Abstract
We present the first direct comparison of the major candidates proposed to underlie the slow phase of the force increase seen following myocardial stretch: (i) the Na+–H+ exchanger (NHE) (ii) nitric oxide (NO) and the ryanodine receptor (RyR) and (iii) the stretch-activated channel (SAC) in both single myocytes and multicellular muscle preparations from the rat heart. Ventricular myocytes were stretched by approximately 7% using carbon fibres. Papillary muscles were stretched from 88 to 98% of the length at which maximum tension is generated (Lmax). Inhibition of NHE with HOE 642 (5 μm) significantly reduced (P < 0.05) the magnitude of the slow force response in both muscle and myocytes. Neither inhibition of phosphatidylinositol-3-OH kinase (PtdIns-3-OH kinase) with LY294002 (10 μm) nor NO synthase with l-NAME (1 mm) reduced the slow force response in muscle or myocytes (P > 0.05), and the slow response was still present in the single myocyte when the sarcoplasmic reticulum was rigorously inhibited with 1 μm ryanodine and 1 μm thapsigargin. We saw a significant reduction (P < 0.05) in the slow force response in the presence of the SAC blocker streptomycin in both muscle (80 μm) and myocytes (40 μm). In fura 2-loaded myocytes, HOE 642 and streptomycin, but not l-NAME, ablated the stretch-induced increase in [Ca2+]i transient amplitude. Our data suggest that in the rat, under our experimental conditions, there are two mechanisms that underlie the slow inotropic response to stretch: activation of NHE; and of activation of SACs. Both these mechanisms are intrinsic to the myocyte.
When cardiac muscle is stretched, the force of contraction increases allowing the intact heart to adjust cardiac output to meet demand. The change in force upon stretch is biphasic (for recent reviews see Calaghan et al. 2003; Cingolani et al. 2003b). Contractility increases within one beat following stretch; this is often referred to as the Frank-Starling mechanism and is due primarily to an increase in the sensitivity of the myofilaments to Ca2+ (Allen & Kurihara, 1982; Kentish & Wrzosek, 1998). Over the following minutes there is a further slow increase in force that is associated with an increase in the magnitude of the intracellular Ca2+ ([Ca2+]i) transient (Allen & Kurihara, 1982).
This slow positive inotropic response to stretch is seen in the intact heart (Tucci et al. 1984), in isolated ventricular and atrial muscle (Parmley & Chuck, 1973; Tavi et al. 1998), and in single ventricular myocytes (Hongo et al. 1996). Thus, the mechanism underlying the slow response is intrinsic to the cardiac cell itself, although in intact cardiac muscle it may be modified by non-myocytes such as fibroblasts and endothelial cells. There is evidence that cyclic AMP contributes to the slow response to stretch (e.g. Calaghan et al. 1999), although the target of protein kinase A phosphorylation has yet to be identified. More recently, two candidate mechanisms for the slow response have received attention: the Na+–H+ exchanger (NHE; Alvarez et al. 1999; Perez et al. 2001; von Lewinski et al. 2003) and nitric oxide (NO; Vila-Petroff et al. 2001).
Inhibition of NHE reduces the magnitude of the slow response in ventricular muscle from the rat, cat and rabbit (Alvarez et al. 1999; Perez et al. 2001; von Lewinski et al. 2003) and in the failing human myocardium (von Lewinski et al. 2004). Stretch-activation of NHE will raise [Na+]i and there is evidence to support a subsequent stimulation of Ca2+ influx via reverse-mode Na+–Ca2+ exchange (NCX) (Perez et al. 2001; von Lewinski et al. 2003, 2004). We have previously shown that endothelin 1 plays a role in the slow response in ferret cardiac muscle (Calaghan & White, 2001), and it has been suggested that activation of NHE is secondary to stimulation by endothelin 1 of protein kinase C (Alvarez et al. 1999; Perez et al. 2001). However, in rabbit cardiac muscle and failing human myocardium, activation of NHE following stretch is independent of endothelin 1 (von Lewinski et al. 2003, 2004).
Vila-Petroff et al. (2001) have presented evidence that NO is important during the slow response. These workers observed a slow increase in Ca2+ spark frequency and [Ca2+]i transient amplitude in single rat ventricular myocytes stretched within an agarose gel, which was sensitive to inhibitors of NO synthase and PtdIns-3-OH kinase. A NO-dependent stimulation of RyR activity via s-nitrosylation was proposed as the mechanism of action.
We consider a third contributor to the slow response to stretch deserves attention: non-selective cationic stretch-activated channels (SACs) (see Calaghan et al. 2003). Like the NHE and NCX, non-selective cationic SACs may be responsible for bringing Na+ and/or Ca2+ into the cardiac myocyte. Several studies have used gadolinium (Gd3+) to block SACs and from these there is evidence to both support (Lab et al. 1994; Tavi et al. 1996) and refute (Lamberts et al. 2002b; von Lewinski et al. 2003) the role of the SAC in the length-dependent modulation of force.
Comparison of previous studies is hampered by differences in species, preparation, parameters measured and mechanisms tested for. The effect on the slow inotropic response to stretch of blocking NHE, NO signalling, the sarcoplasmic reticulum (SR) or SACs in myocytes has not been measured to date. Perhaps because of this, a hypothesis has arisen that the major mechanisms underlying the slow response are different in single and multicellular preparations (Kentish, 1999; Vila-Petroff et al. 2001; Calaghan et al. 2003; Cingolani et al. 2003b). Furthermore, there are inconsistencies in the literature regarding the role of the SR; Vila-Petroff et al. (2001) and von Lewinski et al. (2004) suggested a major role for the SR in the slow response, whereas others (e.g. Bluhm & Lew, 1995; Kentish & Wrzosek, 1998) showed that the slow response is not attenuated by inhibition of SR function.
In order to resolve the above issues we have compared the involvement of NHE, NO signalling and SACs, under the same experimental conditions, in both single myocytes and multicellular preparations from the rat heart.
Methods
Male Wistar rats (250–400 g) were killed humanely by cervical dislocation following stunning and hearts were removed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act of 1986.
