Abstract
Environmental factors in early life are clearly established risk factors for cardiovascular disease in later life. Most studies have focused on nutritional programming and analysed basal cardiovascular parameters rather than responses. In the present study we have investigated whether prenatal stress has long-term effects on cardiovascular responses in adult offspring. Female pregnant Sprague-Dawley rats were subjected to stress three times daily from day 15 to day 21 of gestation. Litters from stressed and control females were cross-fostered at birth to control for mothering effects. When the offspring were 6 months old, blood pressure was measured in the conscious rats through implanted catheters at rest, during restraint stress and during recovery. Basal haemodynamic parameters were similar in the different groups but the pattern of cardiovascular responses during stress and recovery differed markedly between prenatally stressed (PS) and control animals. PS rats had higher and longer-lasting systolic arterial pressure elevations to restraint stress than control animals. They also showed elevated systolic and diastolic blood pressure values during the recovery phase. PS rats demonstrated a greater increase in blood pressure variability compared with control animals during exposure to restraint stress, and showed more prolonged heart rate responses to acute stress and delayed recovery than controls. There was no effect of prenatal stress on baroreflex regulation of heart rate. PS females showed a greater increase in systolic arterial pressure and blood pressure variability and delayed heart rate recovery following return to the home cage then did PS males. These findings demonstrate for the first time that prenatal stress can induce long-term, sex-related changes in the sensitivity of the cardiovascular system to subsequent stress.
There is currently great interest in fetal ‘programming’ of adult systemic and behavioural disorders. The most frequently investigated association is the relationship between low birthweight and cardiovascular disease in later life (Barker, 1995; Law & Shiell, 1996) which has given rise to the ‘fetal origins of adulthood disease’ hypothesis. Animal studies to test the programming hypothesis have used perturbations such as maternal undernutrition (Ozaki et al. 2001) or placental insufficiency (Alexander, 2003) and confirmed that low birthweight or thinness at birth strongly predicts subsequent cardiovascular and metabolic disease. However, it is unlikely that low birthweight per se causes these increased risks. Studies in nutritional (Jansson & Lambert, 1999; Khan et al. 2003) and hormonal programming show that body weight at birth does not necessarily predict such poor outcomes in later life. Rather, there may be a common factor that influences both intrauterine growth and the set point of certain adult physiological systems. It has been recently proposed that the link between the size at birth and altered hypothalamic—pituitary—adrenal axis function in later life is one such mechanism by which programming of adult cardiovascular and metabolic disease occurs (Dodic et al. 1999; Seckl, 2001).
In parallel to the low birthweight/cardiovascular risk literature there is also a considerable body of work which has focused on neuroendocrine and behavioural aspects of fetal programming. In these studies, a protocol frequently used to investigate long-term effects of early life environmental factors on the behavioural development of the offspring is that of prenatal stress (PS). In animal models, there is strong evidence that prenatal stress can reduce birthweight and also lead to developmental and behavioural disorders (Homer et al. 1990; Copper et al. 1996; Lordi et al. 1997; Schneider et al. 1999). However there are also some studies which describe higher birthweight in PS offspring (Szuran et al. 1991) and some which find no differences in birthweight between the groups (Weinstock et al. 1998a). Among the behavioural attributes of adult PS rats, increased emotionality, defensive behaviour and anxiety have been shown (Chapillon et al. 2002). These are associated with long-term changes in the adult offspring hypothalamic—pituitary—adrenal axis, in general programming a persistently hyperactive system and impaired negative feedback regulation (Barbazanges et al. 1996; Welberg & Seckl, 2001; Matthews, 2002). Offspring exposed to chronic stress during pregnancy have elevated basal and stress-induced plasma concentrations of adrenocorticotropin, corticosterone and a prolongation of stress-induced corticosterone and catecholamine responses (Weinstock et al. 1998a; Matthews, 2002).
Although it has been shown that intrauterine events can permanently affect the neuroendocrine and behavioural reactivity of the offspring, the extent to which prenatal stress may affect cardiovascular reactivity to stress in adulthood has not been studied. One small study showed that stressing the mother rat throughout pregnancy significantly increased systolic arterial pressure (SAP) in adult male offspring but not in females; there was no effect on diastolic pressure. Cardiovascular responses to stress were not analysed in this study (Holst et al. 2002).
There is growing evidence that the behavioural and neuroendocrine consequences of prenatal stress may differ significantly depending upon the sex of the offspring (McCormick et al. 1995; Szuran et al. 2000; Sternberg & Ridgway, 2003). However, sex-specific effects of prenatal stress on the cardiovascular system stress responsiveness have not been investigated.
Thus, the present experiments were designed to determine whether prenatal stress influences cardiovascular function in adult offspring and both female and male offspring from stressed dams were studied.
Methods
Experimental protocols were in accordance with European legislation on the use and care of laboratory animals (EEC N 086/608) and approved by the Ethical Committee of the University of Saratov, Russia.
Animals and housing conditions
Sprague-Dawley rats (250–300 g) were housed with free access to food and water, under a constant light—dark cycle (light on from 07.00 h to 20.00 h), with controlled temperature (22 ± 2°C). All female rats were handled for 2 weeks before starting the experimental protocol. For breeding, the virgin female rats were caged with mature males overnight during a whole oestrus cycle. A vaginal smear was examined the next morning. Day 0 of pregnancy was marked by the appearance of a copulation plug. Females who failed to become pregnant after developing a copulation plug were excluded from the study. Each pregnant female was separated and kept in an individual cage under standard conditions. Pregnant females were assigned randomly to prenatal stress (n = 8) or control (n = 8) groups. In the rat the duration of pregnancy is about 21 days and rat dams typically give birth to 12 pups per litter.
Prenatal stress protocol
Pregnant rats were stressed daily from day 15 to day 21 of pregnancy. They were exposed to a regimen of heat, light and restraint stress by placing the female into a Plexiglas restraint tube (20 cm × 6 cm × 8 cm) over which were poised two 100 W flood lights. This procedure produced no more than 60 lux direct exposure and an ambient temperature within the restraint tube of approximately 38°C. Animals were submitted to three 30 min stress sessions at 10.00, 13.00 and 18.00 h (Ward, 1984). Control mothers were left undisturbed for the duration of their pregnancies. This protocol was chosen because it has been used in many previous studies and shown to significantly affect neuroendocrine and behavioural stress reactivity in offspring (Insel et al. 1990; Henry et al. 1994; Barbazanges et al. 1996; Day et al. 1998; Williams et al. 1999; Szuran et al. 2000; Sternberg & Ridgway, 2003) It also produces permanent alterations in brain neurotransmitter systems which are involved in cardiovascular control (Insel et al. 1990; Cratty et al. 1995; Edwards et al. 1999).
