Abstract
Activation of CB1 cannabinoid receptors in the cerebellum acutely depresses excitatory synaptic transmission at parallel fibre–Purkinje cell synapses by decreasing the probability of glutamate release. This depression involves the activation of presynaptic 4-aminopyridine-sensitive K+ channels by CB1 receptors, which in turn inhibits presynaptic Ca2+ influx controlling glutamate release at these synapses. Using rat cerebellar frontal slices and fluorometric measures of presynaptic Ca2+ influx evoked by stimulation of parallel fibres with the fluorescent dye fluo-4FF, we tested whether the CB1 receptor-mediated inhibition of this influx also involves a direct inhibition of presynaptic voltage-gated calcium channels. Since various physiological effects of CB1 receptors appear to be mediated through the activation of PTX-sensitive proteins, including inhibition of adenylate cyclases, activation of mitogen-activated protein kinases (MAPK) and activation of G protein-gated inwardly rectifying K+ channels, we also studied the potential involvement of these intracellular signal transduction pathways in the cannabinoid-mediated depression of presynaptic Ca2+ influx. The present study demonstrates that the molecular mechanisms underlying the CB1 inhibitory effect involve the activation of the PTX-sensitive Gi/Go subclass of G proteins, independently of any direct effect on presynaptic Ca2+ channels (N, P/Q and R (SNX-482-sensitive) types) or on adenylate cyclase or MAPK activity, but do require the activation of G protein-gated inwardly rectifying (Ba2+- and tertiapin Q-sensitive) K+ channels, in addition to 4-aminopyridine-sensitive K+ channels.
In the cerebellar cortex, activation of presynaptic type 1 cannabinoid receptors (CB1) (Herkenham et al. 1991) inhibits excitatory synaptic transmission at parallel fibre (PF)–Purkinje cell (PC) synapses by decreasing the probability of glutamate release (Levenes et al. 1998). This results from the activation of presynaptic 4-aminopyridine-sensitive K+ channels which in turn inhibit presynaptic Ca2+ influx controlling glutamate release at these synapses (Daniel & Crepel, 2001). However, in the latter study, we did not determine whether this CB1 receptor-mediated inhibition of presynaptic Ca2+ influx also implies a direct inhibition of presynaptic voltage-gated calcium channels (VGCCs). At PF–PC synapses there are at least three pharmacologically distinguishable types of VGCCs that synergistically control glutamate release (Mintz et al. 1995). In these fibres, presynaptic Ca2+ influx occurs through (i) ω-agatoxin IVA (ω-Aga-IVA)-sensitive P/Q-type VGCCs, (ii) ω-conotoxin GVIA-sensitive N-type VGCCs and (iii) other VGCCs resistant to ω-Aga IVA, ω-conotoxin GVIA and dihydropyridines (Mintz et al. 1995). These other VGCCs might correspond to R-type channels, or to more than one type of as-yet-unidentified VGCC (see Wu & Sagau, 1997). In heterologous expression systems and cell cultures, cannabinoid receptor activation has been shown to inhibit P/Q- and N-type VGCCs (Caulfield & Brown, 1992; Mackie & Hille, 1992; Mackie et al. 1995; Pan et al. 1996; Twitchell et al. 1997; Sullivan, 1999; Nogueron et al. 2001). On the other hand, the inhibitory effect of cannabinoids in rat striatal slices involves N- but not L-, P- or Q-type VGCCs (Huang et al. 2001), while in slices from the mouse nucleus accumbens or lateral amygdala the same effect does not involve any of these channels (Robbe et al. 2001; Azad et al. 2003). Finally, in hippocampal brain slice preparations, endocannabinoids, acting as retrograde messengers, mediate a selective presynaptic inhibition of N-type Ca2+ channels (Wilson et al. 2001). Such direct inhibition of presynaptic VGCCS might also be operational at cerebellar PFs following activation of presynaptic CB1 receptors, and thus might participate in the reduction of glutamate release at PF–PC synapses.
Cannabinoid CB1 receptors have an amino acid sequence typical of G protein-coupled receptors (Matsuda et al. 1990), and many physiological effects of cannabinoids appear to be mediated through activation of PTX-sensitive Gi/Go proteins (Caulfield & Brown, 1992; Mackie & Hille, 1992; Holwett, 1995; Mackie et al. 1995; Pan et al. 1996; Twitchell et al. 1997; Prather et al. 2000; Nogueron et al. 2001) and negative coupling to adenylate cyclase (for a review see Childers & Deadwyler, 1996; also Holwett, 1985; Pacheco et al. 1993; Jung et al. 1997). However, other studies have reported that CB1 receptor activation can also stimulate adenylate cyclase activity, probably through the Gs subclass of G proteins (Glass & Felder, 1997; Maneuf & Brotchie, 1997; Felder et al. 1998). Finally, agonist stimulation of CB1 receptors may activate other signal transduction pathways via the PTX-sensitive Gi/Go proteins. In particular, evidence that the CB1 receptor is functionally coupled through these proteins to the mitogen-activated protein kinases (MAPKs) (Bouaboula et al. 1995, 1999; Derkinderen et al. 2001, 2003) or to the inwardly rectifying K+ channels (Mackie et al. 1995; McAllister et al. 1999) has been reported.
Here we show that CB1 inhibition of presynaptic Ca2+ transients evoked by PF stimulation is mediated through the activation of the PTX-sensitive Gi/Go subclass of G proteins. This effect is independent of any direct inhibition of presynaptic Ca2+ channels (N, P/Q and R (SNX-482-sensitive) types) and of any modulation of adenylate cyclase or MAPK activity, but does involve activation of G protein-gated inwardly rectifying (Ba2+ and tertiapin-sensitive) K+ channels, in addition to 4-aminopyridin-sensitive K+ channels. These presynaptic molecular events were explored in rat cerebellar slices using fluorometric methods and the fluorescent low affinity Ca2+-sensitive dye fluo-4FF.
Methods
Preparation of cerebellar slices
Animal care and all experimental procedures used during experiments were in accordance with CNRS national guidelines. Coronal or saggital slices were prepared from the vermis of the cerebellum of male Sprague-Dawley rats aged 17–36 days. In brief, the rats were stunned and decapitated, and cerebellar slices (200–250 μm thick) were cut with a vibroslicer (Campden Instruments Ltd). The slices were kept at room temperature for at least 1 h before recording in a storage chamber of saline solution saturated with 95% O2–5% CO2. This solution contained (mm): NaCl, 124; KCl, 3; NaHCO3, 24; KH2PO4, 1.15; MgSO4, 1.15; CaCl2, 2; glucose, 10 (330 mosmol l−1, pH 7.35 at 25°C). For experiments with the K+ channel blocker Ba2+, slices were perfused with an external Hepes-based Ringer solution saturated with O2 that contained (mm): NaCl, 138; KCl, 3; Mg Cl2, 2, CaCl2, 2; glucose, 10; Hepes 10 (330 mosmol l−1, adjusted to pH 7.35). The recording chamber was perfused at a rate of 1.8 ml min−1 with the saline solutions and the GABAA receptor antagonist bicuculline methiodide (10 μm, Sigma Aldrich, St Quentin Fallavier, France) added.