Cell isolation
Ventricular myocytes were isolated enzymatically according to established methods (Calaghan et al. 1998). Excised hearts were perfused sequentially with Hepes-buffered physiological isolation solution at 37°C containing 0.75 mm Ca2+ (to clear the coronary circulation), 0.1 mm EGTA (4 min), 0.8 mg ml−1 Type 1 collagenase and 0.08 mg ml−1 type XIV protease (7 min). Ventricles were then excised from the heart, minced and gently shaken at 37°C in collagenase/protease-containing isolation solution supplemented with 1% bovine serum albumin (BSA). Myocytes were filtered from this solution at 5 min intervals and re-suspended in isolation solution containing 0.75 mm Ca2+.
Stretch of single myocytes
Carbon fibres were used to stretch single myocytes (see Le Guennec et al. 1990; Hongo et al. 1996; Belus & White, 2003) in the experimental chamber of an inverted microscope. The base of the chamber was pre-coated with a 10% solution of BSA to reduce adherence of cells to the chamber. One end of each myocyte was attached to a flexible single carbon fibre (diameter, 12 μm; length, 1.3 mm; compliance, 22 mN−1). The other end of the myocyte was attached to a stiff double fibre (length, 0.8 mm; compliance, 0.3 mN−1) (see Fig. 1). Fibres were mounted in the ends of microelectrodes which were attached to micromanipulators. The degree of displacement of the single fibre during auxotonic contractions was used to calculate the force developed. The cell was stretched by displacement of the stiff fibre. An image of the cell from a CCD camera enabled the position of fibres to be continuously monitored via a video edge-detection system (Crescent Electronics, UT, USA). On-line fast Fourier transformation of the cell image gave a measure of sarcomere length (Gannier et al. 1993). Change in sarcomere length was used as our index of stretch.
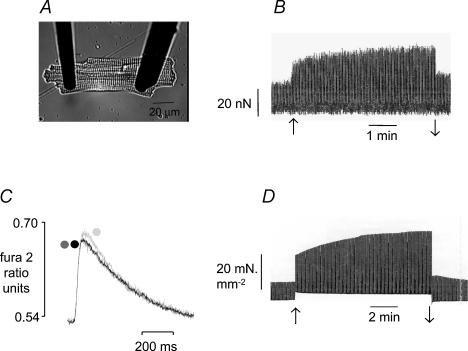
Figure 1. The inotropic response to stretch in a ventricular myocyte and papillary muscle from the rat.
A, myocyte attached to a single supple carbon fibre (left) and a double stiff fibre (right). See Methods for further details. B, representative response to stretch in a myocyte, recorded through a sample and hold circuit to show only changes in active force. Sarcomere length was increased from 1.85 to 2.0 μm (↑) and released (↓) at 5 min. C, representative changes in [Ca2+]i (expressed as fura-2 ratio units) in a myocyte following stretch from 1.81 to 1.90 μm. Traces represent an average of 10 transients recorded at short length (dark grey), between 5 and 15 s following stretch (mid grey) and at 5 min following stretch (light grey). Transients at short length and immediately following stretch are virtually superimposed. D, representative force response to stretch in a papillary muscle. Muscle length was increased from 88 to 98%Lmax (↑) and released to 88%Lmax at 10 min (↓). All these data were obtained in the presence of physiological bicarbonate-buffered bathing solution.
In some cells, simultaneous measurements of [Ca2+]i and force were made upon stretch. For these experiments, myocytes were incubated in a 2.4 μm solution of the acetoxymethyl ester form of fura 2 (fura 2-AM; Molecular Probes, OR, USA) for 10 min at room temperature, followed by resuspension in fura 2-free solution for 30 min (see Calaghan et al. 1998). Fura 2 fluorescence was measured using an Optoscan Monochromator (Cairn Research, Kent, UK). As each cell acted as its own control, [Ca2+]i was expressed as the ratio of fluorescence measured at 510 nm following excitation at 340 nm and 380 nm.
Cells were stimulated to contract at 1 Hz. We stretched myocytes to the greatest extent possible (within a period of 5 s); this typically increased sarcomere length by around 7%. Stretch was maintained for 5 min, after which time the cell was returned to its pre-stretch length. Figure 1B shows a representative force recording from a single myocyte stretched in this way. The rapid response was calculated from the increase in force 10 s after completion of stretch as a percentage of that recorded immediately prior to stretch. The slow response was calculated as the increase in force 5 min after stretch as a percentage of that recorded 10 s after completion of stretch. In fura 2-loaded myocytes, to minimize bleaching of the dye, fluorescence was measured only for short periods before and during stretch, at 2.5 min after stretch, and at 5 min after stretch and during release.
All experiments were paired. After a slow response to stretch was observed under control conditions, cells were perfused with drug for 15 min, and re-stretched in the presence of drug to the same degree as that under control conditions. Because these experiments are technically difficult, it proved impossible to routinely perform a further stretch after washout of drug. However, we are confident that the magnitude of the response to stretch did not change with consecutive stretches. There was no significant difference (P > 0.05) in the magnitude of the slow response to two consecutive stretches (17 ± 7 versus 20 ± 9%) of the same magnitude (6.1 ± 0.7%; n = 5) under control conditions.
Stretch of papillary muscles
Papillary muscles were dissected from excised hearts and mounted in a muscle bath between a fixed hook and lever system (Series 300B, Cambridge Technology) that acts as a force transducer and is also capable of altering muscle length. Muscles were stimulated to contract at 0.5 Hz via 5 ms duration pulses at a voltage 10% above threshold (the stimulation rate was lower than that used for single myocytes in order to optimize muscle function). Muscles were stretched to determine the length at which maximum tension is generated (Lmax). Muscles were maintained at 98% Lmax until the force of contraction stabilized (45 min–1 h), muscle length was then reduced to 88% Lmax.
Muscles were stretched from 88 to 98% Lmax between two contractions and the rapid and slow increases in force recorded at 10 s and 10 min after stretch, respectively. Figure 1D shows a representative response to stretch of a muscle in this way. After release, muscles were perfused with drug for 20 min and re-stretched in the presence of drug. Muscles were washed for at least 30 min before re-stretching.
