Abstract
Phosphoinositides are key regulators of synaptic vesicle cycling and endocytic traffic; the actin cytoskeleton also seems to be involved in modulating these processes. We investigated the effects of perturbing phosphoinositide signalling and actin dynamics on vesicle cycling in frog motor nerve terminals, using fluorescence and electron microscopy, and electrophysiology. Antibody staining for β-actin revealed that actin surrounds but does not overlap with synaptic vesicle clusters. Latrunculin A, which disrupts actin filaments by binding actin monomers, and wortmannin, an inhibitor of phosphatidyl inositol-3-kinase (PI3-kinase), each disrupted the pattern of presynaptic actin staining, but not vesicle clusters in resting terminals. Latrunculin A, but not wortmannin, also reduced vesicle mobilization and exocytosis. Both drugs inhibited the stimulation-induced uptake of the styryl dye FM1-43 and blocked vesicle reformation from internalized membrane objects after tetanic stimulation. These results are consistent with a role of PI3-kinase and the actin cytoskeleton in the slow pathway of vesicle endocytosis, used primarily by reserve pool vesicles.
During repetitive nerve stimulation, synaptic vesicles must undergo repeated rounds of exocytosis, endocytosis, refilling, and mobilization, a phenomenon called recycling. While some aspects of the cycle, particularly exocytosis, can be addressed in molecular detail, other features, especially vesicle trafficking mechanisms, are less well understood. One complicating factor is the existence of multiple vesicle pools, which appear to be recruited by biochemically distinct mechanisms and are refilled by distinct routes of endocytosis (Kuromi & Kidokoro, 1998, 2000, 2002; Richards et al. 2000, 2003). At the frog neuromuscular junction (NMJ), vesicles are divided into a readily releasable pool (RRP) and a reserve pool. RRP vesicles cycle rapidly (∼1 min) via a fast endocytic route, while reserve pool vesicles use a slow pathway (∼10 min) that relies on formation of endosomal intermediates (Heuser & Reese, 1973; Richards et al. 2000; see also Teng & Wilkinson, 2000). These are long tortuous infoldings that retain contact with the extracellular space for many minutes (Richards et al. 2000), and are eventually segregated into new synaptic vesicles, via clathrin-coat-dependent mechanisms.
Phosphoinositides (PI) are important modulators of vesicle cycling in a variety of systems including the presynaptic terminal (reviewed by Cremona & de Camilli, 2001). Recent findings suggest a strong involvement of PI molecules in endocytosis, particularly in clathrin-coat-dependent processes. Formation of clathrin-coated vesicles requires PI binding by adaptor proteins such as AP-2 and AP-180; interactions between PI molecules and proteins like epsin and the GTPase dynamin are also necessary for endocytic vesicles to pinch-off (reviewed by Osborne et al. 2001; see also Hurley & Wendland, 2000). A role for PI in synaptic vesicle exocytosis is also emerging; the putative calcium sensor of synaptic release, synaptotagmin (Geppert & Sudhof, 1998), has been shown to bind PI molecules in vitro (Schiavo et al. 1996), and the interaction between synaptotagmin and PIP2 steers vesicle exocytosis toward the plasma membrane (Bai et al. 2004) and promotes Ca2+-triggered exocytosis in PC12 cells (Tucker et al. 2003), and may also play a role in endocytosis (Littleton et al. 2001).
The actin cytoskeleton is also regarded as an important participant in vesicle trafficking. Filamentous actin is found in presynaptic nerve terminals of the frog (Dunaevsky & Connor, 2000) and snake (Cole et al. 2000) neuromuscular junctions (NMJ), and at mammalian hippocampal neurones (Morales et al. 2000). It surrounds the clusters of synaptic vesicles found at frog NMJ and hippocamapal nerve terminals (Dunaevsky & Connor, 2000; Sankaranarayanan et al. 2003). It may play a role in vesicle endocytosis, perhaps moving endosomes and nascent vesicles back into vesicle pools (Shupliakov et al. 2002), which is consistent with evidence suggesting that clathrin accessory factors regulate actin dynamics (Slepnev & de Camilli, 2000). Also, during coated pit formation, actin is recruited shortly after dynamin, and immediately prior to internalization (Merrifield et al. 2002). Previous work has also suggested that actin might also be involved in exocytosis, and it has been proposed to act as a scaffold for the mobilization of vesicles from the reserve pool (Ryan, 1999; Cole et al. 2000). At a physiological level, the results of actin disruption vary between a reduction in release (Cole et al. 2000), no significant effect (Job & Lagnado, 1998; Holt et al. 2003), and a transient increase in release (Morales et al. 2000). The effect of actin disruption on vesicle clustering has not been investigated at the neuromuscular junction, although studies at synaptic terminals of goldfish retinal bipolar cells (Job & Lagnado, 1998) and rat hippocampal synapses (Sankaranarayanan et al. 2003) suggest that disruption does not affect the localization of synaptic vesicles.
In the present work we have combined fluorescent imaging, electrophysiology, and electron microscopy to investigate the effects of phosphoinositide signalling and the actin cytoskeleton on synaptic vesicle cycling. We perturbed actin dynamics using latrunculin A, a member of a family of toxins from the Red Sea sponge that sequester G-actin (Coue et al. 1987), thus preventing actin polymerization and consequently disrupting the ‘treadmilling’ of actin filaments. We also perturbed PI signalling by inhibiting PI3-kinase using wortmannin (Arcaro & Wymann, 1993). We show that both drugs disrupt the actin cytoskeleton and that wortmannin selectively blocks the slow pathway of endocytosis, while latrunculin A affects both exocytic and endocytic processes. A preliminary report of some of these findings has appeared previously (Richards & Betz, 2000).
Methods
Fluorescence microscopy
Following anaesthesia with ethyl 3-aminobenzoate methanesulphonate salt (Tricaine), frogs (Rana pipiens) were decapitated, and the cutaneous pectoris muscle preparation dissected out and mounted in Sylgard-lined chambers as previously described (Betz & Bewick, 1992; Richards et al. 2000). All procedures were carried out with the approval of the University of Colorado Health Sciences Center Institutional Animal Care and Use Committee. The presynaptic actin isoform (β-actin) was visualized using a fluorescein-conjugated antibody (Sigma, St Louis, MO, USA). This labelled actin within nerve terminals but did not label the underlying muscle fibre. Preparations were fixed with ice-cold methanol for 5 min, and then washed in phosphate buffer solution (PBS) (0.1 m, pH 7.3, 4°C). They were blocked with BSA dissolved in PBS for 1 h at 4°C, and then incubated with antibody (1: 200) for a further hour. They were then washed in PBS for a further hour prior to imaging.
