Abstract
Phytochrome (phy) A mediates two distinct photobiological responses in plants: the very-low-fluence response (VLFR), which can be saturated by short pulses of very-low-fluence light, and the high-irradiance response (HIR), which requires prolonged irradiation with higher fluences of far-red light (FR). To investigate whether the VLFR and HIR involve different domains within the phyA molecule, transgenic tobacco (Nicotiana tabacum cv Xanthi) and Arabidopsis seedlings expressing full-length (FL) and various deletion mutants of oat (Avena sativa) phyA were examined for their light sensitivity. Although most mutants were either partially active or inactive, a strong differential effect was observed for the Δ6-12 phyA mutant missing the serine-rich domain between amino acids 6 and 12. Δ6-12 phyA was as active as FL phyA for the VLFR of hypocotyl growth and cotyledon unfolding in Arabidopsis, and was hyperactive in the VLFR of hypocotyl growth and cotyledon unfolding in tobacco, and the VLFR blocking subsequent greening under white light in Arabidopsis. In contrast, Δ6-12 phyA showed a dominant-negative suppression of HIR in both species. In hypocotyl cells of Arabidopsis irradiated with FR phyA:green fluorescent protein (GFP) and Δ6-12 phyA:GFP fusions localized to the nucleus and coalesced into foci. The proportion of nuclei with abundant foci was enhanced by continuous compared with hourly FR provided at equal total fluence in FL phyA:GFP, and by Δ6-12 phyA mutation under hourly FR. We propose that the N-terminal serine-rich domain of phyA is involved in channeling downstream signaling via the VLFR or HIR pathways in different cellular contexts.
Phytochromes (phy) comprise a family of photoreceptors that help adjust plant growth and development to the ambient light environment. These photoreceptors sense red light (R) and far-red light (FR) through photo-interconversion between two stable conformations, an R-absorbing Pr form and an FR-absorbing Pfr form. In seed plants such as Arabidopsis, as many as five phy isoforms are present (Mathews and Sharrock, 1997). One of the more influential is phyA, the most abundant isoform in dark-grown seedlings (Hirschfeld et al., 1998). phyA helps perceive (a) the brief light pulses that can promote seed germination (Botto et al., l996; Shinomura et al., 1996), (b) the difference between darkness and the FR-rich environment that initiates de-etiolation beneath dense canopies (Yanovsky et al., 1995), (c) the changes in irradiance associated with the presence of neighboring vegetation (Yanovsky et al., 1998), and (d) the duration of the photoperiod (Johnson et al., 1994).
phyA can initiate two photobiologically distinct responses, the very-low-fluence responses (VLFRs) and the high-irradiance responses (HIRs). The VLFR can be achieved by short intermittent pulses of R or FR. For example, the VLFR that inhibits hypocotyl growth can be saturated in Arabidopsis by a 3-min pulse of FR every 2 h with the half-maximal effect requiring 0.1 μmol m−2 s−1 of FR (Casal et al., 2000). In contrast, the HIR that inhibits hypocotyl growth requires sustained activation of phyA triggered by higher fluences of FR (half-maximal effect requiring 3 μmol m−2 s−1) and does not occur under R (Casal et al., 2000). Genetic analyses indicate that the VLFR and HIR are mediated by different transduction pathways. For example, in Arabidopsis, the VLF1 and VLF2 loci, polymorphic between Landsberg erecta and Columbia ecotypes affect VLFR but not HIR (Yanovsky et al., 1997), whereas the fhy3-1 mutant retains VLFR but is severely deficient in HIR (Yanovsky et al., 2000). Furthermore, the interaction between phyA and phyB can be either synergistic or antagonistic, depending on the action of phyA in the VLFR or HIR modes, respectively (Cerdán et al., 1999). Using Lhcb1*2 fused to a reporter, Cerdán et al. (2000) showed that one region of this promoter is required for the HIR but not for the VLFR.
The levels and activity of phyA are regulated at numerous levels, which ultimately facilitates phyA response toward changing light conditions. The PHYA gene is under negative feedback control by Pfr (Quail et al., 1995). Combined with the high turnover rate of the PHYA mRNA, the transition from darkness to the light environment causes a rapid drop in the levels of the PHYA mRNA and the synthesis of the PHYA apoprotein. There is also a rapid degradation of the phyA holoprotein as Pfr, with light decreasing the half-life of the chromoprotein by at least 100-fold (Clough and Vierstra, 1997). Thus, although dark-grown plants have a high concentration of the phyA photoreceptor, most of it is rapidly lost upon transfer to light. A number of studies have implicated the ubiquitin/26S proteasome pathway in this selective removal (Clough and Vierstra, 1997). Finally, the intracellular distribution of phyA changes upon transformation of Pr to Pfr. The Pr of phyA appears to be uniformly distributed in the cytosol in dark-grown plants. Upon photoconversion to Pfr, a majority rapidly aggregates in the cytoplasm (Pratt, 1994) with the remaining entering the nucleus and coalescing into small foci (Kircher et al., 1999; Hisada et al., 2000; Kim et al., 2000). The functions of these nuclear foci are not yet known.
The signaling activity of phyA is apparently regulated by a process involving the Ser-rich domain near the N terminus. Removal or modification of this domain generates a phyA that is physiologically hyperactive under continuous light (Stockhaus et al., 1992; Emmler et al., 1995; Jordan et al., 1995, 1997). One or more Sers in this region are modified with phosphate in vivo (Lapko et al., 1997). Thus, in an analogous manner to the animal photoreceptor rhodopsin, phosphorylation of these Sers may help inactivate phyA, possibly by promoting its association with an inhibitor (Jordan et al., 1997).
Signaling via the VLFR or HIR pathways emanating from phyA may differentially require the various process that regulate the levels, activity, and/or location of the photoreceptor and hence may involve different regions within the phyA holoprotein. To help define these domains, we examined a series of phyA deletions for their ability to trigger the VLFR and HIR in transgenic tobacco (Nicotiana tabacum cv Xanthi) and Arabidopsis. One important domain appears to require the N-terminal Ser-rich region between residues 6 and 12. In several VLFRs, the N-terminal deletion missing this stretch (Δ6-12 phyA) behaved as a hyperactive phyA. However, a dominant negative effect was seen for the HIR. The nuclear accumulation of Δ6-12 phyA also differed from that of full-length (FL) phyA.
RESULTS
Δ6-12 phyA Is Hyperactive in VLFR of Hypocotyl Growth and Cotyledon Unfolding in Tobacco
In previous studies, a collection of oat (Avena sativa) phyA mutants was created in an attempt to define domains essential for chromoprotein assembly and activity (Cherry et al., 1992, 1993; Jordan et al., 1995). These mutants included a series of deletions progressively truncating phyA from the N terminus (Δ7-69, NA; Δ49-62, NB; Δ6-47, NC; Δ7-21, ND; Δ2-5, NE; and Δ6-12, NF) and from the C terminus (Δ1094-1129, CB; and Δ786-1129, CD). All assembled with the phytochromobilin chromophore when expressed in tobacco to generate spectrally active photoreceptors that could accumulate to high levels. Using the light effects on tobacco stem growth as an assay, the Δ1094-1129, Δ786-1129, Δ7-69, and Δ6-47 mutants were inactive photoreceptors, Δ49-62 showed partial activity, Δ2-5 and Δ7-21 showed activity similar to FL phyA, and Δ6-12 behaved as a hyperactive phyA (Cherry et al., 1992, 1993; Jordan et al., 1995). Collectively, the data highlighted the importance of a small Ser-rich region near the N terminus of phyA for controlling photoreceptor activity.