Solutions
Experiments were performed with a bicarbonate-buffered solution containing (mm): NaCl 118.5; NaHCO3 14.5; KCl 4.2; KH2PO4 1.2; MgSO4 1.2; glucose 11.1 and CaCl2 1; equilibrated with 95% O2–5% CO2 (pH 7.4), except for single cell experiments investigating the NO signalling pathway. For these we used a Hepes-buffered solution to mimic the conditions of Vila-Petroff et al. (2001). Our Hepes-buffered solution contained (mm): NaCl 136.9; KCl 5.4; NaH2PO4 0.33; MgCl2 0.5; Hepes 5; glucose 5.6 and CaCl2 1 (pH 7.4). All experiments were performed between 22 and 25°C.
Drugs
Thapsigargin, LY294002 and l-NAME were from Calbiochem (UK), ryanodine was from Sigma Chemical Co (UK) and streptomycin sulphate was from Fluka (Germany). HOE 642 (Cariporide) was a kind gift from Aventis Pharma (Frankfurt, Germany). Stock solutions of drugs were made in water or DMSO.
Statistical analysis
Statistical analysis was performed using the paired or unpaired Student's t test as appropriate.
Results
Effect of stretch on the [Ca2+]i transient
Figure 1C shows representative [Ca2+]i transients recorded at short length, 10 s after stretch and at 5 min after stretch in a population of fura 2-loaded myocytes studied in bicarbonate-buffered solution. The immediate increase in force was associated with a small decrease (P < 0.05) in [Ca2+]i transient amplitude (−3.0 ± 1.0%, n = 13), whereas the slow response was associated with a significant increase (P < 0.001) in [Ca2+]i transient amplitude (10.6 ± 1.1%, n = 13). The kinetics of the [Ca2+]i transient (time to peak and time to half decay) were not altered (P > 0.05) immediately upon stretch or during the slow response (data not shown). Diastolic [Ca2+]i did not change during the slow response (0.504 ± 0.02 ratio units immediately after stretch versus 0.504 ± 0.02 at 5 min after stretch, n = 13).
In this population of fura 2-loaded cells, the slow force response to stretch was 49.4 ± 8.6% (n = 13). This relationship between the magnitude of changes in [Ca2+]i and force during the slow response is similar to that seen by Kentish & Wrzosek (1998) in rat trabeculae. The force–Ca2+ relationship is steep at longer lengths, and in bicarbonate-buffered solution the slow response is not associated with a change in myofilament Ca2+ sensitivity (e.g. Kentish & Wrzosek, 1998; Cingolani et al. 2003a). Therefore we consider that the change in [Ca2+]i that we record is of an amplitude to account for the increase in force during the slow response.
Effect of drugs on contraction at short length
Table 1 shows the effect of all drugs used on the force of contraction at short length and on the rapid response to stretch in muscle. None of these agents increased the force of contraction at short length or changed the magnitude of the rapid response to muscle stretch (P > 0.05). Therefore, we are confident that an increase in the level of muscle activation (Allen & Kentish, 1985) cannot account for any decrease in the magnitude of the slow response in the presence of antagonist.
Table 1.
The effect of antagonists on the force of contraction at short length and the rapid response to stretch in rat papillary muscle
| Force at short length (mN mm−2) | Rapid response to stretch (%) | |||||
|---|---|---|---|---|---|---|
| Drug | Conc (μm) | n | Control | Drug | Control | Drug |
| HOE 642 | 5 | 6 | 11.2 ± 3.6 | 7.1 ± 5.7* | 186 ± 58 | 156 ± 64 |
| l-NAME | 1000 | 6 | 11.2 ± 2.3 | 10.4 ± 2.0 | 163 ± 39 | 161 ± 36 |
| LY294002 | 10 | 6 | 7.9 ± 1.8 | 7.0 ± 1.4 | 220 ± 32 | 256 ± 50 |
| Streptomycin | 80 | 7 | 9.3 ± 2.1 | 8.1 ± 2.1 | 132 ± 24 | 130 ± 19 |
All data are mean ± s.e. The only significant effect was seen with HOE 642 which reduced the force of contraction in muscles held at 88% Lmax
P < 0.05 compared with respective control group, paired Student's t test. This effect of HOE 642 on basal contractility may reflect the relatively high rate of Na+ influx via NHE at physiological pH in the rat ventricle (Bers et al. 2003)
Na+–H+ exchange
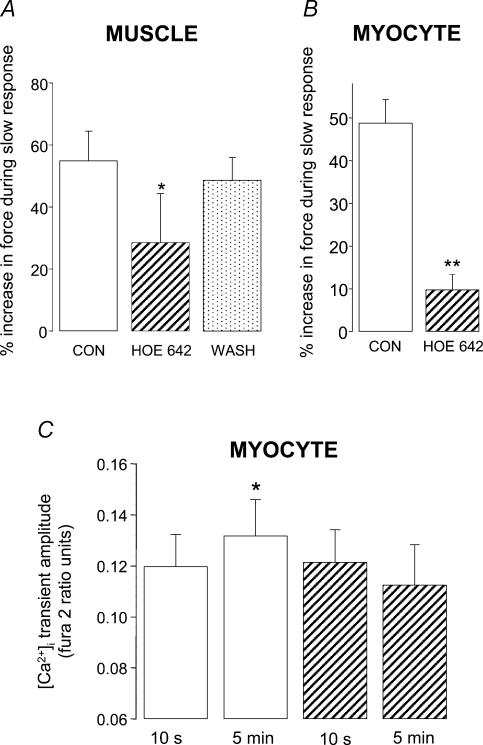
We found that activation of NHE plays an important role in the slow response of both papillary muscles and single myocytes. The slow force response to stretch was significantly attenuated by around 50% in papillary muscles (P < 0.05; Fig. 2A) and by around 80% in single myocytes (P < 0.01; Fig. 2B) in the presence of the NHE inhibitor HOE 642 (5 μm: the IC50 of HOE 642 for NHE1 is in the nm range; Kawamoto et al. 2001).
