Abstract
This study was designed to test the hypothesis that the frequency response of the systems controlling the motor activity of breathing and walking in quadrupeds is compatible with the idea that supra-spinal locomotor centres could proportionally drive locomotion and ventilation. The locomotor and the breath-by-breath ventilatory and gas exchange (CO2 output (V̇CO2) and O2 uptake (V̇CO2)) responses were studied in five sheep spontaneously walking on a treadmill. The speed of the treadmill was changed in a sinusoidal pattern of various periods (from 10 to 1 minute) and in a step-like manner. The frequency and amplitude of the limb movements, oscillating at the same period as the treadmill speed changes, had a constant gain with no phase lag (determined by Fourier analysis) regardless the periods of oscillations. In marked contrast, when the periods of speed oscillations decreased, the amplitude (peak-to-mean) of minute ventilation (V̇E) oscillations decreased sharply and significantly (from 6.1 ± 0.4 l min−1 to 1.9 ± 0.2 l min−1) and the phase lag between ventilation and treadmill speed oscillations increased (to 105 ± 25 ° during the 1 min oscillation periods). V̇E response followed V̇CO2 very closely. The drop in V̇E amplitude ratio was proportional to that in V̇CO2 (from 149 ± 48 ml min−1 to 38 ± 5 ml min−1) with a slightly longer phase lag for ventilation than for V̇CO2. These results show that beyond the onset period of a locomotor activity, the amplitude and phase lag of the V̇E response depends on the period of the walking speed oscillations, tracking the gas exchange rate, regardless of the amplitude of the motor act of walking. Locomotion thus appears unlikely to cause a simple parallel and proportional increase in ventilation in walking sheep.
Walking, like any sort of muscular activity, increases breathing (Dejours, 1959; Kay et al. 1975; Loring et al. 1990). The idea that the motor activity of walking could, per se, account significantly for such a rise in ventilation assumes that either the consequences of the contractions, through mechanosensitive muscle afferent fibres (Comroe & Schmidt, 1943; Dejours, 1959), or the central mechanisms of motor control involved in the generation of the rhythmic movements increase breathing (Eldridge & Waldrop, 1991). Belonging to this category of mechanisms, a very well-accepted hypothesis proposes that the supra-spinal sites, which may control the spinal pattern generators for locomotion, also stimulate breathing (Eldridge & Waldrop, 1991 for review). Direct evidence for such a simple parallel activation has been obtained by stimulating electrically or chemically the hypothalamic and mesencepahlic locomotor regions in decerebrate cats, which indeed results in a combined activation of locomotion and ventilation (Eldridge et al. 1981). As the increase in the ventilatory and locomotor activities during stimulation of the hypothalamic and mesencepahlic locomotor regions resembles the response observed in spontaneously walking animals, it was proposed that a major central feedforward command of ventilation – and circulation (Smith et al. 1960) – originates from these supra-spinal structures coupling the motor act of locomotion and breathing.
One of the main arguments formulated against an important role for such a control mechanism (Whipp, 1981) is that it does not fit in with: (1) the description of the kinetics of the V̇E response to a dynamic exercise of moderate intensity; and (2) its close link to the change in the gas exchange rate, thus maintaining arterial blood gas homeostasis (Casaburi et al. 1978; Whipp et al. 1982; Whipp & Ward, 1991). Indeed, unlike the steady hypocapnic V̇E – or phrenic nerve – response to actual or fictive locomotion triggered by hypothalamic stimulation (HS), the V̇E on- and off-transient response to a dynamic exercise of constant and moderate intensity displays a progressive increase (V̇E phase II) and decline which has a 60–75 s time constant, regardless of the time course of the locomotor response (Fujihara et al. 1973a,b; Casaburi et al. 1977; Bakker et al. 1980; Whipp et al. 1982; Wasserman et al. 1986; Haouzi et al. 1992).
However, it has been argued that an important role for hypothalamic command is not incompatible with such a long time constant of the ventilatory outcome. Indeed the existence of a short-term potentiation (STP) phenomenon at respiratory neurone level may induce a progressive rise in V̇E towards a steady state despite the stimulus to breath being constant (Waldrop et al. 1996 for review). This phenomenon has been demonstrated in several species during and following various types of peripheral stimulation (Eldridge & Gill-Kumar, 1980; Millhorn et al. 1980; Eldridge et al. 1982; Wagner & Eldridge, 1991; Engwall et al. 1994).
If the concept of a central command that could couple locomotion and ventilation applies to physiological conditions, it implies that a significant component of the ventilatory response to locomotion should be proportional to, and in phase with, the motor response. Previous attempts to identify such a component in other forms of muscular activity than locomotion have not been very conclusive (Whipp, 1981 for review). Indeed, as illustrated by the analysis of the V̇E response of human subjects exercising on a cyclo-ergometer with sinusoidal changes in work rate (WR), the amplitude of the V̇E response decreases and the phase lag between work rate oscillations and ventilation increases when the WR oscillation periods decrease despite similar WR amplitude (Casaburi et al. 1977; Bakker et al. 1980; Haouzi et al. 1992). No evidence for a ventilatory component in phase with the WR oscillations has been found in these studies.
The central respiratory drive associated with locomotion has been described in quadrupeds (mainly in cats) during subthalamic stimulation (Eldridge et al. 1981). The question therefore remains open as to whether the mechanisms that control breathing in quadrupeds during walking could rely on more important central feedforward components linked to locomotor activation than in cycling humans.
