Abstract
We tested the hypothesis that in humans, carotid-baroreflex dynamic responses (evaluated by examining the time course of the carotid-baroreflex-induced alterations in muscle sympathetic nerve activity (MSNA), mean arterial blood pressure (MAP) and heart rate (HR)) would be altered during mild orthostatic stress in ways that serve to limit orthostatic hypotension. In 12 healthy subjects (10 male, 2 female), 5-s periods of neck pressure (NP) (50 mmHg) and neck suction (NS) (− 60 mmHg) were used to evaluate carotid baroreflex function at rest (CON) and during lower body negative pressure (LBNP) (−15 mmHg). During LBNP (as compared with CON) (a) the augmentations in MSNA and MAP elicited by NP were greater, (b) the NS-induced period of MSNA suppression was, if anything, shorter, (c) the peak decrement in MAP elicited by NS, although not different in amplitude, occurred earlier and recovered to its initial level more quickly after NS, and (d) the HR responses to NP and NS were greater. These results suggest that during mild orthostatic stress, carotid-baroreflex dynamic responses are modulated in ways that should help maintain blood pressure and limit orthostatic hypotension.
The nature of the mechanisms involved in blood pressure regulation under conditions of orthostatic stress is an important research issue in physiology, especially in humans who usually adopt an upright posture (Rowell, 1986). With the body in an upright posture, blood in the central circulation is pooled in the peripheral veins, thus decreasing the cardiac filling pressure and leading to a decrease in arterial blood pressure. Increases in peripheral vascular resistance and heart rate (HR), major cardiovascular adjustments to orthostatic stress mediated by the autonomic nervous system (Rowell, 1986), form part of the reflex response patterns elicited via the carotid sinus and aortic baroreceptors (arterial baroreflex) and via stretch receptors in the cardiopulmonary regions (cardiopulmonary baroreflex) (Zoller et al. 1972; Johnson et al. 1974; Sundlof & Wallin., 1978; Mark & Manica, 1983; Manica & Mark, 1983; Rowell, 1986; Pawelczyk & Raven, 1989; Eckberg & Sleight, 1992; Nishiyasu et al. 1993, 1999; Taylor et al. 1995).
It has been hypothesized that arterial-baroreflex-mediated cardiovascular control is modulated by the cardiopulmonary baroreflex (Bevegård et al. 1977; Ebert, 1983; Mark & Manica, 1983; Manica & Mark, 1983; Victor & Mark, 1985; Pawelczyk & Raven, 1989; Shi et al. 1993, 1997) and, indeed, ample evidence of such modulation exists in animals (Koike et al. 1975; Mark & Manica, 1983; Manica & Mark, 1983) and in humans (Bevegård et al. 1977; Ebert, 1983; Victor & Mark, 1985; Pawelczyk & Raven, 1989; Shi et al. 1993, 1997; Ogoh et al. 2002). In humans, for example, Victor & Mark (1985) showed that the forearm vasoconstriction induced by neck pressure (i.e. carotid-baroreceptor unloading) was greatly augmented during −10 mmHg lower body negative pressure (LBNP), the augmented response greatly exceeding the sum of the individual reflex responses (i.e. that to LBNP alone plus that to neck pressure alone). In addition, Pawelczyk & Raven (1989) demonstrated that the maximum gain of the carotid-baroreflex control of blood pressure and R-R interval (as calculated from the entire carotid-baroreflex stimulus–response curve) increased progressively with the decreases in central venous pressure induced by increasing the LBNP level (0 to −50 mmHg LBNP). Recently, Ogoh et al. (2002) reported that although the sensitivity of the carotid baroreflex control of HR and mean arterial blood pressure (MAP) was not altered, the contribution made by the evoked change in total vascular conductance to the peak MAP response to 5 s neck pressure or neck suction was greater when the subjects were in the upright, seated position (in which the cardiopulmonary baroreceptors were likely to be unloaded) than when they were supine. Although some doubt exists as to whether a mild, non-hypotensive level of LBNP (less than −20 mmHg) selectively unloads the cardiopulmonary baroreceptors without changing afferent activity from the arterial baroreceptors (carotid and aortic arch baroreceptors) (Taylor et al. 1995), the above studies at least raise the possibility that those carotid-baroreflex responses that are neurally mediated may be modulated by mild orthostatic stress. An enhanced arterial-baroreflex control in situations in which the cardiopulmonary baroreceptors are significantly unloaded ought to be an excellent defence for the organism against orthostatic hypotension. However, it is still uncertain whether and to what extent there is in humans modulation (a) of the carotid-baroreflex regulation of sympathetic nerve activity during mild, non-hypotensive orthostatic stress and (b) of carotid-baroreflex dynamic responses (Ichinose et al. 2002) – which can be evaluated by examining the time course of the carotid-baroreflex-induced alterations in muscle sympathetic nerve activity (MSNA), mean arterial blood pressure (MAP) and HR – during such stress.
The purpose of this study was to investigate the modulation of carotid-baroreflex dynamic responses, during orthostatic stress; specifically, we tested the hypothesis that in humans under a mild (non-hypotensive) level of orthostatic stress, the carotid-baroreflex dynamic responses in MSNA, MAP and HR are altered in ways that serve to limit orthostatic hypotension.
Methods
Subjects
We studied 12 healthy volunteers (10 men and 2 women) with a mean age of 24 ± 2 years, a body weight of 61.8 ± 4.0 kg and a height of 170.1 ± 3.0 cm (mean ±s.e.m.) The subjects were non-smokers and none was taking any medication. The study was in accordance with the Declaration of Helsinki and approved by the Human Subjects Committee of the University of Tsukuba, and each subject gave informed written consent.