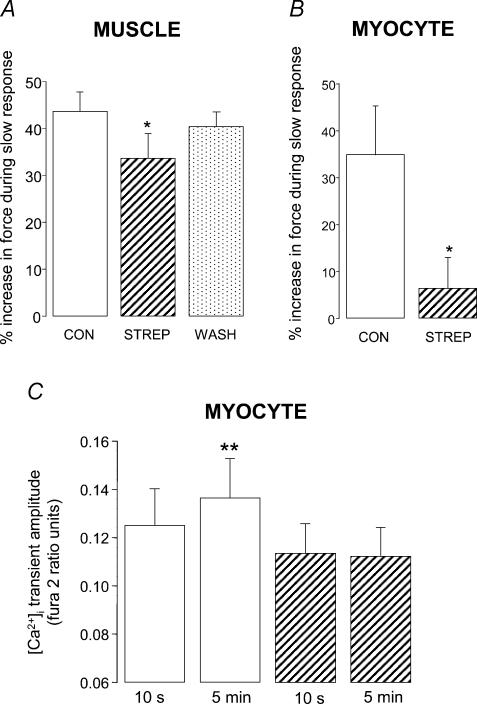
Figure 2. Stimulation of Na+–H+ exchange contributes to the slow response to stretch in both papillary muscle and ventricular myocytes from the rat.
Magnitude of the slow force response in muscle (A) and myocytes (B) under control conditions (open bars), after exposure to the NHE inhibitor HOE 642 (5 μm; hatched bars), and following washout of HOE 642 (spotted bar). Myocytes were stretched by 7.9 ± 1.5% from a resting sarcomere length of 1.84 ± 0.01 μm; *P < 0.05, **P < 0.01 compared with control group (paired Student's t test) C, [Ca2+]i transient amplitude at 10 s and 5 min after stretch in fura 2-loaded myocytes stretched by 5.7 ± 0.7% from a resting sarcomere length of 1.82 ± 0.01 μm; *P < 0.05 compared with control group at 10 s (paired Student's t test). All data are mean + s.e. (n = 4–6).
In fura 2-loaded cells, the significant increase (P < 0.05) in [Ca2+]i transient amplitude seen during the slow response under control conditions was absent in the presence of HOE 642 (Fig. 2C).
NO signalling pathway
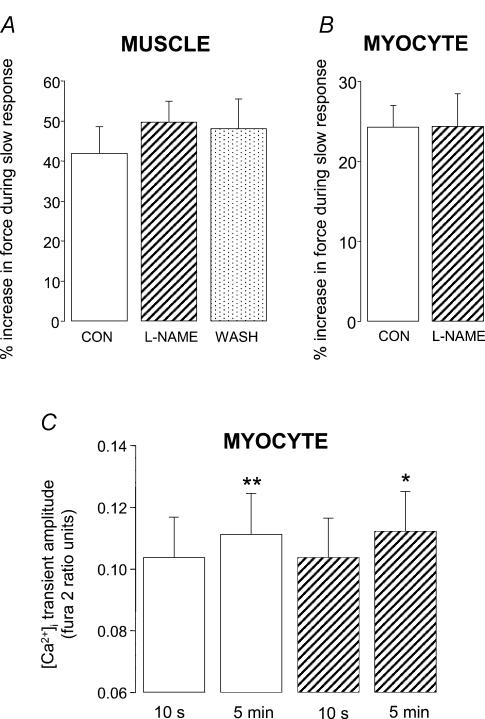
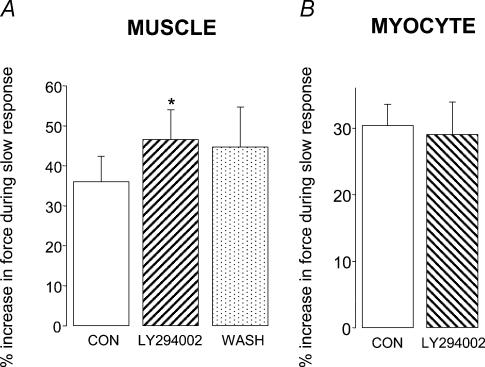
In the presence of the nitric oxide synthase inhibitor l-NAME (1 mm) we saw no reduction in the magnitude of the slow force response in either papillary muscle or myocytes (Fig. 3A and B). Furthermore, we still saw a significant increase (P < 0.05) in [Ca2+]i transient amplitude when fura 2-loaded myocytes were stretched in the presence of l-NAME (Fig. 3C). PtdIns-3-OH kinase played a pivotal role in the stretch-dependent stimulation of NO signalling reported by Vila-Petroff et al. (2001). We therefore examined the consequences of PtdIns-3-OH kinase inhibition using LY294002. Under the conditions of our experiments, LY294002 (10 μm) did not reduce the slow response to stretch in either papillary muscle or myocytes (Fig. 4A and B). Indeed we saw a small, but significant (P < 0.05), increase in the magnitude of the slow response with LY294002 in muscles (Fig. 4A).
Figure 3. Nitric oxide signalling does not contribute to the slow inotropic response to stretch in papillary muscle or ventricular myocytes from the rat.
The magnitude of the slow response to stretch under control conditions (open bars), in the presence of the NO synthase inhibitor l-NAME (1 mm; hatched bars) and following washout (spotted bar) in muscle (A) and myocytes (B). Myocytes were stretched by 8.3 ± 1.2% from a resting sarcomere length of 1.83 ± 0.01 μm. C, [Ca2+]i transient amplitude at 10 s and 5 min after stretch in fura 2-loaded myocytes stretched by 7.3 ± 1.0% from a resting sarcomere length of 1.87 ± 0.01 μm; *P < 0.05, **P < 0.01 compared with respective group at 10 s (paired Student's t test). All bars are mean + s.e. (n = 6–8).
Figure 4. PtdIns-3-OH kinase does not contribute to the slow inotropic response to stretch in papillary muscle or ventricular myocytes from the rat.
Magnitude of the slow response in muscle (A) and myocytes (B) under control conditions (open bars), after exposure to the PtdIns-3-OH kinase inhibitor LY294002 (10 μm; hatched bars), and following washout of LY294002 (spotted bar). Myocytes were stretched by 8.2 ± 0.01% from a resting sarcomere length of 1.81 ± 0.02 μm. All data are mean + s.e. (n = 6–8). *P < 0.05 compared with control group (paired Student's t test).
