Abstract
The present study tested the hypothesis that intense interval training enhances human skeletal muscle blood flow and oxygen uptake (V̇O2) at the onset of dynamic exercise. We also investigated whether possible training effects were dependent on exercise intensity. Six habitually active males carried out 7 weeks of intermittent-exercise one-legged knee-extensor training at an intensity corresponding to ∼150% of peak thigh V̇O2 on three to five occasions per week. After the training period, cardiovascular and metabolic measurements were performed during knee-extensor exercise with the trained leg (TL) and the control leg (CL) for 10 min at intensities of 10 and 30 W, and also for 4 min at 50 W. Femoral venous blood flow was higher (P < 0.05) in TL than CL from 75 to 180 s at 30 W (∼75 s: 3.43 ± 0.20 versus 2.99 ± 0.18 l min−1) and from 40 to 210 s at 50 W (∼75 s: 5.03 ± 0.41 versus 4.13 ± 0.33 l min−1). Mean arterial pressure was not different between legs. Thus, thigh vascular conductance was higher (P < 0.05) in TL than CL from 35 to 270 s at 30 W and from 150 to 240 s at 50 W. Femoral arterial–venous (a-v) O2 difference was higher (P < 0.05) in TL than CL from 20 to 70 s at 30 W, but not different between TL and CL at 50 W. Thigh V̇O2 was higher (P < 0.05) in TL than CL from 20 to 110 s at 30 W (∼45 s: 0.38 ± 0.04 versus 0.30 ± 0.03 l min−1), and from 45 to 240 s at 50 W (∼45 s: 0.64 ± 0.06 versus 0.44 ± 0.08 l min−1). No differences were observed between TL and CL during exercise at 10 W. The present data demonstrate that intense interval training elevates muscle oxygen uptake, blood flow and vascular conductance in the initial phase of exercise at high, but not at low, intensities.
Krogh & Lindhard (1913) observed in a pioneering work that pulmonary oxygen (O2) uptake in the transition from rest to constant cycle exercise ‘does not rise instantaneously though certainly very rapidly to a level corresponding to the amount of work performed’. Since then, the transient lag in O2 uptake and the temporal pattern of O2 uptake has been studied extensively by the use of pulmonary measurements (see Tschakovsky & Hughson, 1999). These studies have established that training status, exercise intensity and previous intense exercise all affect O2 uptake at the onset of dynamic exercise (Hagberg et al. 1978; Cerretelli et al. 1979; Phillips et al. 1995; Engelen et al. 1996; MacDonald et al. 1997; McKenna et al. 1997; Carter et al. 2002).
Cross-sectional and longitudinal studies have shown that O2 uptake kinetics is faster for endurance-trained than untrained subjects during submaximal running and cycle exercise (Hagberg et al. 1978; Hickson et al. 1978; Cerretelli et al. 1979; Overend et al. 1992; Phillips et al. 1995; Womack et al. 1995). Moreover, McKenna et al. (1997) observed that the pulmonary oxygen uptake during maximal 30 s cycle exercise was higher after a period of intense intermittent training. However, it is uncertain to what extent these findings are applicable to the exercising muscles, since pulmonary measurements represent an integrated response of the whole body. Little knowledge has been obtained about training effects on transient O2 uptake at the muscular level. Shoemaker et al. (1996) showed that a short period of endurance training resulted in a faster femoral artery blood velocity at the onset of knee-extensor exercise, but thigh arterial–venous (a-v) O2 difference was not determined. Over the last decade techniques have been developed to determine transient muscle O2 uptake, i.e. frequent measurements of muscle blood flow, arterio-venous O2 difference and assessment of transient time delays from the capillaries to the sampling sites (Grassi et al. 1996; Hughson et al. 1996; Bangsbo et al. 2001; Krustrup et al. 2001, 2003). A combined use of these techniques makes it is possible to examine the effect of exercise training on transient O2 uptake at the muscular level. Furthermore, such measurements can elucidate whether any training-induced changes in muscle O2 uptake are caused by alterations in vascular resistance, blood perfusion and/or O2 extraction.
Several studies have shown that pulmonary O2 uptake kinetics is slower at high compared to low exercise intensities (Hagberg et al. 1978; Paterson & Whipp, 1991; Engelen et al. 1996; Carter et al. 2002). This finding may be explained by a gradually elevated fast-twitch (FT) fibre recruitment with increasing intensities (Gollnick et al. 1974; Vøllestad & Blom, 1985; Krustrup et al. 2004), as in vitro studies and cross-sectional in vivo studies have provided evidence that O2 uptake kinetics are slower in FT than in slow-twitch (ST) fibres (Crow & Kushmerick, 1982; Barstow et al. 1996). Nevertheless, other studies report no differences in the time constants of the rapid phase of O2 uptake between exercise intensities (Barstow & Molé, 1991; Ozyener et al. 2001; Rossiter et al. 2002) and it may be speculated that fibre type-specific differences in O2 uptake kinetics are related to training status. It is well known that muscular adaptations to training are dependent on the exercise regime with continuous endurance training improving oxidative enzyme activity mainly in ST fibres and intense intermittent training predominantly affecting FT fibres (Saltin et al. 1976; Henriksson & Reitman, 1976). However, it has yet to be elucidated whether intense intermittent training specifically enhances muscular O2 uptake at exercise intensities sufficiently high to involve FT fibre recruitment.
Thus, the aim of the present study was to investigate the effect of intense interval one-legged knee-extensor training on muscle O2 uptake and cardiovascular response at the onset of low, moderate and high intensity submaximal exercise.
Methods
Subjects
Six healthy habitually active male subjects participated in the study. They had an average age, height, weight and peak pulmonary O2 uptake (V̇O2peak) of 25.3 ± 2.9 (± s.d.) year, 185.0 ± 3.9 cm, 82.8 ± 11.8 kg and 50.2 ± 1.2 ml O2 min−1 kg−1, respectively. The subjects were fully informed of any risks and discomforts associated with the experiments before giving their written informed consent to participate. The study was approved by the Ethical Committee of the Copenhagen and Frederiksberg Communities, Denmark, and was performed according to the code of ethics of the World Medical Association (Declaration of Helsinki).