All dams delivered at term (on 21st—22nd day of gestation). The offspring were left undisturbed on the day of birth. On the postnatal day 2 the offspring born to stressed mothers were cross-fostered to recently parturient control dams to control for any possible mothering effects. During this procedure, stressed and control mothers were removed form their cages for less than 1 min and then the pups born to stressed mothers were exchanged for the same age litter born to control non-stressed mothers. Likewise, litters of control dams were cross-fostered to control dams who gave birth within the same 48-h period to control for any possible effects of a single separation and pup exchange. Litters were adjusted to 10 pups with equal numbers of males and females to standardize conditions and ensure an adequate milk supply. The offspring were weaned at 21 days. Upon weaning, litters were mixed so that the same-sex rats exposed to the same conditions (but from different litters) were housed together in groups of three to five subjects per cage. Animals were tested at 6 months of age. Two animals of each sex from any one litter were studied at a given age, and these were randomly chosen to remove any litter effects.
Cardiovascular parameters in the adult offspring
Surgical procedures.
At 6 months animals of both groups (PS and control, n = 7 per group) were anaesthetized with an intraperitoneal injection of a mixture of ketamine hydrochloride (40 mg kg−1), xylazine (8 mg kg−1) and chlorpromazine hydrochloride (4 mg kg−1) and instrumented with femoral arterial and venous catheters (PE-50 heat-fused to PE-10) for the recording of arterial pressure and intravenous drug administration. Before surgery the rats were pretreated with the analgesic buprenorphine (0.1 mg kg−1s.c.) and received antibiotics gentamicine sulphate (0.2 ml kg−1s.c.). Supplemental anaesthesia was given when necessary as shown by marked changes in blood pressure, heart rate (HR), or respiration during surgery or in response to a pinch of the hind paw. The catheters were tunnelled subcutaneously and exteriorized at the back of the neck. They were flushed daily with heparinized saline, filled with heparin (1000 U ml−1), and plugged with a plastic obturator. The animals were given subcutaneous fluids and buprenorphine (0.1 mg kg−1s.c.) for postoperative analgesia before being placed in a recovery box. After immediate recovery from anaesthesia, animals were returned to their cages for 24 h.
Experimental protocols
All experiments were conducted between 09.00 and 11.00 h. On day 1 the rat was transferred to the testing room and the arterial catheter was connected to a sterile disposable blood pressure transducer (ADInstruments, Australia). The output of the transducer passed through an analog-to-digital converter, whose signal was then fed into a computerized data acquisition system PowerLab/4SP (ADInstruments). The system was set to sample the blood pressure waveform at 200 Hz. A 30-min acclimatization period allowed arterial pressure (AP) and HR to reach a steady state and baseline haemodynamic parameters were recorded for 30 min. Baroreflex function was assessed in freely moving rats by recording the maximum HR changes at the time of maximum increase and decrease in arterial blood pressure produced by intravenous bolus injections of phenylephrine (PE; 1, 2, 4 μg kg−1) or sodium nitroprusside (SNP; 5, 10, 20 μg kg−1; each in 100 μl of 0.9% saline followed by 200 μl of 0.9% saline) at increasing rates. The doses of nitroprusside and phenylephrine were chosen according to the results of previous experiments so that the linear range of the relationship between mean arterial pressure (MAP) and HR was covered, i.e. changes of basal MAP up to ± 35 mmHg. Approximately 60 s was required to increase and decrease MAP. Subsequent injections of nitroprusside or phenylephrine were only performed when MAP and HR had returned to baseline. Baroreceptors were always uploaded first (PE administration) before unloading (SNP administration) to minimize any potential effects of reflexly released humoral agents, such as vassopressin or angiotensin II, on baroreflex function. The data from each animal were fitted to a sigmoid logistic function as described by Kent et al. (1972), using a non-linear regression program (GraphPad Prism v. 3.00 for Windows, GraphPad Software, San Diego, CA, USA). This analysis evaluates the relationship between HR and MAP, where
This yielded the following baroreflex curve parameters: P1 is the range of HR (maximum response minus minimum response), P2 is the coefficient used to calculate the gain as a function of pressure, P3 is the pressure at the mid-range of the curve, and P4 is the minimum response of HR. The gain at any given MAP was calculated from the first derivative of the sigmoid function using the following equation:
Experimental protocol for effects of acute restraint stress
On day 2, the reactivity of PS and control rats was tested with restraint stress. Blood pressure was recorded continuously in the rat during 30 min of placement in a Plexiglas restraining tube (19 cm × 6 cm × 9 cm), and 30 min following return to the home cage. In order to assess the baroreflex control of heart rate during restraint stress, an intravenous bolus injection of PE (2 μg kg−1) and SNP (10 μg kg−1) was given 10 min after the onset of restraint stress. Only single doses of each drug were given to avoid overlapping stress-induced and drug-induced cardiovascular responses.
The values of diastolic (DAP) and systolic arterial pressure (SAP) and HR were measured and analysed with PowerLab, Chart™ software (PowerLab, ADInstruments). In addition, the standard deviation of mean arterial pressure (s.d.MAP) was calculated for every minute of the recording period. The mean s.d.MAP was used as a measure of the short-term variability of blood pressure, i.e. the extent of the variation in blood pressure within every minute (Parati et al. 1987; Van Vliet et al. 1996).
Animals were killed by sodium pentobarbitone overdose (i.p.).
Statistical analysis
Results are expressed as means ± standard error of the mean (s.e.m.) with P < 0.05 taken as the minimum level of significance. Baseline resting haemodynamic parameters were analysed using Student's unpaired t test. Haemodynamic data were analysed using mixed design repeated measures ANOVA with prenatal condition (prenatal stress and control) and sex (males, females) as between-subject factors and time as a within-subject factor with time being the repeated measure. Separate two-way repeated measures anovas were performed on cardiovascular responses during the restraint stress and during recovery from restraint in the home cage. anovas were performed on both absolute values and percentage changes in cardiovascular variables. A two-way analysis of variance was also used to evaluate differences in baroreflex function parameters (P1, P3 and P4), maximum HR, maximum gain of arterial baroreflex regulation of HR (Gmax), and the average rate of change in blood pressure during generation of baroreflex curves. Finally, where appropriate, post hoc comparisons were undertaken, using Duncan's multiple range method. All statistical analyses were performed using the Statistica 5.0 software package (StatSoft Inc., Tulsa, OK, USA).