For live imaging of FM1-43 and FM4-64 (Molecular Probes, Eugene, OR, USA), methods were modifications of those previously described (Betz & Bewick, 1992; Richards et al. 2000). Briefly, frog cutaneous pectoris nerve muscle preparations were dissected and mounted in a Sylgard-lined chamber containing frog Ringer solution (115 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 2.4 mm NaHCO3). FM1-43, was used at 3.2 μm. When stimulation and imaging took place simultaneously, curare (10 μm) was added to the chamber to prevent muscle contraction. In these experiments images were acquired every 1.4 s. The nerve was stimulated with a suction electrode. Postsynaptic receptors were visualized using rhodamine-conjugated α-bungarotoxin (Molecular Probes), as previously described (Wu & Betz, 1999).
Fluorescence images were acquired using a Nikon Optiphot 2 upright epifluorescence microscope equipped with a 63×, 0.9 NA, water immersion objective lens (Zeiss). Excitation light came from a 100 W Hg lamp, through 5–50% neutral density transmission filters, excitation filters (435/10 nm or 480/30 nm), dichroic mirror (505 or 565 nm), and emission filters (500/10 nm or 650/10 nm). Images were acquired with a Photometrics (Tucson, AZ, USA) SenSys 0400 camera (0.5–2 s exposure) connected to a computer running V for windows imaging software (Digital Optics, Auckland New Zealand). Image analysis was performed using software from G.W. Hannaway (Boulder, CO, USA).
Electrophysiology
For intracellular recording we used sharp microelectrodes (20–40 MΩ) pulled with a box filament on a Flaming-Brown micropipette puller (Sutter, Novato, CA, USA). Muscle fibres had an input resistance of 1–2 MΩ and a resting membrane potential more negative than −70 mV. Endplate potentials (EPPs) were recorded with a concentration of curare sufficient to prevent muscle fibres from reaching firing threshold (1 μm). Signals were amplified through an Axoclamp-2A (Axon Instruments) and digitized using a MIO-16E interface (National Instruments). Recording and analysis software was either custom written, running in the LabView environment (National Instruments; Austin, TX, USA), or WCP for windows (Dr John Dempster, University of Strathclyde, UK).
Electron microscopy
Preparations were fixed in cold PBS (0.1 m, pH 7.3, 4°C) containing paraformaldehyde (1.6%) and glutaraldehyde (2%) for 20 min. The muscles were then isolated from the skin and the sternum and washed vigorously with PBS (0.1 m, 4°C) for 45 min. Preparations were osmium postfixed (2% OsO4 in 0.1 m PBS, pH 7.3, 4°C) for 1 h, dehydrated through an ascending series of ethanol solutions and stained en block with uranyl acetate (2% in 50% ethanol for 30 min). After dehydration tissue was embedded in Epon. The blocks were cross-sectioned in silver-grey (80–90 nm), collected and viewed in a CM-10 Philips electron microscope. Electron micrographs of nerve terminals were scanned from the original negatives, and analysed using Image J (NIH) on a Windows NT workstation.
Statistics were calculated using two-tailed Student's t tests in Microcal Origin. Errors are given as s.e.m.
Results
β-Actin localizes around FM4-64-labelled vesicle clusters
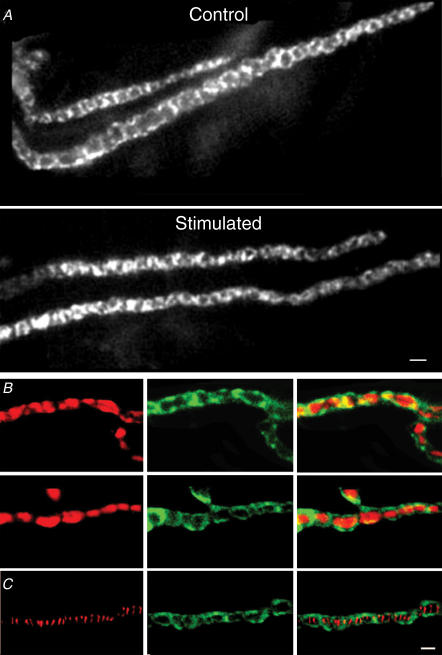
We used a fluorescein isothiocyanate (FITC)-conjugated antibody to β-actin (Cole et al. 2000), to visualize the pattern of actin staining in frog cutaneous pectoris motor nerve terminals. As shown in Fig. 1A, the staining resembled ring-like links in a bracelet, each ring on the order of a micron in diameter. Tetanic nerve stimulation (5 min at 30 Hz) caused a subtle disorganization of this arrangement (Fig. 1A, lower panel). The crisp, regular appearance of actin staining in controls became less ordered following stimulation; the rings had gaps, were not as uniform in thickness, and were interspersed with regions where the staining between gaps was widened. The pattern of β-actin staining was indistinguishable from phalloidin staining in this preparation (Dunaevsky & Connor, 2000), and, as shown in Fig. 1B, staining synaptic vesicles with FM4-64 showed that the rings of β-actin surrounded the vesicle clusters. Finally, Fig. 1C shows a typical result from a preparation stained with rhodamine-conjugated α-bungarotoxin (which labels postsynaptic acetylcholine receptors, left panel) and β-actin (middle panel). The overlay (right panel) shows that the α-bungarotoxin stripes, like vesicle clusters, lie within the ‘holes’ of the actin staining.
Figure 1. β-Actin surrounds synaptic vesicle clusters.
A, an example of anti-β-actin staining in control (upper) and stimulated (lower) nerve terminals. The staining forms regular rings along the length of the control nerve terminal. The right panel shows a nerve terminal stimulated for 5 min at 30 Hz immediately prior to fixation; here the actin staining is less regular, as though it has been partially disrupted. Scale bar = 2 μm. B, two examples of nerve terminals loaded with FM4-64 (left-hand column), then fixed and stained for β-actin (middle column). The images are merged in the images in the right-hand column, which show little overlap. C, staining of postsynaptic acetylcholine receptors with rhodamine-conjugated α-bungarotoxin (left-hand panel) and of β-actin (middle panel). The right-hand panel is an overlay image, showing that the actin surrounds areas that lie directly above the postsynaptic folds. Scale bar = 1 μm for B and C.
