Abstract
Using the isolated juvenile (7–14 days) mouse whole hippocampus preparation, which contains intact complex local circuitry, 145 dual whole cell recordings were made from stratum oriens (s.o.) interneurones under infrared microscopy. In 11.7% of paired recordings, evidence for direct electrotonic coupling between the s.o. interneurones was obtained from the response of one interneurone to a long (400–600 ms) constant current pulse passed into the coupled interneurone. When specifically orienting the dual recordings in the transectional plane of the hippocampus, 18.5% of paired recordings showed electrotonic coupling. The coupling coefficient, estimated from averaged data, was 6.9 ± 4.7%, ranging from 1.3 to 17.6%. The time constant of the electrotonically transmitted hyperpolarization was inversely related to the coupling coefficient between the two neurones. The electrotonic responses of one neurone to constant current pulses injected into the other coupled neurone were intermittent. Spikes in one of the coupled neurones were associated with small electrotonic EPSPs (spikelets) in the other coupled neurone, in those neuronal pairs with coupling coefficients greater than 10%. Failure of spikelet production following a spike in the coupled cell occurred 5–10% of the time. Electrotonic coupling and spikelets persisted in the presence of chemical synaptic transmission blockade by CNQX, APV and bicuculline, or in zero Ca2+ perfusate, but were abolished by carbenoxolone (100 μm), a gap junctional blocker. These data confirm the existence of electrotonic coupling between s.o. interneurones, presumably via gap junctions located in dendrites.
Inhibitory interneurones play a crucial role in regulating the complex activity of principal cells, including population oscillations (Buzsaki & Chrobak, 1995; Whittington et al. 1995; Freund & Buzsaki, 1996; Traub et al. 1996, 1997; Zhang et al. 1998; McBain & Fisahn, 2001; Blatow et al. 2003), neuronal plasticity (Lambert & Grover, 1995; Miles et al. 1996), and epileptic synchronization (Wong et al. 1986; Traub et al. 1999; Carlen et al. 2000; Perez-Velazquez & Carlen, 2000; Ross et al. 2000; Uusisaari et al. 2002). Hippocampal GABAergic interneurones are heavily interconnected by both chemical (Acsady et al. 1996; Hajos et al. 1996) and electrotonic synapses (Fukuda & Kosaka, 2000a,b; Rozental et al. 2000b; Venance et al. 2000; Hormuzdi et al. 2001). Gap junctions (GJs) comprise a direct intercellular electrical and metabolic communication system regulating cellular functions. In nervous tissue, gap junctions are the structural correlate of electrotonic synapses (Bennett, 1997, 2000a; Rozental et al. 2000a). This kind of electrical synapse has been shown to exist throughout the mammalian central nervous system (CNS) (MacVicar & Dudek, 1981; Gibson et al. 1999; Galarreta & Hestrin, 1999, 2001a,b; Mann-Metzer & Yarom, 1999; Rash et al. 2000; Rozental et al. 2000a; Venance et al. 2000; Deans et al. 2001; Landisman et al. 2002; Long et al. 2002; Blatow et al. 2003; De Zeeuw et al. 2003; Friedman & Strowbridge, 2003), playing a significant role in neuronal oscillations (Traub et al. 1996; Deans et al. 2001; Hormuzdi et al. 2001; Blatow et al. 2003; Buhl et al. 2003; Friedman & Strowbridge, 2003).
Unlike pyramidal neurones, which are tightly packed into a thin layer, hippocampal interneurones are widely dispersed throughout the hippocampus. Such a widespread distribution pattern renders somato-somatic electrotonic synapses between interneurones impossible, but dendro-dendritic gap junctions have been seen (Fukuda & Kosaka, 2000a). However, in the in vitro slice preparation, the network formed by such large dendritic trees of interneurones can be easily severed or damaged, interfering with direct gap junctional communication between hippocampal interneurones. In the present study, we took advantage of the isolated mouse hippocampus preparation (IHP) which avoids slicing artifacts inherent in the preparation of brain slices and maintains the entire hippocampal network intact (Khalilov et al. 1997; Wu et al. 2002). Dual simultaneous electrophysiological whole cell recordings of stratum oriens (s.o.) interneurones were made to explore the direct electrical interactions between these interneurones. Our experimental data not only provide direct electrophysiological evidence for the existence of electrotonic coupling between s.o. interneurones, but also suggest that signal conduction between s.o. interneurones via gap junctions may be intermittent in the intact neuronal circuitry of the hippocampus.
Methods
Hippocampus preparation and solutions
All electrophysiological experiments in this study were carried out in the isolated hippocampus preparation (IHP) from 7- to 14-day-old C57/BL mice (Charles River, Quebec, Canada), in accordance with the Canadian Animal Care Guidelines. After the mice were anaesthetized with halothane, they were decapitated. The brains were quickly removed and immersed for dissection into ice cold (2–4°C) oxygenated (95% O2, 5% CO2) standard artificial cerebrospinal fluid (ACSF), which contained (mm): 126 NaCl, 3.5 KCl, 2.0 CaCl2, 1.3 MgCl2,25 NaHCO3, 1.2 NaH2PO4 and 11 glucose. The brain was cut into two pieces in the horizontal plane with a vibratome to expose the hippocampus and dentate gyrus. The dorsal cortex surface of the hemi-sectioned brain was then attached to a Petri dish with Crazy glue. The dentate gyrus was carefully dissected out from the hemi-sectioned brain with fine painting brushes and custom microglass probes under a microsurgical scope. Finally, the hippocampus was taken out. All the procedures were performed in the ice cold (2–4°C) oxygenated standard ACSF. By doing so, we obtained a relatively flat tissue of ∼0.6 mm thick that included the CA1, CA2 and CA3b–c areas. After dissection, the hippocampal isolate was maintained in the ACSF at room temperature (21–23°C) for at least 1 h before recordings.
Electrophysiological recordings
After the IHP was transferred into the recording chamber, it was continuously perfused with ACSF at a speed of 4–6 ml min−1. The recording chamber was mounted on a Zeiss Axioskop FS upright microscope (Neumann/Zeiss). We used infrared differential interference contrast (IR-DIC) microscopy to visualize individual interneurones and guide pipette to make a whole cell patch clamp recording. IR-DIC microscopy has previously been reported to enable the visualization of neurones up to a depth of 100 μm in living brain tissues (Dodt & Zieglgansberger, 1990), which is sufficient for covering the depth of the stratum oriens layer in young mice. Patch clamp electrodes were then readily positioned onto the cell membrane under visual guidance using a Newport crossed roller bearing an XYZ translation stage equipped with motorizers.
Whole cell recordings were performed using an Axoclamp 2A, Axopatch 1D or 200B amplifier from Axon Instruments (Union City, CA, USA). The components of the patch pipette (intracellular) solution were (mm): 150 potassium gluconate, 2 Hepes, 0.1 EGTA (pH 7.25 and 280–290 mosmol l−1). Initially Mg2+-ATP was added to the pipette solution to reduce possible rundown of cellular functions (in ∼20 cells), but we then ceased adding ATP because we found no evident differences in the recordings, as noted previously with or without ATP in the basic intracellular parameters (Zhang et al. 1994; Wu et al. 2002) or dye coupling characteristics (Zhang et al. 1998). In some recordings, 0.05% neurobiotin was added to the intracellular solution and the concentration of potassium gluconate was reduced to 120 mm to balance osmolarity. Patch pipettes were pulled from borosilicate capillary tubing (World Precision Instruments, Sarasota, FL, USA) with a Narashige pipette puller (NG-811). Electrodes had tip resistances ranging from 4 to 6 MΩ when filled with internal solution. The resistance to ground of the whole-cell seal was 2–4 GΩ before breaking through the membrane and the series resistance was less than 20 MΩ. Two adjacent s.o. interneurones were simultaneously recorded with whole cell configuration of current clamp mode. Data acquisition, storage and analyses were performed using pCLAMP software (version 8, Axon Instruments). Digitization was through a 12-bit A/D board (Digidata 1200, Axon Instruments).
