Abstract
Activation of microglial cells, the resident macrophages of the brain, occurs rapidly following brain injury. De-ramification, i.e. transformation from ramified into amoeboid morphology is one of the earliest manifestations of microglial activation. In the present study, we identified the physiological mechanisms underlying microglial de-ramification induced by lysophosphatidylcholine (LPC). Patch-clamp experiments revealed activation of non-selective cation currents and Ca2+-dependent K+ currents by extracellular LPC. LPC-activated non-selective cation channels were permeable for monovalent and divalent cations. They were inhibited by Gd3+, La3+, Zn2+ and Grammostola spatulata venom, but were unaffected by diltiazem, LOE908MS, amiloride and DIDS. Ca2+ influx through non-selective cation channels caused sustained increases in intracellular Ca2+ concentration. These Ca2+ increases were sufficient to elicit charybdotoxin-sensitive Ca2+-dependent K+ currents. However, increased [Ca2+]i was not required for LPC-induced morphological changes. In LPC-stimulated microglial cells, non-selective cation currents caused transient membrane depolarization, which was followed by sustained membrane hyperpolarization induced by Ca2+-dependent K+ currents. Furthermore, LPC elicited K+ efflux by stimulating electroneutral K+–Cl− cotransporters, which were inhibited by furosemide and DIOA. LPC-induced microglial de-ramification was prevented by simultaneous inhibition of non-selective cation channels and K+–Cl− cotransporters, suggesting their functional importance for microglial activation.
Formation of lysophosphatidylcholine (LPC) is enhanced in the pathological central nervous system. Increased concentrations of LPC were detected following ischaemia (Sun & Foudin, 1984; Kinouchi et al. 1990), under inflammatory conditions (Kabarowski et al. 2002) and in epileptic tissue (Yegin et al. 2002). LPC is generated by hydrolysis of phosphatidylcholine (PC) via the action of phospholipase A2 (PLA2). Under normal physiological conditions, the majority of LPC formed in the tissue is rapidly metabolized or reacetylated. In pathological situations, overstimulation of PLA2 results in PC breakdown and subsequent accumulation of LPC in the damaged tissue (Kabarowski et al. 2002; Xu, 2002; Yegin et al. 2002). Increases in PLA2 activity occur in various brain diseases, such as ischaemia, epilepsy, Alzheimer's disease, schizophrenia and spinal cord injury (Farooqui et al. 1999).
A wide variety of in vitro and in vivo studies has demonstrated that LPC promotes the activation of microglia and other immune cells. LPC-induced activation processes include shape changes (Ousman & David, 2000), stimulation of cell motility (Quinn et al. 1988), expression of surface antigens (Kume et al. 1992), release of proinflammatory cytokines (Huang et al. 1999) and others. Although the capability of LPC to activate immune cells has been well demonstrated, the underlying physiological mechanisms of these activation processes are unknown.
LPC has been found to modulate ion channel activity in various cell preparations. In neurones, LPC causes prolonged membrane hyperpolarization due to the activation of the two-pore domain K+ channels TREK-1, TREK-2 and TRAAK (Lesage et al. 2000; Maingret et al. 2000). By shifting the steady-state inactivation curve in depolarizing direction, LPC induced increases of HERG K+ currents in HEK cells (Wang et al. 2001). Furthermore, LPC has been shown to evoke sustained intracellular Ca2+ increases, which were linked to the activation of either voltage-gated L-type Ca2+ channels (Golfman et al. 1999) or non-selective cation channels (Terasawa et al. 2002; Yokoyama et al. 2002). In cardiac myocytes, LPC enhances slow Na+ currents (Burnashev et al. 1989; Undrovinas et al. 1992). Besides the up-regulation of certain types of ion channels, LPC has also been demonstrated to inhibit ion channel activity. In coronary smooth muscle cells, LPC causes inhibition of delayed rectifier K+ currents by shifting their steady-state activation curve in depolarizing direction (Yeon et al. 2001). LPC-induced reduction of inward rectifier K+ currents was detected in cardiac cells by Kiyosue & Arita (1986). Furthermore, inhibitory effects of LPC on fast Na+ currents (Burnashev et al. 1989; Sato et al. 1992) and on NMDA-gated currents (Ikeuchi et al. 1995) were previously described. Although numerous studies report LPC-induced effects on electrophysiological properties of various cell types, data on immune cells, including microglia, are not available.
In the present study, we have identified the ion channels and transporters, namely non-selective cation channels, Ca2+-dependent K+ channels and K+–Cl− cotransporters, which are activated by extracellular LPC in microglia. Furthermore, we provide evidence that non-selective cation channels and K+–Cl− cotransporters are involved in the transformation of microglial cells from ramified into amoeboid morphology.
Methods
Cell culture
Microglia were obtained from brain cell cultures of newborn NMRI mice, supplied by Charles River (Sulzfeld, Germany). Mixed brain cell cultures were prepared as previously described (Eder et al. 1997). Brain cortices were enzymatically dissociated (15 min at 37°C with 0.125% trypsin, type XI, Sigma, Germany) and a single-cell suspension was achieved by repeated triturations. Cells were seeded into tissue culture flasks at a density of 2−4 × 106 (10 ml)−1 in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco; Germany) and 30% supernatant of L-929 fibroblasts as a source of macrophage colony-stimulating factor (M-CSF). After at least 10 days of incubation, microglia were harvested by shaking the cultures (30 min, 300 r.p.m) to detach weakly adherent microglial cells from the astrocytic monolayer. Isolated microglia were seeded on glass coverslips in 24 well Costar plates (3 × 104 (0.5 ml)−1). In order to induce ramification, microglial cells were cultured in astrocyte-conditioned medium (ACM) as previously described (Eder et al. 1999). Experiments were performed on microglial cells, which had been exposed to ACM for at least 3 days. The immortalized mouse microglial cell line BV-2 (kindly provided by Dr E. Blasi, Perugia, Italy) was used in some experiments. BV-2 microglial cells were cultured permanently in DMEM supplemented with 10% FCS and 2 mm l-glutamine. BV-2 cells were split twice a week, and were plated on glass coverslips at a density of 3 × 103 (0.5 ml)−1 for subsequent patch-clamp experiments. BV-2 cells on coverslips were cultured in ACM for at least 3 days. In all experiments performed, no differences were found between cultured primary microglial cells and BV-2 microglial cells regarding their responses to LPC. Therefore, data of all cells were pooled and analysed together.
