Abstract
We have previously demonstrated that trinitrobenzene sulphonic acid (TNBS)-induced colitis in guinea pig is associated with hyperexcitability of myenteric AH neurones, enhanced synaptic activity in the myenteric plexus, increased serotonin (5-HT) availability in the mucosa, and decreased propulsive motor activity. The current study tested the hypothesis that the activation of cyclooxygenase (COX) contributes to these alterations in bowel functions. DFU inhibition of COX-2, but not SC-560 inhibition of COX-1, restored to normal levels the electrical properties of myenteric AH neurones, the proportion of S neurones exhibiting slow EPSPs, and the rate of propulsive motor activity. Neither inhibitor was effective in altering the level of inflammation, the increased availability of mucosal 5-HT, or the enhanced fast EPSPs in myenteric AH and S neurones. COX-2 expression is enhanced in the myenteric plexus and cells within the smooth muscle layers during colitis, possibly reflecting the site at which COX-2 inhibition acts to allow recovery of motor function. In support of this concept, COX-1, but not COX-2, inhibition was effective in restoring normal mucosal prostaglandin levels. These results indicate that the various changes that occur in the motor neural pathways of the distal colon in TNBS-induced colitis do not involve a single neuroimmune mechanism. COX-2 activation is a critical step in the enhanced excitability of AH neurones as well as diminished propulsive motility in TNBS colitis, whereas other yet to be resolved pathways, that do not involve COX-1 or COX-2 activation, lead to altered 5-HT content in the mucosa and an augmentation of fast EPSPs.
Propulsive motor activity in the gut involves the activation of peristaltic reflex circuitry that is present along the length of the small and large intestines. At a given site, stretch and/or release of 5-HT from enterochromaffin (EC) cells activates intrinsic primary afferent neurones (IPANs) which, in turn, spread the signal by activating other IPANs as well as ascending and descending interneurones. Interneurones projecting orally activate excitatory motor neurones, and those projecting aborally activate inhibitory motor neurones, resulting in a pressure gradient and propulsive motor activity. Recent progress in enteric neurobiology has greatly improved our understanding of the key elements of enteric neural networks such that it is now possible to identify the various types of neurones that comprise the peristaltic reflex circuitry. For example, neurones that are classified as AH neurones on the basis of their electrical properties serve as IPANs (Furness et al. 1998), whereas neurones with the electrical properties of S neurones can be identified as interneurones or motor neurones depending on their projections and the neuronal markers that they express (Brookes, 2001). These advances have paved the way for investigations of what changes occur in precise components of gut reflex circuits under pathological conditions such as inflammation. This is clinically quite relevant because motor and secretory activities are altered in the inflamed gut.
We have recently reported that in a guinea pig model of immune-mediated colitis, precise alterations can be detected at various sites along the peristaltic reflex circuit. In the mucosal layer, there is an increase in 5-HT availability due to EC cell hyperplasia and a decrease in the 5-HT selective reuptake transporter (Linden et al. 2003a). In the myenteric plexus, there is an increase in the excitability of AH neurones, and there is an augmentation of synaptic activity in AH neurones and S neurones (Linden et al. 2003b). Furthermore, the rate of propulsive motor activity is reduced in the inflamed region of the colon suggesting that inflammation-induced alterations in 5-HT availability and/or neuronal electrical and synaptic properties could contribute to altered motility in colitis. We are therefore interested in exploring what aspect of the inflammatory response results in the changes that we have observed.
Inflammation involves a complex assortment of interdependent responses, including infiltration of leucocytes and macrophages, release of proinflammatory mediators, and changes in the synthetic machinery of resident cells. In order to begin to elucidate the mechanisms by which enteric motor reflex circuitry is altered in colitis, we have tested the hypothesis that the inflammation-mediated changes in these cells involve the activation of cyclooxygenase-2 (COX-2). There are a number of lines of evidence that support this theory: (1) COX-2 expression is induced by proinflammatory cytokines, including interleukin (IL) 1β and tumour necrosis factor (TNF)-α (Maier et al. 1990); (2) COX-2 expression is up-regulated in tissues of the inflamed colon, including epithelial cells (Reuter et al. 1996; Sakamoto, 1998; Singer et al. 1998); (3) COX-2 is believed to be responsible for the increased production of prostaglandins (PGs) associated with inflammation (Eberhart & Dubois, 1995; Sakamoto, 1998); (4) PG levels are markedly elevated in the mucosa of the inflamed colon (Sharon & Stenson, 1984; Schumert et al. 1988; Sakamoto, 1998); and (5) prostaglandins have an excitatory effect on intestinal enteric neurones where they have been tested (Frieling et al. 1994b, 1995; Dekkers et al. 1997a, b; Manning et al. 2002). Furthermore, prolonged exposure of AH neurones to a stable analogue of PGE2 leads to changes in the accommodation and after-hyperpolarization properties that are comparable to those of AH neurones in inflamed tissue (Manning et al. 2002).
Prostaglandins are products of arachidonic acid metabolism via the COX enzymes (Seeds & Bass, 1999; Iversen & Kragballe, 2000). The COX enzymes exist as two distinct genes, COX-1 which is constitutively expressed in many cell types, and COX-2, which is expressed following induction by proinflammatory cytokines (Williams & DuBois, 1996; Dubois et al. 1998; Sakamoto, 1998), as well as constitutively in some tissues (Jackson et al. 2000; Porcher et al. 2002). Recent advances in the development of selective inhibitors of COX-1 and COX-2 have provided tools for the pharmacological analysis of the contribution of either or both of the COX enzymes to pathophysiological conditions. The goal of this study was to determine whether selective blockade of either COX-1 or COX-2 leads to an attenuation of colitis-induced myenteric neurone excitability, altered mucosal 5-HT signalling, and/or decreased propulsive motor activity.
Methods
Animal preparations
All methods used in this study were approved by the University of Vermont and University of Calgary Animal Care and Use Committees. Adult albino guinea pigs (Charles River, Montreal, Canada) of either sex, weighing 250–350 g, were housed in metal cages with soft bedding. The animals had access to food and water ad libitum and were maintained at 23–24°C on a 12 h: 12 h light–dark cycle.
In order to generate inflammation in the distal colon, guinea pigs were anaesthetized with isoflurane (induced at 4%, maintained on 1.5% in oxygen) and 0.3 ml of trinitrobenzene sulphonic acid (TNBS; 25 mg ml−1) in 30% ethanol was delivered into the lumen of the colon through a polyethylene catheter inserted rectally 7 cm proximal to the anus. Control animals received 0.3 ml of intracolonic saline (0.9% NaCl) under anaesthesia. Animals were maintained in a controlled environment for 6 days after TNBS or saline administration.
The focus of this study was to determine the effect of cyclooxygenase (COX) inhibition on previously observed changes that accompany colitis. For this reason, animals injected with TNBS were separated randomly into one of three groups, those treated with 5,5-dimethyl-3-(3-fluorophenyl)-4(4-methyl-sulphonyl)-phenyl-2-(5H)-furanone (DFU; Merck Frosst, Quebec, Canada) to selectively inhibit COX-2, those treated with [5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethyl-pyrazole] (SC-560; Cayman Chemical, Ann Arbor, MI, USA) to selectively inhibit COX-1, and those to serve as a vehicle control. These animals are referred to as TNBS/DFU, TNBS/SC-560 and TNBS animals, respectively. The COX inhibitors were dissolved in ethanol and injected daily (s.c.) at a dose of 5 mg kg−1, on days 2–6 post-TNBS. At this dose, DFU reverses the increase in PG production that is caused by intestinal manipulation (Schwarz et al. 2001). The IC50 values for DFU and SC-560 are comparable for COX-2 and COX-1, respectively, and each is at least 1000-fold more selective for their respective target enzymes (Riendeau et al. 1997; Smith et al. 1998). The TNBS animals received the same schedule of daily injections (s.c.) of ethanol. In experiments where COX inhibition was responsible for significant functional differences in TNBS-treated animals, the measures were repeated in two additional control groups. These animals received an intracolonic administration of saline followed by daily injections (s.c.) of DFU or SC-560 as described above, and are referred to as saline/DFU and saline/SC-560 animals, respectively.
