Abstract
Transient inhibition of gap junction (GJ)-mediated communication with heptanol during myocardial reperfusion limits infarct size. However, inhibition of cell coupling in normal myocardium may be arrhythmogenic. The purpose of this study was to test the hypothesis that the consequences of GJ inhibition may be magnified in reperfused myocardium compared with normal tissue, thus allowing the inhibition of GJs in reperfused tissue while only minimally modifying overall macroscopic cell coupling in normal myocardium. Concentration–response curves were defined for the effects of heptanol, 18α-glycyrrhetinic acid, halothane, and palmitoleic acid on conduction velocity, tissue electrical impedance, developed tension and lactate dehydrogenase (LDH) release in normoxically perfused rat hearts (n = 17). Concentrations lacking significant effects on tissue impedance were added during the initial 15 min of reperfusion in hearts submitted to 60 min (n = 43) or 30 min (n = 35) of ischaemia. These concentrations markedly increased myocardial electrical impedance (resistivity and phase angle) in myocardium reperfused after either 30 or 60 min of ischaemia, and reduced reperfusion-induced LDH release after 1 h of ischaemia by 83.6, 57.9, 51.7 and 52.5% for heptanol, 18α-glycyrrhetinic acid, halothane and palmitoleic acid, respectively. LDH release was minimal in hearts submitted to 30 min of ischaemia, independently of group allocation. In conclusion, the present results strongly support the hypothesis that intercellular communication in postischaemic myocardium may be effectively reduced by concentrations of GJ inhibitors affecting only minimally overall electrical impedance in normal myocardium. Reduction of cell coupling during initial reperfusion was consistently associated with attenuated lethal reperfusion injury.
Chemical coupling through gap junctions (GJs) has been proposed to allow the spreading of cell death in different situations, including apoptosis of tumoral cells, either spontaneous or induced by anti-neoplastic treatments (McMasters et al. 1998; Krutovskikh et al. 2002), and cell death secondary to ischaemia–reperfusion in astrocytes (Rawanduzy et al. 1997; Lin et al. 1998) and cardiomyocytes (Garcia-Dorado et al. 1997; Garcia-Dorado et al. 2004). In myocardium, reperfusion-induced hypercontracture can spread due to the passage of Na+ through GJs, followed by reverse Na+–Ca2+ exchange (Ruiz-Meana et al. 1999). Heptanol, a standard GJ inhibitor, prevented cell-to-cell progression of hypercontracture and reduced cell necrosis in different models, when given at the time of reperfusion (Garcia-Dorado et al. 1997). More recently, it has been shown that GJ-mediated intercellular communication may persist during ischaemia and allow propagation of rigor contracture (Ruiz-Meana et al. 2001). In support of a role of GJ-mediated communication in the progression of ischaemic injury, it has been shown that pretreatment with heptanol (Saltman et al. 2002) or underexpression of Cx43 in heterozygous connexin43-deficient mice (Kanno et al. 2003) was associated with reduced infarct size after transient or permanent coronary occlusion, respectively. However, other studies have failed to find a protective effect by pretreatment with heptanol (Gysembergh et al. 2001) or underexpression of Cx43 (Schwanke et al. 2002) in isolated mice hearts submitted to transient coronary occlusion.
The therapeutic exploitation of the protective effect of inhibition of GJ-mediated communication with heptanol against ischaemia–reperfusion injury is limited by its relatively low specificity as a GJ inhibitor (it has dose-dependent effects on Na+ channels and other ion transport systems (Niggli et al. 1989; Nelson & Makielski, 1991; Takens-Kwak et al. 1992), and by the undesirable effects of inhibition of GJ-mediated communication in normal myocardium, in particular, electrophysiological alterations causing arrhythmogenicity. This limitation is particularly serious because it may apply not only to heptanol but also to any other GJ inhibitor.
Previous studies have shown the existence of a high ‘safety factor’ in normal myocardium (i.e. that the number of available GJ channels is much higher than those strictly necessary to maintain conduction). This allows important reductions in the number of open junctional channels and/or their average conductivity to have undetectable effects on macroscopic electrical coupling (Jongsma & Wilders, 2000). However, ischaemia and reperfusion have been shown to induce changes in cytoplasmic ionic composition (Maurer & Weingart, 1987; Noma & Tsuboi, 1987; Calero et al. 1998), in phosphorylation of connexin43 proteins (Beardslee et al. 2000; Schulz et al. 2003), and in ATP availability (Sugiura et al. 1990). All these changes reduce the number of available channels, which, in turn, may cause the effect of GJ inhibitors on macroscopic electrical properties to be different in normal and ischaemic–reperfused tissue. A previous study has shown that the effects of heptanol on conduction may be different in normal and diseased myocardium (Spear et al. 1990). We propose the hypothesis that the different effects on electrical behaviour of GJ uncouplers in normal and ischaemic–reperfused myocardium could allow interference with GJ-mediated communication in reperfused myocardium without significantly altering overall macroscopic electrophysiological behaviour of control tissue. To test this hypothesis, the electrophysiological effects of four widely used GJ inhibitors have been characterized in normoxic and reperfused tissues, and correlated with their possible protective effect on cell death.
Methods
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and was performed in accordance with European legislation. The study was approved by the Ethics Committee of our institution.
Experimental preparation
One hundred adult male Sprague-Dawley rats (250–350 g) were killed by an intraperitoneal overdose of pentobarbital. The hearts were quickly removed and retrogradely perfused through the aorta with an oxygenated (95% O2–5% CO2) Krebs solution at 37°C (mm: NaCl 118, KCl 4.7, MgSO4 1.2, CaCl2 2.5, NaHCO3 25, KH2PO4 1.2 and glucose 11, pH 7.4). After removing both atria, complete atrioventricular conduction block was induced by crushing the His bundle with fine forceps. Immediately, the right ventricle and interventricular septum were opened by a longitudinal incision from the cardiac base to the apex. Opened hearts were pinned to a silicone membrane placed at the bottom of an organ bath, exposing the endocardial surface of the left ventricle (Rodriguez-Sinovas et al. 2003). A 2.0 silk-snare was placed in the septum of the hearts and connected to an isometric force transducer (FSG-01, SG-M DC bridge amplifier module, Experimentia, London, UK). Resting tension was 1 g. Preparations were paced from the base of the hearts using rectangular pulses of 2.5 ms duration and 4 V amplitude, at 400 ms basic cycle length.
Transmembrane action potential recordings
Transmembrane action potentials were recorded from the apical region of the heart as previously described (Rodriguez-Sinovas et al. 2003). Conduction time was measured as the time between the stimulus artefact and the onset of the rapid depolarization of the action potential. Using this method, the estimated conduction velocity represents an averaged velocity between the stimulus site and the recording site, as the pathway of activation is not known.
