Abstract
We measured intra- and extravascular body-fluid compartments in 12 resting males before (day 1; control), during (day 8) and after (day 22) a 3-week, exercise–heat acclimation protocol to investigate plasma volume (PV) changes. Our specific focus was upon the selective nature of the acclimation-induced PV expansion, and the possibility that this expansion could be sustained during prolonged acclimation. Acclimation was induced by cycling in the heat, and involved 16 treatment days (controlled hyperthermia (90 min); core temperature = 38.5°C) and three experimental exposures (40 min rest, 96.9 min (s.d. 9.5 min) cycling), each preceded by a rest day. The environmental conditions were a temperature of 39.8°C (s.d. 0.5°C) and relative humidity of 59.2% (s.d. 0.8%). On days 8 and 22, PV was expanded and maintained relative to control values (day 1: 44.0 ± 1.8; day 8: 48.8 ± 1.7; day 22: 48.8 ± 2.0 ml kg−1; P < 0.05). The extracellular fluid compartment (ECF) was equivalently expanded from control values on days 8 (279.6 ± 14.2versus 318.6 ± 14.3 ml kg−1; n = 8; P < 0.05) and 22 (287.5 ± 10.6 versus 308.4 ± 14.8 ml kg−1; n = 12; P < 0.05). Plasma electrolyte, total protein and albumin concentrations were unaltered following heat acclimation (P > 0.05), although the total plasma content of these constituents was elevated (P < 0.05). The PV and interstitial fluid (ISF) compartments exhibited similar relative expansions on days 8 (15.0 ± 2.2% versus 14.7 ± 4.1%; P > 0.05) and 22 (14.4 ± 3.6%versus 6.4 ± 2.2%; P = 0.10). It is concluded that the acclimation-induced PV expansion can be maintained following prolonged heat acclimation. In addition, this PV expansion was not selective, but represented a ubiquitous expansion of the extracellular compartment.
Previous heat adaptation experiments have focused almost exclusively upon the physiological changes accompanying repeated exposure to constant exercise and thermal forcing functions. While this model is of considerable practical significance, it has resulted in the upper limits of human physiological adaptation remaining largely unexplored. For instance, it is well established that the plasma volume (PV) is expanded following heat adaptation (Bass et al. 1955; Senay et al. 1976; Nielsen et al. 1993), and it is generally accepted that the PV regresses towards baseline during the course of constant exercise and thermal load exposures (Bass et al. 1955; Wyndham et al. 1968; Shapiro et al. 1981; Aoyagi et al. 1995). However, we suspected this regression merely represented a progressive reduction in the physiological impact of the forcing function as adaptation progressed, in much the same manner that occurs when these loads are removed. Thus, the PV regression under this constant-load model would not form part of the positive adaptation process, but would instead reflect a negative adaptation, being analogous to a gradual stimulus removal. As an alternative to this consensus, and in an attempt to expand our knowledge of thermal adaptation, we hypothesized that this PV expansion could be sustained beyond the 10–14 days over which such measures are routinely taken, if the thermal loading driving adaptation could be maintained. To test this hypothesis we used the controlled-hyperthermia technique (Fox et al. 1961; Regan et al. 1996), in which subjects performed daily exercise in the heat to obtain, and hold, a target core temperature of 38.5°C for 90 min, over 3 weeks.
Plasma volume expansion is commonly reported following heat adaptation (acclimation; Bass et al. 1955; Senay et al. 1976; Nielsen et al. 1993) and endurance training in humans (Convertino et al. 1980; Convertino et al. 1991). However, it is uncertain whether or not the entire extracellular compartment is expanded following heat acclimation. Senay et al. (1976) reported an expanded PV and an unaltered body mass following 10 days of heat acclimation, and concluded that total body water (TBW), and consequently the extracellular fluid volume (ECF), remained constant, while the PV was selectively expanded at the expense of the interstitial fluid volume (ISF), due to an elevated intravascular protein content. Conversely, Convertino (1991) reported an increase in TBW accompanying the PV expansion induced by 10 days of heat acclimation. However, only two groups have simultaneously measured changes in the intra- and extravascular compartments of the extracellular space accompanying heat acclimation (Bass et al. 1955; Wyndham et al. 1968). This was undertaken using limited subject numbers (n = 5 and 3, respectively), and resulted in conflicting findings. While Bass et al. (1955) reported elevations in both the PV and ECF, Wyndham et al. (1968) observed a PV expansion, and a constant ECF. In attempting to resolve this uncertainty, we have used radioisotope-tracer methods, refined in our laboratory (Maw et al. 1996), to simultaneously quantify the plasma and extracellular compartment fluid changes accompanying humid-heat acclimation.