At the time of tissue collection, animals from which the tissue was used for electrophysiological, colonic propulsion or release experiments were anaesthetized with isoflurane and exsanguinated. Animals from which the tissue was used for immunohistochemistry and to measure serotonin and eicosanoid content were anaesthetized with isoflurane and perfused transcardially with ice-cold phosphate-buffered saline (PBS; 0.1 m, pH 7.4). The distal colon, identified as the part of the colon between the hypogastric flexure and the pelvic brim, was removed and used for experimental studies. The severity of colitis was assessed in three ways: changes in the weight of the animals, scoring of macroscopic damage, and histological evaluation (Linden et al. 2003a). Macroscopic scoring was based on wall thickness and the presence and extent of adhesions, ulceration, hyperaemia and diarrhoea. Histological scoring was based on the following features: extent of destruction of normal mucosal architecture, cellular infiltration, muscle thickening, crypt abscesses, and goblet cell depletion. All animals treated with TNBS lost weight and exhibited macroscopic and histopathological damage scores that were consistent with previous reports (Linden et al. 2003a,b). Selective inhibition of COX-1 or COX-2 did not alter any of these measures of the extent of inflammation (Fig. 1).
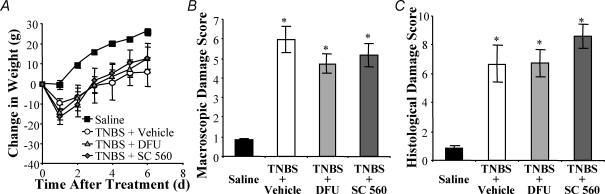
Figure 1. Inflammatory damage and weight loss are not altered by cyclooxygenase (COX) inhibition.
A, TNBS-treated Inflammation is induced by a single intracolonic administration of trinitrobenzene sulphonic acid (TNBS). Animals (○) lost weight initially but began gaining weight at a normal rate 2 days after TNBS, whereas control animals (▪) did not lose weight. Inhibition of COX-2 with DFU (Δ, 5 mg kg−1), or COX-1 with SC-560 (♦, 5 mg kg−1), did not alter the weight profiles of TNBS-treated animals. The gross damage score (B) and histopathology score (C) were significantly increased in colons of TNBS, TNBS/DFU and TNBS/SC-560 animals as compared to controls. P < 0.001 compared to controls; ANOVA, Newman-Keuls multiple comparisons test (for A and B: n = 14–22 animals; for C: n = 4–6 animals).
Electrophysiological recordings
The distal colon was removed and placed in iced Krebs solution (mm: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose, 8; aerated with a 95%O2–5%CO2; all from Sigma, St Louis, MO, USA). Nifedipine (5 μm) and atropine (200 nm) (Sigma) were added to eliminate smooth muscle contraction. The mucosa, submucosa and circular muscle of the colon were removed with forceps exposing the myenteric plexus on the longitudinal smooth muscle. The preparation was then moved to a 2.5 ml recording chamber. Preparations were continuously perfused at 7 ml min−1 with Krebs solution containing nifedipine and atropine maintained at 37°C. Glass microelectrodes used for recording were filled to the shoulder with 1% (w/v) Neurobiotin (Vector Laboratories, Burlingame, CA, USA) in 1.0 m KCl, and the remainder filled with 2.0 m KCl, and had resistances in the range of 50–150 mΩ. Myenteric ganglia were visualized at ×200 with Hoffman modulation contrast optics through an inverted microscope (Nikon Diaphot, Melville, NY, USA) and individual myenteric neurones were randomly impaled. Transmembrane potential was measured with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA, USA) and electrical signals were acquired and analysed using MacLab Chart or Scope software (AD Instruments, Castle Hills, Australia). Synaptic activation of neurones was elicited by direct stimuli (a single pulse of 0.5 ms or a train of 0.5 ms pulses at 20 Hz for 2–4 s) applied to fibre tracts in interganglionic connectives with monopolar extracellular electrodes made from Teflon-insulated platinum wire. Unhealthy cells were excluded from the study if the input resistance was below 50 mΩ or had an action potential that peaked at a level less than 0 mV.
Using criteria previously described for classifying neurones in the guinea pig small intestine (Bornstein et al. 1994; Wood, 1994), neurones evaluated in this study were classified as AH or S. The most important criterion for classifying an individual neurone as AH or S was the presence or absence, respectively, of a shoulder on the repolarizing phase of the action potential (Schutte et al. 1995). Morphological characterization, achieved by incubation of the fixed tissue with AMCA-conjugated strepavidin (1: 500; Jackson ImmunoResearch) and visualized on an Olympus AX70 fluorescence photomicroscope, supported the electrophysiological classification. AH neurones always had a Dogiel type II morphology (multiaxonal) and S neurones always had a Dogiel type I or filamentous morphology (monoaxonal).
All electrophysiological properties were analysed offline on MacLab Chart or Scope software and were characterized with minor changes as previously described (Linden et al. 2003b). In S neurones, the maximum amplitude of the fast excitatory postsynaptic potential (fEPSP) in response to a supramaximal stimulus was determined by averaging the difference between the resting membrane potential (saline: −66 ± 4 mV, n = 21; TNBS + vehicle: −63 ± 3 mV, n = 35; TNBS + DFU: −63 ± 3 mV, n = 38; TNBS + vehicle: −63 ± 4 mV, n = 20) and the peak of the fEPSP of at least three evoked potentials elicited by focal stimulation of fibre tracts with single pulses (0.5 ms, 1–10 V). After-hyperpolarization (AHP) magnitude in AH neurones was determined by integrating the voltage more negative than resting membrane potential (RMP) over time until the membrane potential returned to resting levels (data points at 1 ms intervals; Prism version 3.0a for Macintosh, GraphPad Software, La Jolla, CA, USA). The measured AHP was generated following a single action potential that peaked only after the offset of a single depolarizing current pulse (0.4–0.7 nA; 0.2–5 ms).
Measurement of 5-HT or eicosanoid content of the colon
For experiments to measure serotonin content, a segment of colon was removed from the animal and homogenized in 0.5 ml of iced 0.2 m perchloric acid, and centrifuged at 10 000 g for 10 min. The supernatant was neutralized with 0.5 ml 1.0 m borate buffer (pH 9.25), and centrifuged at 10 000 g for 10 min. The 5-HT content of an aliquot of the sample was analysed by enzyme immunoassay with a kit used according to the manufacturer's instructions (Beckman Coulter, Fullerton, CA, USA).
For experiments to measure eicosanoid content, a segment of distal colon was isolated and homogenized in 50 μl PBS (0.1 m, pH 7.4) containing 1 mm EDTA and 100 μm ibuprofen. Samples were diluted 1: 100 in immunoassay buffer and eicosanoid levels were determined by enzyme immunoassay according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI, USA).
Serotonin or eicosanoid content of the tissue was expressed as a function of the length (in cm) of the colon to compensate for the increased protein and mass due to oedema and infiltration of inflammatory cells that occurs as a result of inflammation. This method has been determined to be a more accurate measure of neuroactive chemical content in the inflamed colon (Linden et al. 2003a).