Myocardial electrical impedance
Measurement of myocardial electrical impedance and its two components, tissue resistivity (R) and phase angle (τ), was performed using a four-electrode probe placed in the septum, at 7 kHz, as previously described (Rodriguez-Sinovas et al. 2003). The impedance probe consisted of a linear array of four platinum electrodes (length: 5 mm, diameter: 0.4 mm), placed at an interelectrode distance of 2.5 mm. An alternating current (10 μA) was applied through the outer pair of electrodes, and the in-phase components of voltage were recorded continuously through the inner pair of electrodes (high-input impedance lock-in amplifier, Princeton Applied Research, model 5110, USA).
Lactate dehydrogenase (LDH) release and myocardial water content
Enzyme release was determined in samples taken from the effluent by a spectrophotometric method at 340 nm, as previously described (Rodriguez-Sinovas et al. 2003). Water content was measured in hearts submitted to ischaemia–reperfusion at the end of the experiments. Tissue samples were weighed before and after desiccation at 100°C for 24 h, and myocardial water content was determined as the difference between wet and dry weight, and was expressed as millilitres of water per 100 g dry weight.
Experimental protocol
Effects of GJ inhibitors during normoxia
After 30 min of equilibration, cmulative concentration–response curves to heptanol (n = 6, from 10−4 to 1.78 × 10−2 m), 18α-glycyrrhetinic acid (n = 4, from 10−6 to 5.62 × 10−5 m), halothane (n = 3, from 10−4 to 5.62 × 10−2 m), and palmitoleic acid (n = 4, from 10−6 to 10−4 m) were performed. Each concentration was applied for 15 min, recording continuously developed tension, myocardial electrical impedance and transmembrane action potentials. LDH release was determined at the end of each concentration. Those concentrations causing LDH release and rigor development were excluded from the analysis. Concentration–response curves to the different GJ inhibitors were adjusted to sigmoid curves to determine the concentration that caused half-maximal effects (EC50). Three additional hearts were used to assess stability of the preparation during 2 h of normoxic perfusion.
Effects of GJ inhibitors after ischaemia–reperfusion
After 30 min of equilibration, 45 hearts were submitted to 1 h of no-flow true global ischaemia followed by reperfusion for 60 min. During the ischaemic period, preparations were immersed in Krebs solution (bubbled with 95% N2–5% CO2, pH 7.4, no glucose), in order to maintain temperature (36.5–36.8°C). GJ inhibitors were added to the oxygenated Krebs solution during the first 15 min of reperfusion. The concentrations used were chosen based on their effects during normoxia: well-characterized inhibitory effects on single-channel microscopic GJ conductance in normal myocytes and negligible actions on tissue impedance (myocardial resistivity and phase angle) in normoxically perfused hearts. These concentrations were 10−3 m for heptanol (n = 5), 10−5 m for 18α-glycyrrhetinic acid (n = 5), 10−3 m for halothane (n = 7), and 3 × 10−5 m for palmitoleic acid (n = 6). Each group of treatment had its paired control group (n = 5–7). In order to dissociate the effects of GJ inhibitors on electrical impedance during reperfusion from their potential effects on the extent of cell death, a second series of experiments (n = 35) was performed in which the duration of ischaemia was reduced to 30 min, a protocol that results in only minimal necrosis (heptanol: n = 6; 18α-glycyrrhetinic acid: n = 4; halothane: n = 4; palmitoleic acid: n = 4, each group with its corresponding control group). In these last hearts, the extent of necrosis was determined after 2 h of reperfusion. Hearts were cut into 2–3 mm slices perpendicular to the longitudinal axis. The slices were weighed and immersed during 15 min in a 1% triphenyltetrazolium chloride (TTC) solution, buffered at pH 7.4, at 37°C, and then imaged using a digital camera under white light. The areas with negative reaction to the TTC were identified as necrotic areas. Infarct mass was determined using the commercially available software MicroImage (Olympus Optical, Hamburg, Germany), as previously described (Garcia-Dorado et al. 1997), and expressed as a percentage of the total weight of the heart.
Changes in developed tension, conduction velocity and myocardial electrical resistivity and phase angle were continuously recorded during the whole experiments, and cell injury was evaluated by measurement of LDH release during reperfusion as previously described (Rodriguez-Sinovas et al. 2003).
Chemicals
Heptanol (1-heptanol), 18α-glycyrrhetinic acid (3β-hydroxy-11-oxo-18α,20β-olean-12-en-29-oic acid) and palmitoleic acid (cis-9-hexadecenoic acid) were obtained from Sigma Chemical (St Louis, MO, USA). Halothane (Fluothane®, 2-bromo-2-chloro-1,1,1,-trifluoroethane) was obtained from Zeneca Farma (Pontevedra, Spain). Palmitoleic acid and 18α-glycyrrhetinic acid were initially dissolved in dimethyl sulfoxide (DMSO), and then dissolved in Krebs solution to the desired concentration. Final DMSO concentration was 0.15 and 0.1%, respectively. Heptanol and halothane were directly dissolved in Krebs solution. All other reagents were commercially obtained.
Statistical analysis
Statistical analysis was performed using commercial available software (SPSS for Windows 8.0). Data are expressed as the mean ± s.e.m. Repeated measures analysis of variance (MANOVA) and Dunnett's post hoc test were used to assess differences in the time course of the changes in tissue electrical impedance, LDH release, developed tension and conduction velocity during reperfusion in the distinct treatment groups. Changes in tissue electrical impedance were analysed at baseline and at minutes 1, 2, 3, 4 and 5 of reperfusion. In the groups of 30 min of ischaemia, statistics for electrical impedance were one tailed, as the direction of the drug effect was previously demonstrated in hearts submitted to 60 min of ischaemia. Student's t test was used to analyse differences in accmulated LDH release, rigor onset and functional recovery. A P value lower than 0.05 was considered to be significant.
Results
Effects of GJ inhibitors during normoxia
In the absence of GJ inhibitors, all three hearts perfused under normoxic conditions for 2 h remained stable, without significant changes in contractile function (developed tension at baseline 380.3 ± 137.6 versus 339.6 ± 101.0 mg at 2 h) or electrophysiological variables (resistivity: 122.8 ± 12.9 versus 123.0 ± 12.5 Ω cm; phase angle: −2.4 ± 0.2 versus −2.2 ± 0.2 deg; conduction velocity: 33.2 ± 9.4 versus 30.1 ± 8.5 cm s−1).
