Abstract
There are two divergent fructokinase isozymes, Frk1 and Frk2 in tomato (Lycopersicon esculentum Mill.) plants. To investigate the physiological functions of each isozyme, the expression of each fructokinase mRNA was independently suppressed in transgenic tomato plants, and the respective phenotypes were evaluated. Suppression of Frk1 expression resulted in delayed flowering at the first inflorescence. Suppression of Frk2 did not effect flowering time but resulted in growth inhibition of stems and roots, reduction of flower and fruit number, and reduction of seed number per fruit. Localization of Frk1 and Frk2 mRNA accumulation by in situ hybridization in wild-type tomato fruit tissue indicated that Frk2 is expressed specifically in early tomato seed development. Fruit hexose and starch content were not effected by the suppression of either Frk gene alone. The results collectively indicate that flowering time is specifically promoted by Frk1 and that Frk2 plays specific roles in contributing to stem and root growth and to seed development. Because Frk1 and Frk2 gene expression was suppressed individually in transgenic plants, other significant metabolic roles of fructokinases may not have been observed if Frk1 and Frk2 play, at least partially, redundant metabolic roles.
Suc translocated from source leaves to sink tissue is first metabolized by Suc synthase (SS) and/or invertase (INV) to form a pool of hexose. Fru is subsequently phosphorylated to Fru-6-phosphate by fructokinase (EC 2.7.1.4) and used as the substrate for respiration and biosynthesis of starch and the other complex carbohydrates. Fructokinase has been characterized from various plant tissues such as pea (Pisum sativum) seeds (Copeland et al., 1978), avocado (Persea americana) fruit (Copeland and Tanner, 1988), maize (Zea mays) kernels (Doehlert, 1990), potato (Solanum tuberosum) tubers (Gardner et al., 1992; Renz and Stitt, 1993), taproots of sugar beet (Beta vulgaris; Chaubron et al., 1995), barley (Hordeum vulgare) leaves (Baysdorfer et al., 1989), spinach (Spinacia oleracea) leaves (Schnarrenberger, 1990), rice (Oryza sativa) embryo (Guglielminetti et al., 2000), and tomato (Lycopersicon esculentum) fruit (Martinez-Barajas and Randall, 1996). The purified fructokinases have been characterized by a generally high affinity for Fru and ATP and, in some cases, two to three fructokinase isoforms have been identified.
It has been proposed that fructokinase may regulate starch synthesis coordinately with SS in sink tissue such as potato tubers and tomato fruit. Potato tubers accumulate starch throughout development (Ross et al., 1994), whereas in tomato fruit, starch is transiently accumulated in young fruit and then degraded to a negligible level in mature fruit (Schaffer and Petreikov, 1997a). In both sink tissues, the activities of fructokinase and SS parallel starch content (Ross et al., 1994; Appeldoorn et al., 1997; Schaffer and Petreikov, 1997a). In addition, the localization of mRNA of both enzymes is closely associated with starch-accumulating cells in young tomato fruit (Wang et al., 1994; Kanayama et al., 1998). Schaffer and Petreikov (1997b) suggested that the cellular concentration of Fru was sufficient to inhibit the activities of SS and fructokinase in vivo and that these two enzymes potentially limited starch synthesis in young tomato fruit. These findings strongly suggest that fructokinase and SS play a key role in starch accumulation in tomato fruit.
Fructokinase cDNAs have been cloned from potato (Smith et al., 1993) and tomato (Kanayama et al., 1997; Martinez-Barajas et al., 1997). In tomato plants, two divergent fructokinase genes, Frk1 and Frk2, have been shown to be differentially expressed (Kanayama et al., 1997). Frk1 mRNA is expressed at a relatively constant level throughout fruit development, whereas Frk2 mRNA transiently accumulates to a very high level in young developing fruit but decreases in later stages of fruit development. Localization of Frk1 and Frk2 mRNA by in situ hybridization in young tomato fruit also indicated that expression of these two genes is spatially distinct (Kanayama et al., 1998). The Frk1 and Frk2 enzymes also exhibit distinct enzymatic properties. Most notably, Frk2 has a much higher affinity for Fru than Frk1, and Frk2 activity is inhibited by high concentration of Fru, whereas Frk1 activity is not. These data collectively suggest that the two tomato fructokinases play unique physiological roles.
Transgenic plants expressing sense or antisense genes for some enzymes involved in Suc metabolism have recently been used to assess the physiological role of each enzyme. Klann et al. (1996) reported that sugar composition and fruit size were altered in transgenic tomato plants expressing an antisense gene encoding intracellular acid INV. A key role for SS in starch synthesis was confirmed in transgenic potato (Zrenner et al., 1995), although D'Aoust et al. (1999) reported that the enzyme was not essential for starch synthesis in tomato fruit. The physiological functions of hexokinase (HK) have also been investigated by transformation of tomato and potato with sense and antisense genes (Dai et al., 1999; Veramendi et al., 1999). To assess the physiological functions in vegetative and fruit development, we independently suppressed the expression of each tomato fructokinase gene and evaluated the resulting transgenic plants for alterations in development and in carbohydrate metabolism.