Immunohistochemistry
Changes in the EC cell population of the colonic epithelium were quantified by immunohistochemically demonstrating 5-HT in transverse sections of the colon. Tissue sections were prepared as previously described (Linden et al. 2003a). Colonic segments from different groups of animals were embedded in OCT compound (Miles, Elkhardt, IN, USA) in the same blocks, so that further processing occurred under identical conditions. 5-HT-containing epithelial cells were counted and normalized as functions of the circumferential length of the muscularis mucosa, which does not change with colitis (Linden et al. 2003a). These determinations were made at a magnification of ×400 from three random locations in each transverse section, taking care to ensure that the area contained intact glands. Three tissue sections, at least 500 μm apart, were assessed from each animal.
The distal colon from some naive (n = 3) and TNBS-treated animals (n = 3) were prepared for whole-mount and transverse section immunohistochemistry for COX-1 and COX-2. For whole mounts, the colons of these animals were opened along the mesenteric border, pinned flat and fixed with Zamboni's fixative overnight. The mucosa, submucosa and circular muscle of the colon were removed with forceps exposing the myenteric plexus on the longitudinal smooth muscle. For tissue sections, fixed colons were sectioned on a cryostat (14 μm) and thaw-mounted onto glass slides. Whole mounts and tissue sections were washed with PBS and incubated for 3 × 15 min at room temperature with PBS containing 0.1% Triton X-100. This solution was removed, and the sections were incubated for 48 h at 4°C with a 1: 500 dilution of rabbit anti-COX-1 or COX-2 antiserum (Merck Frosst, Dorval, QC, USA; Reuter et al. 1996) in PBS containing 0.1% Triton X-100. Following 3 × 15 min washes with PBS, tissue sections were incubated with a 1: 50 dilution of donkey antirabbit antiserum conjugated to CY3 (Jackson ImmunoResearch, West Grove, PA, USA) for 2 h. Following 3 × 15 min washes with PBS, the tissue was mounted on glass slides and viewed on a Zeiss Axioplan Fluorescence microscope and Olympus FV300 confocal microscope. 1024 × 1024 pixel images were obtained using Fluoview software (Olympus) under identical exposure conditions (pinhole aperture, laser strength, scan speed, Kalman averaging ×2) and were processed identically using Adobe Photoshop 7, where only changes to contrast and brightness were made. Each optical section was 1 μm thick. Images consist of 6–23 optical sections in a Z stack.
Measurement of the release of 5-HT or eicosanoids from the mucosa of the colon
Two segments of colon (0.5 cm in length) were opened along the mesenteric border and placed in a 1.5 ml tube containing 0.5 ml of a 37°C Hepes-based buffer (mm: NaCl, 110; KCl, 5.4; CaCl2, 1.8; MgCl2, 1.0; Hepes, 20; glucose, 5; sucrose, 60; pH 7.4). One segment remained undisturbed in the bathing solution for a total of 5 min. The tissue in the second tube was sheer force stimulated on a Vortex Genie2 (Scientific Industries Inc., Bohemia, NY, USA) set at the lowest level that achieved a vortex during minutes 2 and 4 of a 5 min period. A volume of 0.3 ml of the bathing solution was removed from the tube and stored at −20°C until further analysis. The levels of 5-HT or eicosanoids released into the bathing solutions of both tubes were measured by enzyme immunoassay as described above.
In vitro pellet propulsion
The methods used to measure the rate of propulsion of fecal pellets in the isolated distal colon of the guinea pig have been previously described (Linden et al. 2003a). Briefly, a segment of distal colon with attached mesentery was removed from the animal, placed in a Sylgard-coated chamber, and straightened by placing pins every 2 cm along the mesentery. The isolated colon was maintained in circulating (10 ml min−1) 37°C Krebs solution (mm: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose, 8; aerated with a 95%O2–5%CO2). After an equilibration period of 15 min, during which natural pellets were expelled from the colon, silicon-coated fecal pellets were placed into the lumen at the oral end of the colonic segment and moved about 1 cm along the segment with a glass rod. The rate of motility for each trial was calculated by recording the time taken by the isolated colon to propel the pellet a distance of 3 cm in the centre of each segment. The mean rate of propulsion was calculated from at least five trials for each condition conducted with 5 min rest periods between trials.
Data analyses
Statistical analyses were performed using Prism software. For electrophysiological experiments, differences between treatment groups were determined using a one-way ANOVA for continuous data and χ2 for proportional data with a significance level of P < 0.05. N values represent the number of cells tested under each condition. For 5-HT or eicosanoid release experiments, differences between animal treatment groups as well as differences between basal and stimulated conditions were determined using a two-way ANOVA for repeated measures. Statistical analyses for all other experiments were conducted using one-way ANOVA. Non-linear sigmoidal concentration–response regression curves were plotted and IC50 values were determined by using Prism. The data presented are means ± s.e.m. for n animals.
Results
COX-2 inhibition restores electrical and synaptic excitability of myenteric neurones in inflamed distal colon
Intracellular recordings were obtained from 39 neurones in 13 saline-treated colons, 25 neurones in 6 saline/DFU colons, 32 neurones in 5 saline/SC-560 colons, 51 neurones in 11 TNBS colons, 58 neurones in 11 TNBS/DFU colons, and 32 neurones in 9 TNBS/SC-560 colons. Neurones were classified as AH or S neurones as described in Methods. The electrophysiological properties of neurones from saline-treated animals were similar to previously reported myenteric neurones from control guinea pig distal colon (Wade & Wood, 1988a, b; Lomax et al. 1999; Wada-Takahashi & Tamura, 2000; Tamura et al. 2001; Linden et al. 2003b). Likewise, the properties of neurones from TNBS/vehicle animals were similar to properties that we have previously reported for neurones from animals treated with TNBS alone (Linden et al. 2003b).
S neurones.
The electrical properties of S neurones were not altered by TNBS-induced colitis when compared to saline-treated controls (Table 1). In addition, treatment of animals with saline/DFU, saline/SC-560, TNBS/DFU or TNBS/SC-560 did not alter any of these measures (Table 1). The synaptic properties of S neurones were altered by TNBS-induced colitis when compared to saline-treated control animals (Table 2). These changes included a higher proportion of S neurones exhibiting slow excitatory synaptic potentials (sEPSPs), and an increase in fast EPSP amplitude. No changes were detected in the proportions of S neurones that exhibited stimulus-induced fast EPSPs or slow inhibitory postsynaptic potentials (sIPSPs).
Table 1. Effects of COX inhibition on electrical properties of S neurones from inflamed distal colon.
| Controls | Inflamed | |||||
|---|---|---|---|---|---|---|
| Saline | Saline/DFU | Saline/SC-560 | TNBS/vehicle | TNBS/DFU | TNBS/SC-560 | |
| Resting membrane | ||||||
| potential (mV) | −53 ± 3 (27) | −53 ± 2 (21) | −55 ± 2 (27) | −52 ± 2 (37) | −51 ± 2 (48) | −52 ± 3 (23) |
| Input resistance (MΩ) | 153 ± 17 (27) | 154 ± 27 (21) | 136 ± 13 (27) | 129 ± 12 (37) | 150 ± 15 (48) | 175 ± 26 (23) |
| Maximum number of action potentials during a 500 ms current pulse | 4 ± 1 (27) | 9 ± 3 (21) | 5 ± 2 (27) | 4 ± 1 (37) | 8 ± 2 (48) | 7 ± 2 (23) |
| Neurones exhibiting spontaneous activity | 9/27 (33%) | 5/21 (24%) | 8/27 (30%) | 11/37 (30%) | 24/48 (50%) | 7/23 (30%) |
| Neurones exhibiting anodal break action potentials | 11/26 (42%) | 7/21 (33%) | 7/27 (26%) | 18/37 (49%) | 29/48 (60%) | 8/23 (35%) |
Data are the mean ± s.e.m. for (N) cells, or the proportion of cells exhibiting the given property. Continuous data were statistically analysed using ANOVA and proportional data were statistically analysed by χ2.