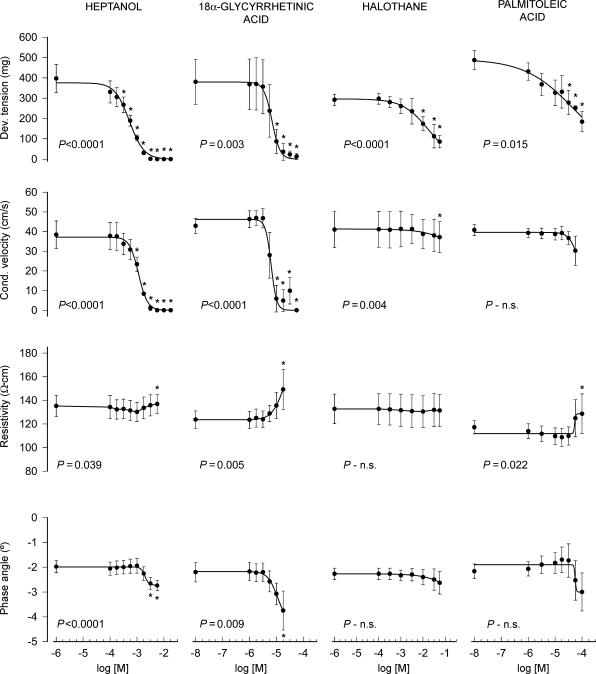
As seen in Fig. 1 and Table 1, all GJ inhibitors induced a concentration-dependent reduction in developed tension. Heptanol and 18α-glycyrrhetinic acid had also a prominent effect on conduction velocity, whereas the effects of palmitoleic acid and halothane were less pronounced (Fig. 1 and Table 1). In contrast to their effects on developed tension and conduction velocity, all four GJ inhibitors investigated induced only minor changes in myocardial resistivity and phase angle (Fig. 1 and Table 1).
Figure 1. Changes in developed tension (Dev. tension), conduction velocity (Cond. velocity), electrical resistivity and phase angle in normoxic isolated rat hearts treated with heptanol, 18α-glycyrrhetinic acid, halothane and palmitoleic acid.
*P < 0.05 indicates significant differences with respect to initial values. The P value shown in each graph indicates the MANOVA P value.
Table 1.
Concentrations (m) causing half-maximal effects on developed tension and conduction velocity in normoxic isolated rat hearts treated with heptanol, 18α-glycyrrhetinic acid (GA), halothane and palmitoleic acid (PA)
| Developed tension | Conduction velocity | |
|---|---|---|
| Heptanol | (5.1 ± 0.7) × 10−4 | (1.2 ± 0.1) × 10−3 |
| 18α-GA | (8.4 ± 1.8) × 10−6 | (8.1 ± 2.9) × 10−6 |
| Halothane | (2.0 ± 1.0) × 10−2 | — |
| PA | (7.5 ± 4.3) × 10−5 | — |
Effects of GJ inhibitors after ischaemia–reperfusion
Changes in tissue electrical impedance during ischaemia–reperfusion
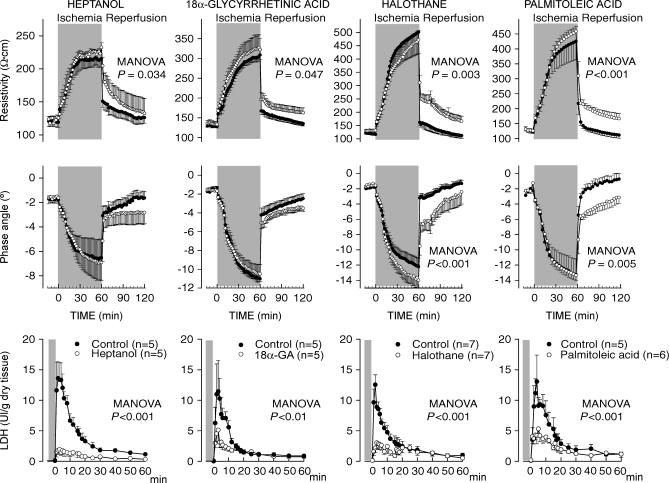
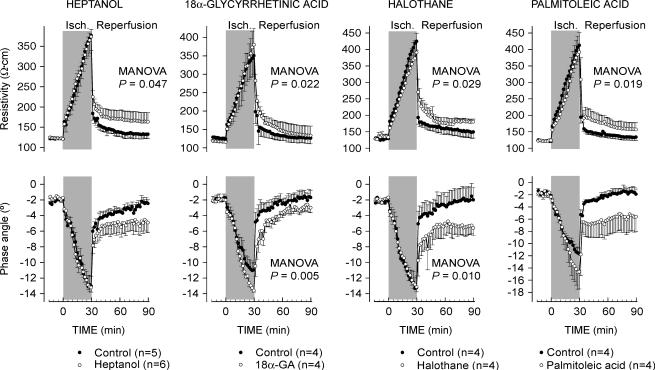
As previously described (Rodriguez-Sinovas et al. 2003), 60 min of ischaemia induced a marked increase in myocardial resistivity (from 120.6 ± 2.9 to 369.0 ± 36.2 Ω cm at 60 min of ischaemia, pooled data from all controls, P < 0.001) and an accentuated decrease in phase angle (from −1.8 ± 0.1 to −11.1 ± 0.8 deg, P < 0.001). Reperfusion caused a quick recovery in both resistivity and phase angle values in all control hearts (Fig. 2). However, hearts treated with any of the GJ inhibitors investigated, heptanol, 18α-glycyrrhetinic acid, halothane or palmitoleic acid, showed marked attenuation of the recovery of myocardial electrical impedance during initial reperfusion (Fig. 2). Similar results were observed when duration of ischaemia was set at 30 min, a protocol that induced only minimal cell death (see below) (Fig. 3).
Figure 2. Changes in electrical resistivity and phase angle during ischaemia–reperfusion, and LDH release during reperfusion, in control isolated rat hearts submitted to 60 min of ischaemia–reperfusion and in hearts treated with different inhibitors of GJ mediated communication during the first 15 min of reperfusion.
Concentrations of GJ inhibitors were as follows: heptanol: 10−3 m; 18α-glycyrrhetinic acid: 10−5 m; halothane: 10−3 m; and palmitoleic acid: 3·10−5 m. The P value shown in each graph indicates the MANOVA P value.
Figure 3. Changes in electrical resistivity and phase angle during ischaemia–reperfusion in control isolated rat hearts submitted to 30 min of ischaemia–reperfusion and in hearts treated with different inhibitors of GJ-mediated communication during the first 15 min of reperfusion.
