Abstract
During maximal exercise in humans, fatigue is preceded by reductions in systemic and skeletal muscle blood flow, O2 delivery and uptake. Here, we examined whether the uptake of O2 and substrates by the human brain is compromised and whether the fall in stroke volume of the heart underlying the decline in systemic O2 delivery is related to declining venous return. We measured brain and central haemodynamics and oxygenation in healthy males (n = 13 in 2 studies) performing intense cycling exercise (360 ± 10 W; mean ±s.e.m.) to exhaustion starting with either high (H) or normal (control, C) body temperature. Time to exhaustion was shorter in H than in C (5.8 ± 0.2 versus 7.5 ± 0.4 min, P < 0.05), despite heart rate reaching similar maximal values. During the first 90 s of both trials, frontal cortex tissue oxygenation and the arterial–internal jugular venous differences (a-v diff) for O2 and glucose did not change, whereas middle cerebral artery mean flow velocity (MCA Vmean) and cardiac output increased by ∼22 and ∼115%, respectively. Thereafter, brain extraction of O2, glucose and lactate increased by ∼45, ∼55 and ∼95%, respectively, while frontal cortex tissue oxygenation, MCA Vmean and cardiac output declined ∼40, ∼15 and ∼10%, respectively. At exhaustion in both trials, systemic 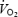 declined in parallel with a similar fall in stroke volume and central venous pressure; yet the brain uptake of O2, glucose and lactate increased. In conclusion, the reduction in stroke volume, which underlies the fall in systemic O2 delivery and uptake before exhaustion, is partly related to reductions in venous return to the heart. Furthermore, fatigue during maximal exercise, with or without heat stress, in healthy humans is associated with an enhanced rather than impaired brain uptake of O2 and substrates.
declined in parallel with a similar fall in stroke volume and central venous pressure; yet the brain uptake of O2, glucose and lactate increased. In conclusion, the reduction in stroke volume, which underlies the fall in systemic O2 delivery and uptake before exhaustion, is partly related to reductions in venous return to the heart. Furthermore, fatigue during maximal exercise, with or without heat stress, in healthy humans is associated with an enhanced rather than impaired brain uptake of O2 and substrates.
During maximal exercise, the human brain integrates input from chemo-, mechano- and barosensitive sensory endings in many regions in the body including skeletal muscle, the heart and the vasculature to generate the neuronal signals that help divert the majority of systemic O2 delivery to contracting skeletal muscles at an enhanced perfusion pressure. This paramount neural process requires appropriate provision of O2 and substrates. Notably, the arterial–internal jugular venous concentration difference (a-v diff) for O2 increases 9–13% during incremental exercise to exhaustion, whereas the a-v diff for glucose remains unchanged or increases and the a-v diff for lactate increases drastically (Ide et al. 2000; Dalsgaard et al. 2002, 2004). Moreover, results from both animal and human studies indicate that regional and global brain blood flow increases 20–30% on the transition from rest to moderate exercise, with no further enlargement when exercise intensity increases to elicit maximal O2 uptake (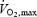 ) (Herlund et al. 1962; Thomas et al. 1989; Huang et al. 1991; Linkis et al. 1995; Hellström et al. 1996; Ide et al. 1998; Delp et al. 2001). Although there are no conclusive haemodynamic data from the human brain, the increased blood flow observed in most studies, together with the increased a-v diff for O2, glucose and lactate (Dalsgaard et al. 2004), suggest that the metabolic activity of the brain as a whole is enhanced during intense exercise. An unresolved question is whether the uptake of O2 and substrates by the human brain is compromised during exhausting maximal exercise, which may bring about a drop in systemic O2 delivery.
) (Herlund et al. 1962; Thomas et al. 1989; Huang et al. 1991; Linkis et al. 1995; Hellström et al. 1996; Ide et al. 1998; Delp et al. 2001). Although there are no conclusive haemodynamic data from the human brain, the increased blood flow observed in most studies, together with the increased a-v diff for O2, glucose and lactate (Dalsgaard et al. 2004), suggest that the metabolic activity of the brain as a whole is enhanced during intense exercise. An unresolved question is whether the uptake of O2 and substrates by the human brain is compromised during exhausting maximal exercise, which may bring about a drop in systemic O2 delivery.
During maximal exercise, exhaustion, with and without heat stress, is tightly linked to reductions in systemic and locomotive skeletal muscle blood flow, O2 delivery and uptake (González-Alonso & Calbet, 2003). Skeletal muscle is clearly the main vascular bed accounting for the reductions in peripheral blood flow and 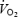 . Notwithstanding, blood flow and O2 delivery to the brain may also become attenuated with a falling cardiac output, as middle cerebral artery mean flow velocity (MCA Vmean) is diminished when cardiac output is suppressed by either cardioselective β1-adrenergic blockade (Ide et al. 1998) or severe heat stress (Nybo & Nielsen, 2001). A more rapid alteration in brain metabolism would then be anticipated with heat stress compared to normal conditions, as heat stress more quickly pushes the cardiovascular system to its regulatory limit, when cardiac output and systemic O2 delivery can no longer be maintained (González-Alonso & Calbet, 2003).
. Notwithstanding, blood flow and O2 delivery to the brain may also become attenuated with a falling cardiac output, as middle cerebral artery mean flow velocity (MCA Vmean) is diminished when cardiac output is suppressed by either cardioselective β1-adrenergic blockade (Ide et al. 1998) or severe heat stress (Nybo & Nielsen, 2001). A more rapid alteration in brain metabolism would then be anticipated with heat stress compared to normal conditions, as heat stress more quickly pushes the cardiovascular system to its regulatory limit, when cardiac output and systemic O2 delivery can no longer be maintained (González-Alonso & Calbet, 2003).
The decline in stroke volume is the cause of the drop in cardiac output and systemic O2 delivery during maximal exercise, because heart rate keeps increasing until exhaustion, whereas the arterial O2 content remains stable (González-Alonso & Calbet, 2003). The drop in stroke volume is likely to be the result of the interaction of several factors diminishing venous return and/or cardiac contractility. Augmented ventricular afterload and skin blood pooling are unlikely explanations, stroke volume declines similarly during heat stress and normal conditions when arterial blood pressure declines (González-Alonso & Calbet, 2003). Rather, a reduction in ventricular preload could contribute to the drop in stroke volume by diminishing end-diastolic ventricular filling when ventricular emptying is at its zenith (Poliner et al. 1980; Higginbotham et al. 1986).
To further understand the factors limiting 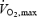 , we determined: (1) whether brain metabolism is impaired in conditions of declining systemic O2 delivery and uptake at exhaustion, (2) whether the precipitated fatigue with heat stress is associated with accelerated alterations in brain metabolism, and (3) whether the decline in stroke volume, which underlies the drop in systemic O2 delivery during maximal exercise, is associated with a concomitant reduction in venous return to the heart. To accomplish these aims, brain and central haemodynamic and metabolic variables were measured at rest, during submaximal and maximal exercise and during recovery in human subjects, both in the presence and in the absence of exogenous heat stress.
, we determined: (1) whether brain metabolism is impaired in conditions of declining systemic O2 delivery and uptake at exhaustion, (2) whether the precipitated fatigue with heat stress is associated with accelerated alterations in brain metabolism, and (3) whether the decline in stroke volume, which underlies the drop in systemic O2 delivery during maximal exercise, is associated with a concomitant reduction in venous return to the heart. To accomplish these aims, brain and central haemodynamic and metabolic variables were measured at rest, during submaximal and maximal exercise and during recovery in human subjects, both in the presence and in the absence of exogenous heat stress.