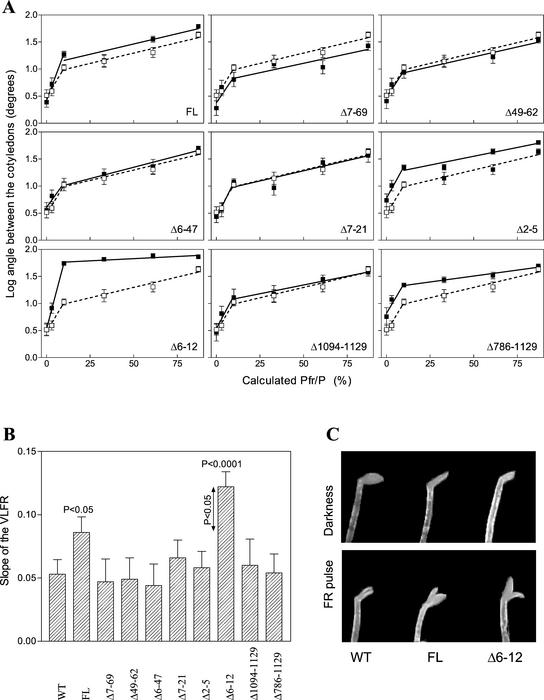
By using tobacco lines expressing similar amounts of chromoprotein (Cherry et al., 1993; Jordan et al., 1995), we tested the effects of the various oat phyA mutants on VLFR of etiolated seedlings. After a single 5-min pulse containing various mixtures of R/FR calculated to establish a range of Pfr/P, the seedlings were incubated for 24 h in darkness before measuring the extent of cotyledon unfolding. Similar to previous studies (Casal et al., 1994), cotyledon opening showed two response phases: a VLFR requiring 10% or less of Pfr/P, and a low-fluence response (LFR) effective between 10% and 87% Pfr/P (Fig. 1A). Expression of FL phyA specifically increased the slope of the VLFR but not of the LFR (Fig. 1, A and B). For most of the transgenic lines, the slopes of the VLFR and LFR were indistinguishable from non-trans-formed plants, indicating that the introduced chromoproteins were not active (Fig. 1, A and B). In contrast, the Δ6-12 mutant dramatically increased the slope of the VLFR (Fig. 1, A–C), consistent with a hyperactive behavior (Jordan et al., 1995).
Figure 1.
Δ6-12 phyA is hyperactive in the VLFR of cotyledon unfolding in etiolated tobacco. Six days after sowing, wild-type (WT) seedlings (white squares) and transgenic seedlings (black squares) expressing oat phyA of FL or carrying deletions in the N- or C-terminal domain were exposed to a single pulse (5 min) of R/FR predicted to establish a series of calculated Pfr/P (A). The angle between cotyledons was measured 24 h later. Data are means and se of nine replicate boxes. B, Slope (and se) of the VLFR. C, Detail of the cotyledons in WT, FL, and Δ6-12 seedlings grown in darkness or exposed to a pulse of FR.
To investigate the effects of the mutants on the VLFR of hypocotyl growth, etiolated tobacco seedlings were exposed to hourly pulses of FR for 48 h (hourly pulses saturate the VLFR but do not initiate the HIR; Casal et al., 2000). As with cotyledon opening, the Δ6-12 mutant behaved as a hyperactive phyA for this VLFR. Relative to dark grown plants, hypocotyl lengths of FR-treated seedlings were 66% ± 2% for WT seedlings, 47% ± 2% for seedlings expressing FL phyA, and 31% ± 1% for seedlings expressing the Δ6-12 mutant.
Δ6-12 phyA Is Not Hyperactive in VLFR of Hypocotyl Growth or Cotyledon Unfolding in Arabidopsis
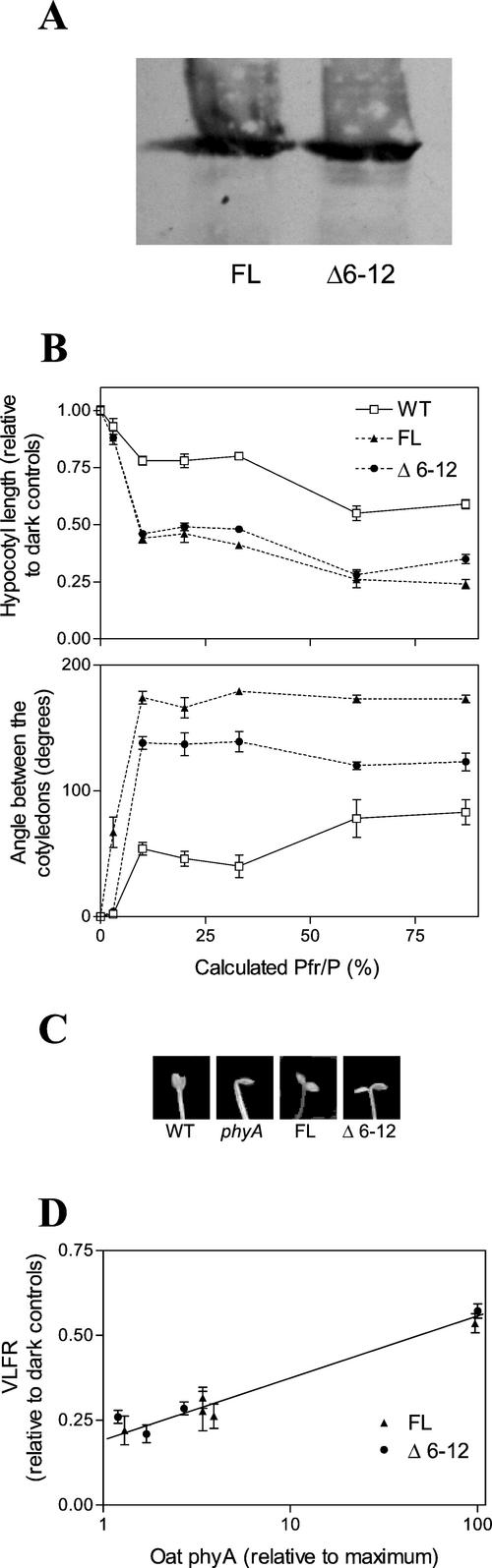
FL and Δ6-12 phyA were also introduced in Arabidopsis and lines expressing comparable levels of these constructions (Fig. 2A) were analyzed under hourly light pulses. Previous studies in Arabidopsis indicated that the VLFRs of hypocotyl growth and cotyledon unfolding (saturating with 10% Pfr/P) are mediated by phyA, whereas the LFRs (above 30% Pfr/P) are mediated by phyB (Yanovsky et al., 1997). As can be seen in Figure 2, B and C, both FL and Δ6-12 phyA enhanced the VLFR to a similar degree, indicating that Δ6-12 phyA is active but not hyperactive in these responses. To investigate this effect in more detail, a series of transgenic lines expressing a range of FL or Δ6-12 phyA levels were exposed to hourly pulses of FR and the VLFR for hypocotyl growth was measured. A similar concentration effect was observed (Fig. 2D), indicating that FL and Δ6-12 phyA had the same activity.
