be involved the regulation of contractility (Ashley et al. 2002), highlighting the site-specific action of this isoform.
Although evidence for the shared functionality of eNOS and nNOS in the regulation of heart rate may be weak, there may be important similarities in the factors responsible for triggering an increase in gene expression during exercise. Despite extensive knowledge about the promoters of eNOS expression during exercise, those for the nNOS gene are not as well documented. Unlike eNOS which responds to vascular shear during exercise (Ziegler et al. 1998), direct evidence for mechanical stimuli in the regulation of nNOS during exercise has not been established. The global perfusion with chemical stimuli such as H2O2 regulates eNOS in cultured endothelial cells (Drummond et al. 2000). Likewise, indirect evidence suggests that nNOS responds to the increased oxidative stress experienced during myocardial infarction (Takimoto et al. 2002) and in hypertension (Piech et al. 2003). This implicates a role for free radical production in the regulation of nNOS which takes place during exercise (Ji et al. 1998). In addition, there is evidence that the reduction in plasma angiotensin II associated with exercise training (Kiyonaga et al. 1985) may be coupled to the NO-dependent actions on the autonomic nervous system (Zucker et al. 2001). Increases in angiotensin II result in both hypertension and interruption of the trafficking of eNOS in vascular myocytes (Gerzanich et al. 2003) indicating a strong regulatory link between these two signalling pathways.
The resemblance in the dysfunctional regulation of eNOS and nNOS in the absence of one allele also suggests that there may be involvement of similar allele silencing and recruitment mechanisms during rest and exercise. The heterozygous eNOS knockout mouse shows unperturbed regulation of blood pressure (Kurihara et al. 1998) and vascular reactivity compared to its wild-type control and exercise capacities also appear to be identical during the first 3 weeks of training (Kojda et al. 2001). However, the physiological responses to exercise training brought about by augmented NO production appear ablated in both eNOS+/− and nNOS+/− mice. In a similar study, Kojda et al. (2001) elegantly described the ablated response to exercise in aorta and coronary vessels from the eNOS+/− mouse compared to its wild-type control, despite there being no difference in either vascular reactivity to ACh or basal eNOS expression before exercise. Although underscoring the importance of cardiac NO in preserving homeostasis during stress, this suggests that the functional role played by one NOS allele whilst the animal remains relatively unstressed may be minimal or redundant.
Studies using the homozygous NOS gene knockout mouse have proved useful in delineating the roles of separate isoforms in the heart, particularly with reference to the microdomain-specific mechanism of actions of NOS in cardiomyocytes (Barouch et al. 2002). However, the effects of NOS knockout on cardiac homeostasis associated with age or stress in these animals may be ultimately more interesting. The absence of dramatic differences observed between NOS homozygous – and even more commonly heterozygous – gene knockouts and wild-type mice may also be because the expression of NOS is low in certain tissues before stress (Kurihara et al. 1998), or that the mutant models commonly develop compensatory mechanisms which mask the effects of gene ablation (Huang et al. 2001).
In this respect, NOS gene polymorphisms may have very little effect in the young, unstressed heart, but cardiac autonomic signalling may be regulated very differently by physiological stresses such as exercise or by ageing. At present, eNOS polymorphisms identified in humans have been implicated in the impairment of endothelium-dependent vasodilatation in coronary artery disease (CAD) patients and may have an adverse effect on training-induced improvement in endothelial function (Erbs et al. 2003). Despite extensive investigation into eNOS polymorphisms with often striking results, research into polymorphisms of nNOS and the consequences of being a carrier of these has received relatively little attention. Recent investigations have found associations between hypertropic pyloric stenosis (Saur et al. 2004), depression (Yu et al. 2003), Parkinson's disease (Levecque et al. 2003) and acute chest syndrome in sickle cell patients (Sullivan et al. 2001). Since we know that nNOS plays a central role in cardiac parasympathetic neural modulation, it is conceivable that polymorphisms of the nNOS gene are associated with reduced parasympathetic activity. Impaired vagal function predisposes mice to arrhythmia (Cogliati et al. 2002) and is strongly correlated with mortality in humans (Cole et al. 1999). It remains to be established whether nNOS gene mutations and polymorphisms interfere with nNOS regulation during exercise, since this could affect cardiac rehabilitation programmes that employ training.