RESULTS
Fructokinase Gene Expression and Enzyme Activity
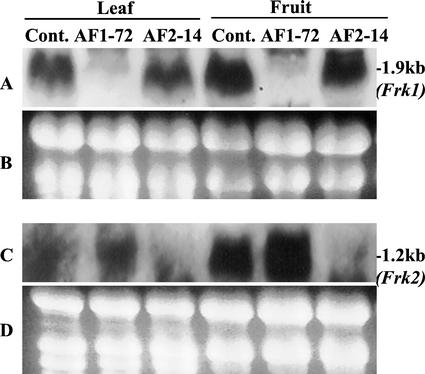
To independently suppress the expression of Frk1 and Frk2, antisense gene constructs were derived from the divergent 3′ region of the respective cDNAs. The nucleotide sequence identity over this region was only 40%. To assess the specificity of each antisense gene construct, mRNA levels in control and transgenic plants were assessed, as shown in Figure 1. RNA gel-blot analysis showed that the level of Frk1 mRNA was suppressed to an undetectable level in the Frk1 antisense line AF1-72, whereas the Frk2 mRNA level was unaffected (Fig. 1). Similarly, in the Frk2 antisense line AF2-14, Frk2 mRNA was undetectable, but Frk1 mRNA was similar to levels in control tomato tissues. These results confirmed that the 3′ antisense gene constructs were sufficiently divergent to suppress the expression of each Frk gene independently.
Figure 1.
RNA gel-blot analysis of Frk1 and Frk2 mRNA in leaf and pericarp tissue of transgenic and control plants. A, Frk1 cDNA was used as a probe. Thirty-eight micrograms of total RNA for leaves and 20 d after flowering (DAF) fruits were loaded on each lane. The x-ray film was exposed for 45 min. C, Frk2 cDNA was used as a probe. Forty-eight micrograms of total RNA for leaves and 41 μg for 20 DAF fruits were loaded on each lane. The x-ray film was exposed for 15 min. B and D, Ethidium bromide-stained RNA as a control for loading.
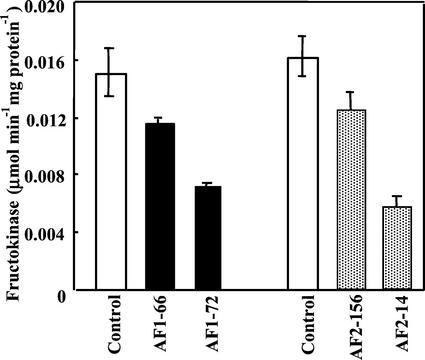
The effectiveness of Frk antisense genes was further examined by assaying fructokinase enzyme activity (Fig. 2). Total fructokinase activity probably comprises the combined activity of both Frk1 and Frk2 but was assayed using substrate conditions that are partially specific for each isoform. Thus, fructokinase activity was measured at the optimal concentration of Fru for Frk1 in Frk1 antisense lines (AF1 lines, 50 mm) or for Frk2 in Frk2 antisense lines (AF2 lines, 0.5 mm) to at least partially resolve changes in the activity of each Frk isoform. The residual activity of Frk1 at 0.5 mm Fru and of Frk2 at 50 mm are approximately 20% and 10% of the activity at the optimal Fru concentration for each isoform, respectively (Martinez-Barajas and Randall, 1996; Kanayama et al., 1998). The results indicate that Frk1 and Frk2 activity was substantially suppressed in transgenic lines AF1-72 and AF2-14, respectively, and moderately suppressed in lines AF1-66 and AF2-156 that also exhibited an intermediate phenotype. The assay conditions used (e.g. 50 mm Fru for Frk1 and 0.5 mm for Frk2) are only partially specific for each fructokinase isoform. In addition to fructokinase activity, Fru phosphorylation when assayed at high concentration (50 mm) may also be contributed by HK (Renz and Stitt, 1993). However, HK activity, measured with Glc as substrate, was much lower (0.00504 ± 0.00082 μmol min−1 mg−1 protein) than fructokinase activity in control lines and present at similar levels in AF1 plants. Thus, the residual activity in measured in AF1 lines is most likely contributed by the Frk2 isoform and HK that are not influenced by Frk1 antisense gene. The residual activity in AF2 lines most likely represents activity of the Frk1 isoform.
Figure 2.
Fructokinase activity in leaves of transgenic and control plants. Fru concentration in the assay was 50 mm (antisense Frk1 lines, black columns) and 0.5 mm (antisense Frk2 lines, gray columns) that were optimal for Frk1 and Frk2 activity, respectively. Bars represent ±se calculated from six times extraction from leaves of three to four plants per line.