Procedures
After entering the test room, which was maintained at 25°C, each subject adopted the supine position with the lower torso, up to the iliac crest, enclosed in the LBNP box. A small door was created at the bottom of the LBNP box to allow recording of MSNA by the microneurographic technique from the tibial nerve at the popliteal fossa (Saito et al. 1990). After identifying MSNA (see below for criteria), the neck chamber and respiratory mask were fitted. Then, a rest period of at least 15 min was allowed before data collection began.
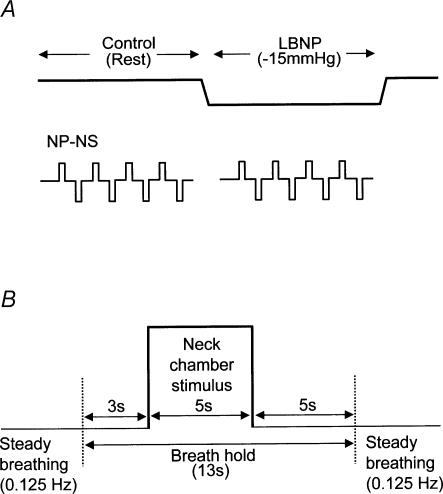
The experimental protocol is illustrated in Fig. 1. The subject was instructed to maintain a constant rate of breathing throughout the experiment, with auditory signals being supplied to assist the subject in controlling breathing frequency at 7.5 cycles min−1. Carotid-baroreflex control of HR, MAP and MSNA was assessed by the use of 5-s periods of neck pressure (50 mmHg) and neck suction (−60 mmHg). To minimize the respiratory-related modulation of HR, MAP and MSNA, each neck-chamber stimulus (neck pressure or neck suction) was applied during a voluntary apnoea (breath-hold) at end-expiration (Fig. 1B).
Fig. 1. General experimental protocol (A) and sequence used for application of each neck-chamber stimulus (B).
NP-NS, neck-chamber stimuli (neck pressure or suction).
While the subject was at rest, both types of neck-chamber stimulus were applied. After an interval of about 2 min, the pressure in the LBNP box was decreased slowly to −15 mmHg, then kept constant for 11–13 min. During this constant-pressure period, the neck-chamber stimuli were applied again. Once all the required neck-chamber stimuli had been applied, the LBNP-box pressure was returned to ambient pressure. In the course of the experiment, four episodes each of neck pressure and neck suction were delivered at rest and again during LBNP.
Neck pressure and suction
A Silastic neck chamber (Sprenkle et al. 1986) was used to load and unload the carotid baroreceptors. The chamber encased the front half of the neck, an airtight seal being made between the mandible and the clavicles and sternum. One part of the chamber was connected to a blower device that could apply either suction or pressure to left and right carotid regions simultaneously. Carotid baroreceptor activity was changed as abruptly as possible by applying 5 s neck pressure (50 mmHg pressure) or 5 s neck suction (60 mmHg suction) via the neck chamber. Neck-chamber pressure was measured using a pressure transducer mounted on the chamber. Each individual stimulus lasted 5 s and the interstimulus interval was 30 s. The order of stimuli was randomized. The neck-chamber stimuli were applied by means of a computer-operated system in which changes in chamber pressure were triggered by the first R wave occurring 3 s or more after the beginning of the breath-hold (to minimize the respiratory-related modulation of HR, MAP and MSNA, all neck-chamber stimuli were delivered during breath-holding (Fig. 1B)). One to two breathing cycles before the beginning of the voluntary apnoea, an investigator signalled to the subject to start breath-holding at the end of the next normal expiration (i.e. without changing the pattern of breathing until the breath-hold itself). The total duration of the voluntary apnoea was about 13 s (a 3 s prestimulus period, a 5 s stimulus and a 5 s poststimulus period; Fig. 1B). To assess the effect of the apnoea itself, measurements were repeated during breath-holding but with neck-chamber pressure kept at ambient pressure. In each subject, four episodes each of neck pressure and neck suction were delivered and four episodes of apnoea alone examined at rest and again during LBNP.
Measurements
HR was monitored via a three-lead electrocardiogram. Beat-to-beat changes in blood pressure were assessed by finger photoplethysmography (Finapres 2300; Ohmeda, USA). The monitoring cuff was placed around the middle finger with the forearm and hand supported so that the cuff was aligned at heart level. The subject wore a mask connected to a respiratory flowmeter (RF-H; Minato Medical Science, Japan). The analog signals representing the ECG, blood-pressure waveforms and respiratory flow were continuously recorded using an FM magnetic-tape data-recorder (MR-30; TEAC, Japan). The data were also digitized at a sampling frequency of 400 Hz through an analog-to-digital converter (Maclab/8e; ADInstruments, Australia) for processing by a personal computer (Powerbook 1400C; Apple, Japan) equipped with an on-line data-acquisition program. In this way, we collected HR, systolic, diastolic and mean arterial blood pressures and MSNA continuously.
Post-ganglionic muscle sympathetic nerve discharges were recorded by the microneurographic technique. A tungsten microelectrode with a shaft diameter of 0.1 mm and an impedance of 1–5 MΩ was inserted manually by an experimenter into the tibial nerve at the popliteal fossa. After insertion, the electrode was adjusted until MSNA was being recorded. The criteria for MSNA were spontaneous burst discharges synchronized with the heart beat and enhanced by Valsalva's manoeuvre or apnoea but showing no change in response to cutaneous touch or arousal stimuli (Delius et al. 1972; Vallbo et al. 1979; Saito et al. 1990). The neurogram was fed into a differential amplifier and amplified 100 000 times through a band-pass filter (500–3000 Hz). The neurogram was full-wave rectified and integrated by a capacitance-integrated circuit with a time constant of 0.1 s. This integrated MSNA was continuously recorded on an FM magnetic-tape data-recorder and also digitized with a sampling frequency of 400 Hz through an analog-to-digital converter for storage on a personal computer (see above). MSNA data were successfully collected in 10 of 12 subjects and the MSNA data shown here are from those 10 subjects (8 male, 2 female).
