Abstract
The termination of obstructive respiratory events is typically associated with arousal from sleep. The ventilatory response to arousal may be an important determinant of subsequent respiratory stability/instability and therefore may be involved in perpetuating obstructive respiratory events. In healthy subjects arousal is associated with brief hyperventilation followed by more prolonged hypoventilation on return to sleep. This study was designed to assess whether elevated sleeping upper airway resistance (RUA) alters the ventilatory response to arousal and subsequent breathing on return to sleep in patients with obstructive sleep apnoea (OSA). Inspired minute ventilation (VI), RUA and end-tidal CO2 pressure (PET,CO2) were measured in 22 patients (11 men, 11 women) with OSA (mean ±s.e.m., apnoea–hypopnoea index (AHI) 48.9 ± 5.9 events h−1) during non-rapid eye movement (NREM) sleep with low RUA (2.8 ± 0.3 cmH2O l−1 s; optimal continuous positive airway pressure (CPAP) = 11.3 ± 0.7 cmH2O) and with elevated RUA (17.6 ± 2.8 cmH2O l−1 s; sub-optimal CPAP = 8.4 ± 0.8 cmH2O). A single observer, unaware of respiratory data, identified spontaneous and tone-induced arousals of 3–15 s duration preceded and followed by stable NREM sleep. VI was compared between CPAP levels before and after spontaneous arousal in 16 subjects with tone-induced arousals in both conditions. During stable NREM sleep at sub-optimal CPAP, PET,CO2 was mildly elevated (43.5 ± 0.8 versus 42.5 ± 0.8 Torr). However, baseline VI (7.8 ± 0.3 versus 8.0 ± 0.3 l min−1) was unchanged between CPAP conditions. For the first three breaths following arousal, VI was higher for sub-optimal than optimal CPAP (first breath: 11.2 ± 0.9 versus 9.3 ± 0.6 l min−1). The magnitude of hypoventilation on return to sleep was not affected by the level of CPAP and both obstructive and central respiratory events were rare following arousal. Similar results occurred after tone-induced arousals which led to larger responses than spontaneous arousals. VI for the first breath following arousal under optimal CPAP was greater in men than women (11.0 ± 0.4 versus 7.6 ± 0.6 l min−1). These results demonstrate that the ventilatory response to arousal is influenced by pre-arousal airway resistance and gender. Whether this contributes to the perpetuation of respiratory events and the pathogenesis of OSA is unclear.
Obstructive sleep apnoea (OSA) is estimated to affect 2–4% of adult Americans (Young et al. 1993) and is characterized by repetitive upper airway obstruction, blood gas disturbance and arousal from sleep. Individuals with OSA demonstrate neurocognitive impairment (Kim et al. 1997), excessive daytime sleepiness (Gottlieb et al. 1999) and have higher rates of motor vehicle accidents than individuals without OSA (Young et al. 1997). There is increasing evidence that OSA is associated with an elevated risk of cardiovascular disease (Peppard et al. 2000; Shahar et al. 2001).
Historically, termination of an obstructive respiratory event by re-opening of the upper airway was thought to be reliant on arousal from sleep. However, recent research suggests that arousal may not be essential for airway opening in OSA (Younes, 2004). Despite this, the termination of respiratory events was found to be associated with arousal (either before or after airway re-opening) in the majority of cases (83% of events). In addition, the magnitude of the increase in airflow following termination of a respiratory event was greater when arousal occurred than when no arousal was identified (Younes, 2004). This led Younes to suggest that arousal from sleep may predispose to further respiratory events in OSA, by exacerbating the ventilatory changes following termination of a respiratory event and promoting hypoventilation/central respiratory instability on return to sleep.
We have recently shown that the ventilatory response to arousal from sleep is greater in healthy young men than women (Jordan et al. 2003), a difference which may potentially contribute to the high male prevalence of OSA (Young et al. 1993). In our previous study, hypoventilation was observed on return to sleep, despite airway resistance being below the pre-arousal level (Jordan et al. 2003). We hypothesize, that this low central respiratory drive, when combined with the elevated upper airway resistance which occurs in untreated or partially treated OSA, may lead to subsequent obstructive apnoea or hypopnoea. We therefore conducted the current study to determine whether the ventilatory response to arousal is greater in men than women with OSA, and whether the ventilatory response following arousal from and return to NREM sleep is altered by the level of pre-arousal airway resistance.
Methods
Subjects
Twenty-five patients (14 men, 11 women) with obstructive sleep apnoea (apnoea–hypopnoea index (AHI) > 20 events h−1) participated in the study, after giving written informed consent. All subjects had normal respiratory function (spirometry, FEV1/FVC > 85% predicted) and were non-smokers with no auditory or sleep problems other than OSA. Eight subjects (2 women) were taking anti-hypertensive medications. Women who were pre-menopausal (n = 7) were studied in the follicular menstrual phase (per history, days 5–11). The study was approved by the Human Research Committee of the Brigham and Women's Hospital and conformed to the standards set by the Declaration of Helsinki.
