Abstract
Prolonged or unaccustomed exercise leads to loss of contractility and muscle cell damage. The possible role of an increased uptake of Ca2+ in this was explored by examining how graded fatiguing stimulation, leading to a graded uptake of Ca2+, results in progressive loss of force, impairment of force recovery, and loss of cellular integrity. The latter is indicated by increased [14C]sucrose space and lactic acid dehydrogenase (LDH) release. Isolated rat extensor digitorum longus (EDL) muscles were allowed to contract isometrically using a fatiguing protocol with intermittent stimulation at 40 Hz. Force declined rapidly, reaching 11% of the initial level after 10 min and stayed low for up to 60 min. During the initial phase (2 min) of stimulation 45Ca uptake showed a 10-fold increase, followed by a 4- to 5-fold increase during the remaining period of stimulation. As the duration of stimulation increased, the muscles subsequently regained gradually less of their initial force. Following 30 or 60 min of stimulation, resting 45Ca uptake, [14C]sucrose space, and LDH release were increased 4- to 7-fold, 1.4- to 1.7-fold and 3- to 9-fold, respectively (P < 0.001). The contents of Ca2+ and Na+ were also increased (P < 0.01), a further indication of loss of cellular integrity. When fatigued at low [Ca2+]o (0.65 mm), force recovery was on average twofold higher than that of muscles fatigued at high [Ca2+]o (2.54 mm). Muscles showing the best force recovery also had a 41% lower total cellular Ca2+ content (P < 0.01). In conclusion, fatiguing stimulation leads to a progressive functional impairment and loss of plasma membrane integrity which seem to be related to an excitation-induced uptake of Ca2+. Mechanical strain on the muscle fibres does not seem a likely mechanism since very little force was developed beyond 10 min of stimulation.
Intense exercise, such as extended periods of running, strength training, sprinting or eccentric exercise, disrupts the normal ultrastructure of skeletal muscle (Waterman-Storer, 1991; Belcastro, 1993; Belcastro et al. 1998). Impaired force recovery seen after intense exercise can be due to structural damage such as myofibrillar and cytoskeletal disruptions often leading to Z-disc streaming (Hoppeler, 1986; Waterman-Storer, 1991; Appell et al. 1992; Gibala et al. 1995), as well as disturbances to mitochondria and the SR–T-tubular system (Hoppeler, 1986; Gibala et al. 1995). Loss of sarcolemmal integrity has also repeatedly been reported after prolonged or unaccustomed exercise (e.g. Stupka et al. 2001; Clarkson & Hubal, 2002), contributing to the functional impairment.
Several mechanisms have been proposed to explain the functional impairment and it is unlikely to be caused by one single factor. The causes of functional impairment could be either mechanical damage due to eccentric contractions (Armstrong, 1986, 1990), loss of excitability (Nielsen & Overgaard, 1996; Clausen, 1996; Overgaard et al. 1999; Carlsen & Villarin, 2002), or Ca2+-induced damage (Gissel & Clausen, 2001; Carlsen & Villarin, 2002; Gissel & Clausen, 2003). Extracellular Ca2+ is important in mediating at least two forms of muscle damage, sarcolemmal leakage (Jackson et al. 1984) and ultrastructural damage to SR, mitochondria and Z-lines (Jones et al. 1984).
During muscle excitation, the influx of Ca2+ is markedly increased, leading to a progressive intracellular accmulation of Ca2+ both in vitro (Bianchi & Shanes, 1959; Curtis, 1966; Gissel & Clausen, 1999, 2000) and in vivo (Sreter et al. 1980; Everts et al. 1993). Ca2+ may enter normal, contracting muscle cells through voltage gated Na+ channels, through voltage gated L-type Ca2+ channels (Gissel & Clausen, 1999, 2001) and through stretch-activated channels (Belcastro et al. 1996; McBride et al. 2000). Store-operated Ca2+ uptake may occur through the transient receptor potential (TRP) channels which can also function as Ca2+ influx channels in skeletal muscle (Kurebayashi & Ogawa, 2001; Vandebrouck et al. 2002). A later increase in cellular Ca2+ could arise from disruption of the sarcolemma, which would allow passive influx of Ca2+ down the electrochemical gradient (Duan et al. 1990; Armstrong et al. 1991; Carlsen & Villarin, 2002). If the Ca2+ influx exceeds the intracellular buffer capacity (SR, mitochondria and cytosolic proteins), resting free intracellular Ca2+ ([Ca2+]i) may rise (Sreter et al. 1987; Lynch et al. 1997), which may in turn lead to cellular damage and thus have long-term consequences for muscle structure and function (Carlsen & Villarin, 2002).
If the cytosolic Ca2+ is elevated to critical levels for sufficient periods of time either globally or within specific compartments of the muscle fibre, Ca2+-activated degradative mechanisms may be initiated. The increased [Ca2+]i may lead to mitochondrial Ca2+ uptake and thus impair respiration (Duan et al. 1990). It may also activate phospholipases (Duncan & Jackson, 1987; Duan et al. 1990) leading to damage to the sarcolemma or Ca2+-dependent proteases, such as calpain (Duan et al. 1990; Belcastro, 1993; Belcastro et al. 1998), resulting in ultrastructural damage to myofibrils, cytoskeleton or triads (Balnave & Allen, 1995; Ingalls et al. 1998). Another effect of increased cytosolic Ca2+ is the production of reactive oxygen species (ROS) leading to peroxidation of membrane lipids (Reid & Li, 2001). It has also been proposed that raised [Ca2+]i plays a role in impairment of excitation–contraction (E-C) coupling (Lamb et al. 1995; Bruton et al. 1998) during extended, vigorous muscle activity (Jones, 1996; Chin & Allen, 1996; Carlsen & Villarin, 2002).
The aim of this study was to further investigate the role of Ca2+ uptake in the development of muscle damage as indicated by loss of force and cellular integrity after an electrical stimulation protocol simulating a fatiguing exercise situation. Since extensor digitorum longus (EDL) has previously been shown to respond to stimulation with a large influx of Ca2+ (Gissel & Clausen, 1999, 2000), this muscle was chosen for the study.
Part of the results of this study have been presented in preliminary versions (Mikkelsen et al. 2003, 2004).
Methods
Animals
All experiments were carried out using 4-week-old female or male Wistar rats weighing 60–70 g (own breed). Animals of this size were chosen to obtain muscles of a relatively small size to improve diffusion and oxygenation during incubation. The rats had free access to food and water and were maintained at a constant temperature (21°C) with constant day length (12 h). All handling and use of animals complied with Danish animal welfare regulations.
Muscle preparation and incubation
Animals were killed by cervical dislocation followed by decapitation and intact extensor digitorum longus muscles, weighing 20–30 mg, were excised as previously described (Chinet et al. 1977). The standard incubation medium was a Krebs-Ringer bicarbonate buffer (pH 7.2–7.4) containing (mm): 122.1 NaCl, 25.1 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.27 CaCl2 and 5.0 d-glucose. After preparation, the muscles were mounted on electrodes for isometric contractions. The buffer was continuously gassed with a mixture of 95% O2 and 5% CO2. All muscles were equilibrated for a minimum of 30 min in the standard medium before further treatment. This procedure has been shown to allow the maintenance of constant membrane potential and Ca2+, Na+ and K+ contents for several hours in vitro (Clausen & Flatman, 1977; Everts & Clausen, 1986). Incubations took place at 30°C in a volume of 5 or 23 ml.
Fatiguing protocol
A standard fatiguing stimulation protocol was applied in all experiments: the muscles were stimulated intermittently (10 s on, 30 s off) at 40 Hz (1 ms pulses of 10 V). In a few experiments a pulse duration of 0.02 ms was used in order to obtain indirect stimulation. The contractions elicited by this pulse duration were completely suppressed by blocking the acetylcholine receptors at the neuromuscular junction by tubocurarine (10−5 m). Stimulation was applied through two platinum electrodes surrounding the central part of the muscle. Different groups of muscles were stimulated for 0, 1, 2, 5, 15, 30, 45 or 60 min so as to follow variations in the measured parameters with stimulation duration. The stimulation protocol was chosen in order to obtain an exercise situation with a reasonably short time horizon to allow comparison of the time course of fatigue development and force recovery, changes in 45Ca influx and loss of cellular integrity.