Figure 2.
Δ6-12 oat phyA is active but not hyperactive in the VLFR of hypocotyl growth and cotyledon unfolding in etiolated Arabidopsis. A, Immunochemically detectable levels of oat phyA in 4-d-old etiolated transgenic seedlings of Arabidopsis. B, One-day-old seedlings of the WT or expressing similar levels of either FL or Δ6-12 oat phyA were exposed for 3 d to hourly R/FR pulses (3 min) predicted to establish the indicated Pfr/P. C, Representative seedlings grown under hourly FR pulses (Pfr/P = 10%). D, Seedlings of independent transgenic lines expressing different levels of FL or Δ6-12 oat phyA (expressed relative to the levels shown in A) were exposed to hourly pulses of FR as in B, and the VLFR was calculated as the difference between hypocotyl length in darkness (1.00) and under hourly FR. Data are means and se of six (B) or five (D) replicate boxes.
Δ6-12 phyA Reduces the HIR of Hypocotyl Growth in Tobacco
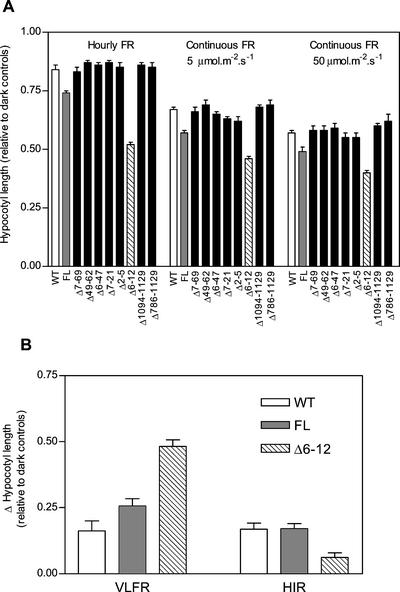
Considering that Δ6-12 phyA behaved as a hyperactive photoreceptor in the VLFR for tobacco (Fig. 1), the light-exaggerated phenotype observed previously under continuous FR (Jordan et al., 1995) could be mediated by the VLFR pathway (that saturates with very low fluence rates but is predicted to operate at higher fluence rates), the HIR pathway (that specifically requires continuous FR to be activated), or both. To distinguish between these possibilities, etiolated seedlings of tobacco expressing the various N- and C-terminal deletions were exposed to continuous FR (5 or 50 μmol m−2 s−1) or hourly pulses of FR (3 min, 100 μmol m−2 s−1) and assayed for the light inhibition of hypocotyl growth. By limiting the irradiations to a 6-h period each day, we avoided hypocotyl growth inhibition from reaching completion. For most of the lines, hypocotyl growth was indistinguishable from WT, indicating that the respective phyA mutants were inactive in this response (Fig. 3A). However, a substantial effect on hypocotyl growth was observed for seedlings expressing FL or Δ6-12 phyA. These seedlings were significantly shorter as compared with WT (P < 0.05) for all three irradiation regimes, with the Δ6-12 mutant conferring a greater response consistent with a hyperactive behavior (Fig. 3A).
Figure 3.
Δ6-12 oat phyA is hyperactive in the VLFR but reduces the HIR of hypocotyl growth in etiolated tobacco. A, Four-day-old seedlings of the WT, or expressing FL or deleted phyA, were exposed for 2 d to 6 h of continuous FR (5 and 50 μmol m−2 s−1) or six hourly pulses of FR (3 min, 100 μmol m−2 s−1). Data are means and se of 27 replicate boxes. B, The VLFR was calculated as the difference (and se) between darkness (1.00) and hourly FR, and HIR was calculated as the difference between hourly and continuous (5 μmol m−2 s−1) FR.
To dissect the response in Figure 3A into the VLFR and HIR components, we calculated the VLFR as the difference between the response toward darkness and hourly FR pulses, and the HIR as the difference between the response toward hourly FR and the lowest fluence rate of continuous FR (5 μmol m−2 s−1; Casal et al., 2000). It should be noted that under 5 μmol m−2 s−1 FR, the inhibition of hypocotyl growth for the Δ6-12 seedlings was not saturated. Interestingly, expression of FL phyA slightly enhanced the VLFR but did not change the HIR, whereas expression of Δ6-12 phyA dramatically enhanced the VLFR but repressed the HIR (Fig. 3B).
Δ6-12 phyA Reduces the HIR of Hypocotyl Growth in Arabidopsis
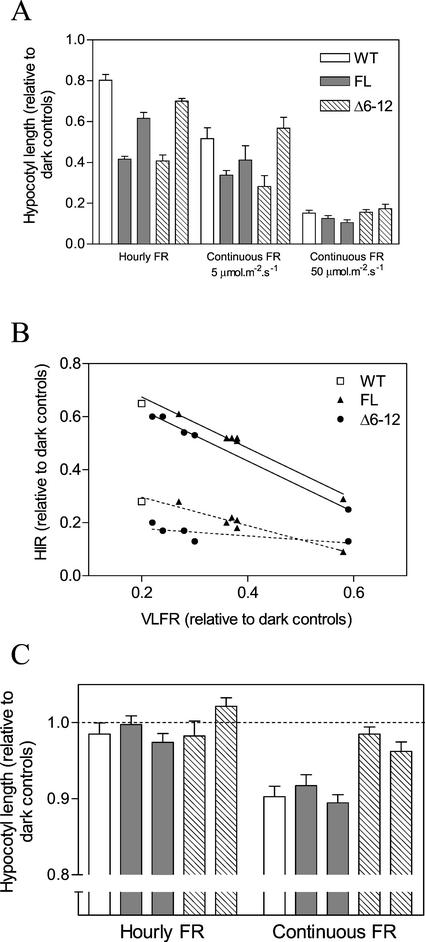
To investigate the HIR in Arabidopsis, the effect of FR on the hypocotyl growth of etiolated seedlings was examined using continuous FR (5 and 50 μmol m−2 s−1) or hourly FR pulses. Under hourly FR pulses, hypocotyl length was reduced by the same amount in FL or Δ6-12 phyA seedlings as compared with WT. The effect was especially obvious with lines containing higher levels of the FL and Δ6-12 chromoprotein (Fig. 4A). However, these differences became less noticeable when continuous high-fluence FR was used (Fig. 4A).
Figure 4.
