Abstract
Glycine is known to modulate immune cell responses. However, the physiological mechanisms underlying inhibitory effects of glycine on macrophages are not well understood. Here we show that glycine is capable of inducing inward currents in brain macrophages (microglia). In contrast to glycine, the glycine receptor agonist taurine failed to elicit currents. Glycine-evoked currents of brain macrophages were unaffected by strychnine, Cl−-free extracellular solution, N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl])sarcosine (NFPS) and amoxapine, but were abolished upon omission of extracellular Na+. Furthermore, glycine caused increases in the intracellular Na+ concentration and pronounced membrane depolarization. Glycine-evoked depolarization was Na+ dependent and occurred independently of the intracellular Cl− concentration. Similarly to glycine, glutamine and α-(methylamino)isobutyric acid (MeAIB) elicited inward currents in brain macrophages. In the presence of either glutamine or MeAIB, glycine-induced currents were inhibited. It is concluded that neither functional glycine receptors nor glycine transporters are expressed in brain macrophages. We suggest that glycine mediates its effects by activation of system A Na+-coupled neutral amino acid transporters.
Glycine has inhibitory effects on immune cells, including macrophages, monocytes, neutrophils and lymphocytes. Numerous in vitro and in vivo studies have demonstrated that millimolar concentrations of glycine reduce agonist-stimulated Ca2+ signals and attenuate the generation of reactive oxygen species, the production of proinflammatory cytokines, phagocytic activity and cell proliferation (for review, see Zhong et al. 2003). The physiological mechanisms by which glycine prevents immune cell activation are not well understood. It has been suggested that glycine causes activation of strychnine-sensitive glycine receptors and subsequent membrane hyperpolarization due to Cl− influx through glycine receptor-coupled Cl− channels. This glycine-induced reduction of agonist-stimulated [Ca2+]i signals has been attributed to inhibition of L-type Ca2+ channels due to membrane hyperpolarization (Ikejima et al. 1997; Wheeler & Thurman, 1999; Zhong et al. 2003).
However, the presence of either functionally active glycine receptors or L-type Ca2+ channels has not been demonstrated convincingly in any type of immune cells by patch-clamp recordings. In contrast, it has been proven that in macrophages and other immune cells the Ca2+ influx from the extracellular space is mediated by either Ca2+ release-activated Ca2+ (CRAC) channels or non-selective cation channels (Lewis & Cahalan, 1989; Nörenberg et al. 1997; Hahn et al. 2000; Lewis, 2001; Prakriya & Lewis, 2003; Schilling et al. 2004). Furthermore, a variety of previous studies has demonstrated that membrane depolarization rather than hyperpolarization down-regulates immune cell activity (for reviews, see Eder, 1998; Cahalan et al. 2001; Lewis, 2001; Chandy et al. 2004).
In order to clarify this controversy, we have investigated the effects of glycine on brain macrophages using patch-clamp and fluorescence imaging techniques. Here we demonstrate for the first time that glycine activates system A Na+-coupled neutral amino acid transporters in macrophages. Functional glycine receptors were not detected in our study. We suggest that membrane depolarization induced by glycine-activated transporter currents leads to immune cell inhibition.
Methods
Cells
The immortalized mouse brain macrophage cell line BV-2 (kindly provided by Dr E. Blasi, Perugia, Italy) was used in all experiments. BV-2 brain macrophages were cultured permanently in tissue culture flasks at a density of 2 × 106 (20 ml)−1 in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco) and 2 mm l-glutamine. BV-2 cell cultures were split twice a week, and were plated on glass coverslips at a density of 5 × 103 (0.5 ml)−1 for subsequent patch-clamp and imaging experiments.
