Abstract
We have shown recently that development from neonatal to adult life affects cerebrovascular tone of mouse cerebral arteries through endothelium-derived vasodilatory mechanisms. The current study tested the hypothesis that development from fetal to adult life affects cerebral artery vascular smooth muscle (VSM) [Ca2+]i sensitivity and tone through a mechanism partially dependent upon endothelium-dependent signalling. In pressurized resistance sized cerebral arteries (∼150 μm) from preterm (95 ± 2 days gestation (95 d)) and near-term (140 ± 2 days gestation (140 d)) fetuses, and non-pregnant adults, we measured vascular diameter (μm) and [Ca2+]i (nm) as a function of intravascular pressure. We repeated these studies in the presence of inhibition of nitric oxide synthase (NOS; with l-NAME), cyclo-oxygenase (COX; with indomethacin) and endothelium removal (E–). Cerebrovasculature tone (E+) was greater in arteries from 95 d fetuses and adults compared to 140 d sheep. Ca2+ sensitivity was similar in 95 d fetuses and adults, but much lower in 140 d fetuses. Removal of endothelium resulted in a reduction in lumen diameter as a function of pressure (greater tone) in all treatment groups. [Ca2+]i sensitivity differences among groups were magnified after E–. NOS inhibition decreased diameter as a function of pressure in each age group, with a significant increase in [Ca2+]i to pressure ratio only in the 140 d fetuses. Indomethacin increased tone and increased [Ca2+]i in the 140 d fetuses, but not the other age groups. Development from near-term to adulthood uncovered an interaction between NOS- and COX-sensitive substances that functioned to modulate artery diameter but not [Ca2+]i. This study suggests that development is associated with significant alterations in cerebral vascular smooth muscle (VSM), endothelium, NOS and COX responses to intravascular pressure. We speculate that these changes have important implications in the regulation of cerebral blood flow in the developing organism.
In the adult cerebral circulation the arteries effectively maintain vascular resistance and autoregulate cerebral blood flow (CBF) over a wide range of pressures (60–180 mmHg) (Lassen, 1959). Compared to the adult, control of CBF in the fetal lamb is over a narrower range of systemic pressures (45–80 mmHg). Moreover, the fetal brain is uniquely susceptible to ischaemic insults because the lower limit of cerebral autoregulation (∼45 mmHg) is nearly identical to normal systemic pressure (44–50 mmHg) of the near-term fetus (Harris et al. 1989; Kitanaka et al. 1989; Helou et al. 1994; Longo & Pearce, 1998).
Compression of the fetal head during normal birth increases intracranial pressure (ICP) to values that approach or exceed cerebral perfusion pressure (Mann et al. 1972; Harris et al. 1989). Elevated ICP activates autoregulatory mechanisms, which reduce cerebral vascular resistance (CVR) and maintain CBF in near-term (∼140 days gestation) but not 0.7 gestation (90–92 days gestation) fetal sheep (Harris et al. 1989). Although CBF autoregulation has developed near-term, the pressure-sensitive mechanisms have yet to be investigated.
To a great extent autoregulation of CBF occurs as a result of pressure-sensitive mechanisms in the cerebral vascular smooth muscle (VSM) cells (Kontos et al. 1978; Harper et al. 1984; Faraci & Heistad, 1990; Davis & Hill, 1999). Myogenic tone is the process by which VSM alters force production in response to transmural pressure in order to maintain appropriate vessel diameter, and thus contribute to autoregulation (Kontos et al. 1978; Davis & Hill, 1999; Osol et al. 2002). This process is thought to involve several mechanisms within VSM including: stretch-activated Ca2+ channels, K+ channels, membrane potential, the actin cytoskeleton, and intracellular signalling via several protein kinases (Osol, 1995; Davis & Hill, 1999).
Numerous studies from our laboratory have shown that fundamental mechanisms of pharmaco-mechanical and electro-mechanical coupling operate very differently in the developing fetus and newborn than in the adult (Akopov et al. 1998; Long et al. 1999, 2002; Geary & Buchholz, 2003; Geary et al. 2003; Lin et al. 2003). In addition, we have recently reported that arteries from neonatal mice respond differently to pressure compared to the adult response (Geary et al. 2003). Our study showed that an immature endothelium elevated vascular response to pressure in neonatal mice. The question thus arises: to what extent does developmental age affect tone and [Ca2+]i in isolated, pressurized ovine cerebral arteries?
The current study tested the hypothesis that vascular smooth muscle [Ca2+]i sensitivity and tone increase with developmental age. The effect of development on [Ca2+]i sensitivity and vascular tone depend in part, on greater endothelium-dependent signalling. To test this hypothesis, resistance sized cerebral arteries from preterm (∼95 days gestation) and near-term (∼140 days gestation) fetuses and non-pregnant adult (18–24 month) were isolated, loaded with fura-2 AM, and subjected to increasing transmural pressures. Diameter and [Ca2+]i responses to pressure were determined in both endothelium-intact and denuded arteries, and following inhibition of nitric oxide synthase (NOS) and/or cyclo-oxygenase (COX).
Methods
General preparation
All surgical and experimental procedures were performed within the regulations of the Animal Welfare Act, and striclty followed guidelines in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and The Guiding Principles in the Care and Use of Animals approved by the Council of the American Physiological Society, and governed by the Animal Care and Use Committee of Loma Linda University. Resistance-sized middle cerebral arteries (MCA; ∼150 μm) were obtained from preterm fetuses (95 ± 2 days gestation, n = 14), near-term fetuses (140 ± 2 days gestation, n = 12), and non-pregnant adult sheep (ages 18–24 month, n = 12) obtained from Nebeker Ranch (Lancaster, CA, USA), as previously described (Longo et al. 1996). The ewes were anaesthetized and killed with 100 mg kg−1 intravenous sodium pentobarbital. We have shown that this method of kill has no significant effect on vessel reactivity, as compared to use of other anaesthetic agents (Pearce et al. 1991). Fetal and adult brains were rapidly removed and placed in cold physiological salt solution (PSS) containing (mm): 130 NaCl, 10.0 Hepes, 6.0 glucose, 4.0 KCl, 4.0 NaHCO3, 1.8 CaCl2, 1.18 KH2PO4, 1.2 MgSO4, and 0.025 EDTA. pH was adjusted to 7.4 using 1 n NaOH. Middle cerebral artery segments (∼150 μm maximum diameter) were dissected and cannulated in an organ chamber (Living Systems, Burlington, VT, USA), followed by placement on the stage of an inverted microscope.
The proximal cannula was connected to a pressure transducer and reservoir of PSS, the pressure of which was controlled by a servo system used to set transmural pressures. The distal cannula was connected to a luer-lock valve that was open to flush the lumen during the initial cannulation. After cannulation, the valve was closed and we conducted all measurements under no-flow conditions. Arterial diameter was recorded using the SoftEdge Acquisition Subsystem (IonOptix Milton, MA, USA).