Concentrations of GJ inhibitors were as follows: heptanol: 10−3 m; 18α-glycyrrhetinic acid: 10−5 m; halothane: 10−3 m; and palmitoleic acid: 3 × 10−5 m. The P value shown in each graph indicates the MANOVA P value.
Functional recovery during reperfusion
Developed tension decreased during ischaemia, to reach a minimal value several minutes later. Rigor contracture, detected as an abrupt increase in diastolic tension, occurred between 14 and 16 min after perfusion cessation, with no differences between groups.
In the absence of GJ inhibitors all hearts submitted to 60 min of ischaemia showed only minor recovery of developed tension during the 60-min period of reperfusion. In contrast, all five hearts treated with heptanol in the first 15 min of reperfusion showed a clear recovery (44.9 ± 9.7% of baseline tension compared with no functional recovery in their 5 paired controls; Student's t test, P < 0.01). The other GJ inhibitors investigated failed to induce any significant improvement in functional recovery during reperfusion.
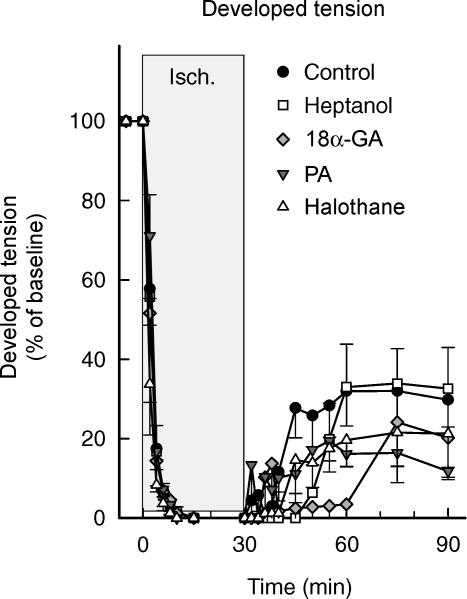
Hearts submitted to 30 min of ischaemia showed marked functional recovery upon reperfusion, with no differences between groups (Fig. 4). Recovery of developed tension occurred at 12.7 ± 1.6 min of reperfusion in control hearts. The time course of recovery was not modified by treatment with palmitoleic acid or halothane, but appeared to be delayed in groups treated with heptanol or 18α-glycyrrhetinic acid. The onset of recovery occurred at 24.8 ± 1.4 and 33.7 ± 9.1 min, respectively (ANOVA and Dunnett's test, P < 0.05) (Fig. 4).
Figure 4. Functional recovery after ischaemia–reperfusion in control isolated rat hearts submitted to 30 min of ischaemia–reperfusion and in hearts treated with different inhibitors of GJ-mediated communication during the first 15 min of reperfusion.
Concentrations of GJ inhibitors were as follows: heptanol: 10−3 m; 18α-glycyrrhetinic acid: 10−5 m; halothane: 10−3 m; and palmitoleic acid: 3 × 10−5 m.
Conduction velocity
In hearts submitted to 30 min of ischaemia, initial conduction velocity ranged between 30 and 45 cm s−1, with no differences between groups. During ischaemia, conduction velocity decreased markedly and reached zero several minutes later. Upon reperfusion, conduction velocity rapidly recovered in control hearts. In heptanol-treated hearts, recovery occurred with some delay (Fig. 5). In hearts treated with 18α-glycyrrhetinic acid, palmitoleic acid or halothane, conduction velocity remained reduced throughout the reperfusion period (Fig. 5) (repeated measures ANOVA and Dunnett's test, P < 0.05).
Figure 5. Recovery of conduction velocity after ischaemia–reperfusion in control isolated rat hearts submitted to 30 min of ischaemia–reperfusion (n = 4) and in hearts treated with different inhibitors of GJ-mediated communication during the first 15 min of reperfusion.
Concentrations of GJ inhibitors were as follows: heptanol: 10−3 m (n = 3); 18α-glycyrrhetinic acid: 10−5 m (n = 3); halothane: 10−3 m (n = 3); and palmitoleic acid: 3 × 10−5 m (n = 3).
Enzyme release
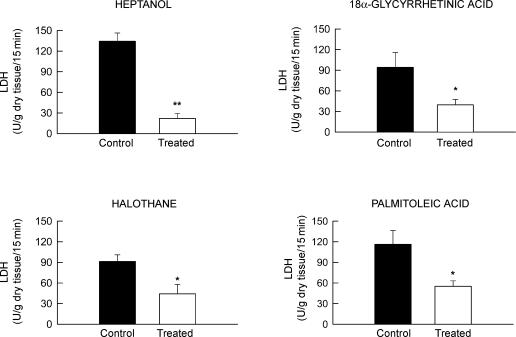
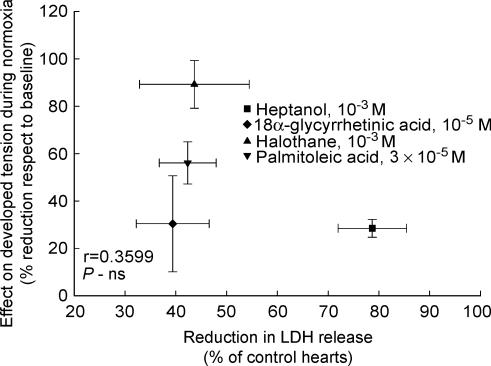
Control isolated rat hearts submitted to 60 min of ischaemia–reperfusion in the absence of GJ inhibitors showed marked LDH release at the time of reperfusion, which was characterized by an early peak occurring in the first 2–4 min of reperfusion, followed by a rapid decay. In contrast, all four GJ inhibitors induced an attenuated enzyme release during the entire reperfusion period (Figs 2 and 6). No significant correlation was observed between the degree of protection afforded by each GJ inhibitor and the effect of the concentrations used on developed tension during normoxia, suggesting that the protective effect is independent of their actions on contractility (Fig. 7).
Figure 6. Accmulated LDH release during the first 15 min of reperfusion in groups treated with different inhibitors of GJ-mediated communication and their respective controls.
Concentrations of GJ inhibitors were as follows: heptanol: 10−3 m; 18α-glycyrrhetinic acid: 10−5 m; halothane: 10−3 m; and palmitoleic acid: 3 × 10−5 m. **P < 0.01 and *P < 0.05 indicate significant differences compared to control hearts.
Figure 7. Correlation between the decrease in developed tension induced by the different drugs when administered during normoxia and the reduction in total LDH release induced when given at the onset of reperfusion.
No clear relationship between both effects was observed.