Therefore this study was designed to determine whether the frequency response of the systems controlling ventilation and locomotion during walking in sheep is compatible with the idea of a neural command signal which could proportionally drive the motor act of walking and breathing. More specifically, we sought to establish whether a temporal relationship exists between the V̇E and the locomotor responses in walking sheep by changing, in a sinusoidal manner, the speed of a treadmill at various periods.
Methods
Five female sheep (age, 13 ± 2 months; weight, 35 ± 3 kg) were studied. All the experiments were done according to the recommendations of the Council of European Communities and under licences from the Ministère de l'Agriculture et de la Pêche (registration number 54–42). In addition, the animals were kept in an animal facility that was approved by the Ministère de l'Agriculture et de la Pêche.
The five animals were selected from a group of nine sheep. They were trained, on a daily basis, for a period of about 2 months before the study, to walk and trot on a treadmill. They were all very well acclimated to the laboratory and familiarized with the equipment, and were able to walk quietly on the treadmill with reliable breath-by-breath ventilatory measurements. Since the end of the experiments, the animals have been kept in the animal facility.
The animals breathed through a low-dead-space mask adapted to each animal. The respiratory masks, which were made of silicone and a plastic frame, were built using a cast for each sheep. The mask was adjusted and attached to two different points behind the occipital bone and the vertex using several elastic straps. A rubber band was applied at the proximal part of the mask to prevent leakage as already reported (Haouzi et al. 2000).
A calibrated pneumotachograph connected to a differential pressure transducer (Valydine MP, 45) allowed the measurement of inspiratory and expiratory airflow. The flow signal was digitized and integrated for breath-by-breath calculation of tidal volume and minute ventilation (V̇E) at body temperature and pressure when saturated with water vapour (BTPS) (Modified CPX, Medical Graphics system, Medical Graphics Corporation, Saint Paul, MN). Gas was drawn from the mid part of the pneumotachograph (at a flow rate of about 110 ml min−1) and the fractions of O2 and CO2 (FO2 and FCO2) were determined by zirconium and infrared fast responding analysers (Datex Analysers, Datex-Ohmeda Division, Instrumentation Corp. Helsinki, Finland). Calibration was performed prior to each test using two gases of known O2 and CO2 concentrations. In addition, the gas transport delay and the response time of the analysers were determined using a step change in gas concentration. V̇O2 and V̇CO2 were determined for each breath at STPD by integrating the product of the fractions of expired O2 and CO2 (FEO2 or FECO2) and flow after correction for the gas transport delay and response time of the gas analysers. The signal controlling the speed of the motor driving the treadmill (modified TMS 2200, Leroy-Somer, Angoulème, France) was delivered by a microcomputer through a digital–analog converter (Digimetrix, PC-DAC12B-4VC, Digimetrie, Perpignan, France).
Protocol
Two questions needed to be clarified prior to the study: (1) the delay between food ingestion and the tests needed to reduce the effects of rumination and eructation, which interferes with the ventilatory measurements; (2) the time needed to reach a steady state before starting the sinusoidal exercise.
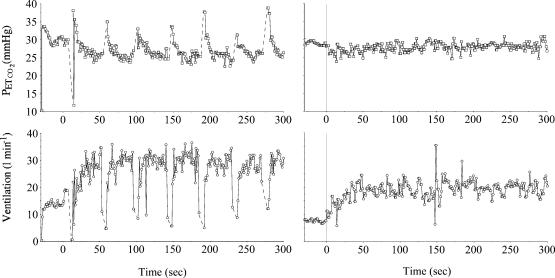
We found that in order to prevent belching and frequent regurgitation episodes, a 30–32 h fasting period was needed. This was very consistent in each sheep. Figure 1 shows typical ventilatory data obtained 12 h and 30 h following food ingestion. The animals were fed in the morning then fasted for 30–32 h before the tests (with water provided ad libitum). Whenever the animals couldn't stay still or had a variable breathing pattern (due to too many swallowing episodes, micturition or defecation during the walking session) the protocol was interrupted. This occurred in about 20% of the tests.
Figure 1. Example of end tidal PCO2 and ventilatory responses during a walking test (4.5 km h−1 from rest) 12 h (right panels) and 30 h (left panels) after food ingestion in one sheep.
Note that within the first 18 h, a large number of episodes of eructation (rise in PETCO2) and swallowing related to rumination provoked rhythmic hypoventilation and hyperventilation. After 30 h, ventilation was stable allowing reproducible measurements.
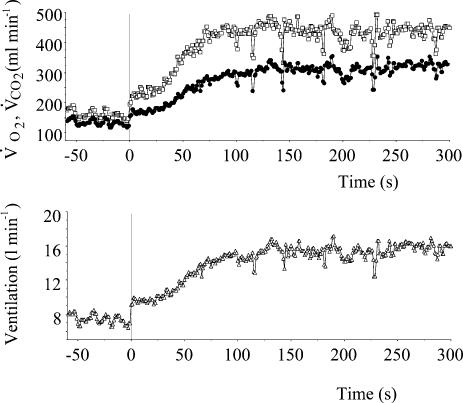
A period of at least 3 min of stable resting ventilation was required before starting the exercise. As illustarted in Figs 1 and 2, the V̇E kinetics were much faster than in humans (Whipp et al. 1982), ventilation reaching a steady state within less than 2 min.