Data analysis
In a 2- to 3-min rest period, during which the subjects breathed at a constant rate, MSNA bursts were identified. Then, the voltage levels in the periods between bursts were averaged and this level was taken as zero. The largest burst occurring in this rest period was assigned a value of 1000 arbitrary units (AU). MSNA data were normalized with respect to this standard in each subject.
The assessment of carotid-baroreflex dynamic responses has been described in detail elsewhere (Ichinose et al. 2002). Briefly, to assess the time course of the MSNA responses to neck-chamber stimuli (MSNA dynamic response), 13 s sequences of MSNA data (3 s prestimulus, 5 s stimulus and 5 s poststimulus) from four trials of neck pressure or suction were ensemble-averaged and, to allow the sequential changes in MSNA to be observed, the averaged data were displayed at 1 s intervals. The MSNA data collected in a given 1 s interval did not strictly correspond to the actual MSNA burst activity (i.e. the averaging method included part of a burst within the 1 s window in some instances and a full burst in others). Nevertheless, the area of MSNA signal calculated automatically for each second is presented unchanged for each 1 s interval and thus each 1 s of MSNA data represents the MSNA level for that second regardless of whether whole or partial bursts were captured (Bath et al. 1981; Wallin & Eckberg, 1982; Rea & Eckberg, 1987; Eckberg & Wallin, 1987; Fritsch et al. 1991; Fadel et al. 2001; Ichinose et al. 2002). For this analysis, the mean voltage neurogram was advanced 1.3 s to allow for the conduction delay between spinal cord and the recording site (Delius et al. 1972; Fagius & Wallin, 1980). This 1.3 s is an average delay time and the variation in the conduction delay in our subjects may have been at most 0.2 s (estimated from the variation in the subjects' height). Hence, only the bottom part of the bell-shaped MSNA burst (if a burst were indeed present) would have been influenced by any error in this value. Furthermore, individual differences in the conduction delay ought not to lead to differences in responses between control and LBNP, because there is little possibility of the conduction delay being greatly changed during LBNP. On this basis, we decided to apply a 1.3 s conduction delay for all subjects. MSNA was found to increase slightly during breath-holding alone. To compensate for this effect, the MSNA responses recorded during four entire periods of breath-holding alone were averaged and the value so obtained was subtracted from the MSNA levels recorded before, during and after each neck-chamber stimulus. Hence, the MSNA responses to the neck-chamber stimuli are expressed as the change from the MSNA level recorded during breath-holding alone. MSNA bursts occurring during 5-s periods of breath-holding in the absence of any neck-chamber stimulus were identified and we then calculated the burst frequency and the mean burst amplitude during each of these periods. The total MSNA activity was obtained as the product of burst frequency and mean burst amplitude for each 5-s period. The total amounts of MSNA activity in all the 5-s periods examined during control and LBNP situations (four records in each) were averaged separately for each situation and these two calculated values were taken as the base-line MSNA values.
The 13 s records of MAP and HR obtained during four trials of neck pressure or neck suction were averaged and integrated over 1-s periods to allow us to assess the time course of MAP and HR responses. The averaged MAP level during the 3 s prestimulus period and the HR at 1 s before the stimulus were taken as the base-line values. MAP and HR responses are expressed as the absolute difference from the base-line value. The main limitation of this analysis is that the exact value and timing for each beat is obscured. Therefore, we also analysed data on a beat-by-beat basis. The peak changes in MAP and HR were averaged for each type of stimulus and taken as the peak response to that stimulus for each subject. Furthermore, two values – the elapsed number of heart beats (EB) from the start of the neck stimulus to the heart beat at the peak of the MAP and HR responses, and the elapsed time (ET) from the beginning of the neck stimulus to the heart beat at the peak of the responses – were averaged for each type of stimulus for each variable. These averaged values were taken as our indices of the timing of the peak responses (on the basis of beat-domain or time-domain data, respectively) for each subject (Potts & Raven, 1995).
Statistical analysis
Data are presented as means ±s.e.m. For base-line values of HR, MAP and MSNA, for the peak responses in HR and MAP and for the indices of the timing of peak responses (EB and ET), comparisons between the control situation and LBNP were made using Student's paired t test. A repeated-measures analysis of variance was performed to compare the time course of HR, MAP and MSNA responses between the control situation and LBNP. When main effects were found to be significant, Fisher's post hoc test was used to assess differences from the value obtained at 3 s prior to the application of neck-chamber stimuli (i.e. 1 s after the start of breath-holding) and Student's paired t test was employed to assess group mean differences. Statistical significance was accepted at a P value of < 0.05.
Results
Table 1 shows the base-line values of HR, MAP and MSNA obtained in the control situation and during LBNP. During LBNP, MSNA total activity was greater than in control, but MAP and HR were not different.
Table 1. Base-line values of HR, MAP and MSNA in control and LBNP situations.
| Control | LBNP | |
|---|---|---|
| MSNA total activity (units (5 s)−1) | 1674 ± 230 | 3033 ± 436 * |
| MAP (mmHg) | 93 ± 3.8 | 92 ± 4.3 |
| HR (beats min−1) | 66 ± 2.2 | 68 ± 1.8 |
Values are means ±s.e.m. MSNA, muscle sympathetic nerve activity (n = 10); MAP, mean arterial pressure (n = 12); HR, heart rate (n = 12).
Significant difference from control, P < 0.05.