Electrophysiological recordings
Whole-cell membrane currents were measured using an EPC-9 patch clamp amplifier (HEKA, Lambrecht/Pfalz, Germany). The amplifier was interfaced to an IBM computer for pulse application and data recording. Series resistance compensation was routinely used to reduce the effective series resistance by about 70%. Patch electrodes of 2–4 MΩ were fabricated on a two-stage puller (Narishige PP-83, Tokyo, Japan) from borosilicate glass (outer diameter 1.5 mm and inner diameter 1 mm; Hilgenberg, Malsfeld, Germany). Intracellular solution I1 contained (mm): KCl, 120; CaCl2, 1; MgCl2, 2; Hepes, 10; EGTA, 11 (pH 7.3). Although no ATP or GTP is included, experiments using perforated-patch recording (Figs 6 and 7) showed identical behaviour with physiological levels of ATP and GTP. Intracellular solution I2 contained (mm): CsCH3SO3, 125; MgCl2, 2; Hepes, 10; BAPTA, 1 (pH 7.3). In some experiments, CsCH3SO3 was substituted by an equimolar concentration of NMGCl. The low Ca2+-buffered intracellular solution I3 contained (mm): KCl, 120; MgCl2, 2; Hepes, 10; BAPTA, 0.1 (pH 7.3). For perforated-patch-clamp experiments, the tips of the pipettes were filled with the following solution (mm): K2SO4, 120; KCl, 16; MgSO4, 5; Hepes, 10 (pH 7.3). A stock solution of nystatin in DMSO (25 mg ml−1) was prepared daily and subsequently diluted in the pipette solution to a final concentration of 150 μg ml−1. After sonication, this solution was used for backfilling the pipettes as previously described (Horn & Marty, 1988). The extracellular solution E1 contained (mm): NaCl, 130; KCl, 5; CaCl2, 2; MgCl2, 1; Hepes, 10; d-glucose, 8 (pH 7.4). In some experiments, 130 mm NaCl were substituted by 70 mm NaCl and 60 mm d-mannitol (extracellular solution E2). In some cases, CaCl2 was omitted from the extracellular solution, while 1 mm EGTA was added and the concentration of MgCl2 was increased to 4 mm (Ca2+-free extracellular solution E3). In some other cases, Cl− was substituted with gluconate (Cl−-free extracellular solution E4). The extracellular solution E5 contained (mm): NaCl, 140; MgCl2, 2; Hepes, 10 (pH 7.4). All recordings were done at room temperature (20–23°C). Whole-cell currents were filtered at 3 kHz and stored on computer disk for subsequent analyses. Analyses were performed on IBM computers with the Pulse/PulseFit programme (HEKA, Lambrecht/Pfalz, Germany). Data were corrected for liquid junction potentials. Data are presented as mean values ± standard error of the mean (s.e.m.). The numbers of experiments (n) are indicated.
Figure 6. Activation of Ca2+-dependent K+ currents by LPC.
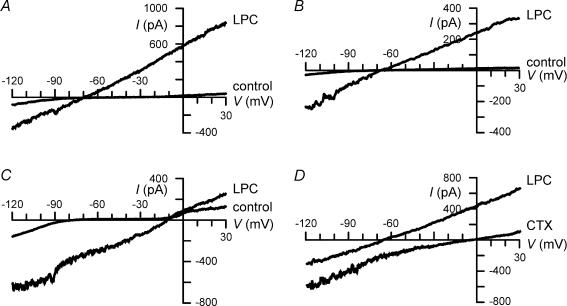
BV-2 microglial cells were held at −60 mV. Voltage ramps were applied from −120 to +30 mV for a duration of 300 ms every 20 s. A–B, measurements were performed either in the whole-cell configuration using low Ca2+-buffered intracellular solution I3 (A) or in the perforated-patch-clamp configuration (B). Currents were recorded before (control) and during application of 15 μm LPC using Ca2+-containing extracellular solution E1. C, whole-cell current recordings before (control) and during application of 15 μm LPC using low Ca2+-buffered intracellular solution I3 and Ca2+-free extracellular solution E3. D, currents evoked by 15 μm LPC were recorded in the absence (LPC) and in the presence of 50 nm CTX. Whole-cell recordings were performed using low Ca2+-buffered intracellular solution I3 and Ca2+-containing extracellular solution E1.
Figure 7. LPC-induced changes in membrane potential of BV-2 microglial cells.
A and B, current clamp recordings were performed using either the whole-cell patch-clamp technique with low Ca2+-buffered intracellular solution I3 (A) or the perforated-patch-clamp technique (B). Following application of 15 μm LPC, cells depolarized transiently, and membrane hyperpolarization occurred subsequently. C, LPC-induced membrane depolarization was inhibited reversibly by Gd3+ and La3+. Recordings were performed using high Ca2+-buffered intracellular solution I1 to prevent activation of Ca2+-dependent K+ channels. D, LPC-induced membrane hyperpolarization was abolished by CTX. Recordings were performed using the perforated-patch-clamp technique. At t = 0 s, the cell was exposed for 220 s to LPC. A–D, cells were superfused with Ca2+-containing extracellular solution E1.
In order to obtain the relative permeability ratio of anions and cations, currents were recorded under biionic conditions and reversal potentials were measured. To determine the permeability of chloride relative to sodium, the extracellular solution contained (mm): NaCl, 40; d-mannitol, 230; Hepes, 10 (pH 7.3), while the intracellular solution contained (mm): NaCl, 150; Hepes, 10; EGTA, 10 (pH 7.3). The permeability of chloride relative to sodium was calculated from the reversal potential using the following equation:
 |
where Erev is the measured reversal potential, P is the permeability, R is the gas constant, T is the absolute temperature, and F is the Faraday constant.
The anion permeability of the channel was very small. Therefore, it was ignored in subsequent calculations of cation permeabilities. Next, the reversal potential of the current was obtained under various ionic conditions and the relative permeability of Na+, Cs+, K+ and NMG+ for the current was calculated in the absence of Ca2+ using the following equation (Hille, 2001):
where C is the test permeant cation.
The permeability of Ca2+ relative to Cs+ was calculated from the reversal potential using the following equation:
Ca2+ imaging
Microglial cells were loaded with 3 μm fura-2 acetoxymethylester (fura-2 AM, Molecular Probes, Eugene, OR, USA) in extracellular solution for 30 min at room temperature (20–23°C). After washing, coverslips were mounted in a chamber on an inverted Olympus IX 50 microscope (Olympus Optical Co. GmbH, Hamburg, Germany) equipped with a water immersion objective 40× UApo/340 (Olympus). The fluorescence imaging system consisted of a monochromator, a charge-coupled device (CCD) camera and the Windows NT-based image processing software (Till Photonics, München, Germany). Microglial cells were exposed to alternating 340 ± 5 nm and 380 ± 5 nm wavelengths of UV light and emission light was passed through a 400 nm dichroic mirror and a 420 nm long pass emission filter (both Olympus) prior to acquisition by the CCD camera. Images were collected every 20 s. In some experiments images were collected every 5 s. The ratio of the two background-corrected fluorescence intensities was converted to the intracellular Ca2+ concentration ([Ca2+]i) of a single cell according to the equation of Grynkiewicz et al. (1985):
where Kd= 224 nm is the dissociation constant of fura and R is the measured ratio. Rmin (0.375) and Rmax (3.930) were determined using 10 μm ionomycin in extracellular solution containing zero Ca2+ (using 2 mm EGTA) or 10 mm Ca2+, respectively, and β (5.66) was calculated as the ratio of the fluorescence intensities at 380 nm in 0 and 10 mm Ca2+. The extracellular solution E1 was used for Ca2+ imaging experiments. Some experiments were performed using Ca2+-free extracellular solution E3.
Morphometric quantification
For statistical evaluation of cell morphology, microglial cells were examined by light microscopy using an inverted Olympus IX 50 microscope (Olympus Optical Co. GmbH) equipped with a water immersion objective 40× UApo/340 (Olympus, Hamburg, Germany) and a CCD camera system (Till Photonics). For each group microglial cells from at least six different coverslips of at least three different cultures were analysed using the programme Image Tool 2.0 (UTHSCSA, San Antonio, TX, USA). Exclusively microglial cells were analysed, which exhibited ramified morphology at the beginning of experiments. Ramified microglia were defined as cells with distinct processes at least greater than one cell diameter in length (Korotzer & Cotman, 1992). Primary cultured microglial cells and BV-2 microglial cells showed a similar degree of ramification before application of LPC under the various experimental conditions. Both cell types were used in experiments studying effects of LPC, LPC + Gd3+ and LPC + Gd3++ DIOA on microglial morphology, while the remaining experiments were performed on BV-2 microglial cells exclusively. Under the various experimental conditions, microglial morphology was unchanged 30 min after exposure to inhibitors in the absence of LPC. For quantification, a ramification index (= form factor) was calculated using the following formula (Wilms et al. 1997):
where RI is the ramification index. Ramification indices for each cell population are presented as mean values ±s.e.m. Amoeboid cells have RI values close to 1, whereas ramified cells show RI values that approach 0. During the process of data analyses experimenters were kept unaware of the experimental conditions and were not informed about any working hypotheses.