Morphological assessment
At the end of those recordings using neurobiotin in one or both of the whole cell recording electrodes, the hippocampus was fixed overnight in 0.1 m phosphate buffer (PB) with 4% paraformaldehyde and 0.05% glutaraldehyde, pH 7.4. Then horizontal sections of 50–100 μm thickness were cut along the temporal-septal axis of the isolated hippocampus with a vibratome. Fixed sections were exposed to a biotin–avidin complex stain, rinsed and reacted with diaminobenzidine tetrohydrochloride and H2O2 (ABC kit, Vector Laboratories Inc., Burlington, Ontario, Canada). The stained sections were then mounted on a glass slide, and a Nikon light microscope (Optishot) was used to visualize and photograph the sample under a 10× or 40× objective. A Zeiss camera lucida drawing device was used to trace the stained neuronal processes. Unfortunately, histological processing of dually recorded, neurobiotin-filled interneurones were not successful, which therefore prevented morphological assessment of possible contact sites between cells.
Membrane time constant estimation
The membrane time constant was measured from responses to small hyperpolarizing current pulses (400 ms; −10 to −40 pA) (Lacaille & Williams, 1990). Responses were evoked within the voltage range free of any observable voltage-dependent rectification with series resistance and capacitance carefully adjusted. The membrane time constant (τm) was determined by fitting an exponential function to the transient voltage responses evoked by hyperpolarizing current pulses.
Spikelet identification
Spikelets were recognized using a commercial software ‘mini-analysis’ (Synaptosoft Inc., Decatur, GA, USA). This program takes a segment of data points indicated by Period-To-Search-Max. We set a window of 10 ms after initiation of individual spikes in the other electrotonically coupled cell as the length of Period-To-Search-Max to find a local maximum within the data chunk. The procedure of spikelet identification is as follow. From the local maximum, it jumps to the left by 5 ms indicated by Period-Start-Baseline. Starting from that data point, the program takes a chunk of data points retrograde for 4 ms and calculates an average baseline. Then the peak amplitude is calculated by taking the amplitude at the local maximum minus the average baseline. The peak amplitude is compared to the Threshold-Amplitude that we set at 0.3 mV. If the peak amplitude is greater than the Threshold-Amplitude, the program proceeds to calculate time to peak and time to decay values. Time to peak is calculated by finding the first data point to the left of the peak that shows 0.5% of the amplitude, and then subtracting the time at this point from the time at the peak. Time to decay is calculated by finding the first data point to the right of the peak that shows 37% of the peak amplitude, and taking a difference between the time at this point and the time at the peak. All data are presented as the mean ±s.d.
Chemicals and pharmacological agents
All external and patch pipette solutions were made with deionized distilled water (resistance > 18 MΩ cm−2; Milli-Q system). Chemicals for making intracellular solutions were purchased from Fluka (New York, USA). The chemicals for making extra- and intracellular solutions were purchased from Sigma. Neurotransmitter receptor antagonists were purchased from Tocris and RBI (Ontario, Canada). Neurobiotin and the ABC kit for morphological studis were purchased from Vector Laboratories.
Viability test of the isolated hippocampus preparation
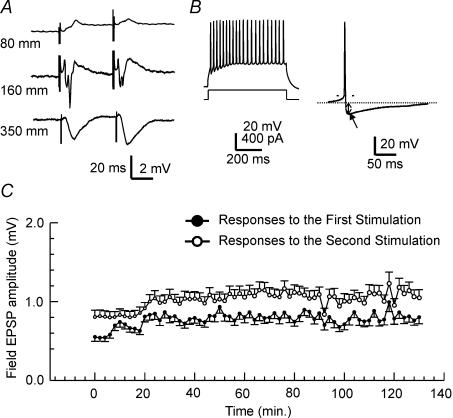
The major concern of using thick tissue in vitro for electrophysiological study is hypoxia. It has been demonstrated in rat hippocampal slices that synaptic field potentials evoked from the CA1 region are highly vulnerable to hypoxia and are readily abolished by brief hypoxic episodes (Zhang & Krnjevic, 1993; Perez-Velazquez & Zhang, 1994; Chung et al. 1998; Ouanounou et al. 1999). Therefore, we monitored the synaptic field potentials as a criterion for possible hypoxia in this unique preparation. To elicit these field potentials, a bipolar tungsten electrode (50 μm in diameter) was placed into the stratum radiatum near the CA2 region. Constant current pulses of 0.1 ms were generated by a Grass stimulator (S88) which was controlled with pCLAMP software (version 8, Axon Instruments). Glass pipettes, used as extracellular recording electrodes, usually had a tip resistance of 1–2 MΩ when they were filled with 2 m NaCl. Evoked field potentials in the CA1 region were sampled through an Axoclamp-2A or Axopatch (200B, or 1D) amplifier (Axon Instruments). As Fig. 1A shows, paired-pulse stimulation of the Schaffer collaterals evoked field excitatory postsynaptic potentials (fEPSPs) in the CA1 region. The polarity change indicated a laminar profile of fEPSPs similar to that observed in in vitro slices (Ouanounou et al. 1999). The evoked CA1 field EPSPs remained at a relatively stable level for a few hours (Fig. 1C), arguing against the deteriorating effects of hypoxia on the glutamate synapses of the CA1 region during the time course of experiments. The amplitudes of the somatic population spike and dendritic field excitatory postsynaptic potential (EPSP) were 4.3 ± 0.4 mV (n = 6) and 2.2 ± 0.2 mV (n = 6), respectively. The paired pulse enhancement of the CA1 field EPSPs was 135.5 ± 5.2% of the control response (n = 6) (interpulse interval, 50 ms). These measurements were comparable to the data collected from rat hippocampal slices, indicating that the afferent stimulation was capable of activating functional glutamatergic synapses in the IHP.
Figure 1. Evoked synaptic field responses and intracellular s.o. interneuronal responses to current injection in the whole hippocampus preparation.
A, evoked field potentials in the CA1 region of the hippocampus. Field potentials were evoked by stimulating the Schaffer collateral pathway to elicit a maximal response. The numbers on the left represent the depth of recording electrode from the top of the IHP into the stratum oriens, the CA1 pyramidal layer, and below into the stratum radiatum. Note the polarity change at the CA1 pyramidal layer. B, left traces, tonic firing in s.o. interneurones. The top trace illustrates an s.o. interneurone firing tonically with little spike frequency adaptation. The lower trace is a schematic description of the depolarizing current intensity. Right trace, average of 5 APs of an s.o. interneurone. AP duration was taken at the baseline (indicated by the thin dotted line). The arrow indicates a large, fast AHP (fAHP). The amplitude of the fAHP was measured from the baseline to the maximal hyperpolarization. C, stable field responses to paired pulse stimulation. Maximal CA1 field potentials evoked by stimulating Schaffer collateral pathway were recorded at a depth of approximately 300 μm from the s.o. side of the hippocampus. The amplitude of the evoked field was measured from the maximal downward voltage deflection. Paired pulse stimuli were delivered every 30 s. The data are presented in the form of mean ±s.d. from 6 IHPs.
Results
Electrophysiological properties of s.o. interneurones in the whole hippocampus
The intrinsic properties of 123 s.o. interneurones without neurobiotin staining were as follows: resting membrane potential, −51 ± 4.3 mV; neuronal membrane input resistance, 283 ± 84 MΩ; action potential (AP) amplitude, 72.5 ± 15 mV; and neuronal time constant (τ0), 29.7 ± 6.5 ms – these suggest a ‘healthy’ neuronal condition during the experiments. All interneurones displayed an afterhyperpolarization (AHP) (Fig. 1B), averaging –9.2 ± 3.7 mV (n = 50) following each individual AP. This afterhyperpolarization, which was referred to as the fast AHP, had a decay time of about 90 ms and was stable throughout the recordings. Moreover, all recorded interneurones manifested minimal spike frequency adaptation (Fig. 1B). These properties are comparable to the properties of s.o. interneurones described by Lacaille et al. in rat hippocampal slices (Lacaille et al. 1987; Lacaille & Williams, 1990).