In the current investigation, we simultaneously measured PV, ECF, ISF, TBW and plasma constituents in resting (seated) subjects on days 1 (control), 8 and 22 of an exercise–heat acclimation protocol to test two hypotheses, (i) that the initial PV expansion accompanying heat acclimation can be maintained following long-term exposure if physiological strain is sustained, and (ii) that this PV expansion is not selective, but a generalized expansion of the extracellular compartment. Subjects acted as their own controls (day 1). We used the controlled-hyperthermia technique to increase and maintain a constant, and elevated core temperature throughout heat adaptation, and applied this treatment for 3 weeks. In addition, we used restricted fluid intake during heat exposures to maximize secretion of the fluid and electrolyte regulatory hormones during exercise.
Methods
Subjects
Twelve healthy, physically active males (age, 21.6 ± 2.9 years; mass, 78.2 ± 8.4 kg; peak oxygen consumption, 4.2 ± 0.9 l min−1 (s.d.)) without a previous history of heat acclimation, were acclimated during the months August–October (southern hemisphere winter–spring). No control group was used for this investigation, since each subject's baseline data were used as the (control) frame of reference, and since we had previously established the reproducibility of these methods in a similar sample, over a 28-day interval (Maw et al. 1996). Resting body-fluid volumes were measured using dye- and radionuclide-dilution techniques. The total radiation dose imposed by these measurements amounted to < 1.5 millisieverts, which is approximately one-thirteenth of the annual dose permitted for an industrial worker (ICRP, 1991). All procedures were approved by the Human Research Ethics Committee, University of Wollongong, and all subjects provided written, informed consent. The experiments were conducted in accordance with the Declaration of Helsinki.
Subjects were carefully informed concerning their daily and pre-experimental requirements. Accordingly, subjects refrained from strenuous exercise and consumption of alcohol, caffeine and tobacco during the 24 h prior to body-fluid measurements on days 1, 8 and 22. The night prior to measuring body-fluid volumes, subjects were instructed to drink 15 ml kg−1 of additional water before retiring, to eat a meal high in carbohydrate and low in fat, and not to consume any food after the evening meal until breakfast was provided at the laboratory.
Experimental design
This experiment consisted of 16 treatment days (exercise and heat acclimation) and three experimental heat exposures (exercising heat stress tests), each preceded by a rest day. However, in this report, we focus only upon the resting body-fluid status, which was determined prior to each heat stress test. Body-fluid compartments were measured in seated subjects, who had rested for at least 4 h prior to data collection, 60 min of which was in a seated posture, in a thermoneutral environment on days 1 (control), 8 and 22 of the experiment. The heat stress tests involved 40 min of seated rest and 110 min of semirecumbent cycling in humid heat, and are described elsewhere in detail (Patterson et al. 2004).
In the treatment exposures, a controlled-hyperthermia technique was used, with the combination of exercise and heat stress used to elevate and maintain a target body core temperature (Tc). On each treatment day, subjects cycled upright (Monark 868 ergometer, Sweden) for 90 min in a hot-humid environment (39.8 ± 0.5°C, relative humidity 59.2 ± 0.8% (s.d.), and wind speed < 0.5 m s−1). The initial work rate was set to elevate Tc to 38.5°C within 30 min (∼44% peak work rate), and then adjusted to maintain the Tc target (Fox et al. 1961; Regan et al. 1996), which was monitored at the auditory canal and rectum, both of which exhibit a close correlation with oesophageal temperature during moderate exercise in the heat (Cotter et al. 1995). Subjects rested for 2 min every 30 min, and consumed 200 ml of water. These treatments produced an average body mass loss of 2.49 ± 0.03%. Subjects were rehydrated before leaving the laboratory, consuming an iso-osmotic drink equivalent to 100% of the body mass change. Exposure time was reduced to 60 min on day 20, due the final assessment of peak aerobic power which preceded the acclimation exposure. The current protocol was designed to facilitate an ECF expansion, via greater electrolyte retention. By restricting rehydration during exercise in the heat, large elevations in the electrolyte-fluid regulatory hormones were expected (Brandenberger et al. 1986).