Determination of extracellular K+ concentration
Microglial cells were seeded in Petri dishes at a density of 5 × 105 and were cultured in ACM for 3 days. Immediately before experiments, cells were washed 2 times with extracellular solution E1. Then cells were treated with 1 ml extracellular solution containing 15 μm LPC in the absence of any inhibitor, in the presence of 100 μm Gd3+, or in the presence of both, 100 μm Gd3+ and 100 μm DIOA. After 30 min, supernatants were collected and the concentration of K+ was determined using an ABL 725 radiometer with a K+ sensitive electrode (Radiometer A/S, Bronshoj, Denmark). Data were obtained from three individual microglia cultures in three different Petri dishes per experimental condition.
Drug application
l-α-lysophosphatidylcholine (LPC), palmitoyl (C16: 0) was dissolved in water at a concentration of 10 mm and stored in aliquots at −20°C. Prior to experiments, aliquots were diluted in bath solution to a final concentration of 15 μm. Preparation and dilution steps were followed by 10 min sonication. In Ca2+ imaging experiments, drug application was achieved by complete exchange of the bath solution. In patch-clamp experiments, cells were superfused continuously with LPC-free control solution or with solutions containing LPC with and without channel inhibitors. These solutions were applied using a four-barrel microperfusion pipette, positioned at a distance of about 30–50 μm from the recorded cell to permit a rapid exchange of solutions. The flow rate was adjusted by hydrostatic pressure. The following inhibitors of ion channels and ion transporters were tested: GdCl3 (10–100 μm); LaCl3 (10–100 μm); ZnCl2 (100–500 μm); amiloride (1 mm), diltiazem (100 μm); 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS; 200 μm); 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB; 200 μm); R(+)-[(2-n-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl), oxy] acetic acid (DIOA; 100 μm); furosemide (1 mm); bumetanide (100 μm); Grammostola rosea brown morph (=G. spatulata) venom (1: 2000; Spider Pharm, Yarnell, USA); LOE908MS (50 μm; kind gift of Boehringer Ingelheim, Biberach, Germany); charybdotoxin (CTX; 50 nm; Latoxan, Rosana, France). If not stated otherwise drugs were obtained from Sigma, Germany.
Statistics
The statistical significance of differences between experimental groups was evaluated by Student's t test and by the Mann–Whitney U test using the SPSS programme. Data were considered to be statistically significant with P < 0.05.
Results
LPC-induced changes in microglial morphology
Application of LPC rapidly activated microglial cells as monitored by changes in cell morphology (Fig. 1A–C). Within the first minutes after exposure to 15 μm LPC, cell processes became thinner and were retracted partially, while cell bodies appeared to increase slightly in size (Fig. 1B). The majority of microglial cells retracted their processes completely, i.e. transformed from their ramified into amoeboid morphology, within 30 min of LPC stimulation (Fig. 1C). Changes in microglial morphology were quantified by determination of the ramification index. As demonstrated in Fig. 1D, the ramification index of microglial cells was increased significantly (P < 0.001) 30 min after treatment with 15 μm LPC, indicating a less pronounced ramification. LPC induced a similar extent of de-ramification in primary cultured microglial cells and in BV-2 microglial cells. In control experiments, we did not observe any significant changes in cell morphology and ramification index of untreated microglia held for 30 min in extracellular solution (Fig. 1D). At LPC concentrations of less than 15 μm, microglial shape changes occurred more slowly than at 15 μm LPC. In the presence of 5–10 μm LPC, the first signs of microglial de-ramification were visible about 30 min after the beginning of LPC application, while no complete transformation of microglial cells from ramified into amoeboid morphology was observed within 60 min of LPC stimulation.
Figure 1. Effects of LPC on microglial morphology.
A–C, primary cultured microglial cells before (A), and 10 min (B) and 30 min (C) following exposure to 15 μm LPC. A, untreated resting microglia exhibited ramified morphology. C, microglial cells stimulated for 30 min with LPC exhibited amoeboid morphology. D, ramification index (mean ±s.e.m.) of microglia exposed for 30 min to extracellular solution in the absence (n = 85) or presence (n = 435) of 15 μm LPC. Open bars (control), mean ramification index of cells determined at the beginning of the measurements. Filled bars, mean ramification index of the same cells held for 30 min under control conditions or stimulated with 15 μm LPC.
LPC-induced membrane current
In order to identify the physiological mechanisms underlying LPC-induced shape changes of microglial cells, patch-clamp experiments were performed. To study LPC-induced changes in microglial physiology over a time course similar to that of LPC-induced changes in microglial morphology, 15 μm LPC was used in further experiments. Figure 2A demonstrates whole-cell recordings of a microglial cell recorded with KCl-containing intracellular solution I1 and extracellular solution E1 (NaCl based, Ca2+ containing) under control conditions and following exposure to 15 μm LPC. Before application of LPC, inward and outward rectifier K+ currents were detected. These two types of microglial K+ currents have been characterized in detail elsewhere (for review, see Eder, 1998). Following extracellular application of LPC, an additional ion current activated. At −60 mV, amplitudes of the whole-cell currents increased from −14 ± 3 to −434 ± 77 pA (n = 5) in primary microglial cells, and from −7 ± 4 to −416 ± 84 pA (n = 9) in BV-2 microglial cells. Amplitudes were determined from ramp currents, because LPC-induced currents exhibited neither time-dependent activation nor inactivation (see below, Fig. 3). Furthermore, in the presence of LPC, the current reversal potential shifted in a depolarizing direction from −64.1 ± 4.2 to −4.4 ± 2.7 mV (n = 5) in primary microglial cells and from −45.7 ± 7.3 to −4.0 ± 2.0 mV (n = 8) in BV-2 microglial cells. Because neither the amplitude nor reversal potential of LPC-induced currents differed significantly (P > 0.8 in each case) between primary microglial cells and BV-2 microglial cells, data from both preparations were pooled in subsequent analyses. Figure 2B illustrates an isolated LPC-induced current, i.e. the difference current of the two currents shown in Fig. 2A.
Figure 2. Activation of LPC-induced membrane current in BV-2 microglia.
Cells were held at −60 mV, and voltage ramps were applied from −90 to +60 mV for a duration of 300 ms every 10 s. Measurements were performed using K+-containing intra- and extracellular solutions (I1 and E1). A, current recordings before (control) and during application of 15 μm LPC (LPC). B, LPC-induced current isolated by subtracting LPC-control from A. C, examples of currents recorded before (1), 160 s (2) and 340 s (3) following the onset of LPC application and 180 s after washout (4) of LPC. D, amplitudes of the current shown in C were measured at −60 mV and plotted as a function of time. Current amplitudes were determined in intervals of 10 s.
Figure 3. Kinetic properties of LPC-induced non-selective cation currents.
A, currents were evoked by 200 ms voltage pulses to potentials between −90 and +60 mV in 10 mV increments. Holding potential was 0 mV. Non-selective cation currents of the BV-2 microglial cell were measured using K+-free intra- and extracellular solutions. B, amplitudes of the current shown in A were plotted as a function of membrane potential. Before LPC treatment, this cell had an input resistance of 4.2 GΩ.