Vegetative Growth
Vegetative growth was severely inhibited in transgenic lines, especially in AF2-14, with suppressed expression of Frk2. AF2-14 plants were visibly stunted compared with the control plants and with lines AF1-66 and AF1-72 with suppressed Frk1 expression. Table I shows the comparison of plant height, node numbers, and root weight in the control and transgenic lines. The data indicate that the decrease in plant height of line AF2-14 was mainly due to the reduction of internodal distances, rather than the number of nodes. The fresh weight of AF2-14 roots was also significantly reduced relative to the control and AF1 plants (Table I). Moderate effects of antisense Frk2 on plant height and root weight were also observed in AF2-156.
Table I.
Comparison of plant growth in control and antisense lines
| Line | Plant Heighta | Node No.a | Root Fresh Weightb |
|---|---|---|---|
| cm | g | ||
| Control | 83.8 ± 2.02 | 34.5 ± 1.32 | 51.9 ± 3.87 |
| AF1-66 | 80.7 ± 1.76 | 34.0 ± 3.00 | 57.6 ± 3.80 |
| AF1-72 | 76.7 ± 3.33 | 34.7 ± 0.67 | 47.0 ± 3.24 |
| AF2-14 | 34.0 ± 2.25 | 30.0 ± 0.75 | 11.0 ± 1.85 |
| AF2-156 | 65.5 ± 3.75 | 40.0 ± 2.12 | 33.9 ± 3.71 |
Mean ± se values were calculated from three to four plants per line.
Four months after sowing.
Approximately 165 d after sowing.
In both AF1-72 and AF2-14 lines, sugar content in leaves was reduced compared with those in the control line (Table II). This reduction was attributed to the reduction of hexose sugars with Suc content unchanged between the transgenic and control lines.
Table II.
Sugar content in leaves
| Line | Sugar Content
|
||
|---|---|---|---|
| Fru | Glc | Suc | |
| mg g fresh wt−1 | |||
| Control | 10.9 ± 0.46 | 3.5 ± 0.18 | 9.4 ± 0.95 |
| AF1-66 | 6.2 ± 0.71 | 3.9 ± 0.63 | 7.0 ± 0.92 |
| AF1-72 | 5.5 ± 0.77 | 2.1 ± 0.27 | 9.5 ± 1.32 |
| AF2-14 | 4.0 ± 0.36 | 1.0 ± 0.18 | 9.1 ± 0.39 |
| AF2-156 | 8.4 ± 1.77 | 2.9 ± 1.04 | 9.4 ± 2.29 |
Mean ± se values were calculated from four to six extractions from three to four plants per line.
Reproductive Development
The suppression of Frk gene expression also had a significant effect on reproductive development in tomato. Table III indicates that the number of flowers per plant was severely reduced in lines AF2-14 and AF2-156. This reduction was attributed to flower abortion because undeveloped inflorescences were observed where flowers did not fully develop. The number of fruit per plant was also reduced in the Frk2-suppressed lines, with the percentage of the number of fruit set per flower being 52%, 32%, and 43% in control, AF2-14, and AF2-156 plants, respectively. Thus, the reduction of fruit number is attributable to the inhibition of fruit setting and of flower development.
Table III.
Number of fruit and flowers per plant, average fruit size, and seed number
| Line | No. per Plant
|
Average Fruit Sizeb | Seed per No. Fruitb | |
|---|---|---|---|---|
| Flowera | Fruita | |||
| g fresh wt | ||||
| Control | 32.3 ± 2.56 | 16.8 ± 1.18 | 30.3 ± 1.56 | 106 ± 7.1 |
| AF1-66 | 34.3 ± 2.85 | 18.7 ± 0.88 | 30.5 ± 1.87 | 97 ± 7.5 |
| AF1-72 | 27.3 ± 1.20 | 8.3 ± 0.33 | 32.2 ± 1.99 | 94 ± 12 |
| AF2-14 | 18.5 ± 2.75 | 6.0 ± 0.82 | 20.7 ± 2.08 | 27 ± 8.0 |
| AF2-156 | 20.8 ± 3.33 | 9.0 ± 1.29 | 36.1 ± 1.26 | 79 ± 8.2 |
Mean ± se values were calculated from the first to the fifth infloresences of three to four plants per line.
Mean ± se values were calculated from eight to 12 mature fruits (first and second inflorescences) of three to four plants per line.
Table III also indicates that the number of seeds per fruit in line AF2-14 was greatly reduced relative to control fruit, whereas seed number in lines AF1-66 and AF1-72 was not affected (Table III). The number of seeds per fruit in line AF2-156 was also reduced moderately. In immature fruits (20 DAF), there were no significant differences in hexoses and starch content between transgenic and control lines, whereas Suc content was increased in both AF1 and AF2 lines (Table IV).
Table IV.