Electrotonic coupling exist between s.o. interneurones
Dual simultaneous whole cell recordings in the ‘bridge mode’ from 145 pairs of s.o. interneurones in the CA1 region were made to explore the existence of electrotonic synapses between these interneurones. The somata of all s.o. interneuronal pairs were separated by 10–100 μm and were located close to the septum. Seventeen of 145 (11.7%) patched s.o. interneurone pairs showed evidence of electrotonic coupling. Most electrotonically coupled cells (14 of 80 pairs, 17.5%) were situated in the transverse plane of the hippocampus. Each electrotonically coupled s.o. interneurone usually had two or three large dendrites visible for more than 50 μm under infrared microscopy. One of the large dendrites ran closely in parallel with the large dendrite of the other cell. When whole cell recording configurations in the ‘bridge’ mode were established in two s.o. interneurones, both cells were hyperpolarized to ∼−65 mV to minimize their spontaneous firing. Stepwise or constant depolarizing or hyperpolarizing current pulses were injected into one interneurone while monitoring the responses from the other cell, and then the reverse procedure was carried out. As shown in Fig. 2, a hyperpolarizing current pulse injected into an s.o. interneurone (cell A, Fig. 2A) produced a simultaneous much smaller hyperpolarization in the non-injected s.o. interneurone (Cell B, Fig. 2A, left panels). Similarly, depolarizing cell B evoked a depolarization in cell A (Fig. 2A, right panels). This phenomenon was best seen by averaging all sweeps with identical stimulations. As expected for remote electrotonic propagation, voltage deflections recorded in the non-injected s.o. interneurone had a much smaller amplitude and slower time course than those in the injected cell. This electrotonic transmission was reciprocal in all recorded electrotonically coupled pairs of s.o. interneurones.
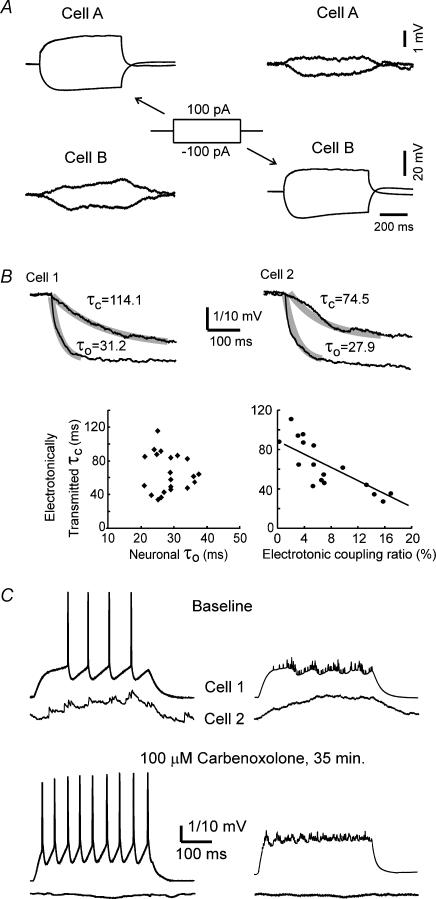
Figure 2. Electrotonic coupling between s.o. interneurones.
A, injecting either depolarizing or hyperpolarizing currents into cell A causes a corresponding passive small depolarization or hyperpolarization in the electrotonically coupled cell B, as seen in the left 2 panels. Conversely, reciprocal electrotonic coupling was demonstrated when cell B was injected with depolarizing or hyperpolarizing currents and the passive voltage responses were measured in cell A (left 2 panels). All traces in A are averaged from 20 sweeps. B, the time course of the hyperpolarization from electrotonically transmitted currents is much slower than that of the hyperpolarization induced by injecting current directly into the recorded neurone. The two examples in the upper 2 panels are from coupled neurones in separate hippocampal preparations. The upper traces in each example show the averaged hyperpolarization response electrotonically transmitted from a coupled cell. The lower traces represent the averaged direct hyperpolarization measured in the current-injected cell. The thick overlapping lines are exponential decay fits for the individual lines. τ0 is the neuronal time constant, while τc is the time constant for the passive electrotonically driven hyperpolarization in the coupled cell. The neuronal time constants (τ0) did not correlate with the time constants of the electrotonically driven hyperpolarizations (τc) (lower right graph), whereas the coupling ratios correlated with the electrotonically driven time constant (τc) (lower right graph). The voltage calibration is 1 mV for the traces represented by τc, and 10 mV for the traces represented by τ0. C, carbenoxolone blocks electrotonic coupling between s.o. interneurones. Top row of traces: simultaneous whole cell recordings from two s.o. interneurones, showing electrotonic coupling between the two cells bathed in normal ACSF. Bottom row of traces: same paired recordings after the hippocampus was exposed to carbenoxolone (100 μm), a gap junctional blocker. The traces on the left show typical traces recorded simultaneously from two electrotonically coupled neurones. The traces on the right show the average of 16 sweeps. Cell 1 is the current injected cell and Cell 2 is the electrotonically coupled cell.
The electrotonic coupling ratio was estimated from the mean maximal voltage deflection of the coupled cell over the mean voltage deflection of the cell injected with a constant current pulse. The average coupling ratio estimated from the mean saturation amplitude of the averaged sweep was 6.9 ± 4.7% (n = 17), ranging from 1.3 to 17.6%. A consistent observation was that the time constant (τc) of the initial charging phase of hyperpolarization in the coupled s.o. interneurone, elicited by injecting hyperpolarizing currents into the other electrotonically coupled s.o. interneurone, was significantly longer than τ0, as estimated from injecting current directly into the cell (Fig. 2B). Moreover, the time constant of the electrotonically driven hyperpolarization had no clear relation with τ0 in the same cell (Fig. 2B, lower left graph). However, as shown in Fig. 2B (lower right panel), the time constant of the electrotonic hyperpolar ization was inversely related to the coupling ratio, suggesting that more electrotonically remote coupling was associated with smaller coupling strength. This could be because of the increased capacitive load imposed by a more electrotonically remote dendritic location of the electrotonic coupling via GJs as frequently seen with EM between hippocampal interneurones (Fukuda & Kosaka, 2000a, 2003).
As shown in Fig. 2C, electrotonic coupling between the two s.o. interneurones was clearly blocked by the gap junctional blocker, carbenoxolone. Although we did not hold these pairs of electrotonically coupled cells long enough to observe the recovery of the gap junctional blockade from carbenoxolone, this result suggests that this electrotonic coupling was mediated by gap junctions.
Electrotonic coupling between s.o. interneurones is intermittent
As mentioned above, the electrotonic coupling between s.o. interneurones was best seen in averaged files. However, in individual sweeps, the coupling was variable and sometimes absent (Fig. 3A). This could be due to the intermittency of electrotonic coupling between s.o. interneurones in our preparation, or due to other factors such as interference from spontaneous EPSPs and IPSPs.
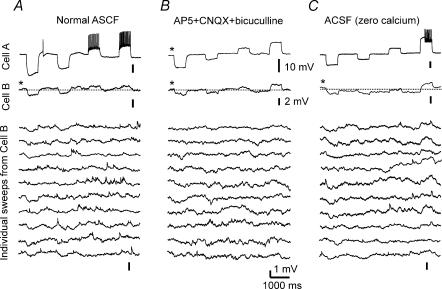
Figure 3. Intermittent weak electronic coupling between s.o. interneurones.
