Abstract
Amphibian metamorphosis includes a complete reorganization of an organism's locomotory system from axial-based swimming in larvae to limbed propulsion in the young adult. At critical stages during this behavioural switch, larval and adult motor systems operate in the same animal, commensurate with a gradual and dynamic reconfiguration of spinal locomotor circuitry. To study this plasticity, we have developed isolated preparations of the spinal cord and brainstem from pre- to post-metamorphic stages of the amphibian Xenopus laevis, in which spinal motor output patterns expressed spontaneously or in the presence of NMDA correlate with locomotor behaviour in the freely swimming animal. Extracellular ventral root recordings along the spinal cord of pre-metamorphic tadpoles revealed motor output corresponding to larval axial swimming, whereas postmetamorphic animals expressed motor patterns appropriate for bilaterally synchronous hindlimb flexion–extension kicks. However, in vitro recordings from metamorphic climax stages, with the tail and the limbs both functional, revealed two distinct motor patterns that could occur either independently or simultaneously, albeit at very different frequencies. Activity at 0.5–1 Hz in lumbar ventral roots corresponded to bipedal extension–flexion cycles, while the second, faster pattern (2–5 Hz) recorded from tail ventral roots corresponded to larval-like swimming. These data indicate that at intermediate stages during metamorphosis separate networks, one responsible for segmentally organized axial locomotion and another for more localized appendicular rhythm generation, coexist in the spinal cord and remain functional after isolation in vitro. These preparations now afford the opportunity to explore the cellular basis of locomotor network plasticity and reconfiguration necessary for behavioural changes during development.
Amphibian metamorphosis involves an extensive transformation in the structure and function of most, if not all, of the animal's physiological systems. During this dramatic transition the organism is faced with the behavioural consequences of a complete alteration in life style that includes changes in body structure, habitat, respiration and diet (for general review, see Shi, 2000). Critically, the organism is also required to change its locomotor strategy from axial-based body undulations to a limb-based mode whilst the tail is being resorbed and limbs are being formed. In contrast to metamorphosis in insects, in which one body format subserving an initial larval lifestyle is dismantled within a cocoon and reassembled into an entirely new adult body format (Consoulas et al. 2000), amphibian metamorphosis is a gradual process during which the extensively changing organism must continue to function and exist in the same environment throughout the transformation from tadpole to frog.
Whilst much is known about the biochemical, molecular and genetic changes that accompany amphibian metamorphosis (Shi, 2000; Beck & Slack, 2001; Shi & Ishizuya-Oka, 2001), and about the ecological factors and evolutionary forces that affect it (Wassersug, 1997), there is a relative paucity of information in the context of the neuronal control of locomotion. Although earlier studies by Stehouwer & Farel (1983, 1985; see also Hughes & Prestige, 1967) provided important insights into the changes in locomotor strategies and interlimb co-ordination during anuran metamorphosis, to date virtually nothing is known about the reconfiguration of locomotor circuitry and neuronal properties that underlie this dynamic process. For this to progress it is critical to have experimental access to physiologically viable in vitro preparations from representative developmental stages during metamorphosis. During this transition the spinal circuitry responsible for controlling axial-based swimming, which is initially assembled during embryonic development and refined in readiness for free-swimming larval life, is gradually replaced by neural control systems appropriate to coordinate movements of the emerging limbs. This situation, in which an adult network is constructed for one function (limb-based kicking) and progressively supersedes a larval network subserving a different function (tail-based swimming), raises several questions of profound neurobiological importance. For example, how is the (secondary) adult locomotor circuitry extricated from its larval precursor as limbs are added and the tail resorbed? To what extent are neuronal elements of the primary network retained and incorporated into the adult circuitry, and how are two neural networks responsible for fundamentally different locomotor behaviours co-ordinated centrally during critical intermediate stages when they coexist and function in the spinal cord?