Drugs and solution
All pharmacological agents were applied to cerebellar slices by direct addition to the saline solution, with the exception of pertussis toxin (PTX). In the experiments involving PTX treatment, the cerebellar slices were incubated in the saline solution containing the toxin (2 μg ml−1) from 1 h after their preparation and for 14–15 h before the recording session. Stock solutions of ω-agatoxin TK (Sigma Aldrich; Alomone Labs, Israel) ω-conotoxin GVIA (Sigma Aldrich; Alomone Labs), SNX-482 (Alomone Labs) and tertiapin-Q (Sigma Aldrich) were prepared in distilled water. Stock solutions of nifedipine (ICN Pharmaceuticals, Orsay, France) were dissolved in ethanol (final concentration of ethanol 0.1%). All these stock solutions were kept at −20°C until the day of experiment, and individual drugs were diluted in saline solution to their final concentration just before application. PD98059 and WIN55,212-2 were purchased, respectively, from Calbiochem and Tocris (Illkirch, France), SR141716-A was a kind gift from Sanofi-Recherche (Montpellier, France). Stocks of PD98059, WIN55,212-2, SR141716-A and forskolin were prepared in dimethylsulfoxide (DMSO), kept at −20°C until the day of experiment, then added to the saline solution at the desired concentration just before application (final concentration of DMSO 0.1%). After each experiment the perfusion system and the recording chamber were carefully washed with dilute ethanol and distilled water, to avoid any carry-over of cannabinoids. All other compounds were obtained from Sigma (Sigma Aldrich), unless stated otherwise.
Calcium-sensitive fluorometric measurements
In coronal slices, presynaptic parallel fibre (PF) tracts were labelled by local application of a saline solution containing the membrane-permeant form of the fluorescent low affinity calcium-sensitive dye fluo-4FF AM (100 μm, Molecular Probes), as previously described (Daniel & Crepel, 2001). Cerebellar slices were positioned on an upright microscope (Nikon or Zeiss) and calcium-sensitive fluorometric changes were measured through the × 40 or × 60 water-immersion objective. The experiments were conducted at 28°C and started at least 45 min after loading. Optical signals were recorded through a 20 μm × 50 μm window, placed on the visible narrow band of labelled PFs, approximately 400–700 μm away from the loading site. Parallel fibres located in the recording window were stimulated every minute, with a single 100 Hz train of five to seven electrical stimuli, through a saline-filled glass electrode positioned in the molecular layer between the loading site and the recording site. A confined region of labelled PFs was illuminated with a xenon or mercury lamp. The fluo-4FF filter sets used were 485DF22 (485 ± 22 nm) for excitation, and DM505 (505 nm) dichroic and 530DF30 (530 ± 30 nm) for emission. Fluorometric signals were collected with a photometer and analysed on- and off-line using the Acquis1 computer program. The fluorescence data were expressed as ΔF/F, where F is the baseline fluorescence intensity, and ΔF is the change induced by PF stimulation. When an unlabelled region of the slice background fluorescence was greater than 5% of the fluorescence of the indicator, the fluorescence data were corrected for background fluorescence.
Electrophysiology
Whole-cell patch-clamp recordings of PCs from sagittal slices were performed at a somatic level with an Axopatch-1D amplifier (Axon instruments). Patch pipettes (2–3.5 MΩ) were filled with an internal solution containing (mm): KCl, 140; NaCl, 8; Hepes, 10; EGTA, 5, CaCl2, 0.5, ATP-Mg, 2 (pH 7.3 with KOH; 300 mosmol l−1). As reported previously (Goossens et al. 2001), PCs were clamped at −70 mV and PFs were stimulated at 0.33 Hz through an extracellular glass saline-filled monopolar electrode, placed at the surface of the slice to evoke PF-mediated excitatory postsynaptic currents (EPSCs).
Results
Consistent with our previous report (Daniel & Crepel, 2001), a train of five to seven stimulations of PFs induced reproducible transient increases in presynaptic fluorescence, which returned to resting levels within a few hundred milliseconds (a in Fig. 1A–B). The peak amplitude of the fluorescence transients was 7.8 ± 0.6% (ΔF/F) (mean ±s.e.m., n = 65).
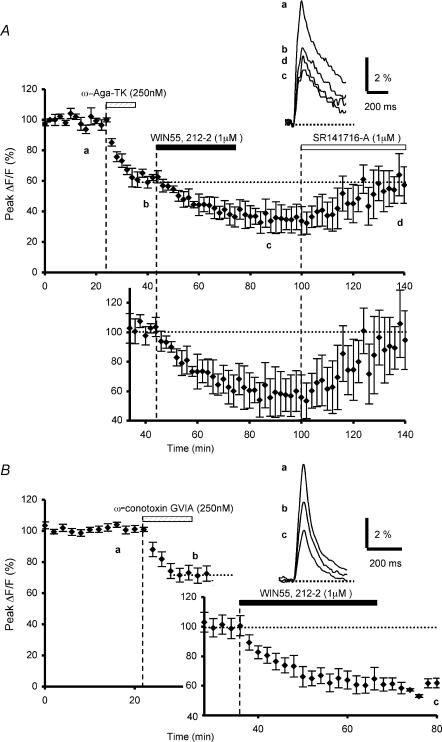
Figure 1. Effects of ω-agatoxin TK or ω-conotoxin GVIA preapplication on WIN55,212-2-mediated inhibition of presynaptic calcium influx.
A, the plot represents the normalized amplitudes of peak fluo-4FF fluorescence transients (ΔF/F) evoked by 5–7 stimulations (delivered at 100 Hz) of the parallel fibres recorded as a function of time, before, during and after sequential bath application of ω-agatoxin TK (250 nm) and WIN55,212-2 (1 μm). Each point is the mean ±s.e.m. of 4 separate experiments. Note that the inhibitory effect of WIN55,212-2 (1 μm) on the normalized amplitude of peak fluorescence transients was reversed by bath application of the selective CB1 antagonist SR141716-A (1 μm, n = 4). The top inset displays superimposed averaged fluorescence changes in one of these experiments, recorded at the indicated times. Each trace is an average of 5–10 consecutive trials. The bottom inset (graph) shows the WIN55,212-2-mediated reduction of the fluorescence transients normalized to the plateau level obtained after application of ω-agatoxin TK. B, the same as in A with sequential application of ω-conotoxin GVIA (250 nm) and WIN55,212-2 (1 μm) (n = 6). Insets as in A. Note that preapplication of ω-agatoxin TK or ω-conotoxin GVIA does not prevent the WIN55,212-2-mediated depressant effect on presynaptic Ca2+ influx.
To test the hypothesis that activation of presynaptic CB1 receptors at PF–PC synapses reduces glutamate release, not only by activation of presynaptic 4-aminopyridine-sensitive K+ channels (Daniel & Crepel, 2001), but also by directly inhibiting presynaptic VGCCs, we used specific toxins that target the different Ca2+ channels known to participate in the neurotransmitter release process at PF–PC synapses (Mintz et al. 1995).