A, the first trace (from top to bottom) represents a single sweep recorded from the current-injected interneurone. The second trace is an averaged sweep of 20 consecutive sweeps from the other coupled interneurone. Electrotonic coupling is clearly seen in this sweep. Below are shown individual sweeps from the coupled interneurone. Note that coupled responses to current injections into the other cell, into which the current pulses are injected, are partially or completely absent from some of the individual traces. B, intermittent electrotonic coupling remains when chemical neurotransmission is blocked by perfusing the hippocampus with ACSF containing CNQX (20 μm), AP5 (25 μm), and bicuculline (20 μm). The top trace represents the averaged sweep of 16 consecutive sweeps from the current-injected cell. The second trace is the average of 16 sweeps from the other coupled neurone, showing electrotonic coupling between the two cells. The lower traces are individual sweeps from this cell. The electrotonic coupling is not obvious in some sweeps. C, the hippocampus was exposed to ACSF without added Ca2+, again to block chemical synaptic transmission. The top trace is a sweep from the current injected cell. The second trace is the average of 16 sweeps from the electrotonically coupled cell. The remaining traces are individual sweeps from the electrotonically coupled cell, again showing that this electrotonic coupling is intermittent.
To address this question, two electrically coupled pairs of s.o. interneurones were exposed to ACSF containing 2-amino-5-phosphonopentanoic acid (AP5) (50 μm), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μm) and bicuculline (10 μm) to block glutamatergic and GABAA receptor-mediated synaptic transmission. As shown in Fig. 3B, we found that the electrotonic coupling remained intermittent when scrutinizing the individual recording traces. Moreover, the coupling ratio, calculated from the integrals of the averaged sweeps from the paired cells, was unchanged. We also analysed five electrotonically coupled pairs of s.o. interneurones which were perfused with ACSF containing 0 added Ca2+ and 4 mm Mg2+, to block chemical transmission presynaptically. Again, the coupling ratio was not significantly affected by this Ca2+ free perfusate (6.1 ± 2.8%versus 6.8 ± 3.0%; P > 0.5), and the intermittency of electrotonic coupling between s.o. interneurones remained in single recording sweeps (Fig. 3C).
This intermittency property was confirmed by further analysis, as shown in Fig. 4. The standard deviations from 20 sweeps of baseline membrane potentials in the electrotonically coupled cell were clearly larger in the time window during which hyperpolarizing current pulses were electrotonically transmitted (Fig. 4A). Moreover, the standard deviations of the membrane potentials in the current-injecting cells were not affected by the hyperpolarizing pulses, remaining relatively stable. We performed a two-way ANOVA on standardized areas under the curve in a total of eight coupled pairs (Table 1). Hyperpolarizing responses to current pulses injected into a coupled s.o. interneurone were associated with significantly increased variability of the membrane potential compared to both the baseline and to the same time window in the other electrotonically coupled s.o. interneurone into which the current was injected directly (F(3, 1, 31) = 6.31, P = 0.018).
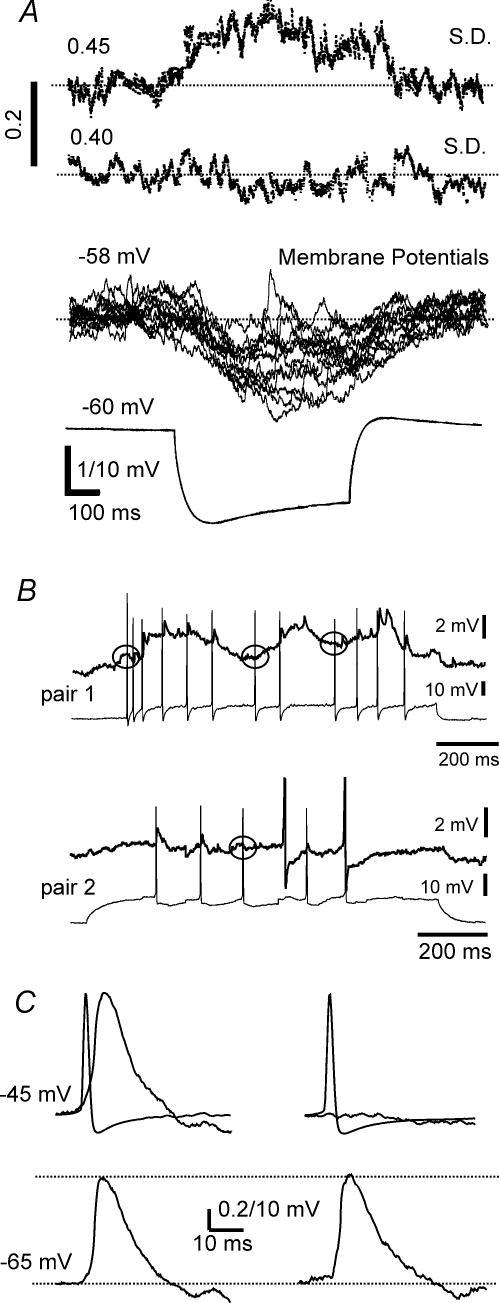
Figure 4. Increased variability of the electrotonically transmitted voltage responses in the coupled cell compared to the current injected cell.
A, increased variance of passive, non-current-injected coupled neurone shown by the standard deviation of the membrane potential calculated from 16 consecutive sweeps. The upper trace represents the standard deviations of the membrane potential in the electrotonically coupled cell, while the second line illustrates those in the current injecting cell. Note the marked increased standard deviations in the upper trace during the time of the current injection into the coupled neurone The lower 2 traces are the corresponding membrane potential responses to hyperpolarizing current pulses in the coupled cells, showing increased voltage fluctuations in the passive non-current injected receiving neurone. B, spikelets could be evoked in s.o. interneurones by spikes in the electrotonically coupled cell, with intermittent failures. The spikes, shown in the lower traces of two separate examples of paired recordings of coupled neurones, evoked corresponding spikelets in the coupled cell shown at a higher gain in the upper traces. However, not all spikes elicited corresponding spikelets in the electrotonically coupled cell as denoted by circles showing absence of evoked spikelets in the electrotonically coupled cell. Pair 1 was in normal ACSF, and pair 2 was perfused with ACSF containing synaptic transmission blockers CNQX (20 μm), AP5 (25 μm) and bicuculline (20 μm). C, spikes in an s.o. interneurone evoke spikelets in the other electrotonically coupled neurone. The upper left traces are averaged data triggered by 110 spikes from the current injected cell. The averaged spike is the briefer event and the mean spikelet is a more prolonged postsynaptic potential shown at a much higher gain (0.2 mV calibration). Failures of the spike to produce a spikelet in the current receiving cell (in this example, 6 out of 110) were rejected from this average and are shown as averaged data in the left upper 2 traces. In the lower 2 traces, spike-triggered average data, each from 50 spikelets at two different membrane potentials in an s.o. interneurone, show that the peak spikelet amplitude is independent of the membrane potential. In these examples, the parental spikes are not superimposed.
Table 1.
Standardized variance of the membrane potential is increased during the period that a cell receives passively electrotonically transmitted current from the other coupled s.o. interneurone
| Before current pulse (n = 8) | During current pulse (n = 8) | |
|---|---|---|
| Current injecting cell | 0.87 ± 0.4 mV | 0.88 ± 0.4 mV |
| E-coupled cell | 0.96 ± 0.4 mV | 1.62 ± 0.6 mV** |
P < 0.05. Note, the area underneath the standard deviation curve, which was calculated from 16–20 sweeps in an individual cell, was analysed before and during the hyperpolarizing current pulse. Because the duration of hyperpolarizing current pulse varied between cells, we standardized the data (area = mV × ms) by dividing by the corresponding time period (ms).
