Abstract
Waves of calcium-induced calcium release occur in a variety of cell types and have been implicated in the origin of cardiac arrhythmias. We have investigated the effects of inhibiting the SR Ca2+-ATPase (SERCA) with the reversible inhibitor 2′,5′-di(tert-butyl)-1,4-benzohydroquinone (TBQ) on the properties of these waves. Cardiac myocytes were voltage clamped at a constant potential between −65 and −40 mV and spontaneous waves evoked by increasing external Ca2+ concentration to 4 mm. Application of 100 μm TBQ decreased the frequency of waves. This was associated with increases of resting [Ca2+]i, the time constant of decay of [Ca2+]i and the integral of the accompanying Na+–Ca2+ exchange current. There was also a decrease in propagation velocity of the waves. There was an increase of the calculated Ca2+ efflux per wave. The SR Ca2+ content when a wave was about to propagate decreased to 91.7 ± 3.2%. The period between waves increased in direct proportion to the Ca2+ efflux per wave meaning that TBQ had no effect on the Ca2+ efflux per unit time. We conclude that (i) decreased wave frequency is not a direct consequence of decreased Ca2+ pumping by SERCA between waves but, rather, to more Ca2+ loss on each wave; (ii) inhibiting SERCA increases the chance of spontaneous Ca2+ release propagating at a given SR content.
Intracellular Ca2+ waves produced by release of Ca2+ from sarcoplasmic or endoplasmic reticulum are responsible for signalling and control of cell function in many tissues including smooth muscle and secretory cells (see Berridge et al. 2000 for a review). In general the wave is produced by Ca2+ release from the store through ryanodine (RyR) and/or IP3 receptors. Calcium must then be reaccmulated into the store via a Ca2+ pump (SERCA). As well as SR or ER transport processes, the surface membrane properties will also play a role in shaping the Ca2+ waves. This is because, in general, some of the Ca2+ released during a wave will be pumped out of the cell and will need to be replaced in the cell between waves. It is therefore of considerable interest to investigate the effects of modifying any of these transport mechanisms on the waves.
Ca2+ waves allow propagation of the Ca2+ transient radially into atrial (Hüser et al. 1996) and Purkinje cells (Boyden et al. 2000) but are not used physiologically in ventricular cells. Rather, Ca2+ release from the SR in ventricular cells is triggered uniformly on each beat by Ca2+ entry into the cell on the L-type Ca2+ current during the action potential. Ca2+ waves can, however, sometimes be observed, if stimulation is stopped (Lakatta & Lappé, 1981). Furthermore Ca2+ waves occur during diastole if the cell is ‘overloaded’ with calcium (Orchard et al. 1983; Wier et al. 1983; Wier et al. 1987). These Ca2+ waves are arrhythmogenic as a consequence of activating inward membrane currents such as Na+–Ca2+ exchange (Ferrier et al. 1973; Lederer & Tsien, 1976; Mechmann & Pott, 1986; Fedida et al. 1987; Berlin et al. 1989). Ca2+ waves can also be seen during systole in ventricular cells during the phenomenon known as alternans (Díaz et al. 2002, 2004) where waves propagate over limited distances following small initial systolic releases.
Given the arrhythmogenic importance of Ca2+ waves, it is important to understand the factors that determine their properties. We have found that metabolic inhibition (to mimic ischaemia) depresses both release and uptake by the SR (Overend et al. 2001). As a result of the decreased release there is an increase of SR Ca2+ content. This effect on release results from a decrease of RyR open probability due in part to intracellular acidification, and also to other factors possibly including increased intracellular Mg2+ or decreased ATP concentration (O'Neill & Eisner, 2003). The effect on release can be mimicked by the local anaesthetic tetracaine or acidosis: both decrease the frequency while increasing the amplitude of Ca2+ waves (Overend et al. 1997; O'Neill & Eisner, 2003).
In contrast, little is known about the effects of inhibition of SERCA on Ca2+ waves. During the later stages of metabolic inhibition the Ca2+ wave is prolonged, an effect that can be mimicked by inhibition of SERCA with thapsigargin (Overend et al. 2001). One might expect that this effect would decrease SR Ca2+ content. Although the observed increase of SR Ca2+ content in metabolic inhibition may be due to decreased opening of the RyR, it is important to establish what effect SERCA inhibition per se has on SR content. Another aim of this work was to establish whether SERCA inhibition has any effects on Ca2+ waves independent of changes of SR content. For example, since Ca2+ release during a wave has to diffuse to neighbouring release sites to allow propagation, it might be that Ca2+ uptake by SERCA will inhibit propagation.