Methods
Thirteen healthy trained males gave written informed consent to participate in two studies, which were approved by the ethical committee of Copenhagen and Frederiksberg (KF j. nr. 01-230/00). The studies were conducted in accordance with the guidelines of the Declaration of Helsinki. The subjects' mean (±s.d.) age, body weight, height, maximal heart rate, and 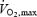 were 25 ±4 years, 77.1 ± 7.4 kg, 181 ± 5 cm, 191 ± 6 beats min−1 and 4.7 ± 0.5 l min−1, respectively. The seven subjects in Study 1 had participated in a previous investigation a year earlier which showed that
were 25 ±4 years, 77.1 ± 7.4 kg, 181 ± 5 cm, 191 ± 6 beats min−1 and 4.7 ± 0.5 l min−1, respectively. The seven subjects in Study 1 had participated in a previous investigation a year earlier which showed that 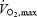 and time to fatigue were lower during heat stress compared to control (González-Alonso & Calbet, 2003). Prior to the invasive experiments, subjects completed four training sessions in the laboratory. On the last practice day, the subjects performed two maximal exercise bouts (see below) separated by 1 h in normal environmental conditions, revealing similar times to exhaustion (in bouts 1 and 2, respectively: Study 1: 8.5 ± 0.6 versus 8.9 ± 0.4 min; Study 2: 8.2 ±1.1 versus 7.6 ± 1.0 min), maximal heart rate (in bouts 1 and 2, respectively: Study 1: 191 ± 2 versus 192 ±2 beats min−1; Study 2: 188 ± 2 versus 189 ± 3 beats min−1) and
and time to fatigue were lower during heat stress compared to control (González-Alonso & Calbet, 2003). Prior to the invasive experiments, subjects completed four training sessions in the laboratory. On the last practice day, the subjects performed two maximal exercise bouts (see below) separated by 1 h in normal environmental conditions, revealing similar times to exhaustion (in bouts 1 and 2, respectively: Study 1: 8.5 ± 0.6 versus 8.9 ± 0.4 min; Study 2: 8.2 ±1.1 versus 7.6 ± 1.0 min), maximal heart rate (in bouts 1 and 2, respectively: Study 1: 191 ± 2 versus 192 ±2 beats min−1; Study 2: 188 ± 2 versus 189 ± 3 beats min−1) and 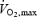 (in bouts 1 and 2, respectively: Study 1: 4.7 ± 0.2 versus 4.6 ± 0.3 l min−1; Study 2: 4.6 ± 0.3 versus 4.7 ± 0.4 l min−1).
(in bouts 1 and 2, respectively: Study 1: 4.7 ± 0.2 versus 4.6 ± 0.3 l min−1; Study 2: 4.6 ± 0.3 versus 4.7 ± 0.4 l min−1).
The subjects reported to the laboratory ∼2 h prior to the experiment after a light breakfast. Upon arrival, catheters were placed under local anaesthesia into the brachial artery and into the internal jugular vein while the subjects were supine. The latter catheter was advanced in the retrograde direction to the bulb (Jacobsen & Enevoldsen, 1989). In the second study, an additional catheter was placed into an anticubital vein and advanced to the right atrium. Following a 30 min rest period in both studies, the subjects completed two maximal cycle ergometer trials at 360 ± 10 W, the first trial starting with a high core and skin temperature (H) and the second trial starting with a normal core and skin temperature (control, C). Each exercise bout was separated by an hour of recovery and was preceded by 15 min of light cycling (∼40% 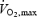 ) and 5 min of rest. In H, internal body and skin temperatures were elevated during submaximal and maximal exercise by perfusing hot water (44°C) into a jacket in contact with the skin of trunk and arms. In C, the subjects wore only shorts while cycling with three fans blowing at an ambient temperature of 12–16°C in the first study and 23–26°C in the second study. In so doing, core temperature at the start of maximal exercise is elevated by ∼1°C in H compared to C, being similar at exhaustion (∼39.5°C), whereas skin temperature remains elevated in H by 6–10°C throughout the entire protocol (González-Alonso & Calbet, 2003). The exercise intensity was selected such that the subjects would become exhausted within 5–10 min and
) and 5 min of rest. In H, internal body and skin temperatures were elevated during submaximal and maximal exercise by perfusing hot water (44°C) into a jacket in contact with the skin of trunk and arms. In C, the subjects wore only shorts while cycling with three fans blowing at an ambient temperature of 12–16°C in the first study and 23–26°C in the second study. In so doing, core temperature at the start of maximal exercise is elevated by ∼1°C in H compared to C, being similar at exhaustion (∼39.5°C), whereas skin temperature remains elevated in H by 6–10°C throughout the entire protocol (González-Alonso & Calbet, 2003). The exercise intensity was selected such that the subjects would become exhausted within 5–10 min and 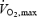 was reached within 4–5 min under normal environmental conditions (80% of 450 ± 46 W peak power in pretests).
was reached within 4–5 min under normal environmental conditions (80% of 450 ± 46 W peak power in pretests).
To restore bodily fluid compartments and energy stores, subjects ingested ∼2 l of a carbohydrate-electrolyte solution (Gatorade®) during the recovery period. During submaximal and maximal exercise as well as recovery, heart rate, arterial and right atrial pressures, carotid artery blood velocity, and MCA Vmean were recorded. During maximal exercise, arterial, jugular and right atrium blood samples (1–5 ml) were drawn simultaneously at 0.5, 1.5, 3.0, 5.8 ± 0.2 and 7.5 ± 0.4 min of exercise.
In the first study (n = 7), internal carotid artery blood flow (BFICA) was determined using Doppler ultrasound (CFM 800 or N-1792, Vingmed Sound, Horten, Norway). Vessel blood flow at rest and during recovery was calculated as the mean blood velocity (VICA, cm s−1; average over 2–3 min) times the cross-sectional area (A) of the internal carotid artery (BFICA=VICA×A =VICA×[(d/2)2×π]. In the second study (n = 6), right and left MCA Vmean were measured using transcranial Doppler ultrasound (Multidop X, DWL, Sipplingen, Germany; Pott et al. 1996), whereas cerebral tissue oxygenation (forehead) was determined using near-infrared spectroscopy (NIRO 500 Hamamatsu photonics, Hamamatsu, Japan) (Ide et al. 1998). During maximal exercise, global brain blood flow was estimated by calculating global brain blood flow at 0 min as [(2 × BFICA) + BFVA], where vertebral blood flow (BFVA) was assumed to be 170 ml min−1 (i.e. ∼24% of global cerebral blood flow) (Schöning et al. 1994; Müller & Schimrigk, 1994; Kashimada et al. 1995; Seidel et al. 1999; Scheel et al. 2000). The 90 s and final values were then estimated using the changes in MCA Vmean. The uptake of O2, glucose and lactate was calculated as blood flow times their respective a-v diff. Heart rate was obtained from an electrocardiogram and arterial and central venous blood pressures were monitored from the brachial artery and right atrium, respectively, with the transducers positioned at the level of the heart (Pressure Monitoring Kit, Baxter). Pulmonary 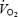 was measured online with a Medgraphics cardiopulmonary exercise testing system CPX/D (Saint Paul, MN, USA). Cardiac output was estimated using the Fick principle (cardiac output =
was measured online with a Medgraphics cardiopulmonary exercise testing system CPX/D (Saint Paul, MN, USA). Cardiac output was estimated using the Fick principle (cardiac output = 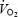 /a-v O2 diff), assuming negligible differences in blood oxygenation between the right atrium and pulmonary artery (Edwards & Mayall, 1998). Haemoglobin, blood gas variables, lactate, and glucose were obtained using an ABL700 analyser (Radiometer, Copenhagen, Denmark). Plasma free fatty acids were determined enzymatically (FFA-C kit, Wako Chemical, Germany) using an automatic analyser (Cobas Fara, Roche, Switzerland). Plasma catecholamines were determined using high performance liquid chromatography with electrochemical detection whereas plasma adenosine triphosphate (ATP) was measured with the luciferin–luciferase technique using an automatic luminometer (ORION Microplate Luminometer, Berthold Detection System GmbH, Pforzheim, Germany; González-Alonso et al. 2002).