In a first step towards addressing such questions, the present study aimed to develop a series of in vitro preparations of the central nervous system of the frog Xenopus laevis at different developmental stages before, during and after metamorphosis and to correlate spinal motor output patterns with locomotor movements in the freely behaving animal. Only once this is achieved will it be possible to elucidate mechanisms of developmental neural plasticity at the cellular and systems levels using a network-based neuroethological approach. In this context the choice of Xenopus in our study is important, since the locomotory system of embryonic and larval stages of this species is already widely characterized at the cellular level and has become established as one of the best understood models of rhythmogenic motor network function (Roberts et al. 1998). Here we show that isolated spinal cord–brainstem preparations of Xenopus remain physiologically viable in vitro and can generate rhythmic patterns of motor output which are appropriate to drive alternating left–right tail oscillations at pre-metamorphic stages and synchronous bilateral limb kicks involving flexion–extension cycles in postmetamorphic froglets. At the critical intermediate stages, both motor patterns can be expressed co-ordinately or independently, indicating the existence of two distinct pattern generating networks in the spinal cord. Some aspects of this work have appeared previously in abstract form (Combes et al. 2002, 2003).
Methods
Experiments were performed on a range of pre-metamorphic, metamorphic climax and post-metamorphic stages of the South African clawed frog, Xenopus laevis, bred from an in-house laboratory colony. Tadpoles were raised to appropriate stages of development (stages 50–64; Nieuwkoop & Faber, 1956) at room temperature. All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and the European Community Council directive of 24 November 1986 (86/609/EEC).
For video recording, spontaneously swimming animals were filmed with a camera (Canon MV630) mounted 0.5 m above a small aquarium. Individual video frames (captured with Microsoft Windows Movie-Maker) were then used to draw the body outline of the animal, so as to chart body movements during episodes of locomotory behaviour.
For in vitro experiments animals were first anaesthetized by placing them in ice-chilled frog Ringer (composition, mm: NaCl, 120; KCl, 2.5; CaCl2, 5; MgCl2, 1; NaHCO3, 15; pH 7.4) containing 230 μg ml−1 of 3-aminobenzoic acid ethyl ester (Sigma-Aldrich, Germany) and secured in a glass Sylgard-lined Petri dish with insect pins. The skin overlying the dorsal aspect of the brain was cut and the forebrain removed and destroyed. The remainder of the nervous system was dissected free from the body. After rinsing in fresh saline it was transferred to a second Petri dish for recording purposes. Saline temperature was maintained at 17°C with a Peltier cooling system.
Extracellular recordings of ventral root motor discharge were made with stainless steel wire electrodes isolated electrically with Vaseline, or glass suction electrodes placed on selected motor roots along the length of the spinal cord. Extensor and flexor motor rootlets were identified by dissecting them distally to their target muscles (gastrocnemius and tibialis anterior, respectively). Spinal motor output patterns, presumed to underlie swimming, occurred either spontaneously, or in the presence of 2–25 μm NMDA. Signals were displayed and recorded on a PC equipped with a data acquisition system (1401 CED; Cambridge Electronic Design, Cambridge, UK) and analysed using Spike2 (CED) software. The results which follow derive from experiments on six pre-metamorphic, 15 metamorphic climax and 7 post-metamorphic stages.