We have therefore examined the effects of the reversible SERCA inhibitor 2′,5′-di(tert-butyl)-1,4-benzohydroquinone (TBQ) (Kabbara & Stephenson, 1997). The results show that SERCA inhibition prolongs the waves thereby increasing the amount of Ca2+ pumped out of the cell and decreases the frequency. In addition we found evidence that uptake of Ca2+ by SERCA increases the requirement for release of Ca2+ by the SR to ensure propagation takes place.
Methods
Myocytes were isolated from rat ventricular muscle using a collagenase and protease technique as previously described (Eisner et al. 1989). Rats were killed by stunning and cervical dislocation. Care and use of animals were in accordance with the Animals (Scientific Procedures) Act 1986. Cells were isolated from a total of four animals.
Cells were voltage-clamped with the perforated-patch technique using the switch clamp mode of the Axoclamp 2B voltage-clamp amplifier (Axon Instruments). Pipettes (< 5 MΩ) were filled with the following solution (mmol l−1): KCH3O3S, 125; KCl, 12; NaCl, 20; Hepes, 10; MgCl2, 5, titrated to pH 7.2 with KOH, and a final concentration of amphotericin B of 240 μg ml−1. The initial bathing solution was as follows (mmol l−1): NaCl, 135; KCl, 4; Hepes, 10; glucose, 11; MgCl2, 1; CaCl2, 1, titrated to pH 7.4 with NaOH.
Ca2+ overload was produced by the combined action of high intracellular [Na+] (resulting from the 20 mmol l−1 level in the pipette solution) and raising external [Ca2+] to 4 mm. The membrane potential was held at a fixed value throughout. The actual value was in the range between −65 and −40 mV and was chosen to produce waves at a rate of about one every 10 s. All solutions contained 5 mm 4-aminopyridine and 0.1 mm BaCl2 to ensure that caffeine-induced current represented only Na+–Ca2+ exchange current. The integral of the caffeine-induced current (corrected for sarcolemmal Ca2+-ATPase) represents the SR Ca2+ content leaving the cell on Na+–Ca2+ exchange and is expressed per litre cell volume, calculated from the cell capacitance (Varro et al. 1993). Effluxes generated by spontaneous waves of Ca2+ release were calculated similarly. The total amount of Ca2+ extruded is expressed per unit cell volume. [Ca2+]i was measured after loading myocytes with the acetoxymethyl ester of fluo-3 or fluo-4 (5 μm) for 5 min. Ca2+ measurements were made using a Bio-Rad 1024 confocal microscope in line scan mode and are presented either as linescan images or as the average signal from each image. Fluorescence signals were calibrated in terms of [Ca2+] using the technique of (Cannell et al. 1994) assuming the control resting [Ca2+]i was 100 nm.
Inhibition of the SR Ca2+-ATPase was produced by application of 100 μm TBQ. All experiments were carried out at room temperature (20°C). All statistics are quoted as means ± s.e.m.; Student's paired t test was used throughout to test statistical significance.
Results
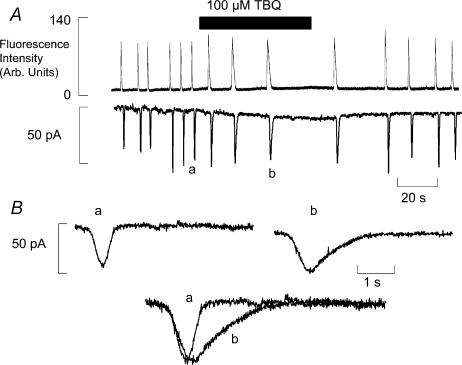
The effects of 100 μm 2,5-di-tert-butyl-1,4-benzohydroquinone (TBQ) on the frequency of spontaneous waves of Ca2+-induced Ca2+ release (CICR) are shown in Fig. 1A. The upper trace is a record of Fluo-4 fluorescence indicating the changes of intracellular Ca2+ concentration ([Ca2+]i). The membrane potential was held at −5 mV to induce spontaneous waves of CICR. On application of 100 μm TBQ there is a reversible reduction of wave frequency and an increase of the resting fluorescence. Converting the fluorescence change to [Ca2+] showed a rise on average from 100 nm to 147 ± 11 nm (n = 6). In addition there is a prolongation of the individual Ca2+ transients. Both of these effects are mirrored in membrane current shown in the panel below. Each transient rise of [Ca2+]i activates a Na+–Ca2+ exchange transient inward current. The increased duration is more clearly seen in Fig. 1B. This shows two representative transient inward currents from control (a) and in TBQ (b). Superimposing these currents in the bottom panel shows a clear prolongation of the transient inward current in the presence of TBQ. This increased duration of the activation of Na+–Ca2+ exchange leads to an increased efflux of Ca2+ from the cell. On average, efflux activated by each wave increased by 2.53 ± 0.20 times (n = 7, P < 0.001) in TBQ (see Fig. 2B). Current amplitude was unchanged at 32.9 ± 3.2 pA in control and 35.7 ± 4.5 pA in TBQ (P > 0.2). The inward shift of holding current was present in 7 of 8 cells studied, and in all cells studied the final effect of 100 μm TBQ was to abolish spontaneous waves.