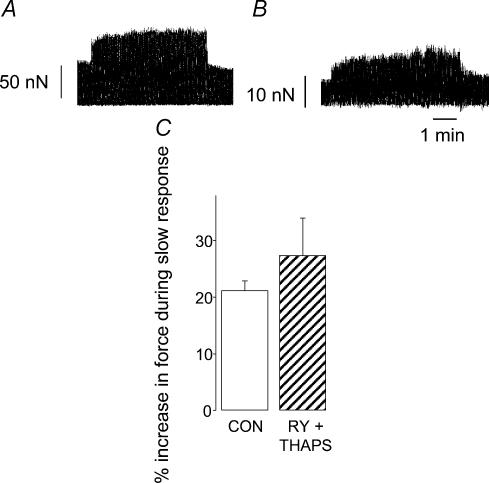
The action of NO described by Vila-Petroff et al. (2001) was linked to SR function, but other evidence from rabbit, ferret and rat ventricular muscle suggests that the slow response to stretch does not require a functional SR (Bluhm & Lew, 1995; Hongo et al. 1995; Kentish & Wrzosek, 1998). In the present study, when single rat ventricular myocytes were exposed to 1 μm ryanodine and 1 μm thapsigargin to inhibit SR function, the force of contraction at short length was reduced to 22 ± 4% (n = 5) of that recorded under control conditions. The relative magnitude of the slow response upon stretch was not significantly different (27 ± 6%; n = 5) compared with that recorded under control conditions (21 ± 2%; P > 0.05; Fig. 5). In absolute terms, the slow response was 16.2 ± 4.2 nN under control conditions and 4.2 ± 1.6 nN with ryanodine and thapsigargin. Thus, in agreement with multicellular studies, SR inhibition in single myocytes reduces the absolute, but not the relative, size of the slow response (see Discussion).
Stretch-activated channels
Finally, we looked at the contribution of SACs to the slow response to stretch. Our agent of choice for SAC blockade was streptomycin which has been shown to be selective for stretch-activated events in single myocytes (at 40 μm) (Belus & White, 2001, 2003). In our hands, streptomycin had no significant effect (P > 0.05) on the force of contraction or on [Ca2+]i transient amplitude prior to stretch (data not shown). We used a higher concentration of streptomycin in muscle (80 μm), as there is evidence that intact muscle is less sensitive to streptomycin than myocytes (see Salmon et al. 1997). In papillary muscle, 80 μm (but not 40 μm) streptomycin significantly reduced the magnitude of the slow response to stretch (34 ± 5%; n = 6, P < 0.05) compared with that recorded under control conditions (44 ± 4%; Fig. 6A). In single myocytes, the slow response was markedly attenuated in the presence of 40 μm streptomycin (6 ± 6%; n = 7, P < 0.05) compared with that recorded under control conditions (35 ± 10%; Fig. 6B).
Figure 6. The stretch activated channel (SAC) contributes to the slow response to stretch in papillary muscle and ventricular myocytes from the rat.
Magnitude of the slow force response in muscle (A) and myocytes (B) under control conditions (open bars), after exposure to the SAC blocker streptomycin (STREP; hatched bars), and following washout of streptomycin (spotted bar). Muscles were exposed to 80 μm streptomycin; myocytes were exposed to 40 μm streptomycin. Myocytes were stretched by 6.7 ± 0.8% from a resting sarcomere length of 1.85 ± 0.01 μm *P < 0.05 compared with control group (paired Student's t test). C, [Ca2+]i transient amplitude at 10 s and 5 min after stretch in fura 2-loaded myocytes stretched by 5.7 ± 0.7% from a resting sarcomere length of 1.90 ± 0.02 μm **P < 0.01 compared with control group at 10 s (paired Student's t test). All data are mean + s.e. (n = 5–7).
In fura 2-loaded myocytes, the significant increase (P < 0.01) in [Ca2+]i transient amplitude seen during the slow response under control conditions was absent in the presence of 40 μm streptomycin (Fig. 6C).
Discussion
We have directly compared three candidates proposed to underlie the slow response to stretch in single cardiac myocytes and intact muscle from the rat heart. Measurement of force and [Ca2+]i following stretch suggest that both NHE and SAC activation, but not NO signalling, contribute to this phenomenon.
Na+–H+ exchange
In rat papillary muscle, we observed an attenuation of the slow response to stretch in the presence of the NHE inhibitor HOE 642. Our data agree with those of others; in cardiac muscle from the rat (Alvarez et al. 1999), cat (Perez et al. 2001) and rabbit (von Lewinski et al. 2003), and support the hypothesis that NHE activation contributes to the slow response to stretch in multicellular preparations. We have also shown the first evidence for activation of NHE by stretch in single myocytes; both the slow increase in [Ca2+]i transient and force are reduced when the NHE is inhibited.
This latter observation argues against the hypothesis that the slow response seen at the level of the single myocyte is Na+-independent (see Kentish, 1999; Vila-Petroff et al. 2001; Calaghan et al. 2003; Cingolani et al. 2003b). This hypothesis was based on reports of raised global [Na+]i in rat cardiac muscle (Alvarez et al. 1999) but not in rat cardiac myocytes (Hongo et al. 1996) during the slow response. The NHE and NCX are thought to be concentrated in the t-tubular portion of the sarcolemmal membrane (Petrecca et al. 1999; Yang et al. 2002). Evidence suggests that a steep [Na+]i gradient exists between the subsarcolemmal space and bulk cytosol in the cardiac cell (for review see Bers et al. 2003). Increases in subsarcolemmal [Na+]i following stretch, which are sufficient to activate reverse-mode NCX activity, may not be detected in single cells as a change in bulk cytosolic [Na+]i. Indeed such spatial inhomogeneity of changes in [Na+]i has been demonstrated recently in murine myocytes (Isenberg et al. 2003).
An important study by von Lewinski et al. (2004) has recently found that the slow response is functionally relevant in the failing human myocardium. These workers showed that in the presence of disease and prior pharmacological treatment, the slow response in human cardiac muscle is dependent upon NHE, rather than NO signalling or SACs.
