Abstract
The aim of this study was to determine if episodic hypoxia evokes persistent increases of genioglossus muscle (GG) activity, termed long-term facilitation (LTF), in neonatal rats in vivo. Experiments were performed on anaesthetized, spontaneously breathing, intubated neonatal rats (postnatal days (P) 3–7), divided into three groups. The first group (n = 8) was subjected to three 5-min periods of hypoxia (5% O2–95% N2) alternating with 5 min periods of room air. The second group (n = 8) was exposed to 15 min of continuous hypoxia. The third (n = 4) group was not exposed to hypoxia and served as a control. GG EMG activity and airflow were recorded before, during and for 60 min after episodic and continuous hypoxic exposure. During hypoxia, GG EMG burst amplitude and tidal volume (VT) significantly increased compared to baseline levels (episodic protocol: mean ±s.e.m.; 324 ± 59% of control and 0.13 ± 0.007 versus 0.09 ± 0.005 ml, respectively; continuous protocol: 259 ± 30% of control and 0.16 ± 0.005 versus 0.09 ± 0.007 ml, respectively; P < 0.05). After the episodic protocol, GG EMG burst amplitude transiently returned to baseline; over the next 60 min, burst amplitude progressively increased to levels significantly greater than baseline (238 ± 40% at 60 min; P < 0.05), without any significant increase in VT and respiratory frequency (P> 0.05). After the continuous protocol, there was no lasting increase in GG EMG burst amplitude. We conclude that LTF of upper airway muscles is an adaptive respiratory behaviour present from birth.
One form of plasticity in breathing adult mammals is long-term facilitation (LTF), a persistent increase in respiratory motor output resulting from episodic, but not continuous, hypoxia (Bach & Mitchell, 1996; Baker & Mitchell, 2000) or from electrical stimulation of the carotid sinus nerve (Millhorn et al. 1980). In humans, LTF cannot be elicited during wakefulness (Jordan et al. 2002), whereas during sleep LTF is seen as an increase in ventilation in individuals who snore and have inspiratory flow limitations (Babcock & Badr, 1998). Depending on the adult rodent experimental model, LTF can manifest as an increase in ventilation and phrenic nerve activity (Bach & Mitchell, 1996; Turner & Mitchell, 1997; Fuller et al. 2000; Mitchell et al. 2001), an increase in hypoglossal nerve activity (Fuller et al. 2001a), or an increase in genioglossus muscle activity (Mateika & Fregosi, 1997). Several factors influence LTF, including age, gender and genetics (Zabka et al. 2001a,b). LTF can also be modified by prior experience; for example, LTF of phrenic amplitude is enhanced following pretreatment with nocturnal chronic intermittent hypoxia (Ling et al. 2001).
Whether neonatal mammals respond to episodic hypoxia to produce LTF is unknown. LTF in neonatal mammals could serve a critical role in regulating responses to the particular ventilatory challenges of early postnatal life. Moreover, since ventilatory stress during fetal and early postnatal life can have long-term effects on respiratory regulation, e.g. exposure to chronic hypoxia in the first week of life alters the hypoxic ventilatory response in adults (Okubo et al. 1990), abnormal LTF responses in neonates could contribute to the development of respiratory dysfunction in adults. The aim of this study was to determine in neonatal rats in vivo if episodic hypoxia evokes LTF of the genioglossus muscle (GG), the principal tongue protruder muscle. Activity of this muscle, innervated by the hypoglossal nerve, affects upper airway resistance.
Methods
Experimental groups
Experiments were carried out on Sprague—Dawley (Holister colony) neonatal rats of both sexes, P3–7. The Chancellor's Animal Research Committee (ARC) at the University of California Los Angeles approved all experimental procedures (protocol no. 1994-159-31E).
