Abstract
The electrophysiological properties of acutely isolated canine articular chondrocytes have been characterized using patch-clamp methods. The ‘steady-state’ current—voltage relationship (I—V) of single chondrocytes over the range of potentials from −100 to +40 mV was highly non-linear, showing strong outward rectification positive to the zero-current potential. Currents activated at membrane potentials negative to −50 mV were time independent, and the I—V from −100 to −60 mV was linear, corresponding to an apparent input resistance of 9.3 ± 1.4 GΩ (n = 23). The outwardly rectifying current was sensitive to the K+ channel blocking ion tetraethylammonium (TEA), which had a 50% blocking concentration of 0.66 mm (at +50 mV). The ‘TEA-sensitive’ component of the outwardly rectifying current had time- and membrane potential-dependent properties, activated near −45 mV and was half-activated at −25 mV. The reversal potential of the ‘TEA-sensitive’ current with external K+ concentration of 5 mm and internal concentration of 145 mm, was −84 mV, indicating that the current was primarily carried by K+ ions. The resting membrane potential of isolated chondrocytes (−38.1 ± 1.4 mV; n = 19) was depolarized by 14.8 ± 0.9 mV by 25 mm TEA, which completely blocked the K+ current of these cells. These data suggest that this voltage-sensitive K+ channel has an important role in regulating the membrane potential of canine articular chondrocytes.
Articular chondrocytes play a central role in the production and maintenance of the cartilage in synovial joints. The metabolic state of the chondrocytes, which are solely responsible for the synthesis and degradation of the extracellular collagen and proteoglycan matrix of the cartilage, is strongly influenced by mechanical loading and the extracellular ionic and osmotic environment of the cell (Urban et al. 1993; Mobasheri et al. 2002). The mechanotransduction processes that link mechanical loading to the metabolic response of the chondrocytes are not completely understood, but factors such as mechanical distortion of the chondrocyte membrane and nucleus (Guilak, 2000), electric stimuli from piezoelectric effects and streaming potentials (Gu et al. 1998; Mow et al. 1999; Lai et al. 2000), effects produced by mechanical load-induced changes in matrix water content, ionic concentrations and pH (Mobasheri et al. 1998; Mow et al. 1999), and changes in chondrocyte membrane potential (Wright et al. 1996, 1997) have all been implicated.
A wide variety of ion channels and transporters are expressed in articular chondrocytes (Mobasheri et al. 1998, 2002). For example, electrophysiological studies have identified voltage-gated Na+ channels (Sugimoto et al. 1996), voltage-gated and stretch-activated K+ channels (Vittur et al. 1994; Sugimoto et al. 1996; Martina et al. 1997; Mozrzymas et al. 1997; Tsuga et al. 2002), and voltage-gated Cl− channels (Sugimoto et al. 1996; Tsuga et al. 2002) in articular chondrocytes. Indirect evidence, based primarily on the effects of pharmacological blockers, has also been obtained for the presence of both N- and L-type voltage-gated Ca2+ channels (Martina et al. 1997; Zuscik et al. 1997; Mow et al. 1999; Millward-Sadler et al. 2000) and stretch-activated cation channels (Wright et al. 1996; Yellowley et al. 1997, 2002; Guilak et al. 1999; Erickson et al. 2001) in articular and growth plate chondrocytes. Immunostaining studies have shown that mouse limb-bud chondrocytes express both epithelial Na+ and voltage-gated Ca2+ channels (Shakibaei & Mobasheri, 2003). Articular chondrocytes also express a variety of electrogenic and non-electrogenic Na+, K+ and Cl− transporters, including Na+−K+-ATPase, Na+−H+ exchanger and Na+−K+−2Cl− cotransporter (Mobasheri et al. 1998; Trujillo et al. 1999). Despite this information, the ionic basis of the membrane potential in articular chondrocytes is not well understood at present.
The membrane potential of cell cultured rabbit articular chondrocytes was reported to be solely due to membrane Cl− permeability, and changes in extracellular [K+] did not influence membrane potential (Tsuga et al. 2002). In contrast, the K+ channel blocker tetraethylammonium (TEA) and high concentrations (150 mm) of extracellular K+ depolarized human articular chondrocytes (Wohlrab & Hein, 2000; Wohlrab et al. 2002), implying that K+ permeability made a contribution to the membrane potential in these cells. The membrane potential of chondrocytes in tissue culture has been shown to be sensitive to mechanical activity (Wright et al. 1992, 1996). Brief episodes of cyclic pressurization of human and sheep articular chondrocytes produced membrane hyperpolarization, an effect that was blocked by quinidine and apamin. The interpretation of these results was that pressurization induced activity of Ca2+-dependent K+ channels, which produced membrane hyperpolarization (Wright et al. 1992, 1996).
In this study, the membrane currents in acutely isolated canine articular chondrocytes have been studied using patch clamp methods. The use of acutely isolated cells reduces the influences of cell dedifferentiation that may occur in cell culture conditions. The current—voltage relation of these acutely isolated cells showed strong outward rectification, which was produced by a time- and voltage-dependent K+ current that was sensitive to relatively low concentrations of TEA (Kd= 0.66 mm). The biophysical properties of this current were characterized and these data suggested that this K+ current could contribute significantly to the membrane potential of acutely isolated canine articular chondrocytes. This was confirmed by showing that concentrations of TEA which were sufficient to block the K+ current resulted in significant membrane depolarization.