Isolated rat hearts submitted to 30 min of ischaemia showed only minimal LDH release during reperfusion, with no significant differences between groups (U (g dry tissue)−1 (15 min)−1: 10.9 ± 1.8 in control hearts, and 9.7 ± 1.3, 9.3 ± 4.3, 9.0 ± 3.1 and 4.6 ± 0.7 in heptanol, 18α-glycyrrhetinic acid, palmitoleic acid and halothane-treated hearts, respectively). The area of necrosis determined by TTC staining in these hearts was also small and homogeneous among groups (7.8 ± 1.0% of total cardiac weight in control hearts, and 5.5 ± 1.2, 11.5 ± 1.8, 9.0 ± 2.9 and 6.8 ± 1.2% in heptanol, 18α-glycyrrhetinic acid, palmitoleic acid and halothane-treated hearts, respectively).
Myocardial water content
Both heptanol and 18α-glycyrrhetinic acid caused a reduction in myocardial water content in hearts submitted to 60 min of ischaemia followed by reperfusion (484.0 ± 18.9 ml (100 g dry tissue)−1 at the end of the reperfusion in heptanol-treated hearts versus 588.4 ± 30.2 in control hearts, P = 0.021; and 515.7 ± 15.5 ml (100 g dry tissue)−1 in 18α-glycyrrhetinic acid-treated versus 597.6 ± 17.3 in controls preparations, P < 0.01). In contrast, hearts treated with halothane or palmitoleic acid did not show any significant change in water content at 1 h of reperfusion. Hearts submitted to 30 min of ischaemia followed by 1 h of reperfusion showed a less severe degree of oedema without differences between groups (ml (100 g dry tissue)−1: 510.4 ± 14.0 in control hearts and 507.9 ± 15.3, 467.3 ± 29.7, 504.2 ± 12.0 and 487.6 ± 21.1 in heptanol, 18α-glycyrrhetinic acid, palmitoleic acid and halothane-treated hearts, respectively).
Discussion
This study demonstrates that four chemically unrelated GJ inhibitors increase electrical impedance during myocardial reperfusion at concentrations lacking measurable effects on electrical resistivity and phase angle under normal conditions. This effect was consistently associated with a marked attenuation of myocardial necrosis induced by ischaemia–reperfusion. These results extend previous observations showing a protective effect of heptanol against ischaemia–reperfusion injury to other GJ inhibitors, and open the possibility of devising pharmacological strategies to inhibit GJ-mediated communication in reperfused myocardium without altering overall macroscopic electrical coupling in control tissue.
The present results provide further support for the hypothesis that GJ-mediated spread of cell death significantly contributes to the final extent of necrosis induced by transient myocardial ischaemia. First, the protection observed with heptanol is extended to other chemically unrelated widely used GJ inhibitors such as halothane, 18α-glycyrrhetinic acid and palmitoleic acid. This is important because of the relative lack of specificity of heptanol, and to a lesser extent of the other GJ inhibitors. Second, it links for the first time the protective effect to the effects on cell coupling, as assessed by analysis of tissue electrical impedance.
The protective effect of GJ uncoupling against lethal reperfusion injury is not exclusive of heptanol
Heptanol, a polyalcohol, has been so far the most widely used GJ inhibitor. Its mechanism of action seems to be related to the modification of the physical properties of the cholesterol-rich domains of the plasma membrane resulting in a reduction of the open probability of junctional channels (Takens-Kwak et al. 1992; Bastiaanse et al. 1993).
Previous studies demonstrated that heptanol reduces cell-to-cell progression of hypercontracture in isolated cardiomyocytes and LDH release and infarct size in intact hearts when given at the time of reperfusion (Garcia-Dorado et al. 1997). Pretreatment with heptanol (Saltman et al. 2002) or infusion of this alcohol during ischaemia (Miura et al. 2003) has been shown to reduce infarct size in isolated rabbit hearts submitted to coronary occlusion followed by reperfusion. Heptanol and other polyalcohols have been found to be protective against cell death induced by ischaemia in brain and in vitro neuronal preparations (Rawanduzy et al. 1997; Rami et al. 2001). In contrast to the general belief that heptanol is protective in the ischaemia–reperfusion setting, some authors found no significant attenuation of infarct size in isolated rabbit hearts (Gysembergh et al. 2001), although this lack of effect might be explained by the lower heptanol concentration used in their study.
In addition to its known effects on GJ (Takens-Kwak et al. 1992; Bastiaanse et al. 1993), heptanol reduces other non-junctional membrane ionic currents, including Na+ and Ca2+ inward currents (Niggli et al. 1989; Nelson & Makielski, 1991; Takens-Kwak et al. 1992). These actions of heptanol may contribute to the observed effects on developed tension and conduction velocity observed in this and other studies (Balke et al. 1988; Keevil et al. 2000), and could contribute to its protective effect against reperfusion injury by GJ-independent mechanisms. However, the observation that other inhibitors of GJ communication, chemically unrelated to heptanol, share its protective effect during myocardial reperfusion is against this possibility.
The effect of halothane, a halogenated anaesthetic, on GJ-mediated intercellular communication has been characterized in detail (Burt & Spray, 1989; He & Burt, 2000) and seems to be due to a reduction in the open probability of junctional channels without change in their unitary conductance. Halothane also acts on several other cell systems, such as the sarcoplasmic reticulum (Wheeler et al. 1997) and different non-junctional currents (Terrenoire et al. 2000). As in the case of heptanol, these actions could contribute to the observed protective effect of halothane against ischaemia and reperfusion injury. Previous studies have shown that pretreatment with halothane protects against cell death secondary to transient ischaemia (Cope et al. 1997; Coetzee et al. 2000). Halothane has been shown to protect also against cell death when given at the time of reperfusion in the in situ rabbit heart (Schlack et al. 1997), in isolated rat hearts (Schlack et al. 1996) and in isolated rat cardiomyocytes (Siegmund et al. 1997). The protective effect of pretreatment with halothane has been related to preconditioning, as it depended on adenosine receptors and protein kinase C (Cope et al. 1997) and ATP-dependent potassium channels (Coetzee et al. 2000). It has been proposed that the protection afforded during reperfusion is due to attenuation of Ca2+ oscillations between cytosol and sarcoplasmic reticulum (Siegmund et al. 1997), and had not been previously related to the effect of the drug on GJs.
18α-Glycyrrhetinic acid and palmitoleic acid are widely used GJ inhibitors. Both are effective in the micromolar range, and are supposedly more specific than heptanol or halothane, effective only in the millimolar range. 18α-Glycyrrhetinic acid seems to act through changes in phosphorylation and/or connexin assembly, while palmitoleic acid is supposed to act, like heptanol, as a lipophilic drug (Burt et al. 1991; Guo et al. 1999; Dhein, 1998), but both substances have also other effects, such as inhibition of sarcolemmal Ca2+ currents and of mRNA synthesis (Shimada & Somlyo, 1992; Guo et al. 1999).