Table 2. Effects of COX inhibition on synaptic properties of S neurones from inflamed distal colon.
| Controls | Inflamed | |||||
|---|---|---|---|---|---|---|
| Saline | Saline/DFU | Saline/SC-560 | TNBS/vehicle | TNBS/DFU | TNBS/SC-560 | |
| Neurones exhibiting fEPSPs | 24/27 (89%) | 20/21 (95%) | 27/27 (100%) | 35/35 (100%) | 48/48 (100%) | 21/23 (91%) |
| fEPSP amplitude (mV) | 13 ± 1 (21) | 11 ± 1 (20) | 11 ± 1 (27) | 20 ± 1 (35)a | 19 ± 1 (38)a,c | 19 ± 1(20)a,c |
| Neurones exhibiting sEPSPs | 13/25 (52%) | 9/19 (47%) | 14/26 (54%) | 23/30 (77%)a | 26/45 (58%)b | 17/20 (85%)a,c |
| Neurones exhibiting sIPSPs | 2/25 (8%) | 4/19 (21%) | 3/26 (12%) | 5/30 (17%) | 6/45 (13%) | 0/20 (0%) |
Data are the mean ± s.e.m. for (N) cells, or the proportion of cells exhibiting the given property. Continuous data were statistically analysed using ANOVA with Newman-Keuls multiple comparisons test and proportional data were statistically analysed by χ2(
P < 0.05 compared to neurones from saline-treated animals;
P < 0.05 compared to neurones from TNBS + vehicle-treated animals;
P < 0.05 compared to neurones from corresponding saline/COX inhibitor-treated animals).
Treatment of animals with the COX-1 inhibitor, SC-560, or the COX-2 inhibitor, DFU, did not affect the synaptic properties of these cells (Table 2). However, treatment of animals with TNBS/DFU was effective in reversing the increased incidence of S neurones that exhibited sEPSPs observed in the TNBS group. On the other hand, COX-2 blockade with DFU did not alter the effect of TNBS-induced inflammation on the amplitude of fEPSPs. The synaptic properties of S neurones in TNBS/SC-560 animals were comparable to those in TNBS animals.
AH neurones
Consistent with previous findings (Linden et al. 2003b), AH neurones from TNBS-treated animals exhibited altered electrical properties consistent with enhanced excitability when compared to saline-treated controls (Table 3). These properties include the following: increased maximum number of action potentials during a depolarizing current pulse; a smaller after-hyperpolarization (Fig. 2); and an increased incidence of AH neurones that exhibit spontaneous activity and an increased proportion of neurones with anodal break action potentials. Treatment of animals with TNBS/DFU but not TNBS/SC-560 attenuated these enhanced properties to levels that were not different from saline-treated controls. Furthermore, AH neurones of TNBS/DFU animals were significantly less excitable than those of TNBS animals with regard to the number of action potentials, magnitude of the after-hyperpolarization, and occurrence of spontaneous activity. The electrical properties of AH neurones in saline/DFU and saline/SC-560 animals were identical to those of saline controls (Table 3).
Table 3. Effects of COX inhibition on electrical properties of AH neurones from inflamed distal colon.
| Controls | Inflamed | |||||
|---|---|---|---|---|---|---|
| Saline | Saline/DFU | Saline/SC-560 | TNBS/vehicle | TNBS/DFU | TNBS/SC-560 | |
| Resting membrane potential (mV) | −68 ± 2 (14) | −76 ± 3 (6) | −68 ± 5 (5) | −67 ± 3 (14) | −70 ± 3 (10) | −69 ± 3 (9) |
| Input resistance (MΩ) | 130 ± 17 (14) | 90 ± 16 (6) | 83 ± 11 (5) | 101 ± 12 (14) | 115 ± 19 (10) | 141 ± 19 (9) |
| AHP magnitude (mV s) | 20 ± 3 (14) | 18 ± 3 (6) | 25 ± 5 (5) | 10 ± 2 (14) a | 18 ± 3 (10) b | 6 ± 2 (9) a,c |
| Maximum number of action potentials during a 500 ms current pulse | 1.6 ± 0.4 (14) | 1.2 ± 0.2 (6) | 1.6 ± 0.6 (5) | 10 ± 2 (14) a | 2.3 ± 0.6 (10) b | 10 ± 2 (9) a,c |
| Neurones exhibiting spontaneous activity | 0/14 (0%) | 0/6 (0%) | 0/5 (0%) | 5/14 (36%) a | 0/10 (0%) b | 4/9 (44%) a,c |
| Neurones exhibiting anodal break action potentials | 2/14 (14%) | 0/6 (0%) | 0/5 (0%) | 7/14 (50%) a | 3/10 (30%) | 8/9 (89%) a,c |
Data are the mean ±s.e.m. for (N) cells, or the proportion of cells exhibiting the given property. Continuous data were statistically analysed using ANOVA with Newman-Keuls multiple comparisons test and proportional data were statistically analysed by χ2(
P < 0.05 compared to neurones from saline-treated animals
P < 0.05 compared to neurones from TNBS + vehicle-treated animals
P < 0.05 compared to neurones from corresponding saline/COX inhibitor-treated animals). AHP, after-hyperpolarization.
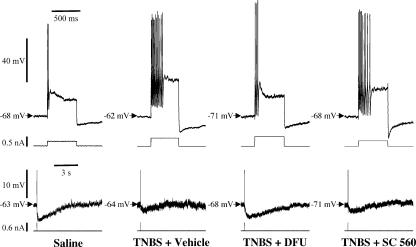
Figure 2. Inflammation-induced changes in the electrical properties of AH neurones are restored to normal by COX-2, but not by COX-1, inhibition.
Representative examples of repetitive action potentials during 500 ms depolarizing current pulses (top traces), and long-lasting after-hyperpolarization (lower traces) following single action potentials (truncated) elicited by brief depolarizing current pulses in AH neurones. These examples illustrate that the ability of AH neurones to accommodate to sustained depolarization is impaired in TNBS animals, and restored to normal in TNBS/DFU but not TNBS/SC-560 animals. The accommodation properties are probably related to the after-hyperpolarization, which was smaller in neurones from TNBS and TNBS/SC-560 animals as compared to those of control and TNBS/DFU animals. Compiled data are presented in Table 3.
While the TNBS colitis-induced changes in electrical properties were attenuated by DFU, the TNBS-induced changes in synaptic activity of AH neurones were not affected by either of the COX inhibitors. The increased proportion of AH neurones from TNBS animals exhibiting fEPSPs in response to fibre tract stimulation compared to saline-treated controls were not attenuated in either TNBS/DFU or TNBS/SC-560 animals (Table 4). The synaptic properties of AH neurones in saline/DFU and saline/SC-560 animals were identical to those of saline controls (Table 4).
Table 4. Effects of COX inhibition on synaptic properties of AH neurones from inflamed distal colon.
| Controls | Inflamed | |||||
|---|---|---|---|---|---|---|
| Saline | Saline/DFU | Saline/SC-560 | TNBS/vehicle | TNBS/DFU | TNBS/SC-560 | |
| Neurones exhibiting fEPSPs | 2/14 (14%) | 1/6 (17%) | 0/5 (0%) | 9/14 (64%) a | 6/10 (60%) a,b | 5/9 (56%) a,b |
| Neurones exhibiting sEPSPs | 14/14 (100%) | 6/6 (100%) | 5/5 (100%) | 14/14 (100%) | 10/10 (100%) | 9/9 (100%) |
| Neurones exhibiting sIPSPs | 0/14 (0%) | 0/6 (0%) | 0/5 (0%) | 0/14 (0%) | 1/10 (10%) | 0/9 (0%) |
Data are the mean ±s.e.m. for (N) cells, or the proportion of cells exhibiting the given property and were statistically analysed by χ2(
P < 0.05 compared to neurones from saline-treated animals
P < 0.05 compared to neurones from corresponding saline/COX inhibitor-treated animals).
