Abstract
The role of P2 receptors in synaptic transmission to the rat medial nucleus of the trapezoid body (MNTB) was studied in an in vitro brain slice preparation. Whole-cell patch recordings were made and spontaneous synaptic responses studied under voltage clamp during application of P2X receptor agonists. ATPγS (100 μm) had no effect on holding current, but facilitated spontaneous excitatory postsynaptic current (sEPSC) frequency in 41% of recordings and facilitated spontaneous inhibitory postsynaptic currents (sIPSCs) in 20% of recordings. These were blocked by the P2 receptor antagonist suramin (100 μm). α,β-meATP also facilitated sEPSC and sIPSC frequency, while l-β,γ-meATP facilitated only sIPSCs. The sEPSC facilitation by ATPγS was blocked by TTX (but did not block facilitation of sIPSCs). sEPSC facilitation was blocked by PPADS (30 μm) and the selective P2X3 receptor antagonist A-317491 (3 μm), suggesting that modulation of sEPSCs involves P2X3 receptor subunits. α,β-meATP-facilitated sIPSCs were also recorded in wild-type mouse MNTB neurones, but were absent in the MNTB from P2X1 receptor-deficient mice demonstrating a functional role for P2X1 receptors in the CNS.
ATP is released from neurones along with a range of classical neurotransmitters (Richardson & Brown, 1987; von Kugelgen et al. 1998; Jo & Schlichter, 1999), from glial cells and following tissue damage (Inoue, 1998; Queiroz et al. 1999), and acts via P2X and P2Y receptors. P2X receptors are ligand-gated non-selective cation channels with significant calcium permeability (Evans et al. 1996; Garcia-Guzman et al. 1997; Virginio et al. 1998a; Surprenant et al. 2000). Seven receptor subunits (P2X1–7) have been identified at the molecular level which associate as homo- and hetero-trimeric channels (e.g. P2X2/3, P2X4/6, P2X1/5) with a range of phenotypes (see review see North, 2002). Seven P2Y G-protein-coupled receptor genes have been identified (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13) with an array of signalling and pharmacological properties (Chang et al. 1995; Rice et al. 1995; Tokuyama et al. 1995; Communi et al. 1997; Bogdanov et al. 1998; Hollopeter et al. 2001; Zhang et al. 2002).
P2 receptors mediate a broad range of effects in the nervous system. For example, presynaptic P2X receptors regulate transmitter release (von Kugelgen et al. 1999; Nakatsuka & Gu, 2001; Smith et al. 2001) both through their depolarizing action and through direct calcium influx (Lalo & Kostyuk, 1998; Shibuya et al. 1999; Khakh & Henderson, 2000). Postsynaptic P2X receptors mediate fast excitatory transmission as well as having roles in sensory transduction and neuronal excitability (Jang et al. 2001; Vlaskovska et al. 2001). P2Y receptors exert both excitatory and inhibitory influences, regulating ion channels and transmitter release and mediating calcium waves in glial cells (Boehm et al. 1995; Harden et al. 1995; Ikeuchi & Nishizaki, 1995; Fam et al. 2000; Filippov et al. 2000).
The medial nucleus of the trapezoid body (MNTB) forms an inverting relay in the binaural auditory pathway (Barnes-Davies & Forsythe, 1995; Forsythe et al. 1998). It receives an excitatory glutamatergic input via the calyx of Held (Forsythe, 1994) and provides an inhibitory projection (Smith et al. 2000) to ipsilateral medial and lateral superior olives (MSO and LSO, respectively). MNTB neurones also receive excitatory glutamatergic inputs from non-calyceal terminals and glycinergic/GABAergic inhibitory inputs (Forsythe & Barnes-Davies, 1993; Hamann et al. 2003). Immunohistochemical, in situ and electrophysiological studies show that P2X receptors are expressed in the auditory system (Nikolic et al. 2001; Housley et al. 2002), hair cells (Glowatzki et al. 1997; Raybould & Housley, 1997; Housley et al. 1998), spiral ganglion (Salih et al. 1999) and brainstem, including the trapezoid nucleus and cochlear nucleus (Yao et al. 2000). There is also evidence for cochlea expression of P2Y receptors (Housley et al. 2002).
There are many regions in the CNS where P2 receptors have been localized but comparatively few specific functional roles have been identified. In this study, we have explored the role of P2 receptors in rat auditory brainstem. We have shown that ATP enhances excitatory and inhibitory transmission in the MNTB via distinct P2X receptor-ion channels and demonstrated a functional role for P2X1 receptor subunits in the CNS.
Methods
Brain slice preparation
Transverse brainstem slices including the MNTB, were prepared as previously described (Barnes-Davies & Forsythe, 1995; Smith et al. 2000). In brief, 9- to 13-day-old Lister Hooded rats or mice (wild-type or P2X1 receptor deficient as previously described; Mulryan et al. 2000) were killed by decapitation and the brainstem removed into cooled (0–4°C) low-Na+, high-sucrose artificial cerebrospinal fluid (aCSF; see below). Transverse slices (120 μm thick) were cut sequentially in the rostral direction from the level of the 7th nerve. The slices were then incubated for 1 h at 37°C in normal aCSF (see below) bubbled with 95% O2–5% CO2, giving a pH of 7.4. Following incubation, the slice maintenance chamber was allowed to cool to room temperature.
For recording, one slice was transferred to a Peltier controlled environmental chamber mounted on the stage of an upright Axioskop microscope (Zeiss, Germany). The microscope was fitted with differential interference contrast (DIC) optics and individual cells were visualized with a × 40 water-immersion objective (Zeiss, NA 0.75). The environmental chamber (300–400 μl volume) was continuously superfused with normal aCSF (bubbled with 95% O2–5% CO2) at a rate of 0.7–1.0 ml min−1 using a peristaltic pump (Gilson, Minipuls 3), at a temperature of 27°C. Drugs were applied by switching between one of four perfusion lines, all of which entered directly into the recording chamber so as to minimize dead space.
Cell culture
Human embryonic kidney 293 (HEK-293) cells stably expressing the recombinant rat glycosilated P2X6 receptor were a gift from Dr I. P. Chessell (GlaxoSmithKline, UK). Cells were maintained in Eagle's medium supplemented with 10% fetal bovine serum, 1% nonessential amino acids and 0.6 mg ml−1 Geneticine (Gibco BRL, UK) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. When required for study, cells were attached to glass coverslips (13 mm) and used for experiment the next day.