Endothelium removal
We removed the endothelium by rubbing the artery lumen over a glass cannula. Successful removal of the endothelium was verified by the absence of a vasodilator response to 10 μm ADP, an endothelium-dependent vasodilator in this preparation (Long et al. 1999, 2000, 2002).
Measurement of smooth muscle Ca2+ in pressurized arteries
To obtain measurements of [Ca2+]i in vascular smooth muscle cells, cannulated arteries were loaded with fura-2 acetoxymethyl ester (AM), a Ca2+-sensitive fluorescent dye. Fura-2 (10 μl), from 1 mm stock dissolved in dimethyl sulfoxide (DMSO), was premixed with 1% pluronic acid (10% solution in H2O) and then diluted in physiological salt solution (PSS) to yield a final fura-2 AM concentration of 1 μm. The cannulated middle cerebral artery segment was incubated in the fura 2 AM–PSS loading solution at room temperature in the dark for 20 min. Fura-2-loaded arteries were then washed with PSS and bath temperature was increased to 38°C. Fura-2 fluorescence was measured using a photomultiplier system (IonOptix) in which background-corrected ratios (collected once every 10 s) were subtracted from the 510 nm emission from arteries alternatively excited at 340 and 380 nm (fura-2 ratio). The sampling rate was 3 Hz. The 380 nm signals among artery groups were similar, suggesting that fura-2 loading was not affected by development (Long et al. 2000). The experimental protocol started after a 60 min equilibration period at 30 mmHg.
Experimental protocols
In all protocols, artery diameter (μm) and smooth muscle Ca2+ (nm) were measured in response to a series of 10 mmHg pressure steps. Diameter responses to step changes in pressure were highly reproducible and fully reversible in both adult and fetal arteries. Where indicated, NG-nitro-l-arginine-methyl ester (l-NAME), indomethacin, and EGTA were added to the organ chamber 20 min before commencing a series of pressure steps, and each pressure was maintained for 5–10 min to allow vessel diameter to stabilize before measurement. All drugs were added, individually or in combination, to the organ chamber in their final optimally effective concentrations (Geary et al. 2003). We conducted three separate protocols.
Protocol 1
This protocol was designed to determine pressure–diameter and pressure–[Ca2+]i relationships in the absence or presence of nitric oxide synthase inhibitors in endothelium-intact arteries from both fetal and adult sheep. As previously described (Geary et al. 2003), the first series of pressure steps was completed in PSS, and a second series was completed in the presence of l-NAME (100 μm). To assess the combined effect of NOS and cyclo-oxygenase inhibition, a third series of pressure steps was completed in the presence of 100 μml-NAME plus 10 μm indomethacin (Indo). To determine the maximum passive diameter and minimum [Ca2+]i a final pressure–diameter and pressure–[Ca2+]i relationship was conducted in Ca2+-free [Ca2+]o PSS with 3 mm EGTA.
Protocol 2
This protocol was designed to determine pressure–diameter and pressure−[Ca2+]i relationships in the absence and presence of cyclo-oxygenase inhibitors in endothelium-intact arteries from both fetal and adult sheep. As described for Protocol 1, the first series of pressure steps was completed in PSS, after which a second series was completed in the presence of 10 μm indomethacin. To assess the combined effect of NOS and cyclo-oxygenase inhibition, a third series of pressure steps was completed in the presence of 10 μm Indo plus 100 μml-NAME. Final pressure–diameter and pressure–[Ca2+]i relationships were plotted in Ca2+-free PSS with EGTA (3 mm) to determine the maximum passive diameter and minimum [Ca2+]i.
Protocol 3
This protocol determined the pressure–diameter and pressure−[Ca2+]i relationships in the absence of the endothelium, and thus assessed cerebral artery smooth muscle tone in fetal and adult sheep. Endothelium-denuded arteries from both fetal and adult sheep were exposed to 100 μml-NAME plus 10 μm Indo throughout these experiments. Following development of a stable diameter, a series of pressure steps were completed to determine both the pressure–diameter and pressure−[Ca2+]i relationships. After this run, a second series of pressure steps were completed in Ca2+-free PSS with EGTA (3 mm) to determine the maximum passive diameter and minimum [Ca2+]i.
Chemicals
Pluronic acid and fura-2 AM were purchased from Molecular Probes (Eugene, OR, USA). All other drugs were purchased from Sigma Chemical (St Louis, MO, USA) and were added, individually or in combination, to the organ chamber in their final optimally effective concentrations. Stock indomethacin (50 mm) was dissolved in Na2CO3 (0.1 m).
Data analysis and statistics
Percentage tone was determined in endothelium-intact (or denuded) arteries by subtracting the diameter (±ENDO) at any given pressure from the maximum passive diameter (zero calcium with 3 mm EGTA), dividing the difference by the maximum passive diameter and multiplying by 100. Percentage [Ca2+]i in response to increasing pressure was determined in endothelium-intact (or denuded) arteries by subtracting [Ca2+]i (±ENDO) at any given pressure from minimum [Ca2+]i (in zero Ca2+ with 3 mm EGTA), dividing the difference by minimum [Ca2+]i and multiplying by 100.
Arterial wall [Ca2+]i was calculated as previously described (Osol et al. 2002) using the following equation (Grynkiewicz et al. 1985): [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where R is the experimentally measured ratio (340 nm : 380 nm) of fluorescence intensities, Rmin is the ratio in the absence of [Ca2+]i, Rmax is the ratio at Ca2+-saturated fura-2 conditions, and Kd is the dissociation constant of fura-2. β is a ratio of the fluorescence intensities at 380 nm excitation wavelength at Rmin and Rmax.
The contractile effects of l-NAME or Indo were determined by subtracting artery diameter after drug treatment from artery diameter in PSS at any given pressure. To determine whether development affects an interaction between NOS- and COX-sensitive substances, artery diameters in the presence of Indo +l-NAME were subtracted from artery diameters in Indo alone. Similarly, artery diameters in the presence of l-NAME + Indo were subtracted from artery diameters in l-NAME.
Data are expressed as means ± s.e.m. Statistical significance was determined using ANOVA with Scheffe's test for post hoc comparisons. Statistical significance implies P < 0.05, unless otherwise stated.