Colitis-induced enhanced serotonin availability is not altered by COX inhibition
We have previously determined that TNBS-induced colitis is associated with an increased availability of serotonin (5-HT) in the epithelium (Linden et al. 2003a). We tested the hypothesis that 5-HT availability could be decreased to normal levels by inhibiting COX-1 or COX-2. The amount of 5-HT in the colon was measured by enzyme immunoassay (Fig. 3A). As previously described, TNBS animals in this study had elevated levels of 5-HT in the colon when compared to saline-treated controls. This elevated level of 5-HT was not altered in TNBS/DFU or TNBS/SC-560 animals when compared to the TNBS group.
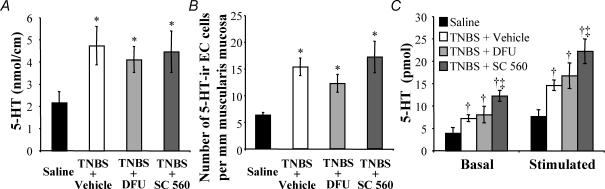
Figure 3. Enhanced 5-HT availability in inflamed colon is not reduced by COX inhibition.
A, examined as a function of length, the amount of 5-HT was significantly increased in TNBS compared to control animals. This increase in 5-HT content was sustained in animals treated with the COX-2 blocker DFU (5 mg kg−1) or the COX-1 blocker SC-560 (5 mg kg−1). B, the number of 5-HT-immunoreactive enterochromaffin (EC) cells per length of muscularis mucosa was increased in TNBS compared to control animals. This EC cell hyperplasia persisted despite inhibition of COX-2 or COX-1. C, mechanical agitation of isolated segments of colon caused a significant increase in 5-HT released into the bathing media regardless of animal treatment (significant difference not shown graphically). TNBS animals released significantly more 5-HT under basal and stimulated conditions when compared to controls. Stimulus-induced 5-HT release from colons of TNBS/DFU animals was not different from TNBS animals, but remained significantly greater than control animals. Interestingly, treatment of animals with TNBS/SC-560 significantly increased basal and stimulated 5-HT release compared to both control animals and TNBS animals. P < 0.05 compared to controls; ANOVA, Newman-Keuls multiple comparisons test. †P < 0.05 compared to controls; ‡P < 0.05 compared to TNBS/vehicle; two-way ANOVA for repeated measures, Bonferroni's multiple comparisons test (n = 4–6 animals).
Consistent with the inability of COX inhibition to alter the effect of TNBS colitis on 5-HT content, COX inhibition did not modify the colitis-induced EC cell hyperplasia in animals treated with TNBS (Fig. 3B). TNBS, TNBS/DFU and TNBS/SC-560 animals all had more 5-HT-immunoreactive EC cells per circumferential length of muscularis mucosa than controls. Furthermore, the change in mucosal architecture that is associated with chronic TNBS-induced inflammation was not altered by COX inhibition. Neither the increased colonic gland depth, nor the increased number of epithelial cells per colonic gland was altered by COX-1 or COX-2 inhibition (saline: 155 ± 6 μm, 49 ± 2 cells gland−1; TNBS + vehicle: 273 ± 30 μm, 74 ± 7 cells gland−1; TNBS/DFU: 306 ± 32 μm, 73 ± 7 cells gland−1; TNBS/SC-560: 353 ± 63 μm, 88 ± 16 cells gland−1; P < 0.05 ANOVA, Newman-Keuls multiple comparisons test for each measure and each treatment group compared to the appropriate saline-treated control group).
There is an increase in 5-HT release from TNBS-inflamed tissue as compared to control tissue under basal and stimulated conditions (Linden et al. 2003a). In the current study, 5-HT release from segments of distal colon was higher in all three TNBS treatment groups, as compared to controls (Fig. 3C). Interestingly, inhibition of COX-1 by treatment of animals with SC-560, after administration of TNBS, significantly increased the levels of 5-HT in the bathing media of both basal and stimulated tissue compared to tissue from TNBS animals.
COX-2 inhibition restores normal rates of colonic propulsive motility
Colitis induced by TNBS is associated with a significant reduction in the rate of fecal pellet propulsion in isolated segments of guinea pig distal colon (Linden et al. 2003a). In the current study, the effects of COX inhibition on colonic propulsion in the TNBS-inflamed distal colon were evaluated. The propulsion rate of fecal pellets was measured in segments of colon isolated from animals in each of the four treatment groups (Fig. 4). The colons of TNBS animals exhibited a significant decrease in the rate of pellet propulsion compared to saline-treated controls. The inhibition of COX-2 in TNBS/DFU animals restored near normal rates of propulsion compared to TNBS animals. In contrast, inhibition of COX-1 in TNBS/SC-560 animals did not alter colonic propulsion compared to TNBS animals. The rate of pellet propulsion was not altered, relative to control values, in colons from saline/DFU or saline/SC-560 animals (saline control, 1.7 ± 0.1 mm s−1; saline/DFU, 1.68 ± 0.04 mm s−1; saline/SC-560, 1.9 ± 0.1 mm s−1).
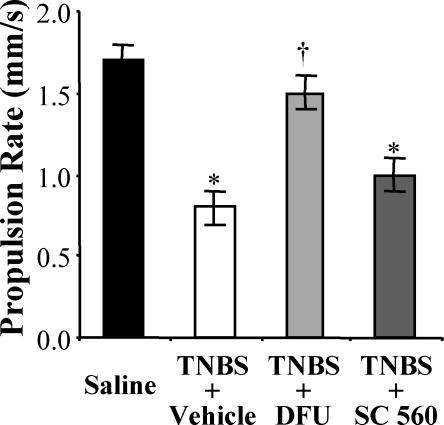
Figure 4. Colonic propulsive motor activity, reduced during inflammation, is restored by COX-2 inhibition.
Colons of TNBS animals had significantly slower fecal pellet propulsion rates compared to control animals. Inhibition of COX-2 in TNBS/DFU animals restored the pellet propulsion to a rate comparable to the control level and a rate that was significantly faster than TNBS animals. By contrast, inhibition of COX-1 in TNBS/SC-560 animals resulted in pellet propulsion rates that were similar to TNBS animals, and slower than controls. P < 0.05 compared to control; †P < 0.05 compared to TNBS/vehicle; ANOVA, Newman-Keuls multiple comparisons test (n = 6 animals).
Eicosanoid levels in the inflamed colon with and without COX inhibition
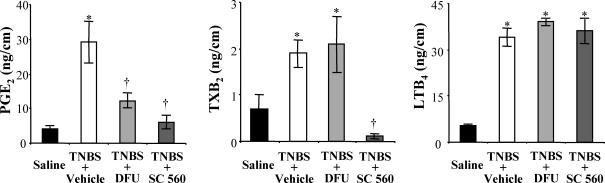
The reduction of myenteric AH neurone excitability and restored colonic pellet propulsion by COX-2, but not COX-1 inhibition in TNBS-treated animals lead us to investigate the action of DFU and SC-560 treatment on colonic eicosanoid levels. COX-1 and COX-2 are rate-limiting enzymes in the synthesis of prostaglandins (PGs) and thromboxanes (TXs) from arachidonic acid (Seeds & Bass, 1999; Iversen & Kragballe, 2000). Another class of eicosanoids generated from arachidonic acid by the enzyme lipoxygenase are the leukotrienes (LTs). In TNBS animals, there was a significant increase in total colonic levels of PGE2, TXB2 and LTB4 compared to saline-treated controls (Fig. 5). When compared to levels in TNBS animals, PGE2 and thormboxane B2 (TXB2) were reduced in TNBS/SC-560 animals, but the leukotriene B4 (LTB4) level was not affected by COX-1 inhibition (Fig. 5). COX-2 inhibition in the TNBS/DFU animals reduced PGE2 but not TXB2 nor LTB4 relative the levels of these eicosanoids in TNBS animals (Fig. 5).