Evoked changes in MSNA
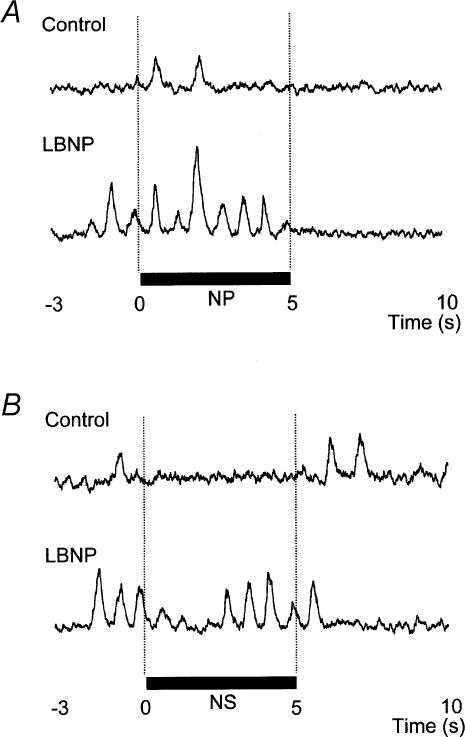
MSNA was increased (transiently) by NP and reduced by NS both in control and during LBNP. However, both the time course and the magnitude of the responses differed between these two situations. In original recordings of the MSNA responses shown by one subject to NP and NS in control and during LBNP (Fig. 2), NP induced a larger increment in MSNA during LBNP than in control. In addition, whereas in the control situation MSNA was depressed for almost the whole 5-s period of NS, during LBNP the depression of MSNA was transient, with a recovery beginning even during the NS stimulus itself.
Fig. 2. MSNA response in one subject to application of neck pressure (A) and neck suction (B) in control and LBNP situations.
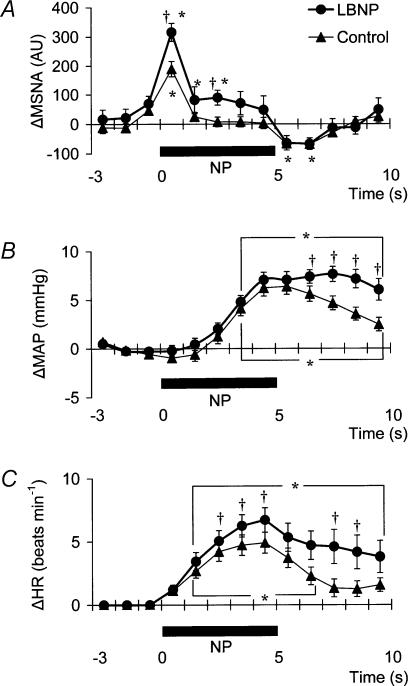
The time course of the MSNA response to NP in control and during LBNP is illustrated in Fig. 3A. In both control and LBNP situations, the increment in MSNA evoked by 5 s of NP was very transient, with a recovery occurring while the level of neck-chamber pressure was still elevated. Moreover, there was an undershoot after neck-chamber pressure had been returned to the ambient level. At both 1 s and 3 s into the response to NP, MSNA was significantly greater during LBNP than in control (Fig. 3A). Since the MSNA data were advanced by 1.3 s to allow for conduction delays, the timing of this enhanced MSNA response is consistent with a reasonable baroreflex latency and it therefore can be taken to represent a carotid-baroreflex-mediated effect.
Fig. 3. Averaged reflex alterations (n = 10–12 subjects) in MSNA (A), MAP (B) and HR (C) elicited by neck pressure in control and LBNP situations.
Significant difference from value obtained at 3 s prior to application of neck pressure, P < 0.05. †Significant difference from control, P < 0.05.
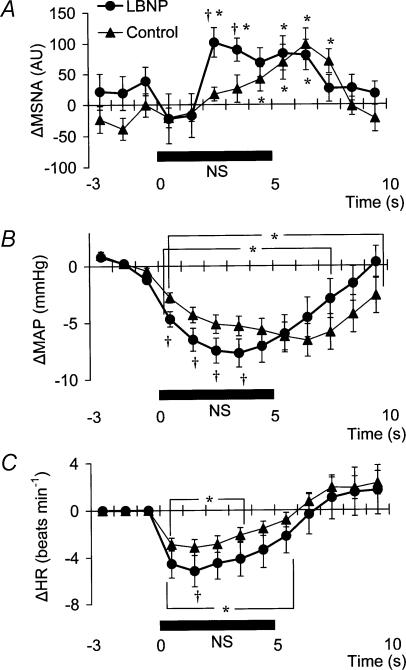
Examination of the time course of the MSNA responses to NS (Fig. 4A) reveals that both in control and during LBNP, MSNA tended to be reduced (relative to the level immediately before NS) for the first 2 s of the time for which NS was applied. Unfortunately, the mean group data in Fig. 4A do not display a significant suppression during the first 2 s or so of the period of NS (although a suppression is clear in Fig. 2). This is at least partly due to differences among individual tests in the timing of MSNA bursts relative to the start of the NS period. Subsequently, the time course of the changes in MSNA differed between control and LBNP, the pattern of change during LBNP being shifted to the left (i.e. occurring earlier). Indeed, MSNA was significantly higher during LBNP than in control at both 3 s and 4 s after the start of NS (Fig. 4A). This leftward shift in the time course of the MSNA elevation implies that the duration of any MSNA suppression induced by NS may be shortened during LBNP.
Fig. 4. Averaged reflex alterations (n = 10–12 subjects) in MSNA (A), MAP (B) and HR (C) elicited by neck suction in control and LBNP situations.
Significant difference from value obtained at 3 s prior to application of neck suction, P < 0.05. †Significant difference from control, P < 0.05.
Evoked changes in MAP and HR
MAP and HR were increased by NP and decreased by NS in both situations (control and LBNP). Figure 3B shows that at 7–10 s after the start of NP (at or after the time at which the peak MAP response occurred), MAP was significantly higher during LBNP than in control.