The activation time course of the LPC-induced current is shown in Fig. 2C–D. Following application of LPC, current activation was delayed by a minute or more. The delay in the onset of current activation ranged from 60 to 300 s after addition of 15 μm LPC and was about 2 min (128 ± 22 s; n = 13) on average. The mean time required to activate the current maximally and to reach stable reversal potentials was about 4.5 min (265 ± 62 s; n = 13) after the beginning of LPC application. Effects of LPC were completely reversible. As shown for the example in Fig. 2D, currents declined rapidly after washout of LPC. After recovery, currents could be activated again by LPC. Amplitudes of the currents induced by a second LPC stimulus did not differ significantly from those of currents activated by the first LPC application (Fig. 2D).
Ion selectivity and kinetics of the LPC-induced membrane current
Almost identical currents were activated by LPC with K+-containing (Fig. 2) and K+-free (Figs 3 and 4) intracellular solutions, regardless of whether potassium ions were substituted by either NMG+ or Cs+. The reversal potential of LPC-induced currents measured with NMGCl in the pipette was −3.2 ± 0.6 mV (n = 5), while currents reversed at −4.2 ± 0.7 mV (n = 20) using Cs+-based intracellular solution I2. These data suggest that LPC-activated ion channels were not highly selective for K+. Furthermore, they also suggest that LPC had little effect on pre-existent K+ currents, or that any such effect was outweighed by the appearance of a new conductance. LPC-induced currents were also detected after lowering the intracellular Cl− concentration from 126 mm (Fig. 2) to 4 mm (Figs 3 and 4) (n = 26), or by equimolar substitution of extracellular Cl− with gluconate (n = 4, data not shown), suggesting that LPC-induced currents were not carried exclusively by chloride ions. This finding is supported by the observation that extracellular application of the chloride channel inhibitors DIDS (200 μm; n = 7; data not shown) or NPPB (200 μm; n = 2; data not shown) did not have any effect on LPC-induced currents. Using CsCH3SO3-based intracellular solution I2, neither the mean amplitude determined at −60 mV (−484 ± 106 pA; n = 20) nor the mean reversal potential (−4.2 ± 0.7 mV; n = 20) of LPC-induced currents differed significantly from the corresponding mean values of LPC-induced currents measured with KCl-based intracellular solution I1 (see above).
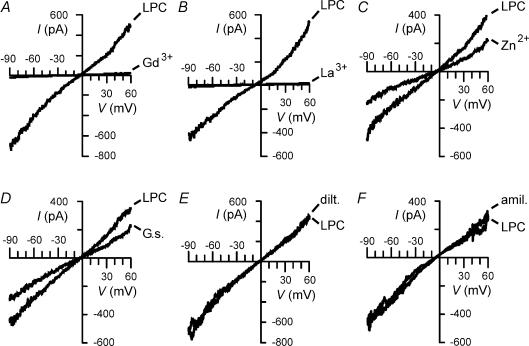
Figure 4. Pharmacological properties of LPC-induced non-selective cation currents.
Currents of BV-2 microglial cells evoked by 15 μm LPC were recorded in the absence (LPC) and in the presence of 100 μm Gd3+ (Gd3+; A), 100 μm La3+ (La3+; B), 500 μm Zn2+ (Zn2+; C), Grammostola spatulata venom, diluted 1: 2000 (G.s.; D), 100 μm diltiazem (dilt.; E), or 1 mm amiloride (amil.; F).
The reversal potential of the LPC-induced membrane current implied that this current was non-selective to cations or anions. After decreasing the ionic strength by superfusing cells with extracellular solution E2, the current reversal potential shifted in negative direction from −3.2 ± 0.9 to −14.2 ± 1.6 mV (n = 7). These data suggest that LPC-induced currents are more selective for cations than for anions. The permeability of the LPC-induced channel for Cl− was very small, i.e. the permeability ratio P Cl: P Na was 0.03 (n = 6). Next, we obtained the relative permeability ratio of various cations for the LPC-induced current by determination of the reversal potentials under various ionic conditions (see Methods). The permeability ratios PCs: PK: PNa: PNMG: PCa was calculated to be 1.54: 1.36: 1: 0.39: 0.22 (n = 8 for K+; n = 11 for Na+; n = 9 for NMG+; n = 5 for Ca2+).
In order to study isolated non-selective cation currents in more detail, we used CsCH3SO3-based intracellular solution I2 and Ca2+-free extracellular solution E5 in further experiments. Cells were held at a holding potential of 0 mV, i.e. close to the reversal potential of the LPC-induced currents, in order to prevent continuous ion flux through LPC-activated channels and subsequent cell swelling.
To evaluate time-dependent gating of the current, hyperpolarizing and depolarizing voltage steps were applied to the cells. As shown for the example in Fig. 3, LPC-induced currents exhibited neither time-dependent activation nor inactivation at potentials between −90 and +50 mV. At +60 mV, slight current decay was detected in 3 out of 19 cells, while the remaining 16 cells did not show any time dependence at all test potentials.
Pharmacological properties of the LPC-induced non-selective cation current
Lanthanides have been reported to be effective inhibitors of non-selective cation currents in a variety of cell preparations (Sachs & Morris, 1998). In microglia, amplitude of LPC-induced currents was reduced by 61.8 ± 21.7% (n = 6) and by 91.2 ± 6.9% (n = 11) in the presence of 10 and 50 μm Gd3+, respectively. The currents were abolished by 100 μm Gd3+ in primary cultured microglia (n = 5) and in BV-2 microglial cells (n = 14) as demonstrated in Fig. 4A. La3+ was slightly more effective than Gd3+ in blocking LPC-induced cation currents. Complete current inhibition was detected at 50 μm (n = 13) and 100 μm La3+(n = 10) (Fig. 4B), while currents were reduced by 83.9 ± 9.5% (n = 6) in the presence of 10 μm La3+. LPC-induced currents were also inhibited by the divalent cation Zn2+, although microglial non-selective cation channels exhibited a lower sensitivity to Zn2+ than to Gd3+ and La3+. LPC-induced currents were reduced by 58.1 ± 12.3% (n = 4) in the presence of 500 μm Zn2+ (Fig. 4C), while they were not significantly affected by 100 μm Zn2+ (n = 4; data not shown). In some cell preparations, G. spatulata spider venom (e.g. Hu & Sachs, 1996; Suchyna et al. 2000), LOE908 (e.g. Krautwurst et al. 1993), diltiazem (e.g. Hashimoto et al. 2000) or amiloride (e.g. Lane et al. 1991; Duranton et al. 2002) have been shown to inhibit non-selective cation channels. In our study on microglia, G. spatulata spider venom induced partial inhibition of LPC-induced currents when applied at high concentration, i.e. at a dilution of 1: 2000 (Fig. 4D). On average, G. spatulata spider venom reduced LPC-induced currents by 39.2 ± 15.3% (n = 7). Microglial non-selective cation currents were unaffected by 50 μm LOE908MS (n = 7; data not shown), 100 μm diltiazem (n = 7; Fig. 4E) or 1 mm amiloride (n = 7; Fig. 4F).