Experimental design
The subjects performed intense intermittent knee-extensor exercise training for 6–8 weeks with one leg (TL), whereas the other leg served as control (CL). A muscle biopsy was obtained from each leg before and after the training programme for determination of local muscular adaptations. After the training period two identical main experiments were performed, one with CL and one with TL. These included measurements of femoral venous blood flow and femoral arterial–venous blood samples before and during submaximal exercise of different intensities.
Exercise model
Subjects performed dynamic one-legged knee-extensor exercise in the spine position on an ergometer that permitted the exercise to be confined to the quadriceps muscle (Andersen et al. 1985). Before the preliminary testing procedures the subjects were familiarized with the exercise model on four separate occasions. Force tracings and kicking frequency were monitored.
Preliminary testing
Each subject underwent preliminary exercise testing consisting of two incremental knee-extensor exercise tests by each leg and an incremental cycle test for determination of peak pulmonary V̇O2 (MedGraphics, Saint Paul, Minneapolis, USA). The incremental knee-extensor exercise test consisted of 4 min at 50 W, then 2 min at 60 W, whereupon the load was increased by 10 W every 2 min until exhaustion. The exercise test was terminated when the kicking frequency reached values below 55 kicks min−1. The exercise time was recorded as the test performance. The two incremental tests for the same leg were separated by one week. Subjects that had a peak pulmonary V̇O2 ranging from 45 to 55 ml O2 min−1 kg−1 and less than 15% differences between the legs in the incremental test performance were included in the study. The leg for training was selected randomly. Three subjects trained with the right leg and three subjects trained with the left leg. The quadriceps muscle masses of TL and CL before initiation of the training period were 2.44 (range: 2.24–3.05) and 2.48 (2.29–2.92) kg, respectively. The knee-extensor test performance times before the training period were 7.65 (5.49–9.22) and 7.80 (5.31–10.16) min for TL and CL, respectively.
Training
The training lasted 7.0 (range: 6.0–7.8) weeks. Each training session was initiated by a 5 min warm-up at 10 W followed by 5 min of rest. Then, the subjects performed 15 × 1 min exercise bouts at an intensity corresponding to ∼150% of thigh V̇O2peak, separated by 3 min rest periods. The load was selected to exhaust the subject at the end of the last bouts of each training session. A muscle biopsy was obtained before and after the first training session in order to determine muscle metabolite changes and fibre type-specific glycogen depletion during training. Every second week the subjects performed an incremental exercise test identical with the one performed prior to the training period and the training load was adjusted upwards based on the result. The initial training load was 92 ± 3 (± s.e.m.) (80–100) W and the load was elevated by 16 ± 6% to 106 ± 4 (96–118) W during the training period. The weekly training frequency was 3 in week 1–2, 4 in week 3–4 and 5 for the last 2–4 weeks. The total number of training sessions was 29 ± 3 (24–32).
Main experiments
The main experiment for TL was performed 40–48 h after the last training session. The main experiment for CL was carried out 10–14 days before (n = 3) or after (n = 3) the experiment with TL. Before each of the main experiments the subjects refrained from strenuous exercise and intake of alcohol for 48 h, as well as from tobacco and caffeine for 12 h. On the morning of the experiment, subjects had a light breakfast and arrived at the laboratory about 2 h prior to the experiment. After 30 min of rest in the spine position, a catheter was placed under local anaesthesia with the Seldinger technique in the femoral vein of the experimental leg with the tip positioned 1–2 cm proximal to the inguinal ligament. This catheter was used for femoral venous blood sampling. Through the catheter a thermistor (Edslab, T.D. Probe, 94-030-2.5F, Baxter A/S, Allerød, Denmark) was placed and advanced 8–10 cm proximal to the tip of the catheter for measurement of femoral venous blood flow by constant infusion thermodilution (Andersen & Saltin, 1985; González-Alonso et al. 2000). A second catheter was inserted in the femoral artery of the resting leg. This catheter was used for arterial blood sampling and measurements of arterial blood pressure. After insertion of the catheters, the subjects were placed in the spine position in the knee-extensor model. A resting muscle biopsy was obtained from the medial part of m. vastus lateralis under local anaesthesia (1 ml of lidocaine (lignocaine); 20 mg ml−1 without adrenaline). Electrocardiogram electrodes were placed on the chest for continuous measurements of heart rate.
Exercise protocol
The exercise protocol consisted of a 10 min bout of exercise at 30 W, followed by 60 min of rest. Then, exercise was performed for 10 min at 10 W, followed by a 10 min rest period and a 4 min exercise bout at 50 W. The exercise intensities were chosen to represent moderate, low, and high intensity submaximal exercise, respectively. Proceeding on from the 50 W exercise the subjects carried out an exhaustive incremental exercise test identical to the one performed prior to the training period.
Femoral blood flow was measured at rest, during the passive exercise, from 0 to 10, 25–40, 75–90, 115–130 and 175–190 s at all intensities as well as after 6 and 10 min during the 10 and 30 W exercise bout. Arterial and venous blood samples were collected at rest, during the passive exercise, and after 11, 18, 45, 70, 110 and 170 s at all intensities, as well as after 3, 6 and 10 min during the 10 and 30 W exercise, after 210 s at 50 W and frequently during the rest of the exhaustive exercise. An occlusion cuff placed below the knee was inflated to 250 mmHg 30 s prior to each exercise bout and remained inflated throughout exercise in order to avoid contribution of blood from the lower leg. For 5 s prior to the onset of each exercise the leg was passively moved in order to accelerate the ergometer flywheel and ensure a constant power output from the onset of exercise.
Muscle mass
The mass of the quadriceps femoris muscle group was estimated using measurements of thigh length, three thigh circumferences and skin-fold thickness, and corrected based on a comparison between anthropometry and MRI-scanning (ratio 1 : 0.8, Rådegran et al. 1999).
Analysis
Blood analysis
All blood samples were collected in 2 ml syringes and immediately placed in ice-slurry until analysed. Oxygen saturation of blood and haemoglobin concentration were determined spectrophotometrically (Radiometer OSM-3 Hemoximeter, Copenhagen, Denmark). The Hemoximeter was calibrated using the cyanomethaemoglobin method (Drabkin & Austin, 1935). Haematocrit (Hct) determinations were made in triplicate, using microcentrifugation. PO2, PCO2 and pH were measured with the Astrup technique (ABL 30, Radiometer, Copenhagen, Denmark). A 100 μl aliquot of each blood sample was haemolysed within 10 s of sampling using a 1 : 1 dilution with a buffer solution containing 20 g l−1 of Triton X-100 (YSI, Yellow Springs, OH, USA) in order to determine blood lactate (YSI Lactate Analyser, Model 23).