Spikes could generate spikelets in the coupled cells
Two types of fast pre-potentials or spikelets, namely rapid biphasic potentials and more prolonged EPSP appearing potentials, have been recorded in CA1 neurones (Perez-Velazquez et al. 1994; Valiante et al. 1995). Neuronal modelling has led to the conclusion that the fast biphasic potential represents the electrical field effects from the synchronous firing of an adjacent cluster of neurones, and the more prolonged potential is an electrotonic EPSP evoked by an action potential from an electrotonically coupled neurone (Valiante et al. 1995; Vigmond et al. 1997). Our dual whole cell recordings showed that both evoked and spontaneous action potentials in one s.o. interneurone had corresponding prolonged EPSP-like spikelets in the electrotonically coupled interneurone (Fig. 4B) in those cases (4/17) where the coupling ratio was greater than 10%.
We set up a time window of 40 ms duration, starting 5 ms prior to the peak of an action potential in one cell, to analyse any spikelet related to the action potential in the other electrically coupled cell. In the windows that contained a spikelet successfully picked up by ‘Mini-Analysis’, it was clearly shown, by averaging these spikelets within this time window, that the spikelet was closely time-locked, following the parental action potential from the other cell by 3–5 ms (peak to peak). Both the rising and decaying times of spikelets were significantly longer than their parental spikes (Fig. 4C). In our quantitative analyses, the rising time of 10% to 90% amplitude was approximately two times longer in spikelets than that in the parental spikes (1.7 ± 0.4 ms versus 0.8 ± 0.2 ms, n = 4). However, the decay time of the spikelets was significantly slower than the decay time of the parental spikes (decay 90–37% of amplitude: 12.2 ± 2.1 ms for the spikelet versus 0.6 ± 0.1 ms for the spike), rendering spikelets much longer half-width than spikes. However, the decay constant of the spikelet (17.8 ± 4.5 ms) was smaller than the membrane time constant in the four pairs of s.o. interneurones (22.3 ± 5.4 ms) as estimated from hyperpolarizing pulses of low intensity and long duration. This result suggests that the large AHP with slow decay following a ‘parental’ spike in the coupled s.o. interneurone accelerates the decay time of the spikelet in the other electrotonically coupled s.o. interneurone. The spikelet amplitude was low (0.85 ± 0.21 mV) and independent of the membrane potential (Fig. 4C). Moreover, the spikelet amplitudes were relatively constant in an s.o. interneurone if the spikelets had the same parental spikes. Comparing the amplitudes of spikelets (usually less than 1 mV) to those of their ‘parental’ spike (usually more than 70 mV) from the other cell, the coupling ratio between the spikelet and its parental spike was significantly lower (1.2 ± 0.2%, n = 4) than the one estimated from long (400–600 ms) current pulses (13.8 ± 2.7%). This difference presumably reflects the low pass filter properties of electrotonic signal conduction via dendritic gap junctions. It also explains why spikelets were often not seen. Another important phenomenon, as shown in Fig. 4B and C, is that about 5–10% of spikes in the ‘parent’ cell did not produce spikelets in the coupled s.o. interneurone. This can be clearly shown by the average analysis triggered by the spikes that do not seem to evoke corresponding spikelets in the electrotonically coupled interneurones (Fig. 4C), again showing the intermittent feature of this electrotonic coupling.
Physiological significance of electrotonic coupling between s.o. interneurones
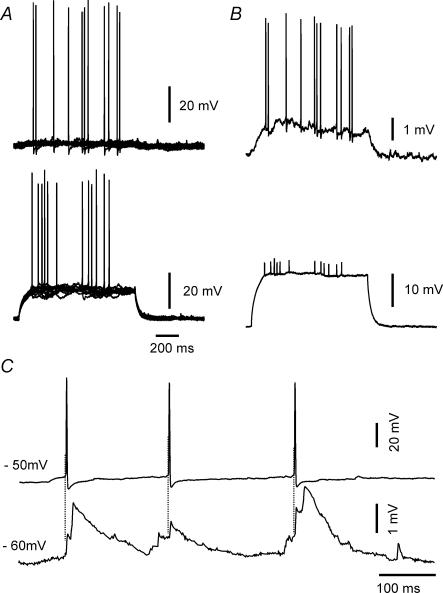
Our experiments showed that the coupling ratio between s.o. interneurones was generally low. However, an s.o. interneurone may exert a significant effect on the other s.o. interneurone through this weak coupling, because the action potential threshold in these interneurones is much lower than in principal cells (Zhang et al. 1998). As Fig. 5A illustrates, depolarization of an s.o. interneurone dramatically increases the firing probability of the other coupled s.o. interneurone. This enhancement was due to a 1 mV depolarization which was caused by the much stronger depolarization in the coupled s.o. interneurone (Fig. 5B). This phenomenon actually reflects the low pass filter properties of electrotonic signal conduction between the coupled s.o. interneurones, i.e. low frequency signals in one s.o. interneurone can affect the other coupled cells, particularly if their resting membrane potential is near spike threshold. However, apparent fast frequency events representing spikelets, following spikes in the electrotonically coupled cell, were sometimes superimposed on the spontaneous EPSPs as demonstrated in Fig. 5C, which shows the spontaneous activities of coupled s.o. interneurones. Because of their morphological and spontaneous features, these EPSPs are presumably mediated by chemical synaptic transmission. Hence, electrotonic synapses can play an important role in synchronizing and augmenting chemical synaptic events between s.o. interneurones.
Figure 5. Electrophysiological effects of electrotonic coupling in individual s.o. interneurones.
Increased cell excitability due to electrotonic coupling. A, 12 sweeps are overlapped to show that depolarization of one interneurone increases the firing probability of the other cell. B, average of all traces, showing, at higher gain, the electrotonically mediated depolarization. C, spontaneous activity between two electrotonically coupled s.o. interneurones. The lower trace shows spontaneous EPSPs in one interneurone. Apparent spikelets, which are closely time-locked to a spike from the coupled cell, are summated onto what appears to be an underlying chemical EPSPs.
Dye coupling using neurobiotin in s.o. interneurones was not apparent
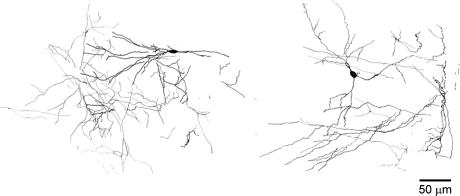
Sixteen s.o. interneurones, loaded with 0.05% neurobiotin, were morphologically recovered. Surprisingly, unlike the dye coupling noted between these neurones in hippocampal slices (Zhang et al. 1998), we did not find any evidence for dye coupling. The consistent characteristics of the reconstructed morphological data is that the dendritic trees of these s.o. interneurones are confined in a narrow region 200 μm thick from the top of the hippocampus. This suggests that the dendrites of most, if not all, s.o. interneurones we selected under infrared differential interference contrast (IR-DIC) videomicroscopy, were located in the stratum oriens–alveus layer, and did not penetrate the pyramidal layer, a morphological characteristic of horizontal s.o. interneurones (Freund & Buzsaki, 1996). Although it has been demonstrated that interneurones located in stratum oriens can form axonal arborizations in every hippocampal layer (Freund & Buzsaki, 1996; Maccaferri et al. 2000), we, unfortunately, were unable to clearly observe the axons of the studied s.o. interneurones, due to horizontally slicing the hippocampus perpendicular to the projections of these axons. Nevertheless, as shown in Fig. 6, it is often seen that the dendritic trees of these interneurones formed a fine mesh after the second or third branching in the stratum oriens layer of the hippocampus. Therefore, lack of somatic dye coupling between s.o. interneurones may be because the coupling occurs in the distal dendrites of s.o. interneurones, too far for neurobiotin diffusion to one soma to the soma of the other coupled cell. Dye coupling between these interneurones in slices may reflect dendritic damage from the slicing procedure.
Figure 6. Morphology of s.o. interneurones.
