Abstract
The effect of intense training on endothelial proliferation, capillary growth and distribution of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) was examined in human skeletal muscle. Two intermittent knee extensor training protocols (at ∼150% (Study 1) versus ∼90% (Study 2) of leg V˙O2max) were conducted. Muscle biopsies were obtained throughout the training periods for immunohistochemical assessment of capillarization, cell proliferation (Ki-67-positive cells), VEGF and bFGF. In Study 1, microdialysis samples were collected from the trained and untrained leg at rest and during exercise and added to endothelial cells to measure the proliferative effect. After 4 weeks of training there was a higher (P < 0.05) capillary-to-fibre ratio (Study 1: 2.4 ± 0.1 versus 1.7 ± 0.1) and number of Ki-67-positive cells (Study 1: 0.18 ± 0.05 versus 0.00 ± 0.01) than before training. Neither the location of proliferating endothelial cells nor capillarization was related to muscle fibre type. The endothelial cell proliferative effect of the muscle microdialysate increased from rest to exercise in both the untrained leg (from 262 ± 60 to 573 ± 87% of control perfusate) and the trained leg (from 303 ± 75 to 415 ± 108% of perfusate). VEGF and bFGF were localized in endothelial and skeletal muscle cells and training induced no changes in distribution. The results demonstrate that intense intermittent endurance training induces capillary growth and a transient proliferation of endothelial cells within 4 weeks, with a similar growth occurring around type I versus type II muscle fibres.
Endurance training can induce growth of new blood vessels (angiogenesis), but the effect is dependent of the type and intensity of training. An increased capillarization has been observed in training studies performed at 70–80% of V˙O2max (Andersen & Henriksson, 1977; Denis et al. 1986) whereas training at an intensity of 45% of V˙O2max has been shown to have no effect on capillarization (Schantz et al. 1983). Little is known about the effect of high intensity endurance training on muscle capillarization. In a study by Daub et al. (1982), involving intense ice-hockey training performed by athletes, no increase in capillary-to-fibre ratio was found; the only increase observed was in capillaries per fibre area of type I fibres. There is reason to believe, however, that the skeletal muscle does adapt to high intensity intermittent training by an increase in capillaries, as oxidative energy metabolism is high both during exercise and in the recovery phase between exercise bouts (Bangsbo, 1999).
The capillary supply to type I and II muscle fibres has been observed to increase equally in response to exercise training at moderate intensities, during which mainly type I fibres are recruited (Andersen & Henriksson, 1977; Saltin et al. 1977; Klausen et al. 1981). However, as exercise at higher intensities causes marked activation of type II muscle fibres, training at high intensities may lead to an enhanced number of capillaries supplying in particular this fibre type. A sensitive method for assessing whether new capillaries are formed in relation to a specific fibre type is immunohistochemical determination of proliferating endothelial associated cells. The monoclonal antibody Ki-67 detects a proliferation-associated nuclear antigen and colocalization of endothelial cell staining and Ki-67 staining provides a powerful tool for assessing the location of proliferating endothelial cells and, thus, the location of new capillaries (Gerdes et al. 1984). This method, which has not previously been used on human muscle to study capillary growth in response to training, was utilized in the present study to test the hypothesis that intense intermittent training, requiring a substantial activation of type II muscle fibres in addition to type I muscle fibres, leads to growth of capillaries associated with type II fibres.
In order to elicit their effects on vascular endothelial cells, compounds responsible for capillary growth in skeletal muscle must be released from cells into the interstitial space or be produced extracellularly (Folkman & Klagsbrun, 1987). Accordingly, we have shown that the concentration of endothelial cell proliferative compounds increases in the human skeletal muscle interstitium during an exercise bout (Hoffner et al. 2003). In the present study we hypothesized that, if there is a direct relationship between the amount of endothelial cell proliferative compounds released and growth of capillaries, the exercise-induced increase in the release of proliferative compounds would be transient during a training period of several weeks. Thus, once the need for an enhanced capillarization is satisfied the exercise-induced release of proliferative compounds would be reduced.