Electrophysiological study
Recordings were made using the whole-cell patch-clamp technique as previously described (Barnes-Davies & Forsythe, 1995; Smith et al. 2000). Patch pipettes were made with a two stage vertical pipette puller (PP-83, Narishige, Japan) from standard walled filamented borosilicate glass (Clark Electromedical, GC150F-7.5) Pipette resistances were around 5 MΩ when filled with intracellular solution (see below). Recordings were made from visually identified MNTB principal neurones. An Axopatch 200B patch-clamp amplifier (Axon Instruments, CA, USA) was used. Series resistances were under 20 MΩ and 70–80% compensation was used with 10 μs lag. Membrane currents were acquired by Digidata 1322A interface (Axon Instruments) with a PC computer using pCLAMP8 software (Axon Instruments). Data were filtered at 5 kHz with a low-pass Bessel filter and digitized at between 5 and 20 kHz. Spontaneous currents (sEPSCs and sIPSCs) were recorded at a holding potential of −70 mV. Analysis of sEPSCs and sIPSCs was conducted using the whole-cell analysis programs WinEDR and WinWCP (John Dempster, University of Strathclyde) with a detection amplitude threshold of 29.3 pA. For analysis of spontaneous currents we measured the number of events in 30 s intervals; control currents were measured 60-30 s before drug application, a test period was measured 30 s after the commencement of agonist perfusion and recovery was measured 5 min after drug washout. For experiments with the antagonist suramin the frequency of spontaneous events was measured 150 s after the start of suramin application. Pharmacological studies were conducted only on those MNTB neurones showing spontaneous IPSCs or EPSCs under control conditions. Resting average spontaneous rates could vary by up to 20%, hence only enhancements of over 2-fold and where the rate on washout returned to control levels were analysed further. Recordings from HEK-293 cells expressing recombinant rat P2X6 receptors were made using whole-cell patch-clamp and agonists were applied using a U-tube (Evans & Kennedy, 1994). Cells were perfused with an Etotal solution (see below). Whole-cell currents were recorded at holding potential of −60 mV.
Solution and drugs
The low-Na+, high-sucrose aCSF used for slice preparation contained (mm): 250 sucrose, 2.5 KCl, 10 glucose, 1.25 NaH2PO4, 26 NaHCO3, 2 sodium pyruvate, 3 myo-inositol, 0.5 ascorbic acid, 4 MgCl2 and 0.1 CaCl2. The normal aCSF used for incubation and control perfusion media for slices contained (mm): 125 NaCl, 2.5 KCl, 10 glucose, 1.25 NaH2PO4, 2 sodium pyruvate, 3 myo-inositol, 0.5 ascorbic acid, 2 CaCl2 and 1 MgCl2. The normal aCSF solutions were bubbled with 95% O2–5% CO2, giving a pH of 7.4. The patch solution for slices, contained (mm): 110 CsCl, 40 Hepes, 10 TEA-Cl, 1 EGTA (pH 7.3 adjusted by CsOH). The external solution for the HEK-293 cell line contained (mm): 150 NaCl, 2.5 KCl, 10 Hepes, 2.5 CaCl2 and 1 MgCl2 (pH 7.3 adjusted by NaOH). The patch solution for the HEK-293 cell line contained (mm): 140 potassium gluconate, 5 NaCl, 10 Hepes, 10 EGTA (pH 7.3 adjusted by KOH).
ATP, UTP, adenosine-5′-O-(3-thiotriphosphate) (ATPγS), α,β-methyleneATP (α,β-meATP), adenosine, suramin, pyridoxal-phosphate-6-azophenyl-2′,4′-di-sulphonate (iso-PPADS) and tetrodotoxin (TTX) were purchased from Sigma (UK). 6-Cyano-7-nitro-quinoxaline-2,3-dione (CNQX), strychnine and bicuculline were purchased from Tocris Cookson (St Louis, MO, USA). l-β,γ-Methylene ATP (L-β,γ-meATP) was purchased from RBI (UK). A-317491 was a gift from Abbott Laboratories, USA.
Statistical analyses
Statistical analyses were performed with the Dunnett multiple comparisons or Chi-squared tests, with P < 0.05 considered significant.
Results
Spontaneous excitatory and inhibitory synaptic currents
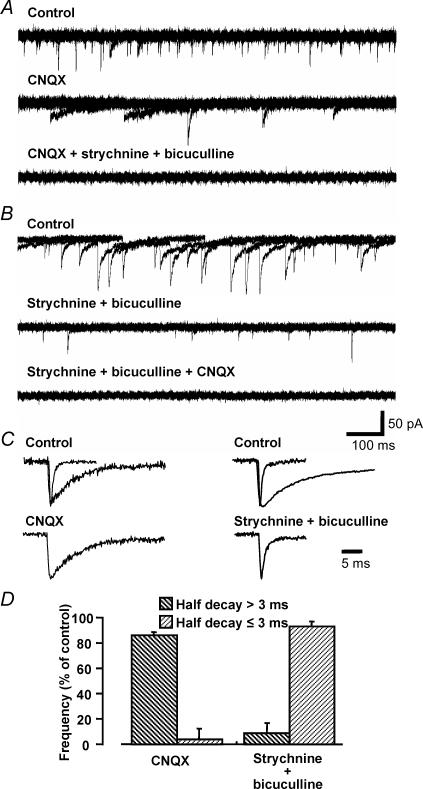
Two distinct types of spontaneous synaptic transient inward currents were recorded from MNTB neurones in the brain slice; (i) fast currents with a half-decay time of ≤ 3 ms and (ii) slow currents that decayed with a half-time of > 3 ms. Using a CsCl2-based internal solution and holding potential of −70 mV, both excitatory and inhibitory synaptic inputs gave rise to inward currents. The MNTB expresses AMPA receptors of the GluRD ‘flop’ spliced-variant that have rapid kinetics and are calcium permeable (Geiger et al. 1995), giving fast EPSC decay time constants (Barnes-Davies & Forsythe, 1995; Taschenberger & von Gersdorff, 2000). AMPA receptor-mediated currents were blocked by the glutamate receptor antagonist CNQX (10 μm) in all cells tested (5/5) (Fig. 1A, C and D). The slow currents mediated by corelease of GABA and glycine (Jonas et al. 1998; Smith et al. 2000) were abolished by combined application of the glycine receptor antagonist strychnine (1 μm) and the GABAA receptor antagonist bicuculline (10 μm) in all cells tested (5/5) (Fig. 1B, C and D). TTX (0.5 μm) had no effect on basal rates of spontaneous IPSCs or EPSCs in all cells tested (17/17). Co-application of all three antagonists blocked all spontaneous synaptic currents (Fig. 1A and B). Therefore spontaneous synaptic currents in the MNTB arise from glutamatergic ‘fast’ sEPSCs and mixed glycine–GABAergic ‘slow’ sIPSCs.
Figure 1. Characteristics of spontaneous excitatory and inhibitory synaptic currents in MNTB neurones.