Experimental procedures
Neonatal rats were anaesthetized with ketamine (20 mg kg−1) and xylazine (10 mg kg−1) injected subcutaneously. A cannula (polyethylene tubing, inner diameter: 0.030 in, outer diameter: 0.048 in, AM Systems) was inserted into the trachea, via the mouth, to measure airflow. To measure GG electromyographic activity (EMG), the muscle was exposed by dissecting out the digastric and mylohyoid muscles, and two insulated stainless steel wires (Cooner wire, Chatsworth, CA, USA) with the last 2 mm uninsulated were sutured into the muscle, which was covered with mineral oil to avoid desiccation. The lateral branches of the hypoglossal nerve, which innervate the styloglossus and hyoglossus extrinsic tongue muscles, were cut prior to the beginning of the protocol to ensure collection of uncontaminated GG EMG. At the end of the protocol, cutting the medial branches of the hypoglossal nerve abolished EMG activity, showing that recordings were from the GG. To prevent movement affecting the recordings, rats were maintained in a supine position throughout the study. Rectal temperature was monitored and maintained around 34°C with a heating pad. At the conclusion of all experiments, rats were killed by decapitation.
Experimental protocol
The preparation was allowed to stabilize for approximately 30 min after electrode implantation. GG EMG activity and airflow were recorded before (starting at —15 min), during and for 60 min post-hypoxia. In the first group, rats (n = 8) were subjected to three 5-min periods of hypoxia (5% O2–95% N2) alternating with equal periods of normoxia, i.e. room air; the final hypoxic episode was followed by 60 min of normoxia. Gas mixtures were delivered via a facemask at 1 l min−1. In the second group, rats (n = 8) were exposed to one continuous 15-min period of hypoxia (equivalent to the sum of the three hypoxic periods in the episodic protocol), followed by 60 min of normoxia. In this study we used 5% O2 as the hypoxic stimulus, in contrast to previous LTF studies in adult rats, where the stimulus was 11% O2 (Baker & Mitchell, 2000; Fuller et al. 2000). We chose to use 5% O2 because it is well documented that the hypoxic ventilatory response in neonates is smaller than that of adults of the same species (Mortola, 1999). In the third group of rats (n = 4), airflow and GG EMG activity were recorded for 90 min under normoxic conditions, i.e. with no exposure to hypoxia, as a time control for natural drift in breathing variables in anaesthetized neonatal rats.
Data acquisition and analysis
All signals were recorded on a laboratory computer via an analog-to-digital interface (Powerlab 16SP, AD Instruments, USA). EMG signals were amplified and filtered (100–1000 Hz; Grass Model P511K, Grass Instrument Co., USA) and sampled at 2000 Hz. Changes in airflow were measured using a respiratory flow head (1 litre, AD Instruments, USA) positioned at the end of the tracheal cannula and attached to a differential pressure transducer (Honeywell Data Instruments). The airflow signal was high-pass filtered (0.2 Hz) to remove DC components and digitally integrated to determine tidal volume (VT).
Integrated GG activity, VT, and respiratory frequency were analysed in 60 s time bins during baseline, hypoxia, and 15, 30, 45 and 60 min post hypoxia. Changes in integrated GG EMG amplitude were calculated as a percentage change from normalized baseline. Normalized baseline was calculated by rescaling baseline integrated EMG amplitude 15 min prior to hypoxia exposure to ‘100’; all data were rescaled relative to this baseline level. Statistical analyses were performed using a two-way repeated measures analysis of variance (ANOVA). Post hoc analyses based on Dunnett were used for individual comparisons (SAS V8.2, SAS Institute). Differences were regarded significant if P < 0.05.
Results
Episodic hypoxia
Each hypoxic episode evoked a significant increase in GG EMG burst amplitude compared to baseline levels (mean ±s.e.m.; first hypoxic episode 324 ± 59% of control; P < 0.05). Immediately after the third and final hypoxic stimulus, the burst amplitude transiently decreased to baseline, then increased progressively over the next 15 min to levels significantly greater than baseline (195 ± 33% at 15 min; P < 0.05). This elevated level of EMG activity was sustained for at least 45–60 min (238 ± 40% at 60 min; P < 0.05; Fig. 1A). This pattern of LTF expression in GG EMG was seen in all rats and is reflected in the group data (Fig. 2A).
Figure 1. Respiratory and GG EMG changes following exposure to episodic or continuous hypoxia.
A, episodic hypoxia. Integrated GG EMG burst amplitude and VT significantly increase during each hypoxic episode (denoted by bars). Post hypoxia, GG EMG burst amplitude transiently decreases; increasing over the next 60 min to a level significantly greater than baseline with no significant increase in VT. B, continuous hypoxia. GG EMG burst amplitude and VT significantly increase during the 15 min exposure to hypoxia (denoted by bar). Post hypoxia, GG EMG burst amplitude decreases, remaining at this lower level over the next 60 min. VT transiently decreases post hypoxia, before returning to and remaining at baseline levels. a.u., arbitrary units.