Wortmannin and latrunculin A disrupt presynaptic actin but not vesicle clusters
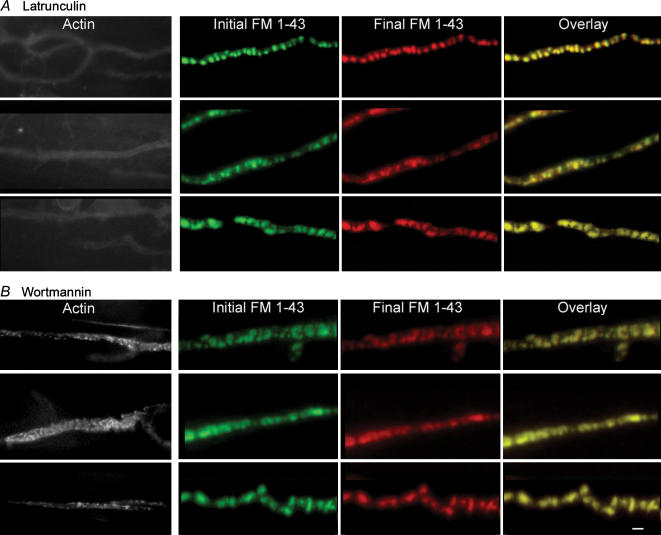
We next examined the effects of latrunculin and wortmannin on the β-actin staining pattern. Figure 2 shows that both drugs profoundly disrupted the pattern of β-actin staining without affecting synaptic vesicle clusters. Preparations were stained with FM1-43 and imaged (Fig. 2, second column), then treated with drug (100 nm wortmannin or 15 μm latrunculin A) for 1 h and reimaged (Fig. 2, third column), which revealed that neither drug caused significant changes in FM1-43 staining pattern (Fig. 2, last column). Preparations were then fixed and stained for β-actin (Fig. 2, first column); the normal pattern (Fig. 1) was greatly disrupted. These results show that β-actin and PI3-kinase signalling are not required for the maintenance of synaptic vesicle clusters in resting nerve terminals.
Figure 2. Disruption of β-actin does not affect vesicle clustering in resting terminals.
A, three typical examples show that latrunculin A destroyed actin staining (left-hand column; images were acquired with increased illumination and longer exposure times). Images of FM1-43 staining patterns before (second column) and after (third column) latrunculin A treatment are overlaid (right-hand column), and show virtually no change. B, wortmannin disrupted actin staining (layout like A) in a manner similar to nerve stimulation, although more severe. Vesicle clusters were not affected. Scale bar = 1 μm.
Wortmannin and latrunculin A reduce evoked but not spontaneous release of acetylcholine
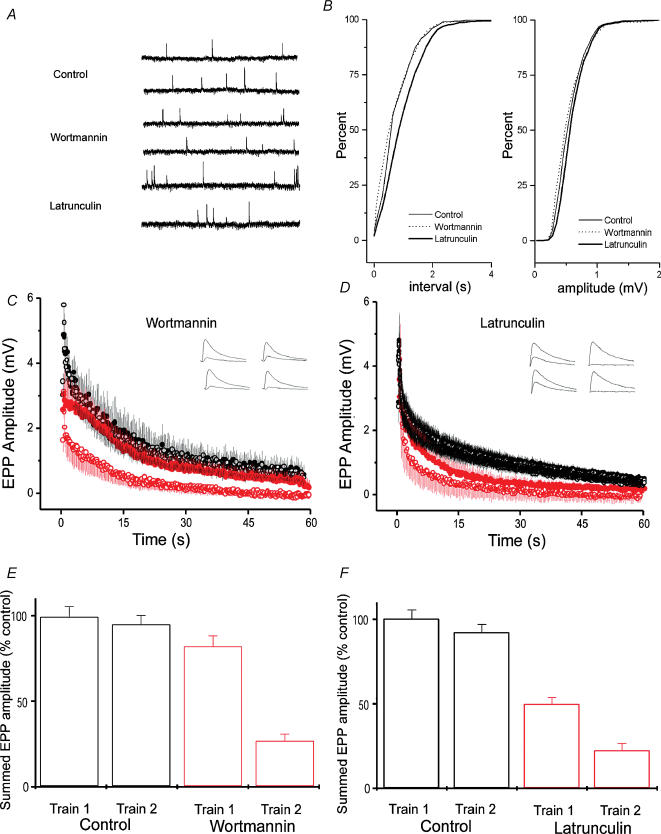
To examine transmitter release, we made intracellular recordings from muscle fibres under various conditions. The amplitudes (Fig. 3A) and frequency (Fig. 3B) of spontaneous miniature endplate potentials (mEPPs) were not significantly altered by either 100 nm wortmannin or 15 μm latrunculin A. The amplitudes of EPPs evoked by low frequency nerve stimulation in curarized preparations were also not affected (data not shown). It was only with repetitive stimulation at high frequency that the effects of drug treatment became apparent. In these experiments, two trains (each 30 Hz for 60 s) were given 20 min apart. In controls, the responses of the two trains were indistinguishable (that is, the terminals recovered completely from synaptic depression during the 20 min rest; see Richards et al. (2003) for a detailed characterization of this recovery). In preparations poisoned with wortmannin for 1 h before any stimulation, transmitter release during the first train was not different from controls, but release during the second train was profoundly depressed (Fig. 3C and E). This suggests that wortmannin did not affect vesicle mobilization, but interfered with vesicle recycling. In contrast, treatment with latrunculin reduced release during both trains (Fig. 3D and F), suggesting interference with both mobilization and recycling. These hypotheses were tested in further experiments, as described below.
Figure 3. Disruption of presynaptic actin and PI signalling reduces transmitter release during tetanic stimulation.
A, examples of mEPPs recorded from muscles from control preparations, following wortmannin treatment and following latrunculin A treatment. B, analysis of mEPPs, recorded from 5–7 preparations. Cumulative frequency plots for inter-event interval (left-hand panel) and amplitude (right-hand panel). No significant changes in amplitude were observed in latrunculin- or wortmannin-treated preparations, although a small reduction in frequency was observed in latrunculin A-treated preparations. C, EPP amplitudes during tetanic stimulation in control (black) and wortmannin-treated (red) preparations. Synaptic depression during a 1 min, 30 Hz train was normal during a first train in wortmannin-treated preparations (upper red curve). However, a second train applied 20 min after the first showed severe depression (lower red curve; n = 8, P < 0.001). Control responses during the two trains superimpose (n = 8). D, synaptic rundown in latrunculin A-treated preparations. Synaptic depression during a 1 min, 30 Hz train was faster during a first train in latrunculin A-treated preparations (n = 8, P < 0.01). A second train applied 20 min after the first showed greatly enhanced rundown (P < 0.001). E, summed EPPs, normalized to the amount of release in control preparations, are plotted for first and second trains in control and wortmannin-treated preparations. Release was normal during the first train in wortmannin, but was greatly reduced during a second train given 20 min after the first. F, summed EPPs, normalized to control preparations, are plotted for first and second trains in control and latrunculin A-treated preparations. Release was reduced during the first train in latrunculin A-treated preparations, and reduced even more during a second train given 20 min after the first.
