Abstract
Activation of colonic proteinase-activated receptor-2 (PAR2) caused inflammation and increased mucosal permeability in mouse colon. The present study was aimed at characterizing the possible links between these two phenomena. We evaluated the effects of intracolonic infusion of PAR2-activating peptide, SLIGRL, on colonic paracellular permeability and inflammation at two different doses, 5 and 100 μg per mouse, in an attempt to discriminate between both PAR2-mediated effects. We further investigated the possible involvement of interferon γ (IFN-γ) and calmodulin-dependent activation of myosin light chain kinase (MLCK), and alterations of zonula occludens-1 (ZO-1) localization in PAR2-induced responses. Thus, at the lower dose, SLIGRL increased colonic permeability without causing inflammation. Western blotting showed phosphorylation of mucosal myosin light chain (MLC) expression after both doses of SLIGRL. Moreover, while the MLCK inhibitor, ML-7, abolished the permeability effects of the low dose of SLIGRL, it only partially inhibited that of the high dose. In IFN-γ-deficient mice (B6 ifng−/−), the increases in permeability were similar for both doses of SLIGRL and prevented by ML-7. In addition, MLCK immunoprecipitation revealed an increase of calmodulin binding to MLCK in the mucosa of mice treated with either dose of SLIGRL. Finally, we have shown that direct activation of PAR2 on enterocytes is responsible for increased permeability and ZO-1 disruption. Moreover, SLIGRL at a dose that does not produce inflammation increases permeability via calmodulin activation, which binds and activates MLCK. The resulting tight junction opening does not depend upon IFN-γ secretion, while the increased permeability in response to the high dose of PAR2 agonist involves IFN-γ secretion.
A major function of gastrointestinal epithelial cells is to form a physical barrier between the gastrointestinal lumen and subepithelial tissues. The apico-laterally located tight junctions (TJs) form a paracellular seal between the lateral membranes of adjacent cells. Lateral contacts, which may be visualized by electron microscopy and freeze-fracture analysis, act as a structural barrier against paracellular permeation (Jinguji & Ishikawa, 1992). Epithelial TJs are composed of at least three families of transmembrane proteins (occludins, claudins and adhesion proteins) and a cytoplasmic ‘plaque’ consisting of many different proteins that form large complexes. The transmembrane proteins mediate cell adhesion and are thought to constitute the intramembrane and paracellular diffusion barriers. The cytoplasmic plaque of TJs is formed by different types of proteins that include adaptors, such as the zonula occludens (ZO) proteins and other proteins that contain PDZ domains, as well as regulatory and signalling components. Adaptor proteins are thought to be connected to transmembrane proteins and to recruit other cytosolic components to TJs, such as protein kinases, GTPases and transcription factors (Mitic & Anderson, 1998). Several components of the cytoplasmic plaque, in addition to the transmembrane protein occludin, also function as cytoskeletal linkers that interact with the cytoskeleton (Mitic & Anderson, 1998). There is a high density of cytoskeletal actin and myosin filaments, which encircle the intestinal epithelial cells near the apical cellular borders at the level of TJs. The disruption of perijunctional actin–myosin filaments allows an increase in epithelial penetration (Yamaguchi et al. 1991). The activity of myosin is regulated by the opposing actions of myosin light-chain phosphatase and myosin light-chain kinase. Myosin light chain (MLC) phosphorylation by MLC kinase (MLCK) is one of the regulators of TJ permeability (Turner et al. 1997). In mice, stress-induced opening of colonic epithelial TJ requires CD4+/CD8+ T cells and IFN-γ, and involves MLCK activation (Ferrier et al. 2003). In the colon, a leaky TJ barrier allows penetration of toxic luminal substances, which promote colonic mucosal injury and inflammation (Gassler et al. 2001).
Proteinase-activated receptors (PARs) belong to a family of seven transmembrane domain G-protein-coupled receptors that are activated by cleavage of their N-terminal domain by a proteolytic enzyme (Nystedt et al. 1995). The unmasked new N-terminal sequence acts as a tethered ligand that binds and activates the receptor itself. PAR2 is activated by trypsin, mast cell tryptase and trypsin-like proteins. Synthetic peptides, so-called PAR-activating peptides (PAR-APs) such as SLIGRL for PAR2, corresponding to the amino-acid sequence of the tethered ligand, are also able to selectively activate PARs (Corvera et al. 1997). PAR2 is found expressed throughout the gastrointestinal tract on several cell types, including enterocytes, mast cells, smooth muscle cells, myenteric neurones and endothelial cells (Kong et al. 1997). Moreover, the involvement of PAR2 in different pathophysiological processes has been reported. In fact, in vivo intracolonic activation of PAR2 led to colonic inflammation in mice and increased paracellular permeability, with bacterial translocation into peritoneal organs (Cenac et al. 2002). In this model, colonic infusion of trypsin, tryptase or SLIGRL induced a Th1-like inflammation, in which TNF-α, interleukin (IL)-1β and IFN-γ mRNA levels are elevated, while IL-4 and IL-10 mRNA expression remain stable (Cenac et al. 2002). In vitro, it has been shown that IFN-γ disrupts the epithelial barrier function of T84 cells by decreasing the levels of ZO-1, perturbing the actin cytoskeleton in the tight junction area, and displacing ZO-2 and occludin (Youakim & Ahdieh, 1999). In addition, TNF-α has been reported to promote the disturbing effects of IFN-γ on the epithelial barrier (Mahraoui et al. 1997), while IL-10 blocks these effects (Oshima et al. 2001). Although the expression of PAR2 has been reported on both apical and basolateral surfaces of enterocytes, evidence is lacking so far that direct activation of PAR2 may lead to a dysfunction of the epithelial barrier.
Consequently, the aim of this work was to determine the mechanisms underlying the effects of different concentrations of a PAR2 agonist, the peptide SLIGRL, on colonic paracellular permeability. Specifically, we investigated (i) the involvement of IFN-γ, (ii) the involvement and activation pathway of MLCK, and (iii) the direct effects of PAR2 agonist on enterocyte barrier functions.
Methods
Animals
This study was conducted on the one hand with Swiss 3T3 mice, since all our previous experiments use this strain (Cenac et al. 2002, 2003). On the other hand, to determine the role of IFN-γ we have used B6 infg−/− mice, which are IFN-γ deficient, and their control strain, C57BL/6J mice. Male Swiss 3T3 mice were from Janvier (Le Genest St-Isle, France) and B6 ifng−/− (IFN-γ deficient) and C57BL/6J (control strain of B6 ifng−/−) mice were from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred and maintained in the animal facility of the Institut Fédératif de Recherche Claude De Preval (INSERM IFR 30, Toulouse, France). Mice were housed in polycarbonate cages in a light- (12 h–12 h cycle) and temperature-controlled room (20–22°C) and were fed standard pellets (Safe A03; Epinay-sur-orge, France). Water was provided ad libitum. The experimental protocols described in this study were approved by the local Institutional Animal Care and Use Committee.