Firstly, using ω-agatoxin TK, which blocks presynaptic P/Q-type Ca2+ channels, we investigated the potential involvement of these channels in the cannabinoid-mediated inhibition of presynaptic Ca2+ transients evoked by PF stimulation. In accordance with previous data (Mintz et al. 1995), a 10 min bath application of ω-agatoxin TK (250 nm) irreversibly inhibited the Ca2+-sensitive fluorescence transients elicited by PF, with a resulting mean decrease in amplitude of the fluorescence transients (plateau) of 38.2 ± 3.7% (mean ±s.e.m., n = 4, Fig. 1A). From this plateau, subsequent application of the CB1 receptor agonist WIN55,212-2 (1 μm) further inhibited presynaptic Ca2+ transients with a resulting final mean decrease in amplitude of 63.7 ± 7.0% (n = 4, Fig. 1A). Thus, the WIN55,212-2-mediated reduction of the fluorescence transients, expressed as a percentage of the amplitude of these signals recorded at the plateau level following the ω-agatoxin TK effect, was ≅ 38%. This value was not significantly different (Student's t test; P > 0.6) of our previously published results in which application of this CB1 agonist alone resulted in a ≅ 33% reduction of the presynaptic fluorescence transients (Daniel & Crepel, 2001). Moreover, the depressant effect of WIN55,212-2 on these transients was reversed by subsequent application of the CB1 receptor antagonist SR141716-A (1 μm) (n = 4, Fig. 1A), demonstrating the specific involvement of CB1 receptors.
To study the potential involvement of N-type Ca2+ channels in the cannabinoid-mediated inhibition of presynaptic Ca2+ transients, we employed ω-conotoxin GVIA, which specifically blocks these Ca2+ channels. Again in agreement with the previous report by Mintz et al. (1995), a 10 min bath application of ω-conotoxin GVIA (250 nm) irreversibly inhibited presynaptic fluorescence transients by 27.9 ± 4.7% (n = 6, Fig. 1B). Subsequent application WIN55,212-2 (1 μm) further reduced presynaptic Ca2+ transients, resulting in a final mean amplitude reduction of 56.7 ± 2.3% (n = 6, Fig. 1B). Here again, the WIN55, 212-2-induced inhibitory effect, expressed as a percentage of the amplitude of the fluorescence signals at the plateau level following ω-conotoxin GVIA effect, was ≅ 37%, i.e. a value not significantly different (Student's t test; P > 0.3) to that observed with WIN55,212-2 application alone (Daniel & Crepel, 2001).
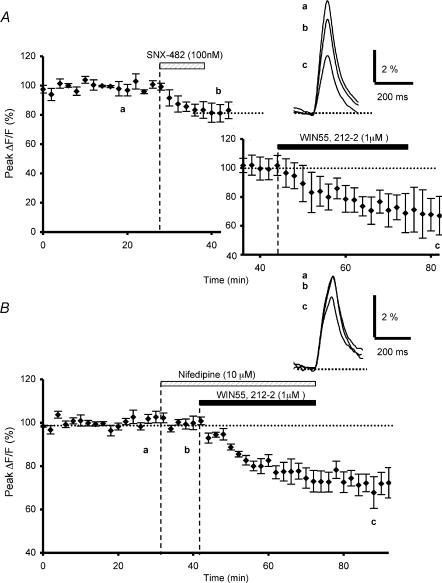
Parallel fibres also possess other VGCCs that control glutamate release and are distinguishable from N and P/Q types, since part of presynaptic Ca2+ influx remains unaffected by combined application of ω-Aga-IVA and ω-conotoxin GVIA (Mintz et al. 1995). Some of these channels resistant to the aforementioned blockers could be R-type Ca2+ channels (Ellinor et al. 1993; Zhang et al. 1993). While these channels have not been formally identified on PFs, their existence has been hypothesized by Randall & Tsien (1995) and demonstrated by Tottene et al. (2000) in rat cerebellar granule neurones in primary culture. In these neurones, the R-type Ca2+ channels are all encoded by the pore-forming α1-E subunit gene (Soong et al. 1993), probably with different isoforms, which could explain their different pharmacological properties (Tottene et al. 2000). Using the recently developed polypeptide toxin SNX-482, a selective antagonist of the α1-E subunit (Newcomb et al. 1998), Tottene et al. (2000) have shown that some of the native R-type Ca2 channels recorded in the soma of cerebellar granule cells are sensitive to this toxin, and some are resistant. Ten minute bath applications of SNX-482 (100 nm) significantly reduced the presynaptic fluorescence transients by 17.5 ± 4.4% (n = 3, Fig. 2A), suggesting that R-type (SNX-482-sensitive) VGCCs are present on PFs. However, this toxin did not abolish the subsequent decrease of fluorescent transients induced by WIN55,212-2. Indeed, the resulting mean decrease in amplitude of these transients was 55.3 ± 9.1% after sequential application of SNX-482 and WIN55,212-2 (n = 3, Fig. 2A). Thus, the WIN55,212-2-induced inhibitory effect, expressed as a percentage of the amplitude of the fluorescence signals at the plateau level following SNX-482 application, was ≅ 34%, a value not significantly different to that observed in control conditions (Student's t test; P > 0.8) (see Daniel & Crepel, 2001) and after application of ω-agatoxin TK (Student's t test; P > 0.7) or ω-conotoxin GVIA (Student's t test; P > 0.5) (see above).
Figure 2. Effects of SNX-482 or nifedipine preapplication on WIN55,212-2-mediated inhibition of presynaptic calcium influx.
A, time course of fluo-4FF fluorescence transients before, during and after sequential bath application of SNX-482 (100 nm) and WIN55,212-2 (1 μm) (n = 3). The insets show fluorescence transients in one of these experiments and the WIN55,212-2-mediated reduction of the fluorescence transients normalized to the plateau level obtained after application of SNX-482. Note that preapplication of SNX-482 does not prevent the WIN55,212-2-mediated depressant effect on presynaptic Ca2+ influx. B, the same as in A with application of nifedipine (10 μm) and WIN55,212-2 (1 μm) (n = 5). Insets as in A. Note that application of nifedipine does not alter the amplitude of presynaptic Ca2+ influx and does not prevent the WIN55,212-2-mediated depressant effect on Ca2+ influx.
Finally, in keeping with previous results by Mintz et al. (1995), presynaptic fluorescence transients evoked by PF stimulation were unaffected (n = 5, Fig. 2B) by a 10 min bath application of the dihydropiridine derivative nifedipine (10 μm), a blocker of L-type Ca2+ channels (Rampe & Triggle, 1990). This confirms that these channels are not involved in the glutamate release process at PF–PC synapses, even if they are present on cerebellar granule neurones in culture (Randall & Tsien, 1995; Pearson et al. 1995). In addition, in the presence of this toxin, the WIN55,212-2-mediated inhibitory effect on presynaptic fluorescence signals was ≅ 29% (n = 5, Fig. 2B), a value not significantly different (Student's t test; P > 0.5) to that observed in control conditions (see Daniel & Crepel, 2001), demonstrating that treatment with an L-type Ca2+ channel blocker did not alter the cannabinoid-mediated depressant effect.