Electrophysiological recordings
Patch-clamp experiments and data analyses were performed as previously described (Schilling et al. 2004). The electrodes were filled with the following solution (high Cl−-containing intracellular solution I1) containing (mm): KCl, 120; CaCl2, 1; MgCl2, 2; Hepes, 10; EGTA, 11 (pH 7.3). In some experiments, this solution contained additionally 4 mm MgATP. In some other cases, the Cl− concentration was reduced from 126 to 4 mm by substitution of KCl with potassium gluconate and CaCl2 with calcium gluconate (low Cl−-containing intracellular solution I2). The extracellular solution contained (mm): NaCl, 130; KCl, 5; CaCl2, 2; MgCl2, 1; Hepes, 10; d-glucose, 10 (pH 7.4). In some cases, Na+ was substituted by N-methyl-d-glucamine (NMG+) or Cl− was substituted by gluconate. The change of the extracellular Cl− concentration around the cell did not affect the extracellular Cl− concentration around the ground electrode, because solutions were applied by a local microperfusion pipette (see below). Electrode junction potentials (−3 mV for the KCl-based internal solution I1 and −8 mV for the potassium gluconate-based internal solution I2) were routinely compensated for. All recordings were done at room temperature (20–23°C). Data are presented as mean values ± standard error of the mean (s.e.m.). The numbers of experiments are indicated.
Glycine concentration–response data were fitted by the following Michaelis-Menten equation:
where I is the current, Imax is the maximum current, [Gly]o is the glycine concentration and Km is the concentration that generates half-maximal current. Before curve fitting, current amplitudes were normalized to the current induced by 10 mm glycine.
Na+ imaging
Brain macrophages were loaded with 10 μm sodium-binding benzofuran-isophthalate acetoxymethyl ester (SBFI-AM, Molecular Probes, Eugene, OR, USA) in extracellular solution for 60 min at room temperature (20–23°C). After washing, coverslips were mounted in a chamber on an inverted Olympus IX 50 microscope equipped with a water immersion objective (40× UApo/340; Olympus Optical Co. GmbH, Hamburg, Germany). The fluorescence imaging system consisted of a monochromator, a charge-coupled device (CCD) camera and the Windows NT based image processing software (Till Photonics, München, Germany). Brain macrophages were exposed to alternating 340 ± 5 and 380 ± 5 nm wavelengths of UV light and emission light was passed through a 400 nm dichroic mirror and a 420 nm long pass emission filter (both Olympus) prior to acquisition by the CCD camera. Images were collected every 20 s. The ratio of the two background-corrected fluorescence intensities was converted to the intracellular Na+ concentration ([Na+]i) of a single cell according to the following equation:
where Kd = 11.3 mm is the dissociation constant of SBFI and R is the measured ratio. Rmin (0.759) and Rmax (1.639) were determined using 5 μm gramicidin, 10 μm monensin and 10 μm ouabain in extracellular solution containing either 0 or 130 mm Na+, and β (2.018) was calculated as the ratio of the fluorescence intensities at 380 nm in 0 and 130 mm Na+.
Drug application
In patch-clamp experiments, cells were superfused continuously with control solution or with solutions containing glycine, taurine, strychnine, l-glutamine, N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy)propyl]) sarcosine (NFPS), amoxapine or α-(methylamino)isobutyric acid (MeAIB) (all from Sigma, Germany). These solutions were applied using a four-barrel microperfusion pipette, positioned at a distance of about 30–50 μm from the recorded cell to permit a rapid exchange of solutions. The flow rate was adjusted by hydrostatic pressure. In Na+ imaging experiments, drug application was achieved by complete exchange of the bath solution.