Figure 4B shows the time course of the MAP responses to NS. In the first 4 s of the NS period, the MAP decrease was greater during LBNP than in control. At 9 s after the beginning of NS, MAP had already recovered to its base-line level during LBNP, while in the control situation it was still significantly lower than base-line. In addition, the peak MAP response to NS was observed 2–4 s earlier during LBNP than in control.
Examination of the time course of the HR responses to NP (Fig. 3C) reveals that the peak HR response occurred within 5 s after the start of NP in both control and LBNP situations and that the HR response was greater during LBNP than in control.
Figure 4C, which shows the time course of the HR response to NS, reveals that the HR decrease was greater during LBNP than in control.
Table 2 shows the magnitude and the timing of the peak MAP and HR responses to each neck-chamber stimulus. The peak change in MAP induced by NP was significantly greater during LBNP than in control, whereas the peak change in MAP induced by NS was not significantly different between control and LBNP. In contrast, the peak changes in HR induced by both NP and NS were significantly greater during LBNP than in control. The EB and ET values for the MAP response to NP were not significantly different between control and LBNP. By contrast, the EB and ET values for the MAP response to NS were, respectively, significantly lower and significantly shorter in LBNP than in control. The EB and ET values for the HR responses to NP and NS were not different between control and LBNP.
Table 2. Magnitude and timing of peak MAP and HR responses to neck pressure and neck suction in control and LBNP situations.
| NP | NS | |
|---|---|---|
| ΔMAP (mmHg) | ||
| Control | 6.2 ± 0.8 | −8.0 ± 1.0 |
| LBNP | 9.1 ± 0.8* | −8.8 ± 1.2 |
| Timing of peak MAP response | ||
| Control | ||
| EB (beats) | 7.3 ± 0.4 | 7.4 ± 0.6 |
| ET (s) | 6.1 ± 0.3 | 6.7 ± 0.5 |
| LBNP | ||
| EB (beats) | 8.7 ± 0.7 | 4.7 ± 0.3 * |
| ET (s) | 7.2 ± 0.5 | 3.9 ± 0.3 * |
| ΔHR (beats min−1) | ||
| Control | 5.5 ± 0.9 | −3.7 ± 0.6 |
| LBNP | 7.5 ± 0.9* | −5.8 ± 1.3 * |
| Timing of peak HR response | ||
| Control | ||
| EB (beats) | 4.9 ± 0.4 | 2.1 ± 0.3 |
| ET (s) | 4.2 ± 0.3 | 1.5 ± 0.25 |
| LBNP | ||
| EB (beats) | 5.7 ± 0.3 | 2.4 ± 0.4 |
| ET (s) | 4.6 ± 0.2 | 1.7 ± 0.3 |
Values are means ±s.e.m.ΔMAP, change in mean arterial pressure from base-line value. ΔHR, change in heart rate from base-line value. EB, elapsed number of heart beats from the start of the neck stimulus to the heart beat at the peak of the response. ET, elapsed time from the start of the neck stimulus to the heart beat at the peak of the response.
Significant difference from control, P < 0.05.
Discussion
The major finding made in this investigation was that in humans, the dynamic MSNA, MAP and HR responses mediated by the carotid baroreflex (as evaluated by analysing the time course of the responses to neck-chamber stimuli) are modulated during non-hypotensive orthostatic stress in ways that would effectively limit orthostatic hypotension. In detail, both the MSNA augmentation and MAP increment induced by carotid compression were greater during LBNP than at rest. On the other hand, the recovery in MSNA that occurred during carotid stretch began earlier during LBNP, indicating a shortening of any period of MSNA suppression that may have been induced by carotid stretch. Moreover, although the peak MAP decrement induced by carotid stretch was not different between control and LBNP, it occurred earlier during LBNP and, moreover, MAP recovered to its initial level more quickly. Thus, the interesting conclusion is that the modulation of MSNA and MAP responses seems to differ between carotid baroreflex loading and unloading. By contrast, both the HR increment elicited by carotid compression and the HR decrement elicited by carotid stretch were greater during LBNP. Thus, our findings indicate that the carotid-baroreflex dynamic controls of MSNA, blood pressure and HR are all modulated under conditions of mild orthostatic stress, although an important proviso is that the nature of the modulation seems to be different between neural vascular control and neural HR control.
A modulation of carotid-baroreflex function at levels of LBNP insufficient to cause hypotension (less than −20 mmHg) has been reported before; indeed, such levels of LBNP have been presumed to unload cardiopulmonary baroreceptors with little or no effect on arterial baroreceptor afferent activity (Zoller et al. 1972; Johnson et al. 1974; Rowell, 1986). Some previous reports have concluded that the augmented response to carotid distension or compression seen during LBNP is evidence of the existence of a tonic inhibitory influence of the cardiopulmonary baroreflex over carotid-baroreflex-mediated cardiovascular adjustments (Ebert, 1983; Victor & Mark, 1985; Pawelczyk & Raven, 1989). However, it should be noted that according to Taylor et al. (1995), the small reduction in central blood volume induced by a mild level of LBNP is sufficient to reduce the dimensions of the aortic baroreceptive areas. If this is so, it is difficult to conclude that the dynamic response induced by a mild level of LBNP results solely from a deactivation (unloading) of cardiopulmonary baroreceptors. Nevertheless, on the basis of the present results we can at least say that in a situation in which mild orthostatic stress is associated with a simultaneous unloading of the cardiopulmonary and arterial baroreceptors, the dynamic alterations in MSNA induced by the carotid baroreflex will be modulated.