Equipment and measurements
A nasal mask (Gel Mask, Respironics, Murraysville, PA, USA) was secured to the subject's face with temporary glue (Spirit Gum, Rubies Costume Co, Richmond Hill, NY, USA) and a head strap. The mouth was taped shut to ensure all breathing occurred through the nasal mask. The mask was attached to a heated pneumotachometer (model 3700A, Hans Rudolf Inc, Kansas City, MO, USA) and a differential pressure transducer (Validyne Corp., Northbridge, CA, USA) for measurement of flow. Inspiratory (TI) and expiratory (TE) times were determined from this signal and it was integrated for calculation of tidal volume (VT). The expirate was continuously sampled to determine the end-tidal partial pressure of CO2 (PET,CO2; Capnograph monitor, BCI, Waukesha, WI, USA) and mask pressure (PMASK) was continuously monitored (Validyne Corp.). Epiglottic pressure (PEPI) was measured with a pressure tipped catheter (model MCP-500, Millar, Houston, TX, USA) advanced through a nostril, which had been previosuly anaesthetized (4% lidocaine HCl), to 1 cm below the tongue base under direct visualization after both nostrils were decongested (0.05% oxymetazoline HCl). Upper airway resistance (epiglottic catheter to mask) was measured at 0.2 l s−1, peak inspiratory flow and at minimum epiglottic pressure (RUA,0.2, RUA,PIF and RUA,MAX, respectively). Both RUA,PIF and RUA,MAX were measured because flow limitation developed with high airway resistance and thus RUA,PIF is difficult to interpret. Surface diaphragm electromyogram (EMGDI) was measured from three sites (the right sixth, seventh and eighth intercostals spaces, adjacent to the costal margin) but only the pair of electrodes with the highest activity during inspiration was recorded. Both the raw and blanked, rectified and moving time averaged EMGDI were recorded and the expiratory tonic and inspiratory phasic (peak inspiratory – expiratory tonic) components of the averaged signal were quantified. Two channel electroencephalography (EEG; C3-A2, O2-A1), left and right electroocculogram (EOG) and submental electromyogram (EMG) were recorded to allow sleep staging and arousal scoring. Electrocardiogram (ECG) was recorded to blank the ECG artefact from the diaphragm signal and to allow the cardiovascular response to arousal to be assessed. Given the previous finding of a smaller ventilatory response following spontaneous arousal than tone-induced arousal from sleep (Jordan et al. 2003), we chose to investigate both arousal types in this study. Subjects were fitted with ear-insert headphones (Model 33-1167, RadioShack Corporation, Fort Worth, TX, USA) for delivery of auditory tones (CoolEdit 2000, Syntrillium Software Corporation, Phoenix, AZ, USA) to induce arousals. Body position was continuously monitored with an infrared camera. All signals (flow, VT, CO2, PEPI, PMASK, EEG, EOG, EMG, ECG, sound (for auditory tones), raw and averaged EMGDI) were simultaneously recorded on a 16 channel Grass model 78 polygraph (Grass Instruments, Astro-Medical Inc, West Warwick, RI, USA) and a personal computer using an analog-to-digital converter (1401plus, Cambridge Electronic Design, Ltd, Cambridge, UK) and data acquisition software (Spike 2, version 3.19, Cambridge Electronic Design, Ltd). Sample rates varied from 125 Hz for respiratory signals to 500 Hz for raw EMGDI.
Protocol
All subjects were fitted with the equipment described above and were asked to perform a Mueller manoeuvre three times to determine the maximal diaphragm activity. This allowed the averaged EMGDI signal to be scaled between electrical zero and the maximum value. Following this, the patients were placed on continuous positive airway pressure (CPAP; BiPAP S/T-D30, Respironics) at their prescribed level (per previous clinical CPAP titration). Each was asked to lay supine, stay awake and keep their eyes open for 10 min before allowing themselves to fall asleep. Once the patient achieved stable NREM sleep (stage 2, 3 or 4 with no spontaneous or induced arousals) the adequacy of the CPAP level was checked and if necessary it was increased to abolish any flow limitation. Flow limitation was defined as a 1 cmH2O drop in epiglottic pressure without further increase in flow (Clark et al. 1998). At this time, CPAP was either maintained at this level (optimal CPAP) or was reduced to the lowest level tolerated without apnoea, hypopnea or repetitive arousal from sleep (sub-optimal CPAP) to randomise conditions. Five minutes of stable NREM sleep was then recorded and the protocol for inducing auditory arousals began. When at least 10 suitable tone-induced arousals had been collected the CPAP condition was switched (optimal to sub-optimal or vice versa) and another 10 or more tone-induced arousals were obtained. Tone-induced arousals were induced in the same manner as previously reported in normal subjects (Jordan et al. 2003). Briefly, tones (55–110 dB, 0.5 s, 1000 Hz) were played to induce brief (3–15 s) EEG-defined arousals. At least 2 min of stable NREM sleep separated each tone and if full awakening occurred (> 15 s wake) a further tone was not played until at least 5 min of stable NREM sleep had occurred. The level of CPAP was held constant during and following tones and arousals. As described below, spontaneous arousals were also evaluated.
Data analysis
The data were analysed in the same manner as previously reported in normal volunteers (Jordan et al. 2003). Briefly, 3–15 s arousals from stage 2–4 NREM sleep and succeeded by stable NREM sleep were identified with the scorer unaware of all signals other than EEG, EOG and submental EMG. Arousals occurring within 5 s of the onset of a tone were assumed to be tone-induced arousals. Arousals occurring with no tone in the 30 s preceding or 60 s following the arousal were designated as spontaneous arousals. Any arousal occurring during a change in the level of CPAP, while there was any leak (detected by positive flow at end expiration), or while not in the supine position was discarded. Breath-by-breath measures of all variables were interpolated at 4 s intervals for 32 s prior to and 60 s following each arousal (start of arousal = time 0). Tone-induced and spontaneous arousals were averaged for each sex in both optimal and sub-optimal CPAP conditions. Heart rate (HR) was measured on a beat-by-beat basis and interpolated at 1 s intervals prior to and following arousal. HR data were only analysed in those subjects not taking anti-hypertensive medications.
Statistical analyses
Baseline anthropometrical data were compared between sexes with Student's t tests. The change in all respiratory variables from wakefulness to stable NREM sleep on optimal CPAP were compared between sexes with repeated measures analysis of variance (ANOVA). Similar analyses were used to compare resting data on optimal CPAP to sub-optimal CPAP. The duration of arousal was compared between sexes, arousal type (spontaneous and tone-induced) and CPAP conditions with paired Student's t tests. Repeated measures ANOVA were used to compare changes in all variables for 60 s following brief arousal from NREM sleep. Tukey's post hoc tests were used when significant ANOVA effects were found. Mean ±s.e.m. are presented, P≤ 0.05 was considered significant. The study was powered to detect differences between the primary variables of interest (ventilatory changes after arousal between sexes and CPAP conditions). Secondary analyses of heart rate, EMGDI, etc. were performed to better understand the cause of the differences found in the primary analyses.
Results
Anthropometric and resting data
Three male subjects slept poorly and had no brief arousals arising from and returning to stable NREM sleep. The remaining 11 men and 11 women did not differ in terms of age (49.3 ± 2.0 versus 46.5 ± 2.9 years, respectively), apnoea–hypopnoea index (AHI, 49.8 ± 8.5 versus 48.0 ± 8.7 events h−1, respectively) or body mass index (BMI, 34.7 ± 1.2 versus 36.4 ± 2.6 kg m−2, respectively). Resting respiratory data during wakefulness and NREM sleep on optimal CPAP are presented in Table 1. While both sex and sleep state differences were observed, the changes with sleep were equivalent in men and women (no sleep stage–gender interaction effects).