Results
Effects of pressure on diameter of endothelium-intact arteries
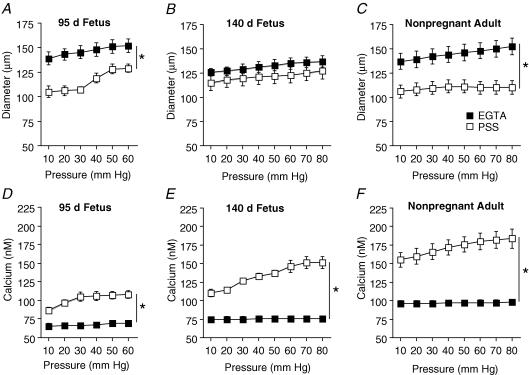
In the absence of extracellular Ca2+ (+ EGTA, 3 mm) in the bath solution (Passive response, Fig. 1A–C), maximal passive diameters of arteries from preterm (152 ± 5 μm, 60 mmHg), near-term (137 ± 6 μm, 80 mmHg), and adult (152 ± 8 μm, 80 mmHg) sheep were not significantly different (P > 0.05, ANOVA). In Ca2+-containing PSS, diameters of endothelium-intact arteries from preterm fetal and adult sheep were significantly smaller than their passive responses (Fig. 1A and C). In contrast, the responses of near-term fetal sheep were not significantly different than their passive responses (10–80 mmHg, Fig. 1B).
Figure 1. Effect of pressure on diameter and [Ca2+]i in endothelium-intact arteries from fetal and adult sheep.
Changes in diameter (A–C) and intracellular Ca2+ (D–F) of endothelium-intact arteries in physiological salt solution (PSS; □) compared to responses in [Ca2+]o plus 3 mm EGTA (▪) from 95 days gestation fetal (A and D, n = 14), 140 days gestation fetal (B and E; n = 12), and non-pregnant adult (C and F; n = 12) sheep. In all experiments, arteries were exposed to EGTA solution for 20 min before determining [Ca2+]o plus 3 mm EGTA responses to pressure. Values are means ± s.e.m. *P < 0.05 compared to respective [Ca2+]o plus 3 mm EGTA response, by ANOVA.
While diameters of arteries from preterm fetal sheep were significantly smaller than their passive responses (10–60 mmHg, Fig. 1A), these arteries began to distend (forced dilatation) at pressures greater than 30 mmHg. At pressures greater than 60 mmHg (70–80 mmHg) diameters were no longer significantly different from their passive responses (Data not shown).
Effects of pressure on [Ca2+]i in endothelium-intact arteries
In the absence of extracellular Ca2+ (+ EGTA, 3 mm in the bath solution), the intracellular Ca2+ concentration did not change with increasing pressure in the arteries from either fetal or adult sheep (Fig. 1D–F). Minimum [Ca2+]i values (10 mmHg) in the absence of extracellular Ca2+ were significantly greater in arteries from the adult (adult 97 ± 3 nm), as compared to either group of fetal sheep (preterm, 69 ± 3 nm; near-term, 75 ± 4 nm) (P < 0.05, ANOVA). In Ca2+-containing PSS, [Ca2+]i responses to pressure were significantly greater than those responses in the absence of extracellular Ca2+ in all groups of endothelium-intact sheep arteries.
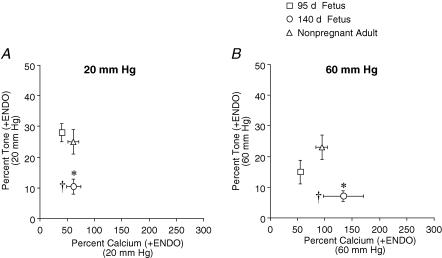
Effects of development on [Ca2+]i sensitivity in endothelium-intact arteries
To determine the effect of development on [Ca2+]i sensitivity, we calculated the tone−[Ca2+]i relationship among groups of endothelium-intact arteries. Arteries from near-term fetal sheep developed significantly less tone ((EGTA diameter – PSS diameter)/EGTA diameter) than arteries from either preterm or adult sheep at either 20 or 60 mmHg (Fig. 2A and B). In addition, the percentage [Ca2+]i (([Ca2+]i PSS – [Ca2+]i EGTA)/[Ca2+]i EGTA) was significantly greater in arteries from near-term fetal sheep compared to arteries from preterm sheep (Fig. 2B). The [Ca2+]i sensitivity (tone−[Ca2+]i relationship) of the contractile apparatus developed a biphasic pattern, decreasing in the near-term fetus and increasing in adulthood.
Figure 2. Effect of development on [Ca2+]i sensitivity in endothelium-intact arteries.
Percentage tone and calcium relationships were determined in endothelium-intact (+ENDO) cerebral arteries from preterm fetal (95 days gestation), near-term fetal (140 days gestation), and adult (non-pregnant) sheep at 20 mmHg (A) and 60 mmHg (B). *P < 0.05 compared to other groups, †P < 0.05 compared to preterm fetal sheep (95 days gestation), determined by repeated-measures ANOVA.
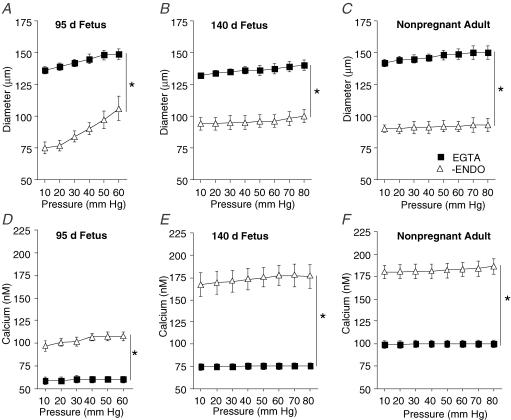
Effects of pressure on diameter in endothelium-denuded arteries
To determine the function of the endothelium in response to pressure, and whether this response was affected by development, we assessed the effect of pressure on artery diameter following disruption of the endothelium. To verify efficient endothelium denudation, we determined responses to the endothelium-dependent dilator adenosine 5′ diphosphate (ADP; 10 μm). In endothelium-intact arteries, the magnitudes of vasodilatation to ADP were similar among arteries from preterm (56 ± 14%, n = 7) and near-term (64 ± 9%, n = 7) and adult (63 ± 25, n = 7) sheep (data not shown). Following endothelium removal, ADP did not produce a significant dilatation in any of the three groups.
In the absence of extracellular Ca2+ in the bath solution (Passive response, Fig. 3A–C), maximal passive diameters of arteries from preterm (149 ± 4 μm, 60 mmHg), near-term (140 ± 3 μm, 80 mmHg), and adult (150 ± 5 μm, 80 mmHg) were not significantly different (P > 0.05, ANOVA). In Ca2+-containing PSS, diameters of endothelium-denuded arteries from each of the groups were significantly smaller than their passive responses (Fig. 3A–C).
Figure 3. Effect of pressure on diameter and Ca2+ in endothelium-denuded arteries from fetal and adult sheep.
Changes in diameter (A–C) and intracellular Ca2+ (D–F) of endothelium-denuded arteries in physiological salt solution (PSS; Δ) compared to responses in [Ca2+]o plus 3 mm EGTA (▪) from 95 days gestation preterm fetal (A and D, n = 14), 140 days gestation near-term fetal (B and E; n = 12), and non-pregnant adult (C and F; n = 12) sheep. In all experiments, arteries were exposed to EGTA solution for 20 min before determining [Ca2+]o plus 3 mm EGTA responses to pressure. Values are means ± s.e.m. *P < 0.05 compared to respective [Ca2+]o plus 3 mm EGTA response, by ANOVA.