In both the control and LBNP situations, the peak MSNA response to NP was observed in the first second after the start of NP (Fig. 3A). During LBNP, in addition to the augmented initial MSNA response, MSNA was maintained at a level above base-line for an additional two seconds after the peak MSNA response, indicating that the MSNA augmentation evoked by NP was more sustainable during LBNP than in control. The greater initial MSNA response and sustained higher MSNA level after the peak MSNA response would be expected to lead, via greater vasoconstriction, to a greater MAP response and to a maintained higher MAP level in the last four seconds of LBNP (as compared with control) (Fig. 3A and B, Table 2). Any HR change induced by NP might alter cardiac output and thus affect the blood-pressure responses (Raven et al. 1997), so the increased HR response to NP seen during LBNP could have contributed to the augmentation of the blood-pressure response. However, the peak blood-pressure response was delayed relative to the peak HR response, particularly in the LBNP situation (Fig. 3B and C). Moreover, according to recent findings by Ogoh et al. (2002), the peak MAP response, normally observed 6–8 s after the start of a 5 s neck stimulus, is mainly due to reflex changes in total vascular conductance, with little contribution being made by changes in cardiac output. Furthermore, the present results directly demonstrate an augmentation of the MSNA response to carotid compression during LBNP (Fig. 3A). Taken together, the above evidence suggests that the augmented MAP response to NP seen during LBNP indicates an augmentation of carotid-baroreflex-induced vasoconstriction. Thus, the present results suggest that during mild orthostatic stress, there is an augmentation of the carotid-baroreflex regulation of blood pressure during NP (unloading) and that this occurs primarily via an augmentation of the baroreflex effect on sympathetic nerve activity.
Although the modulation of the blood-pressure response to arterial-baroreflex loading that occurs during central hypovolaemia has been investigated several times, the results are less conclusive than those demonstrating a modulation of the response to arterial-baroreflex unloading (Koike et al. 1975; Ebert, 1983; Victor & Mark, 1985; Pawelczyk & Raven, 1989). The present results show that mild orthostatic stress affected the time course (latency) of the MSNA and MAP responses evoked by carotid-baroreflex loading (Fig. 4A and B, Table 2). The initial, gradual reduction in MAP that occurred during NS was greater during LBNP than in control. It has been reported that the MAP reduction in this initial period may be due to an altered cardiac output secondary to an HR change (Raven et al. 1997; Ogoh et al. 2002). If so, the increased HR response to NS seen during LBNP may lead to a greater reduction in MAP in the initial period after the start of NS (Fig. 4C). In addition, a briefer MSNA suppression and earlier MSNA elevation during LBNP (Fig. 4A) would counteract any vasodilator response that might have led to a decrement in peak MAP at 6–8 s after the start of NS in the control situation. In this way, the peak MAP response could occur earlier in the LBNP situation. However, Ogoh et al. (2002) reported that a postural change from supine to upright-seated did not change the timing of the peak MAP response to NS (at 6–8 s after the start of NS). The explanation for this discrepancy is not clear, but the different ways used to induce mild orthostatic stress (LBNP versus a postural change) might be relevant. Possibly, the initial decline in systemic blood pressure visible in Fig. 4B may have rapidly caused a reflex reversal of any NS-induced suppression of MSNA via extra-carotid baroreceptors (i.e. aortic baroreceptors) and/or other carotid baroreceptors (Manica & Mark, 1983; Sanders et al. 1989; Kawada et al. 2000). In the control situation, NS tended to suppress MSNA in spite of the falling MAP (which might be expected to exert an MSNA-augmenting effect). During LBNP, any tendency for an NS-induced MSNA suppression lasted only for the first 2 s and this MSNA-suppressing effect was, more quickly than in control, diminished and indeed overcome by an MSNA-augmenting effect (which might have been strengthened by an unloading of cardiopulmonary baroreceptors). This result is consistent with our finding that the peak MSNA response to NP was increased during LBNP (Fig. 3A). Another point is that during LBNP, the greater initial decline in systemic blood pressure seen during NS may enhance the MSNA-augmenting effect. Be that as it may, our results suggest that during mild orthostatic stress, the carotid baroreflex is still capable of suppressing MSNA and reducing blood pressure in response to a hypertensive stimulus but that any MSNA suppression induced by the carotid baroreflex may be overcome sooner, resulting in a more rapid recovery in blood pressure.
It has been suggested that the afferent neural information emanating from the cardiopulmonary baroreceptors inhibits the arterial baroreflex at site(s) within the central nervous system (CNS) (Victor & Mark, 1985; Pawelczyk & Raven, 1989; Shi et al. 1993, 1997; Ogoh et al. 2002, 2003). Since the nucleus of the solitary tract (NTS) receives several viscerosensory afferents, including afferents from arterial and cardiopulmonary baroreceptors, any interaction between those types of baroreflexes may well take place within NTS (Spyer, 1990; Li et al. 1998; Potts et al. 2003). In particular, a modulation of the time course (latency) of the carotid-baroreflex-mediated vascular sympathetic responses during unloading of the cardiopulmonary baroreceptors, as found in this study, could be effected only within such a site of integration. However, the precise mechanisms remain unknown. Further examination of the integration of afferent information from arterial and cardiopulmonary baroreceptors within the CNS (especially at the NTS level) is needed before we can attempt a proper explanation of the modulation of the magnitude and latency of carotid-baroreflex-mediated vascular sympathetic responses.