NO signalling pathway
We were unable to find any evidence for a role of NO signalling in the slow inotropic response to stretch. Neither the PtdIns-3-OH kinase inhibitor LY294002 nor the NO synthase inhibitor l-NAME attenuated the robust slow increase in force seen when muscles or myocytes were stretched. Furthermore, in myocytes, a significant increase in [Ca2+]i transient amplitude following stretch was still detected in the presence of l-NAME. Evidence that NO release upon stretch causes a slow increase in Ca2+ spark frequency was presented by Vila-Petroff et al. (2001) using rat ventricular myocytes stretched within an agarose gel. These observations were supported by a slow stretch-induced increase in peak [Ca2+]i in trabeculae that was sensitive to NO synthase inhibition. Our experiments performed with single myocytes used similar experimental conditions (Hepes-buffered physiological solution at room temperature), rat ventricular myocytes, and the same concentrations of antagonists as those employed by Vila-Petroff et al. (2001).
At present we cannot account for the difference between the present study and that of Vila-Petroff et al. (2001), although two issues are worthy of consideration. The first of these is the index of the slow response used. In our study we compared force (the end-point of the slow response) and the global [Ca2+]i transient immediately after stretch and at 5 min after stretch, whereas Vila-Petroff et al. (2001) measured Ca2+ spark frequency or the [Ca2+]i transient amplitude at slack length and 10–15 min after stretch. It is interesting that the concentration of the NO donor SNAP (100 μm) used to elevate Ca2+ spark frequency in unstretched rat cardiac myocytes (Vila-Petroff et al. 2001), causes a negative inotropic response in these cells (Vila-Petroff et al. 1999).
Secondly, it may be that in single myocytes the method of stretch employed has profound consequences for the signalling pathways activated. Our results were obtained with myocytes that were stretched along one axis with carbon fibres. The majority of data in the study by Vila-Petroff et al. (2001) was obtained using agarose-encased myocytes: this may provide a different mechanical stimulus, such as shear stress (which is well known to activate NO synthase via PtdIns-3-OH kinase; e.g. Dimmeler et al. 1999). Of course shear stress cannot account for the observation that NO synthase inhibition abolishes the slow increase in Ca2+ transient seen following stretch in rat trabeculae (Vila-Petroff et al. 2001). However, our work does not stand alone in refuting the central role of the SR (and by implication NO signalling) in the slow response (see below).
Our data suggest that PtdIns-3-OH kinase, NO and s-nitrosylation of the RyR do not play a pivotal role in the slow inotropic response in the rat heart. In support of this conclusion, the relative magnitude of the slow response is unaffected by inhibition of SR function in intact muscle from the rabbit and rat (Bluhm & Lew, 1995; Kentish & Wrzosek, 1998) and in the single rat cardiac myocyte (the present study). If there were an increase in SR gain as a result of s-nitrosylation of the RyR, we would expect the relative magnitude of the slow response to be reduced when the SR was non-functional. Furthermore, Eisner & Trafford (2000) have shown that enhanced RyR activity results in only transient changes in [Ca2+]i and contraction, and for the temporal characteristics of the slow response to be fulfilled, there would have to be a concurrent increase in SR Ca2+ loading to compensate for the increase in SR Ca2+ release. This could occur through activation of NHE (and SACs, see below) and stimulation of reverse mode NCX activity. However, in that case it is surprising that near total ablation of the slow response is seen when either the NHE (Perez et al. 2001) or the NO pathway (Vila-Petroff et al. 2001) is inhibited. This does not suggest that these two mechanisms are operating in parallel.
Whilst the data obtained under our experimental conditions suggest that the SR is not required for the slow response to stretch, it is logical that a functional SR will handle any extra Ca2+ that is brought into the cell (either via reverse mode NCX or via SACs, see below). This conclusion is supported by the stretch-induced increase in SR load inferred from rapid cooling contracture studies of Bluhm & Lew (1995) and von Lewinski et al. (2003).
Stretch-activated channels
Using a mathematical model of the atrial myocyte, Tavi et al. (1998) have shown that the slow increase in [Ca2+]i seen upon stretch of rat atrial muscle can be mimicked by introducing a non-selective cationic SAC conductance. Experimentally, several studies have relied on the SAC blocker Gd3+ to examine the role of SACs in the stretch-dependent modulation of cardiac contractility. Lab et al. (1994) and Tavi et al. (1996) reported a length-dependent depression of contractility by Gd3+ under conditions where the slow response mechanisms would be activated (i.e. after several minutes of continuous stretch). However, also using Gd3+, Lamberts et al. (2002b) and von Lewinski et al. (2003, 2004) concluded that there was no role for SACs in the slow response in rat, rabbit or failing human cardiac muscle. Interpreting positive and negative results with Gd3+ is difficult because free Gd3+ blocks many types of ion channels and exchangers and binds to anions such as HCO3−, and also because there is evidence from several studies that the Gd-anion complex can block stretch-activated events, perhaps with more specificity than free Gd3+ (see Caldwell et al. 1998; Lamberts et al. 2002a).
In an alternative approach, we elected to use streptomycin to block SACs. Streptomycin blocks stretch-activated currents at 40 μm (Belus & White, 2003), but does not affect the action potential duration (the sum of all electrogenic processes), [Ca2+]i transient or contraction amplitude at short length in myocytes at 50 μm (Belus & White, 2001, 2003). We also saw no significant effect of 40 μm streptomycin on force or [Ca2+]i in unstretched cells. Neither did 80 μm streptomycin modulate contractility in the absence of stretch in muscle; Salmon et al. (1997) have reported similar findings with 200 μm streptomycin in the intact heart.
We saw a significant attenuation of the slow response in both myocytes and intact papillary muscles in the presence of streptomycin. Using arterially perfused rat papillary muscle, Lamberts et al. have shown that the increase in developed force seen in response to increased perfusion pressure (the Gregg effect) is attenuated by 40 μm streptomycin (2002a), although a reduction in the amplitude of the slow response by streptomycin (40 and 100 μm) was not statistically significant (2002b).