In preterm fetal sheep, endothelium-denuded arteries began to distend (forced dilatation) at approximately 30 mmHg. At pressures greater than 60 mmHg (70–80 mmHg) the diameters of endothelium-denuded arteries were no longer significantly different from their passive responses (Data not shown).
Effects of pressure on [Ca2+]i in endothelium-denuded arteries
In the absence of extracellular Ca2+ (+ EGTA, 3 mm) in the bath solution, [Ca2+]i did not change with increasing pressure (Fig. 3D–F). Minimum [Ca2+]i values were significantly different among groups of arteries (preterm, 61 ± 3 nm; near-term, 75 ± 3 nm, adult 102 ± 6 nm) (P < 0.05, ANOVA). In Ca2+-containing PSS, [Ca2+]i responses to pressure were significantly greater in all groups of endothelium-denuded sheep arteries compared to responses in the absence of extracellular Ca2+ (Fig. 3D–F).
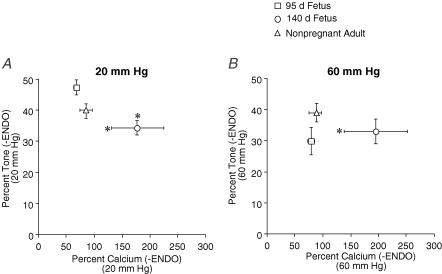
Effects of development on [Ca2+]i sensitivity in endothelium-denuded arteries
To determine the effect of development on [Ca2+]i sensitivity in endothelium-denuded arteries, we recalculated the tone−[Ca2+]i relationship. At 20 mmHg, percentage tone was significantly less in arteries from near-term fetal sheep compared to arteries from preterm and adult sheep (Fig. 4A). In addition, percentage [Ca2+]i was significantly greater in endothelium-denuded arteries from near-term fetal sheep compared to arteries from preterm and adult sheep (Fig. 4A and B). In endothelium-denuded arteries, [Ca2+]i sensitivity of the contractile apparatus developed a biphasic pattern, decreasing in the near-term fetus and increasing in adulthood.
Figure 4. Effect of development on [Ca2+]i sensitivity in endothelium-denuded arteries.
Percentage tone and calcium relationships were determined in endothelium-denuded (−ENDO) cerebral arteries from preterm fetal (95 days gestation), near-term fetal (140 days gestation), and adult (non-pregnant) sheep at 20 mmHg (A) and 60 mmHg (B). *P < 0.05 compared to other groups, determined by repeated-measures ANOVA.
Effects of nitric oxide synthase inhibition on diameter
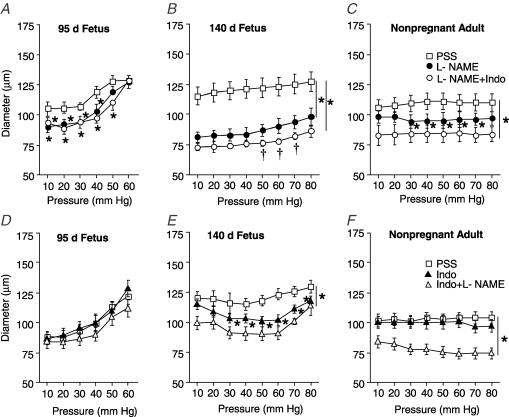
To determine whether development affects nitric oxide-dependent modulation of diameter, we measured diameter in response to pressure in the presence of the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine-methyl ester (l-NAME; 100 μm) (Fig. 5A–C). In arteries from near-term fetal sheep, treatment with l-NAME significantly reduced artery diameters at all pressures compared to responses in PSS (Fig. 5B). In contrast, the effects of l-NAME on diameter were significantly different from those of PSS between 10 and 40 mmHg in arteries from preterm sheep (Fig. 5A) and 30 and 70 mmHg in arteries from the adult (Fig. 5C). As shown in Fig. 6A, the contractile effects of l-NAME were significantly greater in arteries from the near-term fetus compared to the other groups.
Figure 5. Effect of pressure on diameter of endothelium-intact arteries from fetal and adult sheep during inhibition of endothelium-derived substances.
Changes in diameter of endothelium-intact arteries in physiological salt solution (PSS; □) compared to responses in NG.-nitro-L-arginine-methyl ester (l-NAME, •), indomethacin (▴), l-NAME + Indo (○), and Indo +l-NAME (Δ) from 95 days gestation fetal (A and D, n = 14), 140 days gestation fetal (B and E; n = 12), and non-pregnant adult (C and F; n = 12) sheep. In all experiments, arteries were exposed to drug solutions for 20 min before determining responses to pressure. Values are means ± s.e.m. *P < 0.05 compared to PSS response, †P < 0.05 compared to l-NAME response, by ANOVA.
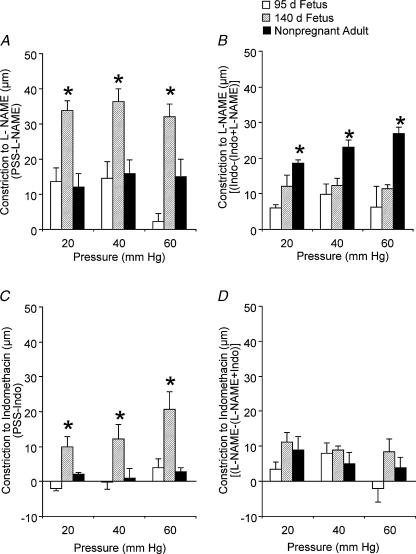
Figure 6. Contractile effects of nitric oxide synthase and cyclo-oxygenase inhibition in endothelium-intact arteries from fetal and adult sheep.
Contractile responses to NG-nitro-l-arginine-methyl ester (l-NAME, 100 μm) or l-NAME in the presence of indomethacin (Indo, 10 μm), are shown at 20, 40 and 60 mmHg in A and B, respectively. Contractile responses to Indo or Indo in the presence of l-NAME are shown at 20, 40 and 60 mmHg in C and D, respectively. Arteries were exposed to drug solutions for 20 min before determination of contractile responses. Values indicate means ± s.e.m., n = 12 − 14. *P < 0.05 compared to other groups, by ANOVA.
In arteries from near-term and adult sheep equilibrated with the cyclo-oxygenase (COX) inhibitor indomethacin (10 μm), the addition of 100 μml-NAME significantly reduced artery diameters compared to responses in PSS (Fig. 5E and F). In contrast, combined treatment with indomethacin plus l-NAME did not significantly affect artery diameters from preterm fetal sheep compared to responses in PSS (Fig. 5D). As determined by repeated measures ANOVA, pretreatment with indomethacin significantly enhanced the effect of l-NAME in arteries from the adult (P < 0.05) (Fig. 5C and F). As shown in Fig. 6B, in the presence of indomethacin, the contractile effects of l-NAME in arteries from the adult were significantly greater than the effects of l-NAME in arteries from the other groups (P < 0.05, ANOVA).