Figure 2. Temporal profile of the averaged pulmonary gas exchange (V̇O2, open symbols V̇CO2 filled symbols) and ventilatory response to a walking test (4.5 km h−1) of the five sheep (10 tests).
Exercise starts at the vertical line. Note that ventilation reached a steady state within less than 2 min
After 3 min of constant speed of walking at the mid-point of the sinusoid (75 m min−1), the treadmill speed was changed with a sinusoidal pattern from 50 to 100 m min−1 at a period (T) of 10, 5, 2 and 1 min and in a stepwise manner (steady state). Each sinusoidal period of oscillation was studied on a separate occasion (one session at a time, two sessions per week for each sheep). At least three tests were performed for each frequency for a duration of 10–15 min.
The amplitude and frequency of the forelimb movements (at elbow level) were determined by goniometry analysis (Two axis goniometer, Penny and Jiles, Blackwood-Gwent, UK). The signals from the treadmill and the goniometer were fed into a data acquisition system (MacLab system, ADinstruments, Castle Hill, Australia) and temporally aligned to the ventilatory data. All the data were analysed using Fourier analysis as previously reported (Haouzi et al. 1992). The variation in the speed of the treadmill was regarded as the input function. The amplitude (A; i.e. mean to peak) and the phase lag (L) of the fundamental component (same frequency as the input function) of the V̇E, V̇O2, V̇CO2 and end tidal PCO2 (PETCO2) responses as well as the locomotor response (movement frequency and amplitude) were computed as follows:
Where Re and Im are the real and imaginary parts of the response determined after second-by-second interpolation of the respiratory and locomotor (x) responses as:
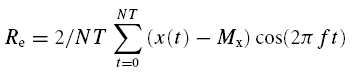 |
and
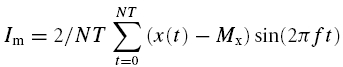 |
Where x (t) is the response value as time t (in seconds), Mx is the mean value of x for an integer number of cycle (N), T is the period of the input signal (in seconds) and f (= 1/T) is its frequency in cycles per second.
Fourier analysis was started 3 min into the onset of the speed oscillations assuming that the factor 2πfe (−t/τ) was close to zero when t = 3 min, that is tau, the time constant of the ventilatory response, being less than 1 min (see Figs 1 and 2).
The fundamental component (F) of the response of each variable was computed as follows:
F(T) was superimposed on the raw data, and the residuals (res) were computed as
In addition to Fourier analysis, a linear regression analysis was applied between the residuals and the treadmill speed oscillations. If a significant correlation is to be found, the correlation coefficient r would be related to the phase lag (L) between these two variables by the relationship:
The amplitude and phase lag were compared between the different frequencies of oscillations for each variable (V̇E, V̇O2, V̇CO2, V̇ETCO2, and the locomotor response) using the non-parametric H test of Kruskall and Wallis (Schwartz, 1986). The value of H (after correction for similar values) and the corresponding P values are given in the text for each variable.
Results
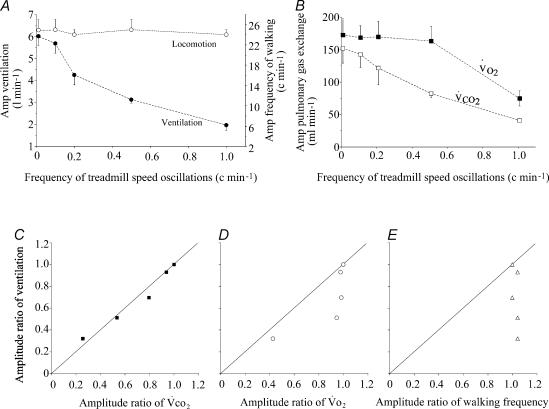
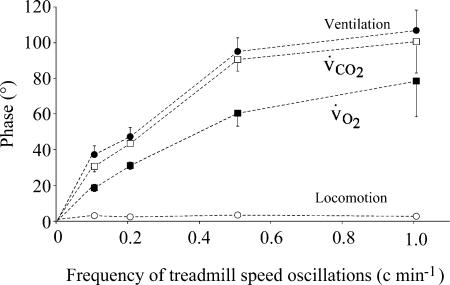
The locomotor response mainly consisted of an increase in the frequency of walking. The frequency of limb movements followed, with a sinusoidal pattern, the changes in treadmill speed (Figs 3 and 4). The amplitude of the walking frequency oscillations was similar at each period (49 ± 2 cycles min−1) and the phase lag between the changes in treadmill speed and walking frequency was virtually zero, even for the fastest oscillations (1.6 ± 0.7 °) demonstrating that the system controlling locomotion has a flat frequency response (Figs 3–5 and 7). The changes in the amplitude of movements during the sinusoidal walking tests were very small and in perfect phase with the speed of the treadmill speed even at the highest frequencies of oscillations (Fig. 3). In contrast, in all the animals and tests, the V̇E response displayed a dramatic and statistically significant reduction in amplitude (H = 16.01, P = 0.0011) and increase in phase lag with the locomotor activity (H = 11.02, P = 0.01) when the period of oscillations of the treadmill speed decreased (Figs 4, 5A and 5B). For instance, during the 1 min oscillation period, the amplitude of the V̇E response decreased from 6.1 ± 0.4 l min−1 (during constant work rate exercise) to 1.9 ± 0.2 l min−1 (−67%) and the phase lag between the locomotor and the ventilatory response increased to 105 ± 25 °.