In the present data, the peak HR response to both NP and NS was increased, with no change in the general time course of the response, during −15 mmHg LBNP (Figs 3C and 4C, Table 2). Our results are inconsistent with previous findings of an unchanged HR response to unilateral carotid-baroreflex unloading or loading during mild levels of LBNP (Takeshita et al. 1979; Victor & Mark, 1985). However, the augmented HR response we saw to both carotid-baroreflex unloading and loading during LBNP is in accord with the finding of Pawelczyk & Raven (1989) that the maximum gain of the carotid-baroreflex control of the R-R interval is increased by a mild level of LBNP (<20 mmHg). It has been shown that the HR responses to neck-chamber stimuli are predominantly mediated by carotid-baroreflex control of cardiac parasympathetic activity (Eckberg, 1980). The arterial baroreceptor projections to the NTS are relayed to the nucleus ambiguus and modulate the activity of preganglionic parasympathetic motor neurones (Spyer, 1990). So, if the afferent inputs from arterial and cardiopulmonary baroreceptors do indeed interact within the NTS, the carotid-baroreflex regulation of cardiac parasympathetic tone may be modulated by an action exerted within the NTS. Another point is that although HR did not change during mild LBNP in this study, cardiac parasympathetic tone has been reported to decrease during mild LBNP (Taylor et al. 1995). Therefore, the change in cardiac parasympathetic activity induced by an abrupt change in the afferent input from the carotid baroreceptors may differ between control and LBNP. Our results do at least suggest that under conditions involving mild orthostatic stress, the carotid-baroreflex control of HR, which is predominantly mediated by a regulation of cardiac parasympathetic tone, would be modulated alongside the regulation of vascular sympathetic activity.
Limitations
One of the limitations of the use of a neck chamber is that it is difficult to quantify transmission of the NP/NS stimuli to the carotid sinus region. Recently, Querry et al. (2001) measured the transmission of external pressure (neck chamber) to the carotid sinus using a balloon-tipped catheter and reported that 89% of the positive pressure and 82% of the negative pressure was transmitted to the carotid sinus region without a kinetic delay. Furthermore, they showed that neither low-intensity exercise (30% maximal oxygen uptake) nor a Valsalva manoeuvre significantly affected the transmission of such stimuli. Their results encouraged us to employ NP/NS stimuli to compare responses between control and a mild level of LBNP in this study.
In conclusion, the results obtained in this study show that the dynamic changes in MSNA, MAP and HR induced by the carotid baroreflex are modulated during non-hypotensive orthostatic stress. During LBNP (as compared with control) (a) the MAP and MSNA responses to NP (baroreceptor unloading) were greater; (b) any MSNA suppression induced by NS (baroreceptor loading) was overcome sooner by MSNA augmentation; (c) the peak decrement in MAP elicited by NS, although not different in amplitude, occurred earlier and recovered to its initial level more quickly after NS; and (d) the HR responses to NP and NS were both greater. Therefore, while the carotid-baroreflex controls of muscle sympathetic nerve activity, blood pressure and HR are all modulated during the imposition of LBNP, the nature of modulations seems to differ between neural vascular control and neural HR control. We suggest that in humans, the modulation of carotid-baroreflex function under conditions of mild orthostatic stress may be one of the mechanisms maintaining blood pressure and limiting orthostatic hypotension.
Acknowledgments
We should like sincerely to thank the volunteer subjects. We also greatly appreciate the help of Dr Robert Timms (English editing and critical comments). This study was supported by grants from Uehara Memorial Foundation, COE projects, and the Ministry of Education, Science, and Culture of Japan.
References
- Bath E, Lindblad LE, Wallin BG. Effects of dynamic and static neck suction on muscle nerve sympathetic activity, heart rate, and blood pressure in man. J Physiol. 1981;311:551–564. doi: 10.1113/jphysiol.1981.sp013604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevegård S, Castenfors J, Lindblad E, Tranesjö J. Blood pressure and heart rate regulation capacity of the carotid sinus during changes in blood volume distribution in man. Acta Physiol Scand. 1977;99:300–312. doi: 10.1111/j.1748-1716.1977.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- Ebert TJ. Carotid baroreceptor reflex regulation of forearm vascular resistance in man. J Physiol. 1983;337:655–664. doi: 10.1113/jphysiol.1983.sp014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg DL. Nonlinearities of the human carotid baroreceptor-cardiac reflex. Circ Res. 1980;47:208–216. doi: 10.1161/01.res.47.2.208. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford, UK: Clarendon Press; 1992. pp. 78–299. [Google Scholar]
- Eckberg DL, Wallin BG. Isometric exercise modifies autonomic baroreflex responses in humans. J Appl Physiol. 1987;63:2325–2330. doi: 10.1152/jappl.1987.63.6.2325. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fritsch JM, Smith ML, Simmons DTF, Eckberg DL. Differential baroreflex modulation of human vagal and sympathetic activity. Am J Physiol. 1991;260:R635–R641. doi: 10.1152/ajpregu.1991.260.3.R635. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during muscle metaboreflex activation in humans. J Physiol. 2002;544:939–948. doi: 10.1113/jphysiol.2002.024794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–524. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- Kawada T, Masashi I, Hiroshi T, Takayuki S, Toshiaki S, Teiji T, Yusuke Y, Masaru S, Kenji S. Counteraction of aortic baroreflex to carotid sinus baroreflex in a neck suction model. J Appl Physiol. 2000;89:1979–1084. doi: 10.1152/jappl.2000.89.5.1979. [DOI] [PubMed] [Google Scholar]