In contrast to heptanol and halothane, data on the potential protective effect of 18α-glycyrrhetinic acid and palmitoleic acid are scant and contradictory. Fatty acids such as oleic acid have been shown to protect cardiac myocytes when given before simulated ischaemia (Mackay & Mochly Rosen, 2001). However, other studies have suggested that they aggravate ATP loss and mitochondrial injury during low-flow anoxia (Piper & Das, 1987). The effects of 18β-glycyrrhetinic acid when infused during ischaemia have been described only very recently in a study by Miura et al. (2003) showing a reduction in infarct size in isolated rabbit hearts. Our present data demonstrate that 18α-glycyrrhetinic acid and palmitoleic acid, like heptanol and halothane, reduce cell death when given at the time of reperfusion.
The GJ inhibitors used in the present study were given during reperfusion at concentrations that depressed developed tension in normoxic hearts. This fact obscures the ascription of the protective effect of these drugs to their effects on cell coupling and, in addition, limits their potential clinical application. However, there are data in the present study that indicate that the protective effect induced by GJ inhibitors is largely independent of their effects on contractility. First, as shown in Fig. 7, there was no significant correlation between the degree of reduction of developed tension during normoxia and the protective effect on LDH release during reperfusion. Second, halothane at 1 mm had nearly undetectable effects on contractile function yet it was very effective in attenuating LDH release.
Finally, the protective effect shared by all four GJ inhibitors was clearly observable in hearts submitted to prolonged (60 min) ischaemia, but not in those submitted to shorter ischaemic periods (30 min), in which no significant effect of the GJ inhibitors on TTC staining or LDH release could be demonstrated. A possible explanation for this lack of significant protection against cell death after 30 min of ischaemia is the very limited extent of necrosis in these experiments.
Effect of inhibitors of GJ-mediated communication on tissue electrical impedance in reperfused myocardium
All four GJ inhibitors investigated markedly reduced conduction velocity and attenuated the recovery of myocardial electrical impedance during the first minutes of reperfusion. This observation is a prerequisite for any attempt to establish a cause–effect relationship between the actions of these drugs on GJ-mediated communication and their protective effects against myocardial necrosis secondary to ischaemia–reperfusion.
A potential limitation of the use of changes in tissue electrical impedance as markers of changes in gap junctional conductance is that they can be influenced by alterations in intracellular and extracellular resistances, as well as by membrane resistance and capacitance (Gebhard et al. 1987). In the present study, a reduced rupture of cell membranes in hearts receiving GJ inhibitors could have contributed to the attenuated recovery of myocardial resistivity observed in hearts submitted to 60 min of ischaemia followed by reperfusion. However, our data obtained in preparations in which the duration of ischaemia was set at 30 min seem to exclude this possibitity as cell death (i.e. LDH release or TTC-negative staining) in these conditions was almost negligible. Moreover, other strongly protective treatments, like cariporide (Rodriguez-Sinovas et al. 2003) or ischaemic preconditioning (Padilla et al. 2003), did not induce any change in the recovery of myocardial electrical resistivity during reperfusion. Reduced tissue oedema could have also contributed to the attenuated reduction of myocardial resistivity during reperfusion observed in the presence of GJ inhibitors, since oedema increases tissue conductivity. However this possibility seems unlikely: although both heptanol and 18α-glycyrrhetinic acid caused a decrease in myocardial water content in hearts submitted to 60 min of ischaemia–reperfusion, the effects of halothane and palmitoleic acid on the recovery of tissue resistivity were not associated with any significant action on myocardial water content. Furthermore, in those hearts submitted to 30 min of ischaemia followed by reperfusion, no difference in tissue water content was observed between control hearts and those treated with GJ inhibitors. Thus, the effects of GJ inhibitors on electrical impedance appear to be dissociated from their effects on tissue oedema.
Another possible limitation of the use of electrical impedance is that, due to the probe's size, it reflects a relatively large area of myocardium around the electrode, which in rat ventricle may include the complete thickness of the ventricular wall. Thus, we cannot exclude the possibility of transmural differences in the response. In addition, our methods do not provide information on changes in the lateral conduction velocity or its dispersion, nor in changes in anisotropy of the tissue. The limited spatial resolution of impedance analysis may, on the other hand, have the adventage of minimizing the influence of small areas of tissue heterogeneities (as in the case of hearts submitted to 30 min of ischaemia showing patchy areas of necrosis) on the final measurement.
Enhanced effect on macroscopic electrical properties of GJ inhibitors in reperfused myocardium
The inhibitory effect of the investigated GJ inhibitors on intercellular communication has been solidly demonstrated (Burt & Spray, 1989; Burt et al. 1991; Takens-Kwak et al. 1992; Bastiaanse et al. 1993; Dhein, 1998; Guo et al. 1999; He & Burt, 2000). The most plausible explanation for the lack of effect of GJ inhibitors on electrical impedance observed in the present study in normoxic myocardium, at concentrations that markedly affect it after ischaemia–reperfusion, is that although the fractional decrease of communication could be the same in normoxic and ischaemic–reperfused myocardium, due to non-linearities in the response, the effects of GJ blockade were more pronounced in reperfused myocardium, due to a strongly reduced number of available GJ channels. This may reflect the high ‘safety factor’ in normal myocardium that allows important reductions in the number of open junctional channels and/or their average conductivity with almost no detectable reduction of electrical coupling (Jongsma & Wilders, 2000). Previous computer simulation studies indicate that even a reduction of 40% in GJ content would induce only moderate effects on conduction velocity in normal tissue, and that a reduction of gap junctional conductance from 7 μS to 3 μS is necessary to induce noticeable effects on gap junctional resistivity (Jongsma & Wilders, 2000). Other authors have suggested that an intervention having physiologically relevant effects on cell-to-cell coupling must result in closure of more than 90% of GJ channels (Christ et al. 1996). It is easily conceivable that the alterations in ionic composition (decrease in intracellular pH and increase in free calcium concentration) (Maurer & Weingart, 1987; Noma & Tsuboi, 1987; Calero et al. 1998) that occur during ischaemia and persist during the initial minutes of reperfusion, together with the dephosphorylation of connexin43 proteins (Beardslee et al. 2000), the reduction in ATP availability (Sugiura et al. 1990), and the increased concentrations of amphipathic catabolites (Wu et al. 1993), factors all known to cause closure of GJs (Dhein, 1998), contribute to reduce the ‘safety factor’. As a consequence, any additional reduction in the open probability and/or average conductance induced by GJ inhibitors would have more apparent effects on electrical tissue impedance in reperfused than in normoxic conditions. Differential effects between normal and diseased myocardium have also been observed by other authors who demonstrated that heptanol depressed conduction in strips from infarcted canine myocardium at concentrations that were without effect in normal tissues (Spear et al. 1990).