Figure 5. Colonic levels of eicosanoids are altered during inflammation and following COX inhibition.
Colonic content of representative members of three of the major classes of eicosanoids were measured in each of the animal groups. Levels of all three eicosanoids were significantly elevated in colons of TNBS animals. In animals treated with the COX-2 inhibitor, DFU (5 mg kg−1), PGE2 levels were reduced compared to TNBS animals, but TXB2 and LTB4 levels were not altered. In animals treated with the COX-1 inhibitor, SC-560, PGE2 and TXB2 levels were reduced compared to those of TNBS animals, but LTB4 content remained the same. P < 0.05 compared to control; †P < 0.05 compared to TNBS/vehicle; ANOVA, Newman-Keuls multiple comparisons test (n = 4–6 animals).
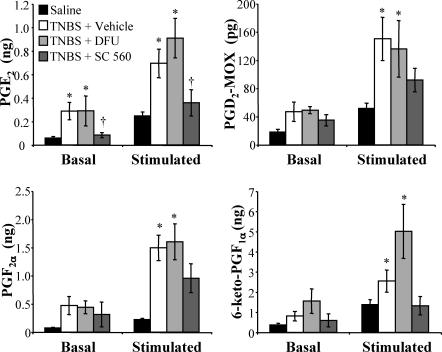
Mechanical stimulation of the intestinal mucosal epithelium causes the release of PGs (Bennett et al. 1967). We tested whether this release of PGs is altered in the inflamed colon, and further whether this release could be inhibited by the inhibition of either COX-1 or COX-2. Agitation of isolated segments of colon caused a significant increase in the levels of PGE2, PGD2 (measured as PGD2-MOX), PGF2α and PGI2 (measured as 6-keto-PGF1α) regardless of animal treatment (Fig. 6). There was enhanced basal and stimulated release of all PGs in TNBS animals compared to saline-treated controls. These TNBS-induced elevated levels of PG release were inhibited in TNBS/SC-560 animals but not in TNBS/DFU animals.
Figure 6. Prostaglandin release from isolated colon is enhanced during inflammation and reduced by COX-1 but not COX-2 inhibition.
Mechanical agitation of isolated segments of colon caused a significant increase in the prostaglandins (PGs) released into the bathing media regardless of animal treatment (significant difference not shown graphically). Under basal conditions, PGE2 release was increased in the TNBS and TNBS/DFU groups relative to control. Under stimulated conditions, release of all forms of PGs tested (PGE2, PGD2-MOX, PGF2α and 6-keto-PGF1α) was increased in TNBS and TNBS/DFU groups when compared to controls. PG release from tissues in the TNBS/SC-560 group was not different from control levels for all PGs tested. In the case of PGE2, the release levels for the TNBS/SC-560 group, under both basal and stimulated conditions, was significantly reduced compared to the TNBS group. P < 0.05 compared to controls; †P < 0.05 compared to TNBS/vehicle; two-way ANOVA for repeated measures, Bonferroni's multiple comparisons test (n = 6 animals).
Expression of COX-2 in the mucosa, myenteric plexus and circular muscle layers in TNBS-treated animals
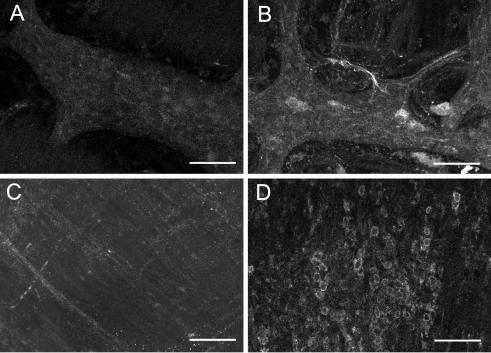
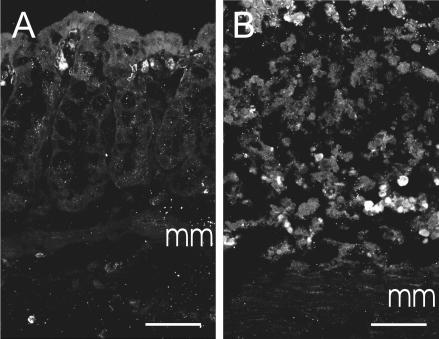
It was somewhat surprising that COX-2 inhibition could restore normal myenteric neurone electrical properties in TNBS-treated animals yet have little effect on mucosal PG levels. We therefore tested the hypothesis that COX-2 is expressed in the deeper layers of the colonic wall during TNBS-induced inflammation. Immunoreactivity for COX-2 was detected in both neuronal and non-neuronal cells in the myenteric plexus and as well as cells within the circular and longitudinal muscle layers in animals treated with TNBS (Fig. 7), but was not observed in untreated animals. Only faint neuronal immunoreactivity for COX-2 was observed in the naive animals. Immunoreactivity for COX-2 was also observed in some infiltrating cells in the mucosa of TNBS-treated animals (Fig. 8). Faint COX-1 immunoreactivity was observed in the mucosa only and was comparable in both groups of guinea pigs (data not shown).
Figure 7. COX-2 immunoreactivity is enhanced in the muscularis externa during inflammation.
Confocal micrographs of colonic myenteric plexus (A and B) and attached circular muscle (C and D). Optical sections of 1 μm thickness were stacked together for each image. A, COX-2 immunoreactivity was barely detectable in the distal colon myenteric plexus of a control animal (12 optical sections). B, in inflamed animals there was an increased intensity and distribution of COX-2 immunoreactivity in neurones and also in some non-neuronal cells (12 optical sections). C, COX-2 immunoreactivity was not observed in circular muscle of the normal colon (13 optical sections). D, COX-2 immunoreactivity was observed in many infiltrating cells in the thickened circular muscle of the inflamed colon (23 optical sections). Scale bars: 100 μm.
Figure 8. COX-2-immunoreactive cells are observed in the mucosa during inflammation.
Confocal micrographs of colonic mucosa (A and B). Optical sections of 1 μm thickness were stacked together for each image. A, COX-2 immunoreactivity was observed in the colonic mucosa of control animals in a few scattered cells immediately deep to the epithelium (6 optical sections). The identity of these cells was not examined, but it is likely that they are subepithelial fibroblasts, which express COX-2 under physiological conditions (Mahida et al. 1997). B, in the inflamed muocsa, COX-2 expression was observed in a large number of cells scattered throughout the mucosa. mm, muscularis mucosa. Scale bars: 50 μm.
Discussion
The data presented here provide the first direct evidence that COX-2 contributes to the hyperexcitability of myenteric neurones in colitis. Most of the profound changes in myenteric neurone function that accompany TNBS-induced colitis can be reduced or reversed by sustained inhibition of COX-2. There was no effect of COX inhibition on the extent of intestinal inflammation, changes in mucosal architecture or increased availability of 5-HT associated with TNBS-induced colitis. However, inhibition of COX-2, but not COX-1, markedly improved the propulsion of fecal pellets in the inflamed colon. Taken together, these studies indicate that COX-2 activation plays a prominent role in AH neuronal hyperexcitability and in decreased propulsive motor activity in the TNBS model of immune-mediated colitis.