/a-v O2 diff), assuming negligible differences in blood oxygenation between the right atrium and pulmonary artery (Edwards & Mayall, 1998). Haemoglobin, blood gas variables, lactate, and glucose were obtained using an ABL700 analyser (Radiometer, Copenhagen, Denmark). Plasma free fatty acids were determined enzymatically (FFA-C kit, Wako Chemical, Germany) using an automatic analyser (Cobas Fara, Roche, Switzerland). Plasma catecholamines were determined using high performance liquid chromatography with electrochemical detection whereas plasma adenosine triphosphate (ATP) was measured with the luciferin–luciferase technique using an automatic luminometer (ORION Microplate Luminometer, Berthold Detection System GmbH, Pforzheim, Germany; González-Alonso et al. 2002).
Statistical analysis
A two-way (trial-by-time) repeated measures analysis of variance (ANOVA) was performed to test significance between and within treatments for each dependent variable. Following a significant F test, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. To determine whether exhaustion was preceded by rapid changes in brain and central haemodynamics and O2 delivery and uptake, final values were compared with peak values during exercise using one-way repeated measures analysis of variance (ANOVA) with Tukey's HSD post hoc procedure. The significance level was set at P < 0.05 and data are means ±s.e.m.
Results
Brain haemodynamics and metabolism
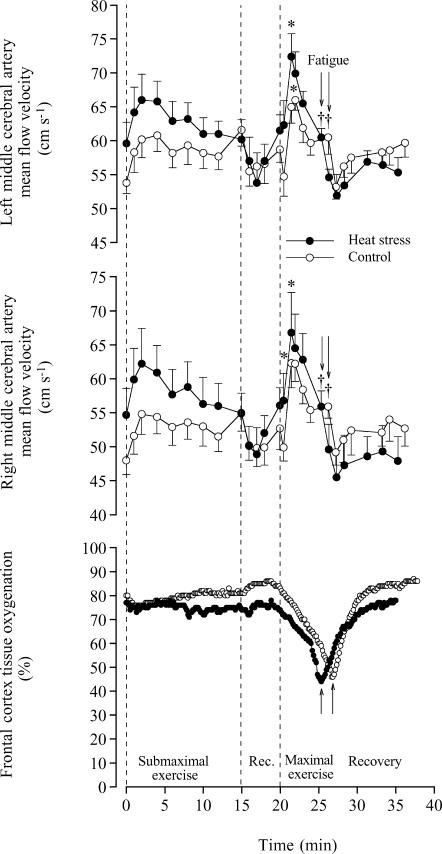
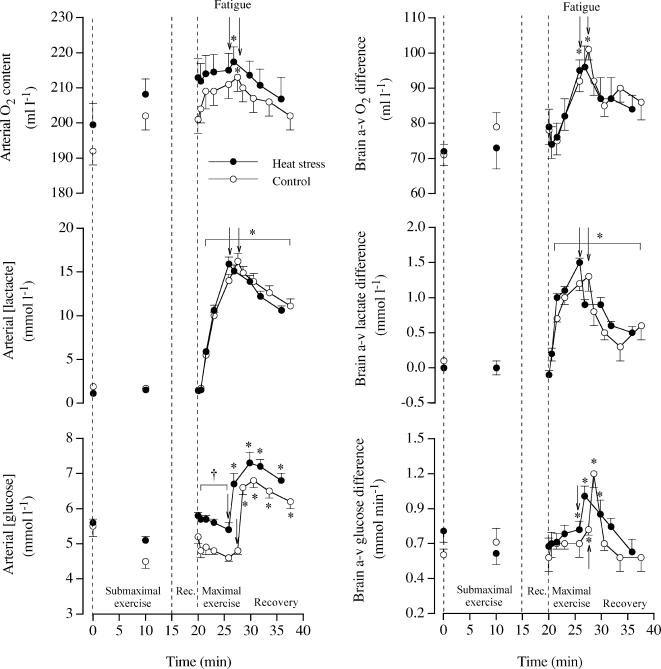
In the two studies, time to exhaustion was 5.8 ± 0.2 min in H and 7.5 ± 0.4 min in C (n = 13). The increase in performance time in C compared to H was greater in the first compared to the second study (2.4 ± 0.4 versus 1.0 ± 0.3 min or 39 ± 8 versus 19 ± 5%, respectively), probably because of a greater difference in exogenous heat stress between conditions. In the first study, we obtained measures of BFICA at rest and in the recovery, but not during vigorous exercise due to body movement artefacts. At rest in H and C, BFICA ranged from 0.32 to 0.35 (± 0.02) l min−1 as VICA and diameter ranged from 16.8 to 17.0 (± 1.3) cm s−1 and 0.64 to 0.67 (± 0.01) cm, respectively. Similar BFICA values were observed during the 5 min rest period before and during the 10 min recovery period following intense exercise. In Study 2, we obtained continuous measures of right and left MCA Vmean at rest and during exercise (Fig. 1). During the first 90 s of maximal exercise, left and right MCA Vmean increased 22 ± 7 and 22 ± 6% in H and by 21 ± 4 and 30 ± 6% in C, respectively. Thereafter, MCA Vmean declined to baseline values on exhaustion in both trials (i.e. 55–61 cm s−1). In contrast, after the initial 11–14% increase during submaximal exercise, left and right MCA Vmean remained unchanged in C or declined slightly in H. During maximal exercise, the progressive decline in arterial saturation and PO2 were accompanied by a greater increase in haemoglobin concentration (Table 1), allowing an increase in arterial O2 content in both trials (Fig. 2). Before and during the first 90 s of maximal exercise, brain a-v diffO2 remained unchanged (72–79 ml l−1). Thereafter, brain a-v diffO2 increased to 97–101 (± 3) ml l−1 on exhaustion in both trials and then decreased to values somewhat higher than at rest after 10 min of recovery. In both trials, peak cerebral O2 extraction before fatigue was ∼47%.
Figure 1. Left and right middle cerebral artery blood flow velocity (MCA Vmean) and near-infrared spectroscopy-determined cerebral tissue oxygenation at rest, during submaximal and maximal exercise and during 10 min of recovery in heat stress and control trials.
MCA Vmean data are means ±s.e.m. for 6 subjects. Tissue oxygenation data are from a representative subject. Higher than value at start of maximal exercise, P < 0.05. † Lower than the peak value during maximal exercise, P < 0.05.
Table 1. Blood variables at rest, during exhaustive maximal exercise and after 10 min of recovery with and without heat stress.