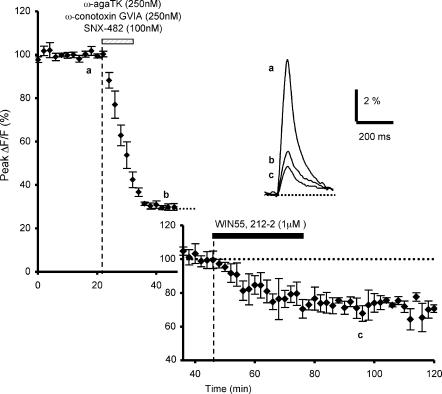
We also investigated possible cross-interactions between CB1 receptors and the different presynaptic Ca2+ channels by combined toxin treatment (ω-agatoxin TK (250 nm), ω-conotoxin GVIA (250 nm) and SNX-482 (100 nm)), followed by addition of WIN55,212-2. Co-application of these blockers decreased fluorescence transients by 70.1 ± 1.2% (n = 4, Fig. 3). Subsequent application WIN55,212-2(1 μm) further reduced presynaptic Ca2+ transients, with a resulting final mean decrease in amplitude of 79.5 ± 1.4% (n = 4, Fig. 3). Thus, the WIN55,212-2-induced inhibitory effect expressed as a percentage of the amplitude of the fluorescence signals at the plateau level reached with the combined blockers, was ≅ 32%, a value not significantly different to that observed in control conditions (Student's t test; P > 0.8) (see Daniel & Crepel, 2001) and after application of ω-agatoxin TK (Student's t test; P > 0.5), or ω-conotoxin GVIA (Student's t test; P > 0.1), or SNX-482 (Student's t test; P > 0.7) alone (see above).
Figure 3. Effects of preapplication of combined toxin treatment on WIN55,212-2-mediated inhibition of presynaptic calcium influx.
Time course of fluo-4FF fluorescence transients before, during and after bath application of combined blockers (ω-agatoxin TK (250 nm), ω-conotoxin GVIA (250 nm) and SNX-482 (100 nm)) followed by application of WIN55,212-2 (1 μm) (n = 4). The insets show the fluorescence transients in one of these experiments (top) and the WIN55,212-2-mediated reduction of the fluorescence transients normalized to the plateau level obtained after combined toxin treatment (bottom).
Taken together, these data demonstrate that the CB1 receptor-mediated depression of presynaptic Ca2+ influx is not due to a direct inhibition of presynaptic P/Q-, N- and R (SNX-482-sensitive)-type VGCCs.
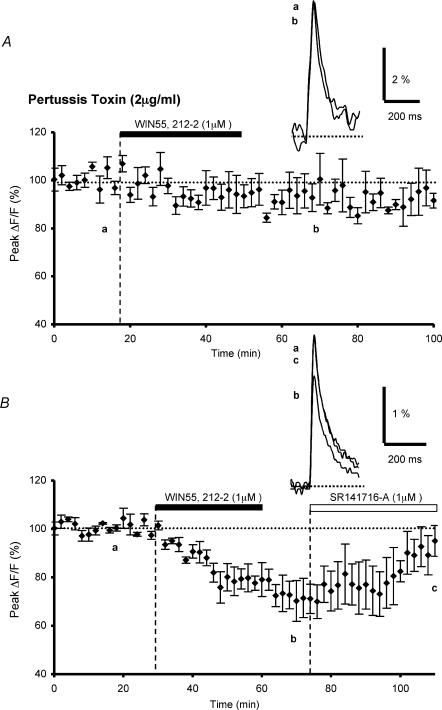
In an attempt to determine whether the intracellular signals mediating the WIN55,212-2-induced inhibition of glutamate release required the activation of the Gi/Go subclass of G proteins, slices were treated with PTX (2 μg ml−1) for 14–15 h before the recording session (see Methods). In these conditions, WIN55,212-2 application had no discernable effect on fluorescence transients, since, on average, 20 min after application of this cannabinoid agonist, the decrease in amplitude of the fluorescence transients was 4.1 ± 5.1% (n = 5, Fig. 4A). In contrast, control slices incubated in the same experimental conditions for an equivalent period of time, but without PTX, showed a clear WIN55,212-2-mediated decrease in the amplitude of fluorescence transients, averaging 29.7 ± 5.9% (n = 3, Fig. 4B). Thus, under these conditions, the magnitude of the WIN55,212-2-mediated inhibitory effect (≅ 30%), was not significantly different (Student's t test; P > 0.4) to that observed when the interval between slice preparation and the recording session was around 3–6 h (see Daniel & Crepel, 2001). Moreover, in the present experiments, the peak amplitude of the fluorescence transients recorded in control slices incubated for long periods of time (8.0 ± 1.9% (ΔF/F), n = 3), was not significantly different from those recorded in control slices without incubation (8.2 ± 0.7% (ΔF/F), n = 46) (Student's t test; P < 0,01). Therefore, these data demonstrate that the inhibitory action of WIN55,212-2 on presynaptic Ca2+ transients is mediated exclusively through the Gi/Go subclass of G proteins.
Figure 4. Effects of pertussis toxin treatment on WIN55,212-2-mediated inhibition of presynaptic calcium influx.
A, plot of normalized amplitudes of peak fluorescence transients before, during and after bath application of WIN55,212-2 (1 μm) (n = 5). Experiments were performed with slices preincubated with pertussis toxin (2 μg ml−1) during 14–15 h. Note that PTX preincubation prevents the WIN55,212-2-induced inhibition of peak fluorescence transients. The insets represent superimposed averaged fluorescence changes in one of these experiments. B, the same as in A with slices incubated in the same experimental conditions for an equivalent period of time, but without PTX (n = 3). Note the clear inhibitory effect of WIN55,212-2 on the fluorescence transients and reversal of the effect by bath application of the selective CB1 antagonist SR141716-A (1 μm, n = 4). Inset as in A.
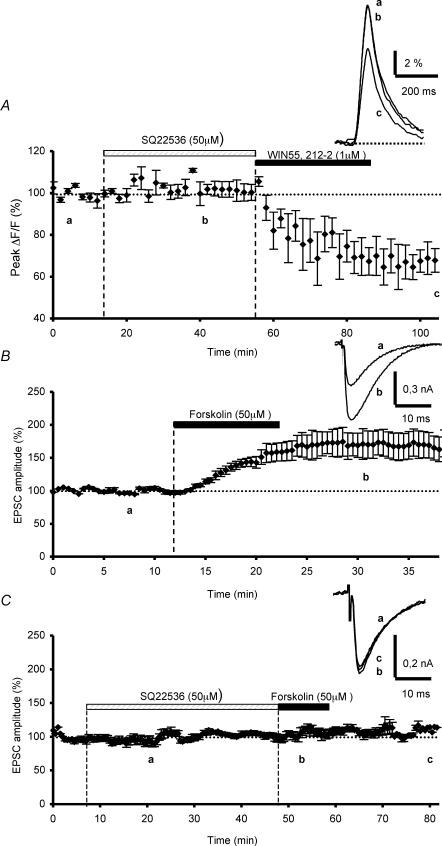
To address the question of a possible participation of adenylate cyclase in the CB1 receptor-mediated depression of presynaptic Ca2+ influx, we carried out experiments with SQ 22,536, an analogue of adenosine that appears to be a broad-spectrum blocker of adenylate cyclase (Harris et al. 1979). A 40 min bath application of SQ 22,536 prior to WIN55,212-2 application, had no effect on control fluorescence transients evoked by PF stimulation (n = 4, Fig. 5A). Moreover, this inhibitor did not prevent the subsequent inhibitory effect of WIN55,212-2 on these transients, since the cannabinoid agonist inhibition of fluorescence transients was 35.2 ± 6.6% on average (n = 4, Fig. 5A), which was not significantly different (Student's t test; P > 0.7) to the WIN55,212-2-mediated inhibitory effect in control conditions (see Daniel & Crepel, 2001).