Results
Pre-metamorphic stages
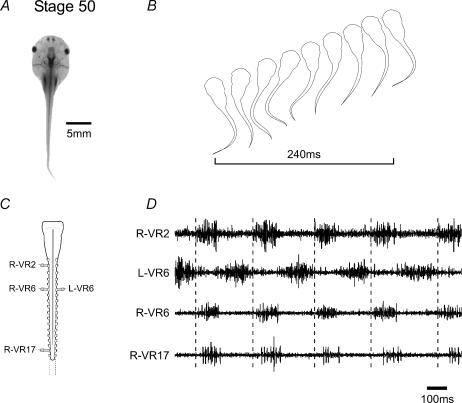
Aside from the increases in the duration and cycle-by-cycle variability of ventral root bursts which occur during early larval development (Sillar et al. 1991), there is no evidence to suggest any further substantial changes in the way swimming is coordinated during the remainder of pre-metamorphic life, although qualitative changes to accommodate further tail growth will almost certainly occur. This was confirmed in the present study in which multiple ventral root recordings were made from the isolated spinal cord–brainstem of late pre-metamorphic larval stages (st. 50–54; Fig. 1A and C). As expected, rhythmic motor bursting, occurring either spontaneously or following the bath application of NMDA, alternated across the spinal cord and propagated rostro-caudally with a clear intersegmental delay (Fig. 1D). Importantly, cycle periods (ca 0.2–0.3 s) and associated intersegmental delays of this rhythm overlapped the ranges of these parameters during swimming in the freely behaving animal at the equivalent stage (Fig. 1B). This in vitro pattern therefore provides a fictive correlate of swimming behaviour, and was generated at the onset of limb bud development (Nieuwkoop & Faber, 1956), but before any evident morphological changes in the spinal cord associated with hindlimb emergence (lumbar enlargement) could be discerned (Fig. 1C).
Figure 1. Axial-based swimming in pre-metamorphic Xenopus larvae.
In a Stage 50 tadpole (A), undulatory swimming movements and forward propulsion are generated by alternate bilateral contractions of axial myotomes with a characteristic rostro-caudal delay along the body (B). When the brainstem and spinal cord are isolated in vitro (C), extracellular recordings from selected spinal ventral roots (D; recorded roots indicated in C) reveal spontaneous bursting in axial motorneurones which, as with swimming in vivo, alternates across the cord and propagates rostro-caudally (dotted lines indicate delay between VR2 and VR17). Note that the horizontal bar in B indicates a complete cycle.
Post-metamorphic climax
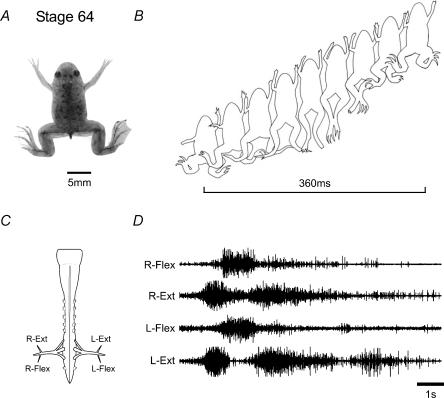
Following the metamorphic climax (stage 65), the locomotory behaviour of now tail-less young froglets (Fig. 2A) involves a periodic but characteristic sequence of movements involving the coordination of the four limbs. In this behaviour the propulsive force is mainly generated by a series of kicking movements of the larger and more powerful hindlimbs (Fig. 2B), consisting of bilaterally synchronous cycles of flexion and extension, which usually begin with extension, and normally last for up to four to five cycles with periods of approximately 1 s. (It is noteworthy here that although the forelimbs play less of a role in propulsive swimming, they nonetheless tend also to move rhythmically, usually in a bilaterally symmetrical fashion and coordinated with the hindlimbs such that they extend forwards in phase with the rearward extensions of the ipsilateral hindlimb.) The in vitro nervous system of these juvenile post-metamorphic froglets, which now possesses a clearly discernible lumbar enlargement that houses the hindlimb pattern-generating circuitry (Fig. 2C), can generate rhythmic motor activity suitable to drive this new behaviour. Thus, recordings made from lumbar root branches that normally innervate flexor and extensor hindlimb musculature displayed occasional spontaneous, but more usually pharmacologically induced, rhythmic discharge lasting one or more cycles (Fig. 2D). In pooled measurements of 27 fictive kick episodes from three preparations, the mean cycle period (±s.e.m.) was 1.31 ± 0.14 s. This intense pattern could begin with either flexor or extensor activity, but in both cases, and in correspondence with actual hindlimb movements, bursts in homologous bilateral ventral roots occurred synchronously whilst activity on the same side alternated between antagonistic motor pools.