Table 1.
Resting data during wakefulness and sleep on optimal CPAP
| Men | Women | |||
|---|---|---|---|---|
| Wake | Sleep | Wake | Sleep | |
| VI (l min−1)*† | 10.6 ± 0.4 | 9.2 ± 0.3 | 7.8 ± 0.4 | 7.2 ± 0.4 |
| VT (l) *† | 0.76 ± 0.05 | 0.65 ± 0.02 | 0.57 ± 0.06 | 0.49 ± 0.03 |
| FB (breaths min−1) | 14.7 ± 0.4 | 14.3 ± 0.3 | 15.2 ± 1.2 | 14.9 ± 0.6 |
| CPAP (cmH2O) | 11.9 ± 0.9 | 11.9 ± 0.6 | 8.6 ± 0.9 | 11.0 ± 1.2 |
| PIF (l s−1) *† | 0.69 ± 0.04 | 0.53 ± 0.02 | 0.51 ± 0.03 | 0.44 ± 0.03 |
| PET,CO2 (Torr) *† | 38.0 ± 1.3 | 41.6 ± 1.0 | 42.0 ± 0.8 | 44.0 ± 1.0 |
| PEPI (cmH2O) † | 2.8 ± 0.4 | 1.8 ± 0.2 | 2.0 ± 0.4 | 1.7 ± 0.2 |
| RUA,0.2 (cmH2O l−1 s) | 1.3 ± 0.3 | 0.9 ± 0.2 | 1.4 ± 0.4 | 1.6 ± 0.2 |
| RUA,MAX (cmH2O l−1 s) | 4.7 ± 1.6 | 2.4 ± 0.5 | 3.6 ± 0.8 | 2.9 ± 0.3 |
| EMGDI Phasic (% max) † | 17.3 ± 2.9 | 7.6 ± 0.9 | 12.8 ± 2.5 | 8.1 ± 2.0 |
| EMGDI Tonic (% max) † | 16.6 ± 4.2 | 14.9 ± 3.8 | 22.9 ± 10.2 | 17.7 ± 5.6 |
Inspired minute ventilation (VI), tidal volume (VT), breathing frequency (FB), continuous positive airway pressure (CPAP), peak inspiratory flow (PIF), end-tidal CO2(PET,CO2), minimum epiglottic pressure (PEPI), upper airway resistance measured at 0.2 l s−1 (RUA,0.2) and at minimum PEPI (RUA,MAX), and inspiratory phasic and expiratory tonic diaphragm electromyogram (EMGDI Phasic and Tonic) during resting wakefulness and stable sleep in 11 men and 11 women with obstructive sleep apnoea. Mean ±s.e.m. presented.
P < 0.05 men different to women,
P < 0.05 wake different from sleep. No gender-by-sleep state interaction effects were observed.
Gender effects
Spontaneous arousal responses on optimal CPAP
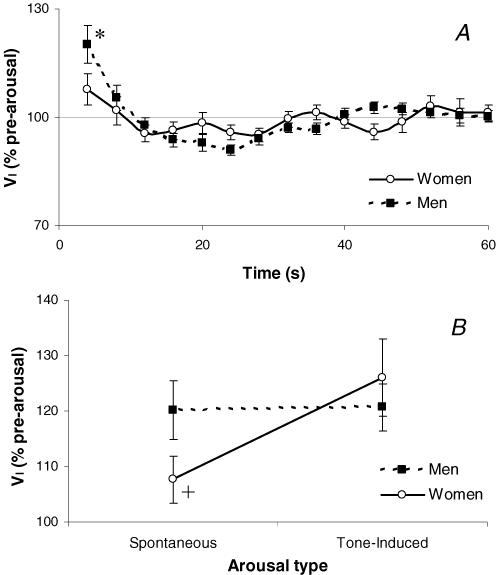
On average each subject had 5.5 ± 1.0 spontaneous arousals that fitted the criteria for analysis (range 3–20 arousals/subject). The mean duration of these arousals (7.1 ± 0.4 s in men and 7.0 ± 0.6 s in women) and the baseline conditions prior to arousal (Table 1) were equivalent in the men and women studied. Despite these similarities, the initial (4 s) increase in ventilation following spontaneous arousal from NREM sleep was greater in men than in women (P < 0.001, Fig. 1A). However, the subsequent (8–60 s) changes in ventilation (Fig. 1A) were not different between sexes. The initial (4 s) difference in VI occurred as VT increased more in men than in women following arousal and TI slightly decreased in men but remained unchanged in women. Significant gender-by-time interaction effects were also observed in PIF, PEPI, RUA 0.2 and phasic EMGDI (data not shown).
Figure 1.
The influence of gender on the ventilatory response to arousal in OSA
Inspired minute ventilation (VI) expressed as a percentage of the pre-arousal sleeping level for 60 s following spontaneous arousal from NREM sleep (A) and at 4 s following spontaneous and tone-induced arousal from sleep (B) in 11 men and 11 women with obstructive sleep apnoea while on optimal CPAP therapy. Mean ±s.e.m. presented.*P < 0.05 different between sexes at the indicated time point, +P = 0.052 trend to a significant interaction between gender and arousal type.
Tone-induced arousal responses on optimal CPAP
All 22 subjects also had tone-induced arousals from NREM sleep (average 11.4 ± 1.3 arousals per subject, range 4–22). The pre-arousal levels of VI, PET,CO2 and RUA were not different between spontaneous or tone-induced arousal types (8.2 ± 0.4 l min−1, 42.7 ± 0.7 Torr, RUA 0.2= 1.3 ± 0.2 cmH2O l−1 s prior to spontaneous arousal and 8.2 ± 0.3 l min−1, 42.7 ± 0.7 Torr, RUA,0.2= 1.2 ± 0.1 cmH2O l−1 s prior to tone-induced arousal). The duration of arousal was also similar between spontaneous and tone-induced arousals (7.0 ± 0.2 versus 7.1 ± 0.4 s, P = 0.9) and was not different between men and women following tone-induced arousal (7.0 ± 0.3 and 7.3 ± 0.4 s, respectively). The ventilatory response to tone-induced arousal was significantly greater than for spontaneous arousal (P = 0.03). However, the sex difference seen in the ventilatory response to spontaneous arousals did not exist with tone-induced arousals. This is likely to be the product of a greater ventilatory response to tone-induced arousal than spontaneous arousal in women, while men had a similar response to both arousal types (Figs 1B, P = 0.052 for gender-by-type of arousal interaction).