| Time (min) | 0 | 0.5 | 1.5 | 3.0 | 5.8 ± 0.2 | 7.5 ± 0.4 | 10 recov. | |
|---|---|---|---|---|---|---|---|---|
| Haemoglobin (g l−1) | ||||||||
| a | Heat stress | 152 ± 4 | 153 ± 4 | 156 ± 4 | 158 ± 4 | 160 ± 4* | — | 149 ± 4 |
| Normal | 144 ± 3† | 147 ± 3† | 152 ± 4* | 154 ± 3* | 157 ± 4* | 160 ± 3* | 146 ± 3 | |
| v | Heat stress | 151 ± 4 | 151 ± 4 | 154 ± 4 | 158 ± 4* | 159 ± 4* | — | 152 ± 4 |
| Normal | 145 ± 4 | 144 ± 4 | 151 ± 5* | 150 ± 4* | 155 ± 5* | 158 ± 5* | 149 ± 5 | |
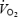 (mmHg) (mmHg) | ||||||||
| a | Heat stress | 111 ± 2 | 98 ± 1 | 93 ± 2* | 90 ± 2* | 91 ± 2* | — | 112 ± 3 |
| Normal | 115 ± 2 | 99 ± 3 | 95 ± 3* | 91 ± 3* | 90 ± 3* | 91 ± 3* | 116 ± 3 | |
| v | Heat stress | 34 ± 2 | 35 ± 2 | 36 ± 2 | 37 ± 2 | 33 ± 1* | — | 35 ± 1 |
| Normal | 32 ± 1 | 33 ± 1 | 35 ± 1 | 35 ± 1 | 33 ± 1 | 33 ± 1 | 35 ± 2 | |
| O2 saturation (%) | ||||||||
| a | Heat stress | 99.0 ± 0.1 | 98.2 ± 0.1 | 97.5 ± 0.2 | 96.6 ± 0.3* | 95.7 ± 0.5* | — | 98.0 ± 0.1 |
| Normal | 98.9 ± 0.1 | 98.1 ± 0.2 | 97.4 ± 0.3 | 96.5 ± 0.4* | 95.6 ± 0.5* | 95.0 ± 0.6* | 97.7 ± 0.3 | |
| v | Heat stress | 63.2 ± 1.8 | 64.5 ± 2.2 | 64.1 ± 2.1 | 60.6 ± 2.5* | 53.3 ± 2.0* | — | 57.5 ± 2.6* |
| Normal | 62.0 ± 2.4 | 65.3 ± 2.9 | 65.1 ± 2.4 | 61.7 ± 2.9 | 54.8 ± 1.8* | 50.8 ± 1.9* | 57.9 ± 3.4 | |
| O2 content (ml l−1) | ||||||||
| a | Heat stress | 213 ± 5 | 212 ± 5 | 214 ± 5 | 215 ± 5 | 215 ± 5 | — | 207 ± 6 |
| Normal | 201 ± 4 | 204 ± 4 | 209 ± 5 | 209 ± 4* | 211 ± 4* | 213 ± 4* | 202 ± 4 | |
| v | Heat stress | 134 ± 6 | 137 ± 6 | 138 ± 6 | 134 ± 6 | 112 ± 7* | — | 123 ± 7* |
| Normal | 125 ± 6 | 128 ± 5* | 133 ± 6 | 127 ± 6* | 117 ± 5 | 112 ± 7* | 118 ± 7* | |
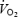 (mmHg) (mmHg) | ||||||||
| a | Heat stress | 39 ± 1 | 39 ± 1 | 39 ± 1 | 36 ± 1* | 30 ± 1* | — | 32 ± 1* |
| Normal | 39 ± 1 | 38 ± 1 | 38 ± 1 | 36 ± 1 | 31 ± 1* | 29 ± 1* | 32 ± 1* | |
| v | Heat stress | 50 ± 1 | 49 ± 1 | 48 ± 1 | 48 ± 1 | 46 ± 1* | — | 45 ± 1* |
| Normal | 51 ± 0 | 50 ± 1 | 51 ± 1 | 49 ± 1 | 47 ± 1* | 47 ± 1* | 46 ± 1* | |
| Noradrenaline (nmol l−1) | ||||||||
| a | Heat stress | 3.4 ± 0.3† | — | — | — | 47.9 ± 7.1† | — | 7.4 ± 0.9 |
| Normal | 2.2 ± 0.2 | — | — | — | 35.7 ± 3.9 | 57.0 ± 7.6‡ | 5.1 ± 0.5 | |
| v | Heat stress | 3.5 ± 0.2† | — | — | — | 41.2 ± 5.3† | — | 7.7 ± 0.8 |
| Normal | 2.3 ± 0.3 | — | — | — | 32.1 ± 3.9 | 52.5 ± 7.5‡ | 5.2 ± 0.5 | |
| Adrenaline (nmol l−1) | ||||||||
| a | Heat stress | 0.9 ± 0.1 | — | — | — | 8.4 ± 1.8 | — | 0.9 ± 0.1 |
| Normal | 0.8 ± 0.2 | — | — | — | 8.0 ± 1.8 | 13.0 ± 2.9 | 1.0 ± 0.2* | |
| v | Heat stress | 0.6 ± 0.1 | — | — | — | 7.5 ± 1.5 | — | 0.9 ± 0.1 |
| Normal | 0.8 ± 0.1 | — | — | — | 7.1 ± 1.4* | 11.4 ± 2.5* | 0.9 ± 0.1* | |
10 recov., after 10 min recovery following exhaustion. Values are means ±s.e.m. for 13 subjects. a, arterial; v, jugular venous.
Different from rest, P < 0.05.
Different from normal, P < 0.05.
Higher than value at 5.8 ± 0.2 min of exercise, P < 0.05.
Figure 2. Arterial concentrations and arterial–internal jugular venous differences (a-v diff) for O2, lactate and glucose at rest, during submaximal and maximal exercise and during 10 min of recovery in heat stress and control trials.
Data are means ±s.e.m. for 13 subjects. Different from the start of maximal exercise, P < 0.05. † Different from control, P < 0.05.
During the 15 min of submaximal exercise in either condition, arterial and internal jugular venous lactate concentrations (∼1.2 mmol l−1) and a-v lactate diff did not change significantly (Fig. 2). During maximal exercise, however, arterial lactate increased to ∼16 mmol l−1, while a-v lactate diff increased from −0.1 to ∼1.5 mmol l−1 in both trials (P < 0.05). After 10 min of recovery, the concentration and the a-v lactate diff remained elevated in both conditions. During maximal exercise, arterial glucose concentration declined and the a-v glucose diff increased in both trials. A marked elevation in the glucose concentration, the a-v glucose diff and global brain uptake was observed immediately in the recovery. Peak cerebral glucose extraction before fatigue amounted to 16%. In contrast, arterial plasma free fatty acids declined from rest to the end of maximal exercise in both conditions (from 0.23 ± 0.02 to 0.14 ± 0.01 μmol l−1 in H and from 0.39 ± 0.05 to 0.18 ± 0.02 μmol l−1 in C; both P < 0.05) whereas the a-v FFA diff was null.
Systemic haemodynamics, O2 transport and O2 uptake
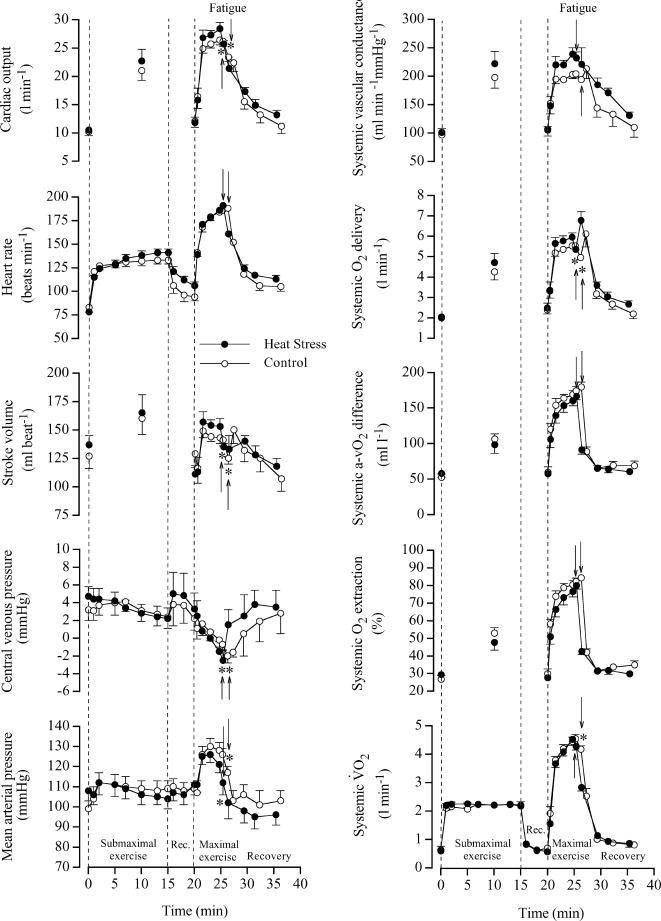
In both H and C, cardiac output and mean arterial pressure increased rapidly during the first minute of exercise, reached a peak value after 3–5 min and declined before exhaustion (pressure declined 2.7–2.8 l min−1 and 13–14 mmHg, respectively; P < 0.05). The drop in cardiac output was associated with a 10 and 6% decline in systemic O2 delivery and 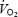 , respectively (Fig. 3). Systemic O2 extraction increased from 30% at the start of exercise to 80 ± 2 and 84 ± 2% upon exhaustion in H and C, respectively. In both trials, the fall in cardiac output before exhaustion was related to the drop in stroke volume (∼20 ml beat−1) as heart rate kept increasing until exhaustion (194 ± 3 and 191 ± 4 beats min−1 in H and C, respectively). Right atrial pressure declined in both H and C from 2 to 3 mmHg to −2 to −3 mmHg upon exhaustion (Fig. 3).