Acknowledgments
This work was supported by grants from the British Heart Foundation and from the Wellcome Trust. E.J.F.D was a James Knott Medical Research Scholar at St Cross College, University of Oxford.
References
- Ashley EA, Sears CE, Bryant SM, Watkins HC, Casadei B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105:3011–3016. doi: 10.1161/01.cir.0000019516.31040.2d. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunner F, Andrew P, Wolkart G, Zechner R, Mayer B. Myocardial contractile function and heart rate in mice with myocyte-specific overexpression of endothelial nitric oxide synthase. Circulation. 2001;104:3097–3102. doi: 10.1161/hc5001.101966. [DOI] [PubMed] [Google Scholar]
- Buch AN, Coote JH, Townend JN. Mortality, cardiac vagal control and physical training – what's the link? Exp Physiol. 2002;87:423–435. doi: 10.1111/j.1469-445x.2002.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Channon KM, Blazing MA, Shetty GA, Potts KE, George SE. Adenoviral gene transfer of nitric oxide synthase: high level expression in human vascular cells. Cardiovasc Res. 1996;32:962–972. [PubMed] [Google Scholar]
- Choate JK, Danson EJ, Morris JF, Paterson DJ. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am J Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- Cogliati T, Good DJ, Haigney M, Delgado-Romero P, Eckhaus MA, Koch WJ, Kirsch JR. Predisposition to arrhythmia and autonomic dysfunction in Nhlh1-deficient mice. Mol Cell Biol. 2002;22:4977–4983. doi: 10.1128/MCB.22.14.4977-4983.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Paterson DJ. Enhanced neuronal nitric oxide synthase expression is central to cardiac vagal phenotype in exercise-trained mice. J Physiol. 2003;546:225–232. doi: 10.1113/jphysiol.2002.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- Erbs S, Baither Y, Linke A, Adams V, Shu Y, Lenk K, Gielen S, Dilz R, Schuler G, Hambrecht R. Promoter but not exon 7 polymorphism of endothelial nitric oxide synthase affects training-induced correction of endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2003;23:1814–1819. doi: 10.1161/01.ATV.0000090128.11465.18. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Ivanova S, Zhou H, Simard JM. Mislocalization of eNOS and upregulation of cerebral vascular Ca2+ channel activity in angiotensin-hypertension. Hypertension. 2003;41:1124–1130. doi: 10.1161/01.HYP.0000066288.20169.21. [DOI] [PubMed] [Google Scholar]
- Gielen S, Hambrecht R. Effects of exercise training on vascular function and myocardial perfusion. Cardiol Clin. 2001;19:357–368. doi: 10.1016/s0733-8651(05)70222-8. [DOI] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Nitric oxide-cGMP pathway facilitates acetylcholine release and bradycardia during vagal nerve stimulation in the guinea-pig in vitro. J Physiol. 2001;535:507–518. doi: 10.1111/j.1469-7793.2001.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Sheseley EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- Ji LL, Leeuwenburgh C, Leichtweis S, Gore M, Fiebig R, Hollander J, Bejma J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998;854:102–117. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- Kiyonaga A, Arakawa K, Tanaka H, Shindo M. Blood pressure and hormonal responses to aerobic exercise. Hypertension. 1985;7:125–131. doi: 10.1161/01.hyp.7.1.125. [DOI] [PubMed] [Google Scholar]
- Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–2844. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- Kojda G, Laursen JB, Ramasamy S, Kent JD, Kurz S, Burchfield J, Shesely EG, Harrison DG. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial cell nitric oxide synthase. Contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc Res. 1999;42:206–213. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Alfie ME, Sigmon DH, Rhaleb NE, Shesely EG, Carretero OA. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32:856–861. doi: 10.1161/01.hyp.32.5.856. [DOI] [PubMed] [Google Scholar]