Figure 1. Inhibition of SERCA reduces the frequency of spontaneous release and prolongs its duration.
A, the time course of the effects of addition and removal of TBQ on Fluo-4 fluorescence (upper trace) and membrane current (lower trace; holding potential −55 mV). B, sample records of individual transient inward currents in control (a) and in TBQ (b) from panel A.
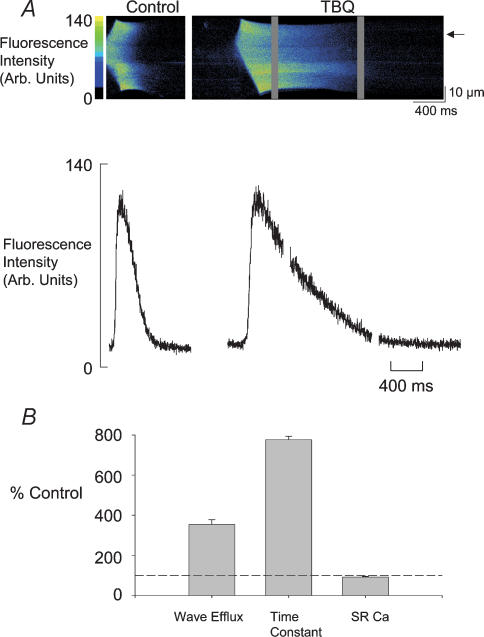
Figure 2. TBQ slows the fall of Ca2+ and prolongs the duration in a spontaneous wave of Ca2+ release.
A, the upper panel shows individual linescan images of Ca2+ waves in control and TBQ. In the lower panel the Ca2+ profile of each wave is shown from the point marked by the arrow. B, percentage change of Ca2+ efflux generated by individual waves, the time constant of recovery of the Ca2+ transient during a wave and SR Ca2+ content. The dashed line marks 100%. In B are shown mean and s.e.m. from 7 cells for wave efflux, 6 cells for time constant and 7 cells for SR content.
We can see in more detail the changes in the [Ca2+]i responsible for the greater duration of the transient inward current in Fig. 2A. The upper panel shows confocal linescan data from a single rat ventricular myocyte. Each panel shows a wave of CICR propagating from a central portion of the cell. In control the wave is complete within the 1 s duration scan period; in TBQ 3 s are required to accommodate the wave (the grey bands in TBQ represent the periods of time between scans). This is shown graphically below where profiles from each of the linescans are shown from the point indicated by the arrow. Single exponential curves fitted to the falling phase of the [Ca2+]i transient had a time constant that increased on average from 0.12 ± 0.01 s in control to 0.75 ± 0.13 s in TBQ (see Fig. 2B). From data such as that in the upper panel we can also calculate the propagation velocity of the wave. In this particular cell, velocity fell from 142 μm s−1 in control to 127 μm s−1 in TBQ; on average the values were 114.2 ± 9.7 μm s−1 in control and 98.1 ± 10.9 μm s−1 in TBQ (n = 5, P < 0.015).