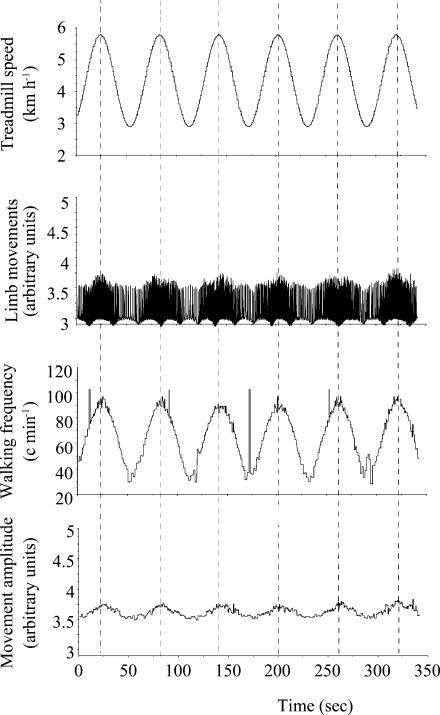
Figure 3. Example in one sheep of the locomotor response during walking with the fastest speed oscillations used in the study, i.e. 1 min period (from 3 to 6 km h−1).
From top to bottom: speed imposed by the treadmill, signal from the goniometers, frequency and amplitude of movement (in arbitrary units) obtained from the goniometric raw data. Note that (1) the increase in walking speed consists mostly of a sinusoidal change in the frequency of walking and (2) both the changes in the frequency and the amplitude of movement are in perfect phase with the treadmill speed oscillations.
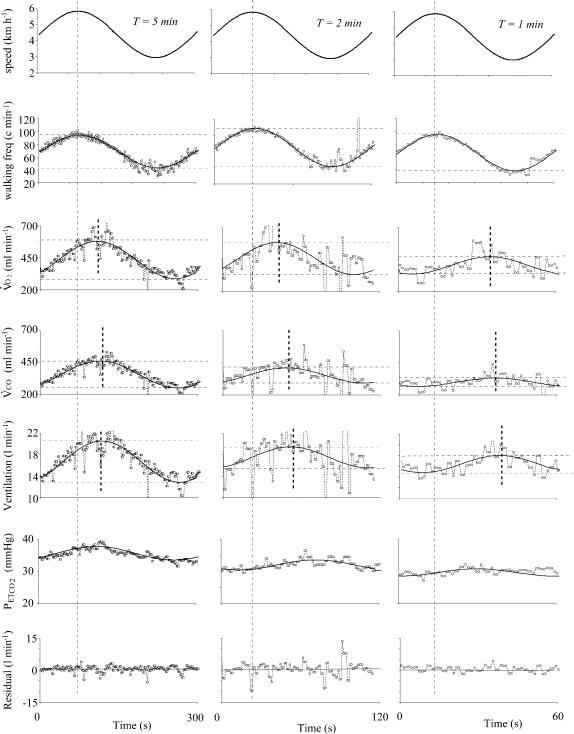
Figure 4. Example in one sheep of the locomotor (frequency of movement), V̇O2, V̇CO2, ventilation and PETCO2 responses to three different treadmill speed oscillation periods.
The fundamental component of the responses computed by Fourier analysis is superimposed on the raw data and the residuals for ventilation (after suppression of the fundamental component of the response) are shown in the lower panel. Note that the change in walking frequency is in phase with the sinusoidal changes in the speed of the treadmill with a constant amplitude. There is a clear reduction in amplitude of both pulmonary gas exchange and ventilation when the oscillation period decreases whereas the phase lag between walking frequency and the respiratory parameters increases. Finally, note that (1) PETCO2 oscillates with a small amplitude in phase with ventilation and (2) the residual data for ventilation are not influenced by the locomotor activity.
Figure 5. Amplitude of the respiratory and locomotor responses as a function of the frequency of the sinusoidal changes in treadmill speed.
A, amplitude of the ventilatory and of the locomotor responses (mean ±s.e.m.) at each frequency of treadmill speed oscillation. Zero frequency corresponds to the steady-state response. Note the dissociation between the lack of change in the oscillations of the frequency of movement and the progressive reduction in V̇E amplitude when the frequency of treadmill speed oscillations increases. B, amplitude of the V̇O2 and V̇CO2 responses at each frequency of treadmill speed oscillation. Note that V̇O2 amplitude starts to decrease at a higher frequency than V̇CO2 (and V̇E), reflecting faster V̇O2 than V̇CO2 kinetics. C–E, relationship between the gain ratio (steady-state response–amplitude response ratio at each frequency) of ventilation and V̇CO2 (C) V̇O2 (D) and the locomotor response (E). Note that the change in amplitude ratio of the locomotor response has no relationship with the ventilatory response, which appears to follow the pulmonary gas exchange amplitude ratio.
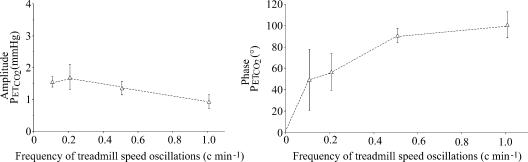
Figure 7. Amplitude and phase of PETCO2 response as a function of the frequency of the sinusoidal changes in treadmill speed.
Note that the amplitude of PETCO2 oscillations is small and is not affected by the frequency of the input.