Upper airway resistance effects (optimal versus sub-optimal CPAP)
Resting data
Sixteen subjects (8 men) had spontaneous arousal from NREM sleep in both optimal and sub-optimal CPAP conditions, while 17 subjects (7 men) had tone-induced arousals in both CPAP conditions. The pre-arousal characteristics were not different between arousal types and so the average data on optimal and sub-optimal CPAP prior to both arousal types are presented in Table 2. Despite only a small reduction in CPAP, upper airway resistance and inspiratory phasic EMGDI were elevated and PEPI more negative on sub-optimal than optimal CPAP. VI and FB remained unchanged despite small decreases in VT and TE and prolongation of TI on sub-optimal CPAP. PET,CO2 was mildly elevated on sub-optimal CPAP.
Table 2.
Resting data during stable sleep on optimal or sub-optimal CPAP
| Optimal CPAP | Sub-optimal CPAP | |
|---|---|---|
| VI (l min−1) | 8.0 ± 0.3 | 7.8 ± 0.3 |
| VT (l) * | 0.56 ± 0.02 | 0.54 ± 0.03 |
| TI (s) * | 1.72 ± 0.05 | 1.85 ± 0.06 |
| TE (s) * | 2.47 ± 0.08 | 2.31 ± 0.08 |
| CPAP (cmH2O) * | 11.3 ± 0.7 | 8.4 ± 0.8 |
| PIF (l s−1) * | 0.47 ± 0.02 | 0.41 ± 0.02 |
| pET,co2(Torr) * | 42.5 ± 0.8 | 43.5 ± 0.8 |
| PEPI (cmH2O) * | 1.8 ± 0.1 | 5.4 ± 0.7 |
| RUA,0.2 (cmH2O l−1 s) * | 1.3 ± 0.2 | 3.8 ± 0.5 |
| RUA,MAX (cmH2O l−1 s) * | 2.8 ± 0.3 | 17.6 ± 2.8 |
| EMGDI Phasic (% max) * | 8.0 ± 1.1 | 9.1 ± 1.7 |
| EMGDI Tonic (% max) | 15.7 ± 3.3 | 17.0 ± 3.4 |
Pre-arousal respiratory data on optimal and sub-optimal CPAP during NREM sleep in 20 patients with OSA. See Table 1 for definitions.
P < 0.05 different between CPAP conditions.
Spontaneous arousal responses on optimal versus sub-optimal CPAP
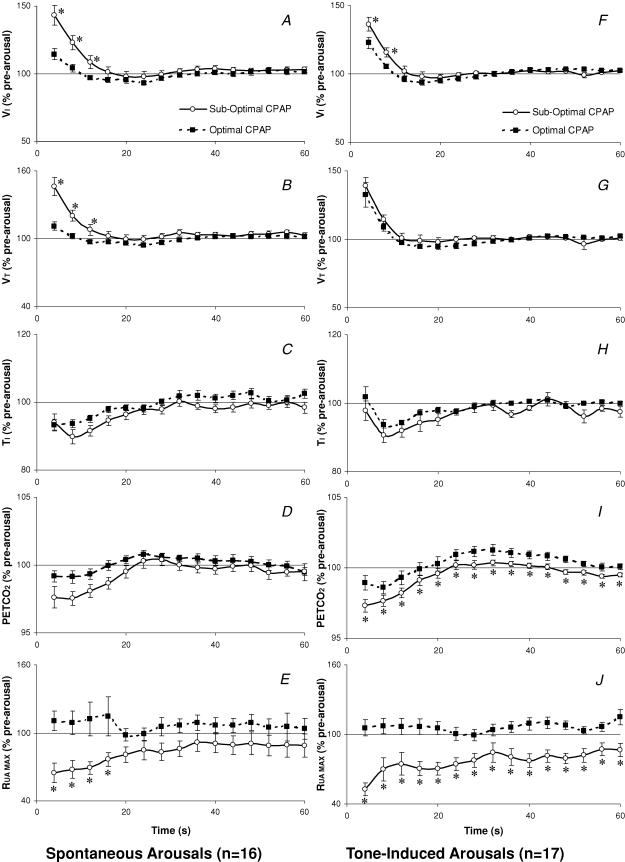
On average these 16 subjects had 4.8 ± 0.5 spontaneous arousals in each CPAP condition (range 1–12) and the mean duration of arousal was not different between CPAP conditions (6.9 ± 0.4 s versus 7.5 ± 0.3 s, P = 0.2). The increase in VI following spontaneous arousal was significantly larger when arousing from sub-optimal CPAP than optimal CPAP (P < 0.001, Fig. 2A). Similar changes were observed in VT (P < 0.001, Fig. 2B), PIF, and RUA MAX (P = 0.002, Fig. 2E). Although a significant change over time was identified for PET,CO2 (Fig. 2D), the difference between CPAP levels failed to reach statistical significance.
Figure 2.
The influence of CPAP condition on arousal responses in OSA
Inspired minute ventilation (VI), tidal volume (VT), Inspiratory Time (TI), end-tidal CO2(PET,CO2) and maximum airway resistance (RUA,MAX) expressed as a percent of the pre-arousal sleeping level for 60 s following spontaneous (A–E) and tone-induced (F–J) arousal from NREM sleep on optimal versus sub-optimal CPAP. Mean ±s.e.m. presented. *P < 0.05 different between CPAP conditions at the times indicated.
Tone-induced arousal responses on optimal versus sub-optimal CPAP
The 17 subjects included in this analysis had on average 9.9 ± 1.0 tone-induced arousals in each CPAP condition (range 1–22). The duration of arousal was not different between CPAP conditions (7.2 ± 0.3 versus 7.3 ± 0.3 s). The ventilatory response to tone-induced arousal was similar to the spontaneous arousal response (P < 0.001, Fig. 2F). Significant differences between CPAP conditions were also found in TI (P = 0.028, Fig. 2H) PIF, PEPI, PET,CO2 (P = 0.003, Fig. 2I) and RUA,MAX (P < 0.001, Fig. 2J).