Methods
Isolation of chondrocytes
Articular cartilage was removed from the stifle joint of healthy, skeletally mature mixed-breed canines of either sex, aged 2–3 years (body mass 15–25 kg). Animals were cared for under the supervision of a veterinarian according to the guidelines of The Canadian Council on Animal Care with the approval of the University of Calgary institutional animal care committee. Animals were killed by intravenous bolus injection of barbiturate (Euthanyl). Chips of articular cartilage were removed and incubated in cell culture medium (DMEM F12 with 10% FBS at 37°C; Invitrogen Corp., Carlsbad, CA, USA) for 12–24 h to ensure sterile conditions. Populations of isolated cells were prepared by digesting chips in a series of solutions containing 0.25% hyaluronidase (room temperature, for 5 min), 0.8% pronase (1 h at 37°C, with stirring) and 0.4% collagenase (20 min at 37°C, with stirring). Aliquots of cell suspension were plated onto glass-bottomed electrophysiological recording chambers for 30–60 min, which allowed the isolated chondrocytes to lightly adhere to the glass before they were superfused with bath solutions. Cell preparations were used for no more than 4 h after isolation.
Electrophysiology
Whole-cell patch-clamp methods (Hamill et al. 1981) were used to measure membrane currents from single voltage-clamped chondrocytes, and to measure membrane potentials in current clamp. Patch pipettes were pulled from 1.5 mm o.d./1.2 mm i.d. borosilicate glass (Sutter Instrument Co., Novato, CA, USA) and lightly fire polished. The resistance of the pipettes when filled with pipette solution was in the range 3–8 MΩ. Membrane currents and potentials were recorded with a MultiClamp 700A amplifier (Axon Instruments, Union City, CA, USA). Currents and voltages were digitized, and voltage-clamp protocols were generated with a Digidata 1322A data-acquisition interface controlled with pCLAMP8 software (Axon Instruments).
The capacitance of acutely isolated canine articular chondrocytes ranged from 1.8 to 11 pF, and averaged 5.78 ± 0.11 pF for a sample of 203 cells from 17 different cell preparations. The pipette-to-cell seal resistance, measured before rupturing the membrane patch in the pipette, averaged 28.2 ± 2.5 GΩ (n = 58).
Cells were superfused with a modified Tyrode solution (see below) at 1–2 ml min−1. The temperature of the superfusion solution was 23 ± 2°C. Solutions containing drugs were delivered to cells using a multibarrelled local superfusion device, which used solenoid valves to control solution flow (ALA Scientific Instruments Inc., Westbury, NY, USA).
Use of unequal [Cl−] in the patch pipette and bath solution (see below) produced a liquid junction potential which resulted in an approximately +10 mV offset of the recorded membrane potentials (Neher, 1992). Note that all membrane potentials in the data presented in Results were ‘corrected’ for the liquid junction potential by subtracting 10 mV from the values recorded during the experiments.
Solutions and drugs
Modified Tyrode solution consisted of (mm): NaCl, 140; KCl, 5; CaCl2, 1.8; MgCl2, 1.0; Hepes, 5; glucose, 10. The pH was adjusted to 7.4 with 1 m NaOH. In solutions containing tetraethylammonium chloride (TEACl), NaCl was replaced by an equal concentration of TEACl. The patch pipette solution consisted of (mm): potassium aspartate, 110; KCl, 20; MgCl2, 4; K2ATP, 4; Na2GTP, 0.1; sodium phosphocreatine, 6.6; EGTA, 5; Hepes, 5. The pH was adjusted to 7.2 with 1 m KOH.
Statistics
Mean values are shown with s.e.m. Sets of data were least-squares-fitted to Hill and Boltzmann equations using ‘Sigmaplot’ (SPSS Inc. Chicago, IL, USA).
Results
Voltage-dependent membrane currents in canine articular chondrocytes
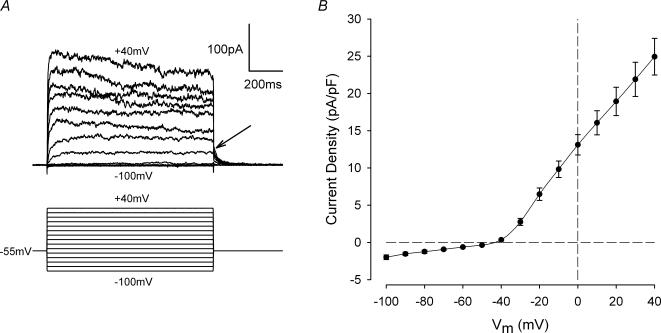
Figure 1A illustrates a representative example of a family of membrane currents from a voltage-clamped articular chondrocyte. The membrane potential was stepped for 1 s from a holding potential of −55 mV to a series of potentials between +40 and −100 mV. Steps to potentials between −50 and −100 mV produced small ‘instantaneous’, time-independent jumps in current. For steps positive to −40 mV, however, outward currents had a time-dependent component which increased in amplitude as the membrane depolarization increased. The rate of activation of the time-dependent current increased with increased membrane depolarization, and over the range of membrane potentials from −30 to +40 mV, the one-half activation time of the time-dependent component of the currents shown in Fig. 1A increased from about 27 ms to 2.2 ms, respectively. After the end of the depolarizing step, the currents declined (deactivated) back to holding current levels within about 50 ms. Figure 1B shows a plot of the mean peak outward current density versus membrane potential (I—V relation) for 24 different cells. Currents from each cell were normalized to cell capacitance, and the current density values were averaged. The I—V plot showed very pronounced outward rectification beginning near −45 mV, and the outward currents monotonically increased as membrane potential became more positive. For membrane potentials negative to about −50 mV, the I—V relation was linear, and the mean apparent input resistance of the cells, from the slope of the I—V between −60 and −100 mV, was 9.3 ± 1.4 GΩ(n = 23).
Figure 1. Current—voltage relation of acutely isolated chondrocytes.