Results
There were no significant differences in basal HR, SAP, DAP and s.d.MAP between control and PS rats. Neither were there any significant differences in these parameters between sexes in the different groups. Body weight was higher in adult PS males than in males of the control group (P < 0.05) (Table 1).
Table 1. Basal haemodynamic parameters and body weight in 6-month-old offspring of control and stressed dams.
| C male (n = 7) | PS male (n = 7) | C female (n = 7) | PS female (n = 7) | |
|---|---|---|---|---|
| Systolic pressure (mmHg) | 102 ± 4 | 104 ± 3 | 98 ± 4 | 100 ± 6 |
| Diastolic pressure (mmHg) | 80 ± 3 | 80 ± 3 | 73 ± 2 | 72 ± 5 |
| Mean arterial pressure (mmHg) | 85 ± 4 | 90 ± 2 | 84 ± 3 | 84 ± 5 |
| Standard deviation of blood pressure (mmHg) | 9 ± 0.9 | 9 ± 1 | 8 ± 0.7 | 10 ± 1 |
| Heart rate (beats min−1) | 344 ± 9 | 330 ± 10 | 345 ± 12 | 329 ± 9 |
| Body weight (g) | 258 ± 34 | 324 ± 25 | 201 ± 25 | 228 ± 27 |
C, control; PS, prenatally stressed. Values are given as means ±s.e.m.
P < 0.05, C versus PS of same sex.
Baroreflex control of HR
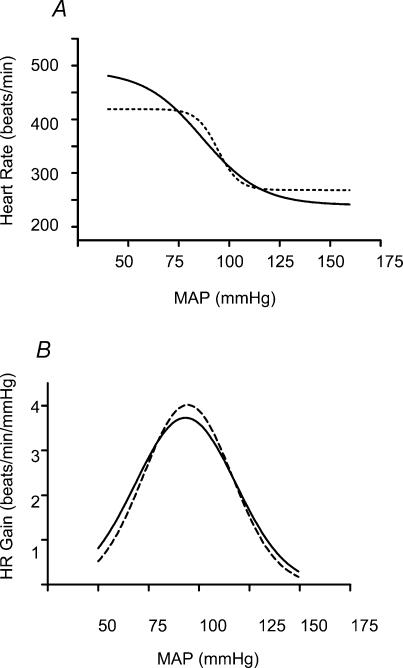
Sex differences in baroreflex parameters were not apparent in either group. Sexes were therefore combined in subsequent analysis of baroreflex gain. Mean sigmoid curves describing the baroreflex control of HR in control and PS rats are shown in Fig. 1A.Figure 1B illustrates the gain of the baroreflex curves as a function of arterial pressure. There were no differences in any baroreflex parameter or the maximum baroreflex gain between control and PS rats (Table 2).
Figure 1. Mean baroreflex curves describing baroreflex control of HR (A) and gain of baroreflex regulation of HR (B).
A, mean baroreflex curves describing reflex control of HR. Both control (n = 14, continuous line) and PS rats (n = 14, dashed line) showed a similar HR response to increase and decreases in MAP. B, mean curves illustrating the maximum gain (Gmax) of the arterial baroreflex regulation of HR for control (n = 14, continuous curve) and PS rats (n = 14, dashed line). Both groups exhibited a similar baroreflex gain throughout the range of MAP.
Table 2. Baroreflex parameters of logistic function for arterial baroreflex regulation of HR in control and PS rats.
| Parameter | C rats (n = 14) | PS rats (n = 14) |
|---|---|---|
| P1 (beats min−1) | 156 ± 26 | 121 ± 14 |
| P3 (mmHg) | 87 ± 3 | 95 ± 5 |
| HR max, beats min−1 | 475 ± 20 | 430 ± 15 |
| P4 (beats min−1) | 244 ± 23 | 275 ± 21 |
| Gmax | 3.7 ± 0.23 | 4.1 ± 0.45 |
C, control; PS prenatally stressed. Values are given as means ±s.e.m. P1, range of HR; P3, pressure at mid-range of the curve; P4, minimum response of HR; Gmax, maximum gain of arterial baroreflex regulation of HR.
Systolic arterial pressure responses to restraint stress and recovery in control and PS rats
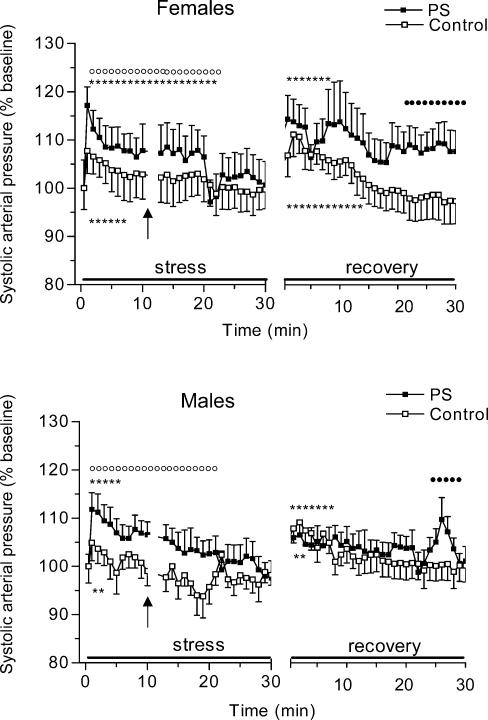
Upon exposure to restraint stress all rats exhibited a marked rise in SAP (F = 4.3, P < 0.01) (Fig. 2). Prenatal stress had a significant main effect (F = 10.2, P < 0.01) on the SAP response evoked by restraint stress whereas sex did not affect acute SAP stress responses. Separate anovas for each sex showed that PS females had a higher SAP increase than control females (F = 5.2, P < 0.05). Significant prenatal stress—time interactions (F = 2.2, P < 0.05) were also found for SAP, indicating that PS and control females changed in different ways over time in response to restraint stress. Thus, post hoc analysis revealed that PS females showed higher SAP values almost across the whole stress period than females of the control group (P < 0.01, minutes 1–20). There was also a significant effect of prenatal stress (F = 11.2, P < 0.01) on SAP responses to restraint stress in males: the magnitude of stress-induced SAP responses was significantly higher in PS males in comparison with control males (P < 0.01, minutes 1–22).
Figure 2. Changes in systolic arterial pressure (SAP) during 30 min period of restraint and for 30 min following return to the home cage in control and prenatally stressed female and male rats.