Δ6-12 oat phyA is active in the VLFR but reduces the HIR of hypocotyl growth in etiolated Arabidopsis. A, One-day-old seedlings of the WT, or expressing either FL or Δ6-12 oat phyA, were exposed for 3 d to continuous FR (5 and 50 μmol m−2 s−1) or hourly pulses of FR (3 min, 100 μmol m−2 s−1). Two FL and Δ6-12 transgenic lines are included. In each case, the left bar is a strong expression line (see Fig. 2A) and the right bar shows a weak expression line (3% oat phyA compared with strong expression lines). B, Seedlings of independent transgenic lines expressing different levels of FL or NF oat phyA (it includes the lines shown in Fig. 2D plus additional lines) were exposed to hourly or continuous FR. The VLFR was calculated as the difference between hypocotyl length in darkness (1.00) and under hourly FR. The HIR for 5 (dotted lines) and 50 (solid lines) μmol m−2 s−1 were calculated as the difference between hourly and continuous FR. C, Four-day-old seedlings were exposed for 8 h to either hourly or continuous FR and hypocotyl length was measured 24 h later (at the beginning of the FR treatments hypocotyl length was approximately 86% of the length of dark controls 24 h later). Data are means and se of five (A and B) or 12 (C) replicates boxes.
When a series of transgenic lines expressing a range of phyA levels was tested (see Fig. 2), a strong negative correlation was observed between VLFR (difference between hourly FR and darkness) and HIR (difference between continuous and hourly FR; Fig. 4B). This correlation implied that increasing amounts of FL or Δ6-12 phyA enhanced the VLFR but repressed the HIR. The negative correlation did not appear to arise from the way in which the HIR is calculated because it was observed for irradiations with 5 μmol m−2 s−1 FR, a fluence rate well below saturation. The calculated HIR was further reduced in the Δ6-12 phyA lines (Fig. 4B), suggesting the HIR in Arabidopsis is negatively affected by Δ6-12 phyA as it is in tobacco.
Arabidopsis seedlings grown for several days in darkness lose their VLFR for some responses such as hypocotyl growth, thus allowing the HIR to predominate (J.J. Casal, unpublished data). By exploiting this phenomenon, we exposed 4-d-old dark-grown seedlings to 8 h of FR treatments and measured the HIR on hypocotyl length 24 h later. As expected, given the absence of the VLFR, hourly pulses of FR had little effect on WT, FL, and Δ6-12 seedlings as compared with those kept in the dark (Fig. 4C). For the continuous FR treatments, the hypocotyl growth of both WT seedlings and seedlings expressing FL phyA were inhibited, indicative of a HIR. However, the HIR was not significant for the Δ6-12 seedlings, suggesting that this mutant chromoprotein negatively interferes with the normal HIR.
Effect of Δ6-12 phyA on Anthocyanin Accumulation and Greening
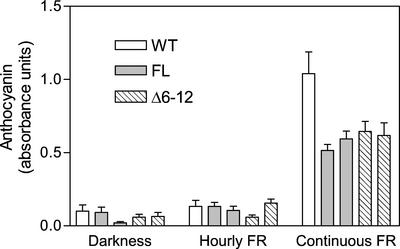
The contribution of VLFR and HIR components to the final outcome of phyA activity varies among the responses. To investigate whether other HIR responses also are repressed by the Δ6-12 phyA mutant, we examined its effect on the increase in anthocyanin synthesis and the inhibition of white light-induced greening, both of which are induced by prolonged exposures to FR (Barnes et al., 1996). In WT plants, these two responses are predominantly a HIR and show little or no VLFR (Yanovsky et al., 2000). For the anthocyanin response, hourly pulses of FR did not increase accumulation in WT, FL, and Δ6-12 plants, demonstrating that the VLFR is negligible (Fig. 5). As expected for a HIR, anthocyanin levels increased in WT plants irradiated with continuous FR. However, this increase was diminished by the introduction of FL or Δ6-12 phyA, indicating that both photoreceptors repress the response.
Figure 5.
Expression of FL or Δ6-12 oat phyA reduces anthocyanin accumulation under continuous FR. One-day-old Arabidopsis seedlings of the WT, or expressing FL or Δ6-12 oat phyA were exposed for 3 d to continuous FR or hourly pulses of FR. Bars corresponding to stronger expression (Fig. 2A) are placed to the left and those corresponding to weaker expression (3% of strong expression lines) are placed to the right. Data are means and se of five or nine (continuous FR) replicate boxes.
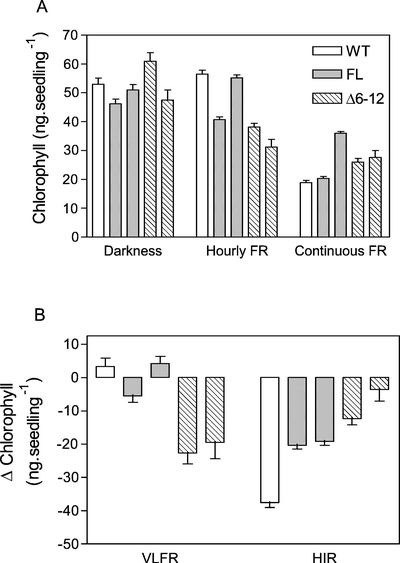
In WT seedlings, greening induced by white light can be substantially inhibited by previous exposure to continuous FR (a typical HIR) but not by hourly pulses of FR, indicative of an HIR with little contribution of a VLFR (Yanovsky et al., 2000; Fig. 6). For seedlings expressing FL phyA, greening was not substantially affected by the hourly pulses of FR but was less repressed by continuous FR than in the WT, indicating that the VLFR was unaffected and the HIR was reduced. For the Δ6-12 phyA plants, both the VFLR and HIR responses were affected, but in opposite ways (Fig. 6). For hourly pulses of FR, Δ6-12 phyA plants greened less (increased VLFR), but for continuous FR, the Δ6-12 phyA plants greened more (reduced HIR).
Figure 6.
Blocking of greening by FR in Arabidopsis. Seedlings expressing Δ6-12 phyA are paler than the WT after hourly FR and greener than the WT after continuous FR. One-day-old seedlings of the WT, or expressing either FL or Δ6-12 oat phyA, were exposed for 3 d to continuous FR or hourly pulses of FR and subsequently transferred to fluorescent white light (100 μmol m−2 s−1) for 24 h to allow greening. Bars corresponding to stronger expression (Fig. 2A) are placed to the left and those corresponding to weaker expression (3% of strong expression lines) are placed to the right. A, Chlorophyll levels. B, VLFR (difference between darkness and hourly FR) and HIR (difference between hourly and continuous FR). Data are means and se of 10 replicate boxes.
Δ6-12 phyA-Green Fluorescent Protein (GFP) Fusions Migrate to the Nucleus
To test if the location of the phyA chromoprotein was altered by the N-terminal deletion that affected physiological responses, its intracellular distribution was examined by expressing similar levels of Δ6-12 or FL phyA fusions to GFP in Arabidopsis (Fig. 7A). The GFP fusions were introduced into the phyA-201 mutant line devoid of phyA proteins to eliminate potential complications from the endogenous photoreceptor. Both the FL and Δ6-12 phyA GFP fusions complemented the phyA-201 phenotype (data not shown), indicating that they can functionally replace Arabidopsis phyA. The seedlings were grown for 3 to 4 d in darkness, irradiated for 24 h with an equal total fluence of continuous or hourly pulses of FR, and the location of the GFP was then examined by fluorescence microscopy (Kircher et al., 1999).