Results
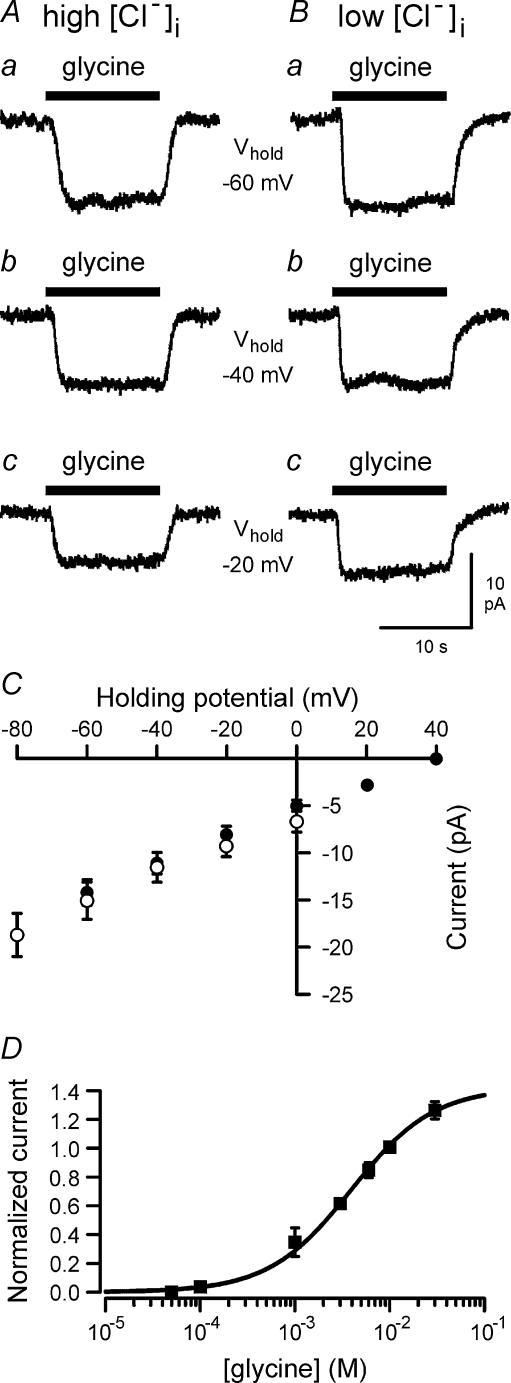
To investigate the effects of glycine on brain macrophages, the patch-clamp technique was applied in the whole cell configuration. Application of glycine to brain macrophages generated inward currents. Figure 1 shows glycine-evoked currents, which were recorded using intracellular solutions I1 (high Cl− containing) or I2 (low Cl− containing), while cells were clamped at different holding potentials. Glycine-evoked inward currents were independent of the concentration of intracellular Cl− ([Cl−]i). After lowering [Cl−]i from 126 to 4 mm by equimolar substitution of Cl− with gluconate, current amplitudes did not differ significantly from those measured with high Cl−-containing intracellular solution (Fig. 1C). At −60 mV, a mean current amplitude of −14.4 ± 1.2 pA (n = 9) was determined using intracellular solution I1 with 126 mm [Cl−]i, which was not significantly different from that of −15.2 ± 2 pA (n = 6) determined for cells measured with intracellular solution I2 with 4 mm[Cl−]i. The current–voltage relationship of glycine-evoked currents was roughly linear between −80 and +40 mV as shown in Fig. 1C. Outward currents were not observed, i.e. glycine-evoked inward currents did not reverse at test potentials of up to +40 mV. Glycine-evoked inward currents were detected first at a concentration of 100 μm glycine. At 50 μm glycine, currents were not generated in cells studied either with (n = 5) or without (n = 4) 4 mm MgATP in the pipette solution. The concentration–response curve for the effects of glycine on brain macrophages is shown in Fig. 1D. Half-maximal current activation occurred at a concentration of 3.9 mm glycine.
Figure 1. Voltage dependence of glycine-evoked inward currents in brain macrophages.
Currents were elicited by 10 mm glycine using either high Cl−-containing intracellular solution I1 (A) or low Cl−-containing intracellular solution I2 (B). Cells were clamped at holding potentials of −60 mV (a), −40 mV (b) or −20 mV (c). C, amplitudes (mean ± s.e.m.) of glycine-evoked currents measured using intracellular solution I1 (n = 9; •) or I2 (n = 6; ○) are plotted as a function of holding potential. D, concentration–response curve for glycine-evoked inward currents. For each cell, current amplitudes were normalized to the current induced by 10 mm glycine. Data points represent mean values (± s.e.m.) of 4–12 individual cells. Cells were held (Vhold) at −60 mV.