A, representative family of membrane currents from a voltage-clamped articular chondrocyte. The voltage-clamp protocol is shown below the current records. Holding potential (Vh) was −55 mV, and the membrane potential was stepped from +40 to −100 mV in 10 mV increments at 3 s intervals. Arrow indicates deactivation of outward currents at the end of the depolarizing steps. Cell capacitance was 6.5 pF. B, mean peak current—voltage relationship from 24 cells (5 different cell preparations). Currents from each cell were normalized to cell capacitance, and then averaged. The mean cell capacitance of the 24 cells was 5.8 ± 0.3 pF.
Outward currents in articular chondrocytes are sensitive to TEA
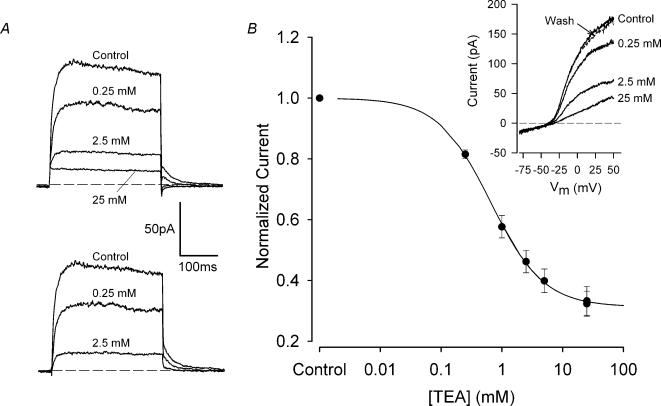
The K+ channel-blocking ion TEA+ strongly inhibited outward membrane currents in articular chondrocytes. Figure 2A illustrates the effect of a wide range of TEA concentrations (0.25–25 mm) on the outward currents produced by a voltage-clamp step to +30 mV. In this chondrocyte, 0.25, 2.5 and 25 mm TEA reduced total outward current amplitude by about 33, 73 and 88%, respectively. The current in the presence of 0.25 and 2.5 mm TEA exhibited time-dependent activation with a time course that was similar to that of the control current, but in the presence of 25 mm TEA, the current appeared to activate ‘instantaneously’ and was constant in magnitude throughout the voltage-clamp step. These results demonstrate that 25 mm TEA completely blocked the voltage-dependent outward current, leaving a time-independent background current. Figure 2A also shows that the ‘TEA-sensitive’ component of the outward current, obtained by subtraction of the current in 25 mm TEA, had a similar time course to the control current, suggesting that the blocking action of TEA was not primarily on open channels. Figure 2B shows the dose—response relation for the blocking action of TEA on the outward current. These data were obtained from I—V relations generated by voltage ramps, as shown in the inset to Fig. 2B. The plot in Fig. 2B is the relationship between normalized total current at +50 mV and concentration of TEA. The current at +50 mV, in each concentration of TEA, was normalized for each cell to its control value, and these normalized values were then averaged. The data in Fig. 2B were pooled from two different batches of cells; in one batch the TEA concentrations used were 1, 10 and 25 mm, and in the other the concentrations were 0.25, 2.5 and 25 mm. The continuous line in Fig. 2B shows the best-fit Hill equation (see figure legend) with n = 1. The 50% blocking dose of TEA from this equation was 0.66 ± 0.02 mm, and 68.7% of the total outward current at +50 mV was TEA sensitive.
Figure 2. Dose—response for the effect of tetraethylammonium (TEA) on outward currents.
A, effect of tetraethylammonium chloride (TEA) on outward currents of a voltage-clamped chondrocyte. Voltage-clamp step was +30 mV, Vh=−55 mV. Currents were recorded in control solution, and solution containing 0.25, 2.5 and 25 mm TEA. Four to six records were averaged at each TEA concentration. The lower set of records shows the ‘TEA-sensitive’ current, obtained by subtraction of the current in 25 mm TEA. B, TEA dose—response relationship. Inset: current—voltage relationships were generated with a 1 s voltage ‘ramp’ from −100 mV to +50 mV (Vh−55 mV), in control and TEA-containing solutions. Plot: mean, normalized current versus TEA concentration. The total current at +50 mV for each TEA concentration was normalized to control for each cell, and normalized values were averaged. Two batches of cells were used; the number of cells at each concentration of TEA was in the range 7–20. Continuous line is best fit of the Hill equation, viz., I =A/(1 +[TEA]/Kd) + (1 −A), where A is the fraction of current sensitive to TEA and Kd is the 50% blocking dose of TEA. The best-fit line had A = 0.687 ± 0.004 and Kd= 0.66 ± 0.02 mm.
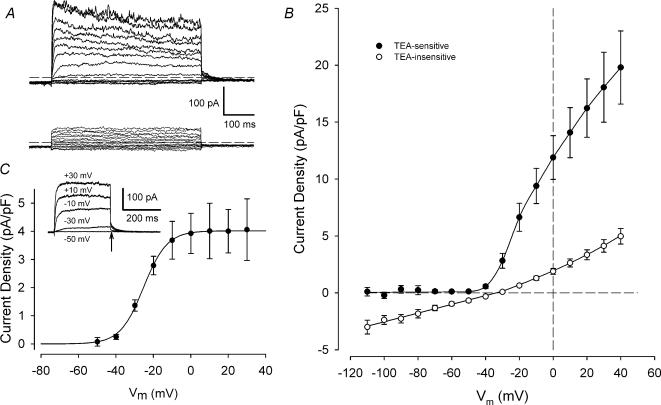
Figure 3A shows the effect of 25 mm TEA on a family of currents produced by voltage steps from +40 to −100 mV. Outward currents were strongly inhibited by TEA, while currents negative to the holding potential, −55 mV, were not significantly affected. Figure 3B compares the I—V relationships for membrane currents that were sensitive and insensitive to TEA. The ‘TEA-insensitive’ current (the current remaining in 25 mm TEA) showed weak outward rectification, with an apparent reversal potential between −30 and −35 mV. Membrane currents at potentials between −50 and −110 mV were unaffected by TEA. The ‘TEA-sensitive’ current (obtained by subtraction of control currents from the ‘TEA-insensitive’ currents) was undetectable at membrane potentials negative to −50 mV, but began to activate near −45 mV. This current increased steeply with membrane depolarization until about −10 mV, and it continued to increase for more positive depolarizations.