PS rats showed an increased peak of SAP responses following the restraint stress and an extended duration of SAP responses during both acute stress and recovery. There were sex-related differences in SAP responses upon return to the home cage with PS females showing a higher SAP increase than PS males. Data are expressed as percentage changes from baseline values to the response for both stress and recovery periods. Control offspring, □, n = 7; PS, prenatally stressed offspring, ▪, n = 7. Values are given as means ±s.e.m.P < 0.01versus basal values; P < 0.05,P < 0.01versus prenatally stressed group. Single doses of phenylephrine and sodium nitroprusside were administered at the time interval depicted by the arrow.
Return to the home cage evoked arousal in rats characterized by increased locomotion, rearing and grooming. This high activity was accompanied by a significant increase in SAP in all animals (F = 4.2, P < 0.01). SAP responses during recovery was modified by prenatal stress (F = 4.1, P < 0.05) and sex (F = 5.7, P < 0.05)). anovas of SAP in females alone revealed that the pattern of SAP responses during recovery differed significantly between control and PS groups (F = 4.7, P < 0.01). Thus, PS females showed higher SAP values than controls over the entire recovery period. These differences reached statistical significance during the last 10 min of the recovery (post hocP < 0.05, minutes 20–30). Similar anovas conducted on males showed a significant interaction of prenatal condition × time on SAP responses during the recovery, with PS males showing higher SAP values at the end of recovery (F = 1.6, P < 0.05, minutes 23–28). There were also significant sex-related differences in SAP in the group of PS animals, with PS females having higher SAP values than PS males at the beginning of the recovery phase (post hocP < 0.05, minutes 1–5).
Diastolic arterial pressure responses to restraint stress and recovery in control and PS rats
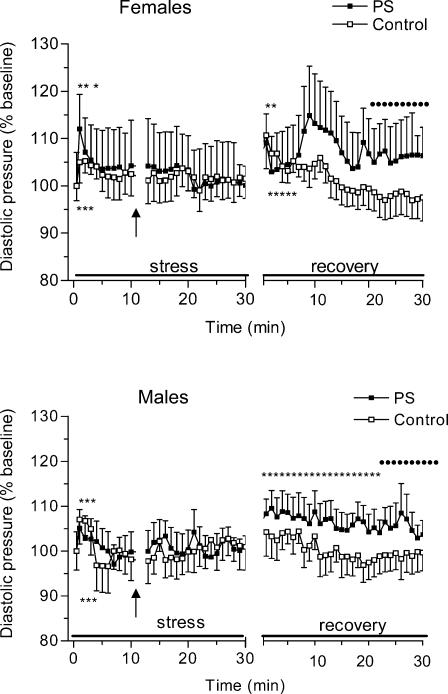
Figure 3 presents changes in DAP in PS and control rats during restraint stress and the recovery period. Restraint stress caused a significant but transient DAP increase in all animals (F = 4.1, P < 0.01, minutes 1–3). anovas yielded no significant effect of either prenatal stress or sex on acute DAP responses to restraint stress.
Figure 3. Changes in diastolic arterial pressure (DAP) during 30 min period of restraint and for 30 min following return to the home cage in control and prenatally stressed female and male rats.
Diastolic arterial pressure responses to the acute restraint did not differ between the groups but the return to rest was delayed in PS rats.
The return to rest was delayed in PS rats in comparison with the control group. anovas for recovery values of DAP showed a significant effect of prenatal condition (F = 4.1, P < 0.05), with PS animals showing higher DAP responses during recovery than their control counterparts (post hocP < 0.05, 20–30 min). Sex had no significant effect on DAP recovery values.
Arterial pressure variability during stress and recovery in control and PS rats
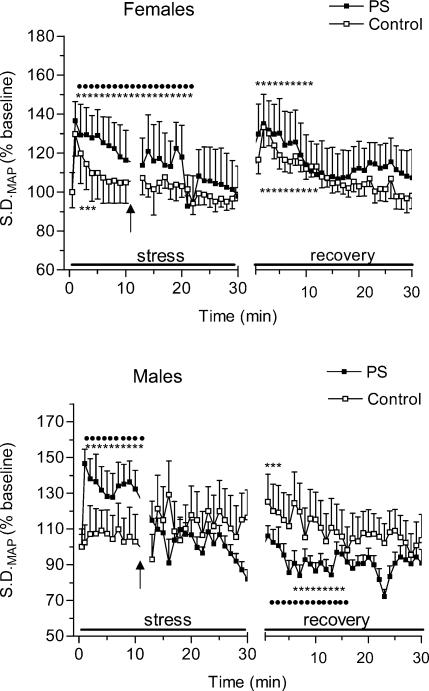
Figure 4 shows changes in standard deviation of blood pressure signal (s.d.MAP) during restraint stress and recovery periods in control and PS rats of both sexes. Following exposure to restraint stress blood pressure variability increased markedly in all animals (F = 3.2, P < 0.01). anovas revealed a significant effect of prenatal stress on blood pressure variability changes during acute restraint stress (F = 5.6, P < 0.02). Post hoc analysis indicated that this effect was due to the higher s.d.MAP values of PS animals compared to that of the control group (P < 0.05, 1−20 min). There were no sex-related differences in s.d.MAP changes during the acute restraint stress period.
Figure 4. Changes in blood pressure variability (s.d.MAP) during 30 min period of restraint and for 30 min following return to the home cage in control and prenatally stressed female and male rats.
Upon exposure to restraint PS rats had a greater increase in s.d.MAP compared with control animals. In the recovery period PS females showed higher s.d.MAP than control females and PS males.
Two-way anovas for recovery values of s.d.MAP revealed a significant effect of prenatal stress (F = 4.2, P < 0.05). Changes in s.d.MAP during the recovery phase were also modified by sex (F = 11, P < 0.001), with PS males showing reduced blood pressure variability compared with PS females over the entire recovery period. Separate anovas for males revealed a significant interaction of prenatal condition × time (F = 4.2, P < 0.05) and a marginal effect of prenatal condition (F = 4.1, P = 0.06). Post hoc analysis indicated that these effects were attributable to reduced s.d.MAP values during the recovery phase in PS males with respect to the control males (P < 0.05, 1−15 min). A similar ANOVA conducted on females alone revealed no effect for prenatal condition.