Wortmannin and latrunculin A reduce uptake of FM1-43
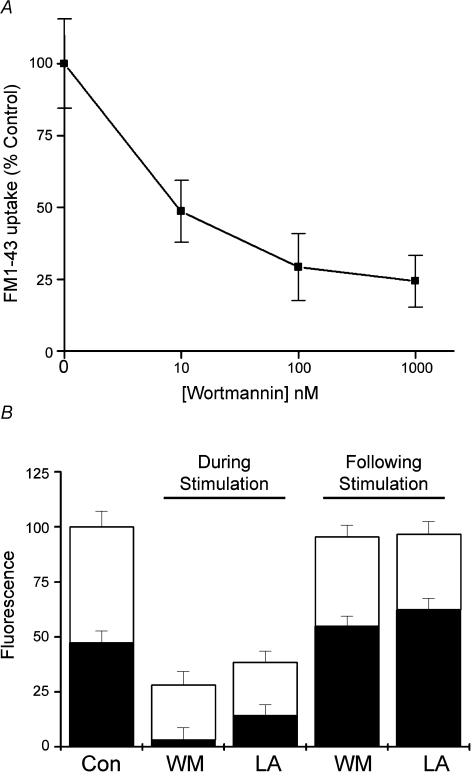
Since wortmannin is known to affect multiple targets depending on the concentration at which it is applied, we measured the dose–response curve for inhibition of FM1-43 uptake. Nerve terminals were stimulated at 30 Hz for 1 min and endocytosis was measured as the fluorescence of internalized FM1-43 (Fig. 4a). Wortmannin is specific for PI3-kinases only at very low concentrations (typical IC50 for PI3-kinases is 10–20 nm; Davies et al. 2000). Our experiments show that for this assay, wortmannin had an IC50 in the low nanomolar range, consistent with much of its effect being due to inhibition of PI phosphorylation, rather than its reported action on myosin light chain kinease (MLCK) (IC50∼1 μm; Davies et al. 2000) or mitogen activated protein (MAP) kinase (also about 1 μm). We also investigated the effect of an unrelated PI3-kinase inhibitor, LY294002 (50 μm), which caused a similar reduction in FM1-43 uptake (30.4 ± 3.2% of control, compared to 100 nm wortmannin, which reduced uptake to 29.3 ± 3.6%, n = 6, P < 0.01). Finally, we examined the effect of 15 μm latrunculin A on FM1-43 uptake; we found that it caused a similar degree of inhibition of FM1-43 uptake as the other agents studied (24.4 ± 6.6% of controls, n = 5, P < 0.01). In Fig. 4B, the height of each bar shows the amount of FM1-43 uptake relative to controls under different conditions. These preparations were then stimulated (30 Hz for 1 min) a second time to release FM1-43; the amount of fluorescence remaining after stimulation is indicated by the black bars. Note the second and third bars from the left: although preincubation with either latrunculin or wortmannin greatly reduced the uptake of FM1-43, the destaining of the small amount of dye taken up was very different. The percentage of destaining seen in latrunculin-treated preparations was similar to control (40.5 ± 5.9, compared to 52.5 ± 6.5), whereas the percentage of destaining in wortmannin-treated preparations was enhanced (88.4 + 9.5, P < 0.01). In other words, what little dye was taken up after wortmannin poisoning was almost totally released by further stimulation. In contrast, terminals loaded prior to exposure to wortmannin showed no difference in their destaining compared to control (42.5 ± 5.9%, compared to 48.5 ± 4.6), and latrunculin-treated preparations showed a small decrease (35.4 ± 7.2), which was not significant.
Figure 4. Disruption of presynaptic actin and PI signalling impairs vesicle cycling.
A, dose–response curve for the effect of wortmannin on the uptake of FM1-43. Terminals were stimulated for 1 min at 30 Hz in the presence of FM1-43, after incubation with various concentrations of wortmannin for 1 h. B, comparison between uptake (total height of bars) and release (open sections of bars) of FM1-43 following treatment with wortmannin (WM) and latrunculin A (LA). Both agents significantly reduced the uptake of FM1-43 compared to control (Con) preparations if applied before loading (n = 5–6, P < 0.01); interestingly, nearly all dye internalized after wortmannin treatment could be released. If the drugs were applied after FM1-43 was loaded, no significant effect was seen.
Effect of wortmannin and latrunculin on nerve terminal ultrastructure before and after stimulation
To understand better the mechanisms by which latrunculin and wortmannin were influencing vesicle recycling, we investigated the effects of these agents on the ultrastructure of nerve terminals at different points in the vesicle cycle. As shown in Fig. 5A–C, neither wortmannin nor latrunculin had much effect on the ultrastructure of unstimulated motor nerve terminals. In a few cases in the latrunculin-treated group we saw unusual membranous structures within the nerve terminal (Fig. 5C). In those sections where an active zone was visible, we quantified the mean distance of synaptic vesicles from the nearest active zone. Results are plotted in Fig. 5D. Little change in the distribution of vesicles was seen between the groups. We also characterized the overall number of synaptic vesicles contained within each nerve terminal (Fig. 5E) and the amount of ‘empty space’ (regions with no discernable structures, and without membrane separating them from the rest of the terminal) within the nerve terminal (Fig. 5F). Latrunculin-treated nerve terminals showed a small reduction in the total number of vesicles within nerve terminals, and a corresponding increase in the amount of empty space. Wortmannin-treated nerve terminals were indistinguishable from control.
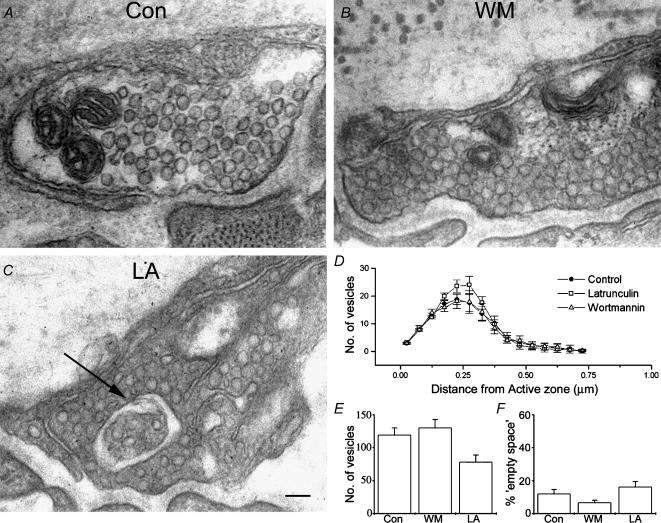
Figure 5. Morphology of resting nerve terminals is normal following treatment with latrunculin and wortmannin.