A, CNQX inhibited only spontaneous currents with a fast time course (half-decay ≤ 3 ms). Strychnine and bicuculline inhibited spontaneous currents with a slow time course (half-decay > 3 ms). Five overlaid traces are shown for control (upper), after application of 10 μm CNQX (middle) and after application of 1 μm strychnine and 10 μm bicuculline in the presence of 10 μm CNQX (bottom). B, in another cell the antagonists were applied in reverse order. All spontaneous activity was abolished in the presence of CNQX, strychnine and bicuculline. C, averaged and expanded traces of the two types of spontaneous currents showing their different decay times. The upper panel shows normalized averaged traces (10 events) of fast and slow events in control conditions. The lower panels show traces following the application of 10 μm CNQX (left panel, data from A) or 1 μm strychnine with 10 μm bicuculline (right panel, data from B). D, summary: CNQX reduced only the fast spontaneous currents (half-decay ≤ 3 ms) and strychnine with bicuculline reduced only slow spontaneous current (half-decay > 3 ms). Each column indicates the mean ± s.e.m. from 5 cells.
ATPγS evoked transient facilitation of sEPSC and sIPSC frequency
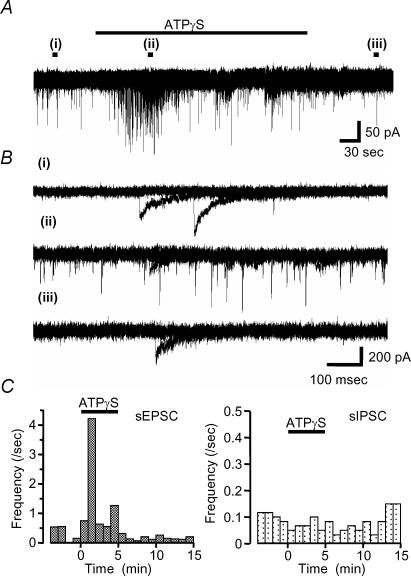
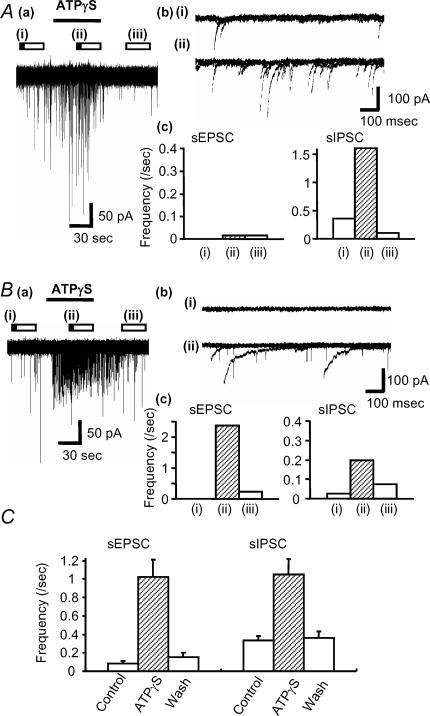
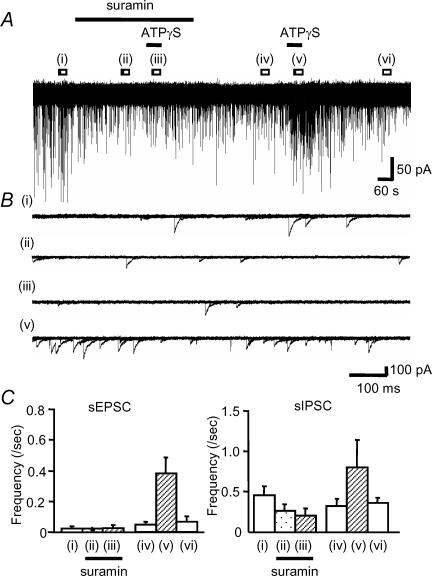
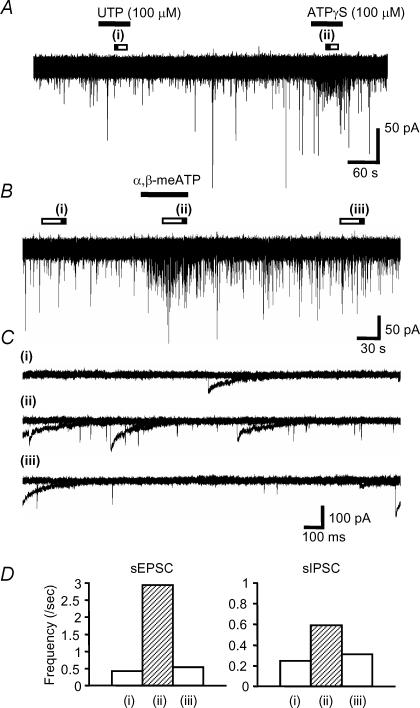
We applied ATPγS, the metabolically stable ATP analogue, to examine the effects of P2 receptor activation on auditory brainstem activity. ATPγS (100 μm) had no effect on the holding current of MNTB neurones (n = 169), indicating that functional P2X receptors are not expressed on the cell body or dendrites of MNTB neurones. However, in 53% of neurones ATPγS showed a dramatic potentiation of spontaneous synaptic current frequency (see Table 1; 47% of neurones showed no change in spontaneous synaptic currents). The effect was transient and decayed during the continued application of ATPγS (over a time course of around 1–2 min). Analysis of this increased activity revealed three different patterns (Figs 2 and 3); (1) the most common response (34%, 57/169) was a transient increase in sEPSC frequency (Fig. 2); (2) 12% (20/169) of MNTB neurones showed a large increase in sIPSC frequency (Fig. 3A), and (3) 8% of neurones (13/169) showed a large increase of both sEPSC and sIPSC frequency (Fig. 3B). Overall, 41% of MNTB neurones showed increased sEPSC frequency and 20% showed increased sIPSC frequency (Table 1, Fig. 3C), however, ATPγS had no effect on the mean current amplitude of sEPSCs (101 ± 8% of control) or sIPSCs (94 ± 7% of control). Following 3 min pre-application of suramin, co-application with ATPγS had no effect on either sIPSC or sEPSC frequency (11/11 neurones) (Fig. 4). As a positive control following washout of suramin, ATPγS increased sIPSC and sEPSC frequency in 27% and 55% of neurones (3/11 and 6/11), respectively. These results indicate that ATPγS mediates the increase in spontaneous synaptic transmission through the activation of P2 receptors.
Table 1.