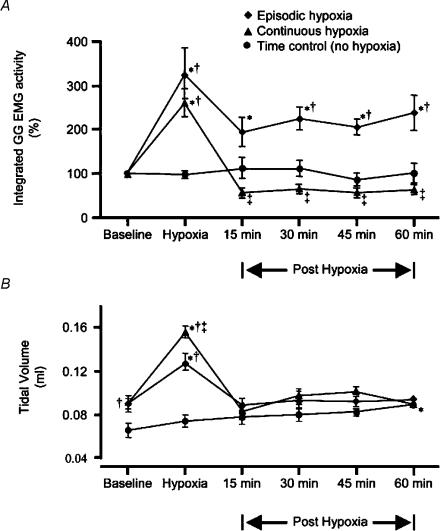
Figure 2. Group GG EMG activity and VT during baseline, hypoxia, and 15, 30, 45 and 60 min post hypoxia.
A, mean integrated GG EMG amplitude (±s.e.m.) expressed as a percentage change from baseline. Episodic hypoxia (♦) elicits a significant increase from baseline in GG EMG amplitude during hypoxia and at all time points post hypoxia. In contrast, continuous (▴) hypoxia elicits a depression of GG EMG amplitude at all time points post hypoxia, which are significantly different to equivalent time points post episodic hypoxia. In the control group (•) there is no significant change in GG EMG amplitude over time. At all time points, except baseline, GG EMG amplitude in the group exposed to episodic hypoxia, is significantly different from the control group. B, mean VT changes from baseline (±s.e.m.). There is a significant increase in VT during hypoxia, which returns to baseline following either episodic or continuous stimuli. In the control group, there is a significant increase in VT at the end of the protocol. Significantly (P < 0.05) different from baseline; † significantly (P < 0.05) different from time control group; ‡ significantly (P < 0.05) different from episodic hypoxia.
VT significantly increased during hypoxic episodes compared to baseline (0.13 ± 0.007 versus 0.09 ± 0.005 ml, respectively; P < 0.05); VT returned to baseline levels immediately post hypoxia (0.09 ± 0.006 ml at 15 min; P > 0.05), with no significant change over the next 60 min (0.09 ± 0.007 ml at 60 min; P > 0.05; Figs 1A and 2B). There was a slight increase in respiratory frequency during hypoxic episodes compared to baseline (106 ± 8 versus 99 ± 7 breaths min−1, respectively; P > 0.05); frequency returned to and remained at baseline levels post hypoxia.
Continuous hypoxia
Unlike episodic hypoxia, continuous hypoxia did not result in a post-stimulus increase in GG EMG burst amplitude; instead it elicited a decrease. During exposure to hypoxia, GG EMG burst amplitude and VT significantly increased compared to baseline (mean ±s.e.m.; 259 ± 30% of control and 0.16 ± 0.005 versus 0.09 ± 0.007 ml, respectively; P < 0.05; Figs 1B and 2). Immediately post hypoxia, GG EMG burst amplitude decreased to below baseline (60 ± 6% at 15 min; P > 0.05), remaining at this lower level for the next 60 min (65 ± 5% at 60 min; P > 0.05; Figs 1B and 2A). VT transiently decreased below baseline (0.08 ± 0.006 ml at 15 min; P > 0.05) before returning to and remaining at baseline levels (0.09 ± 0.005 ml at 60 min; Figs 1B and 2B). As seen in the episodic protocol, respiratory frequency increased during hypoxia, compared to baseline (91 ± 4 versus 74 ± 5 breaths min−1, respectively; P < 0.05), returning to baseline levels post hypoxia.