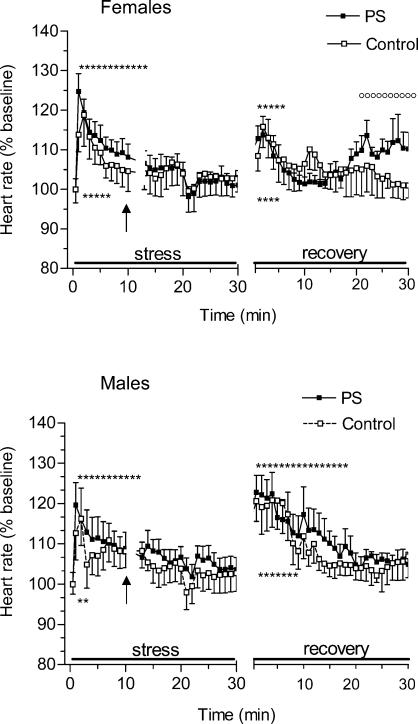
Heart rate responses to restraint stress and recovery in control and PS rats
The time course of changes in HR during restraint stress and recovery is shown in Fig. 5. Upon exposure to restraint all animals exhibited a marked increase in HR (F = 4.5, P < 0.01). This response was similar in magnitude in PS and control rats. anovas for HR stress values also revealed no differences between sexes. There were, however, differences in the time course of HR stress responses: in control rats stress resulted in a short-term transient increase in HR whereas in PS rats stress-induced tachycardia was sustained for 12 min of the stress period.
Figure 5. Changes in heart rate (HR) during 30-min period of restraint and for 30 min following return to the home cage in control and prenatally stressed female and male rats.
The magnitude of HR responses to restraint did not differ between PS and control rats. However, PS offspring rats showed more prolonged HR responses to restraint stress and delayed recovery in comparison with control rats. Male rats showed higher HR responses upon return to the home cages than the females regardless of prenatal condition.
Return to the home cage was accompanied by a significant increase in HR in all animals. Further analysis of the groups revealed a significant interaction of sex × time (F = 2.3, P < 0.01) with the males having a higher HR increase at the beginning of the recovery phase (post hoc P < 0.01, minutes 1–10) than the females regardless of prenatal condition. Separate anovas for each sex revealed a significant effect of the interaction prenatal condition × time (F = 2.1, P < 0.01) for females whereas this effect for the males failed to achieve significance. Inspection of this interaction showed that HR values were elevated in PS females relative to control females at the end of the recovery period (P < 0.01, minutes 20–30).
Arterial baroreflex regulation of HR during restraint stress in control and PS rats
As shown in Table 3 phenylephrine increased mean arterial pressure by approximately 25% in both PS and control animals. A subsequent HR response to baroreceptor uploading did not differ between the groups. The magnitudes of nitroprusside-induced changes in MAP and HR were also similar in PS and control animals. There were no sex-related differences in baroreflex regulation of HR during stress exposure.
Table 3. Effect of restraint stress on phenylephrine and sodium nitroprusside responses in adult offspring of control and stressed dams.
| C rats (n = 14) | PS rats (n = 14) | |
|---|---|---|
| Increase in BP following phenylephrine (%) | 26 ± 4 | 29 ± 5 |
| Fall in HR following phenylephrine (%) | 6 ± 1 | 8 ± 2 |
| Fall in BP following sodium nitroprusside (%) | 25 ± 2 | 30 ± 3 |
| Increase in HR following sodium nitroprusside (%) | 19 ± 2 | 23 ± 3 |
In order to assess the baroreflex control of heart rate during restraint stress, an intravenous bolus injection of phenylephrine (2 μg kg−1) and sodium nitroprusside (10 μg kg−1) was given 10 min after the onset of restraint stress. HR and BP responses to vasoactive drugs are expressed as percentage changes from 60-s averages measured immediately preceding drug administration. C, control; PS, prenatally stressed. Values are given as means ±s.e.m.
Discussion
This study has shown for the first time that exposure to stress early in development can have long-term effects on cardiovascular stress responses in adulthood. In general, the effects were more marked in females than males. Basal haemodynamic parameters were similar in PS and control animals.
Alterations in cardiovascular function of PS rats were evident when the animals were challenged by acute restraint stress. Prenatal stress also determined the duration of the cardiovascular responses during recovery and their return to baseline levels. The results demonstrated that PS rats had an increased peak of SAP responses following the restraint stress and an extended duration of SAP responses during both acute stress and recovery. Diastolic arterial pressure responses to the acute restraint did not differ between the groups but the return to rest was again delayed in PS rats. In addition, PS animals showed more prolonged HR responses to acute stress and delayed recovery in comparison with control rats. Interestingly, although PS animals demonstrated enhanced SAP stress responsiveness, arterial baroreflex control of HR during the restraint stress did not differ between PS and control rats. Finally, our results showed that PS rats had a greater increase in blood pressure variability compared with control animals during exposure to restraint stress. In the recovery period PS females showed higher blood pressure variability than control females and PS males.
At rest, we did not detect any differences in the baroreflex gain between the groups. The baroreflex function curve was shifted to the right in the rats of stressed dams, suggesting resetting of baroreflex regulation to a higher level in PS animals. However, this difference in our experiments was not large enough to be statistically significant. Although we attempted to standardize the baroreflex testing protocol, it is possible that a high degree of variability was introduced using this method and therefore prevented us from detecting any differences in baroreflex function between PS and control animals. We also cannot exclude the possibility that the sample size was not large enough to detect significant differences in baroreflex curve parameters and the baroreflex sensitivity between the groups. The methodology did not provide detailed information regarding the relative contribution of the sympathetic and parasympathetic systems to baroreflex heart rate responses. Further research will be necessary to address these points and gain a more detailed understanding of the underlying mechanisms.
The only previous study, to our knowledge, that has examined prenatal stress in relation to cardiovascular function is that of Holst et al. (2002). However, in that study the authors stressed the pregnant females throughout their pregnancy and only basal cardiovascular parameters were measured in the adult offspring. In contrast to our data they reported increased basal SAP in prenatally stressed males while no effects on females. These different findings may be due to the different prenatal stress protocol; also the timing of prenatal manipulations may affect cardiovascular function differently.
Two recent studies on fetal programming have also found an effect on cardiovascular responses but not basal haemodynamics. Maternal malnutrition (Tonkiss et al. 1998) and prenatal hypoxia (Peyronnet et al. 2002) were used as adverse prenatal factors. Resting cardiovascular parameters were unaltered in the adult offspring but blood pressure responses to acute stress were higher in the groups of animals prenatally exposed to the adverse environment. These data and our own results show that physiological differences between PS and control animals may only become apparent under testing conditions of adult stress (Weinstock, 1997). The similarities between the physiological consequences of malnutrition, prenatal hypoxia and maternal stress suggest that there may be common mechanisms that mediate the effects of the prenatal environment on the cardiovascular responses to stress in adulthood, possibly alterations of the hypothalamic—pituitary—adrenal axis.