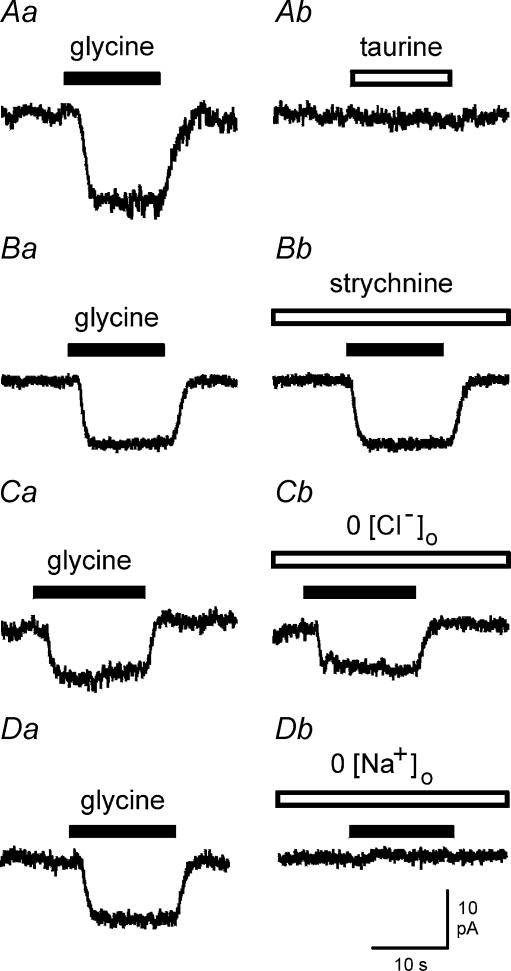
In further experiments brain macrophages were clamped at −60 mV, a potential close to the resting membrane potential of the cells. In contrast to glycine, the glycine receptor agonist taurine failed to induce inward currents when applied at concentrations of up to 10 mm (Fig. 2A). As shown in Fig. 2B, glycine-evoked inward currents of brain macrophages were unaffected by strychnine, a selective inhibitor of glycine receptors. No significant differences were detected between glycine currents recorded in the absence or presence of 1 μm strychnine (n = 5 using ATP-free intracellular solution; n = 3 using ATP-containing intracellular solution). In further experiments, we tested whether glycine-induced currents were influenced by superfusion of brain macrophages with Cl−-free extracellular solution. As shown for the example in Fig. 2C, glycine-evoked inward currents were almost identical in Cl−-containing and Cl−-free extracellular solutions (n = 6). Figure 2D demonstrates effects of glycine on brain macrophages measured in either Na+-containing (Fig. 2Da) or Na+-free (Fig. 2Db) extracellular solution. Glycine failed to generate currents during superfusion of cells with Na+-free solution (n = 9 using ATP-free intracellular solution; n = 4 using ATP-containing intracellular solution). These observations point to a mechanism of Na+-coupled glycine transport in brain macrophages.
Figure 2. Properties of glycine-evoked inward currents in brain macrophages.
A, typical current recordings of a cell exposed to 10 mm glycine (Aa) and 10 mm taurine (Ab). B, current traces elicited by 10 mm glycine in the absence (Ba) and presence (Bb) of 1 μm strychnine. C, glycine-evoked currents measured in Cl−-containing (Ca) and Cl−-free (Cb) extracellular solution. D, effects of 10 mm glycine in Na+-containing (Da) and Na+-free (Db) extracellular solution. Holding potential was −60 mV.
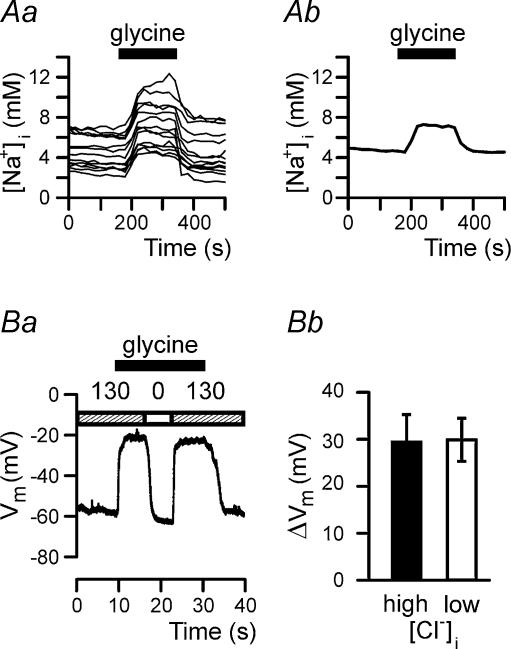
To investigate whether glycine induces Na+ influx in brain macrophages, fluorescence imaging experiments were performed using the ratiometric dye SBFI. As shown in Fig. 3A, SBFI fluorescence is enhanced in the presence of glycine, demonstrating that glycine application caused increases in the concentration of intracellular sodium ions ([Na+]i) in brain macrophages. Increases in [Na+]i were detected at glycine concentrations of at least 100 μm. At 10 mm glycine, [Na+]i increased from 4.95 ± 0.17 to 7.92 ± 0.28 mm (n = 114).