Effects of cyclo-oxygenase inhibition on diameter
To determine whether development affects prostaglandin-dependent modulation of vessel diameter, we measured changes in diameter in response to pressure in the presence of the COX inhibitor indomethacin (10 μm) (Fig. 5D–F). Compared to responses in PSS, indomethacin treatment significantly reduced the diameters of arteries from near-term fetal sheep between 30 and 80 mmHg (Fig. 5E). In contrast, indomethacin treatment did not significantly affect artery diameters from either preterm or adult sheep (Fig. 5D and F). As shown in Fig. 6C, the contractile effects of indomethacin were significantly greater in arteries from near-term fetal sheep, compared to the other groups.
In arteries from near-term and adult sheep equilibrated with the NOS inhibitor l-NAME (100 μm), the addition of indomethacin significantly reduced artery diameters compared to responses in PSS (Fig. 5B and C). Combined treatment with l-NAME plus indomethacin also significantly reduced artery diameters from preterm fetal sheep (10 and 50 mmHg) when compared to responses in PSS (Fig. 5A). In the presence of l-NAME, the contractile effects of indomethacin were not significantly different among groups (Fig. 6D).
Effects of NOS and COX inhibition on [Ca2+]i
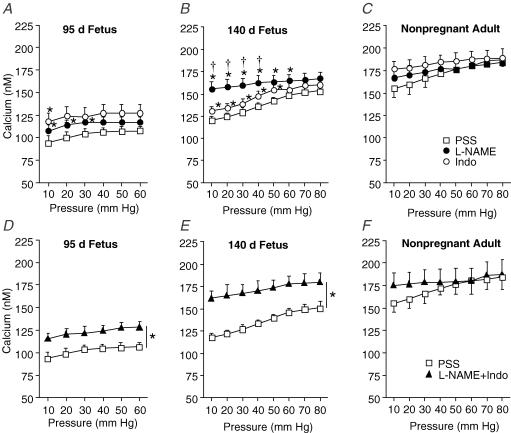
To determine whether development affects NO and prostaglandin-dependent modulation of [Ca2+]i, we measured changes in [Ca2+]i in response to pressure in the presence of (1) l-NAME (100 μm), (2) indomethacin (10 μm), or (3) l-NAME + indomethacin (Fig. 7D–F). Compared to responses in PSS, [Ca2+]i was significantly greater in arteries from preterm (10–30 mmHg) and near-term (10–40 mmHg) fetuses treated with l-NAME (Fig. 7A and B). The absolute magnitude of change in [Ca2+]i caused by l-NAME was significantly greater in arteries from near-term compared to preterm fetal sheep. For example, l-NAME treatment (20 mmHg) increased [Ca2+] by 17 ± 2 μm in arteries from preterm fetal sheep and by 51 ± 9 μm in arteries from near-term fetal sheep. [Ca2+]i was not affected by l-NAME treatment in arteries from the adult (Fig. 7C).
Figure 7. Effect of pressure on [Ca2+]i in endothelium-intact arteries from fetal and adult sheep during inhibition of endothelium-derived substances.
Intracellular Ca2+ concentration of endothelium-intact arteries in physiological salt solution (PSS; □) compared to responses in NG.-nitro-l-arginine-methyl ester (l-NAME, •), indomethacin (Indo, ○), and l-NAME + Indo (▴) from 95 days gestation fetal (A and D, n = 14), 140 days gestation fetal (B and E; n = 12), and non-pregnant adult (C and F; n = 12) sheep. In all experiments, arteries were exposed to drug solutions for 20 min before determining responses to pressure. Values are means ± s.e.m. *P < 0.05 compared to PSS response, †P < 0.05 compared to Indo response, by ANOVA.
Indomethacin treatment significantly increased [Ca2+]i in arteries from the near-term fetus compared to [Ca2+]i responses in PSS (10–50 mmHg, Fig. 7B). Except for an effect of indomethacin at 10 mmHg (preterm fetus), indomethacin did not significantly affect [Ca2+]i in arteries from the preterm fetus or adult (Fig. 7A and C).
Combined treatment with l-NAME plus indomethacin (or indomethacin plus l-NAME, data not shown) significantly increased [Ca2+]i in arteries from both the preterm and near-term fetus when compared to responses in PSS (Fig. 7D and E). The absolute magnitude of the effect of combined l-NAME plus indomethacin on [Ca2+]i was significantly greater in arteries from near-term compared to preterm fetuses (P < 0.05, ANOVA). l-NAME plus indomethacin treatment did not significantly affect [Ca2+]i in arteries from adult sheep (Fig. 7F).
Discussion
The principal findings of this study were that: (1) cerebral artery diameter and intracellular calcium responses to pressure are less in intact vessels from near-term fetuses (140 d) compared to preterm fetuses (95 d) and adult sheep; (2) removal of the endothelium magnified the differences in calcium sensitivity between the near-term fetus and the other age groups; (3) compared to the preterm fetus, arteries from near-term fetuses possessed greater NOS- and COX-sensitive regulation of tone and [Ca2+]i; and (4) arteries from the fetuses underwent forced dilatation at lower pressures than did those from adults, paralleling changes in systemic pressure, which averages 80 mmHg in adults, but only 40–50 mmHg in the fetus (Szymonowicz et al. 1990; Helou et al. 1994; Longo & Pearce, 1998). Together, these data demonstrate developmental differences in myogenic properties, and in the extent and nature of the endothelial influence present as a function of transmural pressure.
Although little is known about cerebral artery responses to pressure in immature or preterm animals compared to adults, it is noteworthy that changes in cerebral autoregulatory capacity have been demonstrated in preterm sheep (Müller et al. 2002). The existence of cerebral autoregulation in utero, and of changes in its pressure dependency are consistent with the active myogenic and endothelial mechanisms observed in this study using isolated in vitro vessels. It is also worth noting that the current studies were carried out in the absence of flow, thereby eliminating a potentially important influence of shear stress on endothelial metabolic and secretory activity.
Only one earlier study (Bevan et al. 1999) has investigated vascular responses to pressure in isolated fetal vessels. In that study, also carried out under no-flow conditions, cerebral arteries (200–500 μm) from preterm (24–37 weeks gestation) human fetuses exhibited a degree of vascular tone that was similar to that observed in preterm (95 d) vessels from sheep. Unfortunately, it is not possible to directly infer the data from near-term sheep, as vascular responses to pressure near the time of birth were not examined by Bevan and coworkers.