Since the distance between the stimulation electrodes was 4 mm, 10 V pulses correspond to an electrical field of 25 V cm−1. This is far below the fields required to produce electroporation and cell damage. We found that in the same muscle preparation, this requires around 300 V cm−1 (Gissel & Clausen, 2003).
Force measurement
The experimental set-up used for force measurements allowed for the simultaneous stimulation of four muscles in 23 ml incubation chambers and recording of the force developed. EDL muscles were mounted on electrodes and during repeated stimulation with single pulses adjusted to optimal length for twitch force development. Isometric force development was measured using a force transducer (Grass FTO3) and recorded with Servogor chart recorders. Prior to stimulation all four muscles were tested at 90 Hz (1 ms pulses of 10 V) for 0.5 s to achieve maximal tetanic activation of all muscle fibres. Testing was done three times at 15 min intervals before the fatiguing stimulation to define the starting level. Force development was recorded during the fatiguing stimulation. Subsequently, force recovery was tested using the same 90 Hz, 0.5 s pulse trains as prior to stimulation at time 0, 15, 30, 60, 90 and 120 min after cessation of stimulation. Finally, the muscles were taken down, the tendons removed and the muscles blotted, weighed and stored at −50°C until Ca2+, Na+ and K+ contents could be measured (see later section). Experiments with different Ca2+ concentrations were performed using an almost identical procedure. After testing three times at 90 Hz the buffer was changed to buffer containing either low (0.65 mm) or high (2.54 mm) concentration of Ca2+ and testing was done three more times to ensure that the change in buffer did not directly affect the force development. Muscles were then stimulated intermittently (10 s on, 30 s off) at 40 Hz for 30 min and immediately after cessation of stimulation the buffer was changed back to normal Ca2+ before testing at 90 Hz.
In parallel experiments, 45Ca uptake, [14C]sucrose space, release of lactate dehydrogenase (LDH) and changes in the contents of Ca2+, Na+ and K+ were examined. The experimental set-up used in these studies allowed for the simultaneous stimulation of 12 muscles in 5 ml incubation tubes. EDL muscles were mounted at resting length on electrodes for isometric contractions and slightly stretched to be comparable to those mounted in the force transducers. Force development was not measured in these experiments.
45Ca uptake
During the last 15 min of fatiguing stimulation the muscles were incubated in buffer containing 45Ca (0.5 μCi ml−1). In some experiments the Na+ channel blocker tetrodotoxin (TTX; 10−6 m) was added at the same time as incubation with 45Ca or 15 min before the onset of stimulation. In order to obtain an accurate measurement of the uptake of 45Ca into the intracellular compartment within this relatively short period, the muscles were subsequently washed in ice-cold Ca2+-free buffer containing 0.5 mm EGTA for 4 × 30 min to remove extracellular 45Ca. Finally the tendons were removed, and the muscles were blotted, weighed and soaked overnight in 3 ml 0.3 m trichloroacetic acid (TCA) in a refrigerator at 5°C to extract all ions from the muscle. The next day 45Ca activity of the TCA extract was determined by liquid scintillation counting (Packard, Tri-Carb 2100 TR) and 45Ca uptake was calculated on the basis of this and the specific activity of 45Ca in the incubation medium. The uptake was corrected for loss of intracellular 45Ca during the washout in ice-cold Ca2+-free buffer using a previously determined correction factor of 1.6 (Gissel & Clausen, 1999).
The initial phase of electrical stimulation was examined by incubating muscles for 15 min in buffer containing 45Ca (1 μCi ml−1). During the last 2 min of this incubation, the fatiguing 40 Hz stimulation was applied (10 s on, 30 s off, corresponding to 3 × 10 s of stimulation). In a few experiments, the effects of indirect stimulation (0.02 ms pulses) and tubocurarine (10−5 m) were examined.
The uptake of 45Ca during 120 min rest after fatiguing stimulation was also followed. 45Ca uptake was determined as described above by incubation with 45Ca for 15 min at chosen time intervals during recovery, followed by washout in ice-cold Ca2+-free buffer.
Resting [14C]sucrose space
In order to detect loss of cellular integrity and ensuing increase in extracellular space, experiments were performed using [14C]sucrose as an extracellular marker. Following fatiguing stimulation the muscles were incubated for 90 min in buffer containing [14C]sucrose (0.1 μCi ml−1) and 1 mm unlabelled sucrose as a carrier. After incubation, the tendons were removed and the muscles were blotted, weighed and soaked overnight in 0.3 m TCA with 0.1 mm sucrose. The next day the activity of [14C]sucrose in the TCA extract was determined by liquid scintillation counting and the uptake of [14C]sucrose in the muscle was calculated by comparison with the specific activity of [14C]sucrose in the incubation medium. TTX (10−6 m) was added in some experiments in order to ascertain that the loss of cellular integrity was elicited by excitation and not by unintentional electroporation. In a few experiments the effects of indirect stimulation were examined.
LDH release
Muscle cell integrity was monitored by measuring the release of LDH into the incubation medium. LDH release is expressed as units (U, see below) per gram wet weight per 30 min (Gissel & Clausen, 2000). After the muscles had been mounted on the electrodes they were pre-washed for 4 × 30 min to wash out any LDH released from cells damaged during excision of the muscles. Buffer samples (500 μl) for determination of the pre-level of LDH release were taken from the last of the four pre-wash tubes. Then the muscles were stimulated intermittently as described above. After stimulation recovery was followed for up to 240 min. The muscles were moved to new tubes every 15 or 30 min, and buffer samples (500 μl) were taken immediately after removal of the muscle. Bovine serum albumin (BSA) was added to the buffer samples at a final concentration of 0.1% and the samples were kept on ice until measurement. A 250 μl sample was mixed with 2.65 ml phosphate buffer (0.1 m K2HPO4 titrated with KH2PO4 to pH 7.0) containing NADH (0.3 mm) and pyruvate (0.8 mm). Then the absorbance of NADH was monitored at 340 nm for 3.5–6 min (Perkin Elmer, Lambda 20) at 30°C. The activity of LDH in the buffer was determined by measuring the decrease in the concentration of the substrate NADH caused by conversion of pyruvate to lactate (expressed as U (g wet wt)−1, 1 unit (U) being the amount of enzyme that catalyses the utilization of 1 μmol substrate min−1). Spontaneous release of LDH was monitored in resting control muscles. At the end of the experiment the tendons were removed, and the muscles were blotted, weighed and soaked overnight in 0.3 m TCA for later determination of Ca2+, Na+ and K+ contents.
Ca2+, Na+ and K+ contents
Whether muscles were frozen or taken directly after the experiment, they were all soaked overnight in 3 ml 0.3 m TCA to extract all ions. Earlier studies showed that using this procedure the extraction of these ions from the whole muscle was as complete as that achieved by homogenization and subsequent centrifugation of the TCA extract (Clausen et al. 1993; Gissel & Clausen, 2000). Ca2+ content was determined by atomic absorption spectrophotometry (Philips PU 9200, Pye Unicam, Cambridge, UK) using 1.5 ml of the TCA extract mixed with 150 μl of 0.27 m KCl. The muscle extracts were measured against a blank and standards containing 12.5, 25 or 50 μm Ca2+ and the same amount of TCA and KCl. To obtain the total intracellular Ca2+ content, the values from atomic absorption were corrected in the following ways: When no washout in ice-cold Ca2+-free buffer had been applied the values were corrected for the Ca2+ content in the extracellular space by subtraction of the concentration of Ca2+ in the buffer, 1.27 mm, multiplied by the extracellular fraction (water space) of the muscle determined as the [14C]sucrose space. When the muscles had been washed in ice-cold Ca2+-free buffer the values were multiplied by 1.6 to correct for the loss of intracellular Ca2+ during washout. Na+ and K+ contents of the TCA extracts were determined using a Radiometer FLM3 flame photometer (Copenhagen, Denmark) with lithium as internal standard. For each sample 0.5 ml of the TCA extract, 1.5 ml 5 mm LiCl and 0.5 ml 0.3 m TCA were used.