It is not known what mechanisms underlie the capillary growth process in skeletal muscle, but reduced oxygen tension and related metabolic consequences have been suggested as possible stimuli (Hudlicka et al. 1992). Furthermore, growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), have been proposed to be of importance for angiogenic processes (Morrow et al. 1990; Breen et al. 1996; Annex et al. 1998). VEGF mRNA expression in human skeletal muscle is up-regulated by a single bout of dynamic exercise (Gustafsson et al. 1999; Richardson et al. 1999). With regard to the location of the increases in VEGF with training, studies using in situ hybridization of human muscle have revealed that 7 days of one-legged exercise training increases the expression of VEGF mRNA between and within skeletal muscle fibres (Gustafsson et al. 2002). VEGF protein has by immunohistochemical analysis in rodents been shown to increase in the matrix between the muscle cells and in endothelial cells after 3–4 days of electrical stimulation (Annex et al. 1998; Milkiewicz et al. 2001). In contrast, Rissanen et al. (2002) failed to detect immunostaining of VEGF protein in non-atrophic chronically ischaemic human skeletal muscle. However, the latter observation may have been due to the ischaemic state of the muscle.
The aim of the present study was to determine the effect of high intensity intermittent endurance training on the presence of proliferating endothelial cells and capillaries in skeletal muscle and their location in relation to muscle fibre types. A further aim was to evaluate the effect of training on the endothelial cell proliferative effect of muscle interstitial fluid obtained at rest and during exercise, as well as on the distribution of VEGF and bFGF, assessed immunohistochemically, in skeletal muscle. Two training protocols, both involving intermittent one-legged knee extensor exercise, but of different intensities (150% and 90% of leg V˙O2max), were employed for comparison, and biopsies as well as microdialysis samples were obtained from the trained as well as the control leg.
Methods
Subjects
Six healthy young men with a mean age and weight of 25.3 ± 1.2 years and 82.8 ± 4.8 kg participated in Study 1. The subjects did not participate in regular physical activity and had a maximal oxygen uptake (V˙O2max) of 50.2 ± 0.5 ml kg−1 min−1. In Study 2, seven untrained young men with a mean age of 23.9 ± 1.4 years and mean body weight of 76.1 ± 2.7 kg participated. The subjects' habitual engagement in exercise training ranged from none to light bicycling five times per week. Their mean V˙O2max was 45.4 ± 1.9 ml kg−1 min−1 as determined during cycle exercise. Before participation, the subjects were informed of the experimental procedure, the potential risks, and that they could withdraw from the experiment at any time. The subjects gave their informed consent prior to participation in the experiment. All procedures used conformed to the Declaration of Helsinki and both studies were approved by the Ethical Committee of Copenhagen and Frederiksberg.
Experimental design
For both training and the main experiments the subjects used a one-legged knee extension ergometer (Andersen et al. 1985). The kicking frequency was maintained at 60 r.p.m. In both studies the leg to be trained was randomly selected and the untrained leg functioned as control.
In Study 1, each subject underwent preliminary exercise testing consisting of two incremental knee extensor exercise tests for each of the legs and an incremental cycle test for determination of maximal pulmonary VO2 (MedGraphics, Saint Paul, Minneapolis, USA). Only subjects with a pulmonary peak oxygen uptake ranging from 45 to 55 ml O2 min−1 kg−1 were included in the study. The incremental knee extensor exercise test consisted of 4 min at 50 W, then 2 min at 60 W, whereupon the load was increased by 10 W every 2 min until exhaustion. The exercise test was terminated when the kicking frequency reached values below 55 kicks min−1 and the exercise time was recorded as the test performance. The two incremental tests were separated by at least 1 week. The training consisted of intermittent one-legged knee extensor exercise at an intensity of about 150% of leg V˙O2max. Every training session lasted for 1 h with 1 min exercise periods separated by 3 min of rest. The duration of the training period was 6.8 ± 0.3 weeks and training sessions were performed three times per week for 2 weeks, four times a week for the next 2 weeks and five times a week for the last 3 weeks. Every second week an incremental knee extensor exercise test was performed on the trained leg to evaluate how much exercise intensity should be increased to maintain the same relative exercise intensity. A muscle biopsy was obtained from m. vastus lateralis at rest before the training period as well as after 2, 4 and 6.8 weeks of training. The biopsies were obtained 24 h after a training session to focus on training and not adaptations resulting from the last exercise bout. Control biopsies were obtained from the untrained leg before and after the training period