The effect of streptomycin that we observe is consistent with activation of non-selective cationic SACs which conduct Na+, K+ and Ca2+. In the present study we saw no change in diastolic [Ca2+]i during the slow response in the rat, in agreement with Kentish & Wrzosek (1998), Hongo et al. (1996) and Alvarez et al. (1999). This reinforces the idea that increased Ca2+ loading takes place during systole. The effects of KB-R7943 on the slow response from both feline and rabbit cardiac muscle (Perez et al. 2001; von Lewinski et al. 2003) are consistent with increased Ca2+ influx via reverse-mode NCX, secondary to Na+ loading (potentially through both SACs and the NHE). The sequential change in [Na+]i then [Ca2+]i may account for the temporal characteristics of increased [Ca2+]i and force during the slow response (see Bluhm et al. 1998). Han et al. (2002) have shown, using computer modelling, that activation of non-selective cationic SACs and stimulation of NHE have qualitatively similar effects on Ca2+ handling in cardiac cells. Ca2+ which enters through SACs may also contribute to the slow increase in Ca2+ transient seen following stretch.
Conclusions
We have compared directly the major candidate mechanisms for the slow response in both multicellular and single myocyte preparations. Under our experimental conditions we find that the slow response to stretch is a sarcolemmal-based response that relies on activation of two pathways, NHE and SACs. The mechanisms that underlie the slow response are qualitatively similar in the single myocyte and intact muscle.
Figure 5. The slow inotropic response to stretch in rat ventricular myocytes does not require a functional sarcoplasmic reticulum.
A, the response of a myocyte to stretch from a sarcomere length of 1.85 to 2.0 μm under control conditions. B, the response of the same myocyte to the same degree of stretch following 10 min exposure to 1 μm thapsigargin and 1 μm ryanodine. Traces show active force only, note the difference in force scale between A and B. C, comparison of the magnitude of the slow inotropic response to stretch under control conditions (open bar) and in the presence of 1 μm ryanodine and 1 μm thapsigargin (RY + THAPS; hatched bar). Myocytes were stretched by 6.7 ± 0.7% from a resting sarcomere length of 1.84 ± 0.01 μm. Data are mean + s.e. (n = 5 cells).
Acknowledgments
This work was sponsored by the British Heart Foundation.
References
- Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985;17:821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE. Mechanisms underlying the increase in force and Ca2+ transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res. 1999;85:716–722. doi: 10.1161/01.res.85.8.716. [DOI] [PubMed] [Google Scholar]
- Belus A, White E. Effects of antibiotics on the contractility and Ca2+ transients of rat cardiac myocytes. Eur J Pharmacol. 2001;412:121–126. doi: 10.1016/s0014-2999(01)00717-8. [DOI] [PubMed] [Google Scholar]
- Belus A, White E. Streptomycin and intracellular calcium modulate the response of single guinea-pig ventricular myocytes to axial stretch. J Physiol. 2003;546:501–509. doi: 10.1113/jphysiol.2002.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912. doi: 10.1016/s0008-6363(02)00656-9. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Lew WYW. Sarcoplasmic reticulum in cardiac length-dependent activation in rabbits. Am J Physiol. 1995;269:H965–H972. doi: 10.1152/ajpheart.1995.269.3.H965. [DOI] [PubMed] [Google Scholar]
- Bluhm WF, Lew WY, Garfinkel A, McCulloch AD. Mechanisms of length history-dependent tension in an ionic model of the cardiac myocyte. Am J Physiol. 1998;274:H1032–H1040. doi: 10.1152/ajpheart.1998.274.3.H1032. [DOI] [PubMed] [Google Scholar]
- Calaghan SC, Belus A, White E. Do stretch-induced changes in intracellular calcium modify the electrical activity of cardiac muscle? Prog Biophys Mol Biol. 2003;82:81–95. doi: 10.1016/s0079-6107(03)00007-5. [DOI] [PubMed] [Google Scholar]
- Calaghan SC, Colyer J, White E. Cyclic AMP but not phosphorylation of phospholamban contributes to the slow inotropic response to stretch in ferret papillary muscle. Pflugers Arch. 1999;437:780–782. doi: 10.1007/s004240050846. [DOI] [PubMed] [Google Scholar]
- Calaghan SC, White E. Contribution of angiotensin II, endothelin 1 and the endothelium to the slow inotropic response to stretch in ferret papillary muscle. Pflugers Arch. 2001;441:514–520. doi: 10.1007/s004240000458. [DOI] [PubMed] [Google Scholar]
- Calaghan SC, White E, Colyer J. Co-ordinated changes in cAMP, phosphorylated phospholamban, Ca2+ and contraction following beta-adrenergic stimulation of rat heart. Pflugers Arch. 1998;436:948–956. doi: 10.1007/s004240050728. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Clemo HF, Baumgarten CM. Using gadolinium to identify stretch-activated channels: technical considerations. Am J Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- Cingolani HE, Chiappe GE, Ennis IL, Morgan PG, Alvarez BV, Casey JR, Dulce RA, Perez NG, Camilion de Hurtado MC. Influence of Na+-independent Cl− HCO3− exchange on the slow force response to myocardial stretch. Circ Res. 2003a;93:1082–1088. doi: 10.1161/01.RES.0000102408.25664.01. [DOI] [PubMed] [Google Scholar]
- Cingolani HE, Perez NG, Pieske B, von Lewinski D, Camilion de Hurtado MC. Stretch-elicited Na+/H+ exchanger activation: the autocrine/paracrine loop and its mechanical counterpart. Cardiovasc Res. 2003b;57:953–960. doi: 10.1016/s0008-6363(02)00768-x. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Trafford AW. No role for the ryanodine receptor in regulating cardiac contraction? News Physiol Sci. 2000;15:275–279. doi: 10.1152/physiologyonline.2000.15.5.275. [DOI] [PubMed] [Google Scholar]