Chemicals and isotopes
All chemicals were of analytical grade. BSA, TTX and tubocurarine were purchased from Sigma Chemical Co. (St Louis, MO, USA); NADH and pyruvate were from Boehringer Mannheim (Mannheim, Germany). 45Ca (1.31 Ci mmol−1) and [14C]sucrose (0.6 Ci mmol−1) were obtained from Amersham International (Aylesbury, UK).
Statistics
Results are given as mean values ± s.e.m. The statistical significance of any difference between groups was ascertained using Student's two-tailed t test for unpaired observations. All comparisons made were between separate groups of muscles.
Results
Force development and 45Ca uptake during stimulation
As shown in Fig. 1, during fatiguing stimulation at 40 Hz (10 s on, 30 s off, 10 V, 1 ms pulses), force declined rapidly from the maximum of 0.28 ± 0.01 N (n = 46), reaching a value of 0.031 ± 0.002 (n = 35) after only 10 min of stimulation. Force development then stayed low and declined during the remaining period of stimulation. Using indirect stimulation (0.02 ms pulses) a similar force decline was found although at lower absolute values, starting at a maximum of 0.15 ± 0.02 N (n = 8).
Figure 1. Maximum force developed in EDL during fatiguing 40 Hz stimulation.
Muscles were mounted at optimal length and – after equilibration for 30 min – stimulated at 40 Hz (10 s on, 30 s off, 10 V, 1 ms) for up to 60 min. The maximum force (in newtons) during each 10 s tetanus is shown throughout the stimulation period. All force measurement experiments are included and stimulation time varied from 1 to 60 min. Mean values ± s.e.m. are shown, n = 5–46 muscles.
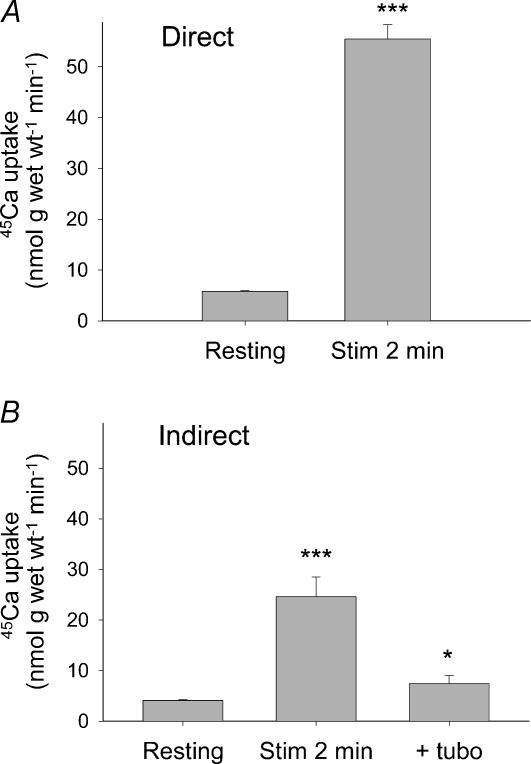
To investigate possible effects of extracellular Ca2+, the uptake of 45Ca was measured during the fatiguing stimulation. As shown in Fig. 2A, only 2 min of direct stimulation increased 45Ca uptake 10-fold, from 5.8 ± 0.2 to 56 ± 3 nmol (g wet wt)−1 min−1. This marked increase is in keeping with earlier studies (Gissel & Clausen, 2000) and demonstrates that the excitation-induced Ca2+ influx is a primary event (rapid in onset) and unlikely to reflect progressive impairment of energy metabolism or structural damage caused by the contractions. Since the stimulation of 45Ca uptake was markedly reduced by TTX (Gissel & Clausen, 2000), it could not be attributed to loss of integrity due to electroporation of the membrane, caused by the stimulation pulses. As shown in Fig. 2B, indirect stimulation also induced a marked (6-fold) rise in 45Ca uptake, which was almost completely (84%) inhibited by tubocurarine and thus not caused by electroporation damage either.
Figure 2. 45Ca uptake during initial stimulation (3 × 10 s).
Muscles were mounted at resting length and – after equilibration for 30 min – incubated for 15 min in buffer containing 45Ca (0.2–1 μCi ml−1). During the last 2 min of 45Ca-incubation the muscles were stimulated at 40 Hz (10 s on, 30 s off) using 1 ms (A, Direct) or 0.02 ms (B, Indirect) pulses. This was followed by washout in ice-cold Ca2+-free buffer. When indicated (B, last column) tubocurarine (10−5 m) was added 15 min before 45Ca incubation and was present throughout the incubation. 45Ca uptake is in nmol (g wet wt)−1 min−1. Mean values with bars denoting s.e.m. are shown. Significance of difference from resting controls is indicated by asterisks: *P < 0.05, ***P < 0.001, n = 5–8 muscles.
Figure 3 shows that during the fatiguing stimulation, 45Ca uptake as measured in time intervals of 15 min underwent a progressive 4- to 5-fold increase during stimulation (open bars) compared to the resting value. In all instances, the increase in 45Ca uptake in stimulated muscles was significant (P < 0.001). During the first 15 min of stimulation the uptake was 213 ± 18 nmol (g wet wt)−1 (15 min)−1. In the following three 15-min periods the uptake was 261 ± 9, 339 ± 16 and 340 ± 17 nmol (g wet wt)−1 (15 min)−1, respectively. The uptake was significantly increased during the last three 15-min periods compared to the first period (P < 0.05). Thus, the excitation-induced increase in 45Ca uptake persisted even after force development had been reduced by 90–95%. In order to assess the role of excitation in the uptake of Ca2+, the specific Na+-channel blocker, TTX, was added to the buffer at the onset of incubation with 45Ca. Previous studies have shown that 79% of the excitation-induced 45Ca uptake in EDL is suppressible by TTX (Gissel & Clausen, 1999, 2000). In the present experiments (Fig. 3, filled bars) TTX caused a significant inhibition (65%) of the excitation-induced 45Ca uptake during the first 15-min period. In the following 15-min periods, the decrease induced by TTX was 70% and 60%, respectively. In the last period, however, TTX no longer induced a significant decrease in 45Ca uptake, suggesting that after 45 min of stimulation a major part of the 45Ca uptake no longer depends on activation of the Na+ channels, but reflects unspecific cell membrane leakage. It should be noted that since TTX was added at the start of incubation with 45Ca, its effect may be underestimated, as diffusion to the core of the muscle causes some delay in the onset of action. From the data in Fig. 3, it could be calculated that, as compared to the first 15-min period, the TTX insensitive uptake of 45Ca into the muscles increased by 13, 111 and 616% in the second, third and fourth 15-min period, respectively.
Figure 3. 45Ca uptake during fatiguing stimulation and effect of TTX.
EDL muscles were mounted at resting length and – after equilibration for 30 min – stimulated for 15, 30, 45 or 60 min. During the last 15 min of stimulation the muscles were incubated in buffer containing 45Ca (0.5 μCi ml−1) and when indicated TTX (10−6 m). This was followed by washout in ice-cold Ca2+-free buffer. In one group of muscles, last column (t), TTX was added 15 min before the onset of stimulation and was present throughout the 60 min of stimulation. 45Ca uptake is in nmol (g wet wt)−1 (15 min)−1. Mean values with bars denoting s.e.m. are shown. Significance of difference between muscles stimulated for the same time period without or with TTX is indicated by asterisks: *P < 0.05, ***P < 0.001, n = 3–9 muscles. The uptake of 45Ca in resting muscles (71 ± 2 nmol (g wet wt)−1 (15 min)−1, n = 37) was measured at representative intervals and the mean value is given as a horizontal line.
In contrast, when TTX (10−6 m) was present 15 min before and throughout the 60 min of fatiguing stimulation (Fig. 3, last column, t), 45Ca uptake measured during the last 15 min of stimulation was reduced by 64% (to 169 ± 12 nmol (g wet wt)−1 (15 min)−1). Since 10−6 m TTX should be sufficient to abolish the action potential in all fibres, it is surprising that 45Ca uptake was not completely suppressed. The remaining rise in 45Ca uptake is likely to represent influx through other channels, particularly Ca2+ channels or unspecific cell membrane leakage.