V̇E response followed more closely V̇CO2 than V̇O2. The drop in V̇E amplitude ratio was proportional to that of V̇CO2 (Fig. 5) with a slightly but significantly longer phase lag for ventilation than for V̇CO2 for each oscillation period, as illustrated in Fig. 6. The significant decrease in amplitude of the V̇O2 response (H = 11.13; P = 0.01) was lower than that of V̇CO2 (H = 14.02; P = 0.003). Indeed, V̇O2 amplitude dropped by 57% from 170 ± 38 ml min−1 (steady state) to 73 ± 26 ml min−1 (1 min period) and V̇CO2 amplitude decreased by 75% from 149 ± 48 ml min−1 (steady state) to 38 ± 5 ml min−1 (1 min period). Finally, the significant increase in the phase lag between the change in treadmill speed and pulmonary gas exchange (H = 12.85, P = 0.005 for V̇O2 and Hcorr = 15.67, P = 0.001 for V̇O2) was greater for V̇CO2 than for V̇O2 at every frequency (Fig. 6), reflecting faster V̇O2 than V̇CO2 kinetics. For instance, for a period of oscillation of 1 min, the phase lag between V̇O2 and motor activity averaged 73 ± 26° for V̇O2versus 99 ± 39° for V̇CO2. After suppression of the fundamental component of the V̇E response (same frequency as the treadmill speed oscillation), no trend for the residuals was found (see example in Fig. 4). It was impossible to identify any component of the residual ventilatory values in phase with the motor act.
Figure 6. Phase lag between treadmill speed oscillations and CO2 output (open squares), ventilation (filled circles), V̇O2 (filled squares) and the locomotor response (frequency of walking, open circles) at each frequency of treadmill speed oscillation.
Note that the phase lag of ventilation follows that of CO2 output very closely. The increase in V̇O2 phase lag is less pronounced than V̇CO2 and V̇E. The frequency response of locomotor response is flat.
Finally, the changes in PETCO2 displayed small amplitude oscillations (between 1 and 2 Torr) occurring in phase with the ventilatory changes (Figs 4 and 7).
Discussion
Whatever the frequency of speed oscillations, we found that the locomotor response, which consisted of changes in stride frequency, had a constant amplitude and was in perfect phase with the treadmill speed changes. The flat frequency response of the locomotor response up to the highest frequencies of speed oscillations simply reflects the immediate and proportional adjustment of the system controlling locomotion (no delay and no time constant) to the changes in speed of walking imposed by the treadmill. In contrast, the frequency response of the ventilatory control system reveals a marked dissociation from the treadmill speed and the locomotor activity. The motor control of the respiratory act of breathing displayed a major drop in amplitude and increase in phase lag when the frequency of oscillations increased, following the pulmonary gas exchange response.
Our approach allowed dissociation of the factors controlling the motor act of walking, which was unaffected by, and able to track, the changes in the frequency of speed oscillations (at least up to a 1 min period sinusoid) from the variations in gas exchange rate which are affected by the frequency of the sinusoidal work rate (having a time constant response of several tenths of seconds). We found that the strategy of the system controlling ventilation is to follow factors related to the gas exchange rate but not to the motor act of walking.
Breath-by-breath ventilatory measurements in sheep
The familiarity of the selected sheep with the equipment and the research team is the main pre-requisite for reliable breath-by-breath measurements of ventilation in unsedated animals. Although sheep have a rather stable breathing pattern, rumination can interfere with flow measurement. Indeed, many factors related to rumination or pseudo-rumination can affect ventilation. Belching can change intra-abdominal pressure and thus the mechanics of breathing as eructation episodes allow a very large volume of gas containing CO2 to be evacuated from the rumen and the reticulum (Dougherty, 1968; Hecker, 1983). Increased inspired PCO2 during eructation can stimulate ventilation (Rollin et al. 1997). Swallowing (Feroah et al. 2002), mastication and contractions and relaxations of the diaphragmatic fibres (Titchen, 1979) during regurgitation provoke short episodes of hypoventilation. All these phenomena are easy to identify on the flow signal and could be limited by increasing the delay between the exercise test and a feeding period. For instance, in most studies performed in walking goats (Bisgard et al. 1982; Smith et al. 1983), a 24 h fasting period was used before ventilatory measurements; although breath-by-breath data are usually not reported in these studies. We found that 30–32 h of deprivation of food (with water provided ad libitum) was always sufficient to get stable ventilatory measurements.
Ventilation, work load and frequency of movements
The V̇E, V̇O2, and V̇CO2 responses to the sinusoidal change in treadmill speed appear to have similar dynamic characteristics to those in humans exercising on a cycle ergometer when the work load was changed while maintaining the pedal rate constant (Casaburi et al. 1977). The increase in V̇O2 phase lag (Fig. 8), as well as the reduction in its amplitude, when the period of speed oscillation decreased, was much less pronounced than that in V̇CO2 and V̇E, reflecting faster O2 uptake than CO2 output and V̇E kinetics. These data are very similar to those already described during a constant work rate exercise of moderate intensity with shorter time constant of V̇O2 and V̇CO2 than V̇E (Whipp et al. 1982; Whipp & Ward, 1991). Finally, we found very small amplitude changes in PETCO2. As in humans, PETCO2 was high when ventilation was high (Whipp, 1981).