Figure 2. Limb-based swimming in post-metamorphic Xenopus froglets.
In a stage 64 juvenile (A), the tail has resorbed and swimming is now produced exclusively by bilaterally synchronous cycles of hindlimb extensions and flexions (B; cycle indicated by bar). The fictive correlate of this behaviour is generated by the isolated spinal cord and brainstem (C), as seen in D, where several cycles beginning with intense coordinately active bursts in left and right extensor lumbar motorneurones alternate with bilateral hindlimb flexor bursts. In this example, 10 µm NMDA was present in the bath. Note that a fictive swim episode could begin with either extensor (as illustrated) or flexor bursting. Note that the rhythm frequency is an order of magnitude slower than the pre-metamorphic tadpole (Fig. 1D).
Metamorphic climax stages
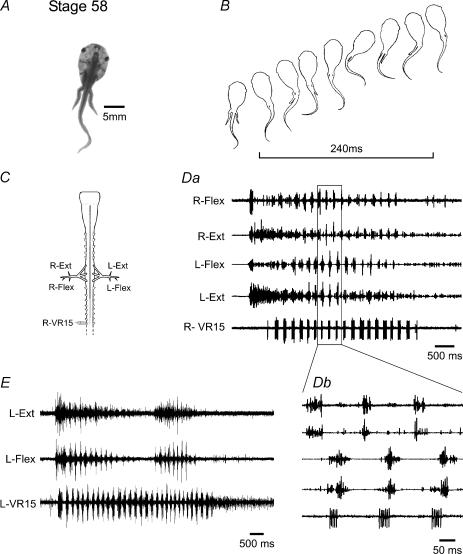
We were next interested in examining the critical intermediate metamorphic stages (58–63) when both the limbs and the tail coexist and participate in locomotion (Figs 3 and 4). The development of the hindlimbs is advanced relative to the forelimbs with the former displaying motility from ca stage 56 and the onset of movement in the latter being delayed until ca stage 59 (Nieuwkoop & Faber, 1956). In stage 58 animals (Fig. 3A) during a swim episode (Fig. 3B), the still-developing hindlimbs are first extended simultaneously and then held close to the tail as it generates larval-like undulatory movements. Consistent with these observations in vivo, simultaneous recordings from caudal tail ventral roots and identified extensor/flexor limb motor nerves in the in vitro spinal cord (Fig. 3C) revealed spontaneous bouts of fictive locomotion (Fig. 3D and E). These often coincided with co-ordinated bursts of hindlimb-nerve discharge that appeared particularly intense in extensor rootlets at the onset of a bout of activity (see 2nd and 4th traces in Fig. 3Da). This initial, predominantly lumbar activity then became progressively and rhythmically modulated by the onset of tail-root bursting (Fig. 3Da, lower trace), with concurrent bursts in ipsilateral flexor and extensor motorneurones now occurring in alternation with their contralateral partners and in strict co-ordination with the more caudal swimming motor pattern (Fig. 3Db). In four preparations (15 episodes) the mean cycle period of this combined rhythm was 0.22 ± 0.01 s. However, discharge in limb motorneurones invariably ceased before an episode of fictive tail-based swimming terminated (Fig 3D and E), and occasionally, distinct bouts of appendicular activity occurred during the course of a given axial swim episode (Fig. 3E). Interestingly, ongoing axial bursting varied significantly (P < 0.05; Student's t test) with the expression of lumbar activity. For example, in three preparations (8 episodes), when axial and appendicular bursting occurred simultaneously, the cycle period of the former rhythm decreased by 21.76 ± 3.37%, whilst burst durations decreased by 39.70 ± 2.20%. Thus, at this early metamorphic climax stage, although signs of functional differentiation are clearly beginning to appear, the limb spinal motor circuitry, whilst capable of producing centrally generated rhythmic activity, cannot yet do so independently of the axial rhythm. Rather the main locomotory mechanism remains an axial-based, tail undulatory system which ‘instructs’ the still-emerging limbs to adopt an extended position, presumably to streamline the body, during the execution of swimming behaviour (Fig. 3B).