Animal studies suggest that glucocorticoids can be important ‘fetal programming’ factors in prenatal stress protocols. In rodent models prenatal stress has been shown to result in up-regulation of glucocorticoid concentrations in both mother and fetus (Dauprat et al. 1984; Cadet et al. 1986) and excessive exposure of the fetus to maternal steroids may constitute at least part of the programming stimulus. (Dodic et al. 1999; Seckl, 2001). Indeed, several studies have demonstrated that prenatal glucocorticoids or stress can affect the development and maturation of specific organs related to blood pressure control and maintenance, such as heart, vasculature, kidney and brain (Seckl, 2001; Welberg & Seckl, 2001; Dodic et al. 2002). Administration of prenatal glucocorticoids have been shown to induce long-term alterations in brainstem, forebrain and hippocampal monoaminergic systems which are key mediators of integrated cardiovascular and autonomic responses to stress (Slotkin et al. 1992; Muneoka et al. 1997). In addition, glucocorticoids can directly regulate blood pressure, increasing sodium and calcium uptake by vascular smooth muscle (Kornel, 1993) and increasing vascular responsiveness to angiotensin II (Provencher et al. 1995) and noradrenaline (Walker & Williams, 1992). These glucocorticoid-induced changes in both central and effector sites of the cardiovascular control may be reflected in enhanced cardiovascular responses to acute stress seen here in PS rats.
A second major mechanism by which prenatal stress may have programmed subsequent enhanced blood pressure responsiveness to stress is by causing direct alteration in the sympathoadrenal system. There is growing evidence that central catecholaminergic areas in the brain can be permanently altered by fetal or neonatal exposures (Peters, 1982; Takahashi et al. 1992; Muneoka et al. 1997; Hayashi et al. 1998; Young, 2002). Catecholaminergic neurones constitute essential components of the central pathways mediating the principal homeostatic cardiovascular reflexes, and any alteration in their metabolism/function may have significant consequences for cardiovascular regulation in adulthood (Dampney, 1994). Other effects of prenatal manipulations on the catecholaminergic system also include alterations in sympathetic efferent pathways (Huff et al. 1991; Bian et al. 1992; Buchholz & Duckles, 2001; Peyronnet et al. 2002) and attenuation of adrenal medullarly function (McMillen et al. 2001).
We found here that PS rats demonstrated a greater increase in systolic arterial pressure and blood pressure variability during restraint stress than control animals. Given that restraint stress has been reported to increase blood pressure largely due to sympathoadrenal system activation (Chen & Herbert, 1995) and the stress-induced increase in blood pressure variability is mediated by sympathetic excitation (Blanc et al. 1991; Gaudet et.al. 1996), it is possible that the increased SAP and BP variability seen in PS rats under stressful conditions is due to higher activation of the sympathetic nervous system. Indeed, Weinstock et al. have reported that prenatal stress induces long-term changes in the sensitivity of the sympathoadrenal system to stress. They showed higher plasma noradrenaline and dihydroxyphenylglycol levels in PS than control offspring following footshock stress (Weinstock et al. 1998a). It seems likely that the sensitivity and/or density of vascular adrenergic receptors rather than cardiac adrenergic receptors is altered in PS rats, since the magnitude of HR stress responses did not differ between PS and control animals. This hypothesis is supported by a recent study (O'Regan et al. 2003). These authors reported enhanced blood pressure responsiveness to stress and increased vascular sensitivity, both in vivo and in vitro, to sympathomimetic and vasoactive agents in adult offspring who had been prenatally exposed to dexamethasone.
The present experiments also demonstrate the sex-specific effects of prenatal stress on the cardiovascular responsiveness to stress in adult offspring. PS females showed delayed HR recovery compared with PS males and a greater increase in SAP and blood pressure variability following return to the home cage. Recent studies on nutritional programming (Ozaki et al. 2001; Khan et al. 2003) and placental insufficiency (Jansson & Lambert, 1999) have also shown that alterations in intrauterine environment can affect cardiovascular function in female and male offspring differently. Sex differences in cardiovascular responses to stress have been demonstrated in both human and animal studies, with greater cardiovascular and plasma catecholamine responses to environmental challenges generally observed in males (Zukowska-Grojec et al. 1991). However, some studies have found greater stress-induced increases in catecholamines in female than in male rats (Livezey et al. 1985; Weinstock et al. 1998b) and higher cardiac stress reactivity in women compared with men (Girdler et al. 1990).
The enhanced cardiovascular sensitivity of PS females resembles other sex-specific effects of prenatal stress. Indeed, sex differences in the neuroendocrine effects of PS are well known in the literature, with the female offspring generally showing larger neuroendocrine and behavioural alterations than males (Kinsley et al. 1989; Rohde et al. 1989; Weinstock et al. 1992; McCormick et al. 1995). There is, however, considerable variability in outcome following PS in male offspring. Thus, in some studies PS males had either attenuated (Reznikov & Nosenko, 1995) or unaffected neuroendocrine responses (Szuran et al. 2000) whereas other studies reported a more marked effect on emotional locomotor activity (Nishio et al. 2001) and spatial learning (Szuran et al. 2000) in male than in female offspring.
The mechanisms underlying the sex-specific effects of prenatal stress on cardiovascular function are unknown but some possible explanations are plausible. The higher and prolonged cardiovascular responses during recovery seen here in PS females may indicate alterations in the mechanisms responsible for returning the system to its basal state, such as prejunctional α2-adrenoreceptors, opioid receptors and endothelium-dependent relaxation. These have now been shown to be involved in fetal programming of vascular disorders (Buchholz & Duckles, 2001; Ghosh et al. 2001).
In the present study we did not weigh the neonates because we have experienced some cases of infanticide in this strain of rats even in control litters. However, our results demonstrated that adult PS males had higher body weight than control males. In contrast, Keshet & Weinstock (1995) showed that the body weight of PS offspring at the age of 60 days was somewhat lower than that of age-matched control animals. This discrepancy may be due to the different prenatal stress protocols used and the fact that the PS male rats in our study were older than those in the previous one.
A possible explanation for a greater increase in the body weight of PS males is that prenatal stress may results in alterations in sex steroid levels, thereby influencing fat metabolism and body composition of adult PS males. Indeed, permanent behavioural feminization and demasculinization have been reported in prenatally stressed male rats. These behavioural abnormalities are associated with reduced prenatal and postnatal testosterone levels in prenatally stressed males (Ward & Weisz, 1980; Anderson et al. 1986). Androgens are important hormonal regulators of protein and lipid homeostasis and their deficiency has been shown to promote visceral fat accumulation and obesity (Tchernof et al. 1996; Jensen, 2000). Since the effect of PS on body weight was evident only in PS male offspring our findings might point to decreased levels of testosterone in PS males and higher sensitivity of PS male offspring to the metabolic effects of prenatal stress.