Carbohydrate content in immature fruit (20 DAF)
| Line | Carbohydrate Content
|
|||
|---|---|---|---|---|
| Fru | Glc | Suc | Starch | |
| mg g fresh wt−1 | ||||
| Control | 11.9 ± 0.63 | 10.7 ± 0.78 | 2.1 ± 0.24 | 9.2 ± 0.79 |
| AF1-66 | 9.0 ± 0.97 | 9.7 ± 0.96 | 6.5 ± 1.32 | 10.2 ± 1.14 |
| AF1-72 | 10.0 ± 1.01 | 10.9 ± 1.07 | 6.2 ± 1.01 | 9.4 ± 0.58 |
| AF2-14 | 10.7 ± 0.66 | 10.9 ± 0.78 | 4.1 ± 0.86 | 10.0 ± 2.83 |
| AF2-156 | 8.7 ± 0.69 | 10.0 ± 0.96 | 5.1 ± 1.01 | 8.4 ± 1.45 |
Mean ± se values were calculated from four to six fruits of three to four plants per line.
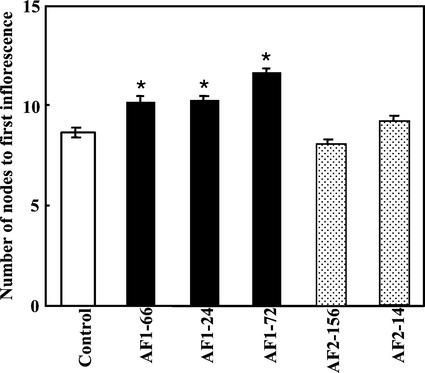
The timing of flowering was affected in some of the transgenic lines suppressed for Frk1 gene expression. The timing of flowering was most readily assessed by examining the number of nodes to first inflorescence. Figure 3 indicates that, by this measure, flowering time was increased by two to three nodes in lines suppressed for Frk1 expression. This delay was most apparent in line AF1-72 compared with the control line. This phenotype of delayed flowering was not evident in transgenic lines, AF2-14 and AF2-156, suppressed for Frk2 expression. After the first inflorescence, node number between inflorescences in Frk1 and Frk2 antisense lines was the same as that in the control line (data not shown).
Figure 3.
Number of nodes to first inflorescence in transgenic and control lines. Bars represent ±se calculated from three to four plants of control and Frk1 antisense lines (black columns), and 10 to 13 plants of Frk2 antisense lines (dotted columns). Asterisks show significantly different lines from control (P < 0.05, t test). Fructokinase activity in leaves of an additional line, AF1-24, was 0.00929 ± 0.00071 μmol min−1 mg−1 protein. The activity in other lines including control plants are shown in Figure 2.
In Situ Localization of Frk1 and Frk2 Transcripts in Fruit Tissue
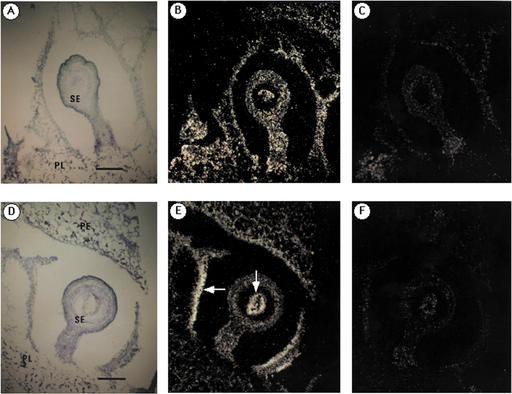
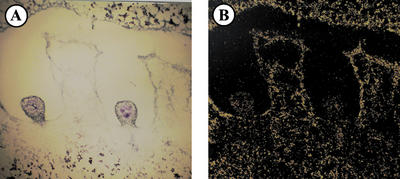
The specific effect of Frk2 suppression on seed number suggested its involvement in early seed development. To assess the relative expression of Frk1 and Frk2 genes in developing seeds, each Frk mRNA was localized in very young wild-type tomato fruit (about 10 mm diameter) by in situ hybridization. To detect the Frk1 transcripts, the sections hybridized with Frk1 probes were exposed for 21 d, whereas the sections for Frk2 were exposed for 10 d because the abundance of Frk2 transcripts is much higher than Frk1 during the early stage of tomato fruit development (Kanayama et al., 1997). The silver grains in the sections hybridized with Frk1 antisense probes were located in most cells of the seed and placental tissue as shown in Figure 4B. In contrast to Frk1, the Frk2 transcripts were concentrated in the developing endosperm of the seed and in the cell layers of placental tissue close to the seed (white arrows in Fig. 4E). However, Frk2 transcripts were not detected in aborted seeds and surrounding tissues (Fig. 5B). The embryo is not readily visible at this developmental stage of tomato fruit, whereas soft endosperm and green testa are observed (Berry and Bewley, 1991). Therefore, most of the cells in which the Frk2 transcripts were located are probably endosperm cells.
Figure 4.
In situ localization of fructokinase mRNA in seeds and surroundings of very young fruit. Fruit of approximately 10 mm in diameter of wild-type tomato was used for the in situ hybridization. Sections hybridized with Frk1 and Frk2 probes were exposed for 21 and 10 d, respectively. A and D, Bright-field microscopy of the sections hybridized with each probe and stained with KI/I2 and toluidine blue. B, Antisense probe of Frk1 was used. C, Sense probe of Frk1 was used to show hybridization background. E, Antisense probe of Frk2 was used. White arrows indicate the concentrated signals of transcripts. F, Sense probe of Frk2 was used to show hybridization background. Bars represent 200 μm. SE, Seed; PE, pericarp; and PL, placenta.