- Levecque C, Elbaz A, Clavel J, Richard F, Vidal JS, Amouyel P, Tzourio C, Alperovitch A, Chartier-Harlin MC. Association between Parkinson's disease and polymorphisms in the nNOS and iNOS genes in a community-based case-control study. Hum Mol Genet. 2003;12:79–86. doi: 10.1093/hmg/ddg009. [DOI] [PubMed] [Google Scholar]
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- Markos F, Snow HM, Kidd C, Conlon K. Nitric oxide facilitates vagal control of heart rate via actions in the cardiac parasympathetic ganglia of the anaesthetised dog. Exp Physiol. 2002;87:49–52. doi: 10.1113/eph8702303. [DOI] [PubMed] [Google Scholar]
- Mohan R, Choate JK, Golding S, Herring N, Casadei B, Paterson DJ. Peripheral pre-synaptic pathway reduces the heart rate response to sympathetic activation following exercise training: role of NO. Cardiovasc Res. 2000;47:90–98. doi: 10.1016/s0008-6363(00)00066-3. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Golding S, Heaton DA, Danson EJ, Paterson DJ. Targeting neuronal nitric oxide synthase with gene transfer to modulate cardiac autonomic function. Prog Biophys Mol Biol. 2004;84:321–344. doi: 10.1016/j.pbiomolbio.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Heaton DA, Danson EJ, Krishnan SP, Cai S, Channon KM, Paterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91:1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- Piech A, Dessey C, Havaux X, Feron O, Balligand JL. Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovasc Res. 2003;57:456–467. doi: 10.1016/s0008-6363(02)00676-4. [DOI] [PubMed] [Google Scholar]
- Rossi GP, Cesari M, Zanchetta M, Colonna S, Maiolino G, Pedon L, Cavallin M, Maiolino P, Pessina AC. The T-786C endothelial nitric oxide synthase genotype is a novel risk factor for coronary artery disease in Caucasian patients of the GENICA study. J Am Coll Cardiol. 2003;41:930–937. doi: 10.1016/s0735-1097(02)03012-7. [DOI] [PubMed] [Google Scholar]
- Saur D, Vanderwinden JM, Siedler B, Schmid RM, DeLaet MH, Allescher HD. Single-nucleotide promoter polymorphism alters transcription of neuronal nitric oxide synthase exon 1c in infantile hypertrophic pyloric stenosis. Proc Natl Acad Sci. 2004;101:1662–1667. doi: 10.1073/pnas.0305473101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CE, Choate JK, Paterson DJ. Effect of nitric oxide synthase inhibition on the sympatho-vagal contol of heart rate. J Auton Nerv Syst. 1998;73:63–73. doi: 10.1016/s0165-1838(98)00123-4. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- Shi X, Gallagher KM, Smith SA, Bryant KH, Raven PB. Diminished forearm vasomotor response to central hypervolemic loading in aerobically fit individuals. Med Sci Sports Exerc. 1996;28:1388–1395. doi: 10.1097/00005768-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Sullivan KJ, Kissoon N, Duckworth LJ, Sandler E, Freeman B, Bayne E, Sylvester JE, Lima JJ. Low exhaled nitric oxide and a polymorphism in the NOS I gene is associated with acute chest syndrome. Am J Respir Crit Care Med. 2001;164:2186–2190. doi: 10.1164/ajrccm.164.12.2012090. [DOI] [PubMed] [Google Scholar]
- Takimoto Y, Aoyama T, Tanaka K, Keyamura R, Yui Y, Sasayama S. Augmented expression of neuronal nitric oxide synthase in the atria parasympathetically decreases heart rate during acute myocardial infarction in rats. Circulation. 2002;105:490–496. doi: 10.1161/hc0402.102662. [DOI] [PubMed] [Google Scholar]
- Wang H, Morris JF. Presence of neuronal nitric oxide synthase in the suprachiasmatic nuclei of mouse and rat. Neuroscience. 1996;74:1059–1068. doi: 10.1016/0306-4522(96)00165-0. [DOI] [PubMed] [Google Scholar]
- Yu YW, Chen TJ, Wang YC, Liou YJ, Hong CJ, Tsai SJ. Association analysis for neuronal nitric oxide synthase gene polymorphism with major depression and fluoxetine response. Neuropsychobiology. 2003;47:137–140. doi: 10.1159/000070582. [DOI] [PubMed] [Google Scholar]
- Ziegler T, Silacci P, Harrison VJ, Hayoz D. Nitric oxide synthase expression in endothelial cells exposed to mechanical forces. Hypertension. 1998;32:351–355. doi: 10.1161/01.hyp.32.2.351. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann N Y Acad Sci. 2001;940:431–443. [PubMed] [Google Scholar]