After the training period, an experiment using a microdialysis technique was performed with the trained (2.3 ± 0.2 days after the last training session to avoid the influence of acute exercise) and the untrained legs on separate days. The semi-permeable fibres (CMA60, CMA microdialysis, Sweden) had a molecular mass cut-off of 20 kDa and were 0.52 mm in diameter. Prior to insertion of the microdialysis probes, the skin, subcutaneous tissue and fascia close to the insertion were anaesthetized with lidocaine (xylocaine; 1 ml; 20 mg ml−1). The microdialysis probes were inserted into m. vastus lateralis of the quadriceps femoris muscle group. The microdialysis probes were aligned with the muscle fibres. The perfusate consisted of Ringer acetate solution containing: 130 mm Na+, 2 mm Ca2+, 4 mm K+, 1 mm Mg2+, 30 mm acetate, with 3 mm glucose and 1 mm lactate added. The microdialysis probes were perfused via a high-precision syringe pump (CMA 102, Carnegie Medicine, Solna, Sweden), at a rate of 5 μl min−1. Samples were collected during 2 × 20 min periods of rest and during 2 × 12 min periods of knee extensor kicking (4 samples) at an intensity of 30 W (collection was performed 5 min after start of exercise). The weight of the sample tubes was determined before and after sampling in order to validate the perfusion rate. Only values from microdialysis probes that had a perfusion flow of 4.5–5.0 μl min−1 throughout the entire experiment were used. Samples were immediately stored at −80°C until added to endothelial cells to analyse the effect on proliferation.
In Study 2 the subjects performed one-legged knee extensor exercise three times per week for 6 weeks. The training consisted of 1 min bouts at an intensity corresponding to about 90% of leg V˙O2max separated by 30 s of rest until the subjects were unable to maintain a kicking frequency above 55 r.p.m. knee extensor after approximately 1 h. The work load was increased successively during the training period to induce fatigue after approximately 1 h of exercise. Muscle biopsies were obtained from both legs (trained and untrained (control)) before and after the 6 weeks of exercise and in addition from the trained leg after 2 and 4 weeks. The biopsies were taken just before a training session and 48 h after the last training session.
Analysis
Immunohistochemistry.
After collection part of the muscle biopsy was mounted in an embedding medium, frozen in isopentane cooled in liquid nitrogen and stored at −80°C for subsequent immunohistochemical analyses.
Transverse sections 8 μm in thickness were cut in a cryostat, placed onto poly-l-lysine-coated glass-slides, fixed by immersion in acetone at −20°C for 10 min and incubated for 2 min in 4%p-formaldehyde at room temperature. The sections were rinsed in 0.01 m TBS–BSA (tris-buffered saline containing 1% bovine serum albumin) and blocked for 1 h with 0.01 m TBS–BSA to avoid non-specific binding of antibodies. The primary anti-human antibodies were diluted in 0.01 m TBS–BSA as follows: CD31 (endothelial cell marker, M0823, DAKO, Denmark) 1: 400; Ki-67 (36521A, Pharmingen, USA) 1: 300; MHC1 (slow myosin heavy chain (type I muscle fibre) marker, M8421, Sigma, MO, USA) 1: 2000; VEGF (7269 Santa Cruz, CA, USA) 1: 100; VEGF (507, Santa Cruz) 1: 100; VEGF (AF-293-NA, R&D Systems, Abingdon, UK) 1: 10; bFGF (79, Santa Cruz) 1: 800; smooth muscle (smooth muscle cell marker, M3558, DAKO) 1: 200. The sections were incubated for 1 h followed by 1 h of incubation with biotinylated secondary antibodies; goat anti-rabbit (E0432, DAKO), rabbit anti-mouse immunoglobulin (E0354, DAKO) or rabbit anti-goat immunoglobulin (E0466, DAKO) diluted in 0.01 m TBS–BSA to 1: 600, 1: 600 or 1: 800, respectively. Between each step in the labelling protocol, sections were rinsed in 0.01 m TBS–BSA. Streptavidin-FITC (F0422, DAKO) was diluted 1: 100 in 0.01 m TBS–BSA and the sections were incubated for 1 h, rinsed in 0.01 m TBS and mounted in Vecta Shield mounting medium (Vector Laboratories, CA, USA). Specificity of the staining was assessed by staining without the primary antibody. Immunoreactive sections were examined in a Zeiss photomicroscope. Localization of proteins was based on identification of the same positively stained structures in serial sections.