Experimental procedures
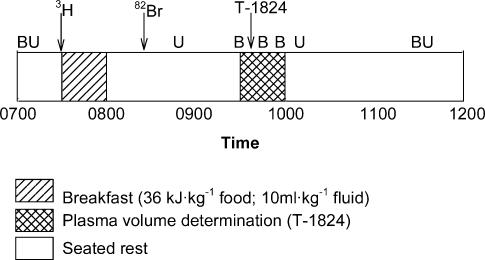
On each experimental day, a urine sample was obtained when the subject awoke. Subjects arrived at the laboratory at 07.00 h in a euhydrated and fasted state, and a resting (seated) blood sample was drawn, without stasis (Fig. 1). These samples were used as background values for subsequent β and γ radiation activity. An aliquot of tritiated water (3H2O; Amersham, UK) was then ingested for determination of TBW. After the commencement of the assessment period, subjects consumed an energy- and fluid-controlled breakfast, high in carbohydrate and low in fat and sodium (38 kJ kg−1), and supplementary water (10 ml kg−1; Fig. 1), and rested for 4 h. At (08.00 h, after breakfast, a catheter was inserted into a left antecubital vein, and an aliquot of sodium bromide (Na82Br; Australian Radioisotopes, Australia) was infused for determination of ECF, followed by a 10-ml saline flush. Between 09.30 and 10.00 h, resting PV was established using the Evans blue dye-dilution technique (T-1824; Ophthalamic Laboratory, Australia). Body mass was then measured (fw-150k, A & D Weighing, USA).
Figure 1. Protocol for the resting assessment of body-fluid volumes.
Arrows indicate the infusion of dilution tracers, with B and U representing blood and urine sampling, respectively. 3H, tritiated water; 82Br, bromide; T-1824, Evans blue dye.
Four hours after 3H2O ingestion, and 3 h after Na82Br infusion, a blood sample was drawn for determination of TBW and ECF, respectively, and plasma concentrations of sodium ([Na+]p), potassium ([K+]p), chloride ([Cl−]p), total protein ([TP]p) and albumin ([Alb]p), and osmolality (Fig. 1). Haematocrit (Hct) and haemoglobin concentrations ([Hb]) were used to derive PV at this blood sampling point (Strauss et al. 1951), since the absolute PV was determined ∼90 min prior to TBW and ECF determination. Subjects urinated after this blood sample, so the renal loss of tracers could be evaluated. All urine produced during the 4-h fluid-volume determination period was also collected to account for tracer loss.
The Na82Br tracer was not available for determination of ECF in four subjects on day 8, due to a non-routine reactor shut down. Thus, for subjects 1–8, we had complete data for the days 1 (control) and 22, but complete data for days 1, 8 and 22 in only four subjects. Consequently, four additional subjects were recruited (subjects 9–12), in whom a longer half-life tracer was used (chromated-ethylenediaminetetra-acetic acid (51Cr-EDTA); Australian Radioisotopes, Australia). This provided data for eight subjects on day 8 (4 Na82Br tracer and 4 51Cr-EDTA tracer), and 12 subjects for days 1 and 22 (8 Na82Br and 4 51Cr-EDTA tracers). The 51Cr-EDTA was infused 1 h prior to drawing the blood sample for TBW determination, allowing a 60-min equilibration period.
Analytical techniques
The determinations of TBW and ECF were made using the dilution techniques we have previously described (Maw et al. 1996). The infused volumes of 3H2O, Na82Br, 51Cr-EDTA and T-1824 were determined gravimetrically (± 0.0001 g). Approximately 150 μCi of 3H2O, 20 μCi of Na82Br, 80 μCi of 51Cr-EDTA and 12.5 g of T-1824 were infused for each assessment. The activity of the standards, and plasma and urine samples, was assessed using a γ counter (Wallac 1480 Wizard™ 3′′, Finland) for Na82Br and 51Cr-EDTA and a β liquid-scintillation counter (Wallac 1409, Finland) for 3H2O. The coefficients of variation of the TBW and ECF techniques for these techniques, in our laboratory, are 3.2% and 4.9%, respectively (Maw et al. 1996).
Intracellular fluid volume (ICF) was derived by subtracting ECF from TBW; likewise ISF was derived by subtracting PV from ECF. Blood volume (BV) was calculated using PV and haematocrit (Hct) data (Greenleaf et al. 1979), after correction for trapped plasma (0.98; Chaplin & Mollison, 1952) and the F-cell ratio (0.94; Maw et al. 1993). Subtracting PV from BV provided red cell volume (RCV).
The measurement of PV was made using Evans blue dye dilution (T-1824; Greenleaf et al. 1979), without extraction. A butterfly needle was placed in a right antecubital vein, through which the T-1824 was infused, after which 15 ml of saline was used to flush the line. At 10, 20 and 30 min after infusion, seated resting venous blood samples were drawn from the left catheter for PV determination, from the change in T-1824 concentration. The absorbance of the standard and plasma samples was determined at 619 nm (Shimadzu UV-1601 spectrophotometer, Japan). The 10-min value was used for all but two subjects, for which the 20-min value was utilized to determine PV.