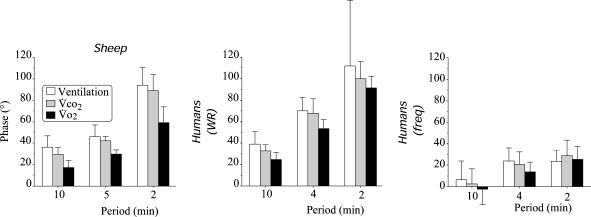
Figure 8. Phase lag between CO2 output, ventilation, oxygen uptake and treadmill speed sinusoidal oscillations in sheep (left panel) work rate sinusoidal oscillations in humans (humans WR, middle panel) and pedalling frequency oscillations in humans (humans freq, right panel) at three different periods of oscillations.
Data in humans were computed from the studies of Casaburi et al. (1977, 1978). The shorter ventilatory phase lag in sheep than in humans (middle panel) was always associated with faster V̇CO2 kinetics in the former. Oxygen uptake phase lags were always much shorter than V̇CO2 in both species. Note the fast dynamics in pulmonary gas exchange and ventilation when fluctuating changes in pedal frequency are applied (see text for comments).
In the present study, the oscillations in work rate were induced by changing the speed of the animals and thus the frequency of movements. There have been many reports in humans, although not always consistent, that increasing predominantly the pedal rate or the frequency of movement could trigger a faster V̇E response and with a larger amplitude than during work load changes only (Kay et al. 1975; Takano, 1988). These observations, which must be distinguished from the phenomenon of entrainment of breathing frequency by the stride frequency (Bechbache & Duffin, 1977; Dempsey et al. 1996 for discussion and review), have been regarded as evidence for rapid neurogenic factors originating in the muscles or controlling ‘centrally’ exercise hyperpnoea (Dejours, 1959). However, few studies have really explored the ventilatory and gas exchange dynamics in response to change in the frequency of movements versus work load. Casaburi et al. (1978) studied subjects who exercised at a ‘constant’ work load with sinusoidally varying pedal rate. They found very fast V̇E response to this type of exercise with a small phase lag between V̇E and the pedal rate fluctuations, the former being largely independent of the frequency of the input forcing (Fig. 8). However, the V̇E response was still coupled to the changes in the pulmonary gas exchange rate. It remains unclear what could have speeded up the kinetics of V̇O2 and V̇CO2 but Casaburi et al. (1978) concluded that the above results do not support an appreciable role for neurally mediated influences related to the motor act of cycling. We are inclined to follow this contention during walking as although the ventilatory response in our trotting sheep was somewhat faster than in cycling humans (with constant pedal rate), it was always associated with fast V̇O2 and V̇CO2 kinetics, resulting in apparent similar coupling between these factors (Fig. 8).
Involvement of the central command in the control of breathing during spontaneous locomotion in sheep
The central command hypothesis assumes that exercise hyperpnoea and locomotion are activated in a simple parallel manner through signals coming from the hypothalamic or the mesencephalic locomotor regions (Eldridge et al. 1981). This coupled respiratory and locomotor response has been shown experimentally to persist after suppression of the motor cortex and afferent feedback signals from the lungs, the skeletal muscles, the central or peripheral chemoreceptors and the arterial baroreceptors. The resulting V̇E or phrenic response was found to be immediate and steady, persisting throughout the entire period of hypothalamic stimulation with the same magnitude (Eldridge & Waldrop, 1991).
The absence of a ventilatory component in phase with the motor act and the fact that almost 70% of the V̇E response was absent when the frequency of speed oscillation increased, implies that experimental stimulation of the hypothalamus produces qualitatively different V̇E responses than during spontaneous walking in quadrupeds. In other words, the fact that the V̇E response during walking at a constant speed in intact animals resembles the responses triggered by hypothalamic stimulation does not mean that the hypothalamic or mesencephalic locomotor regions contribute to the control of ventilation during walking. This also shows that cortical as well as sub-cortical structures, involved during walking in the sheep does not produce a simple parallel activation of ventilation and locomotion.
The present approach does not allow the description of the transient phase from rest (Dejours, 1959), corresponding to the V̇E phase I of a constant work rate exercise (Whipp, 1981). Indeed, the fact that (1) the locomotor activity does not involve rest–exercise transition periods (Whipp et al. 1982) and (2) the frequency domain chosen prevents the ‘isolation’ of the fast components, precludes the possibility of drawing any conclusion about the systems controlling ventilation during the onset of locomotion (Engeman et al. 1983; Haouzi et al. 1992). However, the present results do show that beyond this initial period, the walking and breathing acts are regulated through different control mechanisms or at least by mechanisms with very different dynamic properties.
It is worth noting that studies on the effects of the destruction of the hypothalamic locomotor regions in animals do not always support the idea of an involvement of these supra-spinal sites in the physiological responses to walking. For instance, Ordway et al. (1989) showed that suppression of the sub-thalamic regions involved in automatic walking did not affect the motor and the circulatory responses during spontaneous walking. This has been interpreted as being due to other structures which compensate for the loss of hypothalamic structures, but it could be explained as well by the small contribution of these structures during spontaneous walking, at least in a laboratory environment. The role of such a central feedforward control of breathing remains to be studied in real-life situations, for example, when exercise is part of a complex behavioural response such as escaping from danger.