Figure 3. Current—voltage relations for ‘TEA-sensitive’ and ‘TEA-insensitive’ membrane currents.
A, family of currents from voltage-clamped chondrocyte before (upper records) and after (bottom records) exposure to 25 mm TEA. Voltage-clamp protocol was similar to that shown in Fig. 1A, except the depolarizing steps were 500 ms in duration. B, mean I—V relationships for ‘TEA-sensitive’ and ‘TEA-insensitive’ currents in 15 chondrocytes. ‘TEA-insensitive’ currents were the currents remaining in the presence of 25 mm TEA; ‘TEA-sensitive’ currents were obtained by subtraction of control currents from ‘TEA-insensitive’ currents. Currents from each cell were normalized to cell capacitance, and then averaged. Mean cell capacitance was 5.3 ± 0.4 pF (n = 15). C, voltage dependence of activation of ‘TEA-sensitive’ current. The amplitude of the deactivating tail current (at a membrane potential of −55 mV) was measured as a function of the depolarizing step. Inset shows an example of a family ‘TEA-sensitive’ currents for a series of 300 ms depolarizing steps from −50 to +30 mV. Tail current amplitude was measured at the end of the depolarizing step (arrow). The plot shows mean (±s.e.m.) tail current density (pA pF−1) as a function of membrane potential for 8 cells. The continuous line is the best-fit Boltzmann function (see text), with V½=−25.2 ± 0.3 mV and S½= 6.3 ± 0.3 mV.
The voltage dependence of activation of the ‘TEA-sensitive’ current is shown in Fig. 3C. Activation was measured from the amplitude of the deactivating tail current, as a function of the depolarizing step (see inset to Fig. 3C). The amplitude of the tail current was nearly zero at −50 mV, but it increased rapidly with membrane depolarization over the range −40 mV to about −10 mV, and then remained constant for potentials more positive than −10 mV. The mean tail current amplitude versus potential was fitted to a Boltzmann function, viz.
where Imax is the maximal tail current amplitude, Vm is membrane potential, V½ is the potential for one-half maximal tail current and Sh is a slope factor at V½. The best fit of this equation to the mean tail current data in Fig. 3C had Imax= 4.0 ± 0.02 pA pF−1, V½=−25.2 ± 0.3 mV and S½= 6.3 ± 0.3 mV.
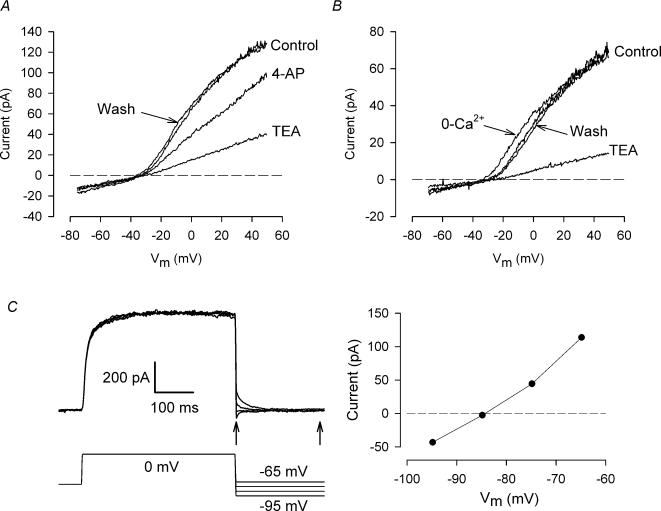
Figure 4A compares the effects on chondrocyte membrane currents of another K+ channel blocker, 4-aminopyridine (4-AP), with TEA. A ‘ramp’ voltage-clamp protocol (cf. Fig. 2B) was used to generate an I—V relation. 4-AP (1 mm) reduced the magnitude of the TEA-sensitive current by about 31% at +50 mV. Similar results were obtained in three other cells; the mean reduction in TEA-sensitive current at +50 mV by 1 mm 4-AP was 26.5 ± 6.3%(n = 4).
Figure 4. Pharmacology and reversal potential of isolated chondrocytes.
A, effect of 4-aminopyridine (4-AP) on currents. I—V relationships were produced with a ramp voltage clamp (cf. Fig. 2). Following the control I—V, the cell was exposed to 25 mm TEA. An I—V was recorded after removing TEA (data not shown), then the cell was exposed to 1 mm 4-AP. ‘Wash’ shows the I—V after washout of 4-AP. B, effect of removing extracellular Ca2+. Ca2+ in control solution (see Methods) was replaced by equimolar Mg2+. Control I—V was followed by 0 Ca2+, wash (data not shown), 25 mm TEA, and second wash. C, reversal potential of current. Tail currents following depolarizing step to 0 mV were recorded at potentials between −95 and −65 mV. The magnitude of the tail current was taken as the difference in current immediately after the end of the depolarizing step, and after the currents had reached a ‘steady state’ (arrows). Plot at right shows tail current magnitude versus membrane potential.