Figure 5. Effects of adenylate cyclase blockade on WIN55,212-2-mediated inhibition of presynaptic calcium influx and on forskolin-induced enhancement of parallel fibre synaptic responses.
A, plot of normalized amplitudes of peak fluorescence transients before, during and after bath sequential application of SQ 22,536 (50 μm) and WIN55,212-2 (1 μm) (n = 4). Note that in these experiments, the adenylate cyclase inhibitor did not prevent the WIN55,212-2-induced inhibition of peak fluorescence transients. The insets represent superimposed averaged fluorescence changes in one of these experiments. B, plot of normalized amplitudes of PF-mediated EPSCs as a function of time before, during and after bath application of forskolin (50 μm) (n = 4). Note the large endurable increase of PF-mediated EPSCs amplitude. Inset, superimposed averaged PF-mediated EPSCs recorded in one of these experiments. Each trace is an average of 10 consecutive trials. C, prevention of the forskolin-induced long-term enhancement of the normalized amplitudes of PF-mediated EPSCs by prior bath application SQ 22,536 (50 μm) (n = 5)
As a control, we performed additional electrophysiological experiments to test the efficiency of this pharmacological inhibitor of adenylate cyclase in our conditions. At PF–PC synapses, elevation of cAMP levels by forskolin, an activator of adenylate cyclase, has been shown to endurably enhance neurotransmitter release (Salin et al. 1996; Chen & Regher, 1997), through a presynaptic mechanism that does not alter presynaptic Ca2+ influx or resting Ca2+ levels, but directly increases the probability of vesicular release (Chen & Regher, 1997). Thus, we reasoned that this long-term enhancement of synaptic strength involving adenylate cyclase, downstream from Ca2+ influx, might provide a sensitive means to test the efficiency of the pharmacological inhibitor SQ 22,536. According to previous reports (Salin et al. 1996; Chen & Regher, 1997), a clear enhancement in the amplitude of PF-mediated EPSCs was seen following 10 min bath applications of 50 μm forskolin, averaging 164.6 ± 16.6% (n = 4, Fig. 5B). In contrast, preapplication of SQ 22,536 (50 μm) for 40 min, rendered forskolin ineffective in enhancing PF-mediated responses (n = 5, Fig. 5C), demonstrating that in our experimental conditions, adenylate cyclase was totally blocked. Taken together, these data establish that an adenylate cyclase-coupled signalling pathway does not mediate the depressant effect of WIN55,212-2 on presynaptic Ca2+ influx.
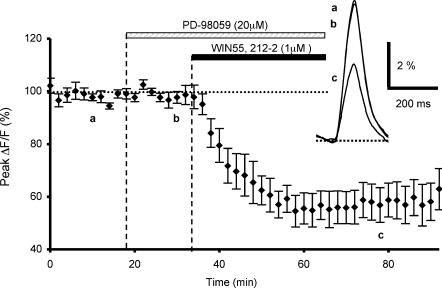
In another set of experiments, we examined the possible involvement of MAPKs in the CB1 receptor-mediated depression of presynaptic Ca2+ influx. We used PD98059, a cell-permeable specific inhibitor of MAPKs (Alessi et al. 1995). PD98059 (20 μm) was bath applied 15 min prior to and during WIN55,212-2 application. This inhibitor affected neither control fluorescence transients evoked by PF stimulation (n = 6, Fig. 6), nor the subsequent depressant effect of WIN55,212-2 on these transients, since the cannabinoid agonist inhibition of fluorescence transients was 40.9 ± 5.8% on average (n = 6, Fig. 6), which was not significantly different (Student's t test; P > 0.2) to the WIN55,212-2-mediated inhibitory effect in control conditions (see Daniel & Crepel, 2001).
Figure 6. Effects of MAPK on WIN55,212-2-mediated inhibition of presynaptic calcium influx.
Plot of normalized amplitudes of peak fluorescence transients before, during bath application of PD-98059 (20 μM) and coapplication with WIN55,212-2 (1 μm), and after this combined treatment (n = 6). Note that in these experiments, the MAPK inhibitor does not prevent the WIN55,212-2-induced inhibition of peak fluorescence transients. The insets represent superimposed averaged fluorescence changes in one of these experiments
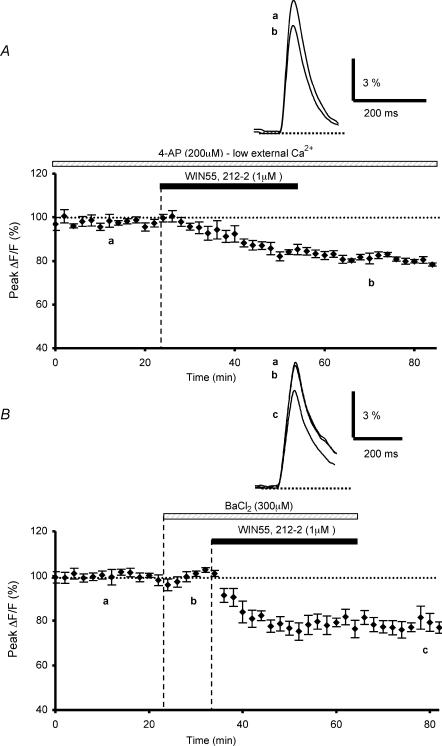
Finally, the actions of K+ channels blockers on the CB1 receptor-mediated inhibition of presynaptic fluorescence transients elicited by PF stimulations, were assessed. We previously found that WIN55,212-2 no longer had any effect on these transients, when presynaptic K+ channels were blocked by application of 1 mm 4-aminopyridine (4-AP) (Daniel & Crepel, 2001). Since, this concentration of 4-AP is fairly unselective (Coetzee et al. 1999), we performed new experiments with this blocker at non-saturating doses (200 μm). As previously described (Daniel & Crepel, 2001), bath application of 4-AP alone increased the amplitude of presynaptic fluorescence transients evoked by PF stimulation compared to those recorded in control conditions, since the resulting mean increase on the amplitude of these transients with 200 μm of this blocker was 625.8 ± 37.1% (n = 8, not illustrated; see Daniel & Crepel, 2001). Thus, in the presence of 200 μm 4-AP, to reduce the amplitude of these transients to values similar to those recorded in control medium, the extracellular Ca2+ concentration was lowered from 2 to 0.2 mm, with corresponding changes in the Mg2+ concentration to maintain the osmolarity (n = 8, not illustrated). In such conditions, when the amplitude of the fluorescence transients was brought back to control levels, the depressant effect of WIN55,212-2 on these transients was 20.4 ± 0.6% on average (n = 4, Fig. 7A), i.e. a value significantly (Student's t test; P < 0,05) smaller than that observed with WIN55,212-2 in control experiments. Thus, the present experiments show that the WIN55,212-2-mediated inhibitory effect on presynaptic Ca2+ influx is partially blocked by 200 μm 4-AP.