, respectively (Fig. 3). Systemic O2 extraction increased from 30% at the start of exercise to 80 ± 2 and 84 ± 2% upon exhaustion in H and C, respectively. In both trials, the fall in cardiac output before exhaustion was related to the drop in stroke volume (∼20 ml beat−1) as heart rate kept increasing until exhaustion (194 ± 3 and 191 ± 4 beats min−1 in H and C, respectively). Right atrial pressure declined in both H and C from 2 to 3 mmHg to −2 to −3 mmHg upon exhaustion (Fig. 3).
Figure 3. Cardiac output, heart rate, stroke volume, central venous pressure, mean arterial pressure, systemic vascular conductance, O2delivery, a-v O2diff, O2 extraction and O2uptake at rest, during submaximal and maximal exercise and during 10 min of recovery in heat stress and control trials.
Data are means ±s.e.m. for 6 subjects. Lower than peak value during maximal exercise, P < 0.05.
Catecholamines and ATP
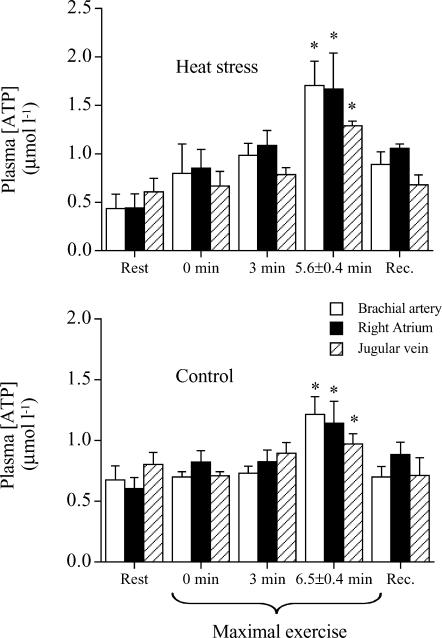
The arterial noradrenaline concentration increased 13- and 25-fold from rest to exhaustion in H and C, respectively, whereas the adrenaline concentration increased 8- and 15-fold (both P < 0.05; Table 1). In H and C, the a-v diff for noradrenaline increased from −0.1 nmol l−1 at rest to 4.5–6.6 nmol l−1 at exhaustion (P < 0.05), whereas the a-v diff for adrenaline increased from 0.1 to 0.4–0.9–1.6 nmol l−1 (P < 0.05), indicating a net brain uptake of both catecholamines. During maximal exercise in both H and C, plasma [ATP] in the brachial artery, right atrium and jugular vein increased (Fig. 4).
Figure 4. Plasma ATP concentration at rest, during maximal exercise and during 10 min of recovery in heat stress and control trials.
Data are means ±s.e.m. for 4–6 subjects. Different from value at the start of maximal exercise, P < 0.05.
Discussion
There were five novel observations in this investigation that provide further insight into the metabolism of the human brain during maximal exercise and the influence of venous return and perfusion pressure on stroke volume and O2 delivery: (1) heat stress accelerates the increases in brain extraction of O2, glucose and lactate and the reductions in blood flow and cerebral tissue oxygenation without altering values at exhaustion; (2) the a-v diff for O2 only increased after 90 s of maximal exercise in either condition when blood flow was declining; (3) at exhaustion in either condition, the estimated uptake of O2, glucose and lactate by the brain increased; (4) in contrast to the limited O2 reserve in contracting skeletal muscle and systemic circulation, the brain maintained more than one-half of its O2 reserve at exhaustion in either condition; (5) the fall in stroke volume in H and C was accompanied by a similar drop in central venous pressure. Together, these results suggest that during maximal exercise, with or without heat stress, fatigue is associated with an enhanced rather than an impaired uptake of O2 and substrates by the brain. Moreover, the reductions in stroke volume, which underlie the fall in systemic O2 delivery and uptake before exhaustion, are in part related to reductions in venous return to the heart.
This study demonstrated that the extraction of O2, glucose and lactate by the human brain increases during maximal exercise, despite a decline in O2 and glucose delivery. The elevations in brain O2 and glucose extraction during maximal exercise, up to ∼47% and ∼16%, respectively, were independent of exogenous heat stress. In both the heat stress and control conditions, the a-v O2 diff across the brain increased (∼45%) after 90 s in association with a decline in brain blood flow and cerebral oxygenation, as suggested by a progressive marked drop in both MCA Vmean and near-infrared spectroscopy-determined tissue oxygenation. During neither submaximal exercise, nor the first 90 s of maximal exercise were there any significant alterations in the a-v O2 diff or frontral cortex tissue oxygenation. During incremental semirecumbent cycling exercise to exhaustion, brain O2 and lactate extractions increase, but glucose extraction does not (Ide et al. 2000; Dalsgaard et al. 2002). With exhaustive combined arm and leg exercise, however, the glucose extraction also increases (Dalsgaard et al. 2004). This study confirms that O2, glucose and lactate a-v diff values increase during intense exercise, yet the ∼45% and 16-fold elevations in a-v diff for O2 and lactate are the highest values reported. With distinct O2, glucose and lactate extractions, the cerebral metabolic uptake ratio (O2/[glucose +½lactate]) decreased drastically during the first 90 s of maximal exercise (from ∼5 to 2.8 to 3.0) and then remained low until several minutes into the recovery, supporting the hypothesis that brain activation is enhanced during maximal exercise (Dalsgaard et al. 2002, 2004). Collectively, the marked increase in O2, glucose and lactate extraction by the brain, together with a robust decline in frontal cortex tissue oxygenation, could reflect two distinct metabolic scenarios, which directly hinge on the magnitude of decline in global brain blood flow during exhausting exercise: (1) brain 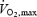 is maintained at levels observed early in exercise if its blood flow declines in proportion to the increase in a-v O2 diff (global brain blood flow declines by 45%), and (2) brain
is maintained at levels observed early in exercise if its blood flow declines in proportion to the increase in a-v O2 diff (global brain blood flow declines by 45%), and (2) brain 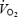 is enhanced prior to exhaustion if the drop in brain perfusion is smaller than the increase in O2 extraction. The possibility that the increase in O2, glucose and lactate extraction observed in the current study is simply a compensatory mechanism initiated by reductions in global cerebral blood flow to sustain metabolic demand (i.e. with no change in overall neural activity) cannot be excluded; however, it would require global cerebral blood flow to decline by 45% after 90 s of maximal exercise, which appears unlikely given that cardiac output and exercising leg blood flow decline only ∼10% during the last 2 min and the right and left MCA Vmean decline only ∼10–15% after 90 s of exercise. Using blood flow in the internal carotid artery and the relative changes in MCA Vmean, we estimated that global cerebral blood flow increased from 0.8–0.9 l min−1 at rest to 1.0–1.1 l min−1 after 90 s of maximal exercise and declined to the resting level on exhaustion (Table 2). This would suggest that global brain uptake of O2, glucose and lactate, with and without heat stress, were enlarged on exhaustion and that only part of the increases in O2 and substrate extraction was associated with the reduction in global cerebral blood flow.
is enhanced prior to exhaustion if the drop in brain perfusion is smaller than the increase in O2 extraction. The possibility that the increase in O2, glucose and lactate extraction observed in the current study is simply a compensatory mechanism initiated by reductions in global cerebral blood flow to sustain metabolic demand (i.e. with no change in overall neural activity) cannot be excluded; however, it would require global cerebral blood flow to decline by 45% after 90 s of maximal exercise, which appears unlikely given that cardiac output and exercising leg blood flow decline only ∼10% during the last 2 min and the right and left MCA Vmean decline only ∼10–15% after 90 s of exercise. Using blood flow in the internal carotid artery and the relative changes in MCA Vmean, we estimated that global cerebral blood flow increased from 0.8–0.9 l min−1 at rest to 1.0–1.1 l min−1 after 90 s of maximal exercise and declined to the resting level on exhaustion (Table 2). This would suggest that global brain uptake of O2, glucose and lactate, with and without heat stress, were enlarged on exhaustion and that only part of the increases in O2 and substrate extraction was associated with the reduction in global cerebral blood flow.