Intracolonic injections
Mice were fasted for 12 h before the beginning of experiments. Under deep xylazine–ketamine (5: 1 ratio, 10 mg and 2 mg per mouse, respectively) anaesthesia, a small polyethylene catheter (0.3/0.07 mm) was inserted intrarectally at 4 cm from the anus. The PAR2 activating peptide SLIGRL, the inactive reverse peptide LRGILS, or their vehicle (saline) was then injected into the colon through the catheter.
Experimental protocols
In previous experiments (Cenac et al. 2002), we demonstrated that peak inflammatory response to PAR2 activation was observed at 4 h after intracolonic administration of the PAR2 agonist (SLIGRL). Peak increase in paracellular permeability was observed from 2 to 4 h after SLIGRL administration. Thus, the 4-h time point was chosen for the present experiments. Four groups of eight Swiss 3T3 mice received intracolonically (50 μl) SLIGRL (5 μg or 100 μg per mouse), LRGILS (100 μg per mouse) or saline (controls). Paracellular permeability, tissue inflammation, and Western-blot analysis of MLCK, MLC and p-MLC in colon, were assessed 4 h after intracolonic injections. Eight groups of eight B6 ifng−/− mice (deficient for functional IFN-γ) and eight groups of eight C57BL/6J mice (control strain for B6 ifng−/−) received intracolonically saline, LRGILS (100 μg per mouse) or PAR2-activating peptide (5 μg or 100 μg per mouse) as previously described. Four groups of IFNγ-deficient mice and four groups of wild-type mice were also treated with the MLCK inhibitor, ML-7 (2 mg kg−1, i.p.), while the remaining groups received the ML-7 vehicle. Here again, paracellular permeability, Western-blot for MLCK, MLC and p-MLC, and tissue inflammation were assessed 4-h after the intracolonic injections. Six groups of eight Swiss 3T3 mice received peptides as described earlier, and were pretreated with chlorpromazine (25 mg kg−1, i.p.), a calmodulin inhibitor, or its vehicle, 30 min before peptide injection and paracellular permeability was assessed. Finally, MLCK immunoprecipitation was studied in four groups of eight Swiss mice that had received SLIGRL intracolonically.
Assessment of inflammation
At 4 h after intracolonic injection of SLIGRL, mice were killed by cervical dislocation and distal colon tissues collected. The severity of intestinal inflammation was evaluated by previously described criteria (Mombaerts et al. 1993) with slight modifications. Briefly, the disease score (0–10) was estimated by a combination of both gross and histological observations. The gross score (0–3) was rated:
presence of normal beaded appearance;
absence of beaded appearance of colon;
focal thickened colon;
marked thickness of the entire colon.
The histological score (0–7) was based upon the extent of:
intestinal wall thickening (0–3);
lamina propria infiltration (0–3);
presence (0–1) of ulceration.
In addition, colonic samples were assayed for myeloperoxidase (MPO) activity and RT-PCR (see below).
Myeloperoxidase activity assay
The activity of myeloperoxidase (MPO), which is found in polymorphonuclear neutrophil granules, was assessed in colon tissues according to the method of Bradley et al. (1982). Briefly, samples of distal colon (1 cm) were suspended in a potassium phosphate buffer (50 mm, pH 6.0) and homogenized in ice. Three cycles of freeze–thaw were undertaken. Suspensions were then centrifuged at 10 000 g for 15 min at 4°C. Supernatants were discarded and pellets were resuspended in hexadecyl trimethylammonium bromide buffer (HTAB 0.5% w/v, in 50 mm potassium phosphate buffer, pH 6.0). These suspensions were sonicated on ice, and centrifuged again at 10 000 g for 15 min at 4°C. The supernatants obtained were diluted in potassium phosphate buffer (pH 6.0) containing 0.167 mg ml−1 of O-dianisidine dihydrochloride and 0.0005% of hydrogen peroxide. Myeloperoxidase from human neutrophils (0.1 units per 100 μl) was used as a standard. The kinetic changes in absorbance at 450 nm, every 10 s over 2 min, were recorded with a spectrophotometer. One unit of MPO activity was defined as the quantity of MPO degrading 1 μmol of hydrogen peroxide min−1 ml−1 at 25°C. Protein concentration was determined with a commercial kit using a modified method of Lowry (Detergent Compatible Assay, Bio-Rad, Marnes la Coquette, France). MPO activity was expressed as units per gram of protein.
RT-PCR detection of β-actin, IL-10, IFN-γ and TNF-α mRNA
For RT-PCR detection of cytokine RNA, total RNA (5–10 μg) was reverse-transcribed into complementary DNA (cDNA) using Superscript II RNase H− RT (Gibco-BRL, Cergy Pontoise, France). The primers used were: β-actin: 5′-GGG TCA GAA GGA TTC CTA TG-3′ and 5′-GGT CTC AAA CAT GAT CTG GG-3′; IL-10: 5′-ATG CAG GAC TTT AAG GGT TACT TG-3′ and 5′-AGA CAG CTT GGT CTT GGA GCT TA-3′; IFN-γ: 5′-GCT CTG AGA CAA TGA ACG CT-3′ and 5′-AAA GAG ATA ATC TGG CTC TGC-3′; TNF-α: 5′-TCT CAT CAG TTC TAT GGC CC-3′ and 5′-GGG AGT AGA CAA GGT ACA AC-3′. Competitive PCR analysis was performed using linearized plasmids: pQB3 for the competition with β-actin, and pMus3 for the competition with IFN-γ, IL-10 and TNF-α (Legoux et al. 1992; Zou et al. 1995). Amplification was performed for 40 cycles consisting of denaturation for 1 min at 94°C, primer annealing for 1 min at 52–55°C, and primer extension for 1.5 min at 72°C. DNA products were then separated on a 3% agarose gel with ethidium bromide and the ratio between amplified molecules for the target cDNA and the competitor, i.e. log (DNA competitor/target cDNA), was calculated for each graded concentration of the competitor, using an image analyser (QuantityOne software; Amersham Pharmacia, Orsay, France). A curve of the ratios was established according to the competitor concentrations. This allowed the calculation of the equivalence point, at which the amount of amplified target mRNA and DNA competitor are equal (log (DNA competitor/target cDNA) = 0). This value corresponds to the concentration of the target cDNA present in the initial sample (Legoux et al. 1992; Zou et al. 1995). To quantify cytokines in samples more accurately, the number of cytokine molecules was expressed in comparisson with the number of cDNA molecules of the internal control, β-actin, in the same sample.