Figure 5.
In situ localization of Frk2 mRNA in aborted seeds and surroundings of very young fruit. Fruit used for the in situ hybridization was the same as Figure 4. A, Bright-field microscopy of the sections hybridized with Frk2 probe, and stained with KI/I2 and toluidine blue. B, Antisense probe of Frk2 was used.
DISCUSSION
Specific Physiological Function of Frk1
Transgenic plants with suppressed Frk1 expression exhibited delayed flowering, based on the increased number of nodes below the first inflorescence. Bernier et al. (1993) suggested that sugar is one of the signal molecules participating in floral induction and demonstrated that in plants induced to flower, Suc was mobilized from starch stored in leaves to the apical meristem. In addition, Arabidopsis mutants unable to mobilize starch showed a late-flowering phenotype (Corbesier et al., 1998). There are several lines of evidence from transgenic plants linking floral induction with transport and/or metabolism of sugars. For example, flowering was delayed in transgenic tobacco plants in which Suc translocation was suppressed by inhibition of expression of a H+-Suc transporter (Burkle et al., 1998) and a plasma membrane H+-ATPase (Zhao et al., 2000). In contrast, an increase in Suc synthesis capacity by the overexpression of Suc phosphate synthase in tomato plants resulted in early flowering (Micallef et al., 1995). In the experiments reported here, although hexose content was decreased in the mature leaves of both AF1-72 and AF2-14 plants compared with control plants, delayed flowering was only observed in the AF1-72 line, suggesting that this effect was not related to leaf carbohydrate status. However, it is possible that Frk1 acts in the apex where it participates in signal transduction to induce flowering.
Jang et al. (1997) suggested that another hexose kinase, HK, has a dual function, both as a hexose-phosphorylating enzyme and as a sugar sensor. The action of HK as a sugar sensor appears not to act through the production of hexose phosphates per se, but through a regulatory activity of the enzyme that is specific for the sugar signaling pathway. By analogy, it is possible that Frk1 plays a similar role as a dual function enzyme in sugar signaling that contributes to floral induction in the shoot apex.
Specific Physiological Function of Frk2
Based on its developmental and spatial pattern of expression, it has been proposed that Frk2 plays a key role in starch biosynthesis in immature tomato fruit (Kanayama et al., 1997, 1998). However, in this study, we observed no significant difference in starch content in immature fruit between AF2 lines and control. A similar result was observed in tomato plants suppressed for SS expression. SS also has been proposed to play an important role in starch biosynthesis in immature tomato fruit (Wang et al., 1994), however, transgenic tomato plants expressing an antisense SS gene showed no apparent difference in starch content in immature fruit compared with control plants (Chengappa et al., 1999; D'Aoust et al., 1999). Therefore, it seems likely that SS is not rate limiting for starch biosynthesis in tomato fruit, and our results suggest that the same is true for Frk2 activity. These results support the proposal of N′tchobo et al. (1999) that the rate of starch biosynthesis in tomato fruit is dependent on Suc unloading into fruit rather than being limited by a downstream metabolic constraint.
A specific phenotype associated with Frk2 suppression was growth inhibition of stems and roots. The critical role of Frk2 in stem and root growth has been proposed in other species. In potato, mRNA and protein corresponding to the Frk2-type fructokinase are induced in growing tubers, organs which are derived from stems (Taylor et al., 1995). In sugarbeet taproots, a single major fructokinase isoform has been purified and characterized (Chaubron et al., 1995) that is very similar to tomato Frk2 and most likely represents the ortholog of tomato Frk2 (Kanayama et al., 1998). The activity of the sugarbeet fructokinase remains high throughout taproot development, suggesting that it may be the major contributor to glycolytic carbon flux during root growth and development. The growth inhibition of stems and roots in Frk2 antisense lines observed in this study confirms an essential role of Frk2 in their growth, as was suggested in potato and sugarbeet.
The second specific phenotype observed in lines suppressed for Frk2 expression was decreased seed number. Both SS and INV have been proposed to play significant roles in maize seed development (Chourey and Nelson, 1976; Hanft and Jones, 1986), but the importance of hexose metabolism in seed development has not been previously addressed. The specific and high expression of Frk2 mRNA in the endosperm of young seeds suggests that fructokinase, potentially in concert with INV and SS, is an important component of active carbohydrate metabolism in early seed development. The reduction of seed number in Frk2-suppressed transgenic plants and the reduced expression of Frk2 in aborted seeds support this hypothesis. The localization of Frk2 mRNA in the placental cell layer close to developing seeds also suggests a unique role for this cell layer in carbohydrate metabolism in the developing seed.