Figure 3. Effect of glycine on intracellular Na+ concentration and membrane potential of brain macrophages.
Aa, changes in intracellular Na+ concentration of 14 individual brain macrophages following application of 10 mm glycine. Ab, averaged fluorescence signal of the 14 cells shown in Aa. Ba, glycine-induced membrane depolarization in the presence (130) and absence (0) of extracellular Na+. The patch pipette contained intracellular solution I2. Bb, changes in membrane potential (mean ± s.e.m.) induced by 10 mm glycine determined in brain macrophages using intracellular solution I1 (high [Cl−]i; filled bar) or I2 (low [Cl−]i; open bar).
To prove further the hypothesis that glycine activates Na+-coupled amino acid transporters rather than glycine receptors, current-clamp recordings were performed to monitor changes in membrane potential of brain macrophages. As expected for an electrogenic Na+-coupled transporter, glycine evoked sustained membrane depolarization, which was reversed in Na+-free extracellular solution (Fig. 3Ba). On average, the membrane potential of brain macrophages depolarized by 29.6 ± 5.9 mV (n = 4) following application of 10 mm glycine using high Cl−-containing intracellular solution I1. Almost identical membrane potential changes (30.0 ± 4.6 mV; n = 6) were determined in cells internally perfused with low Cl−-containing intracellular solution I2 (Fig. 3Bb).
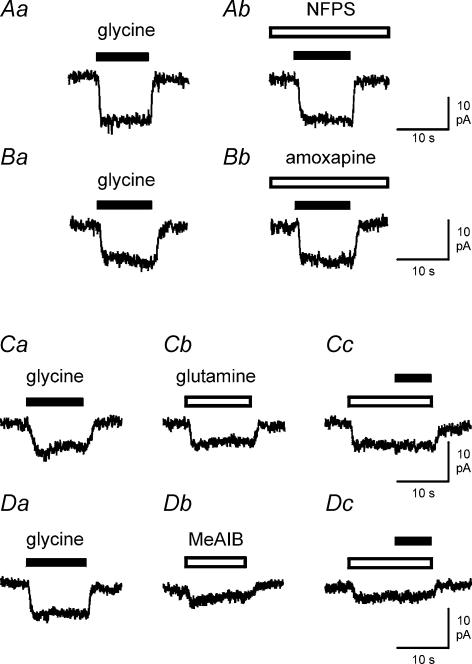
Glycine-evoked currents in brain macrophages were unaffected by inhibitors of glycine transporters. Figure 4A demonstrates the lack of effects of NFPS (1 μm; n = 5), a selective inhibitor of glial glycine transporters GLYT1, on brain macrophages. Amoxapine (100 μm; n = 5), an inhibitor of neuronal glycine transporters GLYT2, also failed to inhibit glycine responses in brain macrophages (Fig. 4B). In further experiments, we investigated effects of glutamine and MeAIB on brain macrophages assuming that glycine-evoked responses were mediated by activation of system A neutral amino acid transporters. As shown in Fig. 4C, 10 mm glutamine elicited inward currents (n = 7) similar to those seen in response to glycine application. In the presence of glutamine, glycine did not evoke additional currents (n = 7; Fig. 4Cc). At 10 mm, MeAIB, a specific substrate of system A neutral amino acid transporters, also generated inward currents in brain macrophages (n = 5; Fig. 4D). In MeAIB-containing extracellular solution, glycine failed to induce further inward currents in brain macrophages (n = 5) as demonstrated in Fig. 4Dc.