Figure 4B shows that the I—V relation was only slightly altered by complete removal of extracellular Ca2+ (replacement with equimolar Mg2+). TEA-sensitive current magnitude was not significantly changed, but the current appeared to activate at slightly more negative membrane potentials. Similar results were obtained in two other cells. The patch-pipette solution contained 5 mm EGTA, without any added Ca2+ (Methods); thus internal free [Ca2+] was very low (<10 nm). Consequently, entry of Ca2+ into the cell cytoplasm via, for example, voltage-gated Ca2+ channels, would be unlikely to result in an increase in intracellular [Ca2+]. In summary, the data in Fig. 4B indicate that it is improbable that activation of the TEA-sensitive current is dependent on intracellular [Ca2+].
TEA-sensitive current is a K+ current
The data in Fig. 4C show that the TEA-sensitive, voltage-gated current is carried by K+. Tail currents which followed a depolarizing step were recorded at membrane potentials between −65 and −95 mV. Tail current polarity reversed at a potential of −84.3 mV. The Nernst potential for K+ in the extra- and intracellular solutions was −84 mV. The only other major ion in the solutions was Cl−, whose Nernst potential was −42 mV. The sensitivity of the voltage-gated current to TEA, and its reversal potential at the K+ Nernst potential clearly identifies it as a K+ current.
TEA produces membrane depolarization of chondrocytes
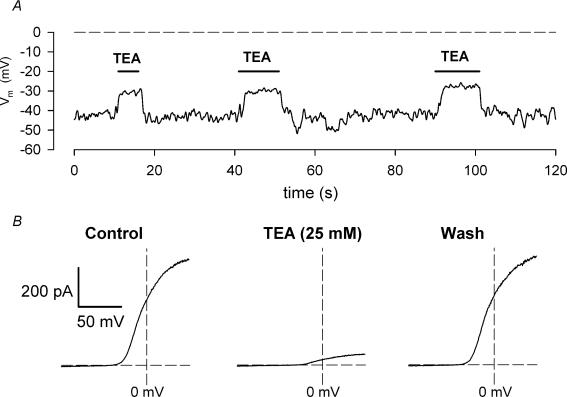
Figure 5A shows the effect of 25 mm TEA on the resting membrane potential of an isolated chondrocyte, recorded in current clamp conditions. The cell was exposed briefly (5–10 s) to solution containing 25 mm TEA during three successive intervals. In each case, exposure of the cell to TEA produced a very rapid depolarization of the membrane from a resting potential of approximately −42 mV to about −30 mV. The depolarization was readily reversible on removal of TEA. Prior to recording the membrane potentials, I—V relations were recorded in the same cell in the absence and presence of 25 mm TEA (Fig. 5B). As shown by data presented above (e.g. Fig. 2) the voltage-dependent outward currents were completely suppressed by TEA. The resting membrane potential of 19 different chondrocytes (from 3 different cell preparations) in control solution was −38.1 ± 1.4 mV. TEA (25 mm) produced an average depolarization of 14.8 ± 0.9 mV in the same 19 chondrocytes.
Figure 5.
A, effect of TEA on resting membrane potential of an isolated chondrocyte. The plot is a continuous recording of the membrane potential. Solution containing 25 mm TEA was applied to the cell for 3 successive intervals, as shown. B, current—voltage relations from the same cell, in control (left), in 25 mm TEA (centre) and after washout of TEA (right). The I—V relations were generated using a voltage ramp protocol (cf. Fig. 2).
Discussion
Properties of voltage-gated K+ current in acutely isolated canine articular chondrocytes
The biophysical properties of a time- and voltage-dependent K+ current in acutely isolated canine articular chondrocytes have been characterized in this study. This K+ current was activated over membrane potentials from about −45 mV to approximately −10 mV. This current exhibited many of the characteristics of a ‘delayed rectifier’ K+ current, with rapid activation and very slow inactivation. Reversal of the current occurred close to the predicted Nernst potential for K+, demonstrating a high selectivity of the channel for K+. Pharmacological studies showed that this current was sensitive to TEA, with a half-blocking concentration of 0.66 mm, and that 4-aminopyridine (1 mm) blocked about 27% of the current. Removal of extracellular Ca2+ had little effect on the current. Since intracellular [Ca2+] was strongly buffered by EGTA in the patch-clamp pipette, this current was probably not activated by changes in intracellular [Ca2+]. Isolated chondrocytes were depolarized by TEA (25 mm), indicating that this K+ current can contribute to the membrane potential of canine chondrocytes.
K+ channels and currents in chondrocytes
Patch-clamp studies have identified a variety of K+ channels and currents in cell cultured articular and growth plate chondrocytes. Large conductance, Ca2+-activated K+ channels were identified in porcine (Grandolfo et al. 1990, 1992) and chicken (Long & Walsh, 1994) growth plate chondrocytes. Two types of voltage-gated K+ channels with different single-channel conductances were recorded from porcine and equine articular chondrocytes (Mozrzymas et al. 1994, 1997; Vittur et al. 1994). A large conductance, stretch-activated K+ channel was recorded from porcine articular chondrocytes (Martina et al. 1997). Whole-cell current recordings from porcine (Mozrzymas et al. 1997) and rabbit (Sugimoto et al. 1996) articular chondrocytes identified K+ currents with delayed rectifier-like properties. The K+ currents in rabbit chondrocytes were partially blocked by 4-AP (5 mm, 92% block) and TEA (5 mm, 58% block). A similar delayed rectifier-like K+ current was recorded from cell cultured chicken growth plate chondrocytes (Walsh et al. 1992); this current was strongly inhibited by 4-AP (82%; 1 mm) and charybdotoxin (∼90%; 10 nm), but was not significantly affected by TEA (10 mm). None of these K+ currents or channels appears to have properties that are identical to the voltage-gated K+ currents recorded from acutely isolated canine articular chondrocytes in this study.