A, a representative electron micrograph of an untreated, unstimulated nerve terminal. B, a representative wortmannin-treated preparation, showing normal ultrastructure. C, an electron micrograph of a latrunculin-treated preparation, illustrating the type of anomaly sometimes seen in these preparations (arrow). Scale bar = 100 nm D, quantification of numbers of vesicles plotted against distance from the active zone. No significant difference was observed between treatments. E, total number of vesicles seen in terminals for each treatment. F, ‘empty space’ observed in resting nerve terminals. No significant difference was seen between preparations. Data plotted in panels B–D are mean values obtained from > 20 terminals, from 5 preparations each.
We next compared the ultrastructure of nerve terminals after intense stimulation (5 min at 30 Hz, fixed about 1 min later). In control preparations the main effect of stimulation was a loss of vesicles coupled to an increase in surface membrane infoldings and cisternae (Fig. 6A). In both wortmannin- and latrunculin-treated preparations, the changes caused by stimulation included additional elements. As shown in Fig. 6B and C, in addition to vesicle depletion, there were few typical cisternae, but many examples of more complex membranous structures, especially regions of stacked membrane. Such structures were never observed in our control preparations, and are rarely seen in normal nerve terminals (S. O. Rizzoli, unpublished observation). Where possible we quantified the distance of synaptic vesicles from release sites. All groups (control, wortmannin- and latrunculin-treated) showed a depletion of synaptic vesicles at sites distal from the release site (Fig. 6B). No significant difference was seen in the number of synaptic vesicles per terminal (Fig. 6C), but as shown in Fig. 6D, complex membrane structures made up a significant fraction of the available cross-sectional area of nerve terminals in both latrunculin- and wortmannin-treated nerve terminals.
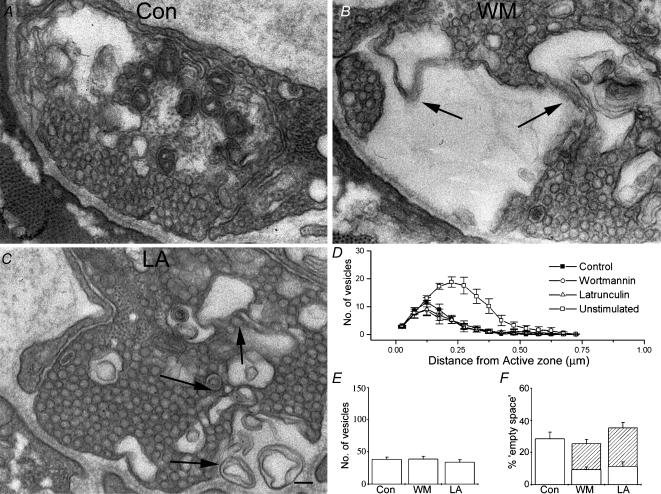
Figure 6. Both latrunculin and wortmannin perturb the morphology of stimulated nerve terminals.
A, a typical electron micrograph of a control nerve terminal following stimulation. B, wortmannin treatment, coupled to stimulation, results in a severe perturbation of nerve terminal ultrastructure. Arrows indicate unusual multilamellar membrane structures seen in these and latrunculin-treated preparations following stimulation. C, an electron micrograph of a latrunculin-treated preparation. Again, arrows indicate morphological abnormalities. In all cases (A, B and C) the ultrastructure is altered compared to resting terminals. In controls this was predominantly seen as a loss of vesicles and the appearance of endocytic intermediates (cisternae). In both wortmannin-treated and latrunculin-treated preparations, in addition to the loss of vesicles, complex membrane structures were often evident. Scale bar = 100 nm D, quantification of numbers of vesicles plotted against distance from the active zone. No significant difference was seen between treatments. Control distribution is plotted again from Fig. 5B for purposes of comparison. E, total number of vesicles seen in terminals for each treatment. F, ‘empty space’ seen in nerve terminals (open sections of bars) and complex membrane regions (hatched sections of bars). The failure of wortmannin- and latrunculin-treated preparations to match the increase in empty space seen in control preparations (P < 0.01) is explained if one considers the area occupied by the complex membrane regions. As in Fig. 5, data plotted in panels B–D are mean values obtained from >20 terminals, from 5 preparations each.
Finally, we examined terminals in muscles that were rested for 15 min after tetanic stimulation (Fig. 7), by which time control terminals fully recover (Richards et al. 2003). Treatment with wortmannin or latrunculin A entirely blocked recovery (Fig. 7D—F). The similarity between the ultrastructure of wortmannin and latrunculin-treated, stimulated, nerve terminals suggests that this block occurred at the same point in both.
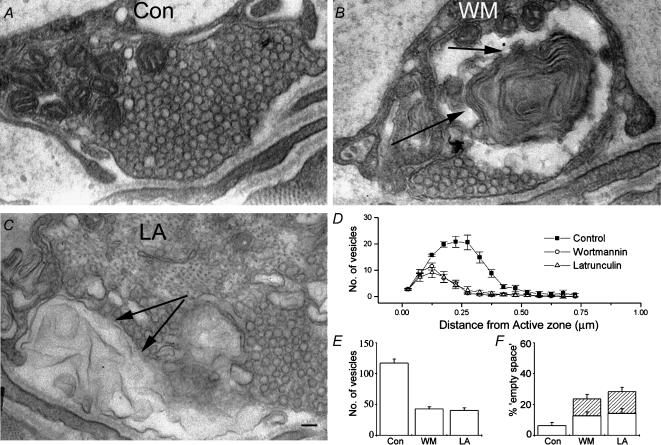
Figure 7. Latrunculin and wortmannin effects were not reversible.
A, a representative electron micrograph showing a nerve terminal which has recovered from stimulation, revealing ultrastructure which is indistinguishable from an unstimulated preparation. B, wortmannin-treated preparations which have been stimulated no longer recover. Multilaminar membrane structures, similar to those seen in treated preparations immediately following stimulation (arrows), persist even 15 min after the end of stimulation. C, similarly to wortmannin-treated preparations, those treated with latrunculin A also fail to recover within 15 min of stimulation. Again, arrows indicate abnormal membraneous structures. Scale bar = 100 nm. D, quantification of numbers of vesicles plotted against distance from the active zone. Control preparations showed full recovery to unstimulated values, whereas treated preparations showed no recovery (P < 0.001). E, total number of vesicles seen in terminals for each treatment. F, ‘empty space’ in nerve terminals (open sections of bars) and complex membrane regions (hatched sections of bars). The wortmannin- and latrunculin-treated preparations retain the complex membrane regions. Controls have recovered to normal. Again, data plotted in panels B–D are mean values obtained from >20 terminals, from 5 preparations each.