Differences in the pressure range and vascular tone among vessels from sheep of different ages might be explained by differences in the development of the endothelium. Numerous studies have shown that the endothelium modulates vascular responses to pressure in arteries from adult animals in general (reviewed by Davis & Hill, 1999), and in cerebral arteries specifically (Geary et al. 2001, 2003; Osol et al. 2002). In the current study, removal of the endothelium enhanced vascular responses to pressure in each age group and, at lower pressures (10–30 mmHg), vascular tone was significantly greater in endothelium-denuded vessels from preterm fetuses compared to either the near-term fetuses or adult sheep.At transmural pressures above 30 mmHg, vascular tone was no longer significantly different between groups. Two important findings derived from this series of experiments. First, vascular responses to pressure were not significantly different between endothelium-denuded arteries from near-term fetuses and adult sheep. Second, endothelium-dependent modulation of cerebrovascular tone increased as a function of fetal developmental age.
Our finding that vascular responses to pressure were similar between endothelium-denuded arteries from near-term fetuses and adult sheep contrast with developmental studies measuring isometric tension (Pearce et al. 1991; Long et al. 2002; Teng et al. 2002). For example, maximal potassium-induced tensions were slightly, but significantly, less in the middle cerebral arteries of fetal compared with adult sheep (Pearce et al. 1991). While this study showed a developmental difference in response to K+ depolarization, it also concluded that smaller, more distal arteries were functionally more mature at term than their larger, more proximal counterparts (Pearce et al. 1991). Unfortunately, major differences between experimental approaches (wire versus pressurized) (Dunn et al. 1994; VanBavel & Mulvany, 1994), contractile stimulus (K+ depolarization versus pressure) (Davis & Hill, 1999; Hill et al. 2001), and vessel diameter (∼500 μm versus 150 μm) (Kontos et al. 1978; Faraci & Heistad, 1990) preclude a detailed comparison between the current study and most developmental studies published previously (Pearce et al. 1991; VanBavel & Mulvany, 1994; Akopov et al. 1997; Long et al. 2002).
The current study also demonstrated that pressure-induced responses were dependent upon intracellular calcium concentration, and associated with calcium elevation within the arterial wall as a function of pressure. The data also showed differences in calcium sensitivity as a function of age, an effect that was determined by examining the relationship between changes in calcium and tone.
Intracellular Ca2+ responses to pressure have been examined in isolated arteries from a number of vascular beds (Davis & Hill, 1999; Hill et al. 2001). These studies have consistently shown that both [Ca2+]i and vascular tone increase with elevation of transmural pressure. Our current findings confirmed that [Ca2+]i increased in response to pressure in endothelium-intact arteries from fetal and adult sheep. Removal of extracellular Ca2+ from the superfusate produced a sharp reduction in [Ca2+]i and abolished fetal and adult cerebrovascular tone. These data strongly suggest that the maintenance of tone requires Ca2+ entry, most likely through voltage-operated and non-selective stretch-operated Ca2+ channels, as observed in adult tissues (Hill et al. 2001). As we have shown for the fetus, this also may involve the L-type Ca2+ channel (Long et al. 1999; Blood et al. 2002).
Normalizing [Ca2+]i concentration and diameter to the zero extracellular [Ca2+]o (+ EGTA) responses allowed us to determine the tone−Ca2+ relationship, i.e. [Ca2+]i sensitivity, in arteries from fetal and adult sheep. This comparison revealed that arteries from endothelium-intact near-term fetuses were significantly less sensitive to [Ca2+]i than those of preterm fetuses or adult sheep. Removal of the endothelium magnified the difference in the tone−Ca2+ relationship between arteries from near-term fetuses and the other groups.
While the current study is the first to report the tone−[Ca2+]i relationship in pressurized, cerebral arteries from fetal and adult sheep, others have used isometric techniques to determine whether development affects [Ca2+]i sensitivity. These studies found that [Ca2+]i sensitivity (pD2 values) of small intracranial arteries (< 500 μm) was not significantly different between arteries from near-term fetal versus adult sheep (Akopov et al. 1997; Long et al. 2000). Conversely, [Ca2+]i sensitivity increased significantly with development in response to noradrenaline (norepinephrine; Long et al. 2002). This difference is not surprising in view of earlier studies that documented a difference in agonist sensitivity and calcium handling in isometric versus isobaric preparations (Dunn et al. 1994; VanBavel & Mulvany, 1994). Furthermore, vessel reactivity to pressure varies with diameter, which can also confound interpretation among studies (Davis & Hill, 1999). In fact, when larger (250 μm) endothelium-intact and denuded cerebral arteries from fetal and adult sheep were used in similar experiments, the tone−[Ca2+]i relationship was unaffected by development (G. G. Geary; unpublished data). Collectively, these studies suggest that [Ca2+]i sensitivity of the sheep cerebral vasculature depends on both vessel diameter and developmental age.
[Ca2+]i responses to pressure (10–40 mmHg) were substantially greater in arteries from near-term fetuses compared to either of the other groups following endothelium denudation. To establish whether endothelium-derived NOS- and/or COX-sensitive substances were responsible for modulating the [Ca2+]i responses to pressure in these arteries, we measured [Ca2+]i in the presence of either l-NAME or indomethacin alone, or in combination. These experiments determined that NOS and COX inhibition elevated [Ca2+]i responses to pressure in arteries from fetal but not adult sheep. More importantly, development from preterm to near-term life significantly increased the effect of l-NAME on [Ca2+]i responses to pressure without affecting the responses to indomethacin. The larger [Ca2+]i responses to pressure in the presence of l-NAME correspond with a greater constrictor sensitivity to l-NAME in arteries from near-term compared to preterm fetuses. Together, our data suggest that development from preterm to near-term life is marked by increasing NO modulation of cerebrovascular tone through Ca2+-dependent mechanisms. Conversely, during postnatal life NO-dependent modulation of tone is via Ca2+-independent mechanisms.
The importance of the present study is underscored by studies showing that NO-releasing vasodilators appear to have greater effects in fetal than in adult cerebral arteries (Pearce et al. 1994). The expression of soluble guanylate cyclase is greater in fetal than adult ovine cerebral arteries (Nauli et al. 2001), which probably accounts for greater cGMP levels in these arteries (Nauli et al. 2001). Another indication that the influence of cGMP on contractility may decrease during cerebral artery development is that intracellular cGMP concentrations increase more rapidly in response to receptor-mediated stimulation and attain higher final values in fetal compared to adult cerebral arteries (Pearce et al. 1994). Combined with the results of this study, these observations suggest that vascular smooth muscle [Ca2+]i extrusion and/or sequestration is influenced to a greater extent by endothelial NOS-dependent mechanisms in preterm fetal (140 d) versus adult cerebral arteries.