Ventilatory response and pulmonary gas exchange
The strong temporal relationship between V̇E and V̇CO2, a tenet of blood gas homeostasis during exercise, has usually been regarded as proof that some CO2 flow-related factors act as a controller of V̇E (Wasserman et al. 1986; Whipp & Ward, 1991 for review). However, the nature of the signals coupling the control of ventilation to pulmonary gas exchange is still open to question and largely debated. The consistent observation that V̇E follows V̇CO2, as in the present study, does not imply that the input signals sent to the brainstem respiratory neurones are related to events occurring at lung level (or in the central circulation). Indeed, one of the fundamental properties of the respiratory centres is to transform (magnify with a slow time constant) the effects of many peripheral stimuli reaching the respiratory neurones (Wagner & Eldridge, 1991; Waldrop et al. 1996). This phenomenon has been demonstrated in de-afferented, anaesthetized or in conscious animals where, despite constant stimulation of the respiratory neurones via the sciatic or sinus nerve, the resulting increase in ventilation is gradual towards steady state (Eldridge & Gill-Kumar, 1980; Millhorn et al. 1980; Eldridge et al. 1982; Wagner & Eldridge, 1991; Waldrop et al. 1996). After the cessation of stimulation, this short-term potentiation component declines exponentially to resting level (Eldridge & Gill-Kumar, 1980). This phenomenon has been accounted for by certain intrinsic properties of the respiratory neurones which may slow down and magnify the effects of any immediate and constant breathing. A given ventilatory outcome during exercise can actually result from a more rapid signal coming from a peripheral source, still proportional to the metabolic load (Haouzi et al. 2004). Our study does not provide additional information on such a putative peripheral signal.
In conclusion, the present results do not support the contention that a proportional and parallel adjustment of the locomotor activity and minute ventilation exists in sheep walking on a treadmill. However, these data suggest that the main component of the V̇E response during locomotion is linked to a signal related to the metabolic load regardless of the motor activity.
Acknowledgments
The authors are indepted to Professor Yoshiyuki Fukuba, School of Health Science, Hiroshima Prefectural Women's University, Japan, for his advice and helpful discussion of the results. We are grateful to Yvonne Bedez, Bernard Tousseul and Françoise Lorrain for their technical assistance and to Dr Noël Martinet for his help with the use of the goniometers. This study was supported by le Ministère de la Recherche, Équipe Contractualisée EA 3450.
References
- Bakker HK, Struikenkamp RS, De Vries GA. Dynamics of ventilation, heart rate, and gas exchange: sinusoidal and impulse work loads in man. J Appl Physiol. 1980;48:289–301. doi: 10.1152/jappl.1980.48.2.289. [DOI] [PubMed] [Google Scholar]
- Bechbache RR, Duffin J. The entrainment of breathing frequency by exercise rhythm. J Physiol. 1977;272:553–561. doi: 10.1113/jphysiol.1977.sp012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Mesina J, Sarazin RG. Role of the carotid body in hyperpnea of moderate exercise in goats. J Appl Physiol. 1982;52:1216–1222. doi: 10.1152/jappl.1982.52.5.1216. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Whipp BJ, Wasserman K, Beaver WL, Koyal SN. Ventilatory and gas exchange dynamics in response to sinusoidal work. J Appl Physiol. 1977;42:300–301. doi: 10.1152/jappl.1977.42.2.300. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Whipp BJ, Wasserman K, Koyal SN. Ventilatory and gas exchange responses to cycling with sinusoidally varying pedal rate. J Appl Physiol. 1978;44:97–103. doi: 10.1152/jappl.1978.44.1.97. [DOI] [PubMed] [Google Scholar]
- Comroe HH, Jr, Schmidt CF. Reflexes from the limbs as a factor in the hyperpnea of muscular exercise. Am J Physiol. 1943;138:536–547. [Google Scholar]
- Dejours P. La régulation de la ventilation au cours de l'exercice musculaire chez l'homme. J Physiol (Paris) 1959;51:163–261. [PubMed] [Google Scholar]
- Dempsey JA, Adams L, Ainsworth DM, Fregosi RF, Gallagher CG, Guz A, Johnson BD, Powers SK. Airway, lung, and respiratory muscle function during exercise. In: Rowell L, Shepherd J, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York, Oxford: The American Physiological Society Oxford University Press; 1996. pp. 448–514. [Google Scholar]
- Dougherty RW. Eructation in ruminants. Ann N Y Acad Sci. 1968;150:22–26. doi: 10.1111/j.1749-6632.1968.tb19026.x. [DOI] [PubMed] [Google Scholar]
- Eldridge F, Gill-Kumar P. Central neural respiratory drive and afterdischarge. Respir Physiol. 1980;40:49–63. doi: 10.1016/0034-5687(80)90004-3. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science. 1981;211:844–846. doi: 10.1126/science.7466362. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Input–output relationships of the central respiratory controller during peripheral muscle stimulation in cats. J Physiol. 1982;324:285–295. doi: 10.1113/jphysiol.1982.sp014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Waldrop TG. Neural control of breathing during exercise. In: Whipp B, Wasserman K, editors. Lung Biology in Health and Disease. Vol. 52. New York Basel: Dekker Inc; 1991. pp. 309–370. Exercise Pulmonary Physiology and Pathophysiology. [Google Scholar]