The increased effect of GJ inhibitors on macroscopic electrical properties of reperfused myocardium opens the possibility of the development of strategies able to interfere with GJ-mediated spread of necrosis in myocardium undergoing reperfusion without significant overall macroscopic effects in myocardium at a distance. This may require the search for new, more specific inhibitors of GJ-mediated intercellular communication lacking the depressor effects on contractility associated with inhibition of sarcolemmal Na+ and Ca2+ currents and with altered function of the sarcoplasmic reticulum (Niggli et al. 1989; Nelson & Makielski, 1991; Takens-Kwak et al. 1992; Shimada & Somlyo, 1992; Wheeler et al. 1997; Guo et al. 1999; Terrenoire et al. 2000).
Acknowledgments
This work was partially supported by grants CICYT SAF 2002-00759, FIS 01/3135. A.R.-S. has a grant from the Ministerio de Sanidad y Consumo (99/3142). The authors acknowledge the excellent technical assistance of Lourdes Trobo.
References
- Balke CW, Lesh MD, Spear JF, Kadish A, Levine JH, Moore EN. Effects of cellular uncoupling on conduction in anisotropic canine ventricular myocardium. Circ Res. 1988;63:879–892. doi: 10.1161/01.res.63.5.879. [DOI] [PubMed] [Google Scholar]
- Bastiaanse EM, van der Jongsma HJ, LA & Takens-Kwak BR. Heptanol-induced decrease in cardiac gap junctional conductance is mediated by a decrease in the fluidity of membranous cholesterol-rich domains. J Membr Biol. 1993;136:135–145. doi: 10.1007/BF02505758. [DOI] [PubMed] [Google Scholar]
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- Burt JM, Massey KD, Minnich BN. Uncoupling of cardiac cells by fatty acids: structure-activity relationships. Am J Physiol. 1991;260:C439–C448. doi: 10.1152/ajpcell.1991.260.3.C439. [DOI] [PubMed] [Google Scholar]
- Burt JM, Spray DC. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res. 1989;65:829–837. doi: 10.1161/01.res.65.3.829. [DOI] [PubMed] [Google Scholar]
- Calero G, Kanemitsu M, Taffet SM, Lau AF, Delmar M. A 17mer peptide interferes with acidification-induced uncoupling of connexin43. Circ Res. 1998;82:929–935. doi: 10.1161/01.res.82.9.929. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, el Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- Coetzee JF, le Roux PJ, Genade S, Lochner A. Reduction of postischemic contractile dysfunction of the isolated rat heart by sevoflurane: comparison with halothane. Anesth Analg. 2000;90:1089–1097. doi: 10.1097/00000539-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- Dhein S. Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol Sci. 1998;19:229–241. doi: 10.1016/s0165-6147(98)01192-4. [DOI] [PubMed] [Google Scholar]
- Garcia-Dorado D, Inserte J, Ruiz-Meana M, Gonzalez MA, Solares J, Julia M, Barrabes JA, Soler-Soler J. Gap junction uncoupler heptanol prevents cell-to-cell progression of hypercontracture and limits necrosis during myocardial reperfusion. Circulation. 1997;96:3579–3586. doi: 10.1161/01.cir.96.10.3579. [DOI] [PubMed] [Google Scholar]
- Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M. Gap junction-mediated spread of cell injury and death during myocardial ischemia-reperfusion. Cardiovasc Res. 2004;61:386–401. doi: 10.1016/j.cardiores.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Gebhard MM, Gersing E, Brockhoff CJ, Schnabel PA, Bretschneider HJ. Impedance spectroscopy: a method for surveillance of ischemia tolerance of the heart. Thorac Cardiovasc Surg. 1987;35:26–32. doi: 10.1055/s-2007-1020192. [DOI] [PubMed] [Google Scholar]
- Guo Y, Martinez-Williams C, Gilbert KA, Rannels DE. Inhibition of gap junction communication in alveolar epithelial cells by 18alpha-glycyrrhetinic acid. Am J Physiol. 1999;276:L1018–L1026. doi: 10.1152/ajplung.1999.276.6.L1018. [DOI] [PubMed] [Google Scholar]
- Gysembergh A, Kloner RA, Przyklenk K. Pretreatment with the gap junction uncoupler heptanol does not limit infarct size in rabbit heart. Cardiovasc Pathol. 2001;10:13–17. doi: 10.1016/s1054-8807(00)00056-9. [DOI] [PubMed] [Google Scholar]
- He DS, Burt JM. Mechanism and selectivity of the effects of halothane on gap junction channel function. Circ Res. 2000;86:E104–E109. doi: 10.1161/01.res.86.11.e104. [DOI] [PubMed] [Google Scholar]
- Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000;86:1193–1197. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- Kanno S, Kovacs A, Yamada KA, Saffitz JE. Connexin43 as a determinant of myocardial infarct size following coronary occlusion in mice. J Am Coll Cardiol. 2003;41:681–686. doi: 10.1016/s0735-1097(02)02893-0. [DOI] [PubMed] [Google Scholar]
- Keevil VL, Huang CL, Chau PL, Sayeed RA, Vandenberg JI. The effect of heptanol on the electrical and contractile function of the isolated, perfused rabbit heart. Pflugers Arch. 2000;440:275–282. doi: 10.1007/s004240000264. [DOI] [PubMed] [Google Scholar]
- Krutovskikh VA, Piccoli C, Yamasaki H, Yamasaki H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene. 2002;21:1989–1999. doi: 10.1038/sj.onc.1205187. [DOI] [PubMed] [Google Scholar]
- Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M. Gap junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- Mackay K, Mochly-Rosen D. Arachidonic acid protects neonatal rat cardiac myocytes from ischaemic injury through epsilon protein kinase C. Cardiovasc Res. 2001;50:65–74. doi: 10.1016/s0008-6363(00)00322-9. [DOI] [PubMed] [Google Scholar]
- Maurer P, Weingart R. Cell pairs isolated from adult guinea pig and rat hearts: effects of [Ca2+]i on nexal membrane resistance. Pflugers Arch. 1987;409:394–402. doi: 10.1007/BF00583793. [DOI] [PubMed] [Google Scholar]