Figure 4. Effects of NFPS, amoxapine, glutamine and MeAIB on glycine-evoked currents in brain macrophages.
A and B, lack of effects of glycine transporter inhibitors. A, inward currents induced by 10 mm glycine in the absence (Aa) or presence (Ab) of 1 μm NFPS. B, current traces elicited by 10 mm glycine in the absence (Ba) or presence (Bb) of 100 μm amoxapine. C, inward currents of a cell exposed to 10 mm glycine (Ca) and 10 mm glutamine (Cb). In the presence of 10 mm glutamine, glycine failed to evoke additional inward currents (Cc). D, effects of 10 mm glycine (Da) and 10 mm MeAIB (Db) on a brain macrophage. During application of 10 mm MeAIB, inward currents could not be augmented by 10 mm glycine (Dc). Holding potential of all cells was −60 mV.
Discussion
In contrast to a previously suggested hypothesis (Zhong et al. 2003), functional glycine receptors were not detected in our study on macrophages. The following experimental evidence argues against the presence of glycine receptors: First, glycine-evoked currents of brain macrophages were Na+ dependent and Cl− independent. Second, the currents were unaffected by the glycine receptor antagonist strychnine. Third, the glycine receptor agonist taurine failed to generate currents. The difference in glycine receptor expression between peripheral (Ikejima et al. 1997; Wheeler & Thurman, 1999) and brain macrophages (present study) can be explained by the difference in cell types investigated. It is also possible that expression of glycine receptors depends on the functional state of macrophages as has been reported for various ion channel types in peripheral and brain macrophages (for review, see Eder, 1998). Further experiments are required to clarify under which conditions functional glycine receptors are expressed by macrophages. In brain macrophages, glycine caused membrane depolarization and increases in the intracellular Na+ concentration. These findings led us further to conclude that glycine activates Na+-coupled amino acid transporters in brain macrophages.
Two major types of specific glycine transporters have been identified, namely GLYT1 and GLYT2 (for reviews, see Roux & Supplisson, 2000; Tunnicliff, 2003). However, it is unlikely that GLYT1 and/or GLYT2 are expressed by brain macrophages. Both glycine transporters are Na+ and Cl− dependent, whereas glycine-evoked currents in brain macrophages did not exhibit any dependence on extra- and intracellular Cl−. Furthermore, glycine-evoked currents in brain macrophages were unaffected by the GLYT1 inhibitor NFPS or by the GLYT2 inhibitor amoxapine. We suggest that the glycine responses of brain macrophages are mediated by activation of system A neutral amino acid transporters, i.e. SNAT1, SNAT2 and/or SNAT4. This conclusion is based on the observations that in brain macrophages (i) glutamine and MeAIB elicited inward currents similar to those evoked by glycine, and (ii) glycine-evoked currents were not induced in the presence of either MeAIB or glutamine. System A transporters are electrogenic, Na+ dependent and Cl− independent. They take up glutamine and MeAIB and are also capable of carrying glycine (for reviews, see Bode, 2001; Mackenzie & Erickson, 2004). Our data do not allow us to identify which of the system A transporters, SNAT1, SNAT2 or SNAT4, is involved in the glycine responses of brain macrophages. None of these transporters has been described before in any macrophage preparation. Further experiments are required to clarify the molecular identity of the system A transporter via which glycine exerts its effects on brain macrophages.
Glycine concentrations required to evoke inward currents and [Na+]i increases in brain macrophages (≥ 100 μm) are relatively high, and are not reached extracellularly in the central nervous system (CNS) under normal physiological conditions (Billups & Attwell, 2003). However, a wide variety of brain disorders, such as trauma, ischaemia, epilepsy and others, are accompanied by neuronal and glial cell death. Intracellular glycine concentrations are assumed to be 10 mm in neurones (Roux & Supplisson, 2000) and 2 mm in glial cells (Attwell et al. 1993; Roux & Supplisson, 2000). Thus, release of glycine from dying cells will most likely cause increases in the extracellular glycine concentration to values that are sufficient to activate system A neutral amino acid transporters in brain macrophages. Thus, we suggest that glycine modulates the immune responses of brain macrophages under pathological conditions of the CNS that are accompanied by cell death. Activated brain macrophages in the injured CNS appear to be less responsive than activated brain macrophages in cell culture (Hurley et al. 1999). In the CNS, glycine may contribute to mechanisms preventing irreversible hyperactivation of brain macrophages, which may cause neurodegeneration or persistent inflammation (Nakamura, 2002). Glycine-mediated reduced production of neurotoxic substances by activated brain macrophages may help to prevent secondary damage to healthy neurones in the immediate vicinity of damaged brain tissue.