In TNBS-induced colitis, which is a model of chronic, immune-mediated inflammation, distinct changes have been detected in the electrical properties of myenteric AH neurones (Linden et al. 2003b). While the resting membrane potential and input resistance of AH neurones are unchanged, these cells exhibit several alterations that are consistent with enhanced excitability, including a decrease in accommodation, an increase in spontaneous activity and an attenuated after-hyperpolarization. In models that reflect acute, infectious or allergenic inflammation, AH neurones exhibit similar changes in excitability, but the membrane potential is depolarized and input resistance is decreased (Frieling et al. 1994a; Palmer et al. 1998; Liu et al. 2003). These differences in altered electrical properties most likely reflect different mechanisms of neuroimmune interaction. In the case of the acute models, histamine released from mast cells is probably the major mediator of the changes in AH neuronal properties, and a decrease in a Ca2+-activated K+ conductance is largely responsible for the electrical changes that are observed. In TNBS colitis, an augmentation of a hyperpolarization-activated cation conductance is a major contributor to the enhanced excitability (Linden et al. 2003b), and results from the current study indicate that COX-2 activation and possibly the release of PGs leads to these changes.
In acute models of inflammation, neurotransmitter release and synaptic potentials are suppressed, probably through the actions of histamine on presynaptic receptors (Collins et al. 1992; Frieling et al. 1993; Liu et al. 2000). In TNBS colitis, synaptic activity is enhanced in both AH and S neurones of the myenteric plexus. The only measured change in AH neurone synaptic activity during inflammation, an increased proportion of neurones that exhibit fEPSPs, was not altered by COX inhibition. In S neurones, inflammation is associated with enhanced fast and slow synaptic activity. Inhibition of COX-2, but not COX-1, restored the proportion of S neurones receiving slow excitatory synaptic input back to the control level. In contrast, the enhanced amplitude of fEPSPs in S neurones during inflammation, is not altered by inhibition of either COX isoform. It is therefore likely that enhancement of sEPSPs in S neurones occurs via a COX-2 mechanism. Furthermore, these findings indicate that, in chronic colitis, augmentation of fEPSPs in both AH and S neurones may occur via a common mechanism that is independent of COX activity.
We originally proposed that mucosal inflammatory mediators were likely to cause the changes we observed in myenteric AH neurones (Linden et al. 2003b). This was because AH, but not S, neurones project to the mucosa and have endings in the lamina propria (Neunlist et al. 1999), and the excitability of AH neurones is more dramatically affected by inflammation than that of S neurones (Linden et al. 2003b). We reasoned that mucosal PGs, a result of infiltrating immune cells in the lamina propria, where the inflammatory response is centred, could selectively affect AH neurones. While this possibility has not been excluded by the current studies, the lack of effect of COX-2 inhibition on mucosal prostaglandin levels sheds doubt on this theory and indicates that COX-2 acting at another site may lead to the changes that are observed in myenteric neurones. The increased COX-2 immunoreactivity within neurones of the myenteric plexus, and the expression of COX-2 in cells within the muscle layers, suggest that the COX-2 affecting myenteric neuronal excitability may reside in the muscularis externa of the colon. If this is the case, it is interesting to note that eicosanoids produced by COX-2 in these deep layers may exert their effects selectively on the electrical properties of AH neurones, as local PGs would be just as likely to contact S neurones as AH neurones. Consistent with this notion, we have recently reported that long-term exposure to PGE2 enhances the excitability of colonic AH neurones whereas the firing properties of S neurones are largely unaffected (Manning et al. 2002).
Results of the current study indicate that administration of a COX-2 blocker to animals with TNBS colitis restored propulsive motor activity to a normal level, whereas administration of a COX-1 inhibitor had no detectable effect on peristalsis in the inflamed colon. Inhibition of COX-2 has been shown to reduce the dysmotility that accompanies mechanical manipulation of the bowel (Schwarz et al. 2001). Intestinal manipulation causes increased PGE2 production that can be completely reduced by COX-2 inhibition with DFU (Kreiss et al. 2003). However, the results of the current study indicate that increased production of prostaglandins and thromboxanes in the mucosa of the inflamed colon is mediated primarily by COX-1. Although it appears counterintuitive that increased COX-2 expression in the mucosa during colitis (Reuter et al. 1996) would not be responsible for increased eicosanoid levels, this effect has been observed in other gastrointestinal distress conditions. Acid challenge of the rat stomach causes an increase in COX-2 expression, but the increased thromboxane and prostaglandin production is reduced by COX-1 inhibition, not COX-2 inhibition (Gretzer et al. 2001). Likewise, Helicobacter pylori infection in humans causes a marked increase in COX-2 expression, but increased eicosanoid levels appear to be due to COX-1 rather than COX-2 (Jackson et al. 2000; Scheiman et al. 2003). Another effect of COX-1 inhibition was an increase in basal and stimulated 5-HT release. It is possible that reducing mucosal eicosanoid levels towards the normal range in colitis may reduce eicosanoid-mediated inhibition of 5-HT release. Although COX-2 inhibition was more effective at restoring normal intestinal propulsive activity, there are clearly distinct roles for COX-1 during inflammation and a more thorough examination of this issue is warranted.
As has been previously reported (Morteau et al. 1993; Martinolle et al. 1995; Reuter et al. 1996; Lesch et al. 1999), inhibition of either COX-1 or COX-2 in the current investigation did not alter the magnitude or extent of TNBS-induced colitis. These and other studies have suggested that leukotrienes are the class of eicosanoids that is responsible for tissue damage in the inflammatory response (Wallace et al. 1989; Wallace & Keenan, 1990a, b; Morteau et al. 1993; Martinolle et al. 1995). Neither SC-560 nor DFU treatment reduced the enhanced levels of colonic LTB4 in the current study. The current study provides no evidence to contradict the hypothesis that leukotrienes contribute to mucosal inflammation, but our findings do suggest that long-lasting PG and thromboxane production, which was attenuated by the COX-1 inhibition, is not involved. We chose to initiate COX inhibition 2 days following TNBS injection to allow the well-documented protective effects of both COX isoforms to occur while inhibiting the subsequent damaging effects of COX (Wallace et al. 1992; Morteau et al. 1993; Reuter et al. 1996; Sakamoto, 1998; Newberry et al. 1999). It was therefore beyond the scope of this study to address the role of either COX isoforms, or the eicosanoids that they produce, in the early stages of the inflammatory response.
In summary, some of the inflammation-induced changes in the neural circuitry, namely increased mucosal 5-HT content, EC cell hyperplasia and increased fast EPSP amplitude, were not altered by COX-2 inhibition. Therefore, it is clear that a single type of neuroimmune interaction does not account for all of the neuroplasticity that occurs in this model of immune-mediated colitis, and future investigations will be necessary to determine the mechanisms responsible for these changes. The findings reported here demonstrate that COX-2 activation is a critical step in the hyperexcitability of AH neurones and reduced propulsive motor activity during chronic colitis in the guinea pig. Although a direct link between these two observations was not established in this study, it is possible that the restored excitability of AH neurones by COX-2 inhibition contributes to the restoration of normal colonic propulsion.
Acknowledgments
This work was supported by NIH grants F32DK60382 (D.R.L.), NS26995 (G.M.M.) and DK62267 (G.M.M.), and a grant from the Crohn's and Colitis Foundation of Canada (K.A.S., G.M.M.). K.A.S. is an Alberta Heritage Foundation for Medical Research Medical Scientist. The authors wish to thank Mr Eric Krauter for his valuable assistance and Dr Alan E. Lomax for his helpful discussion.