Table 2. Estimated O2, glucose and lactate uptakes across the brain during maximal exercise in heat stress and control conditions.
| Start | 90 s | Exhaustion | ||
|---|---|---|---|---|
| Global brain blood flow (l min−1) | Heat stress | 0.90 ± 0.05 | 1.06 ± 0.06* | 0.89 ± 0.05† |
| Control | 0.83 ± 0.06 | 0.99 ± 0.07* | 0.86 ± 0.06† | |
| Brain a-v O2 difference (ml l−1) | Heat stress | 73.6 ± 4.9 | 72.5 ± 5.8 | 100.1 ± 4.4*† |
| Control | 76.1 ± 5.3 | 71.5 ± 4.6 | 100.2 ± 4.0*† | |
Global brain 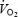 (ml min−1) (ml min−1) |
Heat stress | 69 ± 8 | 78 ± 9* | 90 ± 8*† |
| Control | 63 ± 7 | 71 ± 7 | 87 ± 8*† | |
| Brain a-v glucose difference (mmol l−1) | Heat stress | 0.70 ± 0.10 | 0.67 ± 0.04 | 0.85 ± 0.08*† |
| Control | 0.54 ± 0.09 | 0.66 ± 0.04 | 0.76 ± 0.05* | |
| Global brain glucose uptake (mmol min−1) | Heat stress | 0.63 ± 0.10 | 0.74 ± 0.12 | 0.77 ± 0.09 |
| Control | 0.46 ± 0.09 | 0.65 ± 0.06* | 0.66 ± 0.08* | |
| Brain a-v lactate difference (mmol l−1) | Heat stress | −0.07 ± 0.04 | 1.03 ± 0.16* | 1.37 ± 0.14*† |
| Control | −0.07 ± 0.04 | 0.71 ± 0.06* | 1.50 ± 0.22*† | |
| Global brain lactate uptake (mmol min−1) | Heat stress | −0.1 ± 0.0 | 1.1 ± 0.2* | 1.2 ± 0.1* |
| Control | −0.1 ± 0.0 | 0.7 ± 0.1* | 1.3 ± 0.2*† |
Data are means ±s.e.m. for 7 subjects in study 1. Note that the increase in brain uptake of O2 and substrates on exhaustion is only due to increases in a-v diff. The vast majority of the human cerebral venous blood drainage occurs throughout the internal jugular vein (Schreiber et al. 2003).
Different from start, P < 0.05.
Different from 90 s, P < 0.05.
The measures and estimates of blood flow to the brain are in congruence with evidence in the literature. In both animals and humans global brain blood flow increases 20–30% on the transition from rest (∼0.8 l min−1 in humans; Herlund et al. 1962; Lassen, 1985; Thomas et al. 1989; Schöning et al. 1994; Müller & Schimrighk, 1994; Kashimada et al. 1995; Seidel et al. 1999; Scheel et al. 2000; Delp et al. 2001; Spilt et al. 2002; van Mill et al. 2002) to moderate exercise, with no further increase when exercise intensity increases to elicit 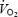 (Herlund et al. 1962; Thomas et al. 1989; Huang et al. 1991; Linkis et al. 1995; Hellström et al. 1996; Delp et al. 2001). This elevation, however, is not uniformly distributed, but it is localized to the regions of the brain related to locomotion, the maintenance of equilibrium, cardiorespiratory control and vision (Delp et al. 2001). In agreement with the above reports and other Doppler ultrasound studies (Huang et al. 1991; Hellström et al. 1996), total BFICA at rest was 0.64–0.70 l min−1 and increased to 0.76 l min−1 during submaximal exercise (n = 2). During the first 90 s of maximal exercise (Study 2), left and right MCA Vmean increased ∼22% when a-v O2 diff was unchanged, suggesting that global brain
(Herlund et al. 1962; Thomas et al. 1989; Huang et al. 1991; Linkis et al. 1995; Hellström et al. 1996; Delp et al. 2001). This elevation, however, is not uniformly distributed, but it is localized to the regions of the brain related to locomotion, the maintenance of equilibrium, cardiorespiratory control and vision (Delp et al. 2001). In agreement with the above reports and other Doppler ultrasound studies (Huang et al. 1991; Hellström et al. 1996), total BFICA at rest was 0.64–0.70 l min−1 and increased to 0.76 l min−1 during submaximal exercise (n = 2). During the first 90 s of maximal exercise (Study 2), left and right MCA Vmean increased ∼22% when a-v O2 diff was unchanged, suggesting that global brain 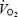 increased early in exercise. Moreover, the large increases in O2 extraction and reductions in frontal cortex tissue oxygenation which only occurred after 90 s of maximal exercise were accompanied by smaller reductions in MCA Vmean (i.e. ∼40−45%versus∼10–15%, respectively), suggesting that global brain metabolism was further enhanced on exhaustion (Table 2). The present results raise the intriguing questions of why global brain energy turnover and neural activation are elevated during maximal exercise and which brain regions are involved.
increased early in exercise. Moreover, the large increases in O2 extraction and reductions in frontal cortex tissue oxygenation which only occurred after 90 s of maximal exercise were accompanied by smaller reductions in MCA Vmean (i.e. ∼40−45%versus∼10–15%, respectively), suggesting that global brain metabolism was further enhanced on exhaustion (Table 2). The present results raise the intriguing questions of why global brain energy turnover and neural activation are elevated during maximal exercise and which brain regions are involved.
A critical question is why blood flow and O2 delivery to the brain decline during maximal exercise. A coupling between reductions in cardiac output and brain circulation during cycling exercise was proposed by Ide et al. (1998), who demonstrated that the lowering in cardiac output by cardioselective β1-adrenergic blockade is associated with a reduction in MCA Vmean. In this study, however, cardiac output increased by 1–2 l min−1 between 1.5 and 4–5 min of each maximal exercise bout, when MCA Vmean was declining, indicating that factors other than cardiac output were involved in the suppression of brain blood flow early in exercise. In support of this, Nybo et al. (2002) showed that a drop in cardiac output is not a prerequisite for brain blood flow to decline with hyperthermia during prolonged exercise, as an 18% decline in flow with heat stress was met by a 33% increase in a-v O2 diff across the brain, leading to a 7% higher brain 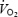 . This observation in the hyperthermic trial contrasted with the unchanged brain blood flow, a-v O2 diff, and
. This observation in the hyperthermic trial contrasted with the unchanged brain blood flow, a-v O2 diff, and 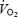 in the control trial (Nybo et al. 2002). An alternative possibility is that reductions in cerebral perfusion pressure contributed to the decrease in brain circulation, since the decreases reported here in left and right MCA Vmean after 90–120 s of exercise were temporally associated with reductions in arterial and central venous pressures. Also, MCA Vmean declined with heat stress during submaximal exercise, in parallel with a drop in arterial and central venous pressures, whereas it did not do so during control exercise when cerebral perfusion pressure was maintained. The role of perfusion pressure on brain circulation has been demonstrated during an orthostatic challenge, when MCA Vmean declined drastically when arterial and central venous pressures were compromised (van Lieshout et al. 2003). A third possibility is that local factors reducing the vasodilator and/or increasing the vasoconstrictor activities suppressed brain perfusion. In this regard, the plasma concentration of the potent vasodilator ATP was elevated in the jugular vein, while the brain was apparently taking up large amounts of catecholamines on exhaustion in both conditions and the arterial and jugular venous PCO2 was declining, suggesting that both vasodilator and vasoconstrictor activities are elevated. Although we are unable to single out the underlying mechanism, it seems that the drop in cerebral perfusion pressure rather than alterations in local vascular tone accounts for the drop in brain circulation during maximal exercise since the declines in blood velocity and perfusion pressure were remarkably similar.