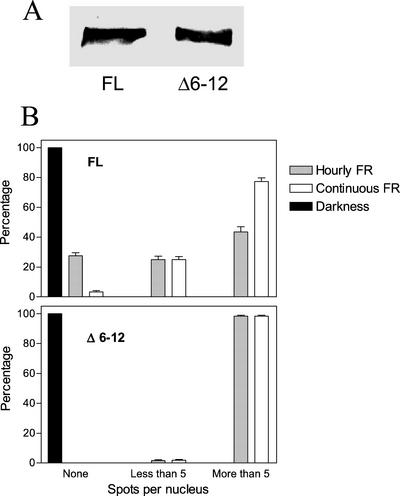
Figure 7.
Continuous FR and the Δ6-12 mutation increase the proportion of nuclei with numerous speckles compared with FL phyA-GFP under hourly FR pulses. A, Immunochemically detectable levels of oat phyA:GFP in 4-d-old etiolated seedlings. B, Proportion of nuclei showing no spots, five or less spots, or more than five spots, in hypocotyl cells from 3- to 4-d-old seedlings of Arabidopsis bearing the oat FL PHYA:GFP or the oat Δ6-12 PHYA:GFP transgene, nonirradiated or exposed to 24 h of either hourly 3-min light pulses of FR (24 μmol m−2 s−1) or continuous FR (1.2 μmol m−2 s−1). Data are means and se of at least 40 seedlings (10 nuclei per seedling).
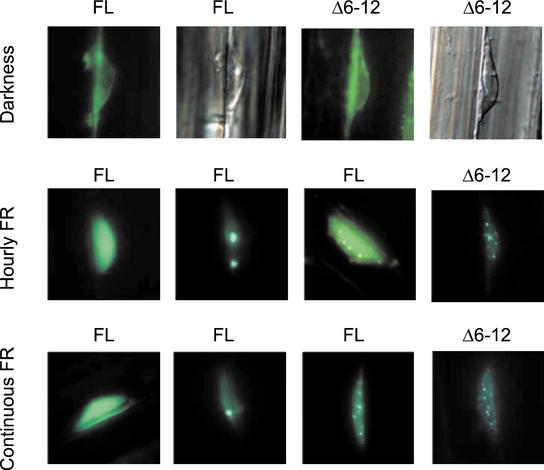
In dark-grown plants, both the FL and Δ6-12 phyA:GFP fusions were predominately located in the cytoplasm with little if any signal present in the nucleus (Fig. 8). Consistent with previous studies, the FL phyA:GFP fusion became concentrated in the nucleus when the plants were exposed to either hourly FR pulses or continuous FR. Three types of nuclear fluorescence were evident (Fig. 8): nuclei with diffuse fluorescence and no concentrated foci, nuclei with most of the FL phyA:GFP aggregated into a few large fluorescent foci with little background fluorescence (2% of the nuclei), and nuclei with a variable number of small fluorescent foci along with a diffuse background fluorescence. When Δ6-12 phyA:GFP was tested, almost all of the nuclei showed the latter pattern, with nuclei containing a number of small fluorescent foci (Fig. 8). No nuclei with a few large foci were evident for Δ6-12 phyA:GFP.
Figure 8.
FL phyA:GFP and Δ6-12 phyA:GFP fusion proteins translocate to the nucleus but show different patterns of nuclear speckle formation under FR. Epifluorescence images of hypocotyl cells from 3- to 4-d-old seedlings of Arabidopsis bearing the oat FL PHYA:GFP or the oat Δ6-12 PHYA:GFP transgene, nonirradiated or exposed to 24 h of either hourly 3-min light pulses of FR (24 μmol m−2 s−1) or continuous FR (1.2 μmol m−2 s−1). The second and fourth pictures of the first row correspond to nuclei that were stained with 4′,6-diamidino-2-phenylindole dihydrochloride to visualize DNA. For both FR conditions, FL oat phyA:GPF transgenics showed (left to right) nuclei with intense background and no spots (the least frequent pattern), nuclei with few small spots, and nuclei with many tiny spots. Δ6-12 oat phyA:GFP fusions only formed numerous tiny nuclear spots.
The proportion of nuclei with no spots, few spots (five or less, regardless of the size), or many spots (more than five) was altered by light conditions and phyA deletion. In FL phyA:GFP transgenics, continuous FR increased the proportion of nuclei with many spots when compared with hourly pulses of FR provided at the same total fluence (Fig. 7B). Compared with FL phyA:GFP, Δ6-12 phyA:GFP increased the proportion of nuclei with many spots under hourly FR (Fig. 7B).
DISCUSSION
Previous studies have shown that the N-terminal Ser-rich domain of phyA is important for proper function of the photoreceptor, possibly acting to down-regulate its activity after Pfr formation (see Jordan et al., 1995, 1997). Here, we show that the effects of this domain on VLFR and HIR are differential. Compared with FL phyA, the Δ6-12 mutant missing this Ser stretch behaved as a hyperactive phyA in VLFR of hypocotyl growth and cotyledon unfolding in tobacco (Figs. 1 and 3). Δ6-12 phyA was also hyperactive with respect to the VLFR blocking greening upon transfer to white light in Arabidopsis (Fig. 6), and was as active as FL phyA in the VLFR of hypocotyl growth and cotyledon unfolding (Fig. 2), and the VLFR or HIR of anthocyanin synthesis (Fig. 5) in this species. Compared with FL phyA, Δ6-12 phyA reduced the HIR of hypocotyl growth in both species and the HIR of the block of greening by FR in Arabidopsis (Figs. 3, 4, and 6). Thus, VLFRs were either enhanced or unaffected, whereas HIRs were either reduced or unaffected in transgenic seedlings containing Δ6-12 phyA as compared with FL phyA.
Expression of oat phyA missing the 52 N-terminal amino acids in Arabidopsis interferes with the inhibition of hypocotyl mediated by endogenous phyA under continuous FR, but it enhances inhibition of hypocotyl growth under R (Boylan et al., 1994). phyA-mediated inhibition of hypocotyl growth is a VLFR under R and predominantly an HIR under continuous FR (Mazzella et al., 1997). Therefore, the effects of oat phyA missing the 52 N-terminal amino acids are also consistent with a differential role of the N-terminal domain on VLFR compared with HIR. Rice (Oryza sativa) phyA missing the 80 N-terminal amino acids also interferes with endogenous phyA responses in tobacco seedlings grown under FR (Emmler et al., 1995). The experiments reported here narrow down the deletion that interfered with the HIR to the same seven amino acids whose deletion enhanced the VLFR.