LPC-induced changes in intracellular Ca2+ concentration and activation of Ca2+-dependent K+ currents
Bath application of 15 μm LPC elicited sustained increases in the intracellular Ca2+ concentration ([Ca2+]i) as shown in Fig. 5A. The time course of LPC-induced [Ca2+]i increases was identical to that of LPC-induced activation of non-selective cation currents. The onset of the Ca2+ signals was delayed by about 2 min (128 ± 3 s; n = 168). During LPC application, Ca2+ signals were detected in all cells investigated. Changes in [Ca2+]i were exclusively caused by Ca2+ influx from the extracellular solution. Ca2+ signals were not detected following bath application of 15 μm LPC (n = 143) in experiments with Ca2+-free extracellular solution E3 (Fig. 5B). Furthermore, LPC-induced [Ca2+]i increases were abolished in the presence of 100 μm La3+(n = 53) or 100 μm Gd3+(n = 230) (Fig. 5C), whereas 1 μm Gd3+ did not affect LPC-induced Ca2+ signals (n = 62; data not shown).
Figure 5. Effects of LPC on intracellular Ca2+ concentration.
Measurements of [Ca2+]i were performed in fura-2 AM-loaded BV-2 microglial cells. A, 15 μm LPC caused sustained increases in [Ca2+]i. B, lack of Ca2+ elevation in Ca2+-free extracellular solution. C, in the presence of 100 μm Gd3+, LPC-induced Ca2+ increases were inhibited. The right panel of the figure represents mean intracellular Ca2+ concentrations of the individual cells (n = 46 cells in A; n = 16 cells in B; n = 23 cells in C), which are shown at the left side of the figure.
To test whether LPC-induced increases in [Ca2+]i are sufficient to activate Ca2+-dependent K+ currents, further patch-clamp experiments were performed using low Ca2+-buffered intracellular solution I3 (KCl-based) and extracellular solution E1 (NaCl based, Ca2+ containing). A current elicited by LPC under these recording conditions is shown in Fig. 6A. The mean reversal potential of the LPC-induced currents was −55.8 ± 6.5 mV (n = 9), suggesting activation of both non-selective cation currents and Ca2+-dependent K+ currents. LPC is also capable of activating Ca2+-dependent K+ currents under conditions of physiological intracellular Ca2+ buffering, as demonstrated by perforated-patch-clamp experiments (n = 5; Fig. 6B). Following omission of Ca2+ from the extracellular solution, LPC failed to induce Ca2+-dependent K+ currents. In the presence of Ca2+-free extracellular solution E3, LPC elicited exclusively non-selective cation currents (Fig. 6C), which reversed at −8 ± 1.9 mV (n = 5). Figure 6D demonstrates the effects of 50 nm charybdotoxin (CTX) on LPC-induced currents in Ca2+-containing extracellular solution. CTX shifted the current reversal potential in a depolarizing direction towards the reversal potential of non-selective cation currents, suggesting that CTX inhibited LPC-induced Ca2+-dependent K+ currents. The mean current reversal potentials were −61 ± 7.1 and −11.1 ± 2.2 mV (n = 7) before and during superfusion of cells with CTX-containing solution, respectively. Properties of Ca2+-dependent K+ currents in murine microglia have been published elsewhere (Eder et al. 1997; Schilling et al. 2002, 2004).
LPC-induced changes in membrane potential
Next, we determined LPC-induced changes in membrane potential. Using high Ca2+-buffered intracellular solution I1 (KCl based), application of LPC elicited membrane depolarization. Using low Ca2+-buffered intracellular solution I3 (KCl based), a more complex pattern of membrane potential changes occurred in response to LPC stimulation. As demonstrated in Fig. 7A, LPC caused first a transient membrane depolarization, which was followed by sustained membrane hyperpolarization. Similar membrane potential changes were observed in perforated-patch-clamp recordings, as shown for the example in Fig. 7B. The onset of the LPC-induced membrane depolarization was delayed on average by 75 ± 15 s (n = 9) in whole-cell recordings using low Ca2+-buffered intracellular solution I3 and by 86 ± 39 s (n = 3) in perforated-patch-clamp recordings. Both values did not differ significantly from each other and from the delay in the onset of the LPC-induced cation current activation and Ca2+ increases (see above). In whole-cell recordings with low Ca2+-buffered intracellular solution I3 we determined a mean resting membrane potential of −45.6 ± 7 mV (n = 4) before application of LPC and mean values of −17 ± 5.7 and −70.9 ± 1.9 mV (n = 4) for LPC-induced membrane depolarization and hyperpolarization, respectively. In perforated-patch-clamp measurements, cells had a mean resting membrane potential of −40.3 ± 10.7 mV (n = 3), depolarized to a mean value of −11.2 ± 2.5 mV and hyperpolarized to −68 ± 2.2 mV (n = 3). Mean values of resting membrane potential, LPC-induced depolarization and LPC-induced hyperpolarization did not differ significantly between whole-cell and perforated-patch recordings. LPC-induced depolarization could be reversed by 100 μm Gd3+(n = 4) or 100 μm La3+(n = 4) (Fig. 7C), while membrane hyperpolarization was abolished by 50 nm CTX (n = 4) (Fig. 7D).
Non-selective cation channels but not Ca2+-dependent K+ channels are important for LPC-induced morphological changes in microglia
To clarify whether LPC-induced de-ramification was due to increases in [Ca2+]i following Ca2+ influx through non-selective cation channels, Ca2+ was omitted from the extracellular solution in some experiments. LPC caused almost identical changes in microglial morphology in measurements with Ca2+-free and Ca2+-containing extracellular solutions. The ramification index of microglial cells treated with LPC in Ca2+-free external solution was slightly larger than that of cells in Ca2+-containing solution (Fig. 8A). These data implied that changes in microglial morphology were not Ca2+-dependent.
Figure 8. Involvement of non-selective cation channels and K+–Cl− cotransporters in LPC-induced microglial de-ramification.
A, partial inhibition of LPC-induced microglial de-ramification by blockers of either non-selective cation channels or K+–Cl− cotransporters. Ramification index (mean ±s.e.m.) of untreated microglia (n = 85) and of microglia exposed for 30 min to LPC in Ca2+-containing (n = 435) or Ca2+-free (n = 150) solution, or in the presence of 100 μm Gd3+(n = 258), 100 μm La3+(n = 176), 1 mm furosemide (n = 116) or 100 μm DIOA (n = 118). B, complete inhibition of LPC-induced microglial de-ramification by combined application of blockers of non-selective cation channels and blockers of K+–Cl− cotransporters. Ramification index (mean ±s.e.m.) of untreated microglia (n = 85) and of microglia exposed for 30 min to LPC and 100 μm Gd3+ in the absence (n = 258) or in the presence of 1 mm furosemide (n = 148), 100 μm DIOA (n = 166), 100 μm bumetanide (n = 244), or in Cl−-free extracellular solution (n = 181). C, example of primary cultured microglial cells exposed to 100 μm Gd3+ and 100 μm DIOA before (C1) and 30 min after (C2) application of 15 μm LPC.
As shown above, Ca2+-dependent K+ channels are not activated by LPC in Ca2+-free extracellular solution. Thus, the lack of inhibitory effects of Ca2+-free extracellular solution on LPC-induced morphological changes of microglia demonstrates further that activity of Ca2+-dependent K+ channels is not required for microglial de-ramification.
In further experiments, we investigated whether inhibition of non-selective cation channels influences LPC-induced shape changes. As demonstrated in Fig. 8A, the ramification index of LPC-treated microglial cells was significantly (P < 0.001) smaller in the presence of 100 μm Gd3+ than in the absence of Gd3+, suggesting that the activity of non-selective cation channels is necessary for LPC-induced morphological changes. Partial inhibition of LPC-induced de-ramification by Gd3+ was observed in both primary cultured and BV-2 microglial cells. Almost identical observations were made when non-selective cation currents were abolished by 100 μm La3+ (Fig. 8A).