The number of flowers per plant was severely reduced in lines AF2-14 and AF2-156, and this phenomenon was due to increased flower abortion rather than a decrease in floral initiation. Fruit setting was also inhibited in the lines. Transgenic tomato plants with suppressed SS expression exhibited almost no difference in the level of starch and sugars in immature and mature fruits compared with controls, but fruit setting was reduced (D'Aoust et al., 1999). These plants also had reduced Suc import capacity in very young fruit (7 DAF), potentially explaining the reduction in fruit set. These results suggested that SS is a major determinant of the carbohydrate supply to fruit and affects the setting activity in very young fruit. The results presented here are very similar to D'Aoust et al. (1999) in that there was no apparent effect of Frk gene suppression on starch and sugar content in fruits, but there was a reduction in flower development and fruit setting. Therefore, it is possible that Frk2 and SS are determinants of the carbohydrate supply to flower and fruit. However, fruit setting was also inhibited in AF1-72 but not in AF1-66, which is moderately suppressed in fructokinase activity by suppression of Frk1 expression. Therefore, it is possible that Frk1 also contributes to fruit setting.
The specific suppression of Frk2 collectively resulted in growth inhibition of both vegetative and reproductive organs. However, in spite of the observed growth inhibition, levels of sugar and starch did not decrease in fruit, suggesting that growth inhibition may be related to carbon flux through Frk2 rather than the steady-state carbohydrate level. This speculation can be tested by the measurement of carbon flux in AF2 plants.
The results presented here reveal a range of physiological roles of the tomato fructokinase isoforms, Frk1 and Frk2, by specific suppression of Frk1 or Frk2 expression and some of the physiological consequences of suppressed activity were specific for each fructokinase. Because each Frk isoform has distinct kinetic properties (Kanayama et al., 1997), it should now be possible to link the biochemical properties of these enzymes with their physiological function.
MATERIALS AND METHODS
Construction of Fructokinase Antisense Genes
The partial sequence between +880 and the 3′ end of the Frk1 cDNA (Kanayama et al., 1997) was amplified using PCR. The primer for +880 contained an engineered SacI site (5′-CTAGCGAGCTCGAGT-GGAATAATGAG-3′). The resulting 700-bp fragment was digested with SacI and BamHI (+1580). The Frk2 cDNA (Kanayama et al., 1997) was digested with SacI (+677) and SpeI (+1244). Each fragment was inserted between the cauliflower mosaic virus 35S promoter and nopaline-synthase termination site in the binary vector pBI121 (CLONTECH, Palo Alto, CA) in which the GUS sequence was removed by digesting with BamHI (Frk1) or XbaI (Frk2) and SacI. Agrobacterium tumefaciens strain LBA4404 (Invitrogen, Carlsbad, CA) was transformed with the respective Frk1 and Frk2 antisense constructs.
Plant Transformation
Transformation was performed using cotyledons of tomato (Lycopersicon esculentum Mill. cv Alisa Craig) as described by McCormick et al. (1986). The regenerated kanamycin resistant plants were grown in a growth chamber. Primary transformants (T0) were identified by PCR analysis and were selected by measuring fructokinase activity in leaves for antisense Frk1 plants, or in immature fruit pericarps for antisense Frk2 plants. The selected plants with low fructokinase activity were self-pollinated. The copy number and segregation of the transgene in T1 and T2 generations were investigated by DNA gel-blot analysis and PCR. The T2 generation was used for detailed analyses.
Plant Material
The plants were grown in the growth chamber with natural day length at 24°C/19°C (day/night) and axillary buds were picked before they grew. The seeds of three Frk1 antisense lines (AF1-24, AF1-72, and AF1-66) and two Frk2 antisense lines (AF2-14 and AF2-156) were sown on February 21, 1999. Azygous plants segregating from the AF1-66 line were used as a control. Immature fruits were harvested 20 DAF. Fully expanded and non-senescent leaves were also harvested for analyses. Roots were sampled by carefully washing in water, weighed, and stored for analyses. All samples were frozen in liquid nitrogen and stored at −80°C.
RNA Gel-Blot Analysis
Total RNA was extracted using RNeasy Mini Kit (Qiagen USA, Valencia, CA). Total RNA was separated on a 1.2% (w/v) denaturing agarose gel containing 10% (v/v) formaldehyde and was transferred to a Hybond-N+ membrane (Amersham Pharmacia Biotech, Uppsala). The membrane was hybridized in DIG Easy Hyb (Roche Molecular Biochemicals, Summerville, NJ) at 50°C overnight, washed with 0.2× SSC containing 0.1% (v/v) SDS at 55°C, and exposed to x-ray film. Probes were made from Frk1 and Frk2 cDNA by using PCR DIG Probe Synthesis Kit (Roche) with the following primers; 5′-AAGTAGTAAACAGGGTGGC-3′ and 5′-CCGAAGAAGC-ATATCAGCAC-3′ for Frk1 (+80 to +690), 5′-GTGCTTCTTCTTCTGGT-TTG-3′ and 5′-TCACATCAGCAGAGTCCC-3′ for Frk2 (+75 to +659). The sequences for the probes were selected not to hybridize with the antisense RNA transcripts.