- Engeman RM, Swanson GD, Jones RH. Optimal frequency locations for estimating model parameters in studies on respiratory control. Comput Biomed Res. 1983;16:531–536. doi: 10.1016/0010-4809(83)90039-3. [DOI] [PubMed] [Google Scholar]
- Engwall MJ, Smith CA, Dempsey JA, Bisgard GE. Ventilatory afterdischarge and central respiratory drive interactions in the awake goat. J Appl Physiol. 1994;76:416–423. doi: 10.1152/jappl.1994.76.1.416. [DOI] [PubMed] [Google Scholar]
- Feroah TR, Forster HV, Fuentes CG, Lang IM, Beste D, Martino P, Pan L, Rice T. Effects of spontaneous swallows on breathing in awake goats. J Appl Physiol. 2002;92:1923–1935. doi: 10.1152/japplphysiol.01079.2000. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Hildebrandt JR, Hildebrandt J. Cardiorespiratory transients in exercising man. I. Tests of superposition. J Appl Physiol. 1973a;35:58–67. doi: 10.1152/jappl.1973.35.1.58. [DOI] [PubMed] [Google Scholar]
- Fujihara Y, Hildebrandt J, Hildebrandt JR. Cardiorespiratory transients in exercising man. II. Linear models. J Appl Physiol. 1973b;35:68–76. doi: 10.1152/jappl.1973.35.1.68. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Allioui EM, Gille JP, Bedez Y, Tousseul B, Chalon B. Stimulation of ventilation by normobaric hyperoxia in exercising dogs. Exp Physiol. 2000;85:829–838. [PubMed] [Google Scholar]
- Haouzi P, Chenuel B, Huzczuk A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: neurphysiological basis and implication in respiratory control. J Appl Physiol. 2004;96:407–418. doi: 10.1152/japplphysiol.00597.2003. [DOI] [PubMed] [Google Scholar]
- Haouzi P, Fukuba Y, Peslin R, Chalon B, Marchal F, Crance JP. Ventilatory dynamics in children and adults during sinusoidal exercise. Eur J Appl Physiol Occup Physiol. 1992;64:410–418. doi: 10.1007/BF00625059. [DOI] [PubMed] [Google Scholar]
- Hecker JF. The Sheep as an Experimental Animal. London: Academic Press; 1983. [Google Scholar]
- Kay JD, Petersen ES, Vejby-Christensen H. Breathing in man during steady-state exercise on the bicycle at two pedalling frequencies, and during treadmill walking. J Physiol. 1975;251:645–656. doi: 10.1113/jphysiol.1975.sp011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring SH, Mead J, Waggener TB. Determinants of breathing frequency during walking. Respir Physiol. 1990;82:177–188. doi: 10.1016/0034-5687(90)90033-u. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Waldrop TG, Iwamoto GA, Gentile BJ. Hypothalamic influences on cardiovascular response of beagles to dynamic exercise. Am J Physiol. 1989;257:H1247–1253. doi: 10.1152/ajpheart.1989.257.4.H1247. [DOI] [PubMed] [Google Scholar]
- Rollin F, Desmecht D, Genicot B, Linden A, Lomba F, Lekeux P. Ventilatory effects of the single-breath CO2 test, compared with eructation, in cattle. Am J Vet Res. 1997;58:310–316. [PubMed] [Google Scholar]
- Schwartz D. Méthodes Statistiques À L'usage Des Médecins et Des Biologistes. Paris: Flammarion Médecine-Sciences; 1986. [Google Scholar]
- Smith CA, Mitchell GS, Jameson LC, Musch TI, Dempsey JA. Ventilatory response of goats to treadmill exercise: grade effects. Respir Physiol. 1983;54:331–341. doi: 10.1016/0034-5687(83)90076-2. [DOI] [PubMed] [Google Scholar]
- Smith O, Rushmer R, Lasher E. Similarity of cardiovascular responses to exercice and to diencephalic stimulation. Am J Physiol. 1960;198:1139–1142. doi: 10.1152/ajplegacy.1960.198.6.1139. [DOI] [PubMed] [Google Scholar]
- Takano N. Effects of pedal rate on respiratory responses to incremental bicycle work. J Physiol. 1988;396:389–397. doi: 10.1113/jphysiol.1988.sp016968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titchen DA. Diaphragmatic and oesophageal activity in regurgitation in sheep: an electromyographic study. J Physiol. 1979;292:381–390. doi: 10.1113/jphysiol.1979.sp012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PG, Eldridge FL. Development of short-term potentiation of respiration. Respir Physiol. 1991;83:129–139. doi: 10.1016/0034-5687(91)90098-4. [DOI] [PubMed] [Google Scholar]
- Waldrop TG, Eldridge F, Iwamoto GA, Mitchel J. Central neural control of respiration and circulation during exercise. In: Rowell L, Shepherd J, editors. Handbook of Physiology, Exercise: Regulation and Integration of Multiple Systems. New York, Oxford: The American Physiological Society Oxford University Press; 1996. pp. 332–380. [Google Scholar]
- Wasserman K, Whipp BJ, Casaburi R. Respiratory control during exercise. In: Macklem PT, Mead J, editors. Handbook of Physiology, The Respiratory System. II. Bethesda MD: The American Physiology Society; 1986. pp. 595–619. [Google Scholar]
- Whipp BJ. The control of exercise hyperpnea. In: Hornbein T, editor. Lung Biology in Health and Disease. Vol. 17. New York Basel: Dekker Inc; 1981. pp. 1069–1139. Regulation of Breathing. [Google Scholar]
- Whipp BJ, Ward SA. Coupling of ventilation to pulmonary gas exchange during exercise. In: Whipp B, Wasserman K, editors. Lung Biology in Health and Disease. Vol. 52. New York Basel: Dekker Inc; 1991. pp. 271–307. Exercise Pulmonary Physiology and Pathophysiology. [Google Scholar]
- Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol. 1982;52:1506–1513. doi: 10.1152/jappl.1982.52.6.1506. [DOI] [PubMed] [Google Scholar]