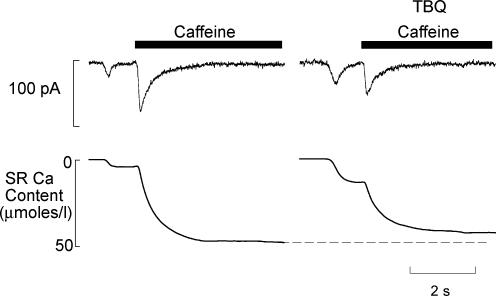
We would expect the extra efflux generated by the longer duration waves in TBQ to reduce the Ca2+ content of the SR. We have examined this by applying caffeine (10 mm) and measuring the integral of the resulting Na+–Ca2+ exchange current as Ca2+ is pumped out of the cell (Varro et al. 1993). In these experiments, caffeine was applied immediately after a spontaneous wave, the integral of the wave-induced current and that produced by caffeine were summed to give the amount of SR Ca2+ at the time the wave began. The integrals in Fig. 3 show that the SR content at this time is reduced. On average SR content in TBQ is 91.7 ± 3.2% of control (P < 0.05, n = 6). It is worth noting that although this is significant statistically, it is a small reduction. In particular it is very small compared to the fractional degree of inhibition of SERCA as estimated from the 680% increase of the time constant of decay of [Ca2+]i (Fig. 2B).
Figure 3. TBQ allows spontaneous waves of Ca release at lower SR content.
The top trace shows membrane current accompanying a wave and then on application of caffeine (10 mm). The lower trace shows the integral of current expressed in terms of micromoles of Ca2+ per litre cell volume. The first deflection represents the loss of Ca2+ associated with the wave, the second the remaining SR Ca2+ content. The left hand panel was obtained in control, the right in 100 μm TBQ. The holding potential was −40 mV throughout.
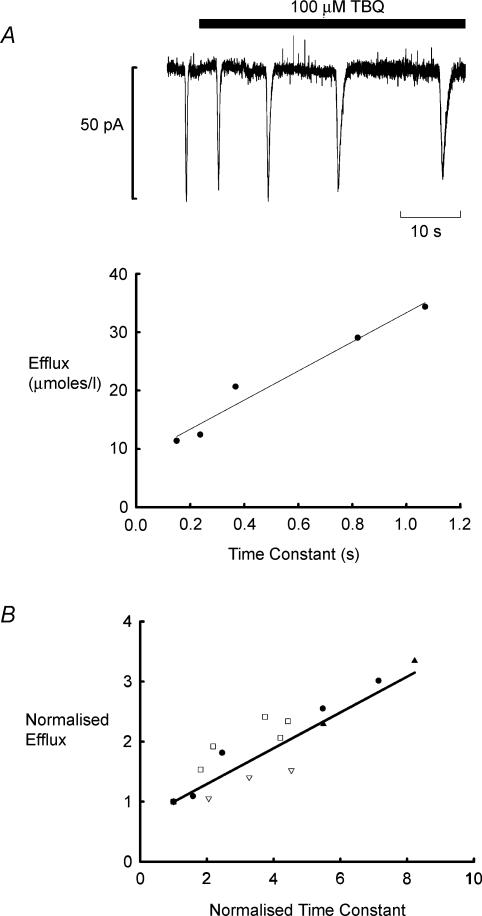
In subsequent experiments, we have investigated the origin of the increased Ca2+ efflux during waves produced by TBQ. Figure 4A shows that the currents activated by waves of CICR become prolonged in the presence of TBQ. Plotted below is the Ca2+ efflux activated by each wave as a function of the degree of inhibition of SERCA as assessed by the time constant of recovery of the current. As the decay phase of the wave slows, so the efflux increases. Since Ca2+ is elevated for longer, the efflux pathways are able to remove more Ca2+ from the cell. Figure 4B demonstrates that similar effects were seen in all four cells studied in this way. We have normalized the effluxes and time constants of decay to the control values and it is clear that a similar linear relation between these parameters exists in each cell.
Figure 4. Ca2+ efflux generated by waves increases as recovery of transient inward current slows in TBQ.
A, transient inward currents associated with waves of Ca2+ release become less frequent and slower to recover in TBQ (100 μm; holding potential −45 mV). The plot below shows efflux as a function of the time constant of recovery for the five currents in the upper panel. B, efflux as a function of time constant of current recovery for 4 cells. Each has been normalized with respect to control.
What is the explanation of the decreased wave frequency in TBQ?