Time controls
At all time points during 90 min of normoxia in control rats, there was no significant change in GG EMG burst amplitude (mean ±s.e.m.; 115 ± 21% of control at 45 min, 101 ± 22% of control at 90 min; P > 0.05; Fig. 2A) and respiratory frequency (93 ± 7 breaths min−1 at 45 min, 106 ± 13 breaths min−1 at 90 min versus 93 ± 6 breaths min−1 baseline; P > 0.05). There was no significant change in VT up to 75 min (0.08 ± 0.006 ml at 45 min, 0.08 ± 0.004 ml at 75 min versus 0.07 ± 0.06 ml baseline; P > 0.05). At 90 min there was a significant increase in VT compared to baseline (0.09 ± 0.003 ml; P < 0.05; Fig. 2B).
Discussion
We show that respiratory-modulated activity of the genioglossus muscle (GG), the principal tongue protruder muscle, in the neonatal rat exhibits LTF in response to episodic, but not continuous, hypoxia. The pattern of LTF in neonatal rats is similar to that of adult rats: small immediately post hypoxia and progressively increasing over the next hour (Baker & Mitchell, 2000; Fuller et al. 2000). Although many studies report LTF as an increase in both respiratory amplitude and frequency, we found LTF to be expressed primarily as an increase in GG EMG burst amplitude, with no significant change in frequency, in common with several previous studies (Cao et al. 1992; Fregosi & Mitchell, 1994; McCrimmon et al. 1995). There was no significant facilitation of tidal volume or respiratory frequency, which are surrogate measures of phrenic nerve activity. As determined by the time control experiments, there is natural drift in EMG amplitude and respiratory variables over time but such changes are insignificant, indicating that episodic but not continuous hypoxia induces a persistent increase in GG EMG amplitude above baseline in subsequent normoxia.
The common experimental model used to investigate hypoxia-induced LTF is the anaesthetized, vagotomised, artificially ventilated adult rat (Baker & Mitchell, 2000; Fuller et al. 2000). In spontaneously breathing mammals, results are variable, with LTF seen in awake goats (Turner & Mitchell, 1997), ducks (Mitchell et al. 2001), dogs (Cao et al. 1992) and rats (Olson et al. 2001) but not in anaesthetized, spontaneously breathing rats (Janssen & Fregosi, 2000). In spontaneously breathing, vagotomised cats at elevated PaCO2, LTF is evoked in GG EMG but not diaphragmatic EMG (Mateika & Fregosi, 1997). The authors postulate that an increase in baseline diaphragm activity due to elevated PaCO2 during control conditions could mask LTF of diaphragmatic EMG, indicating that the level to which PaCO2 is raised above apnoeic threshold may be critical for LTF manifestation (Fregosi & Mitchell, 1994; Mateika & Fregosi, 1997). Here, in spontaneously breathing, anaesthetized neonatal rats, PaCO2 was not monitored because of the technical difficulties of making such measurements in these small mammals. Unclamped fluctuations in PaCO2 may have affected the manifestation of LTF in tidal volume and respiratory frequency
Genetic influences
The Sprague—Dawley rats in this present study were from the Holister colony, a colony not previously studied with respect to LTF. Genetic variability can influence LTF expression (Fuller et al. 2000, 2001a). In rats from two different vendors, Harlan and Charles River Laboratories/Sasco (CRL/S), phrenic nerve LTF is similar in magnitude in both substrains; however, hypoglossal nerve LTF is significant in CRL/S rats, but insignificant in Harlan rats, suggesting that a genetic mechanism influences LTF expression (Fuller et al. 2001a). Genetic variability between colonies, in addition to developmental differences (see below), may account for the contrast between the present finding in neonatal rats, of a significant change in GG EMG amplitude but an insignificant change in ventilation, compared to some studies in adult rats demonstrating LTF of phrenic nerve activity but not hypoglossal nerve activity (Baker & Mitchell, 2000; Fuller et al. 2001a). Other examples of genetic influences in neuroplasticity occur in long-term potentiation and long-term depression associated with learning and memory, which are expressed in various forms across different species of rats (Manahan-Vaughan, 2000).
Developmental considerations
Respiratory regulation during early postnatal development is different from that in adulthood. For example, in the fetus the response to a single episode of hypoxia is ventilatory depression, whereas in the neonate, ventilation initially increases, then is depressed and in mature adults ventilation increases (Mortola, 1999; Moss, 2000; Gozal & Gozal, 2001; Waters & Gozal, 2003). Since the hypoxic ventilatory response in neonates is smaller than that of adults of the same species (Mortola, 1999), we used 5% O2 as the hypoxic stimulus, whereas 11% O2 has been used to elicit LTF in adults (Baker & Mitchell, 2000; Fuller et al. 2000). The larger magnitude of LTF in this present study compared to responses reported in adult rats (Baker & Mitchell, 2000; Fuller et al. 2000) could be due to differences in the levels of hypoxia used.