Heart rate changes following arousal
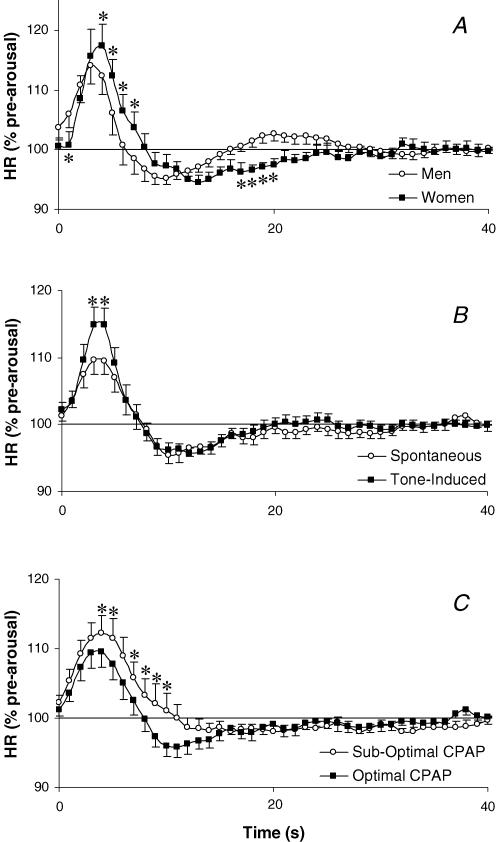
Resting heart rate was not significantly different between men and women in either the optimal (62.5 ± 2.7 and 61.8 ± 2.5 beats min−1, respectively) or sub-optimal (62.2 ± 3.0 and 59.6 ± 1.9 beats min−1, respectively) CPAP conditions. Similarly resting heart rate did not differ between spontaneous and tone-induced arousals (62.1 ± 1.8 and 60.7 ± 1.6 beats min−1, respectively). The change in heart rate following spontaneous arousal was not different between sexes when at optimal CPAP. In contrast to this, however, the heart rate response to tone-induced arousal was slightly but significantly greater and more delayed in women than in men (P = 0.002, Fig. 3A). The heart rate response to arousal was greater following tone-induced than spontaneous arousal (P < 0.001, Fig. 4B) and was also influenced by the CPAP condition, showing a larger initial tachycardia and smaller postarousal bradycardia in the sub-optimal CPAP condition (P < 0.001, Fig. 3C).
Figure 3.
The influence of gender, arousal type and CPAP condition on the heart rate response to arousal from sleep
Heart rate (HR) expressed as a percentage of the pre-arousal sleeping level for 40 s following tone-induced arousal in men and women (A), spontaneous and tone-induced arousal (B) and following spontaneous arousal on optimal and sub-optimal CPAP (C) in OSA patients. Mean ±s.e.m. presented, *P < 0.05 heart rate different between groups at the indicated time points.
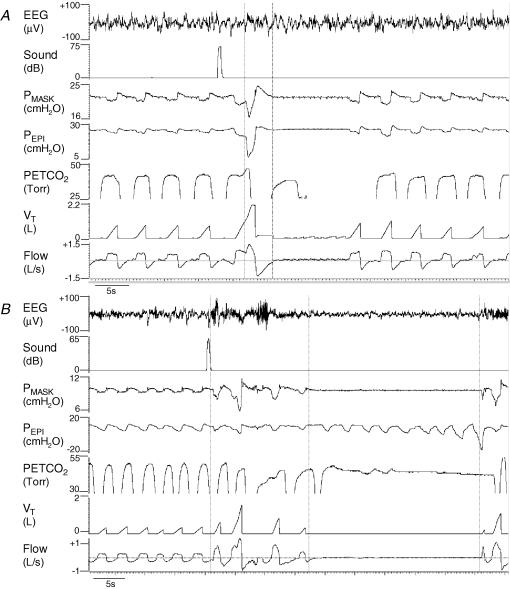
Figure 4.
Examples of a central and an obstructive event following tone-induced arousal from sleep
An example of a central apnoea (A) and an obstructive apnoea (B) in two different women with OSA following tone-induced arousals from sleep. Both women were flow limited (sub-optimal CPAP) but had stable breathing before arousal. Vertical lines indicate the start and end of EEG arousal which are difficult to discern on the compressed time scale.
Respiratory events following arousal
Both obstructive and central respiratory events were rare following arousal from NREM sleep (14 events following 740 arousals). However, four subjects (1 male) had at least one central apnoea (defined as two ‘missed’ breaths: TE > 2 ×TTOT (8.4 s) with no change in PEPI) following return to sleep after arousal. The average central apnoea length was 11.4 ± 1.3 s and these events predominantly (but not exclusively) occurred while on optimal CPAP therapy. An example of one central event is shown in Fig. 4A. Compared to all other arousals in the same subjects and of the same type (CPAP condition, arousal type), the duration of arousal was significantly longer (9.6 ± 1.0 versus 7.0 ± 0.7 s, P = 0.01) and the increase in tidal volume significantly larger (2.1 ± 0.3 versus 1.0 ± 0.1 l, P = 0.02) for those arousals which resulted in central apnoea compared to those which were not followed by any respiratory event. The pre-arousal level of PET,CO2 was not different between arousals resulting in or not resulting in central apnoeas. The magnitude of the reduction in PET,CO2 after the initial hyperventilation was not quantifiable in most situations given the difficulties of measuring PET,CO2 during central apnoea.
Four subjects (all female, one also with central events) had obstructive apnoeas (complete cessation of breathing despite negative PEPI) or hypopneas (50% reduction in flow with increased PEPI) following return to sleep after arousal (example in Fig. 4B). Arousals resulting in obstructive apnoea had larger increases in tidal volume than other arousals in the same subjects which were followed by stable breathing (1.3 ± 0.2 versus 0.5 ± 0.1 l, P = 0.02). There were no other differences between arousals resulting in obstructive apnoea and those resulting in stable breathing. However, when arousals followed by obstructive apnoea or hypopnea were compared to arousals followed by stable breathing, the influence of tidal volume was no longer statistically significant (1.0 ± 0.2 versus 0.5 ± 0.1 l, P = 0.11). Other arousals resulted in increased airway resistance following arousal; however, unless this was accompanied by a 50% reduction in flow, it was not considered to be a respiratory event. This contributes to the variability in the return of resistance in the 20 s following arousal seen in Fig. 2C.