Blood was collected in chilled, lithium-heparin tubes (15 I.U. heparin·ml−1) for analysis of [Na+]p, [K+]p, [Cl−]p, osmolality, [TP]p and [Alb]p, centrifuged at 1500 g for 15 min at 4°C, and stored at −20°C. Blood for Hct and [Hb] determinations was collected in chilled potassium-EDTA tubes (1.6 mg ml−1), with haemoglobin samples stored at −20°C. Samples were measured in duplicate within a single assay to negate interassay variation.
Haematocrit was measured in triplicate, using microcapillary tubes and digital calipers (0.01 mm). Whole blood was used to determine [Hb] using the cyanmethaemoglobin method (Sigma Diagnostics, USA). Plasma sodium ([Na+]p) and potassium concentrations ([K+]p) were determined using a flame photometer (Corning M410, Ciba Corning, UK), and chloride ([Cl−]p) was measured using the thiocyanate technique (Sigma Diagnostics, USA). The vapour-pressure method was used to measure plasma and urine osmolality (Model 5100C, Wescor Inc, USA). Lowry's method (Lowry et al. 1951) was used for [TP]p, and [Alb]p measurement using bromcresol green (Sigma Diagnostics, USA).
Statistical analysis
Statistical analyses were performed on resting, pre-exposure data collected on days 1, 8 and 22 using one-way analysis of variance, with repeated measures. Sources of significant differences were isolated using Tukey's HSD statistic. Student's paired t test was used to compare ECF, ISF and ICF between days 1 and 22 in 12 subjects. α was set at the 5% level for all analyses. Data are presented as means with standard errors of the means (s.e.m.), unless otherwise stated.
Results
Control data
On day 1, all resting body-fluid compartments, except the RCV, were slightly greater (7–11%) than the reported standard reference volumes for healthy males (Fig. 2; ICRP, 1975; standard values: TBW = 600 ml kg−1, ECF = 260 ml kg−1 PV = 40 ml kg−1, ISF = 220 ml kg−1, ICF = 340 ml kg−1), and are similar to those previously reported by our laboratory for lean and physically active subjects (Maw et al. 1996). The plasma constituents and plasma osmolality were within the ranges expected for healthy young males (Table 1), and consistent with data previously reported for the vapour-pressure technique (Grant et al. 1996). These observations verify that our control data were derived from a euhydrated, normal sample.
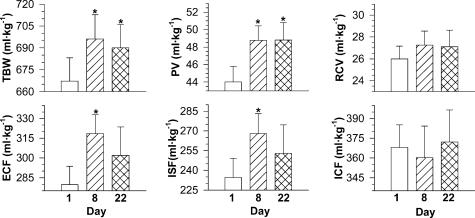
Figure 2. Resting total body water (TBW), plasma volume (PV), red cell volume (RCV), extracellular fluid (ECF), interstitial fluid (ISF) and intracellular fluid (ICF) prior to, and during, a three-week heat acclimation regimen.
Values are means with standard errors of the means. Data in the upper panels are for 12 subjects, while lower panels are for 8 subjects. *Significantly different from Day 1 (P < 0.05).
Table 1.
Resting plasma constituents measured prior to heat acclimation (day 1), and on days 8 and 22 of a three-week exercise and heat acclimation regimen
| Day 1 | Day 8 | Day 22 | |
|---|---|---|---|
| [Na+]p (mEq l−1) | 143.9 ± 0.8 | 143.9 ± 0.9 | 142.4 ± 1.1 |
| Na+ content (mEq kg−1) | 6.32 ± 0.26 | 7.02 ± 0.25* | 6.95 ± 0.28 * |
| [K+]p (mEq l−1) | 4.5 ± 0.1 | 4.5 ± 0.1 | 4.6 ± 0.1 |
| K+ content (mEq kg−1) | 0.20 ± 0.01 | 0.22 ± 0.01* | 0.22 ± 0.01* |
| Osmolality (mosmol (kg H2O)−1) | 276.1 ± 0.5 | 276.1 ± 0.4 | 277.1 ± 0.9 |
| Osmotic content (mosmol kg−1) | 12.2 ± 0.5 | 13.5 ± 0.5 * | 13.6 ± 0.5* |
| [TP]p (g dl−1) | 7.9 ± 0.2 | 7.8 ± 0.2 | 7.9 ± 0.2 |
| TP content (g kg−1) | 3.5 ± 0.2 | 3.8 ± 0.2* | 3.9 ± 0.2 * |
| [Alb]p (g dl−1) | 5.2 ± 0.1 | 5.2 ± 0.1 | 5.2 ± 0.1 |
| Albumin content (g kg−1) | 2.3 ± 0.1 | 2.5 ± 0.1* | 2.5 ± 0.1* |
Values are means with standard errors of the means; n= 12. Abbreviations: [Na+]p, plasma sodium concentration; [K+]p, plasma potassium concentration; [TP]p, plasma total protein concentration; [Alb]p.