Three different antibodies against human VEGF were used to verify that the immunopositive staining reflected VEGF protein. All three antibodies showed similar staining. VEGF sc-7269 was used for the main analyses as it resulted in the strongest signal. Sections stained for VEGF were compared with serial sections where antibodies recognizing endothelial cells, smooth muscle cells and type I muscle fibres had been used.
Capillarization
Number of capillaries and muscle fibre area were determined in the CD31-stained muscle cross-sections in 5–13 fields each covering 5.4 μm2 of muscle cross-sectioned area at a total magnification of ×400. On average 130.5 fibres per biopsy were counted. The number of muscle fibres and capillaries in a field included half the number of fibres and capillaries on the borderline of the field. Fields were distributed randomly throughout the transverse muscle sections. The only criterion for selection of fields was that the tissue had an adequate fixation and staining of the tissue. Averages were computed from all biopsies obtained at the same time point, e.g. after 2 weeks of training. Capillary supply was expressed as capillary-to-fibre ratio (C : F) and capillary density (capillaries per muscle fibre area (cap mm−2)).
In the biopsies obtained in Study 1 additional analyses were performed. At a total magnification of ×200, fields each covering 4.5 μm2 were identified in adjacent serial sections stained for CD31, Ki-67 and MHCI (detecting type I muscle fibres). Two fields from each section were used to create a microscopic video image. The serial sections were used to assess whether the position of Ki-67-positive nuclei colocalized with CD31 staining and hence the site of an endothelial cell. More than 90% of the Ki-67-positive nuclei were localized with endothelial cells and these were identified as developing capillaries per fibre. Each field contained an average of 42 muscle fibres and was used to evaluate localization of capillaries, including Ki-67-positive capillaries, to either type I or II muscle fibres. Each Ki-67-positive capillary in a field was detected and the type of muscle fibres around it determined. Counting of muscle fibre types was used to determine the fibre type ratio (type I: type II). An average from the fields from one biopsy was used to compute an average for each time point. The quantification was performed independently by two investigators.
Endothelial cell proliferation
Human umbilical vein endothelial cells (HUVECs) were supplemented in medium 200 with low serum growth supplement (LSGS) containing fetal bovine serum, fibroblast growth factor, heparin and epidermal growth factor (Cascade Biologics Inc., Portland, OR, USA). The cells were grown on 96-well plates for 24 h before replacing the medium with 50 μl of microdialysate, perfusate or supplemented medium 200. Microdialysate obtained in Study 1 was diluted 1 : 1 with perfusate to obtain a solution that did not provide a response greater than the positive control (supplemented medium 200) and did not reach the maximum level of proliferation. After an additional 24 h of incubation, bromodeoxyuridine (BrdU) was added and then incubated for 12 h. Incorporation of BrdU into the DNA was detected by an immunoassay (Roche, Mannheim, Germany) according to the manufacturer's recommended methods. Addition of perfusate to the proliferation assay was used as control. All measurements were made in duplicate.
Statistical analyses
Results are expressed as means ± s.e.m. Changes in C: F, capillary density, Ki-67/fibre, fibre ratio and mean fibre area in the trained leg were analysed using a one-way repeated measures ANOVA. In the untrained leg these changes were analysed using Student's paired t test. Statistical significance between capillaries in contact with fibre types I and II was calculated by two-way repeated measures ANOVA. Differences in C: F and Ki-67/fibre over time were compared between Study 1 and Study 2 with a two-way repeated measures ANOVA. Values for endothelial cell proliferation were averaged for resting and exercising conditions, respectively, as no changes were found. Changes in endothelial cell proliferation were evaluated using two-way ANOVA, comparing trained and untrained legs over time (rest versus exercise). When significant changes were found the Student-Newman-Keuls method for multiple comparisons was used to determine where the changes occurred. A value of P < 0.05 was accepted as statistically significant.
Results
Performance
Study 1.
After the training period the endurance of the trained leg (10.6 ± 0.7 min) was greater (P < 0.05) in an exhaustive incremental test compared to the untrained leg (8.2 ± 0.7 min). Before the training period the endurance of the trained (7.6 ± 0.6 min) and untrained (7.8 ± 0.7 min) legs were similar and not different (P > 0.05) from that of the untrained leg after the training period. The initial work rate of the experimental leg was 92 ± 8 W and at the end of the training period it had increased (P < 0.05) to 106 ± 9 W. After the training period the V˙O2max of the trained leg (1.10 ± 0.05 l min−1) was higher than V˙O2max of the untrained leg (0.89 ± 0.06) (P < 0.05).