The change in vascular tone caused by endothelium removal was significantly greater in arteries from near-term fetuses compared to either preterm fetuses or adult sheep. In order to identify the endothelium-derived substance(s) modulating diameter, we performed a series of experiments utilizing l-NAME and indomethacin to inhibit endotheliol nitric oxide synthase (eNOS) and COX, respectively. Arteries from each age group were sensitive to NOS and COX inhibition. More importantly, l-NAME and indomethacin treatment revealed that both endothelium-derived NO and an undetermined prostaglandin (perhaps PGI2 or PGE2) were responsible for the reduced vascular tone in arteries from near-term fetal sheep.
Measurable NOS activity has been detected in the brains of fetal (70, 92, 110 and 135 days gestation), newborn (< 7 days old), and adult sheep (Northington et al. 1997). NOS activity reached maximum levels at 135 days gestation, which corresponds with a two-fold increase in CBF during this period. Additionally, maximum NOS activity in near-term fetuses (135 d) also relates to our findings of greater contractile effects of L-NAME in arteries from near-term fetal (140 d) versus preterm (95 d) sheep. Along this line, in common carotid artery eNOS activity is two-fold greater in the near-term fetus, compared to the adult (Hamade et al. 2003).
Although these previous studies have shown that NOS activity is affected by development, we are unaware of any study that has compared NOS- or COX-dependent signalling using isolated arteries from fetal and adult animals. A few studies have determined the effect of development on endothelium-dependent vasodilation. Unfortunately, these studies do not support an effect of development on endothelium-dependent signalling. For example, in preconstricted, wire-mounted middle cerebral arteries, the vasodilatory responses to ADP and A-23187 were not significantly different among fetal (140 days gestation), newborn (3–7 days old), and adult sheep (Pearce & Longo, 1991). Similarly, vasodilatory responses to acetylcholine, examined through a closed cranial window, were similar in arterioles (100–300 μm) from preterm (90–111 days gestation), near-term (128–143 days gestation), and newborn sheep (7–14 days old) (Wagerle et al. 1992).
Several studies support our general hypothesis that postnatal development affects endothelial function. For example, vascular tone declined in isolated mouse cerebral arteries during the transition from neonatal (3–8 days old) to adult life, due to an up-regulation of endothelium-derived NOS- and COX-sensitive substances (Geary et al. 2003). Similarly, the vasodilatory role of endothelial NOS increased in juvenile compared with newborn pig cerebral circulation (Parfenova et al. 2000). Although cyclo-oxygenase-sensitive vasodilatory mechanisms were present in the newborn swine cerebral circulation, these substances were not altered by development (Parfenova et al. 2000). Differences between this, and other studies may be due to species differences or differences in the experimental approach (Faraci & Heistad, 1990; Hutcheson & Griffith, 1996).
The results of combined NOS and COX inhibition revealed some compensatory effects, as have been reported by others. Although investigation of the underlying mechanisms was beyond the scope of this study, the binding of NO to the haeme iron of cyclo-oxygenase represents one potential mechanism by which NO alters the activity of other enzymes (Cooper, 2002; Moncada & Erusalimsky, 2002). Alternatively, up-regulation of the constitutive isoform of COX secondary to NOS inhibition also has been reported (Beverelli et al. 1997). The interactive nature of enzymatic responses may explain the marked difference in arterial responses depending on the order in which l-NAME and indomethacin were applied.
In summary, the current study has shown that vascular and [Ca2+]i responses to pressure were reduced in arteries from near-term fetuses compared to preterm fetuses and adult sheep. Removal of the endothelium magnified differences in [Ca2+]i sensitivity between vessels from the near-term fetus versus the other groups. Further, arteries from near-term fetuses possessed greater NOS- and COX-sensitive regulation of tone and [Ca2+]i compared to those from preterm fetal sheep. These data suggest that endothelium-dependent mechanisms are capable of dramatically altering cerebral vascular tone at term, and during the process of birth. Parturition may be associated with increased intracranial pressure with a reduction in transmural pressure (Mann et al. 1972; Helou et al. 1994), and the potential to reduce CBF. Experiments conducted in utero have shown that raising intracranial pressure decreases cerebrovascular resistance in the near-term (132 d) but not mid-gestation (92 d) fetus. Thus, enhanced NOS- and COX-dependent modulation at term could provide a cerebrovascular reserve capacity, thereby maintaining CBF during reductions in cerebral perfusion pressure via enhanced vasodilatation (Harris et al. 1989).
Acknowledgments
The authors wish to thank Ms Keara McElroy-Yaggy for her exceptional technical assistance, and Brenda Kreutzer for helping to prepare the manuscript. This work was supported by NIH R01 No. HL69078-01 (6, 6, 6), HL 59406 (G.J.O.) and HD03807 (L.D.L.).
References
- Akopov SE, Zhang L, Pearce WJ. Physiological variations in ovine cerebrovascular calcium sensitivity. Am J Physiol. 1997;272:H2271–H2281. doi: 10.1152/ajpheart.1997.272.5.H2271. [DOI] [PubMed] [Google Scholar]
- Akopov SE, Zhang L, Pearce WJ. Regulation of Ca2+ sensitization by PKC and Rho proteins in ovine cerebral arteries: effects of artery size and age. Am J Physiol. 1998;275:H930–H939. doi: 10.1152/ajpheart.1998.275.3.H930. [DOI] [PubMed] [Google Scholar]
- Bevan JA, Dodge J, Walters CL, Wellman T, Bevan RD. As human pial arteries (internal diameter 200–1000 microm) get smaller, their wall thickness and capacity to develop tension relative to their diameter increase. Life Sci. 1999;65:1153–1161. doi: 10.1016/s0024-3205(99)00349-5. [DOI] [PubMed] [Google Scholar]
- Beverelli F, Bea ML, Puybasset L, Giudicelli JF, Berdeaux A. Chronic inhibition of NO synthase enhances the production of prostacyclin in coronary arteries through upregulation of the cyclooxygenase type 1 isoform. Fundament Clin Pharmacol. 1997;11:252–259. doi: 10.1111/j.1472-8206.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Blood AB, Zhao Y, Long W, Zhang L, Longo LD. L-type Ca2+ channels in fetal and adult ovine cerebral arteries. Am J Physiol. 2002;282:R131–R138. doi: 10.1152/ajpregu.00318.2001. [DOI] [PubMed] [Google Scholar]