Muscle analysis
About 30 mg wet weight (ww) muscle tissue of each biopsy was mounted in an embedded medium (OCT Tissue-Tek, Sakura Finetek, Zoeterwoude, The Netherlands) and frozen in isopentane that was pre-cooled in liquid nitrogen. These samples were stored at −80°C until analysed for fibre type distribution (i.e. ST, FTa or FTx fibres) by myosin ATPase staining as previously described (Brooke & Kaiser, 1970). Furthermore, one 16 μm thick transverse section was cut at −20°C and stained for relative glycogen content by the periodic acid–Schiff (PAS) reaction. Under light microscopy the PAS stained fibres were rated as full, partly full, almost empty and empty of glycogen based on the staining intensity. The remainder of the muscle biopsy was frozen in liquid nitrogen, freeze dried and dissected free from blood and connective tissue. About 3 mg dry weight (DW) muscle tissue was homogenized (1 : 400) in a 0.3 m phosphate buffer adjusted to pH 7.7 containing 0.5 mg ml−1 of bovine serum albumin. Citrate synthase (CS) and 3-hydroxyacyl-CoA dehydrogenase (HAD) activity was determined by using fluourometric methods with NAD–NADH coupled reactions as described by Essén-Gustavsson & Henriksson, 1984).
Calculations
Oxygen uptake by and lactate release from the thigh muscles were calculated by multiplying the blood flow with the femoral arterio-venous O2 difference and femoral venous–arterial lactate difference, respectively. A continuous blood flow curve was constructed for each subject using a linear connection of consecutive data points in order to obtain time-matched values for blood flow and blood measurements. Peak thigh V̇O2 for TL and CL was determined in the incremental exercise test as the highest value reached.
To obtain measurements of muscle oxygen extraction, oxygen uptake and lactate release at the capillary level, corrections were made for the blood transit time from the capillaries to the collection points in the femoral artery and vein. Bangsbo et al. (2000) observed that the arterial–venous transit times were 11, 6, 5 and 5 s after 5, 25, 65 and 180 s of intense knee-extensor exercise, respectively, with a third of the time representing the time from artery to capillary. These values were used for TL and CL at all three intensities, as the latter study found no correlation between transit times and training status (peak muscle V̇O2 (kg muscle)−1) and as a pilot experiment (n = 3) indicated that transit times during moderate intensity knee-extensor exercise (30 W) were similar to those during intense exercise, i.e. 10 ± 2, 6 ± 1, 5 ± 0, 4 ± 1 and 4 ± 1 s after 7, 23, 60, 180 and 270 s of exercise, respectively.
Statistics
Differences between TL and CL were tested using a two-way ANOVA for repeated measures. When a significant interaction was detected, data were subsequently analysed using a Newman-Keuls post hoc test to locate differences. A Wilcoxon signed rank-test was used to evaluate differences in fibre type distribution between TL and CL as well as relative fibre type-specific glycogen content before and after the first training session. Values are means ± s.e.m. A significance level of 0.05 was chosen.
Results
Metabolic response to a training session
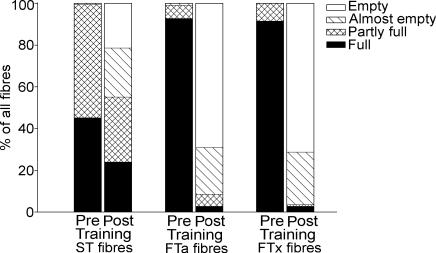
During the first training session, muscle lactate and H+ concentration increased (P < 0.05) 11- and 3-fold, respectively (Table 1). Muscle CP and glycogen decreased (P < 0.05) to 23 and 27% of the pre-training level, respectively (Table 1). After the training session, 22 ± 7, 69 ± 10 and 71 ± 9% of the ST, FTa and FTx fibres, respectively, were depleted for glycogen (Fig. 1).
Table 1.
Muscle CP, lactate, pH and glycogen before (pre-training) and immediately after (post-training) the first training session consisting of 15 × 1 min knee-extensor exercise bouts, at an intensity eliciting ∼150% of peak thigh V̇O2max, separated by 3 min rest periods
| Pre-training | Post-training | |
|---|---|---|
| Muscle CP (mmol (kg DW)−1) | 84 ± 3 | 19 ± 4§ |
| (70–92) | (4–33) | |
| Muscle lactate (mmol (kg DW)−1) | 8.3 ± 1.1 | 91.3 ± 10.5§ |
| (5.2–13.0) | (49.1–134.7) | |
| Muscle pH (– log H+) | 7.14 ± 0.02 | 6.88 ± 0.02§ |
| (7.02–7.21) | (6.82–6.96) | |
| Muscle glycogen (mmol (kg DW)−1) | 494 ± 45 | 133 ± 37§ |
| (289–647) | (41–281) |
Values are means ± s.e.m. and range.
Significantly different (P < 0.05) from pre-training value.
Figure 1. Glycogen depletion pattern during intense interval training.
Glycogen content in ST, FTa and FTx fibres before and after the first training session. Average levels of glycogen depletion are indicated by a filled section (full), a cross-hatched section (partly full), a hatched section (almost empty) and an open section (empty).
Effects of training
The quadriceps muscle mass of TL was 2.55 ± 0.13 kg after the training period, which was 4 ± 1% higher (P < 0.05) than for TL before training (2.44 ± 0.13 kg) and 5 ± 1% higher (P < 0.05) than for CL (2.43 ± 0.11 kg).
The proportion of FTx fibres in TL after training was 9 ± 3%, which was lower (P < 0.05) than in TL before training (20 ± 2%) and in CL (21 ± 3%). The proportions of FTa fibres (36 ± 4, 27 ± 4 and 31 ± 3%, respectively) and ST fibres (54 ± 6, 53 ± 6 and 48 ± 1%, respectively) in TL after training were not significantly different from those in TL before training and CL.