References
- Bennett A, Friedmann CA, Vane JR. Release of prostaglandin E-1 from the rat stomach. Nature. 1967;216:873–876. doi: 10.1038/216873a0. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate. J Auton Nerv Syst. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Collins SM, Hurst SM, Main C, Stanley E, Khan I, Blennerhassett P, Swain M. Effect of inflammation on enteric nerves: cytokine-induced changes in neurotransmitter content and release. Ann N Y Acad Sci. 1992;664:415–424. doi: 10.1111/j.1749-6632.1992.tb39780.x. [DOI] [PubMed] [Google Scholar]
- Dekkers J, Akkermans LMA, Kroese ABA. Effects of the inflammatory mediator prostaglandin E2 on myenteric neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 1997a;272:G1451–G1456. doi: 10.1152/ajpgi.1997.272.6.G1451. [DOI] [PubMed] [Google Scholar]
- Dekkers J, Kroese ABA, Keenan CM, MacNaughton WK, Sharkey KA. Prostaglandin E2 activation of VIP secretomotor neurons in the guinea pig ileum. J Auton Nerv Syst. 1997b;66:131–137. doi: 10.1016/s0165-1838(97)00079-9. [DOI] [PubMed] [Google Scholar]
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. Faseb J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Frieling T, Cooke HJ, Wood JD. Histamine receptors on submucous neurons in guinea pig colon. Am J Physiol. 1993;264:G74–G80. doi: 10.1152/ajpgi.1993.264.1.G74. [DOI] [PubMed] [Google Scholar]
- Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol. 1994a;267:G1087–G1093. doi: 10.1152/ajpgi.1994.267.6.G1087. [DOI] [PubMed] [Google Scholar]
- Frieling T, Rupprecht C, Dobreva G, Haussinger D, Schemann M. Effects of prostglandin F-2x (PGF(2x)) and prostglandin I-2 (PGI(2)) on nerve-mediated secretion in guinea-pig colon. Pflugers Arch. 1995;431:212–220. doi: 10.1007/BF00410193. [DOI] [PubMed] [Google Scholar]
- Frieling T, Rupprecht C, Kroese ABA, Schemann M. Effects of the inflammatory mediator prostaglandin D2 on submucosal neurons and secretion in guinea pig colon. Am J Physiol. 1994b;266:G132–G138. doi: 10.1152/ajpgi.1994.266.1.G132. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gretzer B, Maricic N, Respondek M, Schuligoi R, Peskar BM. Effects of specific inhibition of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the rat stomach with normal mucosa and after acid challenge. Br J Pharmacol. 2001;132:1565–1573. doi: 10.1038/sj.bjp.0703955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L, Kragballe K. Arachidonic acid metabolism in skin health and disease. Prostaglandins Other Lipid Mediat. 2000;63:25–42. doi: 10.1016/s0090-6980(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Wu KC, Mahida YR, Jenkins D, Hawkey CJ. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47:762–770. doi: 10.1136/gut.47.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss C, Birder LA, Kiss S, VanBibber MM, Bauer AJ. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52:527–534. doi: 10.1136/gut.52.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch CA, Kraus ER, Sanchez B, Gilbertsen R, Guglietta A. Lack of beneficial effect of COX-2 inhibitors in an experimental model of colitis. Methods Find Exp Clin Pharmacol. 1999;21:99–104. doi: 10.1358/mf.1999.21.2.529236. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003a;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003b;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Hu HZ, Gao N, Gao C, Wang G, Wang X, Peck OC, Kim G, Gao X, Xia Y, Wood JD. Neuroimmune interactions in guinea pig stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2003;284:G154–G164. doi: 10.1152/ajpgi.00241.2002. [DOI] [PubMed] [Google Scholar]
- Liu S, Xia Y, Hu H, Ren J, Gao C, Wood JD. Histamine H3 receptor-mediated suppression of inhibitory synaptic transmission in the submucous plexus of guinea-pig small intestine. Eur J Pharmacol. 2000;397:49–54. doi: 10.1016/s0014-2999(00)00228-4. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst. 1999;76:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341–G1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990;265:10805–10808. [PubMed] [Google Scholar]
- Manning BP, Sharkey KA, Mawe GM. Effects of PGE2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1388–G1397. doi: 10.1152/ajpgi.00141.2002. [DOI] [PubMed] [Google Scholar]
- Martinolle JP, Garcia-Villar R, More J, Bueno L. Evidence for mast cell, leukotriene and nitric oxide involvement in the regulation of the adrenoceptor number of inflamed small intestine in guinea pigs. Neurogastroenterol Motil. 1995;7:187–195. doi: 10.1111/j.1365-2982.1995.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Morteau O, More J, Pons L, Bueno L. Platelet-activating factor and interleukin 1 are involved in colonic dysmotility in experimental colitis in rats. Gastroenterology. 1993;104:47–56. doi: 10.1016/0016-5085(93)90834-y. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol. 1999;517:533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry RD, Stenson WF, Lorenz RG. Cyclooxygenase-2-dependent arachidonic acid metabolites are essential modulators of the intestinal immune response to dietary antigen. Nat Med. 1999;5:900–906. doi: 10.1038/11341. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Wong-Riley M, Sharkey KA. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am J Physiol. 1998;275:G922–G935. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- Porcher C, Horowitz B, Bayguinov O, Ward SM, Sanders KM. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterology. 2002;122:1442–1454. doi: 10.1053/gast.2002.33065. [DOI] [PubMed] [Google Scholar]
- Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, Ethier D, Evans J, Falgueyret JP, Ford-Hutchinson AW, Gordon R, Greig G, Gresser M, Guay J, Kargman S, Leger S, Mancini JA, O'Neill G, Ouellet M, Rodger IW, Therien M, Wang Z, Webb JK, Wong E, Chan CC, et al. Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto C. Roles of COX-1 and COX-2 in gastrointestinal pathophysiology. J Gastroenterol. 1998;33:618–624. doi: 10.1007/s005350050147. [DOI] [PubMed] [Google Scholar]
- Scheiman JM, Greenson JK, Lee J, Cryer B. Effect of cyclooxygenase-2 inhibition on human Helicobacter pylori gastritis: mechanisms underlying gastrointestinal safety and implications for cancer chemoprevention. Aliment Pharmacol Ther. 2003;17:1535–1543. doi: 10.1046/j.1365-2036.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- Schumert R, Towner J, Zipser RD. Role of eicosanoids in human and experimental colitis. Dig Dis Sci. 1988;33:58S–64S. doi: 10.1007/BF01538132. [DOI] [PubMed] [Google Scholar]
- Schutte IW, Kroese AB, Akkermans LM. Somal size and location within the ganglia for electrophysiologically identified myenteric neurons of the guinea pig ileum. J Comp Neurol. 1995;355:563–572. doi: 10.1002/cne.903550406. [DOI] [PubMed] [Google Scholar]
- Schwarz NT, Kalff JC, Turler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121:1354–1371. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol. 1999;17:5–26. doi: 10.1007/BF02737594. [DOI] [PubMed] [Google Scholar]
- Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–460. [PubMed] [Google Scholar]
- Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Ito H, Wade PR. Morphology, electrophysiology, and calbindin immunoreactivity of myenteric neurons in the guinea pig distal colon. J Comp Neurol. 2001;437:423–437. doi: 10.1002/cne.1293. [DOI] [PubMed] [Google Scholar]
- Wada-Takahashi S, Tamura K. Actions of reactive oxygen species on AH/type 2 myenteric neurons in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G893–G902. doi: 10.1152/ajpgi.2000.279.5.G893. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Electrical behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988a;254:G522–G530. doi: 10.1152/ajpgi.1988.254.4.G522. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Synaptic behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988b;255:G184–G190. doi: 10.1152/ajpgi.1988.255.2.G184. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM. Leukotriene B4 potentiates colonic ulceration in the rat. Dig Dis Sci. 1990a;35:622–629. doi: 10.1007/BF01540411. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990b;258:G527–G534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Keenan CM, Gale D, Shoupe TS. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology. 1992;102:18–27. doi: 10.1016/0016-5085(92)91779-4. [DOI] [PubMed] [Google Scholar]
- Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms. Am J Physiol. 1996;270:G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- Wood JD. Application of classification schemes to the enteric nervous system. J Auton Nerv Syst. 1994;48:17–29. doi: 10.1016/0165-1838(94)90156-2. [DOI] [PubMed] [Google Scholar]