Significantly different from Day 1 (P < 0.05).
Heat acclimation status
Heat acclimation was induced by the current methods, and these data have been reported elsewhere in detail (Patterson et al. 2004). In summary, whole-body sweat rate was elevated by 25% and 33% on days 8 and 22, respectively, while the corresponding mean exercise heart rates were 10 beats min−1 and 14 beats min−1 lower. In addition, the thermoneutral, resting oesophageal temperature was reduced on each heat stress test day, with these decrements being maintained during exercise.
Short-term heat acclimation
On day 8, TBW was expanded by 29.0 ml kg−1 (4.4%; P < 0.05; n = 12; Fig. 2), although body mass was unaltered (78.16 ± 8.37 versus 78.33 ± 8.32 kg; P > 0.05). The resting intracellular fluid compartments were unaffected by heat acclimation on day 8, when compared to day 1, varying by 2.5 ± 3.8% (ICF; P > 0.05) and 5.0 ± 1.7% (RCV; P > 0.05). Each of the resting extracellular body-fluid compartments was elevated relative to day 1 (control; P < 0.05; Fig. 2), and the ECF and ISF increases of 39.0 ml kg−1 (14.4 ± 3.2%) and 33.5 ml kg−1 (14.7 ± 4.1%), respectively, were proportionately similar to the relative PV elevations for these eight subjects (15.0 ± 2.2%; P < 0.05). Thus, the PV/ECF ratio was not altered between days 1 (15.0 ± 0.8%) and 8 (15.1 ± 0.9%; P > 0.05). These data show that the plasma component of the extracellular fluid compartment was not preferentially expanded. Blood volume was elevated from 70.0 ± 2.8 ml kg−1 on day 1 to 76.0 ± 2.8 ml kg−1 on day 8 (P < 0.05), constituting a 9% elevation, and primarily reflecting the PV expansion.
The elevated ECF represented an iso-osmotic expansion, since plasma electrolyte and protein concentrations were unchanged on day 8 (P > 0.05; Table 1). Given that the concentrations of the plasma constituents were unaltered in the presence of an expanded PV, the total intravascular content of each constituent was elevated (P < 0.05; Table 1).
Longer-term heat acclimation
At the conclusion of heat acclimation (day 22), the resting thermoneutral TBW remained significantly greater than control (day 1) values (P < 0.05; Fig. 2), although body mass was still unaltered (78.16 ± 8.37 versus 78.13 ± 8.32 kg; P > 0.05). In accordance with the pattern observed on day 8, neither the ICF nor the RCV was altered on day 22 when compared to days 1 or 8 (P > 0.05). When examining the ECF responses for all 12 subjects, an expansion was also evident on day 22 compared to day 1 (P < 0.05; Table 2). However, for the eight subjects for whom ECF was determined on days 1, 8 and 22, this same trend failed to attain statistical significance (P = 0.10; Fig. 2). Therefore, it would seem that the extracellular space remained expanded, but tended to contract towards control volumes. While a comparison of the relative PV and ISF expansions on day 22 revealed that the PV elevation tended to be greater, this difference was also not significant (14.4 ± 3.6% versus 6.4 ± 2.2%; P = 0.10). As with the short-term acclimation trends, the PV/ECF ratio was not altered between days 1 (15.0 ± 0.8%) and 22 (15.7 ± 0.9%; P > 0.05). The BV was similarly elevated on day 22 (75.9 ml kg−1; P < 0.05).
Table 2.
Resting extracellular fluid (ECF), interstitial fluid (ISF) and intracellular fluid (ICF) prior to and after a 3-week heat acclimation regimen
| Day 1 | Day 22 | |
|---|---|---|
| ECF (ml kg−1) | 287.5 ± 10.6 | 308.4 ± 14.8 * |
| ISF (ml kg−1) | 241.2 ± 10.6 | 257.6 ± 14.8 * |
| ICF (ml kg−1) | 379.7 ± 14.7 | 381.7 ± 16.9 |
Values are means with standard errors of the means; n =12.
Significantly different from Day 1 (P < 0.05).
Plasma electrolyte and protein concentrations did not differ between days 1 and 22 (P > 0.05; Table 1), although the total plasma content of these constituents was elevated on day 22, compared to day 1 (P < 0.05), with day 22 not differing from day 8 (P > 0.05).
Discussion
We have measured resting, steady-state body-fluid compartments before and after extended exercise and heat acclimation. Two novel observations have emerged. First, the initial PV expansion observed on day 8 was maintained through to day 22. Thus, the previously reported PV regression towards basal values (Bass et al. 1955; Wyndham et al. 1968; Shapiro et al. 1981; Aoyagi et al. 1995) may be considered to be more related to experimental design (constant work rate versus controlled hyperthermia) than to a progressive adaptation trend. Second, we have shown this volume expansion to be present across the entire extracellular compartment, and not confined solely to the PV. Therefore, it would seem that the ECF expansion could account for the majority part of the PV elevation following heat acclimation, at least within the first week, with the role of plasma protein content possibly increasing as acclimation progresses.