Discussion
We have investigated the effects of two drugs – latrunculin A and wortmannin – on the actin cytoskeleton and on synaptic vesicle clustering and recycling. The drugs have overlapping but non-identical effects. Both disrupted the normal pattern of actin staining, but neither treatment, however, altered the clustering of synaptic vesicles in resting terminals, indicating that actin is not required for linking vesicles together, a role that may be filled by synapsin molecules (Pieribone et al. 1995). This result also argues against the hypothesis that actin filaments (which are also found within the vesicle clusters; Hirokawa et al. 1989) act as a barrier to synaptic vesicle mobilization from the reserve pool (RP) (Wang et al. 1996), as actin disruption would then result in an increase, not a decrease of vesicle release in our experiments.
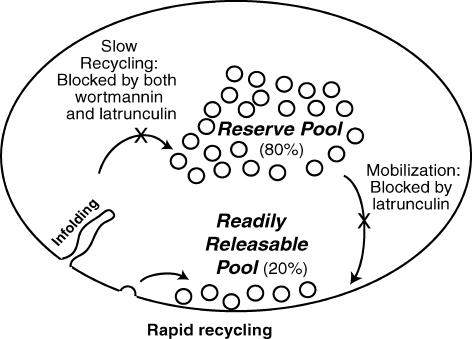
Tetanic nerve stimulation in the presence of the drugs revealed further effects, which we monitored with electrophysiological, fluorescence and electron microscopic techniques. The main results are summarized in Fig. 8. Latrunculin A inhibited both vesicle mobilization and the slow endocytic arm of vesicle recycling, while wortmannin selectively affected vesicle recycling.
Figure 8. Summary cartoon.
Two pathways of endocytosis in nerve terminals are illustrated. Our results are explained by a model in which rapid recycling is unaffected by either latrunculin A or wortmannin, but the slow route of endocytosis is blocked by both drugs. Latrunculin A also impairs release by interfering with vesicle mobilization.
The relatively selective effect of wortmannin on endocytosis was most clearly illustrated with electrophysiological recordings. The total amount of transmitter released during a 30 Hz, 1 min tetanus (measured by summing EPP amplitudes in curarized preparations) was not significantly affected by wortmannin (Fig. 3C and E). However, the terminal was unable to recover; a second tetanus given 20 min later (by which time control terminals had recovered completely) was profoundly depressed. In other words, wortmannin did not interfere with mobilization and exocytosis during the first tetanus, but blocked most recycling and regeneration of vesicles. Electron microscopic observations (Figs 5–7) confirmed this: wortmannin treatment had no discernable effect on the structure of resting nerve terminals, but after tetanic stimulation, membrane recycling was arrested; terminals were replete with cisternae, surface membrane infoldings, and other non-vesicular forms. The reduced uptake of FM dyes (Fig. 4) was further evidence of inhibition of recycling.
Which pool of vesicles is affected?
At the frog neuromuscular junction the population of vesicles comprises a readily releasable pool (RRP) and a reserve pool. These pools were identified and partially characterized in earlier work using different FM dyes; the RRP comprises about 20% of the total vesicle population, and the reserve pool the remainder (Richards et al. 2000). Cycling of reserve pool vesicles is slow, and requires formation of membrane infoldings and membrane-bound endosomal intermediates, while RRP cycling does not. Do latrunculin A and wortmannin affect both pools? At the ultrastructural level, the depletion of synaptic vesicles and their replacement with larger membrane-bound objects indicates that both of these agents have an effect on recycling of the reserve pool. Also, both drugs seem to have little effect on the population of vesicles situated in close proximity to the exocytic active zones, where RRP vesicles are expected to be located (Figs 6B and 7B).
At the functional level, the fact that latrunculin A affects vesicle mobilization as well as recycling makes it difficult to assess its effects on the RRP. Wortmannin, which does not affect mobilization, allows for the continuous cycling of a small population of vesicles that correlates well in size with the RRP: FM dye uptake during a 30 Hz, 1 min tetanus applied after wortmannin treatment was about 25% of control (Fig. 4A); electrophysiologically, the second of a pair of tetani (30 Hz, 1 min) released about 20% as much transmitter as control terminals (Fig. 3E). Also, the dye taken up by loading after wortmannin treatment could be released almost completely by a second tetanus (Fig. 4B), which is characteristic of the RRP (Richards et al. 2000, 2003). These results suggest that both drugs inhibit selectively the slow recycling process that regenerates the reserve pool of vesicles, and not recycling of vesicles into the RRP.
The actin cytoskeleton – a scaffold for vesicle recycling at the frog NMJ?
Disruption of the actin cytoskeleton by latrunculin A resulted in an inhibition of vesicle release, consistent with results obtained at NMJs of the snake (Cole et al. 2000) and Drosophila larva (Kuromi & Kidokoro, 1998), suggesting that actin has a scaffold role in vesicle mobilization (possibly from the RP) to release sites (Ryan, 1999). At the ribbon synapses of bipolar goldfish retina cells actin disruption did not affect vesicle release (Job & Lagnado, 1998; Holt et al. 2003), possibly because of the reliance of these nerve terminals on a different mode of vesicle mobilization or vesicle fusion (ribbon-based motility, Lenzi & von Gersdorff, 2001; or compound vesicle fusion, Parsons & Sterling, 2003). At hippocampal boutons, which rely on release from a limited pool of vesicles (Harata et al. 2001), and seem to frequently use a ‘kiss-and-run’ mode of vesicle cycling (Gandhi & Stevens, 2003; Aravanis et al. 2003) release was also not depressed by latrunculin A (Morales et al. 2000; Sankaranarayanan et al. 2003).
Latrunculin also inhibited vesicle recycling, causing endosomal objects to develop within the terminals after tetanic stimulation. This suggests that membrane infoldings forming at the plasma membrane need the physical support of the actin cytoskeleton in order to progress normally within the nerve terminal. Also, actin polymerization may provide the means for the transport of the resultant endosomes inside the terminal, as is the case for vacuoles formed by macropinocytosis (Merrifield et al. 1999), which are driven through the cells by short-lived actin comets similar to those that propel infectious microorganisms thorough mammalian cells (Tilney & Tilney, 1993). A similar mechanism has been proposed for the transport of newly endocytosed vesicles at lamprey reticulospinal synapses (Shupliakov et al. 2002).