Summarized data of effect of some P2 agonists and antagonists on sEPSC and sIPSC frequency
| Agonist | sEPSC | sIPSC | No change | Total n | |||||
|---|---|---|---|---|---|---|---|---|---|
| (100 μm) | % | (n) | Fold | % | (n) | Fold | % | (n) | |
| ATPγS | 41 | (70) | 10.7 ± 3.7** | 20 | (33) | 4.0 ± 1.0** | 47 | (79) | 169 |
| ATP | 56 | (9) | 9.9 ± 5.4* | 25 | (4) | 4.3 ± 1.7* | 38 | (6) | 16 |
| α,β-meATP | 50 | (7) | 13.3 ± 7.0* | 29 | (4) | 4.5 ± 1.7* | 36 | (5) | 15 |
| l-β,γ-meATP | 0 | — | — | 19 | (5) | 4.0 ± 0.8** | 81 | (21) | 26 |
| UTP | 0 | — | — | 0 | — | — | 100 | (9) | 9 |
| UDP | 0 | — | — | 0 | — | — | 100 | (9) | 9 |
| ADP | 0 | — | — | 0 | — | — | 100 | (9) | 9 |
| ATPγS + | |||||||||
| TTX | 0 | — | — | 24 | (4) | 5.4 ± 1.3** | 76 | (13) | 17 |
| TTX, low [Ca2+]o | 0 | — | — | 0 | — | — | 100 | (16) | 16 |
| Adenosine | 60 | (6) | 9.2 ± 3.7* | 30 | (3) | 3.8 ± 1.8* | 30 | (3) | 10 |
| Suramin | 0 | — | — | 0 | — | — | 100 | (11) | 11 |
| Suramin washout | 55 | (6) | 10.8 ± 0.5* | 27 | (3) | 4.0 ± 0.2* | 36 | (4) | 11 |
| PPADS | 0 | — | — | — | — | — | 100 | (5) | 5 |
| A-317491 | 0 | — | — | — | — | — | 100 | (4) | 4 |
Data show percentages and numbers (n) of neurones responding, and increases in frequency of sEPSCs and sIPSCs relative to control (Fold). Fold data shown as mean ± s.e.m. of effective cells only. No change means that change is less than 1.5-fold of control. (The data for sEPSC and sIPSC included the number of neurones in which both sEPSC and sIPSC frequency was increased).
P < 0.01
P < 0.05, Dunnett's multiple test.
Figure 2. ATPγS evoked a transient facilitation of sEPSC frequency.
In this cell ATPγS facilitated only spontaneous EPSC frequency with no effect on spontaneous IPSCs. A, ATPγS was applied during the period indicated by the horizontal bar. B, overlaid traces (five sweeps) during control (i), during the application of 100 μm ATPγS (ii) and after washout (iii) in the periods indicated by the filled bars in A. C, the time course of changes in sEPSC frequency are shown on the left, no changes were observed in sIPSC frequency (right).
Figure 3. ATPγS can evoke transient facilitation of sEPSC- and sIPSC-frequency.
A, ATPγS increased sIPSCs frequency only in this MNTB neurone. Aa, ATPγS was applied during the period indicated by the horizontal bar. Ab, overlaid traces (5 sweeps) are shown for control (i) and during the application of 100 μm ATPγS (ii). Ac, summary of ATPγS-mediated frequency changes for sEPSCs (left) and sIPSCs (right) in this cell. The bar graphs shows the frequency in control (i), and during the application of 100 μm ATPγS (ii) and after wash (iii) in the period indicated by the bars in a. B, ATPγS evoked facilitation of both sEPSC and sIPSCs frequency in this MNTB neurone. Ba, example traces showing the effect of ATPγS. Bb, overlaid traces (5 sweeps) are shown for control (i) and during the application of 100 μm ATPγS (ii). Bc, bar graphs show the change in sEPSC and sIPSC frequency in control (i), on application of 100 μm ATPγS (ii) and following wash (iii). C, summary: each column indicates the mean ± s.e.mean from 56 (sEPSC) and 24 cells (sIPSC). Open bars above the data trace indicate analysis epochs, with illustrated traces corresponding to times indicated by the filled portion of the bar in this and subsequent figures.
Figure 4. Suramin blocked the ATPγS-evoked facilitation of sEPSC and sIPSC frequency.
A, suramin and ATPγS were applied as indicated by the horizontal bars. B, overlaid traces (5 sweeps) are shown for control (i), on application of 100 μm suramin (ii), during co-application of 100 μm ATPγS and suramin (iii), washout (iv), for 100 μm ATPγS applied following washout of suramin (v). The final wash (vi) data are shown in the averaged graphs. C, summary bar graphs showing the effect of ATPγS on the frequency of sEPSCs (left) and sIPSCs (right) in the presence (ii, iii) or absence of suramin. Each column indicates the mean ± s.e.m. from 6 (sEPSC) and 3 (sIPSC) cells (only those cells that responded to ATPγS following washout of suramin were included).
P2Y receptor and P1 adenosine receptor agonists have no effect on spontaneous activity
ATPγS is an effective agonist at P2X receptors and at some P2Y receptors. To investigate which P2 receptors are expressed in the auditory brainstem we used a range of nucleotide agonists showing some P2Y subtype specificity and with action at P2X receptors. P2Y1, P2Y11, P2Y12 and P2Y13 are ADP sensitive, P2Y2 and P2Y4 receptors are UTP sensitive, and the P2Y6 receptor is UDP sensitive (Webb et al. 1993; Chen et al. 1996; Communi et al. 1996; Nicholas et al. 1996; Bogdanov et al. 1998; Hollopeter et al. 2001; Zhang et al. 2002).
UTP, UDP and ADP (100 μm) preferentially activate specific P2Y receptors but had no effect on the holding current of MNTB neurones or on the frequency of spontaneous synaptic currents (Fig. 5A, Table 1). As a positive control following the washout of ADP, UTP or UDP, ATPγS increased the frequency of spontaneous synaptic events (Fig. 5A). ATP is metabolically unstable and is degraded to ADP, AMP and adenosine by ecto-nucleotidases (Kegel et al. 1997; Cunha et al. 1998; Ohkubo et al. 2000). For example in the caudal regions of the rat nucleus tractus solitarii, ATP mediates excitatory transmission indirectly through breakdown to adenosine and activation of A1 receptors (Cunha et al. 1998; Kato & Shigetomi, 2001). In the present study adenosine (10 μm) had no effect on resting frequency or ATPγS-induced facilitation (Table 1). ATP (100 μm) had similar effects to ATPγS on sEPSC and sIPSC frequency (Table 1), indicating that it was acting directly through P2X receptors (ATP had no significant effect on the mean current amplitude of sEPSCs 92 ± 7% (n = 9) and sIPSCs 97 ± 3% of control (n = 4)). These results indicate that P2Y or P1 adenosine receptors do not regulate MNTB spontaneous activity and that ATPγS effects are most likely to be mediated through P2X receptors. The lack of expression of P2X receptors on acutely dissociated astrocytes suggests that ATPγS is acting directly at neuronal P2X receptors to modulate release of excitatory mediators, e.g. glutamate (Jeremic et al. 2001).