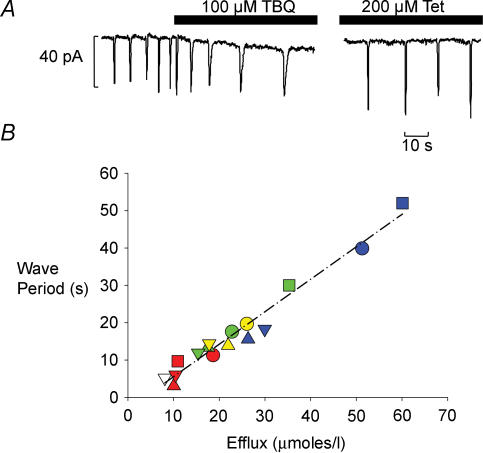
The other major effect of TBQ on waves of CICR is to reduce their frequency. There are two possible explanations for this observation. (1) The decrease of SERCA activity may slow the rate of SR refilling. (2) The increased amount of Ca2+ pumped out of the cell per wave will mean that more time will be required for the cell and the SR to regain this calcium. We can distinguish between these hypotheses by comparing the effects of TBQ with those of tetracaine. Tetracaine does not affect SERCA but decreases the RyR open probability and thereby increases the amplitude of the wave (Overend et al. 1997). On the first hypothesis, tetracaine and TBQ should have different effects whereas, on the second, their effects should be equivalent. In Fig. 5A the two current traces show the effects on transient inward currents of the onset of TBQ application and (on the right) the steady state in tetracaine. In both cases the efflux generated by each wave is increased; in TBQ by prolonging the current, in tetracaine by increasing its amplitude. Figure 5B shows the period between waves as a function of Ca2+ efflux on each wave in four different cells. There is a simple proportional relationship between increasing efflux and increasing wave period; the dashed line shows the regression line through all the data. Importantly, this relationship is the same whether efflux is altered by decreasing the sensitivity of the release mechanism (tetracaine) or inhibiting SR Ca2+ uptake (TBQ). In one cell we also investigated the effect of increasing RyR open probability with caffeine (raw data not shown) and found that this also lies on the same relation between efflux per wave and period (open triangle at extreme left). This proportionality between efflux and period suggests that the rate of refilling is the same in all cases and probably reflects the rate of influx of Ca2+ replacing Ca2+ lost on preceding waves.
Figure 5. Wave period increases as Ca2+ efflux generated by waves increases.
A, the trace to the left shows the effect of TBQ on transient inward current frequency, on the right the steady state effect of tetracaine (holding potential −55 mV). B, plot of wave period as a function of Ca2+ efflux generated by waves in 4 cells. Each symbol type represents an individual cell, the colours code for: 250 μm caffeine (open), control (red), 10 or 50 μm TBQ (green), 200 μm tetracaine (yellow) and 100 μm TBQ (blue).
Discussion
We have examined the effects on properties of Ca2+ waves during the onset of inhibition of SERCA by TBQ. We find an increase of both the duration of individual waves and the period of time between waves. The increase of wave period seems to be entirely due to the increased duration of the individual waves of CICR and the extra efflux this generates.
Inhibition of SERCA has only a small effect on the rate of Ca2+ uptake by the SR
TBQ decreased the rate constant of decay of [Ca2+]i during a wave to 16% of control. If one assumes that SERCA is the only factor responsible for decay of [Ca2+]i in control then this implies that SERCA activity has been decreased to 16% of control. However, given that Na+–Ca2+ exchange contributes about 10% to Ca2+ removal (Negretti et al. 1993) then the fractional inhibition of SERCA is probably even greater. When Ca2+ is released from the SR, there is a competition between SERCA to take the Ca2+ back into the SR and the surface membrane efflux pathways. As these surface membrane pathways are slower in the rat ventricular cell than the SERCA uptake pathway (Negretti et al. 1993; Bassani et al. 1994), the bulk of released Ca2+ returns to the SR. In our experiments, however, inhibiting SERCA prolongs the duration of the Ca2+ transient such that the efflux pathways have a greater period of time in which to remove Ca2+ from the cell. Thus the loss of Ca2+ from the cell generated by each wave increases as inhibition of SERCA develops. Under normal circumstances the Ca2+ lost on an individual wave has to be replaced inside the SR before it is capable of supporting another wave of CICR (Overend et al. 1997). This is also the case in the experiments reported here as the relationship between Ca2+ efflux and the wave period is the same irrespective of the means of changing efflux (Fig. 5). This shows that the reduced frequency of spontaneous waves in TBQ is not due directly to a lower rate of Ca2+ uptake into the SR between waves; rather, it is due to the extra time required for Ca2+ to enter the cell to replace the greater loss generated. This raises the question as to why, despite the substantial inhibition of SERCA, there is apparently no change in the rate of refilling of the SR between waves (as shown by the proportionality between Ca2+ loss and wave period, Fig. 5). There are two possibilities. (1) SR refilling decreases [Ca2+]i below the steady-state level (Baróet al. 1993). This decrease will slow SERCA activity. One would expect that the presence of TBQ would attenuate the decrease of [Ca2+]i during refilling (i.e. [Ca2+]i will be higher, as shown in Fig. 1) and therefore the actual effect on SERCA rate would be less than expected from the TBQ effect alone. (2) The longer lasting Ca2+ release in TBQ will produce a greater depletion of SR content and this may increase the rate of SERCA pumping due to decreased inhibition by lumenal Ca2+.