Discussion
The main findings of this study were that the ventilatory response to spontaneous arousal from NREM sleep is greater in men than in women with OSA and is greater in both sexes when upper airway resistance is increased (sub-optimal CPAP) prior to arousal. Despite the elevated ventilatory response to arousal when upper airway resistance was increased, obstructive and central respiratory events were rare following return to sleep after brief arousal.
The increase in ventilation following arousal from sleep is thought to result from: (1) the restoration of the waking chemoresponsiveness and the increased PCO2 that develops during sleep, (2) the sudden removal of the sleep-related increase in upper airway resistance, and (3) a transient increase in neural drive or ‘waking reflex’ (Phillipson & Bowes, 1986; Khoo et al. 1998; Horner et al. 2001; Trinder et al. 2001). While the sleep-related increase in upper airway resistance was prevented in the current study during the optimal CPAP condition, all three factors contributed to the ventilatory response to arousal on sub-optimal CPAP. However, chemoresponsiveness and the neural waking reflex are not expected to change between CPAP conditions. Thus the elevated ventilatory response following arousal in the sub-optimal CPAP condition is likely to be related to either the increased airway resistance or the mild elevation of PET,CO2 in this condition. The ventilatory response to arousal has been reported to be unchanged with elevated inspired CO2 of between 3 and 7% (Carley et al. 1997) and the magnitude of increase in EMGDI activity following spontaneous arousal was found to be unchanged during mild hypocapnia induced by mechanical hyperventilation (Trinder et al. 2001). These two studies suggest that the influence of PCO2 on the ventilatory response to arousal is small and thus the markedly increased ventilatory response to arousal in the sub-optimal CPAP condition in the current study is likely to be related to increased upper airway resistance. However, the relative role of CO2 and airway resistance could not be distinguished in the current study. Khoo et al. (1996, 1998) has previously suggested that the time course of the ventilatory response to arousal is largely influenced by changes in airway resistance following arousal. The current study suggests that the magnitude of the initial increase in ventilation following arousal is also importantly influenced by airway resistance. However, an alternative explanation is that the sleep-related reduction in end-expiratory lung volume was greater in the sub-optimal CPAP condition, and thus restoration of the waking lung volume at arousal from sleep resulted in a larger increase in ventilation than was observed at optimal CPAP. This possibility clearly requires lung volume measurement for verification.
The increased ventilatory response to arousal found during sub-optimal CPAP, and the concept that an elevated ventilatory response to arousal is more likely to lead to further respiratory instability and obstruction during sleep (Khoo et al. 1996; Younes, 2004) suggest that individuals who have high airway resistance during sleep (snoring and sleep apnoeic patients) may be predisposed to further respiratory events following arousal. However, the observation that arousal from sleep in the sub-optimal CPAP condition was rarely followed by central or obstructive respiratory events argues against this. Arousals that resulted in respiratory events did lead to larger increases in tidal volume than those followed by stable breathing. Specifically, 10 of the 14 respiratory events occurred after arousals with a VT increase that was more than 20% higher than the average increase following other arousals that were not followed by respiratory events (in the same subjects). There were, however, other arousals in these subjects (with occasional respiratory events) that had similar or larger increases in VT that were not followed by apnoea/hypopnea. Thus, while arousal-induced hyperventilation appears likely to be a contributing factor to the development of subsequent respiratory events, we do not think this is the sole cause for such events. It is important to note that in the sub-optimal CPAP condition, patients with OSA were still on an average CPAP of 8.4 ± 0.8 cmH2O as we sought to increase upper airway resistance but still prevent obstructive hypopnea or apnoea during stable sleep. Clearly, many more respiratory events may follow arousal in untreated patients. However, the role of arousal and the subsequent hyperventilation in perpetuating further respiratory events in OSA is still unclear.
The ventilatory response to spontaneous arousal from NREM sleep was observed to be higher in men than women with OSA while on optimal CPAP therapy. This occurred despite the sleep-related changes in ventilation and PET,CO2 being similar in both sexes. Given that upper airway resistance was unchanged during sleep, it appears that the sex difference in ventilatory response to arousal probably results from either a different waking reflex or differing ventilatory responsiveness to CO2. We are unable to dissociate these factors in the current study. Another possibility is related to a previous report of sex differences in startle reflexes (Kofler et al. 2001) that could theoretically have influenced arousal responses. However, Trinder and colleagues (Trinder et al. 2003) have reported that the spontaneous arousal response differed from orienting or startle reflexes. As a result, we believe that sex differences in the startle reflex were not likely to contribute to the observed sex difference in spontaneous arousal response. However, we cannot exclude the possibility that the tones used to induce arousal also elicited a startle response that potentially contributed to the different responses to spontaneous and tone-induced arousals observed in men and women (Fig. 1B). Finally, we have previously reported a sex difference in the ventilatory response to arousal in healthy, young, non-obese individuals (Jordan et al. 2003) that was more marked than the sex difference in OSA patients reported in this study. This may be related to differences in study protocols (such as the CPAP requirement in OSA) or it may indicate that the influence of age, body mass or OSA on the ventilatory response to arousal is different between sexes.