In summary, the present data clearly show that prenatal stress has long-term effects on the cardiovascular function of the offspring. Prenatally stressed rats coped less effectively with acute restraint stress, as demonstrated by enhanced blood pressure and blood pressure variability responses to stress and slow adaptation of stress-induced tachycardia and hypertension in the post-stress period. In general, the female offspring demonstrated greater and more prolonged cardiovascular responses during recovery than males. These findings suggest that PS rats are at high risk for ultimate development of hypertension or other stress-related cardiovascular disorders if exposed to a stressful environment. Further clarification of changes in the central or local control of the cardiovascular system is required to understand the mediating mechanisms.
Such a pattern of haemodymanic responses to stress would have significant consequences for adult cardiovascular health, if also apparent in humans. Thus, in clinical studies, delayed blood pressure (Floras & Senn, 1991) and heart rate (Cole et al. 1999; Watanabe et al. 2001) recovery to basal levels after the stress of exercise has been shown to correlate highly with increased cardiovascular morbidity and mortality. Furthermore, augmented blood pressure variability is found in patients with hypertension and it may play a pathophysiololgical role in the development of end-organ damage (Mancia & Parati, 2003).
Acknowledgments
This study was supported by a Royal Society/NATO Fellowship and the Russian Ministry of Education (grant PD 0.2-1.4-261). We thank Dr O. Khokhlova for her advice and support.
References
- Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Fleming DE, Rhees RW, Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986;370:1–10. doi: 10.1016/0006-8993(86)91098-x. [DOI] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian XP, Seidler FJ, Slotkin TA. Promotional role for glucocorticoids in the development of intracellular signalling: enhanced cardiac and renal adenylate cyclase reactivity to beta-adrenergic and non-adrenergic stimuli after low-dose fetal dexamethasone exposure. J Dev Physiol. 1992;17:289–297. [PubMed] [Google Scholar]
- Blanc J, Grichois ML, Elghozi JL. Effects of clonidine on blood pressure and heart rate responses to an emotional stress in the rat: a spectral study. Clin Exp Pharmacol Physiol. 1991;18:711–717. doi: 10.1111/j.1440-1681.1991.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Buchholz J, Duckles SP. Chronic hypoxia alters prejunctional alpha2-receptor function in vascular adrenergic nerves of adult and fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281:R926–R934. doi: 10.1152/ajpregu.2001.281.3.R926. [DOI] [PubMed] [Google Scholar]
- Cadet R, Pradier P, Dalle M, Delost P. Effects of prenatal maternal stress on the pituitary adrenocortical reactivity in guinea-pig pups. J Dev Physiol. 1986;8:467–475. [PubMed] [Google Scholar]
- Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: a review. Dev Psychobiol. 2002;41:373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FG, Snader CE, Lauer MS. Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;342:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins BA, Johnson F, Jones P, Meier AM. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks' gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dauprat P, Monin G, Dalle M, Delost P. The effects of psychosomatic stress at the end of pregnancy on maternal and fetal plasma cortisol levels and liver glycogen in guinea-pigs. Reprod Nutr Dev. 1984;24:45–51. doi: 10.1051/rnd:19840105. [DOI] [PubMed] [Google Scholar]
- Day JC, Koehl M, Deroche V, Le Moal M, Maccari S. Prenatal stress enhances stress- and corticotropin-releasing factor-induced stimulation of hippocampal acetylcholine release in adult rats. J Neurosci. 1998;18:1886–1892. doi: 10.1523/JNEUROSCI.18-05-01886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodic M, Moritz K, Koukoulas I, Wintour EM. Programmed hypertension: kidney, brain or both. Trends Endocrinol Metab. 2002;13:403–408. doi: 10.1016/s1043-2760(02)00693-8. [DOI] [PubMed] [Google Scholar]
- Dodic M, Peers A, Coghlan JP, Wintour M. Can excess glucocorticoid, predispose to cardiovascular and metabolic disease in middle age. Trends Endocrinol Metab. 1999;10:86–91. doi: 10.1016/s1043-2760(98)00125-8. [DOI] [PubMed] [Google Scholar]
- Edwards E, King JA, Fray JC. Increased basal activity of the HPA axis and renin-angiotensin system in congenital learned helpless rats exposed to stress early in development. Int J Dev Neurosci. 1999;17:805–812. doi: 10.1016/s0736-5748(99)00062-3. [DOI] [PubMed] [Google Scholar]
- Floras JS, Senn BL. Absence of post exercise hypotension and sympathoinhibition in normal subject: additional evidence for increased sympathetic outflow in borderline hypertension. Can J Cardiol. 1991;7:253–258. [PubMed] [Google Scholar]
- Gaudet E, Blanc J, Elghozi JL. Role of angiotensin II and catecholamines in blood pressure variability responses to stress in SHR. Am J Physiol. 1996;270:R1265–R1272. doi: 10.1152/ajpregu.1996.270.6.R1265. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA, Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001;533:815–822. doi: 10.1111/j.1469-7793.2001.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Turner JR, Sherwood A, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosom Med. 1990;52:571–591. doi: 10.1097/00006842-199009000-00009. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Holst S, Uvnas K, Petersson M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Auton Neurosci. 2002;99:85–90. doi: 10.1016/s1566-0702(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Homer CJ, Beresford SA, James SA, Siegel E, Wilcox S. Work-related physical exertion and risk of preterm, low birthweight delivery. Paediatr Perinat Epidemiol. 1990;4:161–174. doi: 10.1111/j.1365-3016.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Huff RA, Seidler FJ, Slotkin TA. Glucocorticoids regulate the ontogenetic transition of adrenergic receptor subtypes in rat liver. Life Sci. 1991;48:1059–1065. doi: 10.1016/0024-3205(91)90507-8. [DOI] [PubMed] [Google Scholar]
- Insel TR, Kinsley CH, Mann PE, Bridges RS. Prenatal stress has long-term effects on brain opiate receptors. Brain Res. 1990;511:93–97. doi: 10.1016/0006-8993(90)90228-4. [DOI] [PubMed] [Google Scholar]
- Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. J Hypertens. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Androgen effect on body composition and fat metabolism. Mayo Clin Proc. 2000;75(Suppl.):S65–S68. [PubMed] [Google Scholar]
- Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology. 1972;57:295–310. doi: 10.1159/000169528. [DOI] [PubMed] [Google Scholar]
- Keshet GI, Weinstock M. Maternal naltrexone prevents morphological and behavioral alterations induced in rats by prenatal stress. Pharmacol Biochem Behav. 1995;50:413–419. doi: 10.1016/0091-3057(94)00289-u. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Mann PE, Bridges RS. Alterations in stress-induced prolactin release in adult female and male rats exposed to stress, in utero. Physiol Behav. 1989;45:1073–1076. doi: 10.1016/0031-9384(89)90240-0. [DOI] [PubMed] [Google Scholar]
- Kornel L. The role of vascular steroid receptors in the control of vascular contractility and peripheral vascular resistance. J Steroid Biochem Mol Biol. 1993;45:195–203. doi: 10.1016/0960-0760(93)90142-j. [DOI] [PubMed] [Google Scholar]
- Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- Livezey GT, Miller JM, Vogel WH. Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett. 1985;62:51–56. doi: 10.1016/0304-3940(85)90283-6. [DOI] [PubMed] [Google Scholar]
- Lordi B, Protais P, Mellier D, Caston J. Acute stress in pregnant rats: effects on growth rate, learning, and memory capabilities of the offspring. Physiol Behav. 1997;62:1087–1092. doi: 10.1016/s0031-9384(97)00261-8. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens. 2003;6(Suppl.):S17–S23. doi: 10.1097/00004872-200307006-00004. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, Owens JA, Robinson JS, Edwards LJ. Fetal growth restriction: adaptations and consequences. Reproduction. 2001;122:195–204. doi: 10.1530/rep.0.1220195. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol. 1997;273:R1669–R1675. doi: 10.1152/ajpregu.1997.273.5.R1669. [DOI] [PubMed] [Google Scholar]
- Nishio H, Kasuga S, Ushijima M, Harada Y. Prenatal stress and postnatal development of neonatal rats – sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int J Dev Neurosci. 2001;19:37–45. doi: 10.1016/s0736-5748(00)00070-8. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Brooker G, Mullins JJ, Seckl JR, Holmes MC. Sympathetic responsivity: the origin of programmed hypertension. J Human Hypertension. 2003;17:S1. [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- Peters DA. Prenatal stress: effects on brain biogenic amine and plasma corticosterone levels. Pharmacol Biochem Behav. 1982;17:721–725. doi: 10.1016/0091-3057(82)90353-7. [DOI] [PubMed] [Google Scholar]
- Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP, Lagercrantz H. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch. 2002;443:858–865. doi: 10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- Provencher PH, Saltis J, Funder JW. Glucocorticoids but not mineralocorticoids modulate endothelin-1 and angiotensin II binding in SHR vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1995;52:219–225. doi: 10.1016/0960-0760(94)00168-l. [DOI] [PubMed] [Google Scholar]
- Reznikov AG, Nosenko ND. Catecholamines in steroid-dependent brain development. J Steroid Biochem Mol Biol. 1995;53:349–353. doi: 10.1016/0960-0760(95)00073-9. [DOI] [PubMed] [Google Scholar]
- Rohde W, Ohkawa T, Gotz F, Stahl F, Tonjes R, Takeshita S, Arakawa S, Kambegawa A, Arai K, Okinaga S. Sex-specific effects on the fetal neuroendocrine system during acute stress in late pregnancy of rat and the influence of a simultaneous treatment by tyrosine. Exp Clin Endocrinol. 1989;94:23–42. doi: 10.1055/s-0029-1210877. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoid programming of the fetus; adult phenotypes and molecular mechanisms. Mol Cell Endocrinol. 2001;185:61–71. doi: 10.1016/s0303-7207(01)00633-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, McCook EC, Tayyeb MI, Eylers JP, Seidler FJ. Glucocorticoids and the development of neuronal function: effects of prenatal dexamethasone exposure on central noradrenergic activity. Biol Neonate. 1992;61:326–336. doi: 10.1159/000243761. [DOI] [PubMed] [Google Scholar]
- Sternberg WF, Ridgway CG. Effects of gestational stress and neonatal handling on pain, analgesia, and stress behavior of adult mice. Physiol Behav. 2003;78:375–383. doi: 10.1016/s0031-9384(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Szuran T, Zimmerman E, Pliska V, Pfister HP, Welzl H. Prenatal stress effects on exploratory activity and stress-induced analgesia in rats. Dev Psychobiol. 1991;24:361–372. doi: 10.1002/dev.420240505. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res. 1992;574:131–137. doi: 10.1016/0006-8993(92)90809-n. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Labrie F, Belanger A, Despres JP. Obesity and metabolic complications: conribution of dehydroepiandrosterone and other steroid hormones. J Endocrinol. 1996;150(Suppl.):S155–S164. [PubMed] [Google Scholar]
- Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension. 1998;32:108–114. doi: 10.1161/01.hyp.32.1.108. [DOI] [PubMed] [Google Scholar]
- Van Vliet BN, Hu L, Scott T, Chafe L, Montani JP. Cardiac hypertrophy and telemetered blood pressure 6 wk after baroreceptor denervation in normotensive rats. Am J Physiol. 1996;271:R1759–R1769. doi: 10.1152/ajpregu.1996.271.6.R1759. [DOI] [PubMed] [Google Scholar]
- Walker BR, Williams BC. Corticosteroids and vascular tone: mapping the messenger maze. Clin Sci (Lond) 1992;82:597–605. doi: 10.1042/cs0820597. [DOI] [PubMed] [Google Scholar]
- Ward IL. The prenatal stress syndrome: current status. Psychoneuroendocrinology. 1984;9:3–11. doi: 10.1016/0306-4530(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Ward I, Weisz J. Maternal stress alters plasma testosterone in fetal males. Science. 1980;207:328–329. doi: 10.1126/science.7188648. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systoloc dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis. Neurosci Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav. 1998a;64:439–444. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 1998b;16:289–295. doi: 10.1016/s0736-5748(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Davis HN, McCrea AE, Long SJ, Hennessy MB. Changes in the hormonal concentrations of pregnant rats and their fetuses following multiple exposures to a stressor during the third trimester. Neurotoxicol Teratol. 1999;21:403–414. doi: 10.1016/s0892-0362(98)00060-9. [DOI] [PubMed] [Google Scholar]
- Young JB. Programming of sympathoadrenal function. Trends Endocrinol Metab. 2002;13:381–385. doi: 10.1016/s1043-2760(02)00661-6. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Shen GH, Capraro PA, Vaz CA. Cardiovascular, neuropeptide Y, and adrenergic responses in stress are sexually differentiated. Physiol Behav. 1991;49:771–777. doi: 10.1016/0031-9384(91)90317-h. [DOI] [PubMed] [Google Scholar]