SERCA inhibition lowers the threshold for Ca2+ waves
If Ca2+ efflux from the cell generated by waves is increased, why is the SR Ca2+ content so little affected (Fig. 3)? Under control conditions, the upper limit to SR Ca2+ content is set by the threshold content that will support propagation of a wave of CICR (Overend et al. 1997). In this study we have examined the effect of TBQ before inhibition of SERCA abolishes waves and therefore the same restriction on content applies. If we assume that TBQ does not alter the sensitivity of RyR, so the threshold Ca2+ required to stimulate release at neighbouring SR release sites would not be expected to change thereby predicting no change of SR content. Nevertheless, there is a consistent small reduction of SR content (Fig. 3). This small fall in SR content we interpret as a change in the efficiency of local release of Ca2+ as a trigger at neighbouring sites. Ca2+ released locally has to diffuse to neighbouring sites past uptake sites, and thus the initial release has to offset loss due to uptake. When SERCA activity is reduced, less uptake takes place and the required trigger concentration of Ca2+ for propagation to proceed can be provided by a smaller initial release, i.e. with a lower SR Ca2+ content. Such an interpretation is supported by the scheme shown in Fig. 6. It is, however, also possible that the observed increase of [Ca2+]i allows propagation of waves at a lower SR Ca2+ content (Davia et al. 2001).
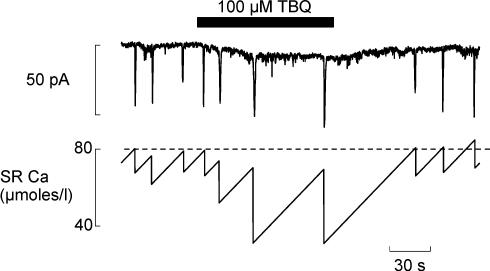
Figure 6. TBQ reduces transient inward current frequency by increasing wave generated efflux.
The upper trace shows the effect of 100 μm TBQ on spontaneous transient inward currents (holding potential −60 mV). The lower trace shows calculations of the changes of SR Ca2+ content obtained as follows. The loss of Ca2+ from the cell (and therefore the SR) on each wave was calculated from the integral of the associated current. The Ca2+ influx was calculated to balance the efflux per unit time (in this experiment 0.76 μmol l−1 s−1).
The baseline shift of current in Fig. 1 is associated with a rise of [Ca2+]i and it may be that some part of the current is due to activation of Na+–Ca2+ exchange. This might be an alternative means by which the cell could meet the demand for Ca2+ efflux. However, it should be noted that TBQ has effects on surface membrane currents that would complicate the interpretation of this current. It is known, for example, that TBQ can inhibit the inward rectifier current (Hassessian et al. 1994). At the holding potentials used in this study (−65 to −40 mV) we would expect any inward rectifier current to be outward, so inhibition would appear as inward current. It is also worth noting that TBQ has been found to inhibit L-type Ca2+ current in vascular smooth muscle cells (Fusi et al. 2001). In this study we have not activated L-type current and our results do not indicate any change in the rate of influx of Ca2+ across the surface membrane (see below).