In vivo permeability studies
Mice were anaesthetized with xylazine–ketamine (5: 1 ratio, 10 mg and 2 mg per mouse, respectively; subcutaneously). To measure colonic paracellular permeability, 0.7 μCi of 51Cr-EDTA (Perkin Elmer Life Sciences, Paris, France) in 0.5 ml NaCl (154 mm) was slowly infused into the colon (0.25 ml h−1). After 2 h, mice were killed by cervical dislocation and colons were removed. Then, colons and remaining bodies were placed in separate counting tubes in a gamma-counter (Packard Cobra II, Packard Bioscience, Meriden CT, USA). The permeability was expressed as the ratio between body and total (body plus colon) radioactivities.
In vitro cell culture
SCBN cells are human non-transformed enterocytes, which form polarized monolayers with functional tight junctions and contain cytokeratins, mucin antigen, and messenger RNA for epidermal growth factor, IL-6, and vascular adhesion molecule-1 (Pang et al. 1996). Cells from passages 22–26 were grown to confluence in Dulbecco's modified Eagle's medium (DMEM, Sigma, St Louis, MO, USA) supplemented with 5% or 10% fetal bovine serum (Sigma), 100 μg ml−1 streptomycin, 100 U ml−1 penicillin, 0.08 mg ml−1 tylosine and 200 mmol l-glutamine (all from Sigma) and incubated at 37°C, 5% CO2 and 96% humidity in chamber slides or Transwells (Costar, Corning, NY, USA).
In vitro permeability measurements
Permeability studies were performed in vitro to further investigate altered barrier function in response to PAR2-AP. SCBN monolayers were grown to confluence on Transwell membranes in 12-well plates (Costar). Five-day-old confluent monolayers develop electrical resistances of > 1200 Ω cm−2 as measured with an electrovoltohmeter (EVOM; World Precision Instruments, Sarasota, FL, USA). Permeability was assessed by measuring apical to basolateral fluxes of fluorescein isothiocyanate (FITC)–dextran (relative molecular mass 3000, 100 μmol l−1; Molecular Probes, Eugene, OR, USA) across the monolayers. Confluent SCBN monolayers on Transwell membranes were washed 3 times in sterile tissue culture-grade PBS (Sigma) to remove residual media. The upper chambers of the Transwells were filled with 300 μl of Krebs buffer. The following treatments were added to either the upper or the lower chamber: Vehicle control (DMEM), LRGILS 25 μm or SLIGRL (25 μm). Then the monolayers were incubated for 2 h in the dark at 37°C, 5% CO2 and 96% humidity. After this incubation, two 300-μl samples were removed from the bottom chamber, and fluorescence was measured on a microplate fluorometer (SpectraMax Gemini; Molecular Devices, Sunnyvale, CA, USA) along with samples of the original apical solution. The FITC–dextran flux was measured as the percentage of the original apical solution that can be detected in the basolateral medium that crossed 1 cm2 in 1 h, as previously described (Chin et al. 2002; Scott et al. 2002).
Immunoprecipitation of MLCK
To investigate the interaction between MLCK and calmodulin, MLCK was immunoprecipited and Western blot analyses of MLCK and calmodulin were performed. For immunoprecipitation, colonic mucosal tissues were scraped into 500 μl of 1% Triton X-100 lysis buffer (50 mm Hepes, 150 mm NaCl, 1% Triton X-100, protease inhibitor cocktail (Sigma, Saint-Quentin Fallavier, France)) and clarified by centrifugation at 4°C for 15 min at 14 0000 g. Before the immunoprecipitation, anti-MLCK IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was bound to protein G–Sepharose (Sigma) in HNT buffer (30 mm Hepes, 30 mm NaCl, 0.1% Triton X-100; pH 7.5). The supernatant was incubated with the antibody–protein G–Sepharose (Sigma) for 7 h at 4°C. Immune complexes were collected by centrifugation, washed 3 times in buffer (30 mm Hepes, 30 mm NaCl, 0.1% Triton X-100; pH 7.5), and treated for Western blot analysis.
Western blot analysis for calmodulin, MLCK, MLC and p-MLC
Mice were killed by cervical dislocation. After laparotomy, colons were collected and washed with saline, and the mucosa was scraped off and recovered. Proteins were extracted with Tri-reagent (Gibco-BRL, Eragny, France) and solubilized with 1% SDS (Sigma). Protein concentration was assessed using the DC protein assay kit (Bio-Rad, France). Equal amounts of each extract were then subjected to SDS-PAGE in 12% (MLC and phospho-MLC determination) or 7.5% (MLCK) slabs, then electro-transferred onto 0.45 mm nitrocellulose membrane. Membrane was blocked with Tris-buffered saline (TBS)–milk 6%, then incubated overnight at 4°C with the primary antibody. We used a 1/1000 dilution for all primary antibodies, i.e. anti-MLC, anti-phospho-MLC, anti-CaM (Santa Cruz Biotechnology), and anti-MLCK (Sigma). Sodium fluoride (10 mm) was added for phospho-MLC determination. After washing, peroxidase-labelled G-protein (Sigma) was added for 1 h at room temperature. The membrane was incubated for 1–5 min with SuperSignal Reagent (Pierce, Bezons, France).
Electron microscopy of tight junction
Three Swiss 3T3 mice per group (treated and controls) were killed 4 h after SLIGRL (5 μg per mouse), LRGILS (100 μg per mouse) or saline infusion. Colons were removed and cut into 2 mm pieces. Tissue samples were fixed in 2.5% glutaraldehyde–2% paraformaldehyde solution (1 h; +4°C), rinsed in 0.1 m cacodylate buffer and post-fixed for 1 h at +4°C in 1% osmium tetroxide. Subsequently, tissue samples were dehydrated by graded ethanol and embedded in Epon-Araldite resin (Euromedex, Mundolsheim, France). Ultra-thin sections (70 nm) were carried out with an ultramicrotome system (Reichert, Leica, Rueil-Malmaison, France) and collected on copper–palladium grids. After staining with 4% uranyl acetate and 0.4% lead citrate, sections were examined with a Hitachi HU11C electron microscope (Hitachi, Paris, France).