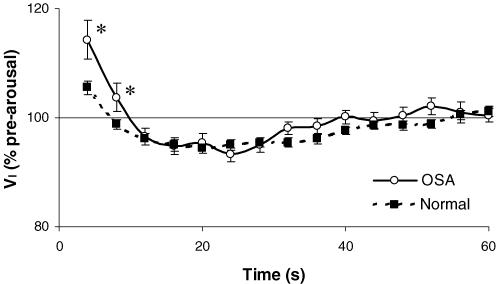
Interestingly, the ventilatory responses to both tone-induced and spontaneous arousal observed in the previous study of healthy subjects (Jordan et al. 2003) were considerably smaller than those seen in OSA patients on optimal CPAP therapy (spontaneous arousal responses for both groups shown in Fig. 5). This occurred despite the sleep-related changes in ventilation and CO2 being equivalent in both groups. The healthy subjects (who were studied in the left lateral position without CPAP) actually had slightly higher airway resistance during sleep than the OSA patients (RUA,PIF= 3.3 ± 0.7 versus 2.3 ± 0.2 cmH2O l−1 s). Thus the different ventilatory response to arousal is not likely to be explained by differing baseline conditions. Although results are mixed, there are also no consistent reports of a difference in chemoresponsiveness between OSA and normal subjects (Verbraecken et al. 1995; Appelberg & Sundstrom, 1997; Sin et al. 2000; Buyse et al. 2003; Mateika & Ellythy, 2003). Therefore, while the different ventilatory response to arousal may be related to differing subject characteristics (age and body mass), or differences between protocols regarding body position or CPAP use, it is also possible that this represents a difference in the neural waking reflex between patients with OSA and healthy volunteers. Khoo and colleagues have previously reported a trend toward a greater ventilatory response to arousal in six OSA patients compared to three healthy controls (Khoo et al. 1998). In Khoo's study, the healthy control subjects were studied both on and off CPAP and the ventilatory response to arousal was found to be reduced in normal subjects when on CPAP (possibly by reducing the small sleep-related increase in airway resistance). Thus CPAP would not appear to explain the difference between groups displayed in Fig. 5. Clearly no firm conclusions can be drawn until a sufficiently large, direct comparison between OSA patients and a suitable control group is made.
Figure 5.
Ventilatory response to arousal in patients with OSA and healthy subjects
Inspired minute ventilation (VI) expressed as a percentage of the pre-arousal sleeping level for 60 s following spontaneous arousal in 22 OSA patients (supine) on optimal CPAP and 25 healthy young subjects (left lateral position) with no CPAP from a previous study (Jordan et al. 2003). Mean ±s.e.m. presented. *P < 0.05 difference between groups at the indicated time points.
In contrast to the ventilatory response to spontaneous arousal, the change in heart rate following spontaneous arousal was not found to be different between sexes in this study. This is also in agreement with our previous study of healthy volunteers (Jordan et al. 2003). Interestingly, while the ventilatory response to tone-induced arousal was not different between men and women with OSA, the heart rate response to tone-induced arousal was significantly higher and more prolonged in women than in men despite the tone volume being equivalent between sexes. This suggests that in OSA patients, the additional cardio-respiratory stimulation following tone-induced arousal (compared to spontaneous arousal) is greater in women than in men. This is not a finding that existed in the healthy subjects previously studied and may represent an age, body mass, or OSA-related modification of the arousal response to auditory stimuli in women.
The cardiovascular system, like the respiratory system, was also influenced by the airflow resistance prior to arousal despite the pre-arousal heart rate being unchanged by CPAP level. The duration of arousal did not differ between sub-optimal or optimal CPAP conditions in the patients included in the heart rate analysis. Thus we expect the state-related changes in autonomic activity to be similar in both conditions. During sub-optimal CPAP, OSA patients developed more negative intrathoracic pressures (PEPI in Table 2) and thus probably had higher baseline vagal tone. The large negative intrathoracic pressure would not be likely to alter sympathetic activity (St Croix et al. 1999). However, lung volume was probably lower at sub-optimal CPAP and may have resulted in higher sympathetic activity compared to the optimal CPAP condition (St Croix et al. 1999). The vagal withdrawal that occurs on arousal from sleep (Horner et al. 1995) may therefore be greater in the sub-optimal CPAP condition and thus explain the greater increase in heart rate following arousal at sub-optimal CPAP. The bradycardia following arousal from sleep is thought to be related to the baroreceptors responding to the arousal-induced increase in blood pressure (Catcheside et al. 2001). In the sub-optimal CPAP condition this bradycardia was smaller (Fig. 3C). It is possible that the reduced bradycardia relates to different blood pressure responses on arousal due to the opposing changes in intrathoracic pressure in the two CPAP conditions. Following arousal at optimal CPAP, epiglottic pressure became more negative whereas at sub-optimal CPAP, epiglottic pressure was less negative after arousal as the airway suddenly re-opened. Thus, the blood pressure rise with arousal may have been reduced at sub-optimal CPAP. Clearly this explanation requires blood pressure measurements for verification. Given that airway resistance/PEPI returned to the pre-arousal level quite slowly after arousal in the sub-optimal CPAP condition, it is also possible that the return of vagal activity to the pre-arousal level is slower in the sub-optimal CPAP condition. This may also contribute to the reduced bradycardia while at sub-optimal CPAP. Whether the alteration of heart rate in response to arousal with high airway resistance has any functional significance is unknown.
There are several methodological considerations that deserve comment. First, EEG arousals were scored visually based on the standard laboratory criteria (American, 1992). We may have missed more subtle subcortical or autonomic arousals. However, we have no reason to suspect that this would preferentially bias one sex, CPAP condition, or arousal type (spontaneous versus tone-induced). In addition, this method seems appropriate given that this is how clinical sleep studies are scored. The second consideration relates to potential confounding by sleep stage, time of night, and the volume of tone required to induce arousal. There were only 16 arousals from stage 3/4 sleep and these events were evenly distributed between sexes, arousal types and CPAP conditions. Thus inclusion of these 16 trials is unlikely to have biased any comparison. Healthy individuals have previously been shown to habituate to arousing tones across a night (Bonnet, 1985). Thus if the ventilatory response to arousal is influenced by tone volume, this could have influenced the study findings. The tone volume required to arouse the OSA patients did increase across the night (Pearson's correlation P < 0.001, R2= 0.067). However, there was no relationship between the magnitude of the increase in ventilation and the volume of the tone used to arouse patients (Pearson's correlation P > 0.05, R = 0.0001). In addition, the order of CPAP conditions was randomised across the night such that any potential time of night effects are unlikely to have importantly influenced our findings. Thirdly, many comparisons were made in this study and therefore the study-wide risk of type I error is relatively high. The primary comparisons of whether gender or upper airway resistance influenced the ventilatory response to arousal in OSA patients were highly significant and therefore of importance. However, the differences observed in the secondary comparisons such as heart rate, airway resistance and PET,CO2 must be interpreted with caution as the study was not designed to test these variables. The fourth consideration regards the sub-optimal CPAP condition. While we set out to investigate the role of upper airway resistance in the ventilatory changes following arousal, we actually altered both airway resistance and PET,CO2 by lowering CPAP. Although the change in airway resistance (192% increase in RUA,0.2) was considerably larger than the change in PET,CO2 (2% increase), we cannot attribute the differences observed between CPAP conditions purely to an effect of airway resistance. Fifth, it is possible that by matching the severity of OSA in men and women, we may have systematically chosen women who are at the more severe end of the spectrum of OSA as women generally have less severe OSA. However, given that the influence of OSA severity on arousal responses is unknown we thought it important to match sexes for AHI. Finally, It is also important to note that seven of the 11 women who participated in this study were pre-menopausal. This is a higher percentage than most typical laboratory or epidemiological studies report and is likely to be related to the exclusion of individuals taking medications that may influence ventilation or sleep (such as hormone replacement therapy). Thus we cannot conclude with certainty that similar results would have been obtained had more postmenopausal women been included.