Estimate of the time course of changes of SR Ca2+ content
The scheme in Fig. 6 shows a simplified version of what we think is happening in TBQ. Integrating the current associated with the waves gives a measure of the amount of Ca2+ lost from the cell (and therefore from the SR) at the end of each wave. This loss must be compensated for by a Ca2+ influx (assumed constant). The dashed line represents the threshold Ca2+ content required for propagation and the time taken to refill the SR determines the frequency of waves. We assume that the rate of Ca2+ entry into the cell across the surface membrane is what limits the rate of refilling of the SR and that this remains constant. As TBQ lowers the rate of SR Ca2+ pumping more efflux of Ca2+ from the cell is generated and the SR is depleted more. Assuming a constant rate of Ca2+ influx, the time required for refilling of the SR has therefore to increase. However, in TBQ a wave occurs before the SR has time to refill to the previous threshold content. This is the equivalent of the reduction of SR Ca2+ content shown in Fig. 3, i.e. waves can propagate at a lower SR Ca2+ content due to reduced uptake between release sites. On removal of TBQ, the original threshold content of SR Ca2+ is re-established, as is the amount of efflux generated by each wave.
Our data show that in TBQ the SR is capable of refilling to virtually the same level as in control despite the rate of pumping being reduced to between a fifth and an eighth of the control level (Fig. 4B). This suggests that the pump under control conditions is operating very far from its dynamic equilibrium and that the limit on SR Ca2+ content is not set by the pump but by the ability of the RyR to retain the content. This is consistent with our previous reports (Overend et al. 1998) that by inhibiting RyR activity it is possible to increase the SR Ca2+ content by as much as 500%.
Comparison with previous work
A previous study has examined the effects of another SERCA inhibitor, thapsigargin, on Ca2+ waves evoked by focal caffeine application (Lukyanenko et al. 1999). In those experiments the SR was pre-loaded with Ca2+ and then caffeine applied. It was found that thapsigargin increased the wave propagation velocity. This contrasts with the decreased propagation velocity seen in the present work. It is likely that the increase of velocity is due to removing the buffering effects of SERCA and allowing Ca2+ release from one site to more quickly activate release from the next site. The decrease of velocity found in this paper is most likely to be due to the small decrease of SR Ca2+ content. Effects on spontaneous Ca2+ waves of SERCA inhibition have been reported in skinned ventricular myocytes (Kawai et al. 1998) using cyclopiazonic acid (CPA). It was observed that the frequency of waves was reduced with little change in the amplitude while the decay time of the Ca2+ transient (averaged over the whole cell) was increased. The authors concluded that CPA increased the time required for Ca2+ in the SR to rise to the threshold concentration required for wave propagation. Although they do not make clear whether this is a direct effect of reducing the rate of uptake or indirect by increasing the amount to be replaced, in the skinned preparation, prolonging the Ca2+ transient would also lead to greater depletion of the SR by allowing more time for diffusion of Ca2+ out of the cell.
The relationship between SERCA expression and arrhythmias
Previous work has shown that overexpressing SERCA decreases the probability of aftercontractions that accompany arrhythmogenic diastolic Ca2+ release (Davia et al. 2001) and also decreases the occurrence of ventricular arrhythmias on reperfusion (Del Monte et al. 2004). Although there are other explanations, this may be related to our observation that a decrease of SERCA activity lowers the SR Ca2+ threshold for waves.
In a wider sense the factors discussed here should also be important when considering any cell in which oscillations of intracellular Ca2+ take place. In many cell types the load of the store is important in determining the timing and/or amount of release. As an example, a recent study carried out in Xenopus oocytes (Falcke et al. 2003) has shown that overexpression of SERCA does indeed increase the frequency of waves of Ca2+ release presumably by reducing the time required for refilling of the store following release. The competition between return of Ca2+ to the store following release and, in the case of cardiac myocytes, loss from the cell will determine the depletion suffered by the store and therefore the profile of subsequent release of Ca2+.
Acknowledgments
This work was supported by the BHF. L.M. holds a MRC studentship.