Recovery of force and cellular integrity after stimulation
To evaluate the effects of stimulation for 1–60 min on muscle function and integrity, a variety of parameters were measured during subsequent recovery periods of up to 240 min. After cessation of stimulation the recovery of force was followed for 90 min as shown in Fig. 4. No further improvement was found when force recovery was followed longer. As the duration of stimulation was increased, the muscles recovered gradually less of their initial force. After 1–5 min of stimulation, 97–58% force recovery was seen, but after 60 min of stimulation, there was less than 10% force recovery. In contrast, the maximum tetanic contractions of the unstimulated control muscles remained at about 100%, confirming that the viability of the muscles was maintained. The total cellular Ca2+ content of the muscles (corrected for Ca2+ content in the extracellular space) is also shown in Fig. 4. It should be noted that it increases with increasing duration of stimulation.
Figure 4. Force recovery after fatiguing stimulation.
Force is given as a percentage of the mean initial force tested before the onset of the 40 Hz fatiguing stimulation using 0.5 s pulse trains of 90 Hz. EDL muscles were mounted at optimal length and – after 30 min of equilibration – tested 3 times at 90 Hz. After 40 Hz fatiguing stimulation of the indicated duration, recovery was tested at 90 Hz at 0, 15, 30, 60 and 90 min after cessation of stimulation. Different symbols designate the different durations of stimulation, and the stimulation time is given. Total cellular Ca2+ content after 120 min recovery is given as well. Mean values ± s.e.m. are shown. Significance of difference in cellular Ca2+ content from resting control muscles is indicated by asterisks: ***P < 0.001, n = 3–22.
To elucidate the possible role of the excitation-induced Ca2+ uptake (Fig. 3) in reducing force recovery, additional contraction experiments were performed with muscles stimulated for 30 min in buffer with either high (2.54 mm) or low (0.65 mm) concentration of Ca2+.
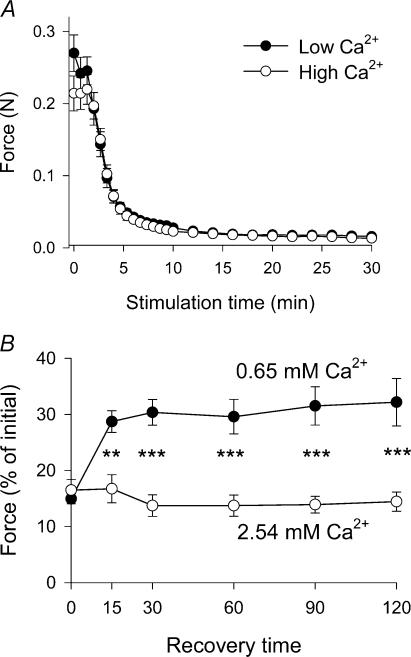
These experiments showed that the tetanic force, elicited by 0.5 s of 90 Hz stimulation, was the same at these two Ca2+ concentrations (data not shown). Likewise, as shown in Fig. 5A, the time course of changes in the force development during fatiguing stimulation at 40 Hz was not significantly different at any time. However, as shown in Fig. 5B, the post-stimulatory force recovery – which was tested in buffer with normal Ca2+ content (1.27 mm) – was significantly improved after stimulation at low [Ca2+]o. On average, the force recovery of muscles stimulated at 0.65 mm Ca2+ was about twofold higher than that of muscles stimulated at 2.54 mm Ca2+. As for total muscle Ca2+ content measured after 120 min of recovery it was significantly lower (P < 0.01) in muscles stimulated at 0.65 mm Ca2+ than in muscles stimulated at 2.54 mm Ca2+ (1.3 ± 0.2 versus 2.2 ± 0.2 μmol (g wet wt)−1, respectively). Ca2+ values were corrected to total cellular Ca2+ content by subtraction of Ca2+ content in the extracellular space.
Figure 5. Force decline (A) and force recovery (B) of muscles stimulated at high (2.54 mm) or low (0.65 mm) [Ca2+]o.
A, force decline during stimulation. Muscles were mounted at optimal length and – after equilibration for 30 min – stimulated at 40 Hz (10 s on, 30 s off, 10 V, 1 ms) for 30 min with either high or low concentration of Ca2+ in the incubation medium. The maximum force during each 10 s tetanus is shown throughout the stimulation period. B, force recovery after stimulation. Force is given as a percentage of the mean initial force, which was tested 3 times at 90 Hz before the onset of fatiguing stimulation. Then buffer Ca2+ was changed to the indicated values and the muscles were stimulated at 40 Hz as shown in A. Immediately after cessation of stimulation the buffer was changed back to standard [Ca2+]o (1.27 mm) before testing force recovery as described in the legend to Fig. 4. Significance of difference between muscles incubated at high and low Ca2+ is indicated by asterisks: **P < 0.01, ***P < 0.001. In both A and B mean values ± s.e.m. are given, n = 7–8.
As seen in Fig. 6 the resting uptake of 45Ca during the first 15 min of recovery showed a marked progressive increase following 15, 30 and 60 min of stimulation (P < 0.001). This increase in 45Ca uptake could be detected long after the cessation of stimulation (data not shown). During the first three 15 min post-stimulatory periods following 60 min stimulation, 45Ca uptake was 504 (Fig. 6), 357 and 355 nmol (g wet wt)−1, respectively, adding up to a total accmulation of 1215 nmol (g wet wt)−1 in the first 45 min post-stimulation. This is well in accordance with atomic absorption measurements of the increase in Ca2+ content during 45 min recovery following 60 min stimulation, showing a net accmulation of 1.4 μmol (g wet wt)−1 (Table 1). Thus, the muscles continue to take up Ca2+ after cessation of stimulation suggesting a general increase in sarcolemmal permeability.
Figure 6. Resting 45Ca uptake during the first 15 min recovery after fatiguing stimulation.
Muscles were mounted at resting length and – after 30 min of equilibration – stimulated for the time indicated (in min). After cessation of stimulation muscles were directly incubated with 45Ca (0.5 μCi ml−1) for 15 min. This was followed by washout in ice-cold Ca2+-free buffer. Ca2+ uptake is given in nmol (g wet wt)−1 (15 min)−1. Mean values are given with bars denoting s.e.m. Significance of difference from resting muscles is indicated by asterisks: ***P < 0.001, n = 9–12.
Table 1.
Total, cellular Ca2+ content after fatiguing stimulation
| Ca2+ content (μmol g wet weight−1) | ||
|---|---|---|
| Stimulation time | 0 min recovery | 45 min recovery |
| 0 min† | 1.4 ± 0.1 (9) | 1.4 ± 0.1 (9) |
| 15 min | 1.5 ± 0.2 (5) | 1.2 ± 0.1 (4) |
| 30 min | 1.4 ± 0.2 (6) | 2.3 ± 0.2 (3)*** |
| 60 min | 1.9 ± 0.2 (6)** | 3.3 ± 0.1 (3)*** |
resting control. Muscles were mounted at resting length and – after 30 min of equilibration – stimulated for the time indicated. Values are corrected for washout in ice-cold Ca2+-free buffer as described in Methods. Mean values ± s.e.m. are given. Significance of difference from resting control muscles is indicated by asterisks:
P < 0.01
P < 0.001. The numbers of observations are given in parentheses.
To further investigate the stimulation-induced permeabilization of the sarcolemma, experiments were conducted using the extracellular marker [14C]sucrose. As shown in Fig. 7, the uptake of [14C]sucrose during the first 90 min of recovery increased with the duration of the preceding fatiguing stimulation. In the unstimulated muscles 22.8 ± 0.8% of the muscle volume was accessible to [14C]sucrose, which is identical to the value previously found for the sucrose space in EDL muscles (Gissel & Clausen, 2003). After 15, 30 or 60 min of stimulation, the [14C]sucrose space increased to 25.5 ± 1.6%, 32.8 ± 2.2% and 39.7 ± 1.4%, respectively. The increases were significant (P < 0.001) after 30 and 60 min of stimulation. To ensure that this did not reflect electroporation damage to muscle fibres, some muscles were stimulated for 60 min with TTX (10−6 m) present 15 min before and throughout the fatiguing stimulation. This entirely suppressed the increase in [14C]sucrose space as also shown in Fig. 7 (last column). As a further evaluation of cellular integrity, Na+ and K+ contents were also measured in these experiments. This showed an increase in Na+ content of 26.3 μmol (g wet wt)−1 and an almost equivalent decrease in K+ content (25.8 μmol (g wet wt)−1) in muscles stimulated for 60 min compared to resting muscles.