Figure 5. Effect of UTP and α,β-meATP on the spontaneous currents.
A, UTP had no effect on the spontaneous currents; however, this cell could respond to ATPγS. B, α,β-meATP evoked transient facilitation of both sEPSC and sIPSC frequency. α,β-meATP was applied during the period indicated by the horizontal bars. C, overlaid traces (5 sweeps) are shown for control (i), during the application of 100 μmα,β-meATP (ii) and after wash (iii). D, summary data for the effect of α,β-meATP on the frequency of sEPSCs (left) and sIPSCs (right). Each column shows the frequency in control (i), on application of 100 μmα,β-meATP (ii) and after wash (iii). Each column shows the mean from 7 (sEPSC) and 4 (sIPSC) cells (including only those cells responding to α,β-meATP).
Subclassification of P2X receptor-mediated increases in synaptic activity
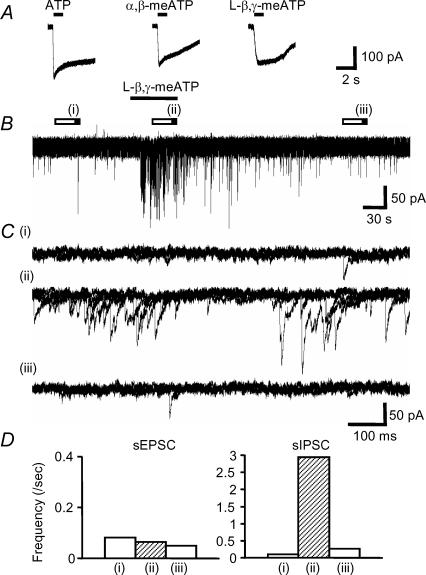
To further characterize the P2X receptor subtypes underlying the ATPγS-mediated responses we used metabolically stable and subtype-selective agonists. α,β-meATP is an agonist at recombinant P2X receptors containing P2X1, P2X3 or P2X6 receptor subunits (e.g. homomeric P2X3 and heteromeric P2X2/3 receptors; North, 2002; Jones et al. 2004 and current study) and evoked increases in sIPSC and sEPSC frequency similar to ATPγS (Table 1, Fig. 5B–D) with no effect on the mean current amplitudes (sEPSCs 100 ± 10% of control (n = 7) and sIPSCs 102 ± 13% control (n = 4)). The percentages of neurones that responded to ATPγS, ATP and α,β-meATP were similar, indicating no additional expression of ATPγS-sensitive but α,β-meATP-insensitive P2X receptors (Table 1). The relative contribution of individual subunits was investigated using L-β,γ-meATP, which acts at P2X1 receptors, but not at P2X3 receptors (Evans et al. 1995; Lewis et al. 1995; Grubb & Evans, 1999). The properties of recombinant P2X6 homomeric receptors have been described recently; this receptor only forms functional channels when correctly glycosylated (Jones et al. 2004). ATP (100 μm), α,β-meATP (100 μm) and L-β,γ-meATP (100 μm) all evoked inward currents at P2X6 receptors (9.9 ± 1.2, 12.0 ± 0.9 and 4.5 ± 0.4 pA pF−1, respectively, Fig. 6A) expressed in HEK-293 cells.
Figure 6. l-β,γ-meATP can induce P2X6 receptor-mediated currents and evoked transient facilitation of sIPSC frequency only.
A, ATP (100 μm), α,β-meATP (100 μm) and l-β,γ-meATP (100 μm) evoked inward currents via P2X6 receptors expressed in HEK-293 cells. B, l-β,γ-meATP facilitated sIPSC frequency onto MNTB neurones. C, overlaid traces (5 sweeps) for control (i), during the application of 100 μml-β,γ-meATP (ii) and after wash (iii). D, summary of the effect of l-β,γ-meATP on the frequency of sEPSCs (left) and sIPSCs (right). Each column shows the frequency in control (i), on application of 100 μml-β,γ-meATP (ii) and after wash (iii). Data are the mean from 26 and 5 cells, respectively.
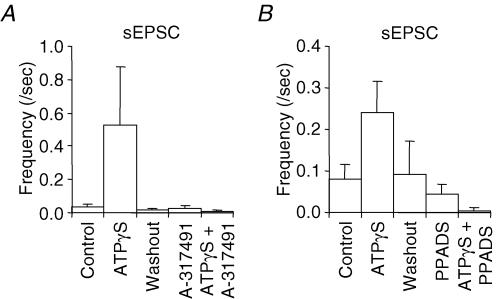
In the MNTB slices l-β,γ-meATP (100 μm) evoked increases in sIPSC frequency (with no effect on the mean current amplitude 104 ± 9% of control (n = 5) but did not change sEPSC frequency (Fig. 6B–D, Table 1). The similar percentage of inhibitory neurones responding to ATPγS, ATP, α,β-meATP and l-β,γ-meATP indicates that there are no additional inhibitory neurones expressing ATPγS-sensitive but l-β,γ-meATP-insensitive P2X receptors. To determine whether the α,β-meATP-sensitive, but l-β,γ-meATP-insensitive increase in sEPSCs was mediated by receptors containing P2X3 receptor subunits we used the selective P2X3 receptor antagonist A-317491 (Jarvis et al. 2002). ATPγS-evoked increases in sEPSCs were abolished by A-317491 (3 μm) and iso-PPADS (30 μm) (Fig. 7).
Figure 7. Summary of P2 antagonism of sEPSC frequency potentiation by ATPγS (100 μm).
A, the P2X3-specific antagonist A-317491 (3 μm) caused a 87.5 ± 12.5%(n = 4) inhibition of the sEPSC potentiation generated by ATPγS. B, 30 μm PPADS reduced the ATPγS-induced potentiation of sEPSCs by 98.8 ± 1.2% (n = 5).
The sensitivity to α,β-meATP and l-β,γ-meATP of the sIPSCs suggested the involvement of either P2X1 or P2X6 receptor subunits and is similar to that described recently in the somatosensory cortex (Pankratov et al. 2003). To determine the contribution of P2X1 receptors we compared responses in MNTB neurones from wild-type and P2X1 receptor-deficient mice (Mulryan et al. 2000). The sIPSC frequency was potentiated by α,β-meATP in 8/8 MNTB neurones from wild-type mice, but α,β-meATP failed to evoke any change in sIPSC frequency recorded from P2X1 receptor-deficient mouse MNTB neurones (0/5) (χ2P < 0.01). These results demonstrate that in the mouse and probably in the rat, P2X1 receptors are expressed on the inhibitory projections to the MNTB.
Is action potential firing required for the modulatory actions of ATPγS on sEPSC and sIPSC frequency in MNTB neurones?