Immunohistochemistry for ZO-1
SCBN monolayers were incubated with 400 μl of SLIGRL (30 μm), LRGILS (30 μm) or DMEM (vehicle control) for 2 h. After permeabilization, monolayers were washed 3 times for 10 min each time in sterile PBS (Sigma) and blocked with fetal bovine serum (FBS, Sigma) for 10 min at room temperature. After three more 10-min washes with sterile PBS, monolayers were incubated with an affinity-purified polyclonal rabbit anti-human ZO-1 antibody (Santa Cruz Biotechnology), 100 μl diluted 1: 100 in PBS, or 100 μl of Cy3-conjugated sheep anti-rabbit IgG (1: 100 in PBS) for 60 min at room temperature. Slides were washed 3 times for 10 min each time in sterile PBS, and 200 μl of Cy3-conjugated sheep anti-rabbit IgG (1: 100 in PBS) was added to each well. After a 60-min incubation in a humidity chamber at room temperature, slides underwent three more 10-min washings in PBS and then were mounted with Aqua Poly Mount (Poly Sciences) and examined on a confocal laser scanning microscope (ViewScan DVD-250; Bio-Rad, Hercules, CA, USA). Micrographs were obtained with a Spot II digital camera (Diagnostic Instruments, Sterling Heights, MI, USA).
Chemicals
Peptides, SLIGRL-NH2 and LRGILS-NH2 (referred to in the text as SLIGRL and LRGILS), prepared by solid phase synthesis, were obtained from Neosystem (Strasbourg, France). The composition and purity of peptides were confirmed by HPLC analysis. SLIGRL and LRGILS were dissolved in saline. The MLCK inhibitor, 1-(5-iodonaphtalene-1-sulphonyl)-1H-hexahydro-1,4-diazepine (ML-7), and the calmodulin inhibitor, chlorpromazine were obtained from Sigma (France). ML-7 was dissolved in 2% ethanol and 98% saline; chlorpromazine was dissolved in saline.
Statistical analysis
Data are presented as means ± standard error of the mean. Analyses were done by running the GraphPad Prism 3.0 software (GraphPad, San Diego, CA, USA). All data were normally distributed. Between-group comparisons were performed by Student's unpaired t test. Multiple comparisons within groups were performed by repeated-measures one-way ANOVA, followed by Student's t test. Statistical significance was accepted at P < 0.05.
Results
A low dose of PAR2-AP increases colonic paracellular permeability without causing inflammation
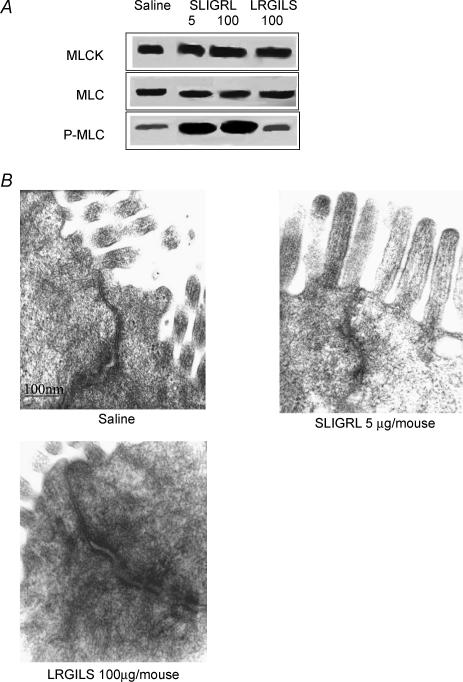
As we have previously shown in Swiss 3T3 mice that PAR2 agonists administered intracolonically caused colon inflammation and increased paracellular permeability, we used the same strain of mice to further study the relationship between inflammation and permeability for different doses of the PAR2 agonist. In colons of Swiss 3T3 mice, the intracolonic administration of SLIGRL (100 μg per mouse, considered as a high dose of PAR2 activating peptide) caused significant increase in MPO activity, damage score, TNF-α and IFN-γ mRNA expressions, caused increased paracellular permeability, and a significant decrease in IL-10 mRNA expression (Table 1). In contrast, a markedly lower dose of SLIGRL (5 μg per mouse) increased paracellular permeability (3.3 ± 0.2% versus 1.4 ± 0.2%; P < 0.05) without concurrent inflammatory reaction: MPO activity, damage score and TNF-α, IFN-γ and IL-10 expression being equivalent to those observed in controls (Table 1). The reverse peptide LRGILS, inactive on PAR2 (100 μg per mouse), had no effect on both inflammatory parameters and paracellular permeability (Table 1). The increase of paracellular permeability mediated by the high dose of SLIGRL was significantly greater than that obtained with the low dose (Table 1). Both doses of SLIGRL increased MLC phosphorylation, without affecting quantities of MLCK and MLC (Fig. 1A). Furthermore, tight junction opening after the low dose of SLIGRL was clearly observed by electron microscopy of colonic epithelial cells (Fig. 1B). The reverse peptide LRGILS had no effect on the expression of MLCK, MLC and p-MLC (Fig. 1A).
Table 1.
Inflammatory parameters in Swiss mice after intracolonic administration of SLIGRL (5 or 100 μg per mouse), LRGILS (100 μg per mouse) or saline
| Saline | 100 μg LRGILS | 5 μg SLIGRL | 100 μg SLIGRL | |
|---|---|---|---|---|
| MPO (U (g of protein)−1) | 84 ± 8 | 49 ± 5 | 78 ± 10 | 638 ± 198* |
| Damage score | 0.5 ± 0.11 | 0.8 ± 0.06 | 1.1 ± 0.12 | 5.8 ± 0.60* |
| TNF-α | 2.36 ± 0.23 | 2.10 ± 0.15 | 2.23 ± 0.38 | 4.39 ± 0.51* |
| IFN-γ | 0.11 ± 0.01 | 0.18 ± 0.04 | 0.13 ± 0.02 | 0.70 ± 0.03* |
| IL-10 | 1.46 ± 0.12 | 1.16 ± 0.09 | 1.28 ± 0.21 | 0.47 ± 0.11* |
| Permeability (%) | 1.8 ± 0.4 | 1.4 ± 0.2 | 3.3 ± 0.2* | 6.6 ± 0.6* |
P < 0.05 compared with ‘saline’ values. The number of cytokine molecules was expressed in comparison with the number of cDNA molecules of the internal control, β-actin, in the same sample. Permeability was expressed in a percentage of 51Cr-EDTA permeability.
Figure 1. MLCK, MLC and p-MLC and electron microscopy of tight junctions.
A, detection of MLCK, MLC and p-MLC proteins by Western blot. Mice (Swiss 3T3) received intracolonocally SLIGRL (5 and 100 μg per mouse), LRGILS (100 μg per mouse) or their vehicle (saline). Mucosa lysates were incubated with anti-mouse MLCK, MLC or p-MLC antibodies. MLCK, MLC and p-MLC were detected as bands with a molecular mass of about 130, 20 and 20 kDa, respectively (n = 5). B, electron microscopy of tight junction observed in mice Swiss 3T3 colon treated with SLIGRL (5 μg per mouse), LRGILS (100 μg per mouse) or saline (n = 3).