Figure 7. Resting uptake of [14C]sucrose during the first 90 min after fatiguing stimulation.
The uptake is expressed as percentage filling (percentage of the muscle volume accessible to [14C]sucrose) and given for muscles either resting throughout the experiment or stimulated for 15, 30 or 60 min prior to incubation with [14C]sucrose. Muscles were mounted at resting length and – after 30 min of equilibration – stimulated as indicated. After cessation of stimulation muscles were incubated in buffer containing [14C]sucrose (0.1 μCi ml−1) and 1 mm sucrose for 90 min. The last column shows results obtained when TTX (10−6 m) was added 15 min before the onset of stimulation and was present throughout 60 min of stimulation. Mean values are given with bars denoting s.e.m. Significance of difference from resting control muscles is indicated by asterisks: ***P < 0.001, n = 9.
Similar experiments performed using indirect stimulation (0.02 ms pulses) also showed an increase in [14C]sucrose space from 22.8% in resting muscles to 29.6% in muscles stimulated for 60 min (P = 0.007, n = 6, data not shown).
To further elucidate the increased sarcolemmal permeability after stimulation, release of the intracellular enzyme LDH was followed for 4 h after stimulation. As shown in Fig. 8, 15 min of stimulation resulted in no increase in LDH release after cessation of stimulation in comparison to resting controls. After 30 min stimulation, a 3-fold increase was found and 60 min of stimulation increased LDH release up to 9-fold. Peak release of LDH was seen 60 min after cessation of stimulation, gradually declining with longer recovery. However, for muscles stimulated for 60 min, LDH release was still increased 6-fold after 240 min of recovery. Cellular Ca2+ content measured after 240 min recovery was significantly increased in muscles stimulated for 30 or 60 min, by 36% and 150%, respectively (Fig. 8).
Figure 8. Release of the intracellular enzyme LDH following stimulation.
Muscles were mounted at resting length and pre-washed for 4 × 30 min before onset of stimulation. A buffer sample was taken from the last pre-wash tube for determination of background LDH activity (pre). Following stimulation the muscles were moved to new tubes every 15–30 min and LDH release measured in the time intervals indicated. Immediately after removal of the muscle, buffer samples were taken for determination of LDH activity. The open symbols are resting controls, and stimulation time is given for the stimulated muscles. Total cellular Ca2+ content of the muscles after 240 min recovery is given as well. Mean values ± s.e.m. are given. For muscles stimulated 30 or 60 min, significance of the difference from resting control muscles at corresponding points of time is indicated by asterisks: *P < 0.05, **P < 0.01, ***P < 0.001, n = 6.
Ca2+, Na+ and K+ contents
As shown in Table 1 stimulation for 30 or 60 min leads to a post-stimulatory accmulation of Ca2+. The cellular Ca2+ content immediately after 60 min stimulation was 1.9 ± 0.2 μmol (g wet wt)−1, increasing to 3.3 ± 0.1 μmol g−1 after 45 min of recovery. Thus an increase in Ca2+ content of 1.4 μmol g−1 was observed in the first 45 min of recovery. As noted above, measurements of 45Ca uptake in the same time period gave a similar value. After 30 min stimulation no increase in cellular Ca2+ content was seen immediately after cessation of stimulation, but after 45 min of recovery cellular Ca2+ content had increased to 2.3 ± 0.2 μmol g−1. It should be noted that the Ca2+ content was still significantly increased after 120 and 240 min recovery in muscles stimulated for 30 or 60 min (Figs 4 and 8).
Figure 9 shows total Na+ and K+ contents after 120 min recovery from 15, 30 or 60 min of fatiguing stimulation. As shown in Fig. 9A the Na+ content in resting EDL muscles was 46 ± 1 μmol (g wet wt)−1. When muscles were stimulated for 30 or 60 min, the value was increased to 66 ± 3 and 75 ± 1, respectively. As shown in Fig. 9B an almost equivalent fall in total K+ content was observed. The K+ content declined from 105 ± 2 μmol (g wet wt)−1 in resting muscles to 92 ± 3 and 81 ± 3 when the muscles were stimulated for 30 or 60 min, respectively. Thus, after 120 min recovery from 60 min stimulation, the Na+ content was increased by 29 and the K+ content decreased by 24 μmol (g wet wt)−1. These values correspond very well with the changes in Na+ and K+ contents observed after 90 min recovery, showing an increase in Na+ and decrease in K+ content of 26 μmol (g wet wt)−1, as described in the experiments with [14C]sucrose.
Figure 9. Total Na+(A) and K+(B) contents after 120 min recovery.
Muscles were mounted at optimal length and – after 30 min of equilibration – stimulated for the time indicated. After cessation of stimulation muscles were resting for 120 min, while force recovery was tested as described in legend to Fig. 3. Finally the muscles were taken for determination of Na+ and K+ content. Mean values are given with bars denoting s.e.m. Significance of difference from resting control muscles is indicated by asterisks: *P < 0.05, **P < 0.01, ***P < 0.001, n = 3–12.
Discussion
A fatiguing stimulation protocol simulating an exercise situation was used to investigate the role of Ca2+ uptake in the impairment of force recovery and the development of muscle cell damage. The main finding was that the loss of contractility and cellular integrity (as indicated by increased resting 45Ca uptake, [14C]sucrose space and LDH release) observed during recovery, increased progressively with the duration of stimulation. This was seen despite very modest force development beyond 10 min of stimulation. Since this was associated with unabated uptake of Ca2+, the loss of contractility and cellular integrity seems to be related at least in part to stimulation-induced Ca2+ uptake rather than to mechanical strain on the muscle fibres.
Force
Force declined rapidly during the fatiguing 40 Hz stimulation, and after 10 and 60 min force development was only 11 and 4% of the initial level, respectively (Fig. 1). This early loss of force is typical of fast-twitch muscles (Nielsen & Clausen, 2000), and has been attributed to loss of excitability (Nielsen & Clausen, 1997, 2000; Overgaard et al. 1999; Clausen et al. 2004), energy depletion (Fitts, 1994) and increased Pi (Westerblad & Allen, 2002). When stimulation was performed using 0.02 ms pulses, causing indirect, tubocurarine-suppressible contractions, max initial force was 47% lower. This may reflect incomplete activation of fibres.
Following 1 min of fatiguing 40 Hz direct stimulation, about 90% force recovery was reached in 15–30 min. With stimulation of longer duration (5–60 min), however, force recovery underwent gradually more pronounced and persistent suppression (Fig. 4). Several factors might be involved in this impairment of force recovery, and the focus of this study is on the role of Ca2+.
45Ca uptake and Ca2+ content
Previous studies have shown that electrical stimulation increases the influx and accmulation of Ca2+ in skeletal muscle (Bianchi & Shanes, 1959; Curtis, 1966; Sreter et al. 1980; Everts et al. 1993; Gissel & Clausen, 1999, 2000, 2003). A maximum of only 20% of the excitation-induced Ca2+ influx occurs via L-type Ca2+ channels, the remaining possibly occurring through voltage gated Na+ channels (Gissel & Clausen, 1999). In the present study we observed a 10-fold increase in 45Ca uptake after only 2 min of direct stimulation. Indirect stimulation caused a 6-fold increase in 45Ca-uptake which was tubocurarine-suppressible. Ongoing studies have shown that total anoxia causes no significant increase in 45Ca uptake until after 15 min of incubation (Fredsted et al. 2004). Taken together these observations indicate that the early influx of Ca2+ is primarily related to the excitation and unlikely to be secondary to electroporation of the membrane or hypoxia.