The activity of CS was 38.0 ± 1.2 μmol g DW−1 min−1 in TL after training, which was 25 ± 10% higher (P < 0.05) than before training and 30 ± 12% (P < 0.05) higher than in CL. The activity of HAD was not different between TL after training, TL before training and CL (49.3 ± 3.1, 44.2 ± 3.5 and 41.5 ± 1.5 μmol g DW−1 min−1, respectively).
Cardiovascular responses
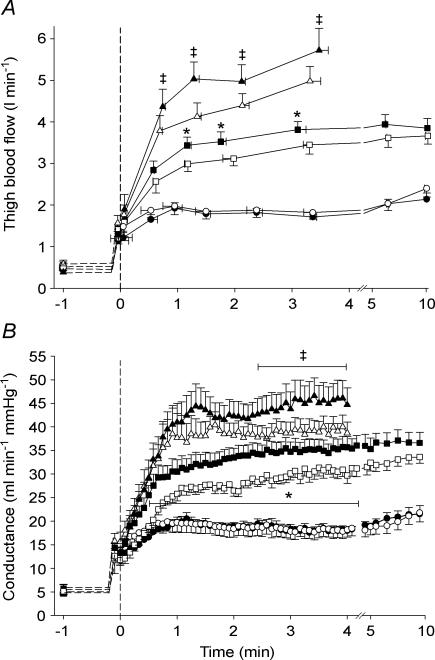
Thigh blood flow was not different between TL and CL during exercise at 10 W, whereas it was higher (P < 0.05) in TL than in CL from 75 to 180 s of exercise at 30 W (∼75 s: 3.43 ± 0.20 versus 2.99 ± 0.18 l min−1), and from 40 to 210 s of exercise at 50 W (∼75 s: 5.03 ± 0.41 versus 4.13 ± 0.33 l min−1 (Fig. 2A).
Figure 2. Effect of intense interval training on thigh blood flow and vascular conductance.
Thigh blood flow (A) and vascular conductance (B) in the trained leg (TL; filled symbols) and control leg (CL; open symbols) before and during exercise at 10 W (circles), 30 W (squares) and 50 W (triangles). Means ± s.e.m. are given for time (x-axis) and thigh blood flow/vascular conductance (y-axis). * TL significantly different from CL during exercise at 30 W ‡ TL significantly different from CL during exercise at 50 W.
Mean arterial pressure (MAP) was not different between TL and CL at any intensity. Pre-exercise MAP was ∼93 mmHg and end-exercise values at 10, 30 and 50 W were 96 ± 1, 101 ± 2, 124 ± 3 mmHg (TL) and 100 ± 3, 104 ± 3, 128 ± 4 mmHg (CL), respectively.
Thigh vascular conductance was not different between TL and CL during the 10 W exercise, whereas it was higher (P < 0.05) in TL than in CL from 35 to 270 s of exercise at 30 W (∼60 s: 31 ± 2 versus 25 ± 1 ml min mmHg−1) and from 145 to 240 s of exercise at 50 W (∼240 s: 45 ± 1 versus 39 ± 2 ml min−1 mmHg−1) (Fig. 2B).
Heart rate was not different between exercise with TL and CL during the 10, 30 and 50 W bouts with end-exercise values of 75 ± 3, 91 ± 4 and 108 ± 4 beats min−1 (TL) and 77 ± 3, 98 ± 3 and 109 ± 6 beats min−1 (CL), respectively.
Thigh oxygen uptake
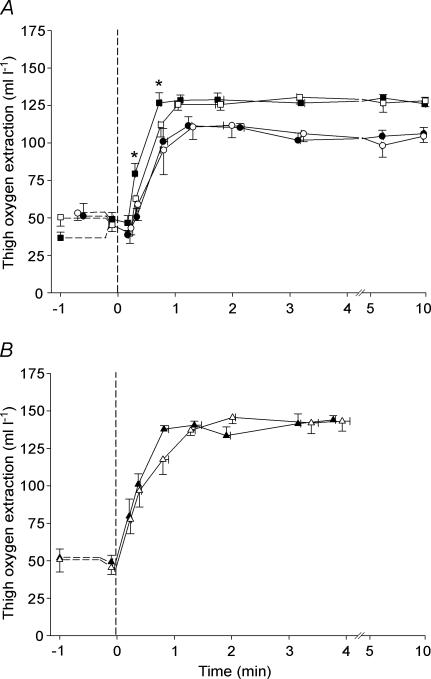
Thigh oxygen extraction (a-v O2 diff) was not different between TL and CL during exercise at 10 and 50 W, whereas it was higher (P < 0.05) in TL than in CL from 20 to 70 s of the 30 W exercise (∼45 s: 127 ± 7 versus 112 ± 8 ml l−1) (Fig. 3A).
Figure 3. Effect of intense interval training on thigh oxygen extraction.
Thigh oxygen extraction in the trained leg (TL; filled symbols) and control leg (CL; open symbols) before and during one-legged knee-extensor exercise at 10 W (A; circles) and 30 W (A; squares), as well as at 50 W (B; triangles). Means ± s.e.m. are given for time (x-axis) and thigh oxygen extraction (y-axis). * TL significantly different from CL during exercise at 30 W.
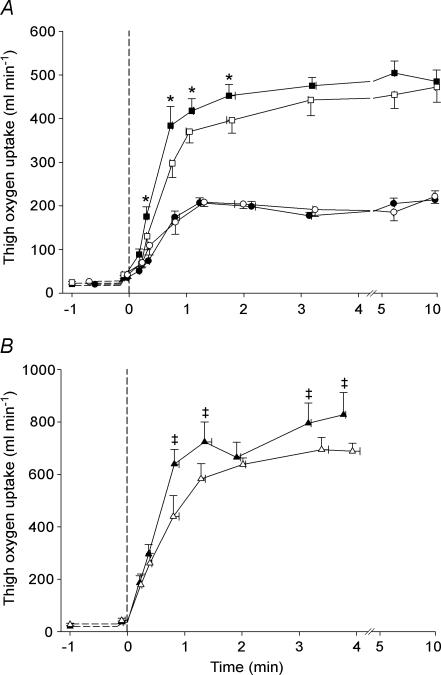
Thigh V̇O2 during exercise at 10 W was not different between TL and CL (Fig. 4A). During exercise at 30 W, thigh V̇O2 was higher (P < 0.05) in TL than in CL from 20 to 110 s (∼20 s: 0.18 ± 0.02 versus 0.13 ± 0.01 l min−1), after which no differences were observed (Fig. 4A). During the 50 W exercise, thigh V̇O2 was higher (P < 0.05) in TL than in CL from 45 to 240 s, except after 2 min (∼45 s: 0.64 ± 0.06 versus 0.44 ± 0.08 l min−1) (Fig. 4B). The mean rate of rise in thigh V̇O2 was higher (P < 0.05) in TL than in CL during the initial 45 s of exercise at 30 W (0.48 ± 0.06 versus 0.34 ± 0.04 l min−2) and 50 W (0.73 ± 0.07 versus 0.50 ± 0.09 l min−2). Peak thigh V̇O2 reached during the incremental exercise test was higher (P < 0.05) in TL than in CL (1.10 ± 0.05 versus 0.89 ± 0.06 l min−1).