The inflammatory reaction mediated by PAR2 activation in wild-type mice is abolished in IFN-γ-deficient mice
To investigate the involvement of IFN-γ in PAR2-induced colitis and increased permeability, we used IFN-γ-deficient mice, which are available only in a C57BL/6J background. Therefore, we reproduced in C57BL/6J mice the experiments previously performed in Swiss 3T3. In C57BL/6J, the intracolonic administration of SLIGRL (100 μg per mouse) also caused a significant increase in MPO activity, damage score and TNF-α mRNA expression, and a significant decrease in IL-10 mRNA expression (Table 2). The reverse peptide LRGILS had no effect on the inflammatory parameters measured (Table 2). In contrast, low doses of SLIGRL (5 μg per mouse) caused no inflammation: MPO activity, damage score and TNF-α and IL-10 expression were similar to those in saline-treated mice (Table 2). Furthermore, in IFN-γ-deficient mice, administration of either SLIGRL (5 and 100 μg per mouse) or the reverse peptide LRGILS (100 μg per mouse) had no effect on the different inflammatory parameters measured (Table 3).
Table 2.
Inflammatory parameters in C57BL/6 J mice (control strain of B6 ifng−/− mice) after intracolonic administration of SLIGRL (5 or 100 μg per mouse), LRGILS (100 μg per mouse) or saline
| Saline | 100 μg LRGILS | 5 μg SLIGRL | 100 μg SLIGRL | |
|---|---|---|---|---|
| MPO (U (g of protein)−1) | 99 ± 21 | 96 ± 9 | 101 ± 13 | 759 ± 71* |
| Damage score | 0.9 ± 0.05 | 1.1 ± 0.03 | 1.5 ± 0.08 | 6.3 ± 0.82* |
| TNF-α | 2.18 ± 0.21 | 2.21 ± 0.19 | 1.95 ± 0.41 | 5.04 ± 0.81* |
| IL-10 | 1.08 ± 0.07 | 0.91 ± 0.08 | 1.21 ± 0.06 | 0.36 ± 0.13* |
P < 0.05 compared with ‘saline’ values. (see Table 1 for legend).
Table 3.
Inflammatory parameters in B6 ifng−/− (IFNγ-deficient) mice after intracolonic administration of SLIGRL (5 or 100 μg per mouse), LRGILS (100 μg per mouse) or saline
| Saline | 100 μg LRGILS | 5 μg SLIGRL | 100 μg SLIGRL | |
|---|---|---|---|---|
| MPO (U (g of protein)−1) | 92 ± 23 | 92 ± 11 | 81 ± 21 | 76 ± 15 |
| Damage score | 0.8 ± 0.06 | 0.6 ± 0.14 | 0.7 ± 0.08 | 1.1 ± 0.12 |
| TNF-α | 1.22 ± 0.18 | 1.32 ± 0.09 | 1.45 ± 0.15 | 1.68 ± 0.13 |
| IL-10 | 0.9 ± 0.04 | 0.9 ± 0.08 | 1.2 ± 0.12 | 1.08 ± 0.10 |
P < 0.05 compared with ‘saline’ values. (see Table 1 for legend).
PAR2 activation increases permeability in wild type and IFN-γ-deficient mice by MLCK activation
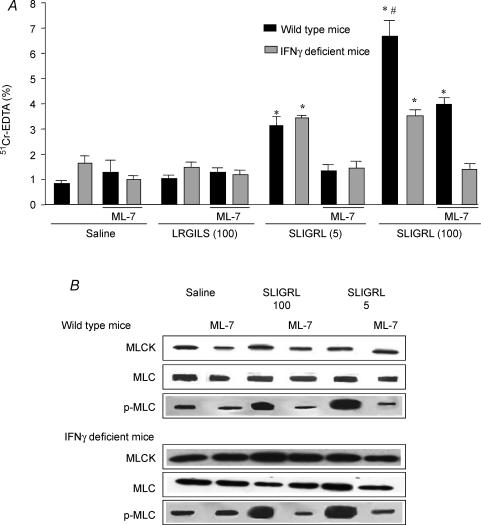
In wild-type mice (C57BL/6J) as well as in Swiss 3T3 mice, the increase of colonic paracellular permeability mediated by the high dose of SLIGRL (100 μg per mouse) was significantly higher than that mediated by the low dose (5 μg per mouse) (Fig. 2A). In contrast, in IFN-γ-deficient mice (B6 ifng−/−), the increased paracellular permeability mediated by SLIGRL was not significantly different for the two doses of SLIGRL (3.3 ± 0.2%versus 3.5 ± 0.2% for 5 and 100 μg per mouse, respectively; P = 0.6), but was significantly higher compared to saline-injected mice (Fig. 2A). The reverse peptide LRGILS had no effect on paracellular permeability in both wild-type and IFN-γ-deficient mice (Fig. 2A). Pretreatment of C57BL/6J by the MLCK inhibitor, ML-7, diminished the increased paracellular permeability mediated by the high dose of SLIGRL (4.0 ± 0.6%versus 5.8 ± 0.5%; P < 0.05) and abolished the effect mediated by the low dose (1.2 ± 0.2%versus 3.1 ± 0.5%; P < 0.05) (Fig. 2A). In contrast, in the IFN-γ-deficient mice, ML-7 prevented the increase of paracellular permeability for both doses of SLIGRL (high dose: 1.7 ± 0.3% versus 3.5 ± 0.2%, P < 0.05; low dose: 1.4 ± 0.2%versus 3.3 ± 0.2%, P < 0.05) (Fig. 2A).
Figure 2. Prevention by MLCK inhibitor of SLIGRL-induced intracellular permeability increase and MLC phosphorylation.
A, prevention by the MLCK inhibitor, ML-7, of SLIGRL-induced colonic paracellular permeability increase. B6 ifng−/− mice (IFN-γ deficient) and their control strain (C57BL/6J) were pretreated by ML-7 (2 mg kg−1) and received intracolonic administration of saline, LRGILS (100 μg per mouse) or SLIGRL (5 and 100 μg per mouse). Intestinal permeability was assessed by measuring the gut lumen-to-body passage of labelled 51Cr-EDTA. Values are means ±s.e.m.; n = 8; *P < 0.05 compared to ‘saline’ values, #P < 0.05 compared to the values of the group treated by 5 μg per mouse of SLIGRL. B, detection of MLCK, MLC and p-MLC proteins by Western blot. IFN-γ-deficient and wild-type mice were treated by SLIGRL (5 and 100 μg per mouse) or its vehicle (saline) and pretreated by a MLCK inhibitor, ML-7 (2 mg kg−1, i.p.), or its vehicle (2% ethanol). Mucosa lysates were incubated with anti-mouse MLCK, MLC or p-MLC antibodies. MLCK, MLC and p-MLC were detected as bands with a molecular mass of about 130, 20 and 20 kDa, respectively (n = 5).