When the fatiguing stimulation was continued for 15–60 min there was a 4- to 5-fold increase in the uptake of 45Ca (Fig. 3). During the first 45 min, 60–70% of the stimulation-induced 45Ca uptake was suppressed by the Na+-channel blocker TTX, whereas in the following and last 15-min period, TTX caused no inhibition. In contrast, muscles exposed to TTX for 15 min before and throughout 60 min of stimulation showed a 64% suppression of 45Ca-uptake in the last 15-min period, indicating that blocking the voltage-sensitive Na+ channels throughout stimulation protects against the late rise in Ca2+ uptake. Thus the initial influx of Ca2+ seems to be associated with the excitation process, whereas during the last 15 min of stimulation Ca2+ influx no longer depends on excitation. This indicates an increasing degree of damage to the plasma membrane and an ensuing acceleration of TTX-insensitive, unspecific leakage of 45Ca into the muscle from the extracellular space. This leakage persists even after the cessation of stimulation. During recovery, the uptake of 45Ca was increased in all stimulated muscles (Fig. 6), the uptake being higher the longer the muscles had been stimulated. Moreover, this increase could be detected long after the cessation of stimulation. This is also evidenced by the concomitant increase in Ca2+ content during the first 45 min of recovery (Table 1). Thus, as noted in Results, these two measures (45Ca uptake and increase in Ca2+ content) give similar values for the Ca2+ accmulation in this period (0–45 min recovery). A post-stimulatory net cellular accmulation of Ca2+ was also observed at later phases of the recovery, that is after 120 min (Fig. 4) and 240 min recovery (Fig. 8). Therefore, even long after stimulation is stopped, further entry of Ca2+ takes place, e.g. by diffusion down the electrochemical gradient through leaks in the plasma membrane. This possibly exacerbates damage processes already initiated during the stimulation. The pronounced accmulation of Ca2+ observed is likely to represent storage in the SR and possibly the mitochondria.
Effects of extracellular [Ca2+] on loss of contractility
Rather direct evidence for the involvement of extracellular Ca2+ in the impairment of force recovery comes from the experiments where the concentration of Ca2+ in the buffer was either halved or doubled during the fatiguing stimulation (Fig. 5). The force recovery of muscles stimulated at low Ca2+ (0.65 mm) was on average about twofold higher than that of muscles stimulated at high Ca2+ (2.54 mm). Muscles showing the best force recovery also had a 41% lower Ca2+ content after 120 min recovery. Although the reduced [Ca2+]o during stimulation does not lead to complete recovery (32% of initial force), it is a remarkable effect on an otherwise rather poor force recovery. The improved force recovery after stimulation at low [Ca2+]o might be attributed to reduced activation of Ca2+-dependent damage processes whether these affect the sarcolemma, the cytoskeleton or the myofibrils. In accordance with this, Jones et al. (1984) found that when removing Ca2+ from the buffer during stimulation, structural damage as well as loss of contractility and membrane integrity were reduced despite the fact that the initial force generated with and without Ca2+ was very similar. The latter was also the case in our experiments. Furthermore, Lowe et al. (1994) found that muscles injured by eccentric contractions, Ca2+ ionophore treatment or muscular dystrophy show increased Ca2+ content. It has repeatedly been indicated that an intracellular accmulation of Ca2+ is important in eliciting different kinds of damage (Armstrong, 1990; Bruton et al. 1998; Gissel & Clausen, 2000; Westerblad et al. 2000; Overgaard et al. 2002). The Ca2+-activated damage processes could involve activation of the neutral protease, calpain, and hence proteolysis of membrane-associated proteins (including some receptors and ion-channel proteins), cytoskeletal proteins (titin, nebulin and desmin) or contractile proteins (troponin T and I, and tropomyosin, but not actin and myosin) (Goll et al. 2003). Such a role for calpain in exercise-induced muscle damage has been demonstrated by Belcastro (1993) and Belcastro et al. (1998). Taken together, these observations indicate that increased cytosolic [Ca2+] leads to activation of calpains, which disrupt the ultrastructure of the fibres leading to impaired force development and loss of membrane integrity. Turner et al. (1993) found that increased [Ca2+]i leads to increased muscle protein degradation, which could be inhibited by the calpain inhibitor, leupeptin. This mechanism recently received strong support by the observation that the calpain inhibitor 3-(4-iodophenyl)-2-mercapto-(Z)-2-propenoic acid improved force recovery and inhibited ultrastructural changes in rat EDL when added prior to fatiguing stimulation (K. Madsen, personal communication). Furthermore, Raastad et al. (2004) found that in human subjects calpain activity in vastus lateralis was increased by > 300% following eccentric exercise and that the time course of force recovery and declining calpain activity were identical.
[14C]Sucrose space and Na+ and K+ contents
[14C]Sucrose was used as a general indicator of plasma membrane permeability (Fig. 7). Following 60 min of fatiguing stimulation, [14C]sucrose space was found to increase from the control level of 22.8% to 39.7%. This is a strong indication of increased unspecific plasma membrane permeability which would also allow free access of extracellular Na+ to the cytoplasm. On the basis of the Na+ concentration in the buffer and the increase in [14C]sucrose space of 16.9%, it could be calculated that this would predict an increase in muscle Na+ content of 25 μmol (g wet wt)−1. The increase in Na+ content measured by flame photometry amounted to 26 μmol (g wet wt)−1. This was accompanied by an equivalent decrease in K+ content (26 μmol (g wet wt)−1). Thus, the changes in Na+ and K+ contents detected 90 min after cessation of the 60 min fatiguing stimulation are accounted for by the concomitant increase in [14C]sucrose space. These values correspond very well to the Na+ and K+ contents found in other experiments (Fig. 9), showing an increase in Na+ content and an almost equivalent decrease in K+ content. After 4 h recovery, Na+ and K+ contents seem to be restored in muscles stimulated for 30 min, but in muscles exposed to 60 min of stimulation, Na+ and K+ contents were not restored, indicating that the fatiguing stimulation had induced a lasting cell damage (data not shown).
It should be noted that the marked increase in [14C]sucrose space elicited by direct stimulation was completely abolished by TTX (Fig. 7), demonstrating that it reflects the result of excitation and not electroporation. Moreover, indirect stimulation also increased [14C]sucrose space, indicating that the cell damage could also be mediated via the normal excitation pathway.
LDH release
The cell damage made evident in the measurements of 45Ca uptake, [14C]sucrose space and Na+ and K+ contents was further explored by measuring the release of a large intracellular protein molecule, LDH (molecular mass 140 kDa), as shown in Fig. 8. After 15 min of fatiguing stimulation, LDH release showed no significant change even though force development was at its maximum during this stimulation period. After 30 or 60 min of stimulation, however, LDH release was increased 3- and 9-fold, respectively, compared to control muscles. During the 240 min of recovery following stimulation, the accmulated release of LDH from muscles stimulated for 60 min was 48 U (g wet wt)−1, which is only 8.3% of their total LDH content of 585 ± 13 U (g wet wt)−1 (Gissel & Clausen, 2000). Muscles stimulated for 30, 15 or 0 min released only 2.5, 1 and 1% of their total LDH content, respectively. Thus it is a fairly small proportion of the total LDH that is released from the muscles, which is in keeping with studies on the increase in plasma LDH and creatine kinase following prolonged exercise in humans (Overgaard et al. 2002). It should be recalled, however, that these averaged values do not permit distinguishing between widespread, but partial cell damage and localized, more complete loss of the integrity of individual cells.
Ca2+, loss of contractility and cellular integrity
We confirm previous observations by Jones et al. (1984) and add the information that fatiguing stimulation increases the uptake of 45Ca and the accmulation of Ca2+ in rat EDL muscle. The increased uptake of Ca2+ is associated with and correlated to the subsequent resting uptake of 45Ca, release of LDH and increased [14C]sucrose space (Figs 6–8), further supporting the idea that Ca2+ is important in the loss of cellular integrity. It is important that during the fatiguing 40 Hz stimulation, the excitation-induced uptake of Ca2+ continues (for at least 45 min) after the force development has declined by 90–95%, indicating that the mechanical strain is of minor importance for the uptake of Ca2+ and the ensuing loss of force and integrity. Moreover, it should be noted that even long after the cessation of stimulation, Ca2+ uptake and LDH release continues, implying that exercise-induced and Ca2+-dependent muscle cell damage may develop into a self-sustaining process. When the various indices of cell damage are compared (increases in [14C]sucrose space, LDH release, Ca2+ content, Na+ content, K+ loss and resting 45Ca uptake), all parameters indicate that clear cut loss of integrity requires 30 min of fatiguing stimulation.