Figure 3. Combined axial- and hindlimb-based locomotion early in metamorphosis.
In a stage 58 tadpole (A), the hindlimbs are present but not yet fully functional. During swimming, they are maintained against the tail which then generates undulatory movements (B; cycle indicated by bar). After isolation (C), the spinal cord can spontaneously generate motor activity (D and E) which, in this example, begins with simultaneous brief flexor and more prolonged extensor discharge, followed ∼500 ms later by rapid bursting in caudal tail segments (VR15). Lumbar activity then becomes coordinated with axial bursting (Db), with ipsilateral extensor and flexor motorneurones firing simultaneously, and in phase-opposition with contralateral partners. Hindlimb motor discharge can wax and wane within a single axial swimming episode (E), the latter invariably outlasting the limb root activity (see also D).
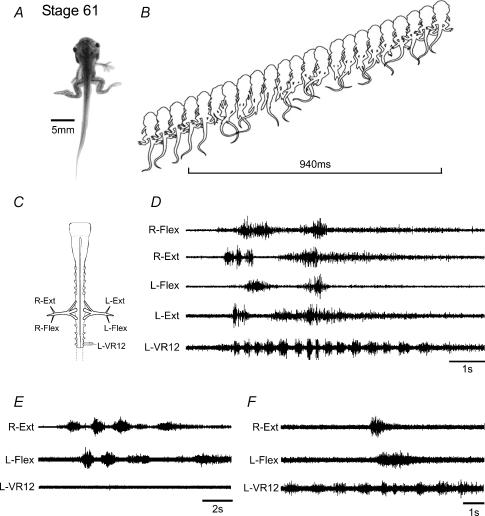
Figure 4. Segregated axial- and hindlimb-based locomotion in intermediate metamorphic climax tadpoles.
At stage 61 (A) the hindlimbs are now fully functional and a combination of rhythmic bilateral limb kicks and tail undulations are used to propel the animal (B). The isolated cord (C) can generate spontaneous motor patterns (D) appropriate for both locomotor modes: higher frequency bursting in tail spinal segments and slower, bilaterally synchronous bursting in lumbar hindlimb motorneurones corresponding to fictive kicking. As in vivo, the two motor programmes may be co-ordinately active (as in D) or can operate independently, with bursting expressed solely in limb motorneurones (E), or more frequently, activity occurring only in axial motorneurones (F). Note that the horizontal bar in B indicates a limb-kick cycle.
By stages 60/61, when the hindlimbs are now well developed (Fig. 4A), as for the earlier stage, during propulsive swimming the tail was recruited to undulatory behaviour and the hindlimbs were again extended and swept back in synchrony, presumably to facilitate forwards thrust. Most significantly, and in contrast to stage 58, the hindlimbs would express repeating cycles of extension and flexion either within a prolonged sequence of axial swimming (Fig. 4B), or independently (not illustrated). As seen in vivo, the spinal cord from stage 60/61 animals (Fig. 4C) was also capable of generating spontaneous bouts of rhythmic motor activity that occurred in the hindlimb and tail ventral roots, either concurrently (Fig. 4D), or independently (Fig. 4E and F). Moreover, the appendicular and axial patterns were radically different; the rhythm subserving the tail was faster (mean period 0.56 ± 0.05 s; 19 episodes from 5 preparations) and involved sequences of many consecutive cycles similar to those seen during fictive axial swimming in younger pre-metamorphic animals (cf. lower traces in Fig. 4D and F with Fig. 3D and E). In contrast, the hindlimb motor rhythm was slower (mean period 1.60 ± 0.08 s) and more closely resembled the appendicular rhythm generated exclusively by older froglets after the tail circuitry has completely disappeared (cf. upper traces in Fig. 4D and E with Fig. 2D). It is noteworthy that in the late metamorphic animal, a temporal interaction is still evident between hindlimb and axial activity, with both the period and duration of tail motor bursts decreasing during concurrent appendicular discharge (see Fig. 4D and F). Thus, stage 60/61 Xenopus represents a later transitional stage in which distinct rhythmic motor patterns for both axial and limb-based systems can be coexpressed and presumably both contribute to the generation of propulsive force.