Δ6-12 phyA repressed the HIR apparently by interfering with normal phyA signaling in the HIR mode. Expression of Δ6-12 phyA does not affect the endogenous phyA levels (Jordan et al., 1995). Although the experiments were done under non-saturating light conditions, the calculation of HIR as the difference between the effects of continuous and hourly FR may have contributed to reduced HIR in Δ6-12 compared with FL transgenics when the effects of hourly FR were enhanced (Figs. 3B and 6). However, authentic interference is demonstrated by the following pieces of evidence. First, reduced HIR was observed for hypocotyl growth in Arabidopsis, a response where Δ6-12 phyA is not hyperactive in the VLFR. Second, the reduction in HIR did not correlate with the magnitude of the VLFR in Δ6-12 phyA plants (Fig. 4B). Third, reduced HIR of hypocotyl growth was also observed under developmental conditions where a VLFR of hypocotyl growth was not evident (Fig. 4C).
Compared with the WT, ectopic expression of FL oat phyA enhanced VLFR (this report; Casal et al., 1994; Clough et al., 1995) but did not increase and in fact may have reduced the HIR in etiolated tobacco or Arabidopsis. These observations are consistent with recent microarray studies showing that 40% of the genes whose expression is enhanced by continuous FR have reduced expression when phyA is overexpressed (Ma et al., 2001). Although oat phyA has the capacity to operate via the HIR mode in rice (Casal et al., 1996) and light-grown tobacco (Casal et al., 1995), in etiolated tobacco and Arabidopsis it appears to be more effective in the VLFR mode. In accordance with this interpretation, the maximum effect of overexpressed oat phyA on hypocotyl growth is already achieved with less than 1 μmol m−2 s−1 of continuous FR in etiolated tobacco and Arabidopsis seedlings (McCormac et al., 1992; Whitelam et al., 1992). For both oat phyA and rice phyA, similar if not greater effects are seen with R than FR irradiations (Boylan et al., 1994; Emmler et al., 1995).
Under continuous or hourly FR, both FL and Δ6-12 oat phyA fused to GFP were translocated to the nucleus and coalesced into foci (Fig. 8). Based on our knowledge in other systems, possible functions of these foci include regulation of transcription by phyA-containing complexes, degradation of phyA as Pfr, and/or sequestration of the phyA molecules within inclusion bodies or aggresomes (Amirand et al., 1998; Kopito and Sitia, 2000). Like phy, it has been reported that the blue-light photoreceptors cryptochromes (Mas et al., 2000), the COP1 nuclear repressor of photomorphogenesis (Stacey and von Arnim, 1999), and the MYB transcription factor LAF1 involved in phyA signaling (Ballesteros et al., 2001) also concentrate into nuclear speckles. In FL phyA:GFP transgenics, the proportion of nuclei with many spots was larger in seedlings exposed to continuous compared with hourly FR at equal total fluence (Fig. 7B). Under hourly FR pulses, Δ6-12 phyA: GFP showed a larger proportion of nuclei with numerous spots than FL phyA:GFP (Fig. 7B). These light and phyA molecule effects could be the result of changes in the intranuclear amount of phyA and/or of special qualitative properties of phyA with regard to interactions with other intranuclear proteins. Available evidence does not allow one to discriminate between these possibilities. Continuous FR is required for both HIR and maximum spot formation and this provides correlative evidence for a role of spot formation in HIR. The significance of enhanced spot formation as a result of the Δ6-12 deletion of phyA is not clear at present. The Δ6-12 phyA transgenics showed enhanced VLFR, but we have recently obtained a missense phyA mutant that retains VLFR and lacks HIR and spot formation (Yanovsky et al., 2002), suggesting that spot formation would not be necessary for VLFR.
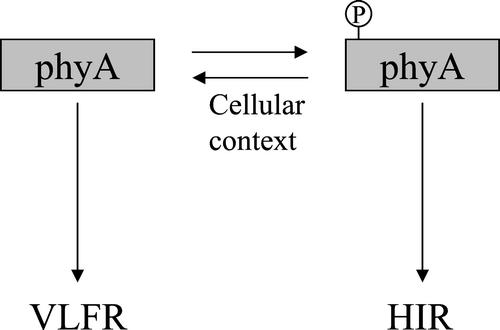
The photomorphogenic responses that can be induced by transient activation of phyA (VLFR) and those that specifically require sustained activation of phyA with FR (HIR) are photobiologically and genetically distinct (Yanovsky et al., 1997, 2000; Casal et al., 2000; Cerdán et al., 2000) and can exert contrasting regulation of phyB signaling (Cerdán et al., 1999). Here, we find that the differences between VLFR and HIR can be traced back to at least one region in the phyA molecule, namely the Ser-rich domain between residues 6 and 12. A likely scenario is that the Ser-rich domain is part of a mechanism that down-regulates the VLFR and up-regulates the HIR possibility by switching the sensory flux between the two systems (Fig. 9). This regulation could involve cellular context-dependent phosphorylation of the domain, a process that could account for the observed dependence of the effects on the host species and the specific physiological processes. If true, such regulation could then add versatility to light perception by the phy family of photoreceptors by governing which response predominates in a given light situation.
Figure 9.
Working model of the regulatory role played by phosphorylation of the Ser-rich N-terminal domain of phyA in the regulation of sensitivity to light.
MATERIALS AND METHODS
Plant Material
The WT lines used were tobacco (Nicotiana tabacum cv Xanthi) and Arabidopsis ecotype RLD. The construction of oat (Avena sativa) phyA deletion mutants Δ6-12, Δ7-69, Δ49-62, Δ6-47, Δ7-21, Δ2-5, Δ1094-1129, and Δ786-1129, their introduction into tobacco, and the initial characterization of the plants have been described previously (Cherry et al., 1993; Jordan et al., 1995). The FL and Δ6-12 phyA constructions described by Jordan et al. (1995) were also introduced in WT Arabidopsis by infiltration of the inflorescence (Chang et al., 1994) with Agrobacterium tumefaciens strain GV3101. To generate the 35S:FL PHYA:GFP and 35S:Δ6-12 phyA:GFP chimeric genes, the rice (Oryza sativa) PHYA cDNA present in the pPCV 812 binary vector (Kircher et al., 1999) was replaced by the FL or Δ6-12 phyA coding regions (Jordan et al., 1995) by removing the rice PHYA fragment via BamHI-SmaI digestion as described by Kim et al. (2000). Transgenic plants harboring GFP constructions were produced in the phyA-201 mutant of the Arabidopsis ecotype Wassilewskija background (Kim et al., 2000) according to the method of Koncz et al. (1994).
Tobacco seeds were sown in clear plastic boxes (40 × 33 mm2, 15-mm height) containing 3 mL of 0.8% (w/v) agar-water. The seeds were exposed to fluorescent white light for 24 h and transferred to darkness for 5 d (cotyledon-unfolding experiments) or 3 d (hypocotyl growth experiments) before the light treatments. Seeds of Arabidopsis were sown in the plastic boxes containing agar-water, incubated at 4°C in darkness for at least 3 d, given an R pulse, and transferred to darkness for 24 h (unless stated otherwise) before light treatments. For all responses, the plants were maintained at 25°C.