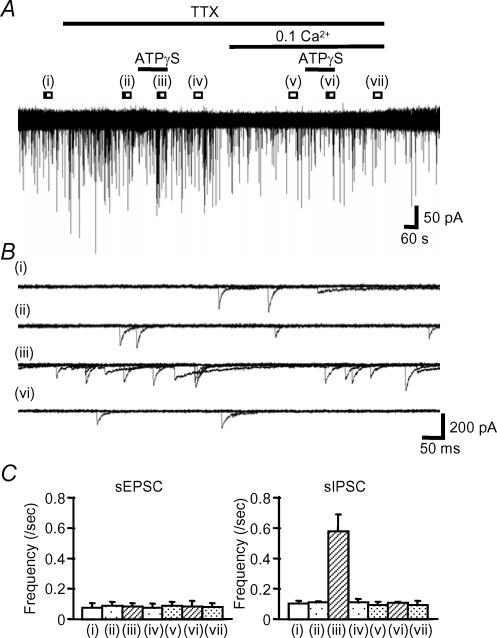
The P2X receptor effects may depend on the cellular localization of the receptors; for example in intrinsic sensory neurones P2X receptors are present on the cell body and their activation leads to depolarization and firing of action potentials (Bertrand & Bornstein, 2002). P2X receptors may also be present at the presynaptic nerve terminal and directly regulate transmitter release via calcium influx through the receptor ion channel (Rhee et al. 2000; Kato & Shigetomi, 2001; Nakatsuka & Gu, 2001). We used the voltage-gated sodium channel blocker tetrodotoxin (TTX) to determine whether action potential propagation was required for P2X receptor-mediated facilitation of spontaneous synaptic transmission. Figure 8 shows the typical effect of TTX (0.5 μm) on spontaneous currents and the ATPγS-evoked facilitation. TTX did not change the frequency of spontaneous currents in any neurones (17/17) and in the presence of TTX, ATPγS no longer facilitated sEPSC frequency (Table 1, Fig. 8C). However the proportion of cells responding to ATPγS with an increase in sIPSCs was unchanged (Table 1, Fig. 8C). These results suggest that P2X receptors are present predominantly on the cell body or fibre tracts in excitatory pathways, while for inhibitory pathways they are located on the presynaptic nerve terminals.
Figure 8. ATPγS applied in the presence of TTX and low [Ca2+]o (0.1 mm).
A, ATPγS evoked facilitation of sIPSC-frequency but not sEPSC-frequency in the presence of 0.5 μm TTX. ATPγS failed to change sIPSC or sEPSC frequency in low Ca2+ conditions (0.1 mm). B, overlaid traces (5 sweeps) in control (i), 5 min following the application of 0.5 μm TTX (ii), during co-application of 100 μm ATPγS and 0.5 μm TTX (iii) and during application of 100 μm ATPγS in low [Ca2+]o and in the presence of 0.5 μm TTX (vi). C, summary bar graphs showing frequency changes for sEPSCs (left) and sIPSCs (right). Each column shows control (i), application of 0.5 μm TTX (ii), co-application of 100 μm ATPγS and 0.5 μm TTX (iii), wash of ATPγS (iv) in TTX, 5 min after the application of low [Ca2+]o (v), on application of 100 μm ATPγS in low [Ca2+]o in the presence of 0.5 μm TTX (vi) and after wash ATPγS in low [Ca2+]o conditions in TTX (iv). Each column indicates the mean ± s.e.m from 16 and 4 cells, respectively (all cells for sEPSCs and only those cells potentiated by ATPγS for sIPSCs).
Is the facilitation of sIPSC frequency dependent on calcium influx?
In order to determine if the facilitation of sIPSC frequency was Ca2+ dependent we tested the effect of ATPγS in low external Ca2+ solution (low [Ca2+]o) and in the presence of TTX. As a positive control in 0.5 μm TTX, ATPγS facilitated sIPSC frequency in 24% (4/17) of cells (Table 1, Fig. 8). After 5 min perfusion of low [Ca2+]o, ATPγS no longer facilitated sIPSC frequency in any of the 16 cells tested (Table 1, Fig. 8), indicating that calcium influx via presynaptic P2X receptors mediates the increase in sIPSCs frequency.
Discussion
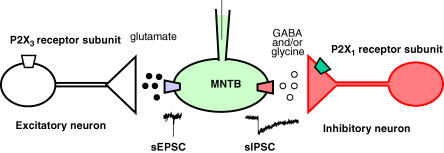
In this study we have shown that functional P2X receptor-ion channels are expressed in the auditory brainstem where they act to facilitate transmitter release in the superior olivary complex. Although ATP potentiates release at both excitatory and inhibitory synapses, it does so by different P2X receptor subtypes expressed at different cellular locations: receptors comprising P2X3 receptor subunits on cell bodies or axons of excitatory pathways and receptors comprising P2X1 receptor subunits directly on the presynaptic terminals of inhibitory pathways (Fig. 9).
Figure 9. Schematic diagram of excitatory and inhibitory inputs innervating rat MNTB neurones and regulated by P2X receptors.
The α,β-meATP-sensitive, l-β,γ-meATP-insensitive, TTX-sensitive excitatory input is consistent with mediation by channels containing P2X3 receptor subunits located on the cell body. Activation of these P2X receptors increases spontaneous glutamate release at non-calyceal synapses. Mediation of the l-β,γ-meATP-sensitive inhibitory input is consistent with expression of channels containing P2X1 receptor subunits located on the nerve terminal. Activation of these receptors leads to an increase in spontaneous IPSCs in a TTX-insensitive manner.
The application of ATPγS (or indeed any of a number of purinergic agonists) had no effect on the holding current of MNTB neurones, suggesting that functional P2X receptor-ion channels are not present on MNTB somas (or in their short dendritic tree). P2X receptors could be present on the axonal projections of the MNTB, but this remains to be determined. In contrast, ATPγS gave a profound increase in spontaneous transmitter release from both excitatory (11.5-fold) and inhibitory inputs (3.6-fold), indicating a high degree of purinergic regulation within the superior olivary complex. However not all inputs responded with an increase in activity (41% of excitatory and 20% of inhibitory inputs showed potentiation), suggesting that there is a heterogeneity in the neuronal input to the MNTB. ATPγS is an agonist at all P2X receptors and many P2Y receptors. We have shown that only P2X receptors were involved in the reported effects since a range of P2Y receptor agonists (selective for all currently identified P2Y receptor subtypes) had no effect on spontaneous synaptic currents in the MNTB. Using subtype-selective purinergic agonists we have demonstrated the presence of molecularly distinct P2X receptors on excitatory and inhibitory inputs.