In contrast to a previous hypothesis (Zhong et al. 2003), we suggest that glycine exerts its inhibitory effects on immune cells by depolarizing the cell membrane. Glycine-induced depolarization will lead to an inhibition of agonist-induced Ca2+ signals that are required for the activation processes of immune cells (for reviews, see Eder, 1998; Cahalan et al. 2001; Lewis, 2001). In brain macrophages and other immune cells, Ca2+ influx through CRAC channels or non-selective cation channels is reduced by membrane depolarization due to the reduction of the driving force for Ca2+ entry (Prakriya & Lewis, 2003; Schilling et al. 2004). Our hypothesis that glycine-induced depolarization down-regulates the activity of brain macrophages is supported by previous findings. It has been demonstrated that membrane depolarization induced by either elevated extracellular K+ concentration or Ca2+-dependent K+ channel blockade causes inhibition of interleukin-1β release and of superoxide production by activated brain macrophages (Sanz & Di Virgilio, 2000; Khanna et al. 2001).
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft grants SFB 507-C7, GRK 238 and a Heisenberg fellowship (all to C.E).
References
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D. Active release of glycine or d-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. Eur J Neurosci. 2003;18:2975–2980. doi: 10.1111/j.1460-9568.2003.02996.x. [DOI] [PubMed] [Google Scholar]
- Bode BP. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131:2475S–2485S. doi: 10.1093/jn/131.9.2475S. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Wulff H, Chandy KG. Molecular properties and physiological roles of ion channels in the immune system. J Clin Immunol. 2001;21:235–252. doi: 10.1023/a:1010958907271. [DOI] [PubMed] [Google Scholar]
- Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am J Physiol. 1998;275:C327–C342. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- Hahn J, Jung W, Kim N, Uhm DY, Chung S. Characterization and regulation of rat microglial Ca2+ release-activated Ca2+ (CRAC) channel by protein kinases. Glia. 2000;31:118–124. [PubMed] [Google Scholar]
- Hurley SD, Walter SA, Semple-Rowland SL, Streit WJ. Cytokine transcripts expressed by microglia in vitro are not expressed by ameboid microglia of the developing rat central nervous system. Glia. 1999;25:304–309. doi: 10.1002/(sici)1098-1136(19990201)25:3<304::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Ikejima K, Qu W, Stachlewitz RF, Thurman RG. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272:G1581–G1586. doi: 10.1152/ajpgi.1997.272.6.G1581. [DOI] [PubMed] [Google Scholar]
- Khanna R, Roy L, Zhu X, Schlichter LC. K+ channels and the microglial respiratory burst. Am J Physiol. 2001;280:C796–C806. doi: 10.1152/ajpcell.2001.280.4.C796. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Annu Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447:784–795. doi: 10.1007/s00424-003-1117-9. [DOI] [PubMed] [Google Scholar]
- Nakamura Y. Regulating factors for microglial activation. Biol Pharm Bull. 2002;25:945–953. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- Nörenberg W, Cordes A, Blohbaum G, Frohlich R, Illes P. Coexistence of purino- and pyrimidinoceptors on activated rat microglial cells. Br J Pharmacol. 1997;121:1087–1098. doi: 10.1038/sj.bjp.0701241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1β release from microglial cells. J Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Schilling T, Lehmann F, Rückert B, Eder C. Physiological mechanisms of lysophosphatidylcholine-induced de-ramification of murine microglia. J Physiol. 2004;557:105–120. doi: 10.1113/jphysiol.2004.060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliff G. Membrane glycine transport proteins. J Biomed Sci. 2003;10:30–36. doi: 10.1007/BF02255994. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Thurman RG. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am J Physiol. 1999;277:L952–L959. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]