Figure 7. Effects of K+ channel blockers 4-AP and Ba2+ on WIN55,212-2-mediated inhibition of presynaptic calcium influx.
A, plot of normalized amplitudes of peak fluorescence transients before, during and after application of WIN55,212-2 (1 μm), in the presence of 4-AP (200 μm) with low extracellular calcium concentration (0.2 mm) (n = 4). Note that in these experiments, bath-applied 4-AP partially prevents the WIN55,212-2-induced inhibition of peak fluorescence transients. The insets represent superimposed averaged fluorescence changes in one of these experiments. B, the same as in A with applications of Ba2+ (300 μm) and WIN55,212-2 (1 μm) (n = 5). Inset as in A. Note that this K+ channel blocker also partially prevents the WIN55,212-2-induced inhibition of peak fluorescence transients.
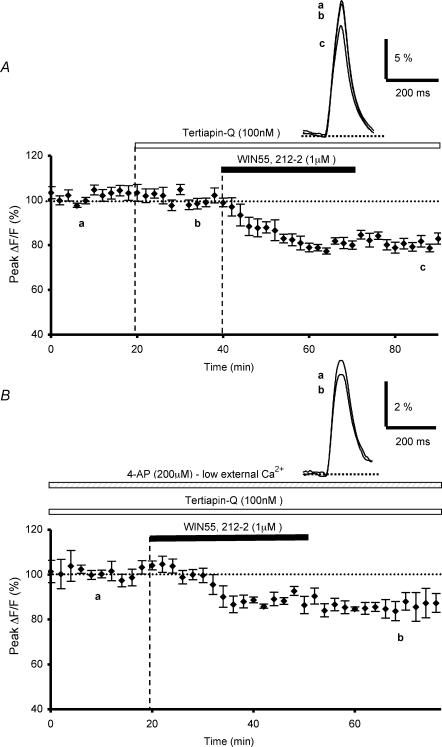
We then tested the hypothesis that CB1 receptor-mediated inhibition of presynaptic Ca2+ influx elicited by PF stimulation involves the activation of G protein-gated inwardly rectifying K+ channels (GIRKs) by determining whether the inhibitory effect of WIN55,212-2 was modulated by Ba2+ or tertiapin-Q, two blockers of this channel family, even though it is known that Ba2+ also affects other K+ channels and that tertiapin only affects some subtypes of GIRKs (Slesinger et al. 1997; McAllister et al. 1999; Jin et al. 1999; Jin & Lu, 1999; Takigawa & Alzheimer, 2002). In these experiments, bath application of 300 μm Ba2+, a non-selective blocker of GIRKs, did not appreciably alter the absolute fluorescence, suggesting that it does not seem to sizeably affect the basal Ca2+ level concentration in PFs (n = 5). Moreover, the amplitude and the duration of presynaptic fluorescence transients evoked by PF stimulations were unaffected by this blocker, compared to those recorded in control conditions (n = 5, Fig. 7B). In contrast; in the presence of 300 μm Ba2+, the depressant effect of WIN55,212-2 on fluorescence transients was only 21.8 ± 2.1% on average (n = 5, Fig. 7B), i.e. a value significantly (Student's t test; P < 0,05) smaller than that observed with WIN55,212-2 in control conditions (see Daniel & Crepel, 2001). These experiments demonstrate the blocking action of Ba2+ on the depressant effect of WIN55,212-2 on presynaptic Ca2+ influx. Since even low concentrations of Ba2+ affect various types of K+ channels in addition to GIRKs (see Discussion), we subsequently employed tertiapin-Q, which selectively inhibits some subtypes of this K+ channel family with nanomolar affinity. A 20 min bath application of tertiapin-Q (100 nm), prior to WIN55,212-2 application, had no effect on control fluorescence transients evoked by PF stimulation (n = 6, Fig. 8A). In contrast, in the presence of this blocker, the depressant effect of WIN55,212-2 on fluorescence transients was only 19.9 ± 1.9% on average (n = 6, Fig. 8A), i.e. a value significantly (Student's t test; P < 0,02) smaller than that observed with WIN55,212-2 in control experiments (see Daniel & Crepel, 2001).
Figure 8. Effects of K+ channel blocker tertiapin-Q alone or combined with 4-APon WIN55,212-2-mediated inhibition of presynaptic calcium influx.
A, plot of normalized amplitudes of peak fluorescence transients before, during bath application of tertiapin Q (100 nm) and coapplication with WIN55,212-2 (1 μm), and after washout of WIN 55, 212-2 (n = 6). Note that this K+ channel blocker partially prevents the WIN55,212-2-induced inhibition of peak fluorescence transients. The inset represents superimposed averaged fluorescence changes in one of these experiments. B, plot of normalized amplitudes of peak fluorescence transients before, during and after application of WIN55,212-2 (1 μm), in the continuous presence of bath applied tertiapin-Q (100 nm) and 4-AP (200 μm) with a low extracellular calcium concentration (0.2 mm) (n = 4). Inset as in A. Note that in these conditions, tertiapin-Q and 4-AP drastically reduce the WIN55,212-2-induced inhibition of peak fluorescence transients.
Finally, we tested whether combined application of tertiapin-Q and 4AP would produce a larger reduction of the WIN55,212-2-induced inhibition on fluorescence transients, than the reduction observed with either blocker applied alone. When 4-AP (used at non-saturating doses of 200 μm with reduced extracellular Ca2+ concentration) and tertiapin-Q (100 nm) were applied together, the depressant effect of WIN55,212-2 on the presynaptic fluorescence transients was only 12.9 ± 3.7% on average (n = 4, Fig. 8B). Together, these data strongly suggest that the inhibitory action of WIN55,212-2 on presynaptic Ca2+ transients can be attributed to the activation of 4-AP-sensitive and tertiapine-Q-sensitive K+ channels.