MNTB neurones receive excitatory input from a single giant synapse (calyx of Held), which covers around half of the somatic surface area, as well as conventional glutamatergic synaptic terminals (Hamann et al. 2003). P2X receptor-mediated potentiation of sEPSCs was sensitive to TTX, indicating that the receptors were located predominantly on cell bodies or axon tracts of excitatory fibres innervating the MNTB (it is unlikely that a polysynaptic pathway is involved due to the orientation during cutting of the brain slice). The giant calyceal input is unlikely to be involved in P2X receptor stimulation as the increases in sEPSCs were blocked by TTX, hence they must require action potential propagation. Such spontaneous action potentials would generate giant sEPSCs at the calyx of Held, which were never observed. However, presynaptic P2X receptors have been observed at other giant synapses (Sun & Stanley, 1996). Previous reports have demonstrated a modest depression of the evoked EPSC at the calyx of Held/MNTB synapse by adenosine (Barnes-Davies & Forsythe, 1995) and in the present study adenosine had no effect on the frequency of sEPSCs. Thus the potentiation of spontaneous excitatory events is probably via non-calyceal high threshold excitatory inputs innervating the MNTB, as previously described (Hamann et al. 2003).
In addition to ATPγS stimulation, transmitter release from the excitatory terminals was potentiated by α,β-meATP (an agonist at recombinant receptors containing P2X1, P2X3 and P2X6 receptor subunits). The potentiation of sEPSCs was abolished by the P2X3 receptor-selective antagonist A-317491 (Jarvis et al. 2002) and insensitive to the P2X1 and P2X6 receptor subunit agonist l-β,γ-meATP (Evans et al. 1995; Buell et al. 1996). These results indicate that the high threshold excitatory neurones express P2X3 receptor subunits. P2X3 receptor subunits are thought to be expressed predominantly, if not exclusively, on sensory nerves, suggesting that either the MNTB receives a sensory afferent input or more likely, given the anatomy of the brainstem, that P2X3 receptors are not expressed exclusively on sensory fibres. The incorporation of P2X3 receptor subunits into heteromeric channels can confer α,β-meATP sensitivity to the resulting channel, e.g. P2X2/3 (where P2X2 receptor homomeric channels are essentially insensitive to α,β-meATP) (Lewis et al. 1995; Le et al. 1998). The desensitizing nature of the response (1–2 min) is considerably slower than that for recombinant P2X3 receptors (1–2 s) and suggests that the P2X3 receptor subunit contributes α,β-meATP sensitivity to a heteromeric P2X receptor on excitatory neurones.
The MNTB receives inhibitory drive from recurrent inputs and from other brainstem nuclei of the superior olivary complex (Guinan & Stankovic, 1996; Kopp-Scheinpflug et al. 2002). The mixed GABA–glycine-mediated sIPSCs reported are similar to those characterized previously in the medial superior olive (Smith et al. 2000). The sensitivity of P2X receptors located on inhibitory inputs to l-β,γ-meATP (P2X1 or P2X6 receptor subunit selective) and the abolition of responses in P2X1 receptor-deficient mice suggest that the P2X channel on these neurones expresses P2X1 receptor subunits. This is consistent with in situ hybridization (Kidd et al. 1995; Collo et al. 1996) and immunohistochemical studies (Xiang et al. 1998; Yao et al. 2000; Rubio & Soto, 2001) that have shown these subunits to be expressed in the CNS and brainstem (Yao et al. 2000). However, it is unlikely that the response corresponds to homomeric P2X1 receptors as these show rapid desensitization (1–2 s) (Evans et al. 1995). It seems more likely that P2X1 receptor subunits contribute α,β-meATP and l-β,γ-meATP sensitivity to a heteromeric P2X receptor on the terminals of inhibitory neurones similar, for example, to the P2X1/2 heteromeric channel in superior cervical ganglion neurones (Calvert & Evans, 2004).
The TTX insensitivity of the P2X agonist effects on inhibitory pathways demonstrates that action potential propagation is not involved and suggests that the P2X receptors are present on the presynaptic nerve terminal. This is consistent with previous studies demonstrating presynaptic P2X receptors regulating transmitter release (Sun & Stanley, 1996; Nakatsuka & Gu, 2001; Smith et al. 2001) and including inhibitory glycinergic (Rhee et al. 2000; Jang et al. 2001) and GABAergic neurones (Hugel & Schlichter, 2000).
In summary we have shown that ATP can regulate both excitatory and inhibitory inputs to MNTB neurones by the discrete localization of functional P2X receptor subtypes in the brainstem; one mechanism is via P2X3-containing receptors located on excitatory neuronal cell bodies and a second is via presynaptic P2X1-containing receptors located on inhibitory neurones. P2X receptors have been described in the primary sensory apparatus of the cochlea and this study demonstrates that these receptors can also play a functional role in the regulation of auditory processing at the level of the brainstem. This is the first time that a functional role of P2X1 receptors has been demonstrated in the central nervous system.
Acknowledgments
We thank Dr I. P. Chessell (GlaxoSmithKline, UK) for providing HEK-293 cells expressing the P2X6 receptor, Abbott Laboratories for the A-317491 and Dr E. P. Seward for useful comments on the manuscript. This work was supported by the Medical Research Council and the Wellcome Trust.