in the control trial (Nybo et al. 2002). An alternative possibility is that reductions in cerebral perfusion pressure contributed to the decrease in brain circulation, since the decreases reported here in left and right MCA Vmean after 90–120 s of exercise were temporally associated with reductions in arterial and central venous pressures. Also, MCA Vmean declined with heat stress during submaximal exercise, in parallel with a drop in arterial and central venous pressures, whereas it did not do so during control exercise when cerebral perfusion pressure was maintained. The role of perfusion pressure on brain circulation has been demonstrated during an orthostatic challenge, when MCA Vmean declined drastically when arterial and central venous pressures were compromised (van Lieshout et al. 2003). A third possibility is that local factors reducing the vasodilator and/or increasing the vasoconstrictor activities suppressed brain perfusion. In this regard, the plasma concentration of the potent vasodilator ATP was elevated in the jugular vein, while the brain was apparently taking up large amounts of catecholamines on exhaustion in both conditions and the arterial and jugular venous PCO2 was declining, suggesting that both vasodilator and vasoconstrictor activities are elevated. Although we are unable to single out the underlying mechanism, it seems that the drop in cerebral perfusion pressure rather than alterations in local vascular tone accounts for the drop in brain circulation during maximal exercise since the declines in blood velocity and perfusion pressure were remarkably similar.
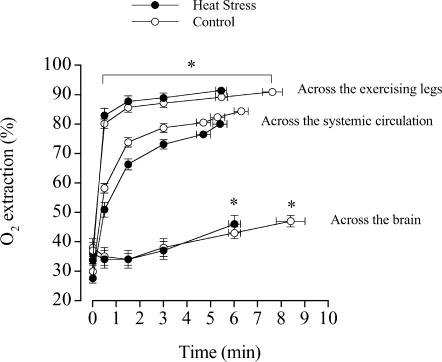
A fundamental difference between the brain and the contracting skeletal muscle with respect to their O2 reserves may explain why the brain is less prone than skeletal muscle to reductions in tissue 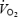 when challenged with a similar relative fall in O2 delivery. During maximal exercise with and without heat stress, we observed that cerebral O2 extraction increased from 36% to 45–48%, suggesting that even at exhaustion the brain possesses a large O2 reserve to compensate for reductions in blood flow. This is in contrast to locomotive leg skeletal muscle, which showed a mean increase in O2 extraction from 89 to 91%, while exercising leg blood flow and
when challenged with a similar relative fall in O2 delivery. During maximal exercise with and without heat stress, we observed that cerebral O2 extraction increased from 36% to 45–48%, suggesting that even at exhaustion the brain possesses a large O2 reserve to compensate for reductions in blood flow. This is in contrast to locomotive leg skeletal muscle, which showed a mean increase in O2 extraction from 89 to 91%, while exercising leg blood flow and 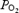 declined before exhaustion in both experimental conditions (Fig. 5) (González-Alonso & Calbet, 2003). Although selected brain regions may increase their O2 extraction during maximal exercise above the levels indicated by our measures of cerebral oxygenation (frontal cortex) and a-v O2 diff, it seems unlikely that they reach the maximal levels attained in contracting skeletal muscle. This concept is supported by the data from simultaneous measures of quadriceps muscle (vastus lateralis) and frontal cortex tissue oxygenation in two subjects showing a much lower tissue oxygenation value at exhaustion in contracting muscle than brain (15 versus 45%, respectively), which is in close agreement with our measures of the blood a-v O2 diff across these tissues (Fig. 5). Together, these results indicate that the human brain possesses a large O2 reserve during maximal exercise, which affords the possibility of increasing brain
declined before exhaustion in both experimental conditions (Fig. 5) (González-Alonso & Calbet, 2003). Although selected brain regions may increase their O2 extraction during maximal exercise above the levels indicated by our measures of cerebral oxygenation (frontal cortex) and a-v O2 diff, it seems unlikely that they reach the maximal levels attained in contracting skeletal muscle. This concept is supported by the data from simultaneous measures of quadriceps muscle (vastus lateralis) and frontal cortex tissue oxygenation in two subjects showing a much lower tissue oxygenation value at exhaustion in contracting muscle than brain (15 versus 45%, respectively), which is in close agreement with our measures of the blood a-v O2 diff across these tissues (Fig. 5). Together, these results indicate that the human brain possesses a large O2 reserve during maximal exercise, which affords the possibility of increasing brain 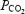 when systemic and exercising leg O2 delivery and uptake are declining.
when systemic and exercising leg O2 delivery and uptake are declining.
Figure 5. Oxygen extraction across the brain, the systemic circulation and the exercising legs during maximal exercise in heat stress and control trials.
Data on the exercising legs and brain were obtained in the same subjects in a previous study (González-Alonso & Calbet, 2003) and the present Study 1. Note the similarity of the average performance times measured in these subjects at two different time points separated by a year. Data on the systemic circulation were obtained in 6 different subjects for Study 2. Different from value at 0 min, P < 0.05.
The present data and previous findings in the same group of human subjects indicate that the cascade of events leading to fatigue during maximal exercise, with or without heat stress, is closely linked to cardiovascular instability and reduced systemic O2 delivery, impairing locomotive skeletal muscle aerobic metabolism but not brain aerobic metabolism (González-Alonso & Calbet, 2003). In keeping with previous results, we found that heat stress caused a more rapid decline in stroke volume, cardiac output, perfusion pressure and O2 delivery in all but two subjects, but the cardiovascular instability preceding exhaustion was similar when subjects were exposed to both heat stress and control conditions. Two observations further link the fatigue process to cardiovascular instability: (1) when exposed to either heat stress or control conditions, two well-trained subjects showed identical time courses for the declines in cardiac output, perfusion pressure and systemic and brain O2 delivery and performance times, and (2) three subjects were able to exercise for 2–6 min longer when the rate of rise in heart rate was slower during the non-invasive maximal pretests. This suggests that any additional stress that quickly pushes the cardiovascular system to its maximal regulatory limit leads to early cardiovascular instability and fatigue.
The decline in stroke volume is the most important component of cardiovascular strain leading to reductions in systemic O2 delivery because heart rate keeps increasing until exhaustion and arterial O2 increases or remains unchanged (González-Alonso & Calbet, 2003). Confirming these results on trained men, we found that cardiac output was elevated with heat stress compared to control early in maximal exercise while stroke volume was similar, yet a more rapid decline in stroke volume was observed with heat stress later in the exercise bout. Several factors altering ventricular preload, ventricular afterload and/or myocardial contractility could underlie the drop in stroke volume late in exercise. A contributory role of augmented ventricular afterload and skin blood pooling appears unlikely, as stroke volume and central venous pressure fall similarly during heat stress and normal conditions when arterial blood pressure declines (González-Alonso et al. 2000; González-Alonso & Calbet, 2003). The drastic decline in central venous pressure during maximal exercise with or without heat stress compared to the slight changes during submaximal exercise could be interpreted to mean that a reduction in venous return contributed to the fall in stroke volume (Rowell et al. 1966; Rowell, 1974). Yet a reduced ventricular filling preload is not the only factor, because stroke volume only declined during the last minute of exercise, but right atrial pressure declined from the start of exercise. The observation that the decline in stroke volume coincided with a drop in mean arterial pressure, central venous pressure of approximately −2 mmHg, near-maximal heart rates and high right atrial plasma ATP, catecholamines and muscle temperature favours the interaction of several factors transiently depressing preload and/or left ventricular function (González-Alonso & Calbet, 2003). In view of the decline in systemic, exercising legs and brain circulations during maximal exercise, it seems plausible that the coronary circulation and left ventricular function are suppressed on exhaustion. Although this possibility awaits confirmation, the large drop in central venous pressure suggests that reduced venous return to the heart contributes to the fall in stroke volume late in exercise.