Horizontal 100 μm slices were made in parallel with the longitudinal axis of the hippocampus. Two successive slices are superimposed for each individual s.o. interneurone. Camera lucida drawings are made from neurobiotin-filled interneurones.
Unidirectional GABAergic coupling
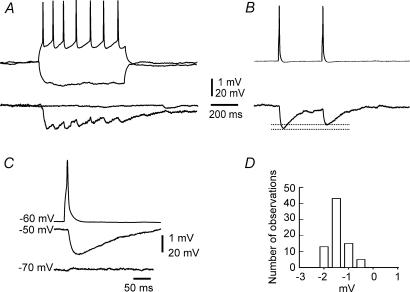
Four pairs of s.o. interneurones showed unidirectional coupling via GABAergic synapses, without concomitant electrotonic coupling (Fig. 7). The average amplitude of the monosynaptic IPSP was −1.6 ± 0.4 mV (n = 72), ranging from −0.4 mV to −2.4 mV. These IPSPs reversed at approximately −70 mV and were blocked by bicuculline (10 μm, n = 2). They had a long rise time (rise 10–90%: 8.6 ± 4.2 ms, n = 3) and decayed slowly (decay 90–37%: 80 ± 18 ms). Failure of action potential evoked IPSPs was not observed in the present study, suggesting that inhibition between s.o. interneurones is highly reliable. Unlike interneurones in layer V of cortex (Galarreta & Hestrin, 1999; Gibson et al. 1999), no neuronal pair coupled by synaptic transmission in our experiments showed electrotonic coupling.
Figure 7. The s.o. interneurone can be coupled by chemical synapses without concomitant electrotonic coupling.
A, the tonic firing of one cell causes repetitive IPSPs in the other cell, whereas a tonic hyperpolarization has no effect on the other cell. B, paired pulse depression is evoked by two action potentials separated by 240 ms, triggered at 0.5 Hz. Data are averaged from 32 sweeps. C, the reversal potential of the synaptic potential is near −70 mV (averaged from 32 sweeps). D, amplitude distribution of the IPSPs (n = 76), showing a relatively stable amplitude.
Discussion
We have demonstrated electrotonic coupling between s.o. interneurones in the in vitro intact hippocampus, a suitable preparation especially for exploring connectivity between completely intact neurones in a complex cellular network. The major concern for using thicker tissues as an experimental preparation is hypoxia. Khalilov et al. (1997) demonstrated, in a similar recording chamber, that hypoxia could occur in in vitro intact rat hippocampi older than 12 postnatal days. We took several measures to prevent hypoxia from occurring in the present study. First, we used mice aged 7–14 days postnatally, which have a considerably smaller hippocampus. Second, the dentate gyrus was removed to make the hippocampal tissue thinner (∼600 mm thick). Third, the experiments were done at room temperature (∼21°C). And fourth, the hippocampus was perfusated at a high rate (∼6 ml min−1). Both the basic electrophysiological parameters of the s.o. interneurones and the field responses were comparable to those measured in slices (Chung et al. 1998; Zhang et al. 1998). Moreover, the dendritic networks of s.o. interneurones are also better preserved than in the more usual hippocampal slice preparation, permitting observation of the electrotonic coupling in a more physiological context.
We selected s.o. interneurones as our experimental target for the following reasons. It has been shown that the dendritic trees of s.o. interneurones predominantly run in parallel within the alveus, and are restricted to the stratum oriens–alveus border zone; axons of these interneurones terminate in the stratum lacunosum-moleculare and at the border of the stratum radiatum, innervating the most distal parts of pyramidal cell dendrites (Gulyas et al. 1993a,b; Sik et al. 1994; Maccaferri & McBain, 1995). The distribution of the dendritic tree of the s.o. interneurone suggests that the major excitatory drive of these interneurones comes from local pyramidal cells, because in the CA1 region, local collaterals of pyramidal cells are restricted to the stratum oriens–alveus border as are the OA interneuronal dendrites (Knowles & Schwartzkroin, 1981). Activation of s.o. interneurones has a profound inhibitory effect on signal transmission in the apical dendritic region of CA1 (Yanovsky et al. 1997). Finally, these interneurones are located in the outermost layer of the hippocampus, which permits easy visualization and recording by microelectrodes under infrared microscopy.
We believe that gap junctions underlie the electrotonic coupling documented in the present study, because this electrotonic coupling is sensitive to the gap junctional blocker, carbenoxolone. Furthermore, we argue that this electrotonic coupling between s.o. interneurones is due to gap junctions linking the distal dendrites of these interneurones. As stated by Bennett (2000b), ‘seeing is believing.’ Gap junctions linking distal dendrites between interneurones have been seen with electron microscopy (EM) (Fukuda & Kosaka, 2000a) in the stratum oriens layer of the CA1 region. However, no EM data are yet available showing axo-axonal or axo-somatic couplings via gap junctions in hippocampal interneurones, although physiological and pharmacological evidence indicated the existence of axo-axonal gap junction coupling between hippocampal pyramidal neurones (Schmitz et al. 2001) and these axonal gap junctions can lead to very fast network oscillations under some conditions (for a review, see Traub et al. 2002). In the present study, the coupling ratio between s.o. interneurones varies widely from 1.3 to 17.6%. Considering the inherent limitation of applying the whole cell voltage clamp technique to a large neurone, especially in this preparation in which the integrity of neurones is preserved, we did not estimate the conductance of this coupling. Recent simulations showed that dendritic gap junctions with relatively low conductance between hippocampal interneurones contribute to the stabilization and enhancement of the synchrony of underlying oscillations in the hippocampal interneuronal network (Traub et al. 2001). In the present study, we did observe that in those coupled pairs with a coupling ratio of greater than 10%, an action potential in one interneurone produced an electrotonic EPSP (spikelet) in the other coupled interneurone. Although dendritic shafts of s.o. interneurones are covered by a high and uniform density of voltage-dependent Na+ channels, and action potentials can be initiated therein (Martina et al. 2000), our data differed from the axo-axonal coupling which showed spikes actively invading and propagating antidromically into the other electrotonically coupled axon (Schmitz et al. 2001). Our data showed that spikelets in s.o. interneurones are apparently propagated passively in the receiving neuronal dendrite, because their amplitude is much smaller and independent of the membrane potential. We presume that in the coupled interneurone in which the spike is generated, the spike amplitude will not be significantly attenuated in its back-propagation into the coupled dendrite, as shown by Martina et al. (2000) measuring spikes from dendrites of this interneuronal type. On the other hand, low spikelet amplitude, as measured in the soma of the receiving neurone, may be due either to low gap junctional conductance, and/or to the capacitive filtering effect caused by a long propagation path along the dendrites with significant attenuation of the voltage as shown in the multicompartmental s.o. interneuronal model of Saraga et al. (2003).
In the present study, we suggested for the first time that neuronal electrotonic coupling may be intermittent. We noted both variability of electrotonic transmission of the small voltage deflections received from the much larger voltage deflections generated in the coupled interneurone (Figs 3 and 4, and Table 1), and the occurrence of failures of spike coupled electrotonic EPSPs (spikelets, Fig. 4). This variability persisted in zero added calcium perfusate or in perfusates containing IPSP and EPSP blockers, suggesting that fluctuating dendritic synaptic potentials do not explain the variability seen. However fluctuating dendritic ion channel function cannot be ruled out since there is evidence for high dendritic densities of active sodium and potassium channels on s.o. interneurones (Martina et al. 2000; Saraga et al. 2003). The back-propagation of action potentials has been measured in s.o. interneurones to be reliable, without any significant decline in amplitude along the dendrite (Martina et al. 2000). If this is the case in the interneurones from which we recorded, then failure of presynaptic input as a cause for spikelet failure is probably ruled out. Because of the limited sample of apparently directly coupled interneurones (17/145 pairs), a more detailed analysis was not possible.