Figure 4. Effect of intense interval training on thigh oxygen uptake.
Thigh oxygen uptake in the trained leg (TL; filled symbols) and control leg (CL; open symbols) before and during one-legged knee-extensor exercise at 10 W (A; circles), 30 W (A; squares) and 50 W (B; triangles). Means ± s.e.m. are given for time (x-axis) and thigh oxygen uptake (y-axis). * TL significantly different from CL during exercise at 30 W ‡ TL significantly different from CL during exercise at 50 W.
Thigh lactate release
No net thigh lactate release was observed in TL or CL during exercise at 10 W. Thigh lactate release was lower (P < 0.05) in TL than in CL from 35 s and throughout the 30 W exercise (∼110 s: 2.2 ± 0.4 and 3.2 ± 0.4 mmol min−1). Thigh lactate release was not different between TL and CL during the 50 W exercise (∼240 s: 6.5 ± 1.1 versus 7.8 ± 1.7 mmol min−1).
Blood gasses and pH
Femoral venous PO2 was not different between TL and CL during exercise at 10 or 50 W with end-exercise values of ∼28 and ∼25 mmHg, respectively. During exercise at 30 W, femoral venous PO2 was lower (P < 0.05) in TL than in CL from 20 to 45 s (∼20 s: 31.6 ± 1.2 versus 37.4 ± 4.3 mmHg). Arterial PO2 was above 100 mmHg throughout the exercises with both legs.
Femoral venous PCO2 during the 10, 30 and 50 W bouts was not different between TL and CL with end-exercise values of 51.7 ± 1.3, 56.6 ± 1.3 and 66.6 ± 4.4 mmHg (TL) and 51.3 ± 1.7, 58.8 ± 1.6 and 67.9 ± 4.5 mmHg (CL), respectively. Arterial PCO2 was ∼40 mmHg throughout exercise at 10, 30 and 50 W with both legs.
Femoral venous pH was not different between TL and CL during exercise at 10 or 50 W with end-exercise values of ∼7.33 and ∼7.24, respectively. During exercise at 30 W, venous blood pH was higher (P < 0.05) in TL than CL from 45 s to 6 min (∼110 s: 7.31 ± 0.01 versus 7.28 ± 0.01). Arterial blood pH was ∼7.40 throughout exercise at 10 and 30 W in both legs, and decreased (P < 0.05) to ∼7.38 in both legs during the 50 W exercise.
Discussion
The major finding of the present study was that a period of intense intermittent knee-extensor training enhanced thigh oxygen uptake at the onset of high intensity submaximal exercise, but had no effect at low intensity. Furthermore, the present study demonstrated that thigh blood flow and vascular conductance were initially elevated when the trained muscle exercised at high intensity.
Effect of training on muscle oxygen uptake
The present study is the first to show that a period of training enhances muscle oxygen uptake at the onset of dynamic exercise. As a result of 7 weeks of intense intermittent exercise training the rate of rise in oxygen uptake was markedly higher in the trained than untrained leg during the initial 45 s of moderate (30 W) and intense (50 W) submaximal dynamic exercise, corresponding to ∼55 and 75% of thigh peak V̇O2 in TL and ∼60 and ∼85% of thigh peak V̇O2 in CL, respectively. It was, moreover, observed that the trained leg had higher oxygen uptakes for at least 2 min during the 30 W exercise and throughout the 4 min bout at 50 W. On the other hand, no differences in muscle oxygen uptake were found between the trained and untrained leg during exercise at a low intensity (10 W: ∼30% (TL) and ∼35% (CL) of thigh peak V̇O2). In the present study efficiency was not determined. Nevertheless, the similar oxygen uptakes in the trained and untrained leg during the last 5–7 min of the 30 W exercise and throughout the 10 W exercise indicates that the training affected the adjustment of O2 uptake to the requirement rather than modifying the actual O2 cost of contractions. This notion is supported by the observation that the higher oxygen uptake in the trained leg during the first minutes of the 30 W exercise was associated with a 50% lower lactate release, indicating a marked attenuation of anaerobic energy turnover. At the end of the 50 W exercise O2 uptake was about 100 ml min−1 higher in the trained than in the untrained leg, whereas lactate release was not significantly lower (6.5 versus 7.8 mmol min−1), which may have been due to the limited number of subjects. Nevertheless, the muscle lactate accmulation may have been lower in the trained compared to the untrained leg because the capacity for lactate transport is elevated after a period of intense training (Juel et al. 2004).