PAR2 activation increases MLC phosphorylation in wild-type and IFN-γ-deficient mice by MLCK activation
In IFN-γ-deficient mice (B6 ifng−/−) and in wild-type mice (C57BL/6J), both doses of SLIGRL induced similar increases of MLC phosphorylation (Fig. 2B) and had no effect on the quantity of MLC and MLCK (Fig. 2B). ML-7 (2 mg kg−1) inhibited MLC phosphorylation in the two strains of mice and had no effect on MLC and MLCK expression (Fig. 2B).
PAR2-induced activation of MLCK is calmodulin dependent
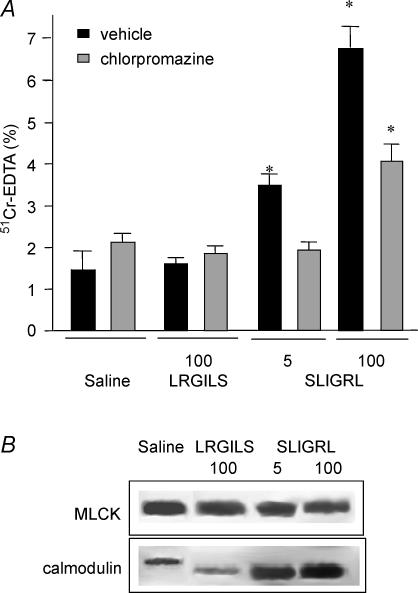
In Swiss 3T3 mice, inhibition of calmodulin by chlorpromazine (25 mg kg−1), diminished the increase of paracellular permeability induced by the high dose (100 μg per mouse) of SLIGRL (3.9 ± 0.4%versus 6.8 ± 0.4%; P < 0.05) and abolished that induced by the low dose (5 μg per mouse) of SLIGRL (1.9 ± 0.2%versus 3.5 ± 0.3%; P < 0.05) (Fig. 3A). Chlorpromazine had no effect per se on paracellular permeability. MLCK immunoprecipitation revealed an increase of calmodulin bound to MLCK in SLIGRL-treated mice compared to those treated with saline or the reverse peptide LRGILS (Fig. 3B).
Figure 3. Interaction between MLCK and calmodulin.
A, inhibition by the calmodulin inhibitor, chlorpromazine, of SLIGRL-induced colonic paracellular permeability increase. Swiss 3T3 mice were pretreated by chlorpromazine (25 mg kg−1, i.p.) (grey bars) or its vehicle (saline) (black bars) and received intracolonically saline, LRGILS (100 μg per mouse) or SLIGRL (5 and 100 μg per mouse). Intestinal permeability was assessed by measuring the gut lumen-to-body passage of labelled 51Cr-EDTA. Values are means ±s.e.m.; n = 8; *P < 0.05 compared to ‘saline’ values. B, calmodulin–MLCK interactions. Four hours after treatment of Swiss 3T3 mice by saline, LRGILS (100 μg per mouse) or SLIGRL (5 and 100 μg per mouse), colons were removed, and colonic mucosa proteins were immunoprecipitated with an anti-MLCK antibody followed by calmodulin and MLCK immunoblotting assays. MLCK and calmodulin were detected as bands with a molecular mass of about 130 kDa and 16 kDa, respectively (n = 5).
In vitro, basal but not apical activation of PAR2 increases epithelial permeability
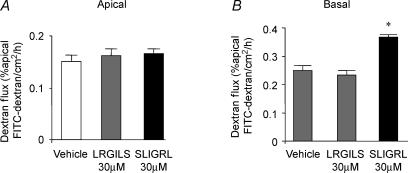
Apical to basolateral fluxes of FITC–dextran 3000 were measured to assess whether apical or basal exposure of SCBN monolayers to SLIGRL (30 μm) for 2 h could reproduce the PAR2-AP-induced loss of intestinal barrier function observed in mice in vivo. Apical exposure of SLIGRL had no effect on transepithelial dextran fluxes (Fig. 4). In contrast, basal exposure of SCBN monolayers to SLIGRL significantly increased dextran fluxes (0.37 ± 0.02 versus 0.23 ± 0.01% apical FITC–dextran cm−2 h−1; P < 0.05). Neither apical nor basal exposure of SCBN monolayers to the reverse peptide LRGILS had any effect on transepithelial dextran fluxes.
Figure 4. Paracellular permeability of SCBN monolayers, measured as transepithelial flux of FITC-dextran 3000.
Values were obtained from control monolayers with vehicle and monolayers incubated with SLIGRL (30 μm) or LRGILS (30 μm) for 2 h (apical, A, and basal, B). Values are means ±s.e.m. of the percentage apical fluorescence that crossed 1 cm2 of the epithelial barrier into the basolateral chamber in 1 h. *P < 0.05 compared to saline values; n = 12 per group.
PAR2 activation induces reorganization of epithelial ZO-1
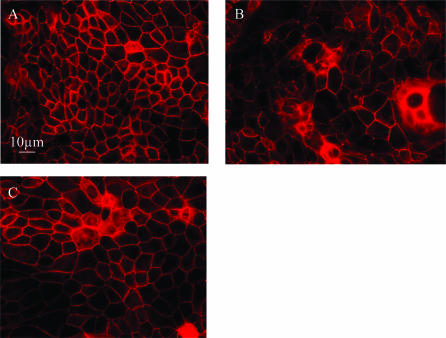
In control SCBN monolayers, tight junctional ZO-1 was characteristically located at the cellular margins (Fig. 5A). After a 2-h incubation with SLIGRL (30 μm), ZO-1 migrated from the cellular margins into the cytoplasm (Fig. 5B). The reverse peptide LRGILS (30 μm) did not change tight junctional ZO-1 localization (Fig. 5C).
Figure 5. Representative confocal laser scanning micrographs of SCBN monolayers stained for tight junctional ZO-1.
A, preparations included unmanipulated control monolayers: typical distribution of ZO-1 in control monolayers can be seen at the periphery of the enterocytes; B, monolayers exposed to SLIGRL (30 μm) for 2 h: exposure to SLIGRL causes a perinuclear relocation of ZO-1; C, monolayers exposed to LRGILS (30 μm) for 2 h: LRGILS had no effect.