In the neonate, exposure to intermittent hypoxia can have long-term effects. For example, in 2- to 3-day-old rats, exposure to repetitive bouts of hypoxia subsequently modifies the late phase of the hypoxic ventilatory response (Gozal & Gozal, 2001); in piglets (10–12 days old), exposure to repetitive, but not continuous, hypoxia attenuates the hyperventilatory response as well as increasing the occurrence of arrested brain activity (Waters et al. 1996, 2003). Taken together, we suggest that developmental respiratory plasticity is related to LTF in neonates. Moreover, dysfunction of the cellular mechanism(s) of LTF could underlie breathing abnormalities in infants, for example, congenital central hypoventilation syndrome (CCHS) (Gaultier, 2001) and sudden infant death syndrome (SIDS) (Kinney et al. 2001).
At present, the cellular basis of LTF is unknown. In vivo in adult rats, initiation, but not maintenance, of respiratory LTF can be abolished by serotonin receptor antagonists (Bach & Mitchell, 1996; Fuller et al. 2001b; Baker-Herman & Mitchell, 2002). In neonatal rat medullary slices generating respiratory rhythm, episodic but not continuous application of a serotonin (5-HT2A) receptor agonist evokes an in vitro form of LTF, i.e. persistent increases of hypoglossal motor output, due to an increase in AMPA-mediated inspiratory drive currents in hypoglossal motoneurones (Bocchiaro & Feldman, 2004).
Conclusion
This study demonstrates the presence of LTF in neonates, indicating that this presumably adaptive respiratory behaviour is present from birth. LTF, manifested as an increase in GG EMG activity resulting from episodic hypoxia, may play a crucial role in minimizing upper airway collapse during sleep, which occurs in humans with obstructive sleep apnoea, a disorder most commonly affecting the elderly but also prevalent in children (Katz & White, 2003). Clearly, a greater understanding of the mechanisms underlying LTF would help to ascertain its relevance to and its possible role in the prevention of disorders of the control of breathing.
Acknowledgments
We thank Max Shao for advice concerning statistical analysis. This work was supported by NIH Grant HL070029.
References
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(1):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independant persistent plasticity in endogenously active mammalian motoneurons. Proceedings of the Natl Acad Sci USA. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477:469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001a;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001b;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Gaultier C. Abnormalities of the chemical control of breathing: Clinical correlates in infants and children. Pediatr Pulmono Suppl. 2001;23:114–117. [PubMed] [Google Scholar]
- Gozal E, Gozal D. Respiratory plasticity following intermittent hypoxia: developmental interactions. J Appl Physiol. 2001;90:1995–1999. doi: 10.1152/jappl.2001.90.5.1995. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol. 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med. 2003;168:664–670. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–247. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Long-term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb Cortex. 2000;10:482–487. doi: 10.1093/cercor/10.5.482. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Deiken MS, Mitchel GS. Glutamate, GABA, and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. Vol. 79. New York: Marcel Dekker Inc.; 1995. pp. 151–218. [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001;124:117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116:95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- Moss IR. Respiratory responses to single and episodic hypoxia during development: mechanisms of adaptation. Respir Physiol. 2000;121:185–197. doi: 10.1016/s0034-5687(00)00127-4. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP, Rezzonico R, Gleed RD. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol. 1990;259:R836–R841. doi: 10.1152/ajpregu.1990.259.4.R836. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, et al. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499:543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KA, Beardsmore CS, Paquette J, Meehan B, Cote A, Moss IR, Turner GA. Respiratory responses to rapid-onset, repetitive vs. continuous hypoxia in piglets. Respir Physiol. 1996;105:135–142. doi: 10.1016/0034-5687(96)00046-1. [DOI] [PubMed] [Google Scholar]
- Waters KA, Gozal D. Responses to hypoxia during early development. Respir Physiol Neurobiol. 2003;136:115–129. doi: 10.1016/s1569-9048(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001a;531:509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol. 2001b;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]