Discussion
Our results show that rhythmic motor patterns responsible for larval axial-based swimming or froglet hindlimb propulsion in vivo are expressed in isolated spinal cord and brainstem preparations of corresponding metamorphic developmental stages. Importantly, these preparations can remain viable in vitro, in the absence of movement-related sensory feedback for over 24 h with the motor patterns generated throughout being appropriate to drive the characteristic locomotory behaviour of that particular stage. An important analytical feature of our in vitro investigation was the use of multiple simultaneous extracellular recordings from ventral motor roots at various points up and down the cord, in addition to those at the level of the hindlimb girdle.
In pre-metamorphic preparations (at or before stage 54; Nieuwkoop & Faber, 1956), spinal motor output corresponded to typical post-embryonic and larval undulatory swimming movements generated by alternate bilateral contractions of axial muscles with a characteristic rostro-caudal delay (see Fig. 1). In post-metamorphic (at or before stage 64) juveniles after the tail has been resorbed, spinal motor output was now appropriate for swimming produced exclusively by slower and bilaterally synchronous cycles of hindlimb extension and flexion (Fig. 2). Thus, in a period of 2–3 weeks the organism's central locomotor circuitry, which is distributed along the spinal cord of pre-metamorphic larvae, is replaced in froglets by a hindlimb-kick network which is now confined to the lumbar region of the neuroaxis.
At intermediate metamorphic stages (54–63) the earliest movements of the emerging hindlimbs consist initially of bilateral extension movements that maintain the legs in a rearward position during an episode of undulatory swimming (Fig. 3). However, that the lumbar motorneurones can also fire in tight co-ordination with the axial rhythm (Fig. 3D and E) suggests that alternating lateral displacement of the hindlimbs may also actively assist tail-based movements. As the hindlimbs and their muscles develop, this essentially auxiliary locomotor role is superseded by synchronous rhythmic leg movements that by ca stage 60 provide in parallel propulsive behaviour. As in the freely behaving animal, the motor patterns for both axial- and limb-based locomotion can be expressed independently or conjointly (albeit at very different frequencies), thereby confirming the coexistence of different rhythm-generating capabilities within the spinal cord. Whether this derives from distinctly different modular neural machinery remains unknown, although evidence of a persistent co-ordination between hindlimb and axial motor activity (Fig. 4D and F) suggests that in the lumbar region of the cord, both locomotor programmes are generated by functionally overlapping neural circuitry. In this context it is interesting that in the metamorphosing bullfrog, Rana catesbeiana, the hindlimbs are capable of both alternate stepping-like movements and bilaterally synchronous kicking, which appears later in metamorphic development. However, for both modes of hindlimb co-ordination, and in contrast to Xenopus, a strict 1: 1 frequency coupling is always observed with bursting in primary axial motorneurones in the isolated spinal cord (Stehouwer & Farel, 1983, 1985).
In stage 58 Xenopus, therefore, the coupling of bilaterally alternating axial and hindlimb motor bursting may represent an early transitional metamorphic phase in which central pattern-generating and co-ordinating circuitry remains shared, before progressive segregation and/or specification of new spinal mechanisms allow independent production of synchronous hindlimb movements. In the developing mammalian spinal cord a broadly similar transition in limb motor coordination occurs due to an inversion in the sign and action of synaptic inputs from depolarizing excitation to hyperpolarizing inhibition (Kudo et al. 2004). Whether such a mechanism could explain phase changes within the emerging limb circuitry of metamorphosing amphibians is not known. Clearly, unravelling such developmental issues in Xenopus spinal locomotor networks now awaits detailed examination at the cellular level, which the new series of in vitro preparations described in the present study now render feasible.