- McMasters RA, Saylors RL, Jones KE, Hendrix ME, Moyer MP, Drake RR. Lack of bystander killing in herpes simplex virus thymidine kinase-transduced colon cell lines due to deficient connexin43 gap junction formation. Hum Gene Ther. 1998;9:2253–2261. doi: 10.1089/hum.1998.9.15-2253. [DOI] [PubMed] [Google Scholar]
- Miura T, Ohnuma Y, Kuno A, Tanno M, Ichikawa Y, Nakamura Y, Yano T, Miki T, Sakamoto J, Shimamoto K. Protective role of gap junctions in preconditioning against myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;286:H214–H221. doi: 10.1152/ajpheart.00441.2003. [DOI] [PubMed] [Google Scholar]
- Nelson WL, Makielski JC. Block of sodium current by heptanol in voltage-clamped canine cardiac Purkinje cells. Circ Res. 1991;68:977–983. doi: 10.1161/01.res.68.4.977. [DOI] [PubMed] [Google Scholar]
- Niggli E, Rudisuli A, Maurer P, Weingart R. Effects of general anesthetics on current flow across membranes in guinea pig myocytes. Am J Physiol. 1989;256:C273–C281. doi: 10.1152/ajpcell.1989.256.2.C273. [DOI] [PubMed] [Google Scholar]
- Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla F, Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M, Inserte J, Soler-Soler J. Protection afforded by ischemic preconditioning is not mediated by effects on cell-to-cell electrical coupling during myocardial ischemia reperfusion. Am J Physiol Heart Circ Physiol. 2003;285:H1909–H1916. doi: 10.1152/ajpheart.00438.2003. [DOI] [PubMed] [Google Scholar]
- Piper HM, Das A. Detrimental actions of endogenous fatty acids and their derivatives. A study of ischaemic mitochondrial injury. Basic Res Cardiol. 1987;82(Suppl. 1):187–196. doi: 10.1007/978-3-662-08390-1_23. [DOI] [PubMed] [Google Scholar]
- Rami A, Volkmann T, Winckler J. Effective reduction of neuronal death by inhibiting gap junctional intercellular communication in a rodent model of global transient cerebral ischemia. Exp Neurol. 2001;170:297–304. doi: 10.1006/exnr.2001.7712. [DOI] [PubMed] [Google Scholar]
- Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg. 1997;87:916–920. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A, Garcia-Dorado D, Padilla F, Inserte J, Barrabes JA, Ruiz-Meana M, Agullo L, Soler-Soler J. Pre-treatment with the Na+/H+ exchange inhibitor cariporide delays cell-to-cell electrical uncoupling during myocardial ischemia. Cardiovasc Res. 2003;58:109–117. doi: 10.1016/s0008-6363(02)00840-4. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Garcia-Dorado D, Hofstaetter B, Piper HM, Soler-Soler J. Propagation of cardiomyocyte hypercontracture by passage of Na+ through gap junctions. Circ Res. 1999;85:280–287. doi: 10.1161/01.res.85.3.280. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Garcia-Dorado D, Lane S, Pina P, Inserte J, Mirabet M, Soler-Soler J. Persistence of gap junction communication during myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H2563–H2571. doi: 10.1152/ajpheart.2001.280.6.H2563. [DOI] [PubMed] [Google Scholar]
- Saltman AE, Aksehirli TO, Valiunas V, Gaudette GR, Matsuyama N, Brink P, Krukenkamp IB. Gap junction uncoupling protects the heart against ischemia. J Thorac Cardiovasc Surg. 2002;124:371–376. doi: 10.1067/mtc.2002.124239. [DOI] [PubMed] [Google Scholar]
- Schlack W, Hollmann M, Stunneck J, Thamer V. Effect of halothane on myocardial reoxygenation injury in the isolated rat heart. Br J Anaesth. 1996;76:860–867. doi: 10.1093/bja/76.6.860. [DOI] [PubMed] [Google Scholar]
- Schlack W, Preckel B, Barthel H, Obal D, Thamer V. Halothane reduces reperfusion injury after regional ischaemia in the rabbit heart in vivo. Br J Anaesth. 1997;79:88–96. doi: 10.1093/bja/79.1.88. [DOI] [PubMed] [Google Scholar]
- Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17:1355–1357. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–H1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- Shimada T, Somlyo AP. Modulation of voltage-dependent Ca channel current by arachidonic acid and other long-chain fatty acids in rabbit intestinal smooth muscle. J General Physiol. 1992;100:27–44. doi: 10.1085/jgp.100.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B, Schlack W, Ladilov YV, Balser C, Piper HM. Halothane protects cardiomyocytes against reoxygenation-induced hypercontracture. Circulation. 1997;96:4372–4379. doi: 10.1161/01.cir.96.12.4372. [DOI] [PubMed] [Google Scholar]
- Spear JF, Balke CW, Lesh MD, Kadish AH, Levine JL, Moore EN. Effect of cellular uncoupling by heptanol on conduction in infarcted myocardium. Circ Res. 1990;66:202–217. doi: 10.1161/01.res.66.1.202. [DOI] [PubMed] [Google Scholar]
- Sugiura H, Toyama J, Tsuboi N, Kamiya K, Kodama I. ATP directly affects junctional conductance between paired ventricular myocytes isolated from guinea pig heart. Circ Res. 1990;66:1095–1102. doi: 10.1161/01.res.66.4.1095. [DOI] [PubMed] [Google Scholar]
- Takens-Kwak BR, Jongsma HJ, Rook MB, van Ginneken AC. Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. Am J Physiol. 1992;262:C1531–C1538. doi: 10.1152/ajpcell.1992.262.6.C1531. [DOI] [PubMed] [Google Scholar]
- Terrenoire C, Piriou V, Bonvallet R, Chouabe C, Espinosa L, Rougier O, Tourneur Y. Opposite effects of halothane on guinea-pig ventricular action potential duration. Eur J Pharmacol. 2000;390:95–101. doi: 10.1016/s0014-2999(00)00019-4. [DOI] [PubMed] [Google Scholar]
- Wheeler DM, Rice RT, duBell WH, Spurgeon HA. Initial contractile response of isolated rat heart cells to halothane, enflurane, and isoflurane. Anesthesiology. 1997;86:137–146. doi: 10.1097/00000542-199701000-00018. [DOI] [PubMed] [Google Scholar]
- Wu J, McHowat J, Saffitz JE, Yamada KA, Corr PB. Inhibition of gap junctional conductance by long-chain acylcarnitines and their preferential accmulation in junctional sarcolemma during hypoxia. Circ Res. 1993;72:879–889. doi: 10.1161/01.res.72.4.879. [DOI] [PubMed] [Google Scholar]