The K+ current recorded from canine articular chondrocytes appears to most closely resemble the K+ currents recorded from an in situ preparation of porcine growth plate chondrocytes (Lee et al. 2001). Chondrocytes embedded in pieces of growth plate were exposed by enzymatic digestion of the surface of the cartilage, and then whole-cell patch clamped. A delayed rectifier-like K+ current was the principal voltage-dependent current recorded from cells in this preparation. The current was half-activated at about −11 mV, and was completely blocked by 20 mm TEA. The current density depended on whether the recordings were made from resting, proliferative or hypertrophic chondrocytes. At a membrane potential of 0 mV, current density varied from about 0.3 pA μm−2 for resting and hypertrophic cells to 0.6 pA μm−2 for proliferative cells. Assuming a specific membrane capacitance of 1 μF cm−2 (Hille, 2001), this corresponds to a current density of about 30–60 pA pF−1. This compares favourably with a current density of ∼12 pA pF−1 for the TEA-sensitive current of canine chondrocytes at 0 mV (Fig. 3B).
Chondrocyte membrane potential — a role for K+ channels?
The ionic basis of chondrocyte membrane potential is not well understood, and has been investigated in detail only for cell cultured rabbit articular chondrocytes (Tsuga et al. 2002). Membrane potential, measured using patch-clamp methods, was determined by the Nernst potential for intra- and extracellular [Cl−]. Single-channel recordings identified large conductance (>200 pS), voltage-gated Cl− channels which were probably the basis for the membrane Cl− permeability (Tsuga et al. 2002). Large changes in extracellular [K+] (5–40 mm) had no significant effect on membrane potential, indicating that K+ ions did not contribute to the membrane potential. This is in contrast to cell cultured human articular chondrocytes in which membrane potential, measured with potential-sensitive optical dyes (Wohlrab & Hein, 2000; Wohlrab et al. 2002), was significantly depolarized by increased extracellular [K+] and high concentrations of TEA (20–40 mm), indicating the involvement of K+ channels in determining membrane potential. Depolarization of acutely isolated canine chondrocytes by 25 mm TEA (Fig. 5), which completely blocked the voltage-gated K+ current in these cells (Figs 2 and 5), provides strong evidence that K+ channels contribute significantly to membrane potential under the conditions of these experiments.
The membrane potential of rabbit growth plate chondrocytes, measured in situ with intracellular microelectrodes in pieces of cartilage (Edelman et al. 1985), was dependent on the stage of differentiation of the chondrocyte, approximately −36 mV for hypertrophic cells and −56 mV for proliferative cells. It is interesting that the density of the delayed rectifier K+ current recorded from in situ porcine growth plate chondrocytes was significantly greater for proliferative cells than for hypertrophic cells (Lee et al. 2001); this suggests that the difference in membrane potential between proliferative and hypertrophic growth plate chondrocytes may result from the difference in current density of the voltage-gated K+ current in these cells. Although the half-activation potential of the current (approximately −11 mV) was much more positive than the membrane potential (i.e. −36 to −56 mV), activation of the current began between −60 and −50 mV (Lee et al. 2001). Because of the very high membrane resistance of chondrocytes, ‘steady-state’ activation of only a very small amount of K+ current would significantly hyperpolarize these cells. It is probable that the TEA-sensitive, voltage-gated K+ current contributes to resting membrane potential of isolated canine chondrocytes by this mechanism. Figure 3C shows that the TEA-sensitive and TEA-insensitive currents were equal and opposite in magnitude near −40 mV, which was about 10 mV more negative than the zero-current potential of the TEA-insensitive current. Hence, block of the TEA-sensitive K+ current would be expected to produce a membrane depolarization of approximately 10 mV, which is in good agreement with the mean TEA-induced depolarization of 14.8 mV (cf. Fig. 5). The data in Fig. 3C shows that only about 12% of the maximal K+ conductance was activated at the mean resting membrane potential, −38 mV.
Limitations of this study
Dissociated chondrocytes readily dedifferentiate in monolayer cell culture conditions, and the expression of cell matrix proteins such as collagen and proteoglycans changes in culture (Mayne et al. 1976; Pacifici et al. 1981; Benya & Shaffer, 1982; Plaas et al. 1983). There are indications that expression and properties of ion channels may also change in cell cultured chondrocytes. For example, the conductance of K+ channels recorded from cultured porcine growth plate chondrocytes increased with time in culture, as the cultures changed from isolated cells to confluence (Grandolfo et al. 1990), and in monolayer cell cultured porcine articular chondrocytes, stretch-activated K+ channels were recorded most frequently in cells that had been in culture for 4–6 days (Martina et al. 1997). In this study, we have attempted to minimize such sources of variability by using chondrocytes which were acutely isolated from intact cartilage for at most a few hours before use. However, mechanical activity also profoundly affects chondrocyte metabolism, changing for example, proteoglycan, DNA and cAMP synthesis (e.g. Bourret & Rodan, 1976; Veldhuijzen et al. 1979; Sah et al. 1989; Buschmann et al. 1995; Lee & Bader, 1997). Consequently, storage of chips of cartilage in culture conditions for 12–24 h (see Methods) without appropriate mechanical stimulation before isolation of the chondrocytes may potentially produce changes in ion channel expression.