The cardiovascular instability observed in trained subjects during constant high intensity exercise differs from the circulatory responses reported in untrained subjects during incremental exercise. Poliner et al. (1980) and Higginbotham et al. (1986) found progressive increases in cardiac output during incremental upright exercise, but a maintained (Higginbotham et al. 1986) or increased (Poliner et al. 1980) stroke volume on increasing work rate from 40 to 50% of 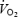 peak-to-peak exercise. One reason for the discrepancy could be that the untrained subjects did not exercise to the maximal levels of exertion needed to show a decline in stroke volume and cardiac output, as peak heart rate ranged from 167 to 182 beats min−1, which is considerably lower that the rate of 192 beats min−1 seen in the trained subjects.
peak-to-peak exercise. One reason for the discrepancy could be that the untrained subjects did not exercise to the maximal levels of exertion needed to show a decline in stroke volume and cardiac output, as peak heart rate ranged from 167 to 182 beats min−1, which is considerably lower that the rate of 192 beats min−1 seen in the trained subjects.
In summary, the present results during maximal exercise in healthy, trained humans show that heat stress accelerates the increases in brain extraction and uptake of O2, glucose and lactate without altering the values measured at exhaustion. In both heat stress and control conditions, the increases in a-v diff for O2 and glucose only occurred after ∼90 s when blood velocity in left and right middle cerebral arteries was declining, yet the uptake of O2, glucose and lactate by the brain was elevated. In the central circulation, the fall in stroke volume with or without extrageneous heat stress was accompanied by a similar drop in central venous pressure, suggesting that reduced venous return to the heart contributed to the reduction in stroke volume. Collectively, these findings suggest that fatigue during maximal exercise, with or without heat stress, is associated with an enhanced rather than an impaired brain metabolic rate for O2 and substrates. In contrast to the limited O2 reserve in contracting skeletal muscle and systemic circulation, the human brain maintains a large O2 reserve on exhaustion, which appears to protect this vital organ against reductions in brain O2 delivery.
Acknowledgments
This study was supported by grants from the Gatorade Sports Science Institute and Team Denmark. J. González-Alonso was supported by grants from The Danish National Research Foundation (504-14) and The Copenhagen Hospital System (Hovedstadens Sygehusfællesskab).
References
- Dalsgaard MK, Ide K, Cai Y, Quistorff B, Secher NH. The intent to exercise influences the cerebral O2/carbohydrate uptake ratio in humans. J Physiol. 2002;540:681–689. doi: 10.1113/jphysiol.2001.013062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the brain. J Physiol. 2004;554:571–578. doi: 10.1113/jphysiol.2003.055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Armstrong RB, Godfrey DA, Laughlin MH, Ross CD, Wilkerson MK. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol. 2001;533:849–859. doi: 10.1111/j.1469-7793.2001.t01-1-00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JD, Mayall RM. Importance of the sampling site for measurement of mixed venous oxygen saturation in shock. Crit Care Med. 1998;26:1356–1360. doi: 10.1097/00003246-199808000-00020. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JAL. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodríguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol. 2000;278:H321–H330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Hellström GW, Fischer-Colbrie NG, Wahlgren, Jögestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- Herlund S, Nylin G, Rengström O. The behaviour of the cerebral circulation during muscular exercise. Acta Physiol Scand. 1962;54:316–324. doi: 10.1111/j.1748-1716.1962.tb02355.x. [DOI] [PubMed] [Google Scholar]
- Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- Huang SY, Tawney KW, Bender PR, Groves BM, McCullough RG, McCullough RW, et al. Internal carotid flow velocity with exercise before and after acclimatization to 4300 m. J Appl Physiol. 1991;71:1469–1476. doi: 10.1152/jappl.1991.71.4.1469. [DOI] [PubMed] [Google Scholar]
- Ide K, Pott F, Van Lieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand. 1998;162:13–20. doi: 10.1046/j.1365-201X.1998.0280f.x. [DOI] [PubMed] [Google Scholar]
- Ide K, Schmalbruch IK, Quistorff B, Horn A, Secher NH. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J Physiol. 2000;522:159–164. doi: 10.1111/j.1469-7793.2000.t01-2-00159.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen M, Enevoldsen E. Retrograde catherization of the right internal jugular vein for serial measurement of cerebral venous oxygen content. J Cereb Blood Flow Met. 1989;9:717–720. doi: 10.1038/jcbfm.1989.101. [DOI] [PubMed] [Google Scholar]
- Kashimada A, Machida K, Honda N, Mamiya T, Takahashi T, Kamano T, Osada H. Measurement of cerebral blood flow with two-dimensional cine phase-contrast MR imaging: evaluation of normal subjects and patients with vertigo. Radiot Med. 1995;13:95–102. [PubMed] [Google Scholar]
- Lassen NA. Normal average value of cerebral blood flow in younger adults is 50 ml/100 g/min. J Cereb Blood Flow Metab. 1985;5:347–349. doi: 10.1038/jcbfm.1985.48. [DOI] [PubMed] [Google Scholar]
- Linkis P, Jørgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean blood flow velocity. J Appl Physiol. 1995;78:12–16. doi: 10.1152/jappl.1995.78.1.12. [DOI] [PubMed] [Google Scholar]
- Müller M, Schimrigk K. A comparative assessment of cerebral haemodynamics in the basilar artery and carotid territory by transcranial Doppler sonography in normal subjects. Ultrasound Med Biol. 1994;20:677–687. doi: 10.1016/0301-5629(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Nybo L, Møller K, Volianitis S, Nielsen B, Secher NH. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J Appl Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol. 2001;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist G, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation. 1980;62:528–534. doi: 10.1161/01.cir.62.3.528. [DOI] [PubMed] [Google Scholar]
- Pott F, Jensen K, Hansen H, Christensen NJ, Lassen NA, Secher NH. Middle cerebral artery blood velocity and plasma catecholamines during exercise. Acta Physiol Scand. 1996;158:349–356. doi: 10.1046/j.1365-201X.1996.564325000.x. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Marx HJ, Bruce RA, Conn RD, Kusumi F. Reductions in cardiac output, central blood volume, and stroke volume with thermal stress in normal men during exercise. J Clin Invest. 1966;45:1801–1816. doi: 10.1172/JCI105484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel P, Ruge C, Petruch UR, Schöning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. 2000;31:147–150. doi: 10.1161/01.str.31.1.147. [DOI] [PubMed] [Google Scholar]
- Schöning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke. 1994;25:17–22. doi: 10.1161/01.str.25.1.17. [DOI] [PubMed] [Google Scholar]
- Schreiber SJ, Lürtzing F, Götze R, Doepp F, Klingebiel R, Valduez JM. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J Appl Physiol. 2003;94:1802–1805. doi: 10.1152/japplphysiol.00782.2002. [DOI] [PubMed] [Google Scholar]
- Seidel E, Eiche BM, Tettenborn B, Krummenauer F. Reference values for vertebral artery flow volume by duplex sonography in young and elderly adults. Stroke. 1999;30:2692–2696. doi: 10.1161/01.str.30.12.2692. [DOI] [PubMed] [Google Scholar]
- Spilt A, Box FMA, van der Geest RJ, Reiber JH, Kunz P, Kamper AM, Blauw GJ, van Buchem MA. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. J Magn Reson Imaging. 2002;16:1–5. doi: 10.1002/jmri.10133. [DOI] [PubMed] [Google Scholar]
- Thomas SN, Schroeder T, Secher NH, Michell JH. Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol. 1989;67:744–748. doi: 10.1152/jappl.1989.67.2.744. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–848. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- van Mill AHM, Spilt A, van Buchem MA, Bollen EL, Teppema L, Westendorp RG, Blauw GJ. Nitric oxide mediates hypoxia-induced cerebral vasodilation in humans. J Appl Physiol. 2002;92:962–966. doi: 10.1152/japplphysiol.00616.2001. [DOI] [PubMed] [Google Scholar]