Recent reviews have commented on the uncertainty of the ECF response following heat acclimation (Convertino, 1991; Mack & Nadel, 1996). This state stems from the contrasting observations of two classical investigations (Bass et al. 1955; Wyndham et al. 1968), and the subsequent work of Senay et al. (1976). Bass et al. (1955) reported similar relative expansions of the PV and ECF after 2 weeks of heat acclimation, while TBW was unchanged. Wyndham et al. (1968) suggested that poor sensitivity of the technique used to measure TBW (anti-pyrene) could have influenced those results. In contrast, they reported an elevated PV and TBW after 5 days of heat acclimation, concluding that electrolyte retention mediated the PV expansion. However, this conclusion was not completely justified, since most of the fluid was retained within the intracellular space, and the ECF was not significantly altered. This implied that the PV expansion was at the expense of the ISF, and Senay et al. (1976) later reported this would occur, yet they did not measure either the ISF or the ECF. Our data conflict with this possibility, and represent the first demonstration of a simultaneous expansion of the resting PV, ISF, ECF and TBW following either short- or longer-term heat acclimation. Convertino & Kirby (1984) reported relative TBW and PV expansions similar to those observed in the current investigation. However, since this group did not measure extracellular water, changes in the ISF and ICF remained unknown. We suggest that, for the current posture and exercise forcing function, an acclimation-induced PV expansion forms part of a ubiquitous expansion of the entire extracellular compartment, evident in the pre-exposure, resting state even after six acclimation exposures (day 8).
The simultaneous measurement of both PV and ECF enables speculation about the possible mechanisms responsible for the PV expansion. Water movement between body-fluid compartments is dependent on the balance of osmotic and hydrostatic forces acting across capillary beds. Altering the hydrostatic forces, by either reducing the capillary hydrostatic pressure or elevating the interstitial hydrostatic pressure, can cause a selective PV increase. Similarly, an elevated intravascular colloid-osmotic pressure, attributable to an elevated plasma protein content, will draw fluid into the intravascular compartment, inducing a selective PV expansion. Based on the assumption that the 14.4% ECF expansion would increase both the PV and ISF by 14.4%, the theoretical PV increase of 5.97 ml kg−1 could account for 98% of the observed PV expansion (6.11 ml kg−1). That is, the PV was not preferentially expanded. Alternatively, and assuming that 1 g of protein binds with 15 ml of water (Scatchard et al. 1944), the 0.38 g kg−1 increase in intravascular protein mass on day 8 would tend to selectively elevate PV (5.69 ml kg−1), and this change would be at the expense of the ISF. This hypothetical fluid shift represents 93% of the observed PV expansion. However, since the ISF and PV exhibited equivalent relative expansions on day 8, it is clear that the elevated intravascular protein content did not selectively expand the PV at the expense of the ISF, as proposed by Senay et al. (1976). Instead, we believe that a generalized ECF expansion was the primary mechanism responsible for the elevated PV.
Following more prolonged heat acclimation (day 22), the relative ECF and ISF expansions seemed to be less than the PV elevation (P = 0.10). Thus, only 64% of the PV enlargement could be explained on the basis of the simultaneous ECF expansion. On the other hand, 131% of the day 22 PV enlargement could be associated with a greater plasma protein content. Therefore, it seems that the ECF expansion did not entirely account for the elevated PV on day 22, and that at least the residual PV expansion (36%) could have occurred at the expense of the ISF, and could be due to an elevated plasma protein content.
Indirect evidence from the literature supports the view that electrolyte retention, and the subsequent ECF expansion, mediates the early PV elevation accompanying exercise or heat adaptation (Armstrong et al. 1987; Armstrong et al. 1993; Luetkemeier et al. 1994). For instance, the observation that acclimation-induced PV expansions are reduced in subjects fed a low- compared to moderate- or high-sodium diet (Armstrong et al. 1987; Armstrong et al. 1993) is consistent with this regulatory role. Furthermore, when spironolactone, an aldosterone inhibitor, was administered to subjects during endurance training, the exercise-induced PV expansion was also restricted (Luetkemeier et al. 1994). That is, in the absence of adequate electrolyte replacement or retention, the PV is not necessarily expanded. Convertino (1991) reported reduced sodium, osmotic and water clearances following heat acclimation, and therefore an elevated net fluid balance. This was consistent with the TBW expansion previously reported by Convertino & Kirby, 1984). Nevertheless, neither investigation measured changes in ECF. We can now, on the basis of our data, speculate that this TBW expansion would most likely have been due to an expanded extracellular space. Thus, when considered with the present observations, one may conclude that both endurance exercise-induced, and heat acclimation-induced PV expansions are associated with an electrolyte-mediated ECF enlargement, to which plasma protein changes may perhaps play a contributory and supportive, but not a dominant, role, particularly during humid-heat acclimation.