References
- Baró I, O'Neill SC, Eisner DA. Changes of intracellular [Ca2+]i during refilling of sarcoplasmic reticulum in rat ventricular and vascular smooth muscle. J Physiol. 1993;465:21–41. doi: 10.1113/jphysiol.1993.sp019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependant differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin JR, Cannell MB, Lederer WJ. Cellular origins of the transient inward current in cardiac myocytes: role of fluctuations and waves of elevated intracellular calcium. Circ Res. 1989;65:115–126. doi: 10.1161/01.res.65.1.115. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Boyden PA, Pu J, Pinto J, terKeurs HEDJ. Ca2+ transients and Ca2+ waves in Purkinje cells. Role in action potential initiation. Circ Res. 2000;86:448–455. doi: 10.1161/01.res.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. Spatial non-uniformities of [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994;67:1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davia K, Bernobich E, Ranu HK, Del Monte F, Terracciano CM, MacLeod KT, Adamson DL, Chaudhri B, Hajjar RJ, Harding SE. SERCA2A overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2001;33:1005–1015. doi: 10.1006/jmcc.2001.1368. [DOI] [PubMed] [Google Scholar]
- Del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz ME, Eisner DA, O'Neill SC. Depressed ryanodine receptor activity increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–593. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- Díaz ME, Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardia alternans. Circulation Research. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Nichols CG, O'Neill SC, Smith GL, Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcke M, Li Y, Lechleiter JD, Camacho P. Modeling the dependence of the period of intracellular Ca2+ waves on SERCA expression. Biophys J. 2003;85:1474–1481. doi: 10.1016/S0006-3495(03)74580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Noble D, Rankin AC, Spindler AJ. The arrhythmogenic transient inward current ITI and related contraction in isolated guinea-pig ventricular myocytes. J Physiol. 1987;392:523–542. doi: 10.1113/jphysiol.1987.sp016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier GR, Saunders JH, Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973;32:600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Fusi F, Saponara S, Gagov H, Sgaragli G. 2,5-Di-t-butyl-1,4-benzohydroquinone (BHQ) inhibits vascular L-type Ca2+ channel via superoxide anion generation. Br J Pharmacol. 2001;133:988–996. doi: 10.1038/sj.bjp.0704183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassessian H, Vaca L, Kunze DL. Blockade of the inward rectifier potassium current by the Ca2+-ATPase inhibitor 2′,5′-di(tert-butyl)-1,4-benzohydroquinone (BHQ) Br J Pharmacol. 1994;112:1118–1122. doi: 10.1111/j.1476-5381.1994.tb13199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol. 1996;494:641–651. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara AA, Stephenson DG. Effects of 2,5-di-tert-butylhydroquinone on rat cardiac muscle contractility. Am J Physiol. 1997;272:H1001–H1010. doi: 10.1152/ajpheart.1997.272.2.H1001. [DOI] [PubMed] [Google Scholar]
- Kawai M, Hussain M, Orchard CH. Cs+ inhibits spontaneous Ca2+ release from sarcoplasmic reticulum of skinned cardiac myocytes. Am J Physiol. 1998;275:H422–H430. doi: 10.1152/ajpheart.1998.275.2.H422. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Lappé DL. Diastolic scattered light fluctuation, resting force and twitch force in mammalian cardiac muscle. J Physiol. 1981;315:369–394. doi: 10.1113/jphysiol.1981.sp013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ, Tsien RW. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibers. J Physiol. 1976;263:73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Subramanian S, Györke I, Wiesner TF, Györke S. The role of luminal Ca in the generation of Ca waves in rat ventricular myocytes. J Physiol. 1999;518:173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S, Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986;319:597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Negretti N, O'Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993;27:1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- O'Neill SC, Eisner DA. pH-dependent and -independent effects inhibit Ca2+-induced Ca2+ release during metabolic blockade in rat ventricular myocytes. J Physiol. 2003;550:413–418. doi: 10.1113/jphysiol.2003.042846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Overend CL, Adams WA, O'Neill SC, Eisner DA. The Ca2+ content of sarcoplasmic reticulum is limited by leak, not uptake, in rat cardiac isolated myocytes. J Physiol. 1998;511.P:85P–86P. [Google Scholar]
- Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J Physiol. 1997;502:471–479. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend CL, Eisner DA, O'Neill SC. Altered cardiac sarcoplasmic reticulum function of intact myocytes of rat ventricle during metabolic inhibition. Circ Res. 2001;88:181–187. doi: 10.1161/01.res.88.2.181. [DOI] [PubMed] [Google Scholar]
- Varro A, Negretti N, Hester SB, Eisner DA. An estimate of the calcium content of the sarcoplasmic reticulum in rat ventricular myocytes. Pflugers Arch. 1993;423:158–160. doi: 10.1007/BF00374975. [DOI] [PubMed] [Google Scholar]
- Wier WG, Cannell MB, Berlin JR, Marban E, Lederer WJ. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987;235:325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Wier WG, Kort AA, Stern MD, Lakatta EG, Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983;80:7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]