- Cooper CE. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem Sci. 2002;27:33–39. doi: 10.1016/s0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Wellman GC, Bevan JA. Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am J Physiol. 1994;266:H147–H155. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Geary GG, Buchholz JN. Selected contribution: Effects of aging on cerebrovascular tone and [Ca2+]i. J Appl Physiol. 2003;95:1746–1754. doi: 10.1152/japplphysiol.00275.2003. [DOI] [PubMed] [Google Scholar]
- Geary GG, Buchholz JN, Pearce WJ. Maturation depresses mouse cerebrovascular tone through endothelium-dependent mechanisms. Am J Physiol. 2003;284:R734–R741. doi: 10.1152/ajpregu.00510.2002. [DOI] [PubMed] [Google Scholar]
- Geary GG, McNeill AM, Ospina JA, Krause DN, Korach KS, Duckles SP. Selected contribution: cerebrovascular NOS and cyclooxygenase are unaffected by estrogen in mice lacking estrogen receptor-alpha. J Appl Physiol. 2001;91:2391–2399. doi: 10.1152/jappl.2001.91.5.2391. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamade MW, White CR, Chang MM, Pearce WJ. Maturation increases ADP and fluid shear-induced release of endothelium derived nitric oxide (NO) from perfused ovine artery segments. FASEB J. 2003;17:566.2. [Google Scholar]
- Harper SL, Bohlen HG, Rubin MJ. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am J Physiol. 1984;246:H17–H24. doi: 10.1152/ajpheart.1984.246.1.H17. [DOI] [PubMed] [Google Scholar]
- Harris AP, Koehler RC, Gleason CA, Jones MD, Jr, Traystman RJ. Cerebral and peripheral circulatory responses to intracranial hypertension in fetal sheep. Circ Res. 1989;64:991–1000. doi: 10.1161/01.res.64.5.991. [DOI] [PubMed] [Google Scholar]
- Helou S, Koehler RC, Gleason CA, Jones MD, Jr, Traystman RJ. Cerebrovascular autoregulation during fetal development in sheep. Am J Physiol. 1994;266:H1069–H1074. doi: 10.1152/ajpheart.1994.266.3.H1069. [DOI] [PubMed] [Google Scholar]
- Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- Hutcheson IR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br J Pharmacol. 1996;118:720–726. doi: 10.1111/j.1476-5381.1996.tb15459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK, Longo LD. Fetal responses to long-term hypoxemia in sheep. Am J Physiol. 1989;256:R1348–R1354. doi: 10.1152/ajpregu.1989.256.6.R1348. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39:183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- Lin MT, Hessinger DA, Pearce WJ, Longo LD. Developmental differences in Ca2+-activated K+ channel activity in ovine basilar artery. Am J Physiol. 2003;285:H701–H709. doi: 10.1152/ajpheart.00138.2003. [DOI] [PubMed] [Google Scholar]
- Long W, Zhang L, Longo LD. Cerebral artery KATP- and KCa-channel activity and contractility: changes with development. Am J Physiol. 2000;279:R2004–R2014. doi: 10.1152/ajpregu.2000.279.6.R2004. [DOI] [PubMed] [Google Scholar]
- Long W, Zhang L, Longo LD. Fetal and adult cerebral artery KATP and KCa channel responses to long-term hypoxia. J Appl Physiol. 2002;92:1692–1701. doi: 10.1152/japplphysiol.01110.2001. [DOI] [PubMed] [Google Scholar]
- Long W, Zhao Y, Zhang L, Longo LD. Role of Ca2+ channels in NE-induced increase in [Ca2+]i and tension in fetal and adult cerebral arteries. Am J Physiol. 1999;277:R286–R294. doi: 10.1152/ajpregu.1999.277.1.R286. [DOI] [PubMed] [Google Scholar]
- Longo LD, Pearce WJ. High altitude, hypoxic-induced modulation of noradrenergic-mediated responses in fetal and adult cerebral arteries. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:683–694. doi: 10.1016/s1095-6433(98)01006-x. [DOI] [PubMed] [Google Scholar]
- Longo LD, Ueno N, Zhao Y, Zhang L, Pearce WJ. NE-induced contraction, alpha 1-adrenergic receptors, and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol. 1996;270:H915–H923. doi: 10.1152/ajpheart.1996.270.3.H915. [DOI] [PubMed] [Google Scholar]
- Mann LI, Carmichael A, Duchin S. The effect of head compression on FHR, brain metabolism and function. Obstet Gynecol. 1972;39:721–726. [PubMed] [Google Scholar]
- Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- Müller T, Löhle M, Schubert H, Bauer R, Wicher C, Antonow-Schlorke I, Sliwka U, Nathanielsz PW, Schwab M. Developmental changes in cerebral autoregulatory capacity in the fetal sheep parietal cortex. J Physiol 539. 2002;3:957–967. doi: 10.1113/jphysiol.2001.012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Zhang L, Pearce WJ. Maturation depresses cGMP-mediated decreases in [Ca2+]i and Ca2+ sensitivity in ovine cranial arteries. Am J Physiol. 2001;280:H1019–H1028. doi: 10.1152/ajpheart.2001.280.3.H1019. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Tobin JR, Harris AP, Traystman RJ, Koehler RC. Developmental and regional differences in nitric oxide synthase activity and blood flow in the sheep brain. J Cereb Blood Flow Metab. 1997;17:109–115. doi: 10.1097/00004647-199701000-00014. [DOI] [PubMed] [Google Scholar]
- Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res. 1995;32:275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- Osol G, Brekke JF, McElroy-Yaggy K, Gokina NI. Myogenic tone, reactivity, and forced dilatation: a three-phase model of in vitro arterial myogenic behavior. Am J Physiol. 2002;283:H2260–H2267. doi: 10.1152/ajpheart.00634.2002. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Massie V, Leffler CW. Developmental changes in endothelium-derived vasorelaxant factors in cerebral circulation. Am J Physiol. 2000;278:H780–H788. doi: 10.1152/ajpheart.2000.278.3.H780. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol. 1991;261:R458–R465. doi: 10.1152/ajpregu.1991.261.2.R458. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Hull AD, Long DM, White CR. Effects of maturation on cyclic GMP-dependent vasodilation in ovine basilar and carotid arteries. Pediatr Res. 1994;36:25–33. doi: 10.1203/00006450-199407001-00005. [DOI] [PubMed] [Google Scholar]
- Pearce WJ, Longo LD. Developmental aspects of endothelial function. Sem Perinatol. 1991;15:40–48. [PubMed] [Google Scholar]
- Szymonowicz W, Walker AMYuVY, Stewart ML, Cannata J, Cussen L. Regional cerebral blood flow after hemorrhagic hypotension in the preterm, near-term, and newborn lamb. Pediatr Res. 1990;28:361–366. doi: 10.1203/00006450-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Teng GQ, Nauli SM, Brayden JE, Pearce WJ. Maturation alters the contribution of potassium channels to resting and 5HT-induced tone in small cerebral arteries of the sheep. Brain Res Dev Brain Res. 2002;133:81–91. doi: 10.1016/s0165-3806(01)00304-2. [DOI] [PubMed] [Google Scholar]
- VanBavel E, Mulvany MJ. Role of wall tension in the vasoconstrictor response of cannulated rat mesenteric small arteries. J Physiol. 1994;477:103–115. doi: 10.1113/jphysiol.1994.sp020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagerle LC, Kurth CD, Busija DW. Cholinergic reactivity of cerebral arteries in the developing fetal and newborn lamb. J Dev Physiol. 1992;17:51–54. [PubMed] [Google Scholar]