- Gannier F, Bernengo JC, Jacquemond V, Garnier D. Measurements of sarcomere dynamics simultaneously with auxotonic force in isolated cardiac cells. IEEE Trans Biomed Eng. 1993;40:1226–1232. doi: 10.1109/10.250578. [DOI] [PubMed] [Google Scholar]
- Han C, Tavi P, Weckstrom M. Modulation of action potential by [Ca2+]i in modelled rat atrial and guinea pig ventricular myocytes. Am J Physiol. 2002;282:H1047–H1054. doi: 10.1152/ajpheart.00573.2001. [DOI] [PubMed] [Google Scholar]
- Hongo K, White E, Le Guennec JY, Orchard CH. Changes in [Ca2+]i, [Na+]i and Ca2+ current in isolated rat ventricular myocytes following an increase in cell length. J Physiol. 1996;491:609–619. doi: 10.1113/jphysiol.1996.sp021243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo K, White E, Orchard CH. Effect of stretch on contraction and the Ca2+ transient in ferret ventricular muscles during hypoxia and acidosis. Am J Physiol. 1995;269:C690–C697. doi: 10.1152/ajpcell.1995.269.3.C690. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Kazanski V, Kondratev D, Gallitelli MF, Kiseleva I, Kamkin A. Differential effects of stretch and compression on membrane currents and [Na+]c in ventricular myocytes. Prog Biophys Mol Biol. 2003;82:43–56. doi: 10.1016/s0079-6107(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Kimura H, Kusumoto K, Fukumoto S, Shiraishi M, Watanabe T, Sawada H. Potent and selective inhibition of the human Na+/H+ exchanger isoform NHE1 by a novel aminoguanidine derivative T-162559. Eur J Pharmacol. 2001;240:1–8. doi: 10.1016/s0014-2999(01)00991-8. [DOI] [PubMed] [Google Scholar]
- Kentish JC. A role for the sarcolemmal Na+/H+ exchanger in the slow force response to myocardial stretch. Circ Res. 1999;85:658–660. doi: 10.1161/01.res.85.8.658. [DOI] [PubMed] [Google Scholar]
- Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol. 1998;506:431–444. doi: 10.1111/j.1469-7793.1998.431bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lab MJ, Zhou BY, Spencer CI, Horner SM, Seed WA. Effects of gadolinium on length-dependent force in guinea-pig papillary muscle. Exp Physiol. 1994;79:249–255. doi: 10.1113/expphysiol.1994.sp003758. [DOI] [PubMed] [Google Scholar]
- Lamberts RR, Rijen MH, Sipkema P, Fransen P, Sys SU, Westerhof N. Increased coronary perfusion augments cardiac contractility in the rat through stretch-activated ion channels. Am J Physiol. 2002a;282:H1334–H1340. doi: 10.1152/ajpheart.00327.2001. [DOI] [PubMed] [Google Scholar]
- Lamberts RR, Van Rijen MH, Sipkema P, Fransen P, Sys SU, Westerhof N. Coronary perfusion and muscle lengthening increase cardiac contraction: different stretch-triggered mechanisms. Am J Physiol. 2002b;283:H1515–H1522. doi: 10.1152/ajpheart.00113.2002. [DOI] [PubMed] [Google Scholar]
- Le Guennec JY, Peineau N, Argibay JA, Mongo KG, Garnier D. A new method of attachment of isolated mammalian ventricular myocytes for tension recording: length dependence of passive and active tension. J Mol Cell Cardiol. 1990;22:1083–1093. doi: 10.1016/0022-2828(90)90072-a. [DOI] [PubMed] [Google Scholar]
- Parmley WW, Chuck L. Length-dependent changes in myocardial contractile state. Am J Physiol. 1973;224:1195–1199. doi: 10.1152/ajplegacy.1973.224.5.1195. [DOI] [PubMed] [Google Scholar]
- Perez NG, de Hurtado MC, Cingolani HE. Reverse mode of the Na+-Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res. 2001;88:376–382. doi: 10.1161/01.res.88.4.376. [DOI] [PubMed] [Google Scholar]
- Petrecca K, Atanasiu R, Grinstein S, Orlowski J, Shrier A. Subcellular localization of the Na+/H+ exchanger NHE1 in rat myocardium. Am J Physiol. 1999;276:H709–H717. doi: 10.1152/ajpheart.1999.276.2.H709. [DOI] [PubMed] [Google Scholar]
- Salmon AH, Mays JL, Dalton GR, Jones JV, Levi AJ. Effect of streptomycin on wall-stress-induced arrhythmias in the working rat heart. Cardiovasc Res. 1997;34:493–503. doi: 10.1016/s0008-6363(97)00024-2. [DOI] [PubMed] [Google Scholar]
- Tavi P, Han C, Weckstrom M. Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes. Role of increased troponin C affinity and stretch-activated ion channels. Circ Res. 1998;83:1165–1177. doi: 10.1161/01.res.83.11.1165. [DOI] [PubMed] [Google Scholar]
- Tavi P, Laine M, Weckstrom M. Effect of gadolinium on stretch-induced changes in contraction and intracellularly recorded action- and afterpotentials of rat isolated atrium. Br J Pharmacol. 1996;118:407–413. doi: 10.1111/j.1476-5381.1996.tb15417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci PJF, Bregagnollo EA, Spadaro J, Cicogna AC, Ribeiro MCL. Length-dependence of activiation in the isovolumic blood-perfused dog heart. Circ Res. 1984;55:59–66. doi: 10.1161/01.res.55.1.59. [DOI] [PubMed] [Google Scholar]
- Vila-Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Vila-Petroff MG, Younes A, Egan J, Lakatta EG, Sollott SJ. Activation of distinct cAMP-dependent and cGMP-dependent pathways by nitric oxide in cardiac myocytes. Circ Res. 1999;84:1020–1031. doi: 10.1161/01.res.84.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lewinski D, Stumme B, Fialka F, Luers C, Pieske B. Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ Res. 2004;94:1392–1398. doi: 10.1161/01.RES.0000129181.48395.ff. [DOI] [PubMed] [Google Scholar]
- von Lewinski D, Stumme B, Maier LS, Luers C, Bers DM, Pieske B. Stretch-dependent slow force response in isolated rabbit myocardium is Na+ dependent. Cardiovasc Res. 2003;57:1052–1061. doi: 10.1016/s0008-6363(02)00830-1. [DOI] [PubMed] [Google Scholar]
- Yang Z, Pascarel C, Steele DS, Komukai K, Brette F, Orchard CH. Na+ −Ca2+ exchange activity is localized in the T-tubules of rat ventricular myocytes. Circ Res. 2002;91:315–322. doi: 10.1161/01.res.0000030180.06028.23. [DOI] [PubMed] [Google Scholar]