Enzyme Activity Analysis
Enzyme extraction was carried out at 4°C according to modifications of the method of Schaffer and Petreikov (1997a). The frozen sample was ground to a powder with liquid nitrogen using a mortal and pestle and was mixed with a vortex mixer in extraction buffer containing 50 mm HEPES-NaOH (pH 7.5), 1 mm MgCl2, 1 mm EDTA, 10 mm KCl, 2.5 mm dithiothreitol, 3 mm sodium N,N-diethyldithiocarbamate trihydrate, and 2% (w/v) polyvinylpolypyrrolidone. After centrifugation at 10,000g for 15 min, the supernatant was adjusted to 80% saturation with ammonium sulfate, incubated on ice for 30 min, and centrifuged at 10,000g for 15 min. The precipitate was resuspended in 1 mL of the extraction buffer without polyvinylpolypyrrolidone and desalted by passage over a NAP10 column (Amersham Pharmacia Biotech). The desalted extract was used for enzyme assays. Fructokinase activity was measured at 25°C according to Martinez-Barajas and Randall (1996) except Fru concentration that was either 0.5 or 50 mm Fru.
Determination of Carbohydrate Content
Sugars were extracted in hot ethanol and analyzed by HPLC equipped with a refractive index detector and NH2 P-50 4E column (Asahipak, Showa Denko, Japan), as previously described by Suzuki et al. (2001). Starch was extracted from the ethanol-insoluble fraction dried to the pellet. Extraction and determination of starch were performed using the Starch Assay Kit (Sigma, St. Louis) according to the manufacturer's instructions.
In Situ RNA Hybridization
In situ hybridizations were carried out as described by Kanayama et al. (1998) using the tissue of wild-type tomato fruit of approximately 10 mm in diameter.
ACKNOWLEDGMENTS
We thank Dr. Masayasu Nagata (National Research Institute of Vegetable and Tea Science, Mie, Japan) for providing of seeds of tomato cv Alisa Craig. We thank Professor Koki Kanahama, Maki Kogawa, and Tomoko Motohashi (Tohoku University, Sendai, Japan) for their help.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.000703.
LITERATURE CITED
- Appeldoorn NJG, Bruijin SM, Koot-Gronsveld EAM, Visser RGF, Vreugdenhil D, Plas LHW. Developmental changes of enzymes involved in conversion of sucrose to hexose-phosphate during early tuberisation of potato. Planta. 1997;202:220–226. [Google Scholar]
- Baysdorfer C, Kremer DF, Sicher RC. Partial purification and characterization of fructokinase activity from barley leaves. J Plant Physiol. 1989;134:156–161. [Google Scholar]
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. Physiological signals that induce flowering. Plant Cell. 1993;5:1147–1155. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry T, Bewley JD. Seeds of tomato (Lycopersicon esculentum Mill.) which develop in a fully hydrated environment in the fruit switch from a developmental to a germinative mode without a requirement for desiccation. Planta. 1991;186:27–34. doi: 10.1007/BF00201494. [DOI] [PubMed] [Google Scholar]
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB. The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol. 1998;118:59–68. doi: 10.1104/pp.118.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubron F, Harris N, Ross HA, Davies HV. Partial purification and characterization of fructokinase from developing taproots of sugar beet (Beta vulgaris) Plant Sci. 1995;110:181–186. [Google Scholar]
- Chengappa S, Guilleroux M, Phillips W, Shields R. Transgenic tomato plants with decreased sucrose synthase are unaltered in starch and sugar accumulation in the fruit. Plant Mol Biol. 1999;40:213–221. doi: 10.1023/a:1006136524725. [DOI] [PubMed] [Google Scholar]
- Chourey PS, Nelson OE. The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet. 1976;14:1041–1055. doi: 10.1007/BF00485135. [DOI] [PubMed] [Google Scholar]
- Copeland L, Harrison DD, Turner JF. Fructokinase (fraction) of pea seeds. Plant Physiol. 1978;62:291–294. doi: 10.1104/pp.62.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland L, Tanner GJ. Hexose kinases of avocado. Physiol Plant. 1988;74:531–536. [Google Scholar]
- Corbesier L, Lejeune P, Bernier G. The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta. 1998;206:131–137. doi: 10.1007/s004250050383. [DOI] [PubMed] [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D. Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell. 1999;11:1253–1266. doi: 10.1105/tpc.11.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aoust MA, Yelle S, Nguyen-Quoc B. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell. 1999;11:2407–2418. doi: 10.1105/tpc.11.12.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert DC. Fructokinases from developing maize kernels differ in their specificity for nucleoside triphosphates. Plant Physiol. 1990;93:353–355. doi: 10.1104/pp.93.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Davies HV, Burch LR. Purification and properties of fructokinase from developing tubers of potato (Solanum tuberosum L.) Plant Physiol. 1992;100:178–183. doi: 10.1104/pp.100.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Perata P, Morita A, Loreti E, Yamaguchi J, Alpi A. Characterization of isoforms of hexose kinases in rice embryo. Phytochemistry. 2000;53:195–200. doi: 10.1016/s0031-9422(99)00541-5. [DOI] [PubMed] [Google Scholar]