Another possibility to explain the intermittency of gap junctional communication is variability of gap junctional conductance. New appreciation of nervous system GJs, stimulated by recent molecular and physiological studies (Spray, 1996; Galarreta & Hestrin, 2001b; Rouach et al. 2002), supports the concept of intermittency for gap junctional communication. Using reverse-transcription PCR, Venance et al. (2000) demonstrated that gap junctions between inhibitory interneurones in various regions of the mouse brain mainly consisted of Cx36. Cx36 has also been identified at ultrastructurally defined neuronal gap junctions (Rash et al. 2000; Venance et al. 2000). In a recent study, Srinivas et al. (1999) showed that connexin36 has a very low unitary conductance with two open states. These intrinsic properties of connexin36, together with other intracellular and extracellular factors such as Ca2+ (Perez-Velazquez et al. 1994; Peracchia et al. 2000), phosphorylation (Lampe & Lau, 2000), and neurotransmitters (Rorig et al. 1995; Rorig & Sutor, 1996; Perez-Velazquez et al. 1997; Smith & Pereda, 2003) that modulate gap junctional conductance, suggest possible mechanisms for the intermittency of the electrotonic coupling observed. Hence, gap junction channels can be regarded as being in a constantly dynamic state, modulated by many extracellular and intracellular factors, rather than as static structures containing a fixed large-diameter, aqueous pore.
Compared to previous similar studies, the mean coupling ratio (11.7%) in our experimental sample is comparable to the mean ratios of 2.6% to 11% documented between basket cells in the hippocampal dentate gyrus (Venance et al. 2000; Bartos et al. 2002), but is significantly lower than those reported in neocortex (62–84.6%) (Galarreta & Hestrin, 1999; Gibson et al. 1999; Tamas et al. 2000; Venance et al. 2000), hippocampal CA3 region (Hormuzdi et al. 2001), thalamus (31%) (Landisman et al. 2002), and inferior olivary (IO) nucleus (50%) (Devor & Yarom, 2002). This low coupling rate agrees with the low expression density of connexin36 in the CA1 region (Deans et al. 2001). However, after summarizing our data, we found that most electrotonically coupled pairs occurred in neurones which were located in the transverse plane, perpendicular to the long axis of hippocampus (18.5% coupling rate). This not only suggests that gap junctions play a particularly important role in signal transmission in a segmentally transverse fashion across the hippocampus, but also indicates that gap junctions connect s.o. interneurones into specific networks. It has been postulated that synaptic activities conducted both via lamellae (layers in the transverse plane) and longitudinal networks are involved in information processing of the hippocampus (Ishizuka et al. 1990; Li et al. 1994). Moreover, computer simulations showed that the role of gap junctional conductance between interneurones for coherent neuronal oscillations depends on the spatial distribution of gap junctions (Traub et al. 2001). Finally, the degree of electrotonic coupling may actually be much higher since spontaneous small depolarizing potentials with the characteristics of electrotonic spikelets were seen in over 70% of recordings (Figs 2, 3, 4, and 5).
In our experiments, we measured chemical synaptic coupling rarely. It was always unidirectional, without concomitant electrotonic coupling, unlike what was seen in the cortex (Galarreta & Hestrin, 1999; Gibson et al. 1999; Galarreta & Hestrin, 2001a). This property may reflect the uniqueness of interneuronal networks in the s.o. region.
Based on the above analyses, we suggest that s.o. interneurones are linked by gap junctions. The gap junctions between s.o. interneurones appear to occur in distal dendrites. Hence, low pass filter properties in electrotonic signal conduction via gap junctions are expected between s.o. interneurones, favouring synchronization of slow oscillations and synaptic events in the distal dendrites between s.o. interneurones. Moreover, the intermittency of this coupling suggests that it is under dynamic control, permitting a wide range and subtlety of this type of interneuronal communication.
Acknowledgments
The want to thank Dr Qiping Wu for processing morphological data. This work is supported by CIHR grants to P.L.C. and L.Z.
References
- Acsady L, Gorcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73:317–334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- Bennett MV. Electrical synapses, a personal perspective (or history) Brain Res Brain Res Rev. 2000a;32:16–28. doi: 10.1016/s0165-0173(99)00065-x. [DOI] [PubMed] [Google Scholar]
- Bennett MV. Seeing is relieving: electrical synapses between visualized neurons. Nat Neurosci. 2000b;3:7–9. doi: 10.1038/71082. [DOI] [PubMed] [Google Scholar]
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Skinner F, Zhang L, Naus C, Kushnir M, Perez Velazquez JL. The role of gap junctions in seizures. Brain Res Brain Res Rev. 2000;32:235–241. doi: 10.1016/s0165-0173(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Chung I, Zhang Y, Eubanks JH, Zhang L. Attenuation of hypoxic current by intracellular applications of ATP regenerating agents in hippocampal CA1 neurons of rat brain slices. Neuroscience. 1998;86:1101–1107. doi: 10.1016/s0306-4522(98)00103-1. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Chorev E, Devor A, Manor Y, Van Der Giessen RS, De Jeu MT, Hoogenraad CC, Bijman J, Ruigrok TJ, French P, Jaarsma D, Kistler WM, Meier C, Petrasch-Parwez E, Dermietzel R, Sohl G, Gueldenagel M, Willecke K, Yarom Y. Deformation of network connectivity in the inferior olive of connexin 36-deficient mice is compensated by morphological and electrophysiological changes at the single neuron level. J Neurosci. 2003;23:4700–4711. doi: 10.1523/JNEUROSCI.23-11-04700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Devor A, Yarom Y. Electrotonic coupling in the inferior olivary nucleus revealed by simultaneous double patch recordings. J Neurophysiol. 2002;87:3048–3058. doi: 10.1152/jn.2002.87.6.3048. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Zieglgansberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Friedman D, Strowbridge BW. Both electrical and chemical synapses mediate fast network oscillations in the olfactory bulb. J Neurophysiol. 2003;89:2601–2610. doi: 10.1152/jn.00887.2002. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kosaka T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci. 2000a;20:1519–1528. doi: 10.1523/JNEUROSCI.20-04-01519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Kosaka T. The dual network of GABAergic interneurons linked by both chemical and electrical synapses: a possible infrastructure of the cerebral cortex. Neurosci Res. 2000b;38:123–130. doi: 10.1016/s0168-0102(00)00163-2. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kosaka T. Ultrastructural study of gap junctions between dendrites of parvalbumin-containing GABAergic neurons in various neocortical areas of the adult rat. Neuroscience. 2003;120:5–20. doi: 10.1016/s0306-4522(03)00328-2. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001a;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001b;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Miles R, Hajos N, Freund TF. Precision and variability in postsynaptic target selection of inhibitory cells in the hippocampal CA3 region. Eur J Neurosci. 1993a;5:1729–1751. doi: 10.1111/j.1460-9568.1993.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Miles R, Sik A, Toth K, Tamamaki N, Freund TF. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993b;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Acsady L, Freund TF. Target selectivity and neurochemical characteristics of VIP-immunoreactive interneurons in the rat dentate gyrus. Eur J Neurosci. 1996;8:1415–1431. doi: 10.1111/j.1460-9568.1996.tb01604.x. [DOI] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Esclapez M, Medina I, Aggoun D, Lamsa K, Leinekugel X, Khazipov R, Ben-Ari Y. A novel in vitro preparation: the intact hippocampal formation. Neuron. 1997;19:743–749. doi: 10.1016/s0896-6273(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Knowles WD, Schwartzkroin PA. Axonal ramifications of hippocampal Ca1 pyramidal cells. J Neurosci. 1981;1:1236–1241. doi: 10.1523/JNEUROSCI.01-11-01236.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Mueller AL, Kunkel DD, Schwartzkroin PA. Local circuit interactions between oriens/alveus interneurons and CA1 pyramidal cells in hippocampal slices: electrophysiology and morphology. J Neurosci. 1987;7:1979–1993. doi: 10.1523/JNEUROSCI.07-07-01979.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Williams S. Membrane properties of interneurons in stratum oriens-alveus of the CA1 region of rat hippocampus in vitro. Neuroscience. 1990;36:349–359. doi: 10.1016/0306-4522(90)90431-3. [DOI] [PubMed] [Google Scholar]