The in situ extracellular osmotic and ionic environment of articular chondrocytes is very different from that of most cells. The presence of immobile negative charges on the cartilage matrix proteoglycans results in cation concentrations in cartilage that are elevated compared to those in serum/synovial fluid, whereas anion concentrations are reduced. The concentrations of Na+ (250–350 mm), K+ (8–10 mm) and Ca2+ (15–20 mm) in articular cartilage are 2–10 times greater than their respective concentrations in serum/synovial fluid, whereas Cl− concentration in cartilage (60–90 mm) is roughly 1.5–2 times less (Maroudas, 1979). Moreover, the pH of cartilage is 6.9–7.1 (Gray et al. 1988), significantly lower than the pH of 7.4 of serum. The elevated cation concentration results in an increased osmotic pressure of the cartilage fluid, which measures 380–450 mosmol (kg H2O)−1: this compares with an osmolality of about 280 mosmol (kg H2O)−1 for the Hepes-buffered solution used in the patch-clamp experiments. It is unclear how the properties of the voltage-gated K+ channel recorded from isolated chondrocytes might be modified in the presence of elevated cation and reduced anion concentrations, and osmotic pressures and pH that are more representative of intact cartilage.
Summary
This study demonstrates that a voltage-gated, Ca2+-independent K+ current with a substantial current density can be recorded from acutely isolated canine articular chondrocytes, and this K+ current makes an important contribution to chondrocyte membrane potential. Further experiments will be necessary to determine the properties and role of this current in regulating chondrocyte membrane potential in ionic and osmotic conditions that more closely mimic those found in situ in cartilage.
The majority of electrophysiological studies of chondrocytes to date have used cells in monolayer culture. It would be of interest in future studies to measure the electrophysiological properties of chondrocytes that have been maintained in three-dimensional culture conditions such as agarose (Benya & Shaffer, 1982) or alginate (Bonaventure et al. 1994) gels that are known to preserve chondrocyte phenotype better than monolayer cultures.
Acknowledgments
This work was supported by research grants from the Natural Sciences and Engineering Research Council of Canada, and a Canada Research Chair in Orthopaedic Bioengineering (N.A.D.), and the Canadian Institutes of Health Research (W.R.G.) and a Research Chair endowed by the Heart and Stroke Foundation of Alberta (W.R.G.). The authors thank M. Chung for isolation of chondrocytes.
References
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, Freisinger P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212:97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- Bourret LA, Rodan GA. Inhibition of cAMP accumulation in epiphyseal cartilage cells exposed to physiological pressure. Calcif Tissue Res. 1976;21(suppl.):431–436. [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Edelman A, Thil CL, Garabedian M, Plachot JJ, Guillozo H, Fritsch J, Thomas SR, Balsan S. vitamin D metabolite effects on membrane potential and potassium intracellular activity in rabbit cartilage. Miner Electrolyte Metab. 1985;11:97–105. [PubMed] [Google Scholar]
- Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces Volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34:1527–1535. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Grandolfo M, D'Andrea P, Martina M, Ruzzier F, Vittur F. Calcium-activated potassium channels in chondrocytes. Biochem Biophys Res Commun. 1992;182:1429–1434. doi: 10.1016/0006-291x(92)91893-u. [DOI] [PubMed] [Google Scholar]
- Grandolfo M, Martina M, Ruzzier F, Vittur F. A potassium channel in cultured chondrocytes. Calcif Tissue Int. 1990;47:302–307. doi: 10.1007/BF02555913. [DOI] [PubMed] [Google Scholar]
- Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- Gu WY, Lai WM, Mow VC. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. J Biomech Eng. 1998;120:169–180. doi: 10.1115/1.2798299. [DOI] [PubMed] [Google Scholar]
- Guilak F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology. 2000;37:27–44. [PubMed] [Google Scholar]
- Guilak F, Zell RA, Erickson GR, Grande DA, Rubin CT, McLeod KJ, Donahue HJ. Mechanically induced calcium waves in articular chondrocytes are inhibited by gadolinium and amiloride. J Orthop Res. 1999;17:421–429. doi: 10.1002/jor.1100170319. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 2001. p. 11. [Google Scholar]
- Lai WM, Mow VC, Sun DD, Ateshian GA. On the electric potentials inside a charged soft hydrated biological tissue: streaming potential vs diffusion potential. J Biomech Eng. 2000;122:336–346. doi: 10.1115/1.1286316. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181–188. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- Lee KM, Ye GL, Yung WH, Leung KS, Leung PC. In situ model for studying potassium currents in various growth plate chondrocyte subpopulations. Life Sci. 2001;69:721–728. doi: 10.1016/s0024-3205(01)01163-8. [DOI] [PubMed] [Google Scholar]
- Long KJ, Walsh KB. A calcium-activated potassium channel in growth plate chondrocytes: regulation by protein kinase A. Biochem Biophys Res Commun. 1994;201:776–781. doi: 10.1006/bbrc.1994.1768. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Physiochemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. London: Pitman Medical; 1979. pp. 215–290. [Google Scholar]
- Martina M, Mozrzymas JW, Vittur F. Membrane stretch activates a potassium channel in pig articular chondrocytes. Biochim Biophys Acta. 1997;1329:205–210. doi: 10.1016/s0005-2736(97)00154-5. [DOI] [PubMed] [Google Scholar]