The higher oxygen uptake after training was a result of both an elevated blood flow and an enhanced oxygen extraction in the initial phase of exercise at 30 W, whereas at 50 W it was only due to a higher blood flow. The latter finding is in agreement with a previous observation of an unaltered arterial–venous oxygen difference during intense cycle exercise after 7 weeks of training (McKenna et al. 1997). Also in accordance with the observations in the present study, Shoemaker et al. (1996) found that femoral arterial blood flow velocity during submaximal knee-extensor exercise was higher after 10 days of training. It has been proposed that oxygen delivery limits the oxygen utilization at the onset of dynamic exercise, as muscle oxygen uptake has shown to be lowered when blood flow and oxygen delivery is reduced (Hughson et al. 1996). There is evidence, however, that an elevated blood and oxygen delivery do not accelerate muscle oxygen uptake kinetics during submaximal dynamic contractions (Grassi et al. 1998a,b). Therefore, it is not likely that the higher oxygen uptake in the initial phase of the 30 and 50 W exercise was caused by the higher blood flow per se. In support of this, during the first 30 s of the 30 W exercise, both with the trained and untrained leg, thigh oxygen delivery exceeded oxygen uptake more than during the next minute of exercise (∼300 versus 220 ml min−1), which is in agreement with findings in a study involving supra-maximal knee-extensor exercise (∼110% of V̇O2max) (Bangsbo et al. 2000). Thus, it appears that there is a local limitation of muscle oxygen utilization in the initial phase of exercise. It should be noted, however, that as the femoral vein also is draining non-muscle tissue and inactive muscles during exercise, the femoral arterial–venous difference may not represent the oxygen extraction of the contracting muscle. The oxygen extraction of the quadriceps muscle can be calculated based on the assumptions that: (1) non-muscle tissue during passive exercise account for blood flow and oxygen uptake values of 0.3 l min−1 and 5 ml min−1, respectively (Savard et al. 1988), (2) the remaining blood flow and oxygen uptake during passive exercise is equally distributed to the quadriceps muscle and hamstring/adductor muscles (Wesche, 1986) and (3) perfusion and oxygen uptake of the hamstring/adductor muscles during exercise is the same as during passive exercise and maintained constant throughout exercise (Bangsbo, 2000). From Fig. 5 it is clear the quadriceps oxygen extraction was far from maximal in the first minute at the 10, 30 and 50 W exercise, supporting a limitation at the muscle level. Apparently, there have to be other explanations than the elevated blood flow for the intensity-dependent effect of training on muscle oxygen kinetics.
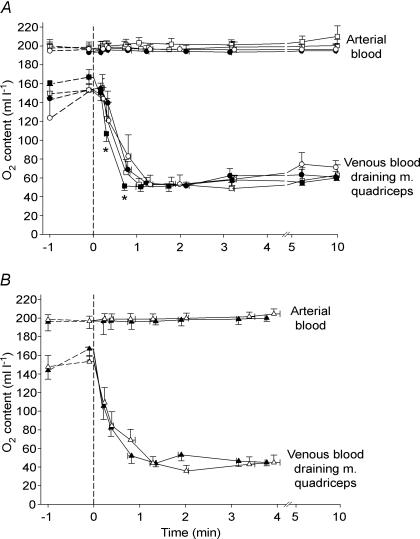
Figure 5. Quadriceps muscle oxygen extraction.
O2 content in femoral arterial blood and estimated O2 content in venous blood coming from m. quadriceps in the trained leg (TL; filled symbols) and control leg (CL; open symbols) before and during one-legged knee-extensor exercise at 10 W (A; circles), 30 W (A; squares) and 50 W (B; triangles). Means ± s.e.m. are given for time (x-axis) and oxygen content (y-axis). * TL significantly different from CL during exercise at 30 W.
Intensity-dependent effects of intense muscular training
An interesting finding of the present study was that training enhanced transient oxygen uptake at the two high intensities but not at the low exercise intensity. It has been proposed that FT fibres have slower oxygen uptake kinetics during dynamic exercise than ST fibres (Barstow et al. 1996) and this may explain that several studies using pulmonary measurements during whole body exercise have found slower oxygen uptake kinetics at high compared to low exercise intensities (Hagberg et al. 1978; Engelen et al. 1996; Carter et al. 2002). Actually, the V̇O2 data from the control leg provides evidence that V̇O2 kinetics is also slower at high compared to low intensities at the muscular level. Thus, the time constant for the rapid phase of oxygen uptake was found to be higher during exercise at 50 W compared to exercise at 10 W (47 versus 21 s), when applying two-component (30 and 50 W) and one-component (10 W) exponential fittings (Table 2). It seems plausible that during the exercise at 10, 30 and 50 W in the control leg none, some and essentially all FT fibres were recruited, respectively (Gollnick et al. 1974; Vøllestad & Blom, 1985; Krustrup et al. 2004). Therefore, a possible explanation for the exercise-dependent effect of training on transient oxygen uptake, could be that the training period leads to less recruitment of FT fibres during exercise at 30 and 50 W as the work loads were relative lower with the trained leg, whereas solely ST fibres were recruited during 10 W exercise with both legs. The training may also have changed the recruitment of subgroups of FT fibres during the 50 W exercise, as a significant fraction of FTx fibres was transformed to FTa fibres during the training period. On the other hand, as the FTx fibres were not likely to be active during the exercise bout performed at 30 W in either leg, the fibre transformation cannot explain the observed difference in oxygen uptake between the untrained and trained leg at this exercise intensity. Furthermore, we observed no difference in oxygen uptake kinetics between the 10, 30 and 50 W exercises for the trained leg (τ: 22–28 s; Table 2), although without doubt a larger fraction of FT fibres were active at the high compared to the low intensity. Therefore a difference in fibre recruitment cannot explain all of the observed training-induced changes in transient muscle oxygen uptake and it is likely that the training resulted in significant local adaptations within the muscle, related either to a greater transport of oxygen from the capillaries to the muscle cells or a more rapid acceleration of oxygen utilization within the mitochondria.
Table 2.
Parameter estimates for exponential curve fitting of muscle V̇O2 response to exercise at 10, 30 and 50 W for the trained (TL) and untrained leg (CL)
| 10 W | 30 W | 50 W | ||||
|---|---|---|---|---|---|---|
| CL | TL | CL | TL | CL | TL | |
| Baseline (BL; ml min−1) | 26 ± 4 | 18 ± 3 | 24 ± 4 | 21 ± 5 | 27 ± 5 | 20 ± 2 |
| Time delay (TD1; s) | 9 ± 3 | 10 ± 2 | 9 ± 2 | 7 ± 1 | 5 ± 2 | 6 ± 1 |
| Gain (G1; ml min−1) | 182 ± 10 | 184 ± 11 | 425 ± 37# | 449 ± 20# | 719 ± 32# | 761 ± 63# |
| Time constant (τ1; s) | 21 ± 4 | 22 ± 3 | 33 ± 5 | 25 ± 5 | 47 ± 9# | 28 ± 4* |
Values are means ± s.e.m. One-component (10 W) or two-component (30 and 50 W) exponential fits were used as described by Barstow & Molé (1991), i.e. VO2(t) = VO2(BL) + G1(1 − exp−(t−TD1)/τ1) + G2(1 − exp−(t−TD2)/τ2), where V̇O2(t) is the V̇O2 at any given time point. The fitting parameters for the primary phase were the time delay (TD1), gain (G1) and time constant (τ1). The least sum of squared error was used as the criterion for convergence.