Acknowledgments
This work was supported by a Research Interchange Grant from the Leverhulme Trust (UK), to whom we are grateful. We also thank John Olthoff for help with artwork.
References
- Beck CW, Slack JM. An amphibian with ambition: a new role for Xenopus in the 21st century. Genome Biol. 2001;2:1029.1–1029.5. doi: 10.1186/gb-2001-2-10-reviews1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes D, Merrywest SD, McLean D, Simmers J, Sillar KT. Society for Neuroscience. Washington, DC: 2002. A novel preparation for the study of neuronal plasticity during amphibian metamorphosis. Program No. 863.16. 2002 Abstract Viewer/Itinerary Planner. [Google Scholar]
- Combes D, Merrywest SD, Sillar KT, Simmers J. Society for Neuroscience. Washington, DC: Development of motor patterns driving limb and axial musculature recorded in vitro during amphibian metamorphosis. Program No. 277.16. 2003 Abstract Viewer/Itinerary Planner. [Google Scholar]
- Consoulas C, Duch C, Bayline RJ, Levine RB. Behavioral transformations during metamorphosis: remodeling of neural and motor systems. Brain Res Bull. 2000;53:571–583. doi: 10.1016/s0361-9230(00)00391-9. [DOI] [PubMed] [Google Scholar]
- Hughes AF, Prestige MC. Development of behavior in the hindlimb of Xenopus laevis. J Zool. 1967;152:347–359. [Google Scholar]
- Kudo N, Nishimaru H, Nakayama K. Developmental changes in rhythmic spinal neural activity in the rat fetus. Brain mechanisms for integration posture movement. Prog Brain Res. 2004;143:49–55. doi: 10.1016/s0079-6123(03)43005-7. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber B. Normal Tables for Xenopus Laevis (Daudin) Amsterdam: North Holland Publishing Co; 1956. [Google Scholar]
- Roberts A, Soffe SR, Wolf ES, Yoshida M, Zhao FY. Central circuits controlling locomotion in young frog tadpoles. Ann NY Acad Sci. 1998;860:19–34. doi: 10.1111/j.1749-6632.1998.tb09036.x. [DOI] [PubMed] [Google Scholar]
- Shi YB. Amphibian Metamorphosis: from Morphology to Molecular Biology. New York: Wiley Liss; 2000. [Google Scholar]
- Shi YB, Ishizuya-Oka A. Thyroid hormone regulation of apoptotic tissue remodelling: implications from molecular analysis of amphibian metamorphosis. Prog Nucl Acid Res Mol Biol. 2001;65:53–100. doi: 10.1016/s0079-6603(00)65002-x. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Wedderburn JF, Simmers AJ. The development of swimming rhythmicity in post-embryonic Xenopus laevis. Proc R Soc Lond B Biol Sci. 1991;246:147–153. doi: 10.1098/rspb.1991.0137. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Farel PB. Development of hindlimb locomotor activity in the bullfrog (Rana catesbeiana) studied in vitro. Science. 1983;219:516–518. doi: 10.1126/science.6600518. [DOI] [PubMed] [Google Scholar]
- Stehouwer DJ, Farel PB. Development of locomotor mechanisms in the frog. J Neurophysiol. 1985;53:1453–1466. doi: 10.1152/jn.1985.53.6.1453. [DOI] [PubMed] [Google Scholar]
- Wassersug RJ. Where the tadpole meets the world – observations and speculations of biomechanical and biochemical factors that influence metamorphosis in anurans. Am Zool. 1997;37:124–136. [Google Scholar]