Inmunological Analysis
Seedlings were frozen and crushed to a powder at liquid nitrogen temperatures and protein was extracted according to Somers et al. (1991). Equal amounts of protein were subjected to SDS-PAGE and electroblotted onto nitrocellulose membranes. After a blocking step using 1% (w/v) skimmed milk, the membranes were probed with the monoclonal antibody Oat-22 specific for oat phyA (Cordonier et al., 1984). Detection of phyA used alkaline phosphatase-conjugated goat antibody to mouse IgG (Sigma, St. Louis) in conjunction with 5-bromo-4-chloro-3-indolyl phosphate, p-toluidine salt, and nitroblue tetrazolium. Staining intensity was quantified by using a Peltier-cooled CCD camera/imager system (Fluor-S MultiImager, Bio-Rad, Hercules, CA).
For the analysis of FL phyA:GFP and Δ6-12 phyA:GFP, 4-d-old dark-grown seedlings of Arabidopsis were homogenized in hot extraction buffer and heated as described by Kircher et al. (1999). The supernatant was denatured by centrifugation (10 min, 20000g, and 25°C) and equal amounts of protein (20 μg) were separated in SDS-PAGE and blotted to polyvinyl difluoride membrane. Immunodetection was performed by using the phyA-specific antibody mAR14 and an alkaline phosphatase-coupled anti-rabbit antiserum (Bio-Rad) as described by Kircher et al. (1999).
Light Sources
Light sources were as described earlier (Yanovsky et al., 2000). FR (calculated to generate a 10% Pfr/P ratio) was provided by incandescent lamps in combination with a water filter, a red acetate filter, and six 2-mm-thick blue acrylic filters (Paolini 2031, La Casa del Acetato, Buenos Aires). For the greening experiments, long-wavelength FR (calculated Pfr/P = 3%) was provided by an incandescent lamp in combination with a water filter and an RG9 filter (Schott, Maintz, Germany). Mixtures of R and FR were provided by incandescent lamps in combination with a water filter and a red acetate filter either alone (calculated Pfr/P = 61%), or in combination with either two blue acrylic filters (20%) or a green acetate (33%). R (calculated to generate a Pfr/P of 87%) was provided by light-emitting diodes.
Physiological Measurements
Hypocotyl length was measured to the nearest 0.5 mm with a ruler. To eliminate aberrant seedlings, only the tallest 10 of 15 Arabidopsis seedlings and six of eight tobacco seedlings were averaged for each box (i.e. one replicate). Hypocotyl length was expressed relative to dark controls. No systematic differences between dark controls were observed. The angle between the cotyledons was measured with a protractor. For measurement of anthocyanin levels, 200 seedlings were extracted in 1 mL of 1% (w/v) HCl methanol. A530 was measured and corrected for chlorophyll absorption (657 nm) as described (Mancinelli et al., 1991). To assess greening under fluorescent white light (100 μmol m−2 s−1) after FR treatments, 20 seedlings were harvested in 1 mL of N,N′-dimethylformamide and incubated in darkness at −20°C for at least 3 d. Chlorophyll levels in the soluble phase were calculated according to the method of Moran (1982).
Epifluorescence and Light Microscopy
Dark-grown seedlings (3–4 d old) were either kept in the dark or exposed to 24 h of either continuous FR (1.2 μmol m−2 s−1) or 3-min light pulses of FR (24 μmol m−2 s−1) given hourly. All subsequent manipulations were performed under dim green light. Pulsed plants were examined just before the 25th pulse. The upper halves of the hypocotyls were collected, transferred to glass slides and analyzed under an Axirovert microscope (Zeiss, Oberkochem, Germany). Excitation of GFP was performed with standard flurescein isothiocyanate filters. Representative cells were photographed with an automatic Contax 167 MT camera containing Eastman Kodak 64T film (Kodak AG, Stuttgart, Germany). Selected nuclei were stained with 50 ng mL−1 4′,6-diamidino-2-phenylindole dihydrochloride to visualize DNA. The slides were scanned and processed for presentation as described (Kim et al., 2000).
Footnotes
This work was supported by the Fondo Nacional de Ciencia y Técnica (grant no. BID 1201/OC–AR PICT 06739 to J.J.C.), by the University of Buenos Aires (grant no. TG59 to J.J.C.), by the Consejo Nacional de Investigaciones Científicas y Técnicas (grant no. PIP 0888/98 to J.J.C.), by the Fundación Antorchas (grant no. A–13622/1–40 to J.J.C.), by the U.S. Department of Energy (grant no. DE–FG02–88ER13968 to R.D.V.), by the Deutsche Forschungsgemeinschaft (grant no. SFB 592 to E.S.), by a Howard Hughes Medical Institute International Scholar Fellowship, by the Hungarian Science Foundation (grant no. OTKAT–02584 to F.N.), and by a Wolfgang Pauls Award of the Alexander von Humboldt Foundation (to F.N.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010977.
LITERATURE CITED
- Amirand C, Viari A, Ballini JP, Rezaei H, Beaujean N, Jullien D, Kas E, Debey P. Three distinct sub-nuclear populations of HMG-I protein of different properties revealed by co-localization image analysis. J Cell Sci. 1998;111:3551–3561. doi: 10.1242/jcs.111.23.3551. [DOI] [PubMed] [Google Scholar]
- Ballesteros ML, Bolle C, Lois LM, Moore JM, Vielle-Calzada J-P, Grossniklaus U, Chua N-M. LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev. 2001;15:2613–2625. doi: 10.1101/gad.915001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Nishizawa NK, Quaggio RB, Whitelam GC, Chua N-H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell. 1996;8:601–615. doi: 10.1105/tpc.8.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto JF, Sánchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan M, Douglas N, Quail PH. Dominant negative suppression of Arabidopsis photoresponses by mutant phytochrome-A sequences identifies spatially discrete regulatory domains in the photoreceptor. Plant Cell. 1994;6:449–460. doi: 10.1105/tpc.6.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Clough RC, Vierstra RD. High-irradiance responses induced by far-red light in grass seedlings of the wild type or overexpressing phytochrome A. Planta. 1996;200:132–137. [Google Scholar]
- Casal JJ, Sánchez RA, Boylan M, Vierstra RD, Quail PH. Is the far-red-absorbing form of Avena phytochrome A that is present at the end of the day able to sustain stem-growth inhibition during the night in transgenic tobacco and tomato seedlings? Planta. 1995;197:225–232. [Google Scholar]
- Casal JJ, Sánchez RA, Vierstra RD. Avena phytochrome A overexpressed in transgenic tobacco seedlings differentially affects red/far-red reversible and very-low-fluence responses (cotyledon unfolding) during de-etiolation. Planta. 1994;192:306–309. [Google Scholar]
- Casal JJ, Yanovsky MJ, Luppi JP. Two photobiological pathways of phytochrome A activity, only one of which shows dominant negative suppression by phytochrome B. Photochem Photobiol. 2000;71:481–486. doi: 10.1562/0031-8655(2000)071<0481:tppopa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cerdán PD, Staneloni RJ, Ortega J, Bunge MM, Rodriguez-Batiller J, Sánchez RA, Casal JJ. Sustained but not transient phytochrome A signaling targets a region of a Lhcb1*2 promoter that is not necessary for phytochrome B action. Plant Cell. 2000;12:1203–1211. [PMC free article] [PubMed] [Google Scholar]