Functional importance of K+–Cl− cotransporters for LPC-induced changes in microglial morphology
Although 100 μm Gd3+ or 100 μm La3+ abolished non-selective cation currents, neither Gd3+ nor La3+ inhibited LPC-induced de-ramification of microglia completely. Therefore, it was investigated whether electroneutral ion transporters were involved additionally in microglial shape changes. As shown in Fig. 8A, inhibition of K+–Cl− cotransporters leads to partial inhibition of microglial de-ramification. Thirty minutes after exposure to LPC, the mean ramification indices of cells treated with either 1 mm furosemide or 100 μm DIOA were significantly smaller than that of cells held in the absence of K+–Cl− cotransporter inhibitors. Figure 8B demonstrates that combined application of 100 μm Gd3+ and 1 mm furosemide prevented LPC-induced transformation of microglial morphology from the ramified to the amoeboid form. LPC-induced de-ramification also did not occur in the presence of 100 μm Gd3+ and 100 μm DIOA in either primary cultured microglia or BV-2 microglia (Figs 8B and C). The mean ramification index of these cell populations was not significantly different from that of untreated cells (Fig. 8B). In contrast, combined application of 100 μm Gd3+ and 100 μm bumetanide failed to prevent microglial de-ramification. LPC-induced shape changes were also not abolished by Gd3+ in Cl− free extracellular solution (Fig. 8B).
To verify the activity of K+–Cl− cotransporters we determined the extracellular K+ concentration ([K+]o) of LPC-treated microglia cultures in the absence and presence of DIOA. We determined a mean [K+]o of 7.2 ± 0.3 mm(n = 4) for LPC-stimulated cultures 30 min after exposure of microglia to LPC. In the presence of 100 μm Gd3+, [K+]o of LPC-stimulated cell cultures was 6.9 ± 0.2 mm(n = 4). During combined application of Gd3+ and DIOA, [K+]o of LPC-stimulated cultures was significantly smaller (P < 0.05) than that of cultures not exposed to DIOA. A mean [K+]o of 6.4 ± 0.1 mm(n = 4) was determined for LPC-stimulated microglial cultures exposed to both 100 μm Gd3+ and 100 μm DIOA. These data suggest that K+–Cl− cotransporters contribute to K+ efflux from LPC-stimulated microglial cells.
Discussion
In this study we demonstrate for the first time that in microglia non-selective cation channels, Ca2+-dependent K+ channels and K+–Cl− cotransporters are activated by LPC. Furthermore, we demonstrate the functional importance of non-selective cation channels and K+–Cl− cotransporters for microglial de-ramification.
Physiological concentrations of LPC are in the range of 5–180 μm (Croset et al. 2000; Xu, 2002), varying in different tissues and body fluids. In pathophysiological situations, such as ischaemia, LPC may reach concentrations of up to 200 μm (Akita et al. 1986; Sedlis et al. 1990). Assuming that ∼90% of LPC is protein bound, it has been suggested that the free concentration of LPC reaches 20 μm in ischaemic tissue (Yu et al. 1998). In microglia, physiological concentrations of LPC evoke membrane depolarization and sustained Ca2+ increases by activating non-selective cation channels. Activation of non-selective cation currents in response to LPC has been reported in ventricular myocytes (Magishi et al. 1996; Hashimoto et al. 2000; Song & Ochi, 2002), arterial smooth muscle cells (Jabr et al. 2000; Terasawa et al. 2002) and endothelial cells (Yokoyama et al. 2002). LPC-activated non-selective cation channels of microglia have similarities with those of other cell preparations. They activated after some delay, were permeable for monovalent and divalent cations and could be inhibited by lanthanides. Diltiazem has been shown to inhibit LPC-induced currents of ventricular myocytes (Hashimoto et al. 2000), but failed to block microglial currents. Grammostola spatulata spider venom had inhibitory effects on LPC-induced currents in microglia similar to those on stretch-activated cation currents in astrocytes and heart cells (Hu & Sachs, 1996; Suchyna et al. 2000). Based on our observations that LPC-induced currents were inhibited by Zn2+ but unaffected by amiloride, it can be excluded that microglial channels are related to the amiloride-sensitive ENaC/DEG channel superfamily (Kellenberger & Schild, 2002). LPC-activated cation currents of microglial cells correspond in some of their properties to the currents that are activated by sustained depolarization, ADP-ribose or βNAD+ in peritoneal macrophages (Campo et al. 2003). Moreover, microglial non-selective cation channels resemble closely stretch-activated/mechanosensitive cation channels described in a variety of cell preparations (Sachs & Morris, 1998). Perozo et al. (2002) have recently elucidated the mechanism by which LPC causes activation of cation channels. According to the authors' model, LPC produces local stress due to its insertion into the outer monolayer of the plasma membrane. By sensing this stress, mechanosensitive channels undergo conformational changes and open subsequently (Perozo et al. 2002; Sachs, 2002). Most likely, microglial cation channels are activated by the same mechanism. However, it cannot be excluded that LPC receptor-coupled intracellular signalling pathways modulate channel activity additionally.
We suggest that LPC-induced sustained Ca2+ increases are mediated by activation of non-selective cation channels. It can be excluded that Ca2+ release-activated Ca2+ (CRAC) currents contribute to the LPC-induced Ca2+ signal, because Gd3+ did not affect LPC-induced Ca2+ increases at a concentration of 1 μm, which is sufficient to block CRAC channels completely (Ross & Cahalan, 1995). The LPC-induced Ca2+ signal appears to consist of two components. The first transient component of LPC-induced Ca2+ signals may reflect swelling-activated Ca2+ (SWAC) transients, which have been described first in thymocytes (Ross & Cahalan, 1995), and can also be elicited in microglia by hyposmotic extracellular solutions (T. Schilling, C.Eder, unpublished observations). However, it is also possible that the LPC-induced Ca2+ signal decreased to some extent after reaching the initial peak due to Ca2+ extrusion mechanisms. Further experiments are required to test whether one of these hypotheses can explain the two components of the LPC-induced Ca2+ signal.
Furthermore, we provide evidence that a Ca2+-dependent K+ current is activated secondary to LPC-induced Ca2+ entry through non-selective cation channels. We have shown previously that Ca2+-dependent K+ currents of murine microglia are voltage independent, sensitive to inhibitors of intermediate conductance (IK) channels, such as charybdotoxin and clotrimazole, but insensitive to an inhibitor of small conductance (SK) channels, apamin, and to an inhibitor of large conductance (BK) channels, paxilline. It was also demonstrated that the channel is encoded by the gene IKCa1 (Eder et al. 1997; Schilling et al. 2002, 2004). The Ca2+-dependent K+ current evoked by LPC was identical to the IKCa1 Ca2+-dependent K+ current described before in murine microglia. Similar to IKCa1 currents, LPC-evoked Ca2+-dependent K+ currents exhibited voltage-independent activation behaviour, reversed at potentials close to the K+ equilibrium potential and were inhibited by charybdotoxin. K+ current activation did not occur by using high Ca2+-buffered intracellular solution or Ca2+-free extracellular solution, suggesting that increases in the intracellular concentration of Ca2+ were responsible for current activation. Importantly, LPC-activated Ca2+-dependent K+ currents caused membrane hyperpolarization, which occurred secondary to the initial depolarization that was evoked by non-selective cation currents. We propose that the membrane hyperpolarization that results from Ca2+-activated K+ channels increases the driving force for Na+ and Ca2+ entry through non-selective cation channels. Thus, Ca2+-dependent K+ channels help maintain substantial cation influx.