In conclusion, we have demonstrated that the ventilatory response to spontaneous arousal from NREM sleep is greater in men than women with obstructive sleep apnoea and is greater when airway resistance is elevated. The heart rate response to arousal is also enhanced with elevated airway resistance. Finally, arousal from NREM sleep was occasionally associated with central and obstructive respiratory events on return to sleep in some subjects even though each was still partially treated with CPAP. Taken together these results suggest that elevated upper airway resistance prior to arousal may predispose to arousal-induced breathing and/or upper airway instability on return to sleep. However, further research is required to more adequately assess the role of arousal in propagating respiratory events in obstructive sleep apnoea.
Acknowledgments
This work was supported by NIH/NHLBI grants: R01 HL48531, P50 HL60292, NCRR GCRC M01 RR02635. Dr Jordan is a recipient of the TSANZ/Allen and Hanbury's respiratory research fellowship.
References
- American Sleep Disorders Association Task Force. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- Appelberg J, Sundstrom G. Ventilatory response to CO2 in patients with snoring, obstructive hypopnoea and obstructive apnoea. Clin Physiol. 1997;17:497–507. doi: 10.1046/j.1365-2281.1997.05353.x. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–19. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- Buyse B, Markous N, Cauberghs M, Van Klaveren R, Muls E, Demedts M. Effect of obesity and/or sleep apnea on chemosensitivity: differences between men and women. Respir Physiol Neurobiol. 2003;134:13–22. doi: 10.1016/s1569-9048(02)00202-1. [DOI] [PubMed] [Google Scholar]
- Carley DW, Applebaum R, Basner RC, Onal E, Lopata M. Respiratory and arousal responses to acoustic stimulation. Chest. 1997;112:1567–1571. doi: 10.1378/chest.112.6.1567. [DOI] [PubMed] [Google Scholar]
- Catcheside PG, Chiong SC, Orr RS, Mercer J, Saunders NA, McEvoy RD. Acute cardiovascular responses to arousal from non-REM sleep during normoxia and hypoxia. Sleep. 2001;24:895–902. doi: 10.1093/sleep/24.8.895. [DOI] [PubMed] [Google Scholar]
- Clark SA, Wilson CR, Satoh M, Pegelow D, Dempsey JA. Assessment of inspiratory flow limitation invasively and noninvasively during sleep. Am J Respir Crit Care Med. 1998;158:713–722. doi: 10.1164/ajrccm.158.3.9708056. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Whitney CW, Bonekat WH, Iber C, James GD, Lebowitz M, Nieto FJ, Rosenberg CE. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol. 1995;79:151–162. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO2 levels between sleep and wakefulness. J Physiol. 2001;534:881–890. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. The ventilatory response to brief arousal from NREM sleep is greater in men than in women. Am J Respir Crit Care Med. 2003;168:1512–1519. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Koh SS, Shin JJ, Westbrook PR, Berry RB. Ventilatory dynamics during transient arousal from NREM sleep: implications for respiratory control stability. J Appl Physiol. 1996;80:1475–1484. doi: 10.1152/jappl.1996.80.5.1475. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Shin JJ, Asyali MH, Kim TS, Berry RB. Ventilatory dynamics of transient arousal in patients with obstructive sleep apnea. Respir Physiol. 1998;112:291–303. doi: 10.1016/s0034-5687(98)00041-3. [DOI] [PubMed] [Google Scholar]
- Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156:1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- Kofler M, Muller J, Reggiani L, Valls-Sole J. Influence of gender on auditory startle responses. Brain Res. 2001;921:206–210. doi: 10.1016/s0006-8993(01)03120-1. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Ellythy M. Chemoreflex control of ventilation is altered during wakefulness in humans with OSA. Respir Physiol Neurobiol. 2003;138:45–57. doi: 10.1016/s1569-9048(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Bowes G. Control of breathing during sleep. In: Cherniack N S, Widdicombe J G, editors. The Respiratory System. Vol. 2. Bethesda: American Physiological Society; 1986. pp. 649–689. part 2. [Google Scholar]
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Sin DD, Jones RL, Man GC. Hypercapnic ventilatory response in patients with and without obstructive sleep apnea: do age, gender, obesity, and daytime PaCO2 matter. Chest. 2000;117:454–459. doi: 10.1378/chest.117.2.454. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.res.85.5.457. [DOI] [PubMed] [Google Scholar]
- Trinder J, Allen N, Kleiman J, Kralevski V, Kleverlaan D, Anson K, Kim Y. On the nature of cardiovascular activation at an arousal from sleep. Sleep. 2003;26:543–551. [PubMed] [Google Scholar]
- Trinder J, Padula M, Berlowitz D, Kleiman J, Breen S, Rochford P, Worsnop C, Thompson B, Pierce R. Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. J Appl Physiol. 2001;90:1455–1463. doi: 10.1152/jappl.2001.90.4.1455. [DOI] [PubMed] [Google Scholar]
- Verbraecken J, De Backer W, Willemen M, De Cock W, Van de Wittesaele W & H. Chronic CO2 drive in patients with obstructive sleep apnea and effect of CPAP. Respir Physiol. 1995;101:279–287. doi: 10.1016/0034-5687(95)00037-e. [DOI] [PubMed] [Google Scholar]
- Younes MK. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- Young T, Blustein J, Finn L, Palta M. Sleep-disordered breathing and motor vehicle accidents in a population- based sample of employed adults. Sleep. 1997;20:608–613. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]