- Koike H, Mark AL, Heistad DD, Schmid PG. Influence of cardiopulmonary vagal afferent activation on carotid chemoreceptor and baroreceptor reflexes in the dog. Circ Res. 1975;37:422–429. doi: 10.1161/01.res.37.4.422. [DOI] [PubMed] [Google Scholar]
- Li J, Potts LT, Mitchell JH. Effects of barodenervation on c-Fos expression in the medulla induced by static muscle contraction in cats. Am J Physiol. 1998;274:H901–H908. doi: 10.1152/ajpheart.1998.274.3.H901. [DOI] [PubMed] [Google Scholar]
- Manica G, Mark AL. Arterial baroreflexes in humans. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, the Cardiovascular System. III. Bethesda, MD, USA: American Physiological Society; 1983. pp. 755–793. Peripheral Circulation and Organ Blood Flow, part 2, [Google Scholar]
- Mark AL, Manica G. Cardiopulmonary baroreflexes in humans. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, the Cardiovascular System. III. Bethesda, MD, USA: American Physiological Society; 1983. pp. 795–813. Peripheral Circulation and Organ Blood Flow, part 2, [Google Scholar]
- Nishiyasu T, Shi X, Mac GW, Nadel ER. Forearm vascular responses to baroreceptor unloading at the onset of dynamic exercise. J Appl Physiol. 1993;75:979–985. doi: 10.1152/jappl.1993.75.2.979. [DOI] [PubMed] [Google Scholar]
- Nishiyasu T, Tan N, Kondo N, Nishiyasu M, Ikegami H. Near-infrared monitoring of tissue oxygenation during application of lower body pressure at rest and during dynamical exercise in humans. Acta Physiol Scand. 1999;166:123–130. doi: 10.1046/j.1365-201x.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Wasmund WL, Raven PB. Haemodynamic changes during neck pressure and suction in seated and supine positions. J Physiol. 2002;540:707–716. doi: 10.1113/jphysiol.2001.013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Volianitis S, Nissen P, Wray DW, Secher NH, Raven PB. Carotid baroreflex responsiveness to head-up tilt-induced central hypovolaemia: effect of aerobic fitness. J Physiol. 2003;551:601–608. doi: 10.1113/jphysiol.2003.046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk JA, Raven PB. Reductions in central venous pressure improve carotid baroreflex responses in conscious men. Am J Physiol. 1989;257:H1389–H1395. doi: 10.1152/ajpheart.1989.257.5.H1389. [DOI] [PubMed] [Google Scholar]
- Potts JT, Paton JFR, Mitchel JH, Garry MG, Kline G, Anguelov PT, Lee SM. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signaling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience. 2003;119:201–214. doi: 10.1016/s0306-4522(02)00953-3. [DOI] [PubMed] [Google Scholar]
- Potts JT, Raven PB. Effect of dynamic exercise on human carotid-cardiac baroreflex latency. Am J Physiol. 1995;268:H1208–H1214. doi: 10.1152/ajpheart.1995.268.3.H1208. [DOI] [PubMed] [Google Scholar]
- Querry RG, Smith SA, Stromstand M, Ide K, Secher NH, Raven PB. Anatomical and functional characteristics of carotid sinus stimulation in humans. Am J Physiol. 2001;280:H2390–H2398. doi: 10.1152/ajpheart.2001.280.5.H2390. [DOI] [PubMed] [Google Scholar]
- Raven PB, Potts JT, Shi X. Baroreflex regulation of blood pressure during dynamic exercise. Exerc Sport Sci Rev. 1997;25:365–389. [PubMed] [Google Scholar]
- Rea EF, Eckberg DL. Carotid baroreceptor-muscle sympathetic relation in humans. Am J Physiol. 1987;253:R929–R934. doi: 10.1152/ajpregu.1987.253.6.R929. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation. New York: Oxford University Press; 1986. [Google Scholar]
- Saito M, Naito M, Mano T. Different responses in skin and muscle sympathetic nerve activity to static muscle contraction. J Appl Physiol. 1990;69:2085–2090. doi: 10.1152/jappl.1990.69.6.2085. [DOI] [PubMed] [Google Scholar]
- Sanders JS, Mark AL, Ferguson DW. Importance of aortic baroreflex in regulation of sympathetic responses during hypotension. Evidence from direct sympathetic nerve recordings in humans. Circulation. 1989;79:83–92. doi: 10.1161/01.cir.79.1.83. [DOI] [PubMed] [Google Scholar]
- Shi X, Foresman BH, Raven PB. Interaction of central venous pressure, intramuscular pressure, and carotid baroreflex function. Am J Physiol. 1997;272:H1359–H1363. doi: 10.1152/ajpheart.1997.272.3.H1359. [DOI] [PubMed] [Google Scholar]
- Shi X, Potts JT, Foresman BH, Raven PB. Carotid baroreflex responsiveness to lower body positive pressure-induced increase in central venous pressure. Am J Physiol. 1993;265:H918–H922. doi: 10.1152/ajpheart.1993.265.3.H918. [DOI] [PubMed] [Google Scholar]
- Sprenkle JM, Eckberg DL, Goble RL, Schelhorn JJ, Halliday HC. Device for rapid quantification of human carotid baroreceptor-cardiac reflex responses. J Appl Physiol. 1986;60:727–732. doi: 10.1152/jappl.1986.60.2.727. [DOI] [PubMed] [Google Scholar]
- Spyer KM. The central nervous organization of reflex circulatory control. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 168–188. [Google Scholar]
- Sundlof G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol. 1978;278:525–532. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita A, Mark AL, Eckberg DL, Abboud FM. Effect of central venous pressure on arterial baroreflex control of heart rate. Am J Physiol. 1979;236:H42–H47. doi: 10.1152/ajpheart.1979.236.1.H42. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. ‘Non-hypotensive’ hypovolemia reduces ascending aortic dimensions in humans. J Physiol. 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Victor RG, Mark AL. Interaction of cardiopulmonary and carotid baroreflex control of vascular resistance in humans. J Clin Invest. 1985;76:1592–1598. doi: 10.1172/JCI112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol. 1982;242:H185–H190. doi: 10.1152/ajpheart.1982.242.2.H185. [DOI] [PubMed] [Google Scholar]
- Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of lower pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest. 1972;51:2967–2297. doi: 10.1172/JCI107121. [DOI] [PMC free article] [PubMed] [Google Scholar]