The refilling of the RP was inhibited by actin disruption at the Drosophila NMJ (Kuromi & Kidokoro, 1998), consistent with our findings, as the RP appears to refill through a slow, endosome-dependent route at this synapse, as at the frog NMJ (Koenig & Ikeda, 1996). Also, actin disruption has also been shown to inhibit bulk membrane endocytosis but not synaptic vesicle cycling in goldfish retinal bipolar cells (Holt et al. 2003; it should be noted that the role of bulk endocytosis is still unclear at this synapse, though it may serve a role similar to that at the frog NMJ, Paillart et al. 2003). At hippocampal synapses actin does not appear to be required for internalization (Sankaranarayanan et al. 2003), consistent with a limited role for slow endocytosis in this system (Murthy & Stevens, 1998; Aravanis et al. 2003; however, see also Takei et al. 1996 for a description of an endosomal intermediate-dependent recycling route at hippocampal synapses).
Wortmannin effects – multiple targets of inhibition?
A tempting hypothesis is that the effects of wortmannin are mediated by its disruption of the actin cytoskeleton (Fig. 2B), as phosphoinositides are known to modulate actin dynamics (reviewed by Takenawa & Itoh, 2001). Wortmannin disrupted the actin staining (though less severely than latrunculin, which abolished staining completely, Fig. 2), and another PI3-kinase inhibitor (LY294002) has also recently been shown to perturb actin dynamics (it stopped the stimulation-dependent growth of the actin cytoskeleton in goldfish retinal bipolar cells; Holt et al. 2003). The similarity between the effects of wortmannin and latrunculin at the ultrastructural level (both cause vesicle depletion and an increase in the number of endosomal objects) also argues in favour of a common mechanism of action.
The effects of latrunculin and wortmannin on vesicle release are more difficult to reconcile, as latrunculin perturbs vesicle mobilization while wortmannin does not. One explanation would be that latrunculin, being a more potent disruptor (see Fig. 2), affects the cytoskeletal elements necessary in vesicle mobilization from the RP, as well as the stimulation-induced actin remodelling responsible for vesicle recycling (Shupliakov et al. 2002; see also Fig. 1A). Wortmannin, inhibiting actin dynamics less potently, may only affect stimulated actin dynamics (as in the experiments of Holt et al. 2003; see above). It is well established that actin undergoing rapid cycling is much more sensitive to perturbations than more stable actin filaments (Hall, 1998). This is supported by Fig. 2, where latrunculin treatment virtually abolishes actin staining, whereas a more complex disruption or derangement of actin is observed following wortmannin treatment.
An equally valid hypothesis is that phosphoinositide signalling may directly affect vesicle cycling. Bulk endocytosis (thought to be clathrin independent) is followed by vesicle reformation through clathrin-coat-dependent mechanisms (Takei et al. 1996), which are regulated by PI molecules (Cremona & De Camilli, 2001). Briefly, clathrin-coat formation begins with the binding of the adaptor protein AP-2 to the membrane, followed by the binding of a number of other proteins (such as the adaptor AP-180, auxilin and amphiphysin) and by clathrin assembly (Brodin et al. 2000). AP-2 and AP-180 are PI-binding proteins, and their recruitment to membranes is modulated by this interaction (reviewed by Cremona & De Camilli, 2001). PI molecules also influence the endocytic vesicle pinch-off (dependent upon the GTPase dynamin). Finally, the uncoating of the endocytosed vesicles is dependent upon PI-(4,5)-P2 dephosphorylation by synaptojanin (Osborne et al. 2001). Thus, it is possible that wortmannin disrupts vesicle reformation from internalized membrane through an inhibition of clathrin-coat-dependent mechanisms.
While the role of clathrin in vesicle reformation from endosomal objects is firmly established (Takei et al. 1996), it is more difficult to assess its importance in RRP recycling. RRP vesicles recycle through endosome-independent mechanisms at the frog NMJ, but their mode of internalization is not yet completely understood. Our model of vesicle cycling (Richards et al. 2000, 2003) can accommodate at least two possible mechanisms: clathrin-mediated internalization directly from the plasma membrane, and ‘kiss-and-run’ (incomplete fusion followed by rapid, clathrin-independent recovery through scission of the fusion pore). Does the wortmannin data argue for a ‘kiss-and-run’ mechanism? A blockage of clathrin-mediated mechanisms, coupled with wortmannin's lack of effects on RRP cycling supports such a hypothesis. However, we cannot exclude the possibility that the wortmannin inhibition of clathrin-mediated mechanisms is incomplete, and that the fraction of the endocytic machinery remaining active is sufficient for the internalization of the RRP, but not for the reformation of RP vesicles from internalized endosomes. The saturating dose dependence of wortmannin on FM1-43 uptake shown in Fig. 4 speaks against this possibility, however.
Does wortmannin affect PI signalling through PI3-kinase inhibition?
Clathrin adaptor molecules have been shown to bind 3-phosphorylated PI molecules (Hao et al. 1997; Gaidarov & Keen, 1999), and the interaction appears functionally relevant (at least for AP-2; Rapoport et al. 1997). Also, clathrin itself has recently been shown to interact directly with an isoform of PI3-kinase (PI3K C2α), recruiting it to coated pits and enhancing its catalytic activity (Gaidarov et al. 2001), and wortmannin has been shown to block PI3-kinase activities associated with purified clathrin-coated vesicles and synaptic vesicles (Prior & Clague, 1999).
Wortmannin has been shown to inhibit another related enzyme, PI4-kinase, which may also contribute to its effects on vesicle cycling. At the frog NMJ we recently investigated the effects of a different PI3-kinase inhibitor, LY294002 (LY; Rizzoli & Betz, 2002), which blocks PI4-kinase less potently (reviewed by Stein & Waterfield, 2000). As LY blocks casein kinase 2 (CK2; IC50 similar to that for PI3-kinase; Davies et al. 2000), not all the effects of LY and wortmannin are directly comparable. However, they both inhibit synaptic vesicle recycling as measured by FM dye uptake. At the ultrastructural level, a depletion of vesicles is found after both wortmannin and LY treatments. Wortmannin induces the formation of endosomal structures after tetanic stimulation, suggestive of defects in vesicle reformation after internalization. Vesicle reformation appears to be blocked by LY, also at a step after internalization, and vesicles are replaced by irregularly sized endosomes. The highly convoluted structures seen with wortmannin were not observed in the LY treatment, but this may depend upon the stimulation method used: tetanic stimulation, releasing most of the vesicles within a few minutes for wortmannin, and spontaneous release (for hours) in the LY treatment (as LY dramatically enhanced spontaneous release, probably through a PI3-kinase independent/CK2-dependent process; Rizzoli & Betz, 2002). Wortmannin appears to affect selectively the slow endocytic route; the increase in spontaneous release by LY made this hypothesis hard to test, but the vesicles persisting after LY treatment were found in the vicinity of the release sites at the ultrastructural level (as for wortmannin, Fig. 7B), and the size of the vesicle population persisting after LY treatment corresponded to the RRP size (Rizzoli et al. 2003).