Significant difference (P < 0.05) between TL and CL.
Significant different (P < 0.05) from 10 W within the same leg.
In the present study the number of capillaries in contact with each fibre increased by 19% for ST fibres and 21% for FT fibres as a result of training (Jensen et al. 2004). This adaptation may have caused a lower blood-to-fibre diffusion distance for oxygen and may have enhanced oxygen extraction in the transient phase of intense dynamic exercise. In addition, the greater number of capillaries may have elevated microcirculatory volume in the trained muscle. Such an elevation in volume would counteract a reduction in mean transit time of blood in the muscle as a result of the higher muscle blood flow, allowing sufficient time for extraction of oxygen. In accordance, the a-v O2 difference was the same in the trained and control leg for the 50 W exercise, and higher in the trained leg in the initial phase of the 30 W exercise bout. The observed higher a-v O2 difference in the trained leg may also be explained by metabolic adaptations that occurred within the muscle fibres. The activity of the oxidative enzyme CS was 30% higher in the trained muscle compared to control. As the FTa and FTx fibres were heavily tasked during each training session (Fig. 1), it may be that especially the FT fibres increased their oxidative potential during the training period. This notion is supported by the observations that intense intermittent exercise training predominantly elevates enzyme activities of FT fibres (Henriksson & Reitman, 1976) and that the oxidative capacity of FTa fibres can reach the level of ST fibres in trained muscles (Proctor et al. 1995). Such an adaptation in the FT fibres may explain why no differences were observed in the fundamental rapid phase of oxygen uptake kinetics between the 10, 30 and 50 W exercises for the trained leg and explain the finding of an intensity-dependent training effect on transient muscle oxygen uptake.
It should also be considered whether the greater oxygen extraction and oxygen uptake in the trained compared to the untrained leg was due to a greater off-loading of oxygen from haemoglobin. A lowering of blood pH as well as an increase in blood temperature and partial pressure of CO2 can cause a rightward shift in the oxygen saturation curve and thereby stimulate a greater oxygen off-loading (Bohr effect). However, femoral venous pH was higher and venous PCO2 was lower in the trained than untrained leg in the initial phase of exercise at 30 W, whereas neither variable were different between legs during exercise at the other intensities. In addition, femoral venous blood temperatures prior to and during each of the exercises were the same in the trained and untrained muscle (data not shown). Thus, it is not likely that the greater muscle extraction and oxygen uptake in the initial phase of exercise at high intensities in the trained leg was caused by a Bohr effect.
Effect of training on the cardiovascular response to exercise
In the present study it was observed that thigh blood flow was higher in the first minutes of exercise at moderate and high exercise intensities in the trained leg, whereas blood flow was similar in the two legs during exercise at 10 W and in the later stages of exercise at 30 W (Fig. 2A). Similarly, thigh vascular conductance was transiently higher during exercise at 30 W and during a phase of the 50 W exercise (Fig. 2B), as mean arterial pressure was unaltered after training. In accordance with these observations, Shoemaker et al. (1996) found an elevated femoral arterial blood velocity and an increased vascular conductance during moderate submaximal knee-extensions after a short period of endurance training. However, the present findings of an intensity-dependant effect on thigh blood flow and vascular conductance are novel. As the local regulation of blood flow during exercise remains unclear (Clifford & Hellsten, 2004), the reason for the training-induced elevation in blood flow can only be speculated on. Muscle blood flow has been shown to be closely related to oxygen utilization during exercise (Andersen & Saltin, 1985; Richardson et al. 1999), and it has been observed that there is a relationship between the oxygen saturation of the haemoglobin molecule and leg vascular conductance in humans (González-Alonso et al. 2001), suggesting that the erythrocyte may be an important mediator of blood flow in skeletal muscle. In the initial phase of the 30 W exercise, the femoral venous oxygen content and PO2 were lower in the trained compared to the control leg, which may reflect a difference in oxygen content and tension at the capillary level also, causing a difference in the conductance. However, significant oxygen gradients exist in the microcirculation (Tsai et al. 2003), which means that venous oxygen content and PO2 may not represent tissue levels. Nevertheless, an explanation of the higher blood flow and conductance after training must also involve other factors because both variables were elevated during periods of the 30 W and 50 W exercises with no differences in venous oxygen content and PO2.
A possible explanation for the increase in blood flow at the high work loads is that the ∼5% increase in muscle mass and the ∼20% greater number of capillaries in the trained leg expanded the microvascular volume at the onset of exercise. The enhanced capillarization in the trained muscle may, moreover, have resulted in a greater total activity of vasodilator enzymes located in the endothelial cells, e.g. nitric oxide synthase (NOS) and cytochrome P450 2C9 (CYP 2C9), which have been shown to be of importance in human skeletal muscle blood flow regulation (Hillig et al. 2003). In accordance with this proposition, an enhanced number of endothelial NOS-containing capillaries has been observed after an 8 week period of intense exercise training in humans (Frandsen et al. 2000). However, further studies are needed to elucidate the causes of training-induced increases in vascular conductance and blood flow in the first phase of intense dynamic exercise.
Summary
The present study demonstrated that intense interval training with an isolated muscle group enhanced human skeletal muscle oxygen uptake at the onset of moderate and high intensity submaximal exercise. In contrast, no training effects were observed during exercise at a low intensity. It was also shown that the training-induced elevation in muscle oxygen uptake was caused by a larger muscle blood flow and oxygen extraction during moderate exercise and solely by an elevated blood flow during high intensity exercise.
Acknowledgments
We would like to acknowledge the subjects for their motivation and attitude during the training sessions and experiments. The excellent technical assistance by Merete Vannby, Ingelise Kring and Winnie Taagerup is appreciated. Furthermore, we would like to thank David Hudson and Christina Klarskov for their great effort in conducting the training sessions. Support was given by Team Denmark and the Danish National Research Foundation (501–14).