It is likely that the loss of force is partly due to loss of cellular integrity, the ensuing depolarization and loss of excitability. It is well documented that following long-term electrical stimulation of intact muscles, the cells undergo depolarization (Locke & Solomon, 1967; Hanson, 1974; Balog et al. 1994).
After 30 or 60 min of fatiguing stimulation, there is only modest reversal of force and LDH release, at least within the time horizon used in the present experiments (120–240 min). This lack of reversibility might reflect cell death, but a prolonged follow-up may allow more complete recovery. Several days of recovery may be needed and this is not possible in the in vitro set-up. In vivo studies on human subjects have shown that following 15–30 min of intense exercise, the quadriceps undergoes up to 30% loss of force lasting up to 4 days (Newham et al. 1983a,b; Raastad et al. 2004). The force recovery may be due to the more efficient oxygenation under in vivo conditions allowing complete, albeit late recovery.
It cannot be excluded, that part of the excitation-induced loss of integrity demonstrated in this study reflects hypoxic damage. It has been shown, however, that hypoxia alone is not sufficient to damage the muscles and that Ca2+ also plays an important role in this type of muscle damage (Jones et al. 1984). On the other hand, ongoing experiments have shown that in resting rat EDL muscles, [14C]sucrose space and LDH release show no significant increase until after 120 min of exposure to total anoxia (Fredsted et al. 2004).
Conclusions
To sum up, fatiguing stimulation leads to impaired force recovery. Furthermore, we see an increased sarcolemmal permeability in terms of an increased post-stimulatory 45Ca uptake, [14C]sucrose space and LDH release. Likewise, the contents of Na+ and Ca2+ were increased, and the K+ content decreased after fatiguing stimulation. The increased sarcolemmal permeability seems to be related to the excitation-induced Ca2+ uptake, rather than to mechanical strain on the muscle fibres, as this Ca2+ uptake persists throughout stimulation, whereas only very modest force is developed beyond 10 min of fatiguing 40 Hz stimulation. The present observations further support the idea that Ca2+ is important in eliciting muscle damage after unaccustomed exercise. Finally, the progressive loss of contractility seen during fatiguing stimulation does not protect against muscle damage.
Perspectives
The excitation-induced Ca2+ uptake observed in isolated rat muscle in this study explains the significant increase in the Ca2+ content of human vastus lateralis after running 20 and 100 km (Overgaard et al. 2002, 2004). The observed release of LDH indicates loss of sarcolemmal integrity and is well in line with observations following prolonged or unaccustomed exercise in humans (e.g. Jones et al. 1986, 1987; Clarkson & Hubal, 2002). The present study adds to a further understanding of the mechanisms of muscle damage. The increase in Ca2+ content suggests that an intracellular accmulation of Ca2+ and a rise in [Ca2+]i may be involved in the development of muscle cell damage. This points to regulation of free Ca2+ levels as an important component in the maintenance of muscle structure and function, particularly during repetitive muscle activity. The therapeutic possibilities of this would be to develop means of reducing the cell membrane damage or alternatively reducing the activity of Ca2+-activated degradative enzymes such as calpains or phospholipases thereby reducing cell damage. This may be important not only in exercise (training and sports performances), but also in other situations where Ca2+ influx is increased, such as crush injury, rhabdomyolysis and muscular dystrophies. On the other hand, it should be recalled that Ca2+ uptake may not only elicit degradation and as such lead to muscle fibre loss, but may also serve as an adaptive response contributing to fibre transformation and replacement.
Acknowledgments
We thank Marianne Stürup-Johansen, Tove Lindahl Andersen, Vibeke Uhre and Ann-Charlotte Andersen for skilled technical assistance. The study was supported by grants from the Danish Medical Research Council (No. 22010189), the Danish Biomembrane Research Centre, Aarhus Universitets Forskningsfond, the Korning Foundation and the Carlsberg Foundation.
References
- Appell HJ, Soares JM, Duarte JA. Exercise, muscle damage and fatigue. Sports Med. 1992;13:108–115. doi: 10.2165/00007256-199213020-00006. [DOI] [PubMed] [Google Scholar]
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370–381. doi: 10.2165/00007256-198603050-00006. [DOI] [PubMed] [Google Scholar]
- Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. 1990;22:429–435. [PubMed] [Google Scholar]
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995;488:25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balog EM, Thompson LV, Fitts RH. Role of sarcolemma action potentials and excitability in muscle fatigue. J Appl Physiol. 1994;76:2157–2162. doi: 10.1152/jappl.1994.76.5.2157. [DOI] [PubMed] [Google Scholar]
- Belcastro AN. Skeletal muscle calcium-activated neutral protease (calpain) with exercise. J Appl Physiol. 1993;74:1381–1386. doi: 10.1152/jappl.1993.74.3.1381. [DOI] [PubMed] [Google Scholar]
- Belcastro AN, Albisser TA, Littlejohn B. Role of calcium-activated neutral protease (calpain) with diet and exercise. Can J Appl Physiol. 1996;21:328–346. doi: 10.1139/h96-029. [DOI] [PubMed] [Google Scholar]
- Belcastro AN, Shewchuk LD, Raj DA. Exercise-induced muscle injury: a calpain hypothesis. Mol Cell Biochem. 1998;179:135–145. doi: 10.1023/a:1006816123601. [DOI] [PubMed] [Google Scholar]
- Bianchi CP, Shanes AM. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J General Physiol. 1959;42:803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. Mechanisms underlying the slow recovery of force after fatigue: importance of intracellular calcium. Acta Physiol Scand. 1998;162:285–293. doi: 10.1046/j.1365-201X.1998.0292f.x. [DOI] [PubMed] [Google Scholar]
- Carlsen RC, Villarin JJ. Membrane excitability and calcium homeostasis in exercising skeletal muscle. Am J Phys Med Rehabil. 2002;81:S28–S39. doi: 10.1097/00002060-200211001-00005. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol. 1996;491:813–824. doi: 10.1113/jphysiol.1996.sp021259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinet A, Clausen T, Girardier L. Microcalorimetric determination of energy expenditure due to active sodium-potassium transport in the soleus muscle and brown adipose tissue of the rat. J Physiol. 1977;265:43–61. doi: 10.1113/jphysiol.1977.sp011704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- Clausen T. The Na+, K+ pump in skeletal muscle: quantification, regulation and functional significance. Acta Physiol Scand. 1996;156:227–235. doi: 10.1046/j.1365-201X.1996.209000.x. [DOI] [PubMed] [Google Scholar]
- Clausen T, Andersen SL, Flatman JA. Na+-K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. J Physiol. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol. 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Overgaard K, Nielsen OB. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol Scand. 2004;180:209–216. doi: 10.1111/j.0001-6772.2003.01251.x. [DOI] [PubMed] [Google Scholar]
- Curtis BA. Ca2+ fluxes in single twitch muscle fibers. J General Physiol. 1966;50:255–267. doi: 10.1085/jgp.50.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Delp MD, Hayes DA, Delp PD, Armstrong RB. Rat skeletal muscle mitochondrial [Ca2+] and injury from downhill walking. J Appl Physiol. 1990;68:1241–1251. doi: 10.1152/jappl.1990.68.3.1241. [DOI] [PubMed] [Google Scholar]
- Duncan CJ, Jackson MJ. Different mechanisms mediate structural changes and intracellular enzyme efflux following damage to skeletal muscle. J Cell Sci. 1987;87:183–188. doi: 10.1242/jcs.87.1.183. [DOI] [PubMed] [Google Scholar]