Discussion
Our study reveals for the first time that (i) a low dose of SLIGRL increases paracellular permeability via MLC phosphorylation by MLCK without causing inflammation, and (ii) that the increase in paracellular permeability mediated by the inflammatory dose of SLIGRL involves an INF-γ-dependent mechanism. Activation of PAR2 by low doses of SLIGRL provoked the activation of calmodulin, which interacts with and stimulates MLCK most likely in epithelial cells, without concomitant influence of inflammatory factors. In fact, for all strains of mice studied here, low doses of PAR2-AP had no effect on inflammatory parameters. By electron microscopy, we have shown that a low dose of SLIGRL opened epithelial cell tight-junctions with a concurrent activation of MLCK, which in turn, led to an increase in p-MLC proteins observed by Western-blot analysis. Moreover, PAR2 agonist relocalized ZO-1 and increased paracellular permeability in epithelial monolayers, further demonstrating a direct effect of PAR2 activation on enterocytes. In C57BL/6J and Swiss 3T3 mice, permeability was dose-dependently increased after SLIGRL. While ML-7 abolished the effect of the low dose of SLIGRL, it only decreased that of the high dose. In these mice, high doses of SLIGRL provoked an inflammatory reaction characterized by increased MPO activity, high damage scores, increased IFN-γ and TNF-α mRNA, and decreased IL-10 mRNA. In IFN-γ-deficient mice, SLIGRL had no effect on inflammatory parameters, but increased intestinal permeability to the same extent for both doses of SLIGRL. This increase in paracellular permeability was linked to MLCK activation. The inflammatory reaction is IFN-γ dependent and for the high dose of SLIGRL the paracellular permeability is IFN-γ and calmodulin–MLCK dependent. In contrast, for the low dose of SLIGRL, the increase in paracellular permeability depends on calmodulin–MLCK activation, but not on IFN-γ.
In previous studies, we have shown that the inflammatory reaction mediated by high doses of SLIGRL was inhibited by chronic pretreatment with either capsaicin, or by NK1 or CGRP receptor antagonist suggesting a neurogenic component of this inflammation (Cenac et al. 2003; Nguyen et al. 2003). While the inflammatory response was inhibited by such treatments, a residual increase of paracellular permeability was still observed and was of the same magnitude as the increase observed for a low dose of SLIGRL (Cenac et al. 2003). The calmodulin–MLCK complex, as well as inflammatory mediators such as IFN-γ are involved in SLIGRL-induced paracellular permeability increase during inflammation. Indeed, in B6 ifng−/− mice, the increase in permeability was similar for both doses of SLIGRL and prevented by ML-7. The high dose of SLIGRL did not increase MPO activity, damage score, or TNF-α and IL-10 mRNA in these IFN-γ-deficient mice.
The increased permeability response to PAR2 activation may not be linked to selective activation of PAR2 located at the apical site of epithelial cell membrane as in vitro, only when PAR2 agonist was added to the basolateral side of SCBN monolayers, permeability to FITC–dextran was increased. However, it is not known whether PAR2 presents the same distribution on apical and basal sides of epithelial cell membranes in vitro and in vivo. We hypothesize that the non-inflammatory permeability response to PAR2 activation is linked to the activation of a subset of receptors, with distinct intracellular pathways. Indeed, in human airway smooth muscle cells, PAR2 activation stimulates phospholipase C (PLC) via its coupled Gq protein (Berger et al. 2001). The activation of the PLC-dependent signalling pathway has been implicated in the assembly, regulation and barrier properties of the tight junction (Balda et al. 1991). In fact activation of PLC releases IP3 into the cytosol, which increases intracellular Ca2+ concentration, which activates MLCK through the activation of calmodulin-dependent kinases (Berridge et al. 1983; Streb et al. 1983). MLCK phosphorylates MLC and thereby induces contraction of the perijunctional actin–myosin ring, which has been reported to provoke the opening of tight junctions (Yamaguchi et al. 1991). The increase in paracellular permeability caused by ethanol (Ma et al. 1999) and medium chain fatty acids, such as capric and lauric acid (Lindmark et al. 1998), as well as that caused by microbes (Scott et al. 2002) are attributed to this mechanism. Ethanol caused disassembly and displacement of perijunctional actin–myosin ring and stimulates MLCK (Ma et al. 1999). The inhibitor of MLCK (ML-7) attenuated both the ethanol-mediated increase in paracellular permeability and MLCK activity (Ma et al. 1999). Similarly, chelation of cytosolic Ca2+ and inhibition of MLCK attenuated the ability of capric and lauric acid to increase paracellular permeability (Lindmark et al. 1998). In our study we show that the activation of PAR2 increases the interaction of calmodulin and MLCK probably by an increase of intracellular Ca2+ concentration. This hypothesis is further supported by the fact that in many cell types, PAR2 activation causes intracellular calcium mobilization. This complex favours MLC phosphorylation and perijunctional actin–myosin ring contraction leading to alteration of paracellular permeability.
At a higher dose, PAR2-AP may be absorbed, and subsequently activates receptors located at the baso-lateral site of epithelial cells or other cell types such as immunocytes or nerves, inducing a neurogenic inflammation (Cenac et al. 2003; Nguyen et al. 2003). In agreement with this hypothesis, capsaicin inhibits the inflammatory reaction and diminishes the increase in permeability (Cenac et al. 2003). Results obtained in IFN-γ-deficient mice confirmed the crucial role of IFN-γ since no inflammation was seen even with a high dose of PAR2-AP in these animals. Disruption of the epithelial barrier in the gut is a hallmark of inflammatory bowel disease (IBD) and intestinal infections (Gitter et al. 2001). Although it remains unclear whether barrier breakdown is an initiating event or a consequence of inflammation, it is obvious that barrier loss contributes to propagation and exacerbation of inflammation (Gassler et al. 2001). Barrier breakdown can be elicited by a number of agents, including bacteria, immunocytes, and proinflammatory cytokines. Previous studies have demonstrated that T84 cells exposed to IFN-γ show decreased barrier function integrity (Youakim & Ahdieh, 1999). IFN-γ is greatly elevated in human intestinal disease and undoubtedly contributes to the inflammatory cascade, which includes barrier disruption. In fact, IFN-γ acts on at least two elements critical to barrier function. The first is to cause a decrease in the expression of ZO-1, a key component of the tight junction (Youakim & Ahdieh, 1999). The second is to alter the organization of the actin cytoskeleton in the apical region of the cells (Youakim & Ahdieh, 1999). TNF-α can enhance these effects, probably due to IFN-γ-dependent increase in TNF receptor gene expression suggesting that, in IBD, TNF-α may have synergistic effects on IFN-γ-mediated alterations of epithelial cell function (Rodriguez et al. 1995). In a recent study carried out on 23 patients with active Crohn's disease, it was demonstrated that treatment with TNF antibodies largely restores the gut barrier (Suenaert et al. 2002). Recent clinical trials provided evidence for a therapeutic effect of IL-10 in patients with Crohn's disease by reducing intestinal inflammation (Schmit et al. 2002). One potential explanation for the observed inhibition of IFN-γ effects on barrier function by IL-10 could be that IL-10 down-regulates receptors for IFN-γ (Oshima et al. 2001). A beneficial effect of IL-10 is its ability to block the reduced expression of occludin induced by IFN-γ (Oshima et al. 2001).