References
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–4775. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm S, Huck S, Illes P. UTP- and ATP-triggered transmitter release from rat sympathetic neurones via separate receptors. Br J Pharmacol. 1995;116:2341–2343. doi: 10.1111/j.1476-5381.1995.tb15075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- Calvert JA, Evans RJ. Heterogeneity of P2X receptors in sympathetic neurons: contribution of neuronal P2X1 receptors revealed using knockout mice. Mol Pharmacol. 2004;65:139–148. doi: 10.1124/mol.65.1.139. [DOI] [PubMed] [Google Scholar]
- Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinol. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastiao AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Kennedy C. Characterization of P2-purinoceptors in the smooth muscle of the rat tail artery: a comparison between contractile and electrophysiological responses. Br J Pharmacol. 1994;113:853–860. doi: 10.1111/j.1476-5381.1994.tb17071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R, Lewis C, Buell G, Valera S, North R, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- Evans R, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North R. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Brown DA, Barnard EA. The P2Y1 receptor closes the N-type Ca2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. Br J Pharmacol. 2000;129:1063–1066. doi: 10.1038/sj.bjp.0703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Barnes-Davies M. The binaural auditory pathway: excitatory amino acid receptors mediate dual timecourse excitatory postsynaptic currents in the rat medial nucleus of the trapezoid body. Proc R Soc Lond B Biol Sci. 1993;251:151–157. doi: 10.1098/rspb.1993.0022. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Soto F, Gomez-Hernandez JM, Lund PE, Stuhmer W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Ruppersberg JP, Zenner HP, Rusch A. Mechanically and ATP-induced currents of mouse outer hair cells are independent and differentially blocked by d-tubocurarine. Neuropharmacology. 1997;36:1269–1275. doi: 10.1016/s0028-3908(97)00108-1. [DOI] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Stankovic KM. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am. 1996;100:1680–1690. doi: 10.1121/1.416066. [DOI] [PubMed] [Google Scholar]
- Hamann M, Billups B, Forsythe ID. Non-calyceal excitatory inputs mediate low fidelity synaptic transmission in rat auditory brainstem slices. Eur J Neurosci. 2003;18:2899–2902. doi: 10.1111/j.1460-9568.2003.03017.x. [DOI] [PubMed] [Google Scholar]
- Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJ, Thorne PR. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol. 2002;7:55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- Housley GD, Luo L, Ryan AF. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. J Comp Neurol. 1998;393:403–414. [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi Y, Nishizaki T. The P2Y purinoceptor-operated potassium channel is possibly regulated by the beta gamma subunits of a pertussis toxin-insensitive G-protein in cultured rat inferior colliculus neurons. Biochem Biophys Res Commun. 1995;214:589–596. doi: 10.1006/bbrc.1995.2326. [DOI] [PubMed] [Google Scholar]
- Inoue K. ATP receptors for the protection of hippocampal functions. Jpn J Pharmacol. 1998;78:405–410. doi: 10.1254/jjp.78.405. [DOI] [PubMed] [Google Scholar]
- Jang IS, Rhee JS, Kubota H, Akaike N. Developmental changes in P2X purinoceptors on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurones. J Physiol. 2001;536:505–519. doi: 10.1111/j.1469-7793.2001.0505c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Jones CA, Vial C, Sellers LA, Humphrey PP, Evans RJ, Chessell IP. Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel α,β-methylene ATP-sensitive phenotype. Mol Pharmacol. 2004;65:979–985. doi: 10.1124/mol.65.4.979. [DOI] [PubMed] [Google Scholar]
- Kato F, Shigetomi E. Distinct modulation of evoked and spontaneous EPSCs by purinoceptors in the nucleus tractus solitarii of the rat. J Physiol. 2001;530:469–486. doi: 10.1111/j.1469-7793.2001.0469k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel B, Braun N, Heine P, Maliszewski CR, Zimmermann H. An ecto-ATPase and an ecto-ATP diphosphohydrolase are expressed in rat brain. Neuropharmacol. 1997;36:1189–1200. doi: 10.1016/s0028-3908(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Henderson G. Modulation of fast synaptic transmission by presynaptic ligand-gated cation channels. J Auton Nerv Syst. 2000;81:110–121. doi: 10.1016/s0165-1838(00)00111-9. [DOI] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PP. Localization of P2X purinoceptor transcripts in the rat nervous system. Mol Pharmacol. 1995;48:569–573. [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dorrscheidt GJ, Rubsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J Assoc Res Otolaryngol. 2002;8:8. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Kostyuk P. Developmental changes in purinergic calcium signalling in rat neocortical neurones. Brain Res Dev Brain Res. 1998;111:43–50. doi: 10.1016/s0165-3806(98)00120-5. [DOI] [PubMed] [Google Scholar]
- Le KT, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- Nikolic P, Housley GD, Luo L, Ryan AF, Thorne PR. Transient expression of P2X1 receptor subunits of ATP-gated ion channels in the developing rat cochlea. Brain Res Dev Brain Res. 2001;126:173–182. doi: 10.1016/s0165-3806(00)00149-8. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ohkubo S, Kimura J, Matsuoka I. Ecto-alkaline phosphatase in NG108-15 cells: a key enzyme mediating P1 antagonist-sensitive ATP response. Br J Pharmacol. 2000;131:1667–1672. doi: 10.1038/sj.bjp.0703750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. P2X receptor-mediated excitatory synaptic currents in somatosensory cortex. Mol Cell Neurosci. 2003;24:842–849. doi: 10.1016/s1044-7431(03)00233-1. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kugelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Raybould NP, Housley GD. Variation in expression of the outer hair cell P2X receptor conductance along the guinea-pig cochlea. J Physiol. 1997;498:717–727. doi: 10.1113/jphysiol.1997.sp021896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee JS, Wang ZM, Nabekura J, Inoue K, Akaike N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J Physiol. 2000;524:471–483. doi: 10.1111/j.1469-7793.2000.t01-1-00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR, Burton FM, Fiedeldey DT. Cloning and expression of the alveolar type II cell P2u-purinergic receptor. Am J Respir Cell Mol Biol. 1995;12:27–32. doi: 10.1165/ajrcmb.12.1.7811468. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Brown SJ. ATP release from affinity-purified rat cholinergic nerve terminals. J Neurochem. 1987;48:622–630. doi: 10.1111/j.1471-4159.1987.tb04138.x. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih SG, Housley GD, Raybould NP, Thorne PR. ATP-gated ion channel expression in primary auditory neurones. Neuroreport. 1999;10:2579–2586. doi: 10.1097/00001756-199908200-00026. [DOI] [PubMed] [Google Scholar]
- Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514:351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Hansen MA, Liu DM, Adams DJ. Pre- and postsynaptic actions of ATP on neurotransmission in rat submandibular ganglia. Neuroscience. 2001;107:283–291. doi: 10.1016/s0306-4522(01)00347-5. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XP, Stanley EF. An ATP-activated, ligand-gated ion channel on a cholinergic presynaptic nerve terminal. Proc Natl Acad Sci U S A. 1996;93:1859–1863. doi: 10.1073/pnas.93.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, Schneider DA, Wilson HL, Galligan JJ, North RA. Functional properties of heteromeric P2X1/5 receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J Auton Nerv Syst. 2000;81:249–263. doi: 10.1016/s0165-1838(00)00123-5. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama Y, Hara M, Jones EM, Fan Z, Bell GI. Cloning of rat and mouse P2Y purinoceptors. Biochem Biophys Res Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- Virginio C, North RA, Surprenant A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurones. J Physiol. 1998a;510:27–35. doi: 10.1111/j.1469-7793.1998.027bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kugelgen I, Goncalves J, Driessen B, Starke K. Corelease of noradrenaline and adenosine triphosphate from sympathetic neurones. Adv Pharmacol. 1998;42:120–125. doi: 10.1016/s1054-3589(08)60710-3. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Norenberg W, Meyer A, Illes P, Starke K. Role of action potentials and calcium influx in ATP- and UDP-induced noradrenaline release from rat cultured sympathetic neurones. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:360–369. doi: 10.1007/pl00005362. [DOI] [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ. Comparative study on the distribution patterns of P2X1-P2X6 receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecholamine cell groups. J Comp Neurol. 2000;427:485–507. [PubMed] [Google Scholar]
- Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, Yang S, Laz TM, Bayne M, Monsma F., Jr P2Y13: identification and characterization of a novel Galphai-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther. 2002;301:705–713. doi: 10.1124/jpet.301.2.705. [DOI] [PubMed] [Google Scholar]