References
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as a model for the study of an isolated exercising muscle in man. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J. Muscle oxygen uptake in humans at onset of and during intense exercise. Acta Physiol Scand. 2000;168:457–464. doi: 10.1046/j.1365-201x.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, González-Alonso J, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, González-Alonso J, Saltin B. ATP production and efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol Endocrinol Metab. 2001;280:E956–E964. doi: 10.1152/ajpendo.2001.280.6.E956. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fibre type and pedal frequency on oxygen uptake kinetics of heavy exercise. J Appl Physiol. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Molé P. Linear and non-linear characteristics of oxygen uptake kinetics during heavy exercise. J Appl Physiol. 1991;71:2099–2106. doi: 10.1152/jappl.1991.71.6.2099. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three myosine adenosine triphosphatase systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Carter H, Pringle JS, Jones AM, Doust JH. Oxygen uptake kinetics during treadmill running across exercise intensity domains. Eur J Appl Physiol. 2002;86:347–354. doi: 10.1007/s00421-001-0556-2. [DOI] [PubMed] [Google Scholar]
- Cerretelli P, Pendergast D, Paganelli WC, Rennie DW. Effects of specific muscle training on VO2 on-response and early blood lactate. J Appl Physiol. 1979;47:761–769. doi: 10.1152/jappl.1979.47.4.761. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J General Physiol. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin DL, Austin JH. Spectrophotometric studies II. Preparations from washed blood cells, nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem. 1935;122:51–65. [Google Scholar]
- Engelen M, Porszasz J, Riley M, Wasserman K, Maehara K, Barstow TJ. Effects of hypoxia on O2 uptake and heart rate kinetics during heavy exercise. J Appl Physiol. 1996;81:2500–2508. doi: 10.1152/jappl.1996.81.6.2500. [DOI] [PubMed] [Google Scholar]
- Essén-Gustavsson B, Henriksson J. Enzyme levels in pools of microdissected human muscle fibres of identified type. Adaptive response to exercise. Acta Physiol Scand. 1984;120:505–515. doi: 10.1111/j.1748-1716.1984.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Høffner L, Betak A, Saltin B, Bangsbo J, Hellsten Y. Endurance training does not alter the level of neuronal nitric oxide synthase in human skeletal muscle. J Appl Physiol. 2000;89:1033–1038. doi: 10.1152/jappl.2000.89.3.1033. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human skeletal muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Gladden B, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998a;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden B, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect VO2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998b;85:1404–1412. doi: 10.1152/jappl.1998.85.4.1404. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Hagberg JM, Nagle FJ, Carlson JL. Transient O2 uptake response at the onset of exercise. J Appl Physiol. 1978;44:90–92. doi: 10.1152/jappl.1978.44.1.90. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Reitman JS. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976;97:392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Hickson RC, Bomze HA, Hollozy JO. Faster adjustment of O2 uptake to the energy requirement of exercise in the trained state. J Appl Physiol. 1978;44:877–881. doi: 10.1152/jappl.1978.44.6.877. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome P450 2C9 plays an important role in the regulation of exercise induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson RL, Shoemaker JK, Tshakovsky ME, Kowalchuk JM. Dependence of muscle VO2 on blood flow dynamics at onset of forearm exercise. J Appl Physiol. 1996;81:1619–1626. doi: 10.1152/jappl.1996.81.4.1619. [DOI] [PubMed] [Google Scholar]
- Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarisation and presence of angiogenic factors in human skeletal muscle. J Physiol. 2004;557:571–582. doi: 10.1113/jphysiol.2003.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–E251. doi: 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjær M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, González-Alonso J, Quistorff B, Bangsbo J. Muscle heat production and anaerobic energy production during repeated intense dynamic exercise in man. J Physiol. 2001;536:947–956. doi: 10.1111/j.1469-7793.2001.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense submaximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004;47:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Pedersen PK, Hughson RL. Acceleration of VO2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJ, McKelvie RS, Obminski G, MacDougall JD, Jones NL. Enhanced pulmonary and active skeletal muscle gas exchange during intense exercise after sprint training in men. J Physiol. 1997;501:703–716. doi: 10.1111/j.1469-7793.1997.703bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend TJ, Paterson DH, Cunningham DA. The effect of interval and continuous training on the aerobic parameters. Can J Sport Sci. 1992;17:129–134. [PubMed] [Google Scholar]
- Ozyener F, Rossiter HB, Ward SA, Whipp BJ. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol. 2001;533:891–902. doi: 10.1111/j.1469-7793.2001.t01-1-00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DH, Whipp BJ. Asymmetries of oxygen uptake transients at the on- and offset of heavy exercise in humans. J Physiol. 1991;443:575–586. doi: 10.1113/jphysiol.1991.sp018852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal exercise. J Appl Physiol. 1995;79:1914. doi: 10.1152/jappl.1995.79.6.1914. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol. 1995;78:2033–2028. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol. 1999;87:2375–2380. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol. 2002;541:991–1002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick PD. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Savard GK, Nielsen B, Laszczynka L, Elmann-Larsen B, Saltin B. Muscle blood flow is not reduced in man during moderate exercise and heat stress. J Appl Physiol. 1988;64:649–657. doi: 10.1152/jappl.1988.64.2.649. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Phillips SM, Green HJ, Hughson RL. Faster femoral artery blood velocity kinetics at the onset of exercise following short-term training. Cardiovasc Res. 1996;31:278–286. [PubMed] [Google Scholar]
- Tsai AG, Johnson PC, Intaglietta M. Oxygen gradients in the microcirculation. Physiol Rev. 2003;83:933–963. doi: 10.1152/physrev.00034.2002. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Wesche J. The time course and magnitude of blood flow changes in human quadriceps muscles following isometric contraction (1986) J Physiol. 1986;377:445–462. doi: 10.1113/jphysiol.1986.sp016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack CJ, Davis SE, Blumer JL, Barrett E, Weltman AL, Gaesser GA. Slow component of O2 uptake during heavy exercise: adaptations to endurance training. J Appl Physiol. 1995;79:838–845. doi: 10.1152/jappl.1995.79.3.838. [DOI] [PubMed] [Google Scholar]