- Hanft JM, Jones RJ. Kernel abortion in maize: I. Carbohydrate concentration patterns and acid invertase activity of maize kernels induced to abort in vitro. Plant Physiol. 1986;81:503–510. doi: 10.1104/pp.81.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama Y, Dai N, Granot D, Petreikov M, Schaffer A, Bennett AB. Divergent fructokinase genes are differentially expressed in tomato. Plant Physiol. 1997;113:1379–1384. doi: 10.1104/pp.113.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama Y, Granot D, Dai N, Petreikov M, Schaffer A, Powell A, Bennett AB. Tomato fructokinases exhibit differential expression and substrate regulation. Plant Physiol. 1998;117:85–90. doi: 10.1104/pp.117.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann EM, Hall B, Bennett AB. Antisense acid invertase (TIV1) gene alters soluble sugar composition and size in transgenic tomato fruit. Plant Physiol. 1996;112:1321–1330. doi: 10.1104/pp.112.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Barajas E, Luethy MH, Randall DD. Molecular cloning and analysis of fructokinase expression in tomato (Lycopersicon esculentum Mill.) Plant Sci. 1997;125:13–20. [Google Scholar]
- Martinez-Barajas E, Randall DD. Purification and characterization of fructokinase from developing tomato (Lycopersicon esculentum Mill.) fruits. Planta. 1996;199:451–458. doi: 10.1007/s004250050357. [DOI] [PubMed] [Google Scholar]
- McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R. Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep. 1986;5:81–84. doi: 10.1007/BF00269239. [DOI] [PubMed] [Google Scholar]
- Micallef BJ, Haskins KA, Vanderveer PJ, Roh KS, Shewmaker CK, Sharkey TD. Altered photosynthesis, flowering, and fruiting in transgenic tomato plants that have an increased capacity for sucrose synthesis. Planta. 1995;196:327–334. [Google Scholar]
- N′tchobo H, Dali N, Nguyen-Quoc B, Foyer CH, Yelle S. Starch synthesis in tomato remains constant throughout fruit development and is dependent on sucrose supply and sucrose synthase activity. J Exp Bot. 1999;50:1457–1463. [Google Scholar]
- Renz A, Stitt M. Substrate specificity and product inhibition of different forms of fructokinases and hexokinases in developing potato tubers. Planta. 1993;190:166–175. [Google Scholar]
- Ross HA, Davies HD, Burch LR, Viola R, McRae D. Developmental changes in carbohydrate content and sucrose degrading enzymes in tuberising stolons of potato (Solanum tuberosum) Physiol Plant. 1994;90:748–756. [Google Scholar]
- Schaffer AA, Petreikov M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 1997a;113:739–746. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Petreikov M. Inhibition of fructokinase and sucrose synthase by cytosolic levels of fructose in young tomato fruit undergoing transient starch synthesis. Physiol Plant. 1997b;101:800–806. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C. Characterization and compartmentation, in green leaves, of hexokinases with different specificities for glucose, fructose, and mannose and for nucleoside triphosphates. Planta. 1990;181:249–255. doi: 10.1007/BF02411547. [DOI] [PubMed] [Google Scholar]
- Smith SB, Taylor MA, Burch LR, Davies HV. Primary structure and characterization of a cDNA clone of fructokinase from potato (Solanum tuberosum L. cv Record) Plant Physiol. 1993;102:1043. doi: 10.1104/pp.102.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Odanaka S, Kanayama Y. Fructose content and fructose-related enzyme activity during the fruit development of apple and Japanese pear. J Japan Soc Hortic Sci. 2001;70:16–20. [Google Scholar]
- Taylor MA, Ross HA, Gardner A, Davies HV. Characterisation of a cDNA encoding fructokinase from potato (Solanum tuberosum L.) J Plant Physiol. 1995;145:253–256. [Google Scholar]
- Veramendi J, Roessner U, Renz A, Willmitzer L, Trethewey RN. Antisense repression of hexokinase 1 leads to an overaccumulation of starch in leaves of transgenic potato plants but not to significant changes in tuber carbohydrate metabolism. Plant Physiol. 1999;121:123–133. doi: 10.1104/pp.121.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith AG, Brenner ML. Temporal and spatial expression pattern of sucrose synthase during tomato fruit development. Plant Physiol. 1994;104:535–540. doi: 10.1104/pp.104.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Dielen V, Kinet JM, Boutry M. Cosuppression of a plasma membrane H+-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell. 2000;12:535–546. doi: 10.1105/tpc.12.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.) Plant J. 1995;7:97–107. doi: 10.1046/j.1365-313x.1995.07010097.x. [DOI] [PubMed] [Google Scholar]