In summary, our results suggest a selective role for the actin cytoskeleton and PI (possibly PI3-kinase) signalling in the slow regeneration of vesicles into the reserve pool, but not the more rapid recapture of vesicles in the RRP. They also argue for a role of actin dynamics in vesicle mobilization. Thus, these data provide further evidence for the existence of two distinct vesicle pools in motor nerve terminals, recycling through functionally and biochemically distinct endocytic routes (Murthy & Stevens, 1998; Kuromi & Kidokoro, 1998, 2000, 2002; Richards et al. 2000, 2003).
Acknowledgments
We thank Dr R. S. Wilkinson for helpful advice on actin staining, and Steve Fadul and Dot Dill for outstanding technical support. This work was supported by research grants to W. J. B. from the NIH and MDA.
References
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nature Struct Mol Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;225:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Brodin L, Low P, Shupliakov O. Sequential steps in clathrin-mediated synaptic vesicle endocytosis. Curr Opin Neurobiol. 2000;10:312–320. doi: 10.1016/s0959-4388(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Cole JC, Villa BR, Wilkinson RS. Disruption of actin impedes transmitter release in snake motor terminals. J Physiol. 2000;525:579–586. doi: 10.1111/j.1469-7793.2000.t01-2-00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaevsky A, Connor EA. F-actin is concentrated in nonrelease domains at frog neuromuscular junctions. J Neurosci. 2000;20:6007–6012. doi: 10.1523/JNEUROSCI.20-16-06007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Keen JH. Phosphoinositide–AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidarov I, Smith ME, Domin J, Keen JH. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7:443–449. doi: 10.1016/s1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Geppert M, Sudhof TC. RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu Rev Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hao W, Tan Z, Prasad K, Reddy KK, Chen J, Prestwich GD, Falck JR, Shears SB, Lafer EM. Regulation of AP-3 function by inositides. Identification of phosphatidylinositol 3,4,5-trisphosphate as a potent ligand. J Biol Chem. 1997;272:6393–6398. doi: 10.1074/jbc.272.10.6393. [DOI] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–643. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sobue K, Kanda K, Harada A, Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989;108:111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, Wendland B. Endocytosis: driving membranes around the bend. Cell. 2002;111:143–146. doi: 10.1016/s0092-8674(02)01044-9. [DOI] [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitve drosophila mutant. Shibire Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron. 2000;27:133–143. doi: 10.1016/s0896-6273(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Selective replenishment of two vesicle pools depends on the source of Ca2+ at the Drosophila synapse. Neuron. 2002;35:333–343. doi: 10.1016/s0896-6273(02)00777-8. [DOI] [PubMed] [Google Scholar]
- Lenzi D, von Gersdorff H. Structure suggests function: the case for synaptic ribbons as exocytotic nanomachines. Bioessays. 2001;23:831–840. doi: 10.1002/bies.1118. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Bai J, Vyas B, Desai R, Baltus AE, Garment MB, Carlson SD, Ganetzky B, Chapman ER. Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nature Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Moss SE, Ballestrem C, Imhof BA, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nature Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- Osborne SL, Meunier FA, Schiavo G. Phosphoinositides as key regulators of synaptic function. Neuron. 2001;32:9–12. doi: 10.1016/s0896-6273(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Sterling P. Synaptic ribbon. Conveyor belt or safety belt. Neuron. 2003;37:379–382. doi: 10.1016/s0896-6273(03)00062-x. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- Prior IA, Clague MJ. Localization of a class II phosphatidylinositol 3-kinase, PI3KC2alpha, to clathrin-coated vesicles. Mol Cell Biol Res Commun. 1999;1:162–166. doi: 10.1006/mcbr.1999.0126. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Betz WJ. A possible link between phosphatidyl-inositol signaling and the acton cytoskeleton in synaptic vesicle cycling at the frog neuromuscular junction. Soc Neurosci Abs. 2000;26:887. [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO, Betz WJ. Synaptic vesicle pools at the frog neuromuscular junction. Neuron. 2003;39:529–541. doi: 10.1016/s0896-6273(03)00405-7. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Effects of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one on synaptic vesicle cycling at the frog neuromuscular junction. J Neurosci. 2002;22:10680–10689. doi: 10.1523/JNEUROSCI.22-24-10680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Richards DA, Betz WJ. Monitoring synaptic vesicle recycling in frog motor nerve terminals with FM dyes. J Neurocytol. 2003;32:539–549. doi: 10.1023/B:NEUR.0000020609.19873.e8. [DOI] [PubMed] [Google Scholar]
- Ryan TA. Inhibitors of myosin light chain kinase block synaptic vesicle pool mobilization during action potential firing. J Neurosci. 1999;19:1317–1323. doi: 10.1523/JNEUROSCI.19-04-01317.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Atluri PP, Ryan TA. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nature Neurosci. 2003;6:127–135. doi: 10.1038/nn1002. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci U S A. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Bloom O, Gustafsson JS, Kjaerulff O, Low P, Tomilin N, Pieribone VA, Greengard P, Brodin L. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc Natl Acad Sci U S A. 2002;99:14476–14481. doi: 10.1073/pnas.212381799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nature Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta. 2001;1533:190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J Neurosci. 2000;20:7986–7996. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS. The wily ways of a parasite: induction of actin assembly by Listeria. Trends Microbiol. 1993;1:25–31. doi: 10.1016/0966-842x(93)90021-i. [DOI] [PubMed] [Google Scholar]
- Tucker WC, Edwardson JM, Bai J, Kim HJ, Martin TF, Chapman ER. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J Cell Biol. 2003;162:199–209. doi: 10.1083/jcb.200302060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Zheng JQ, Poo MM. Effects of cytochalasin treatment on short-term synaptic plasticity at developing neuromuscular junctions in frogs. J Physiol. 1996;491:187–195. doi: 10.1113/jphysiol.1996.sp021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Spatial variability in release at the frog neuromuscular junction measured with FM1-43. Can J Physiol Pharmacol. 1999;77:672–678. [PubMed] [Google Scholar]