- Lambert N, Grover L. The mechanism of biphasic GABA responses. Science. 1995;269:928–929. doi: 10.1126/science.7638614. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Landisman CE, Long MA, Beierlein M, Deans MR, Paul DL, Connors BW. Electrical synapses in the thalamic reticular nucleus. J Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Passive propagation of LTD to stratum oriens-alveus inhibitory neurons modulates the temporoammonic input to the hippocampal CA1 region. Neuron. 1995;15:137–145. doi: 10.1016/0896-6273(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Electrotonic coupling between pyramidal cells: a direct demonstration in rat hippocampal slices. Science. 1981;213:782–785. doi: 10.1126/science.6266013. [DOI] [PubMed] [Google Scholar]
- Mann-Metzer P, Yarom Y. Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci. 1999;19:3298–3306. doi: 10.1523/JNEUROSCI.19-09-03298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Vida I, Jonas P. Distal initiation and active propagation of action potentials in interneuron dendrites. Science. 2000;287:295–300. doi: 10.1126/science.287.5451.295. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Ouanounou A, Zhang L, Charlton MP, Carlen PL. Differential modulation of synaptic transmission by calcium chelators in young and aged hippocampal CA1 neurons: evidence for altered calcium homeostasis in aging. J Neurosci. 1999;19:906–915. doi: 10.1523/JNEUROSCI.19-03-00906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C, Sotkis A, Wang XG, Peracchia LL, Persechini A. Calmodulin directly gates gap junction channels. J Biol Chem. 2000;275:26220–26224. doi: 10.1074/jbc.M004007200. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Carlen PL. Gap junctions, synchrony and seizures. Trends Neurosci. 2000;23:68–74. doi: 10.1016/s0166-2236(99)01497-6. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Han D, Carlen PL. Neurotransmitter modulation of gap junctional communication in the rat hippocampus. Eur J Neurosci. 1997;9:2522–2531. doi: 10.1111/j.1460-9568.1997.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Valiante TA, Carlen PL. Modulation of gap junctional mechanisms during calcium-free induced field burst activity: a possible role for electrotonic coupling in epileptogenesis. J Neurosci. 1994;14:4308–4317. doi: 10.1523/JNEUROSCI.14-07-04308.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Velazquez JL, Zhang L. In vitro hypoxia induces expression of the NR2C subunit of the NMDA receptor in rat cortex and hippocampus. J Neurochem. 1994;63:1171–1173. doi: 10.1046/j.1471-4159.1994.63031171.x. [DOI] [PubMed] [Google Scholar]
- Rash JE, Staines WA, Yasumura T, Patel D, Furman CS, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc Natl Acad Sci U S A. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorig B, Klausa G, Sutor B. Beta-adrenoreceptor activation reduces dye-coupling between immature rat neocortical neurones. Neuroreport. 1995;6:1811–1815. doi: 10.1097/00001756-199509000-00025. [DOI] [PubMed] [Google Scholar]
- Rorig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur J Neurosci. 1996;8:1685–1695. doi: 10.1111/j.1460-9568.1996.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Ross FM, Gwyn P, Spanswick D, Davies SN. Carbenoxolone depresses spontaneous epileptiform activity in the CA1 region of rat hippocampal slices. Neuroscience. 2000;100:789–796. doi: 10.1016/s0306-4522(00)00346-8. [DOI] [PubMed] [Google Scholar]
- Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, Giaume C. Gap junctions and connexin expression in the normal and pathological central nervous system. Bio Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Rozental R, Giaume C, Spray DC. Gap junctions in the nervous system. Brain Res Brain Res Rev. 2000a;32:11–15. doi: 10.1016/s0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]
- Rozental R, Srinivas M, Gokhan S, Urban M, Dermietzel R, Kessler JA, Spray DC, Mehler MF. Temporal expression of neuronal connexins during hippocampal ontogeny. Brain Res Brain Res Rev. 2000b;32:57–71. doi: 10.1016/s0165-0173(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Saraga F, Wu CP, Zhang L, Skinner FK. Active dendrites and spike propagation in multi-compartment models of oriens-lacunosum/moleculare hippocampal interneurons. J Physiol. 2003;552:673–689. doi: 10.1113/jphysiol.2003.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Schuchmann S, Fisahn A, aguhn A, Buhl EH, Petrasch-Parwez E, Dermietzel R, Heinemann U, Traub RD. Axo-axonal coupling. a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsaki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994;265:1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Smith MV, Pereda AE. Chemical synaptic activity modulates nearby electrical synapses. Proc Natl Acad Sci U S A. 2003;100:4849–4854. doi: 10.1073/pnas.0734299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC. Molecular physiology of gap junction channels. Clin Exp Pharmacol Physiol. 1996;23:1038–1040. doi: 10.1111/j.1440-1681.1996.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nature Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, Buhl EH, LeBean FEN, Whittington MA. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neuroscience. 2001;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, Schmitz D. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci. 1997;4:141–150. doi: 10.1023/a:1008839312043. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Functionally relevant and functionally disruptive (epileptic) synchronized oscillations in brain slices. Adv Neurol. 1999;79:709–724. [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493:471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari M, Smirnov S, Voipio J, Kaila K. Spontaneous epileptiform activity mediated by GABA(A) receptors and gap junctions in the rat hippocampal slice following long-term exposure to GABA(B) antagonists. Neuropharmacology. 2002;43:563–572. doi: 10.1016/s0028-3908(02)00156-9. [DOI] [PubMed] [Google Scholar]
- Valiante TA, Perez Velazquez JL, Jahromi SS, Carlen PL. Coupling potentials in CA1 neurons during calcium-free-induced field burst activity. J Neurosci. 1995;15:6946–6956. doi: 10.1523/JNEUROSCI.15-10-06946.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci U S A. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigmond EJ, Perez Velazquez JL, Valiante TA, Bardakjian BL, Carlen PL. Mechanisms of electrical coupling between pyramidal cells. J Neurophysiol. 1997;78:3107–3116. doi: 10.1152/jn.1997.78.6.3107. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wong RK, Traub RD, Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol. 1986;44:583–592. [PubMed] [Google Scholar]
- Wu C, Shen H, Luk WP, Zhang L. A fundamental oscillatory state of isolated rodent hippocampus. J Physiol. 2002;540:509–527. doi: 10.1113/jphysiol.2001.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky Y, Sergeeva OA, Freund TF, Haas HL. Activation of interneurons at the stratum oriens/alveus border suppresses excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience. 1997;77:87–96. doi: 10.1016/s0306-4522(96)00461-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Krnjevic K. Whole-cell recording of anoxic effects on hippocampal neurons in slices. J Neurophysiol. 1993;69:118–127. doi: 10.1152/jn.1993.69.1.118. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Perez Velazquez JL, Tian GF, Wu CP, Skinner FK, Carlen PL, Zhang L. Slow oscillations (≤1 Hz) mediated by GABAergic interneuronal networks in rat hippocampus. J Neurosci. 1998;18:9256–9268. doi: 10.1523/JNEUROSCI.18-22-09256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Weiner JL, Valiante TA, Velumian AA, Watson PL, Jahromi SS, Schertzer S, Pennefather P, Carlen PL. Whole-cell recording of the Ca(2+)-dependent slow afterhyperpolarization in hippocampal neurones: effects of internally applied anions. Pflugers Arch. 1994;426:247–253. doi: 10.1007/BF00374778. [DOI] [PubMed] [Google Scholar]