- Mayne R, Vail MS, Mayne PM, Miller EJ. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976;73:1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Lee HS, Caldwell H, Nuki G, Salter DM. Altered electrophysiological responses to mechanical stimulation and abnormal signalling through α5β1 integrin in chondrocytes from osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:272–278. doi: 10.1053/joca.1999.0301. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; Putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26:1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Mobasheri R, Francis MJ, Trujillo E, Alvarez de la Rosa D, Martin-Vasallo P. Ion transport in chondrocytes: membrane transporters involved in intracellular ion homeostasis and the regulation of cell volume, free [Ca2+] and pH. Histol Histopathol. 1998;13:893–910. doi: 10.14670/HH-13.893. [DOI] [PubMed] [Google Scholar]
- Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Martina M, Ruzzier F. A large-conductance voltage-dependent potassium channel in cultured pig articular chondroctyes. Pflugers Arch. 1997;433:413–427. doi: 10.1007/s004240050295. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Visintin M, Vittur F, Ruzzier F. Potassium channels of pig articular chondrocytes are blocked by propofol. Biochim Biophys Res Commun. 1994;202:31–37. doi: 10.1006/bbrc.1994.1889. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. In: Rudy B, Iverson LE, editors. Methods in Enzymology, vol. 207, Ion Channels. San Diego: Academic Press; 1992. pp. 123–131. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Fellini SA, Holtzer H, De Luca S. Changes in the sulfated proteoglycans synthesized by ‘aging’ chondrocytes. I. Dispersed cultured chondrocytes and in vivo cartilages. J Biol Chem. 1981;256:1029–1037. [PubMed] [Google Scholar]
- Plaas AH, Sandy JD, Muir H. Proteoglycan aggregate formation by articular chondrocytes. Decrease in link-protein synthesis during culture. Biochem J. 1983;214:855–864. doi: 10.1042/bj2140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Mobasheri A. (1-Integrins co-localize with Na,K-ATPase, epithelial sodium channels (ENaC) and voltage-activated calcium channels (VACC) in mechanoreceptor complexes of mouse limb-bud chondrocytes. Histol Histopathol. 2003;18:343–351. doi: 10.14670/HH-18.343. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Yoshino M, Nagao M, Ishii S, Yabu H. Voltage-gated ionic channels in cultured rabbit articular chondrocytes. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:223–232. doi: 10.1016/s0742-8413(96)00091-6. [DOI] [PubMed] [Google Scholar]
- Trujillo E, Alvarez de la Rosa D, Mobasheri A, Gonzalez T, Canessa CM, Martin-Vasallo P. Sodium transport systems in human chondrocytes. II. Expression of ENaC, Na+/K+/2Cl− cotransporter and Na+/H+ exchangers in healthy and arthritic chondrocytes. Histol Histopathol. 1999;14:1023–1031. doi: 10.14670/HH-14.1023. [DOI] [PubMed] [Google Scholar]
- Tsuga K, Tohse N, Yoshino M, Sugimoto T, Yamashita T, Ishii S, Yabu H. Chloride conductance determining membrane potential of rabbit articular chondrocytes. J Membr Biol. 2002;185:75–81. doi: 10.1007/s00232-001-0112-3. [DOI] [PubMed] [Google Scholar]
- Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen JP, Bourett LA, Rodan GA. In vitro studies of the effect of intermittent compressive forces on cartilage cell proliferation. J Cell Physiol. 1979;98:299–306. doi: 10.1002/jcp.1040980206. [DOI] [PubMed] [Google Scholar]
- Vittur F, Grandolfo M, Fragonas E, Godeas C, Paoletti S, Pollesello P, Kvam BJ, Ruzzier F, Starc T, Mozrzymas JW, Martina M, de Bernard B. Energy metabolism, replicative ability, intracellular calcium concentration, and ionic channels of horse articular chondrocytes. Exp Cell Res. 1994;210:130–136. doi: 10.1006/excr.1994.1019. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Cannon SD, Wuthier RE. Characterization of a delayed rectifier potassium current in chicken growth plate chondrocytes. Am J Physiol. 1992;262:C1335–C1340. doi: 10.1152/ajpcell.1992.262.5.C1335. [DOI] [PubMed] [Google Scholar]
- Wohlrab D, Hein W. Der einfluss von ionenkanalmodulatoren auf das membranpotential humaner chondrozyten. Orthopade. 2000;29:80–84. doi: 10.1007/s001320050013. [DOI] [PubMed] [Google Scholar]
- Wohlrab D, Lebek S, Kruger T, Reichel H. Influence of ion channels on the proliferation of human chondrocytes. Biorheology. 2002;39:55–61. [PubMed] [Google Scholar]
- Wright M, Jobanputra P, Bavington C, Salter DM, Nuki G. Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: evidence for the presence of stretch-activated membrane ion channels. Clin Sci (Lond) 1996;90:61–71. doi: 10.1042/cs0900061. [DOI] [PubMed] [Google Scholar]
- Wright MO, Nishida K, Bavington C, Godolphin JL, Dunne E, Walmsley S, Jobnaputra P, Nuki G, Salter DM. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor. J Orthop Res. 1997;15:742–747. doi: 10.1002/jor.1100150517. [DOI] [PubMed] [Google Scholar]
- Wright MO, Stockwell RA, Nuki G. Response of plasma membrane to applied hydrostatic pressure in chondrocytes and fibroblasts. Connect Tissue Res. 1992;28:49–70. doi: 10.3109/03008209209014227. [DOI] [PubMed] [Google Scholar]
- Yellowley CE, Hancox JC, Donahue HJ. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem. 2002;86:290–301. doi: 10.1002/jcb.10217. [DOI] [PubMed] [Google Scholar]
- Yellowley CE, Jacobs CR, Li Z, Zhou Z, Donahue HJ. Effects of fluid flow on intracellular calcium in bovine articular chondrocytes. Am J Physiol. 1997;273:C30–C36. doi: 10.1152/ajpcell.1997.273.1.C30. [DOI] [PubMed] [Google Scholar]
- Zuscik MJ, Gunter TE, Puzas JE, Rosier RN. Characterization of voltage-sensitive calcium channels in growth plate chondrocytes. Biochem Biophys Res Commun. 1997;234:432–438. doi: 10.1006/bbrc.1997.6661. [DOI] [PubMed] [Google Scholar]