Discussion
The primary finding of this study, which extends earlier work on the mechanisms of cannabinoid receptor-mediated inhibition of excitatory synaptic transmission at PF–PC synapses (Levenes et al. 1998; Daniel & Crepel, 2001), is that cannabinoids do not directly inhibit the two major presynaptic N- and P/Q-type VGCCs required for evoked neurotransmitter release at these synapses (Mintz et al. 1995). Cannabinoids have been found to inhibit N-type and/or P/Q-type VGCCs in non-neuronal cell lines (Mackie & Hille, 1992), transfected cells (Mackie et al. 1995), cultured hippocampal neurones (Twitchell et al. 1997; Sullivan, 1999) and primary cultures of cerebellar granule neurones (Nogueron et al. 2001). Since, in brain slice preparations, our findings show that the activation of CB1 receptors by a cannabinoid agonist does not interfere with presynaptic VGCCs, it cannot be excluded that the signal transduction events linked to cannabinoid receptor activation in cell lines or primary cultures may be different. Nevertheless, the molecular mechanisms underlying the cannabinoid-mediated effects studied in brain slice preparations depend on the structure or neuronal cell type studied. For instance, presynaptic CB1 receptor-mediated depression of glutamatergic synaptic inputs involves the inhibition of N-type but not P/Q type Ca2+ channels in the striatum (Huang et al. 2001), whereas such direct inhibition of VGGCs is absent in the nucleus accumbens (Robbe et al. 2001) and lateral amygdala (Azad et al. 2003), as here in the cerebellar cortex. In contrast, blockade of N-type Ca2+ channels (Wilson et al. 2001) or blockade of all subtypes of VGCCs by cadmium a non-selective blocker (Hoffman & Lupica, 2000) abolishes the CB1-mediated inhibition of GABAergic neurotransmission in the hippocampus.
In addition, the present experiments show that application of SNX-482, a blocker of a part of R-type Ca2 channels, is able to reduce the amplitude of presynaptic Ca2+ transients evoked by PF stimulation. This observation strongly supports the fact that PFs bear not only N- and P/Q-type Ca2+ channels, but also R-type (SNX-482-sensitive) Ca2+ channels, which could contribute to fast excitatory synaptic transmission in rat cerebellar cortex, as previously demonstrated in rat hippocampus (Gasparini et al. 2001). Interestingly, our observation demonstrates that these channels are not involved in the cannabinoid receptor-mediated inhibition of presynaptic Ca2+ transients.
Taken together, our data demonstrate that the direct inhibition of presynaptic P/Q-, N- and R (SNX-482 sensitive)-type VGCCs is not involved in cannabinoid receptor-mediated inhibition of excitatory synaptic transmission at PF–PC synapses.
Our finding that PTX pretreatment abolishes the WIN55,212-2-mediated inhibitory effect on presynaptic Ca2+ influx evoked by PF stimulation indicates that CB1 receptor activation inhibits glutamatergic synaptic transmission at PF–PC synapses through a PTX-sensitive Gi/Go subclass of G proteins. A well-documented action of cannabinoids mediated through such proteins, is the inhibition of adenylate cyclase activity. Such inhibition has been described in many different cell types (Holwett, 1995; Childers & Deadwyler, 1996; Shen et al. 1996), including cultured cerebellar granule cells (Pacheco et al. 1993; Jung et al. 1997). Using a pharmacological tool inhibiting this cyclase in cerebellar slice preparations, we have presented evidence that CB1 activation is unlikely to decrease synaptic transmission at PF–PC synapses through modulation of adenylate cyclase activity. This finding agrees with previous reports showing that in nucleus accumbens (Robbe et al. 2001) or in hippocampal slices (Wilson et al. 2001) activation of presynaptic CB1 receptors inhibits, respectively, glutamate or GABA release, independently of an adenylate cyclase-coupled signalling pathway. Since the inhibition of adenylate cyclase activity is one of the predominant effects of cannabinoid receptor activation in primary cultures of neurones, and particularly in cultured cerebellar granule cells (Pacheco et al. 1993; Jung et al. 1997), it cannot be excluded that these cultured cells may develop their own intracellular signal transduction pathways linked to cannabinoid receptors. However, another possibility is that the activation of cannabinoid receptors in cerebellar slices effectively leads to inhibition of adenylate cyclase activity in PFs, but as proposed, this molecular mechanism is independent of the signalling pathway underlying the control of synaptic transmission at PF–PC synapses.
Activation of MAPK, mediated through a PTX-sensitive G protein, is another well-established mechanism induced by CB1 activation in heterologous expression systems (Bouaboula et al. 1995, 1999) and in hippocampal slices (Derkinderen et al. 2001, 2003). However, in the present study, interfering with MAPK activity did not affect the depressant effect of WIN55,212-2 on presynaptic Ca2+ influx, indicating that the MAPK pathway is not involved in the CB1 receptor-mediated decrease of excitatory synaptic transmission at PF–PC synapses.
Finally, although the molecular mechanisms downstream of the PTX-sensitive Gi/Go proteins remain to be elucidated, the present study demonstrates that the CB1-mediated inhibition of synaptic transmission at PF–PC synapses requires activation of GIRKs. However, although such a functional coupling between this subtype of G protein-activated K+ channels (sensitive to PTX and inhibited by Ba2+ ions) and the GABAB receptor has previously been demonstrated in cerebellar granule cells (Slesinger et al. 1997), the exact identity of the K+ channels underlying the CB1-mediated inhibition of presynaptic Ca2+ transients remained to be determined. Indeed, the K+ channel blocker Ba2+, even at low concentrations, not only inhibits GIRKs, but is also known to affect Ca2+-activated K+ channels (Katz et al. 1995), delayed rectifier K+ channels, K+ channels closed by cytosolic ATP (KATP channels) (Hille, 1992) and two-pore-domain K+ channels (Lesage & Lazdunski, 2000; Han et al. 2002). Even though Ba2+ is not a highly selective blocker of GIRKs, our data obtained with tertiapin-Q, a selective inhibitor of some subtypes of this K+ channel family, demonstrates that the activation of these channels participates in the reduction of glutamate release by cannabinoids. Moreover, immunohistological and in situ hybridization studies have demonstrated that GIRK subunits are abundant in the cerebellar cortex, where they are concentrated primarily in the granule cell layer but are also found in the molecular layer (Liao et al. 1996; Ponce et al. 1996; Karschin et al. 1996). However, their precise location on the granule cell axons remains to be established. In addition, the present data together with our previous study (Daniel & Crepel, 2001), demonstrate the participation of 4-AP-sensitive K+ channels in cannabinoid receptor-mediated inhibition of excitatory synaptic transmission at PF–PC synapses. Nevertheless, because the selectivity of 4-AP is not high, even when it is used at low concentrations (Mathie et al. 1998), one cannot unambiguously attribute the inhibition observed with this blocker to a particular K+ channel subtype. Further studies to determine which subtypes of 4-AP-sensitive K+ channels are involved in this inhibition would be worthwhile. Taken together, our results show that the activation of presynaptic CB1 receptors inhibits glutamatergic transmission via voltage-dependent K+ channels (4-AP-sensitive) and G protein-gated inwardly rectifying K+ channels (Ba2+- and tertiapin-Q-sensitive); an effect that is also found in other brain areas (Robbe et al. 2001; Azad et al. 2003).
In conclusion, using the exogenous cannabinoid agonist WIN55,212-2, the present study identifies presynaptic targets which are involved in CB1 receptor-mediated acute inhibition of neurotransmitter release at PF–PC synapses. Further studies will be necessary to show whether the retrograde regulation of excitatory PF–PC synaptic transmission by endogenous cannabinoids (Kreitzer & Regehr, 2001) shares similar molecular mechanisms. It also remains to be determined to what extent the effects of cannabinoids on cerebellar function, leading to impairment of motor performances, are attributable to such modulation of synaptic transmission.