The protracted maintenance of an elevated PV was predicted. Most previous researchers have used a constant workload forcing function throughout the acclimation period, observing progressive reductions in thermal strain and PV as acclimation progressed (Bass et al. 1955; Wyndham et al. 1968; Shapiro et al. 1981; Aoyagi et al. 1995). We hypothesized that the acclimation-induced PV response was strain related, and would remain expanded if the relative intensity of the forcing function could be sustained. The current data supported this prediction, with the early increase in PV being maintained across the 3-week experiment, without evidence of a regression to baseline. Two closely linked outcomes emerge from these data. First, the body-fluid responses accompanying humid-heat adaptation reveal some degree of forcing function specificity. Second, the previously reported PV regression merely represents a tracking of the gradual reduction in physiological strain, which is not too dissimilar to that which would be observed during detraining of an endurance-trained and heat-acclimated individual. However, while we have expanded our understanding of these body-fluid movements, we are, as yet, unable to speculate on how long this ECF expansion could be sustained.
In conclusion, the current investigation has confirmed that the initial PV expansion can be maintained following longer-term heat acclimation when physiological strain is sustained. Furthermore, a generalized ECF expansion has been shown to occur following heat acclimation, such that the PV expansion was not at the expense of the ISF. However, our data do not permit a clear elucidation of the mechanisms responsible for this generalized expansion. These two novel findings are both possibly attributable to our experimental design, such that the controlled-hyperthermia technique resulted in a constant physiological strain being maintained over the 3-week protocol, and minimal hydration was used to optimize stimulation of fluid-regulatory hormone release. Therefore, we conclude that the notion that the PV will return towards baseline during progressive heat acclimation is not universally applicable, but is related only to acclimation methods in which the exercise forcing function remains constant.
Acknowledgments
The authors would like to thank Kylie Mansfield, Sheena McGhee, Arthur Jenkins and Lee Astheimer for their technical assistance, and the subjects for participating in the study. This investigation was supported by funding from the Defence Science and Technology Organization (Australia) and the Australian Institute of Nuclear Science and Engineering. The opinions expressed in this paper are those of the authors and do not reflect the official policy or position of the Defence Science and Technology Organization or the Australian Government. Mark J. Patterson held a Post-Graduate Research Scholarship from the University of Wollongong, and Jodie M. Stocks was funded by Aboriginal and Torres Straight Islander Study Assistance Scheme (ABSTUDY), Department of Employment, Education and Training, Australia.
References
- Aoyagi Y, McLellan TM, Shephard RJ. Effects of 6 versus 12 days of heat acclimation on heat tolerance in lightly exercising men wearing protective clothing. Eur J Appl Physiol. 1995;71:187–196. doi: 10.1007/BF00854978. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Costill DL, Fink WJ. Changes in body water and electrolytes during heat acclimation: effects of dietary sodium. Aviat Space Environ Med. 1987;58:143–148. [PubMed] [Google Scholar]
- Armstrong LE, Hubbard RW, Askew EW, DeLuca JP, O'Brien C, Pasqualicchio A, Francesconi RP. Responses to moderate and low sodium diets during exercise-heat acclimation. Int J Sport Nutr. 1993;3:207–221. doi: 10.1123/ijsn.3.2.207. [DOI] [PubMed] [Google Scholar]
- Bass DE, Kleeman DR, Quinn M, Henschel A, Hegnauer AH. Mechanisms of acclimatization to heat in man. Medicine. 1955;34:323–380. doi: 10.1097/00005792-195509000-00002. [DOI] [PubMed] [Google Scholar]
- Brandenberger G, Candas V, Follenius M, Libert JP, Kahn JM. Vascular fluid shifts and endocrine responses to exercise in the heat. Eur J Appl Physiol. 1986;55:123–129. doi: 10.1007/BF00714993. [DOI] [PubMed] [Google Scholar]
- Chaplin H, Mollison PL. Correction for trapped plasma in the red cell column of the hematocrit. Blood. 1952;7:1227–1238. [PubMed] [Google Scholar]
- Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exercise. 1991;23:1338–1348. [PubMed] [Google Scholar]
- Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE. Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J Appl Physiol. 1980;48:665–669. doi: 10.1152/jappl.1980.48.4.665. [DOI] [PubMed] [Google Scholar]
- Convertino VA, Kirby CR. Expansion of total body water following heat acclimation. Physiologist. 1984;27:230. [Google Scholar]
- Convertino VA, Mack GW, Nadel ER. Elevated central venous pressure: a consequence of exercise training-induced hypervolemia? Am J Physiol. 1991;260:R273–R277. doi: 10.1152/ajpregu.1991.260.2.R273. [DOI] [PubMed] [Google Scholar]
- Cotter JD, Patterson MJ, Taylor NAS. The topography of eccrine sweating in humans during exercise. Eur J Appl Physiol. 1995;71:549–554. doi: 10.1007/BF00238559. [DOI] [PubMed] [Google Scholar]
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization of the sweating mechanism in man. J Physiol. 1961;157:56–57. [Google Scholar]
- Grant SM, Green HJ, Phillips SM, Enns DL, Sutton JR. Fluid and electrolyte hormonal responses to exercise and acute plasma volume expansion. J Appl Physiol. 1996;81:2386–2392. doi: 10.1152/jappl.1996.81.6.2386. [DOI] [PubMed] [Google Scholar]
- Greenleaf JE, Convertino VA, Mangseth GR. Plasma volume during stress in man: osmolality and red cell. J Appl Physiol. 1979;47:1031–1038. doi: 10.1152/jappl.1979.47.5.1031. [DOI] [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection) Report of the Task Group on Reference Man. Oxford: Pergamon Press; 1975. (Publication no. 23) [Google Scholar]
- ICRP (International Commission on Radiological Protection) 1990 Recommendations of the ICRP. Oxford: Pergamon Press; 1991. (Publication no. 60) [PubMed] [Google Scholar]
- Lowry O, Rosebrough NJ, Farr AL, Randall RJ. Protein determination with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luetkemeier MJ, Flowers KM, Lamb DR. Spironolactone administration and training-induced hypervolemia. Int J Sports Med. 1994;15:295–300. doi: 10.1055/s-2007-1021063. [DOI] [PubMed] [Google Scholar]
- Mack GW, Nadel ER. Body fluid balance during heat stress in humans. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, section 4, Environmental Physiology. I. New York: Oxford University Press; 1996. pp. 187–214. [Google Scholar]
- Maw GJ, MacKenzie IL, Comer DA, Taylor NAS. Whole-body hyperhydration in endurance-trained males determined using radionuclide dilution. Med Sci Sports Exerc. 1996;28:1038–1044. doi: 10.1097/00005768-199608000-00014. [DOI] [PubMed] [Google Scholar]
- Maw GJ, Mackenzie IL, Taylor NAS. Changes in the ratio of whole-body to venous haematocrit affect the measurement of plasma volume during exercise. Proc Aust Physiol Pharmacol Soc. 1993;24:150P. [Google Scholar]
- Nielsen B, Hales JRS, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MJ, Stocks JM, Taylor NAS. Humid heat acclimation does not elicit a preferential sweat redistribution towards the limbs. Am J Physiol. 2004;286:R512–R518. doi: 10.1152/ajpregu.00359.2003. [DOI] [PubMed] [Google Scholar]
- Regan JM, Macfarlane DJ, Taylor NAS. An evaluation of the role of skin temperature during heat adaptation. Acta Physiol Scand. 1996;158:365–375. doi: 10.1046/j.1365-201X.1996.561311000.x. [DOI] [PubMed] [Google Scholar]
- Scatchard G, Batchelder A, Brown A. Chemical, clinical, and immunological studies on the products of human plasma fractionation. VI. The osmotic pressure of plasma and of serum albumin. J Clin Invest. 1944;23:458–464. doi: 10.1172/JCI101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senay LC, Mitchell D, Wyndham CH. Acclimatization in a hot humid environment: body fluids adjustments. J Appl Physiol. 1976;40:786–796. doi: 10.1152/jappl.1976.40.5.786. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Hubbard RW, Kimbrough CM, Pandolf KB. Physiological and hematologic responses to summer and winter dry-heat acclimation. J Appl Physiol. 1981;50:792–798. doi: 10.1152/jappl.1981.50.4.792. [DOI] [PubMed] [Google Scholar]
- Strauss MB, Davis RK, Rosenbaum JD, Rossmeisl EC. ‘Water diuresis’ produced during recumbency by the intravenous infusion of isotonic saline solution. J Clin Invest. 1951;30:862–868. doi: 10.1172/JCI102501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham CH, Benade AJA, Williams CG, Strydom NB, Goldin A, Heynes AJA. Changes in central circulation and body fluid spaces during acclimatization to heat. J Appl Physiol. 1968;25:586–593. doi: 10.1152/jappl.1968.25.5.586. [DOI] [PubMed] [Google Scholar]