In our experiments, microglial cells transformed almost completely from a ramified to an amoeboid morphology following stimulation with LPC. Intriguingly, microglial de-ramification occurred independently of intracellular and extracellular Ca2+. This observation appears to be in contrast to some reports linking cytoskeletal reorganization to increases in intracellular Ca2+ (e.g. Onuma & Hui, 1988; Li et al. 2001). LPC-induced morphological changes of microglial cells were partially inhibited in the presence of Gd3+, La3+, furosemide or DIOA, suggesting involvement of non-selective cation channels and K+–Cl− cotransporters in microglial de-ramification. Microglial shape changes were prevented by combined application of Gd3+ and either furosemide or DIOA. This observation led us to conclude that simultaneous activity of both non-selective cation channels and K+–Cl− cotransporters is required for microglial de-ramification. At a concentration of 100 μm, DIOA specifically inhibits K+–Cl− cotransporters without affecting Na+–K+–2Cl− cotransporters (Garay et al. 1988). Although furosemide is capable of inhibiting both K+–Cl− cotransporters and Na+–K+–2Cl− cotransporters (Lauf & Adragna, 2000; Russell, 2000), our data argue against a contribution of Na+–K+–2Cl− cotransporters. Specific inhibition of Na+–K+–2Cl− cotransporters either by using Cl−-free extracellular solution or by application of bumetanide failed to inhibit LPC-induced morphological changes. Further experiments are required to clarify whether K+–Cl− cotransporters are activated by LPC directly or secondarily to other LPC-induced processes. It has been demonstrated for other cell preparations that K+–Cl− cotransporters can be activated by cell swelling, intracellular acidification, reactive oxygen radicals, or intracellular signalling processes, such as protein phosphorylation (Lauf & Adragna, 2000).
In contrast to non-selective cation channels and K+–Cl− cotransporters, Ca2+-dependent K+ channels appear to play only a minor role in regulating microglial shape changes. In experiments at which Ca2+-dependent K+ channel activation was prevented using Ca2+-free extracellular solution, LPC-induced microglial de-ramification was not inhibited. In contrast, under these conditions de-ramification was slightly more pronounced than in experiments using Ca2+-containing solutions. It is possible that Ca2+-dependent K+ channels control the amount of LPC-induced cell body swelling by counteracting the activity of non-selective cation channels. Most likely, osmolarity and subsequent swelling of the cell body is reduced to some extent due to K+ efflux through Ca2+-dependent K+ channels. Loss of up to 20% of the cell volume was attributed to the activity of IKCa1 Ca2+-dependent K+ channels in other cell preparations (Schneider et al. 2000).
We hypothesize that rearrangement of the cytoskeleton following exposure of microglia to LPC is accompanied by changes in osmolarity. To explain the physiological mechanisms underlying LPC-induced microglial de-ramification, we propose the following model (Fig. 9): Extracellular application of LPC leads to simultaneous activation of non-selective cation channels and K+–Cl− cotransporters. We suggest that the expression of cation channels is higher in cell bodies than in cell processes, while K+–Cl− cotransporters are predominantly expressed in cell processes. Cation influx through non-selective cation channels increases the osmolarity inside the cell. The subsequent osmotic entry of water results in swelling of cell bodies. In contrast, KCl efflux due to activity of K+–Cl− cotransporters reduces osmolarity in the processes. Subsequently, water leaves cell processes causing their shrinkage and retraction. These two opposing mechanisms occur simultaneously and lead finally to complete retraction of cell extensions and increased size of cell bodies, i.e. the cells exhibit amoeboid morphology (Fig. 9).
Figure 9.
Proposed model of physiological mechanisms of microglial de-ramification induced by LPC (see Discussion)
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft grants SFB 507-C7, GRK 238 and a Heisenberg fellowship (all to C.E.). The authors wish to thank Dr Thomas DeCoursey for helpful comments on the manuscript. We also thank Boehringer Ingelheim for kindly providing LOE908MS.
References
- Akita H, Creer MH, Yamada KA, Sobel BE, Corr PB. Electrophysiologic effects of intracellular lysophosphoglycerides and their accumulation in cardiac lymph with myocardial ischemia in dogs. J Clin Invest. 1986;78:271–280. doi: 10.1172/JCI112561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev NA, Undrovinas AI, Fleidervish IA, Rosenshtraukh LV. Ischemic poison lysophosphatidylcholine modifies heart sodium channels gating inducing long-lasting bursts of openings. Pflugers Arch. 1989;415:124–126. doi: 10.1007/BF00373151. [DOI] [PubMed] [Google Scholar]
- Campo B, Surprenant A, North RA. Sustained depolarization and ADP-ribose activate a common ionic current in rat peritoneal macrophages. J Immunol. 2003;170:1167–1173. doi: 10.4049/jimmunol.170.3.1167. [DOI] [PubMed] [Google Scholar]
- Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J. 2000;345:61–67. [PMC free article] [PubMed] [Google Scholar]
- Duranton C, Huber SM, Lang F. Oxidation induces a Cl−dependent cation conductance in human red blood cells. J Physiol. 2002;539:847–855. doi: 10.1113/jphysiol.2001.013040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am J Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- Eder C, Klee R, Heinemann U. Pharmacological properties of Ca2+-activated K+ currents of ramified murine brain macrophages. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:233–239. doi: 10.1007/pl00005046. [DOI] [PubMed] [Google Scholar]
- Eder C, Schilling T, Heinemann U, Haas D, Hailer N, Nitsch R. Morphological, immunophenotypical and electrophysiological properties of resting microglia in vitro. Eur J Neurosci. 1999;11:4251–4261. doi: 10.1046/j.1460-9568.1999.00852.x. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Litsky ML, Farooqui T, Horrocks LA. Inhibitors of intracellular phospholipase A2 activity: their neurochemical effects and therapeutical importance for neurological disorders. Brain Res Bull. 1999;49:139–153. doi: 10.1016/s0361-9230(99)00027-1. [DOI] [PubMed] [Google Scholar]
- Garay RP, Nazaret C, Hannaert PA, Cragoe EJ., Jr Demonstration of a [K+,Cl−]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl−]-cotransport system. Mol Pharmacol. 1988;33:696–701. [PubMed] [Google Scholar]
- Golfman LS, Haughey NJ, Wong JT, Jiang JY, Lee D, Geiger JD, Choy PC. Lysophosphatidylcholine induces arachidonic acid release and calcium overload in cardiac myoblastic H9c2 cells. J Lipid Res. 1999;40:1818–1826. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hashimoto Y, Yabana H, Murata S. Electrophysiological effect of 1-cis-diltiazem, the stereoisomer of d-cis-diltiazem, on isolated guinea-pig left ventricular myocytes. Eur J Pharmacol. 2000;391:217–223. doi: 10.1016/s0014-2999(00)00077-7. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 2001. [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J General Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Sachs F. Mechanically activated currents in chick heart cells. J Membr Biol. 1996;154:205–216. doi: 10.1007/s002329900145. [DOI] [PubMed] [Google Scholar]