Acknowledgments
This work was supported by Sanofi Recherche. We thank Gérard Sadoc for the Acquis1 software used in this study.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Bianchini L, McKenzie FR, Pouyssegur J, Casellas P. Cannabinoid receptor CB1 activates the Na+/H+ exchanger NHE-1 isoform via Gi-mediated mitogen activated protein kinase signaling transduction pathways. FEBS Lett. 1999;449:61–65. doi: 10.1016/s0014-5793(99)00395-6. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca 2+ current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992;106:231–232. doi: 10.1111/j.1476-5381.1992.tb14321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regher WG. The mechanism of cAMP-mediated enhancement at a cerebellar synapse. J Neurosci. 1997;17:8687–8694. doi: 10.1523/JNEUROSCI.17-22-08687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Deadwyler SR. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol. 1996;52:819–827. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Daniel H, Crepel F. Control of Ca2+ influx by cannabinoid and metabotropic glutamate receptors in rat cerebellar cortex requires K+ channels. J Physiol. 2001;537:793–800. doi: 10.1111/j.1469-7793.2001.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkinderen P, Ledent C, Parmentier M, Girault JA. Cannabinoids activate p38 mitogen-activated protein kinases through CB1 receptors in hippocampus. J Neurochem. 2001;77:957–960. doi: 10.1046/j.1471-4159.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–2382. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinor PT, Zhang JF, Randall AD, Zhou M, Schwarz TL, Tsien RW, Horne WA. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993;363:455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, Cullinan GJ, Hunden DC, Johnson D, Chaney MO, Koppel GA, Brownstein M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J Pharmacol Exp Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens J, Daniel H, Rancillac A, van der Steen J, Oberdick J, Crepel F, De Zeeuw C, Frens MA. Expression of protein kinase C inhibitor blocks cerebellar long-term depression without affecting Purkinje cell excitability in alert mice. J Neurosci. 2001;21:5813–5823. doi: 10.1523/JNEUROSCI.21-15-05813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DN, Assad MM, Phillips MB, Goldenberg HJ, Antonaccio MJ. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyc Nucl Res. 1979;5:125–134. [PubMed] [Google Scholar]
- Herkenham M, Lynn A, Jonhson M, Melvin L, de Costa B, Rice K. Characterisation and localisation of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates Inc.; 1992. [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holwett AC. Cannabinoid inhibition of adenylate cyclase Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27:429–436. [PubMed] [Google Scholar]
- Holwett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxico. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Klem AM, Lewis JH, Lu Z. Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry. 1999;38:14294–14301. doi: 10.1021/bi991206j. [DOI] [PubMed] [Google Scholar]
- Jin W, Lu Z. Synthesis of a stable form of tertiapin: a high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1999;38:14286–14293. doi: 10.1021/bi991205r. [DOI] [PubMed] [Google Scholar]
- Jung M, Calassi R, Rinaldi-Carmona M, Chardenot P, Le Fur G, Soubrie P, Oury-Donat F. Characterization of CB1 receptors on rat neuronal cell cultures: binding and functional studies using the selective receptor antagonist SR 141716A. J Neurochem. 1997;68:402–409. doi: 10.1046/j.1471-4159.1997.68010402.x. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stuhmer W, Karschin A. IRK(1–3) and GIRK(1–4) inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci. 1996;16:3559–3570. doi: 10.1523/JNEUROSCI.16-11-03559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Ferro PA, Cherksey BD, Sugimori M, Llinas R, Uchitel OD. Effects of Ca2+ channel blockers on transmitter release and presynaptic currents at the frog neuromuscular junction. J Physiol. 1995;486:695–706. doi: 10.1113/jphysiol.1995.sp020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;3:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SD, Griffin G, Satin LS, Abood M. Cannabinoid receptors can activate and inhibit G protein-coupled inwardly rectifying potassium channels in a Xenopus oocyte expression system. J Pharmacol Exp Ther. 1999;291:618–626. [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Nat Acad Sci. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Wenstenbroek R, Mitchell R. Cannabinoids activate a G protein-gated inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM. Paradoxical action of the cannabinoid WIN 55,212–2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br J Pharmacol. 1997;120:1397–1398. doi: 10.1038/sj.bjp.0701101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Wooltorton JR, Watkins CS. Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents. General Pharmacol. 1998;1:13–24. doi: 10.1016/s0306-3623(97)00034-7. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron. 1995;15:675–688. doi: 10.1016/0896-6273(95)90155-8. [DOI] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos J, Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Nogueron MI, Porgilsson B, Schneider WE, Stucky CL, Hillard CJ. Cannabinoid receptor agonists inhibit depolarization-induced calcium influx in cerebellar granule neurons. J Neurochem. 2001;79:371–381. doi: 10.1046/j.1471-4159.2001.00567.x. [DOI] [PubMed] [Google Scholar]
- Pacheco MA, Ward SJ, Childers SR. Identification of cannabinoid receptors in cultures of rat cerebellar granule cells. Brain Res. 1993;603:102–110. doi: 10.1016/0006-8993(93)91304-b. [DOI] [PubMed] [Google Scholar]
- Pan X, Ikeda SR, Lewis D. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;9:707–714. [PubMed] [Google Scholar]
- Pearson HA, Sutton KG, Scott RH, Dolphin AC. Characterization of Ca2+ channel currents in cultured rat cerebellar granule neurones. J Physiol. 1995;482:493–509. doi: 10.1113/jphysiol.1995.sp020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce A, Bueno E, Kentros C, Vega-Saenz de Miera E, Chow A, Hillman D, Chen S, Zhu L, Wu MB, Wu X, Rudy B, Thornhill WB. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J Neurosci. 1996;15:1990–2001. doi: 10.1523/JNEUROSCI.16-06-01990.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather PL, Martin NA, Breivogel CS, Childers SR. Activation of cannabinoid receptors in rat brain by WIN55,212–2 produces coupling to multiple G protein α-subunits with different potencies. Mol Pharmacol. 2000;57:1000–1010. [PubMed] [Google Scholar]
- Rampe D, Triggle DJ. New ligands for L-type Ca2+ channels. Trends Pharmacol Sci. 1990;11:112–115. doi: 10.1016/0165-6147(90)90196-f. [DOI] [PubMed] [Google Scholar]
- Randall A, Tsien WT. Pharmacological dissection of multiple types of calcium channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni O. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of mouse nucleus accumbens. J Neurosci. 2001;15:2995–3012. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fibre synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer S. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger PA, Stoffel M, Jan YN, Jan LY. Defective gamma-aminobutyric acid type B receptor-activated G protein-gated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Nat Acad Sci. 1997;94:12210–12217. doi: 10.1073/pnas.94.22.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal cells. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol. 2002;539:67–75. doi: 10.1113/jphysiol.2001.012883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottene A, Volsen S, Pietrobon D. α1-E subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell WS, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wu LG, Sagau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;132:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]