- Everts ME, Clausen T. Effects of thyroid hormones on calcium contents and 45Ca exchange in rat skeletal muscle. Am J Physiol. 1986;251:E258–E265. doi: 10.1152/ajpendo.1986.251.3.E258. [DOI] [PubMed] [Google Scholar]
- Everts ME, Lømo T, Clausen T. Changes in K+, Na+ and calcium contents during in vivo stimulation of rat skeletal muscle. Acta Physiol Scand. 1993;147:357–368. doi: 10.1111/j.1748-1716.1993.tb09512.x. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fredsted A, Mikkelsen UR, Gissel H, Clausen T. Hypoxia, calcium and muscle damage. Acta Physiol Scand. 2004;181:R153. (abstract) [Google Scholar]
- Gibala MJ, Macdougall JD, Tarnopolsky MA, Stauber WT, Elorriaga A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J Appl Physiol. 1995;78:702–708. doi: 10.1152/jappl.1995.78.2.702. [DOI] [PubMed] [Google Scholar]
- Gissel H, Clausen T. Excitation-induced Ca2+ uptake in rat skeletal muscle. Am J Physiol. 1999;276:R331–R339. doi: 10.1152/ajpregu.1999.276.2.R331. [DOI] [PubMed] [Google Scholar]
- Gissel H, Clausen T. Excitation-induced Ca2+ influx in rat soleus and EDL muscle: Mechanisms and effects on cellular integrity. Am J Physiol. 2000;279:R917–R924. doi: 10.1152/ajpregu.2000.279.3.R917. [DOI] [PubMed] [Google Scholar]
- Gissel H, Clausen T. Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol Scand. 2001;171:327–334. doi: 10.1046/j.1365-201x.2001.00835.x. [DOI] [PubMed] [Google Scholar]
- Gissel H, Clausen T. Ca2+ uptake and cellular integrity in rat EDL muscle exposed to electrostimulation, electroporation, or A23187. Am J Physiol. 2003;285:R132–R142. doi: 10.1152/ajpregu.00196.2002. [DOI] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Hanson J. The effects of repetitive stimulation on the action potential and the twitch of rat muscle. Acta Physiol Scand. 1974;90:387–400. doi: 10.1111/j.1748-1716.1974.tb05600.x. [DOI] [PubMed] [Google Scholar]
- Hoppeler H. Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med. 1986;7:187–204. doi: 10.1055/s-2008-1025758. [DOI] [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Jones DA, Edwards RH. Experimental skeletal muscle damage: The nature of the calcium-activated degenerative processes. Eur J Clin Invest. 1984;14:369–374. doi: 10.1111/j.1365-2362.1984.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Jones DA. High- and low-frequency fatigue revisited. Acta Physiol Scand. 1996;156:265–270. doi: 10.1046/j.1365-201X.1996.192000.x. [DOI] [PubMed] [Google Scholar]
- Jones DA, Jackson MJ, McPhail G, Edwards RH. Experimental mouse muscle damage: The importance of external calcium. Clin Sci (Lond) 1984;66:317–322. doi: 10.1042/cs0660317. [DOI] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Obletter G. Nature of exercise-induced muscle pain. Adv Pain Res Ther. 1987;10:207–218. [Google Scholar]
- Jones DA, Newham DJ, Round JM, Tolfree SE. Experimental human muscle damage: Morphological changes in relation to other indices of damage. J Physiol. 1986;375:435–448. doi: 10.1113/jphysiol.1986.sp016126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke S, Solomon HC. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity and the Na-K pump. J Exp Zool. 1967;166:377–386. doi: 10.1002/jez.1401660310. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Warren GL, Hayes DA, Farmer MA, Armstrong RB. Eccentric contraction-induced injury of mouse soleus muscle: Effect of varying [Ca2+]o. J Appl Physiol. 1994;76:1445–1453. doi: 10.1152/jappl.1994.76.4.1445. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium. 1997;22:373–383. doi: 10.1016/s0143-4160(97)90022-1. [DOI] [PubMed] [Google Scholar]
- McBride TA, Stockert BW, Gorin FA, Carlsen RC. Stretch-activated ion channels contribute to membrane depolarization after eccentric contractions. J Appl Physiol. 2000;88:91–101. doi: 10.1152/jappl.2000.88.1.91. [DOI] [PubMed] [Google Scholar]
- Mikkelsen U, Fredsted A, Gissel H, Clausen T. Excitation-induced Ca2+ influx and skeletal muscle damage. In: Müller E, Schwameder H, Zallinger G, Fastenbauer V, editors. 8th Annual Congress, European College of Sport Science Abstract Book. Austria: Institute of Sport Science, University of Salzburg; 2003. p. 73. [Google Scholar]
- Mikkelsen U, Fredsted A, Gissel H, Clausen T. Reduced external Ca2+ [Ca2+]o improves force recovery in fatigued rat EDL muscles. Acta Physiol Scand. 2004;181:R153. (abstract) [Google Scholar]
- Newham DJ, Jones DA, Edwards RH. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983a;6:380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- Newham DJ, Mills KR, Quigley BM, Edwards RH. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci. 1983b;64:55–62. doi: 10.1042/cs0640055. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, Clausen T. Regulation of Na+–K+ pump activity in contracting rat muscle. J Physiol. 1997;503:571–581. doi: 10.1111/j.1469-7793.1997.571bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen OB, Clausen T. The Na+/K+-pump protects muscle excitability and contractility during exercise. Exerc Sport Sci Rev. 2000;28:159–164. [PubMed] [Google Scholar]
- Nielsen OB, Overgaard K. Ion gradients and contractility in skeletal muscle: The role of active Na+, K+ transport. Acta Physiol Scand. 1996;156:247–256. doi: 10.1046/j.1365-201X.1996.204000.x. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Fredsted A, Hyldal A, Ingemann-Hansen T, Gissel H, Clausen T. Effects of running distance and training on Ca++ content and damage in human muscle. Med Sci Sports Exerc. 2004 doi: 10.1249/01.mss.0000126468.65714.60. in press. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Lindstrøm T, Ingemann-Hansen T, Clausen T. Membrane leakage and increased content of Na+-K+ pumps and Ca2+ in human muscle after a 100-km run. J Appl Physiol. 2002;92:1891–1898. doi: 10.1152/japplphysiol.00669.2001. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. J Physiol. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raastad T, Enns D, Overgaard K, Gylterud S, Ugelstad I, Belcastro A, Hallén J. Activation of proteolytic systems after eccentric exercise. In: Van Praagh E, Coudert J, Fellmann N, Duché P, editors. 9th Annual Congress, European College of Sport Science, Abstract Book. Université Blaise Pascal, Universite d'Auvergne; 2004. [Google Scholar]
- Reid MB, Li YP. Cytokines and oxidative signalling in skeletal muscle. Acta Physiol Scand. 2001;171:225–232. doi: 10.1046/j.1365-201x.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- Sreter FA, Lopez JR, Alamo L, Mabuchi K, Gergely J. Changes in intracellular ionized Ca2+ concentration associated with muscle fiber type transformation. Am J Physiol. 1987;253:C296–C300. doi: 10.1152/ajpcell.1987.253.2.C296. [DOI] [PubMed] [Google Scholar]
- Sreter F, Mabuchi K, Köver A, Gesztelyi I, Nagy Z, Furka I. Effect of chronic stimulation on cation distribution and membrane potential in fast-twitch muscles of rabbit. In: Pette D, editor. Plasticity of Muscle. Berlin, New York: Walter de Gruyter & Co; 1980. pp. 441–451. [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol. 2001;91:1669–1678. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- Turner PR, Schultz R, Ganguly B, Steinhardt RA. Proteolysis results in altered leak channel kinetics and elevated free calcium in mdx muscle. J Membr Biol. 1993;133:243–251. doi: 10.1007/BF00232023. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM. The cytoskeleton of skeletal muscle: Is it affected by exercise? A brief review. Med Sci Sports Exerc. 1991;23:1240–1249. [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Recent advances in the understanding of skeletal muscle fatigue. Curr Opin Rheumatol. 2002;14:648–652. doi: 10.1097/00002281-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Allen DG, Lännergren J. Functional significance of Ca2+ in long-lasting fatigue of skeletal muscle. Eur J Appl Physiol. 2000;83:166–174. doi: 10.1007/s004210000275. [DOI] [PubMed] [Google Scholar]