- Huang YH, Schafer-Elinder L, Wu R, Claesson HE, Frostegard J. Lysophosphatidylcholine (LPC) induces proinflammatory cytokines by a platelet-activating factor (PAF) receptor-dependent mechanism. Clin Exp Immunol. 1999;116:326–331. doi: 10.1046/j.1365-2249.1999.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi Y, Nishizaki T, Matsuoka T. Lysophosphatidylcholine inhibits NMDA-induced currents by a mechanism independent of phospholipase A2-mediated protein kinase C activation in hippocampal glial cells. Biochem Biophys Res Commun. 1995;217:811–816. doi: 10.1006/bbrc.1995.2844. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Yamazaki J, Hume JR. Lysophosphatidylcholine triggers intracellular calcium release and activation of non-selective cation channels in renal arterial smooth muscle cells. Pflugers Arch. 2000;439:495–500. doi: 10.1007/s004249900206. [DOI] [PubMed] [Google Scholar]
- Kabarowski JH, Xu Y, Witte ON. Lysophosphatidylcholine as a ligand for immunoregulation. Biochem Pharmacol. 2002;64:161–167. doi: 10.1016/s0006-2952(02)01179-6. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Imaizumi S, Yoshimoto T, Yamamoto H, Motomiya M. Changes of polyphosphoinositides, lysophospholipid, and free fatty acids in transient cerebral ischemia of rat brain. Mol Chem Neuropathol. 1990;12:215–228. doi: 10.1007/BF03159946. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Arita M. Effects of lysophosphatidylcholine on resting potassium conductance of isolated guinea pig ventricular cells. Pflugers Arch. 1986;406:296–302. doi: 10.1007/BF00640917. [DOI] [PubMed] [Google Scholar]
- Korotzer AR, Cotman CW. Voltage-gated currents expressed by rat microglia in culture. Glia. 1992;6:81–88. doi: 10.1002/glia.440060202. [DOI] [PubMed] [Google Scholar]
- Krautwurst D, Hescheler J, Arndts D, Losel W, Hammer R, Schultz G. Novel potent inhibitor of receptor-activated nonselective cation currents in HL-60 cells. Mol Pharmacol. 1993;43:655–659. [PubMed] [Google Scholar]
- Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JW, McBride D, Hamill OP. Amiloride block of the mechanosensitive cation channel in Xenopus oocytes. J Physiol. 1991;441:347–366. doi: 10.1113/jphysiol.1991.sp018755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem. 2000;10:341–354. doi: 10.1159/000016357. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Li C, Fultz ME, Parkash J, Rhoten WB, Wright GL. Ca2+-dependent actin remodeling in the contracting A7r5 cell. J Muscle Res Cell Motil. 2001;22:521–534. doi: 10.1023/a:1015026530258. [DOI] [PubMed] [Google Scholar]
- Magishi K, Kimura J, Kubo Y, Abiko Y. Exogenous lysophosphatidylcholine increases non-selective cation current in guinea-pig ventricular myocytes. Pflugers Arch. 1996;432:345–350. doi: 10.1007/s004240050142. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Onuma EK, Hui SW. Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol. 1988;106:2067–2075. doi: 10.1083/jcb.106.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousman SS, David S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia. 2000;30:92–104. [PubMed] [Google Scholar]
- Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PE, Cahalan MD. Ca2+ influx pathways mediated by swelling or stores depletion in mouse thymocytes. J General Physiol. 1995;106:415–444. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Sachs F. Retaining your identity under stress. Nat Struct Biol. 2002;9:636–637. doi: 10.1038/nsb0902-636. [DOI] [PubMed] [Google Scholar]
- Sachs F, Morris CE. Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- Sato T, Kiyosue T, Arita M. Inhibitory effects of palmitoylcarnitine and lysophosphatidylcholine on the sodium current of cardiac ventricular cells. Pflugers Arch. 1992;420:94–100. doi: 10.1007/BF00378647. [DOI] [PubMed] [Google Scholar]
- Schilling T, Repp H, Richter H, Koschinski A, Heinemann U, Dreyer F, Eder C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca2+-dependent K+ channels. Neuroscience. 2002;109:827–835. doi: 10.1016/s0306-4522(01)00534-6. [DOI] [PubMed] [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- Schneider SW, Pagel P, Rotsch C, Danker T, Oberleithner H, Radmacher M, Schwab A. Volume dynamics in migrating epithelial cells measured with atomic force microscopy. Pflugers Arch. 2000;439:297–303. doi: 10.1007/s004249900176. [DOI] [PubMed] [Google Scholar]
- Sedlis SP, Sequeira JM, Altszuler HM. Coronary sinus lysophosphatidylcholine accumulation during rapid atrial pacing. Am J Cardiol. 1990;66:695–698. doi: 10.1016/0002-9149(90)91132-p. [DOI] [PubMed] [Google Scholar]
- Song YM, Ochi R. Hyperpolarization and lysophosphatidylcholine induce inward currents and ethidium fluorescence in rabbit ventricular myocytes. J Physiol. 2002;545:463–473. doi: 10.1113/jphysiol.2002.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J General Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, Foudin LL. On the status of lysolecithin in rat cerebral cortex during ischemia. J Neurochem. 1984;43:1081–1086. doi: 10.1111/j.1471-4159.1984.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Terasawa K, Nakajima T, Iida H, Iwasawa K, Oonuma H, Jo T, Morita T, Nakamura F, Fujimori Y, Toyo-oka T, Nagai R. Nonselective cation currents regulate membrane potential of rabbit coronary arterial cell: modulation by lysophosphatidylcholine. Circulation. 2002;106:3111–3119. doi: 10.1161/01.cir.0000039345.00481.1d. [DOI] [PubMed] [Google Scholar]
- Undrovinas AI, Fleidervish IA, Makielski JC. Inward sodium current at resting potentials in single cardiac myocytes induced by the ischemic metabolite lysophosphatidylcholine. Circ Res. 1992;71:1231–1241. doi: 10.1161/01.res.71.5.1231. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang H, Han H, Zhang Y, Yang B, Nattel S, Wang Z. Phospholipid metabolite 1-palmitoyl-lysophosphatidylcholine enhances human ether-a-go-go-related gene (HERG) K+ channel function. Circulation. 2001;104:2645–2648. doi: 10.1161/hc4701.100513. [DOI] [PubMed] [Google Scholar]
- Wilms H, Hartmann D, Sievers J. Ramification of microglia, monocytes and macrophages in vitro: influences of various epithelial and mesenchymal cells and their conditioned media. Cell Tissue Res. 1997;287:447–458. doi: 10.1007/s004410050769. [DOI] [PubMed] [Google Scholar]
- Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim Biophys Acta. 2002;1582:81–88. doi: 10.1016/s1388-1981(02)00140-3. [DOI] [PubMed] [Google Scholar]
- Yegin A, Akbas SH, Ozben T, Korgun DK. Secretory phospholipase A2 and phospholipids in neural membranes in an experimental epilepsy model. Acta Neurol Scand. 2002;106:258–262. doi: 10.1034/j.1600-0404.2002.01238.x. [DOI] [PubMed] [Google Scholar]
- Yeon D, Kwon S, Nam T, Ahn D. Lysophosphatidylcholine decreases delayed rectifier K+ current in rabbit coronary smooth muscle cells. J Vet Med Sci. 2001;63:395–399. doi: 10.1292/jvms.63.395. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Ishibashi T, Ohkawara H, Kimura J, Matsuoka I, Sakamoto T, Nagata K, Sugimoto K, Sakurada S, Maruyama Y. HMG-CoA reductase inhibitors suppress intracellular calcium mobilization and membrane current induced by lysophosphatidylcholine in endothelial cells. Circulation. 2002;105:962–967. doi: 10.1161/hc0802.104457. [DOI] [PubMed] [Google Scholar]
- Yu L, Netticadan T, Xu YJ, Panagia V, Dhalla NS. Mechanisms of lysophosphatidylcholine-induced increase in intracellular calcium in rat cardiomyocytes. J Pharmacol Exp Ther. 1998;286:1–8. [PubMed] [Google Scholar]