PAR2 activation at an ‘inflammatory dose’ of SLIGRL was associated with an increase in TNF-α, IFN-γ and IL-1β expression and a decrease in IL-10 expression. Thus, we can hypothesize that the Th1 profile mediated by PAR2 activation during the inflammatory reaction increased paracellular permeability by a combined action of IFN-γ and TNF-α on the expression of ZO-1 and on the organization of the actin cytoskeleton in the apical region. This hypothesis is supported by the reduction of paracellular permeability increase in mice deficient for IFN-γ.
In summary, in non-inflammatory conditions, PAR2 activation increases paracellular permeability through calmodulin activation, which can bind to and activate MLCK, and then provokes tight junction opening by perijunctional ring myosin phosphorylation. In inflammatory conditions increased paracellular permeability is mediated by the pathway described above and by an IFNγ-dependent pathway.
Acknowledgments
The authors thank B. Joseph for his technical assistance and Institut National de la Recherche Agronomique for its financial support.
References
- Balda MS, Gonzalez-Mariscal L, Contreras RG, Macias-Silva M, Torres-Marquez ME, Garcia-Sainz JA, Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- Berger P, Tunon-De-Lara JM, Savineau JP, Marthan R. Selected contribution: tryptase-induced PAR-2-mediated Ca2+ signaling in human airway smooth muscle cells. J Appl Physiol. 2001;91:995–1003. doi: 10.1152/jappl.2001.91.2.995. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Dawson RM, Downes CP, Heslop JP, Irvine RF. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Cenac N, Coelho AM, Nguyen C, Compton S, Andrade-Gordon P, MacNaughton WK, Wallace JL, Hollenberg MD, Bunnett NW, Garcia-Villar R, Bueno L, Vergnolle N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Garcia-Villar R, Ferrier L, Larauche M, Vergnolle N, Bunnett NW, Coelho AM, Fioramonti J, Bueno L. Proteinase-activated receptor-2-induced colonic inflammation in mice: possible involvement of afferent neurons, nitric oxide, and paracellular permeability. J Immunol. 2003;170:4296–4300. doi: 10.4049/jimmunol.170.8.4296. [DOI] [PubMed] [Google Scholar]
- Chin AC, Teoh DA, Scott KG, Meddings JB, Macnaughton WK, Buret AG. Strain-dependent induction of enterocyte apoptosis by Giardia lamblia disrupts epithelial barrier function in a caspase-3-dependent manner. Infect Immun. 2002;70:3673–3680. doi: 10.1128/IAI.70.7.3673-3680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795–804. doi: 10.1016/s0016-5085(03)01057-6. [DOI] [PubMed] [Google Scholar]
- Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–1328. doi: 10.1053/gast.2001.29694. [DOI] [PubMed] [Google Scholar]
- Jinguji Y, Ishikawa H. Electron microscopic observations on the maintenance of the tight junction during cell division in the epithelium of the mouse small intestine. Cell Struct Funct. 1992;17:27–37. doi: 10.1247/csf.17.27. [DOI] [PubMed] [Google Scholar]
- Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoux P, Minty C, Delpech B, Minty AJ, Shire D. Simultaneous quantitation of cytokine mRNAs in interleukin-1 beta stimulated U373 human astrocytoma cells by a polymerisation chain reaction method involving co-amplification with an internal multi-specific control. Eur Cytokine Netw. 1992;3:553–563. [PubMed] [Google Scholar]
- Lindmark T, Schipper N, Lazorova L, de Boer AG, Artursson P. Absorption enhancement in intestinal epithelial Caco-2 monolayers by sodium caprate: assessment of molecular weight dependence and demonstration of transport routes. J Drug Target. 1998;5:215–223. doi: 10.3109/10611869808995876. [DOI] [PubMed] [Google Scholar]
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- Mahraoui L, Heyman M, Plique O, Droy-Lefaix MT, Desjeux JF. Apical effect of diosmectite on damage to the intestinal barrier induced by basal tumour necrosis factor-alpha. Gut. 1997;40:339–343. doi: 10.1136/gut.40.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Coelho AM, Grady E, Compton SJ, Wallace JL, Hollenberg MD, Cenac N, Garcia-Villar R, Bueno L, Steinhoff M, Bunnett NW, Vergnolle N. Colitis induced by proteinase-activated receptor-2 agonists is mediated by a neurogenic mechanism. Can J Physiol Pharmacol. 2003;81:920–927. doi: 10.1139/y03-080. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Larsson AK, Aberg H, Sundelin J. The mouse proteinase-activated receptor-2 cDNA and gene. Molecular cloning and functional expression. J Biol Chem. 1995;270:5950–5955. doi: 10.1074/jbc.270.11.5950. [DOI] [PubMed] [Google Scholar]
- Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S, Granger DN, Joh T, Alexander JS. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- Pang G, Buret A, O'Loughlin E, Smith A, Batey R, Clancy R. Immunologic, functional, and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology. 1996;111:8–18. doi: 10.1053/gast.1996.v111.pm8698229. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Heyman M, Candalh C, Blaton MA, Bouchaud C. Tumour necrosis factor-alpha induces morphological and functional alterations of intestinal HT29 cl. 19A cell monolayers. Cytokine. 1995;7:441–448. doi: 10.1006/cyto.1995.0060. [DOI] [PubMed] [Google Scholar]
- Schmit A, Carol M, Robert F, Bontems P, Houben JJ, Van Gossum A, Goldman M, Mascart F. Dose-effect of interleukin-10 and its immunoregulatory role in Crohn's disease. Eur Cytokine Netw. 2002;13:298–305. [PubMed] [Google Scholar]
- Scott KG, Meddings JB, Kirk DR, Lees-Miller SP, Buret AG. Intestinal infection with Giardia spp. reduces epithelial barrier function in a myosin light chain kinase-dependent fashion. Gastroenterology. 2002;123:1179–1190. doi: 10.1053/gast.2002.36002. [DOI] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–C1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Dalle-Molle E, Hardison WG. Vasopressin and A23187 stimulate phosphorylation of myosin light chain-1 in isolated rat hepatocytes. Am J Physiol. 1991;261:G312–G319. doi: 10.1152/ajpgi.1991.261.2.G312. [DOI] [PubMed] [Google Scholar]
- Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- Zou W, Durand-Gasselin I, Dulioust A, Maillot MC, Galanaud P, Emilie D. Quantification of cytokine gene expression by competitive PCR using a colorimetric assay. Eur Cytokine Netw. 1995;6:257–264. [PubMed] [Google Scholar]