- Cerdán PD, Yanovsky MJ, Reymundo FC, Nagatani A, Staneloni RJ, Whitelam GC, Casal JJ. Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 1999;18:499–507. doi: 10.1046/j.1365-313x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- Chang SC, Park SK, Kim BC, Kang BJ, Kim DU, Nam HG. Stable genetic transformation of Arabidopsis thaliana by Agrobacterium inoculation in planta. Plant J. 1994;5:551–558. [Google Scholar]
- Cherry JR, Hondred D, Walker JM, Keller JM, Hershey HP, Vierstra RD. Carboxy-terminal deletion analysis of oat phytochrome A reveals the presence of separate domains required for structure and biological activity. Plant Cell. 1993;5:565–575. doi: 10.1105/tpc.5.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JR, Hondred DM, Walker JM, Viestra RD. Phytochrome requires the 6-kDa N-terminal domain for full biological activity. Proc Natl Acad Sci USA. 1992;89:5039–5043. doi: 10.1073/pnas.89.11.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough RC, Casal JJ, Jordan ET, Christou P, Vierstra RD. Expression of functional phytochrome A in transgenic rice. Plant Physiol. 1995;109:1039–1043. doi: 10.1104/pp.109.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- Cordonier M-M, Greppin H, Pratt LH. Characterization by enzyme-linked immunosorbent assay of monoclonal antibodies to Pisum and Avena phytochrome. Plant Physiol. 1984;74:123–127. doi: 10.1104/pp.74.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmler K, Stockhaus J, Chua N-H, Schäfer E. An amino-terminal deletion of rice phytochrome A results in a dominant negative suppression of tobacco phytochrome A activity in transgenic tobacco seedlings. Planta. 1995;197:103–110. doi: 10.1007/BF00239945. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA. Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics. 1998;149:523–535. doi: 10.1093/genetics/149.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada A, Hanzawa H, Weller J, Nagatani A, Reid J, Furuya M. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell. 2000;12:1063–1078. doi: 10.1105/tpc.12.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Bradley M, Harberd P, Whitelam GC. Photoresponses of light-grown phyA mutants of Arabidopsis. Phytochrome A is required for the perception of daylength extensions. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan ET, Cherry JR, Walker JM, Vierstra RD. The amino-terminus of phytochrome A contains two distinct functional domains. Plant J. 1995;9:243–257. doi: 10.1046/j.1365-313x.1996.09020243.x. [DOI] [PubMed] [Google Scholar]
- Jordan ET, Marita JM, Clough RC, Vierstra RD. Characterization of regions within the N-terminal 6-kilodalton domain of phytochrome A that modulate its biological activity. Plant Physiol. 1997;115:693–704. doi: 10.1104/pp.115.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Kiercher S, Toth R, Adam E, Schäfer E, Nagy F. Light induced nuclear import of phytochrome-A:GFP fusions proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 2000;22:125–133. doi: 10.1046/j.1365-313x.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- Kircher S, Kozma-Bognar L, Kim L, Adam E, Harter K, Schäfer E, Nagy F. Light-quality dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell. 1999;11:1445–1456. doi: 10.1105/tpc.11.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz CS, Martini N, Szabados L, Hrouda M, Bachmair A, Schell J. Specialized vectors for gene tagging and expression studies. In: Gelvin BS, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Press; 1994. pp. 1–22. [Google Scholar]
- Kopito RR, Sitia R. Aggresomes and Russell bodies. Symptoms of cellular indigestion? EMBO Rep. 2000;11:225–231. doi: 10.1093/embo-reports/kvd052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapko VN, Jiang X-Y, Smith DL, Song P-S. Posttranslational modification of oat phytochrome A: phosphorylation of a specific serine in a multiple serine cluster. Biochemistry. 1997;36:10595–10599. doi: 10.1021/bi970708z. [DOI] [PubMed] [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chan Z, Zhao H, Deng X-W. Light control of Arabidopsis development entails co-ordinated regulation of genome expression and cellular pathways. Plant Cell. 2001;13:2589–2607. doi: 10.1105/tpc.010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli AL, Rossi F, Moroni A. Cryptochrome, phytochrome and anthocyanin production. Plant Physiol. 1991;96:1079–1085. doi: 10.1104/pp.96.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas P, Devlin PF, Panda S, Kay SA. Functional interaction of phytochrome B and cryptochrome 2. Nature. 2000;408:207–211. doi: 10.1038/35041583. [DOI] [PubMed] [Google Scholar]
- Mathews S, Sharrock RA. Phytochrome gene diversity. Plant Cell Environ. 1997;20:666–671. [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- McCormac A, Whitelam G, Smith H. Light-grown plants of transgenic tobacco expressing an introduced oat phytochrome A gene under the control of a constitutive viral promoter exhibit persistent growth inhibition by far-red light. Planta. 1992;188:173–181. doi: 10.1007/BF00216811. [DOI] [PubMed] [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt LH. Distribution and localization of phytochrome within the plant. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 163–185. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and phytochrome B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1991;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey MG, von Arnim AG. A novel motif mediates the targeting of the Arabidopsis COP1 protein to subnuclear foci. J Biol Chem. 1999;274:27231–27236. doi: 10.1074/jbc.274.38.27231. [DOI] [PubMed] [Google Scholar]
- Stockhaus J, Nagatani A, Halfter U, Kay S, Furuya M, Chua N-H. Serine-to-alanine substitutions at the amino-terminal region of phytochrome A result in an increase in biological activity. Genes Dev. 1992;6:2364–2372. doi: 10.1101/gad.6.12a.2364. [DOI] [PubMed] [Google Scholar]
- Whitelam GC, McCormac AC, Boylan MT, Quail PH. Photoresponses of Arabidopsis seedlings expressing an introduced oat phyA cDNA: persistence of etiolated plant type responses in light-grown plants. Photochem Photobiol. 1992;56:617–621. [Google Scholar]
- Yanovsky MJ, Alconada-Magliano TM, Mazzela MA, Gatz C, Thomas B, Casal JJ. Phytochrome A affects stem growth, anthocyanin synthesis, sucrose-phosphate-synthase activity and neighbour detection in sunlight-grown potato. Planta. 1998;205:235–241. [Google Scholar]
- Yanovsky MJ, Casal JJ, Luppi JP. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia, dissect two branches of phytochrome A signalling pathways that correspond to the very-low fluence and high-irradiance responses of phytochrome. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995;18:788–794. [Google Scholar]
- Yanovsky MJ, Luppi JP, Kirchbauer D, Ogorodnikova OB, Sineshchekov VA, Adam E, Kircher S, Staneloni RJ, Schäfer E, Nagy F, Casal JJ (2002) Missense mutation in the PAS2 domain of phytochrome A impairs subnuclear localization and a subset of responses. Plant Cell (in press) [DOI] [PMC free article] [PubMed]
- Yanovsky MJ, Whitelam GC, Casal JJ. fhy3-1 retains inductive responses of phytochrome A. Plant Physiol. 2000;123:235–242. doi: 10.1104/pp.123.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]