Study 2.
The mean work rate was 51.7 ± 0.7 W during the training sessions at the beginning of the training period and increased (P < 0.05) during the training period to reach 64.6 ± 2.8 W at the end.
Capillarization
Study 1.
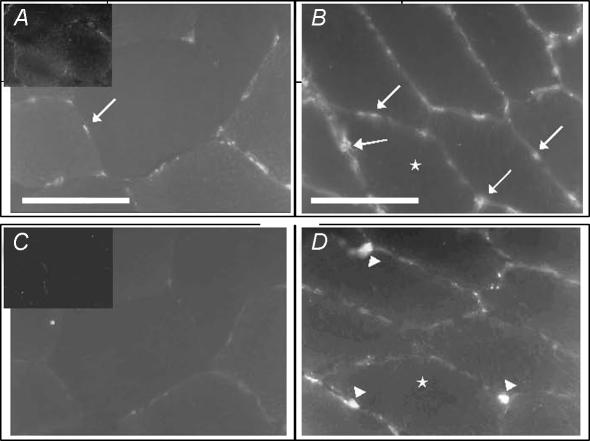
Representative transverse sections immunohistochemically stained for CD31 and Ki-67, before and after 4 weeks of training illustrate the increase (P < 0.05) in capillaries and capillary-associated proliferating cells observed after 4 weeks of training (Fig. 1).
Figure 1. Representative serial sections showing immunohistochemical staining of capillaries and proliferating cells in human skeletal muscle before and after intense intermittent training.
CD31-positive capillaries (arrow) before (A) and after (B) 4 weeks of high intensity intermittent training. Ki-67-positive proliferating cells, indicating developing capillaries (arrowhead) before (C) and after (D) 4 weeks of high intensity intermittent training. Negative control (obtained without primary antibody) is inserted in the upper left corner of micrographs. Scale bars = 50 μm; bar in A applies to C and D.
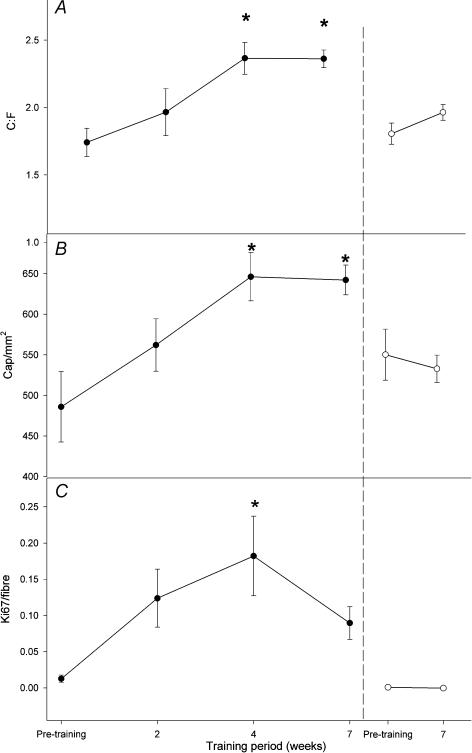
In the trained leg, the C : F ratio increased from 1.74 ± 0.10 before training to 2.37 ± 0.12 after 4 weeks of training and remained elevated after 6.8 weeks (2.36 ± 0.07) (P < 0.05) (Fig. 2A). Similarly, capillary density (cap mm−2) in the trained leg was higher after 4 and 6.8 weeks (646 ± 29 and 642 ± 18 cap mm−2, respectively) compared to before training (551 ± 25 cap mm−2) (P < 0.05) (Fig. 2B). No significant changes were observed for the control leg. The mean fibre areas in the trained and the untrained legs were unaltered throughout the training period (P > 0.05), being 3846.2 ± 89.57 and 3762.5 ± 283 μm2 before and 3692.2 ± 162.5 and 3874.8 ± 171.4 μm2 after the training period in the untrained and trained leg, respectively.
Figure 2. Presence of capillaries and proliferating endothelial cells in human skeletal muscle before and after intense (150% of V˙O2max) intermittent training.
Capillary-to-fibre ratio (C : F; A), capillary density (cap mm−2; B) and endothelial cell associated proliferating cells (C) before, during and after a 6.8 week training period in the trained (•) and the control (○) leg. *P < 0.05versus pre-training, n = 6.
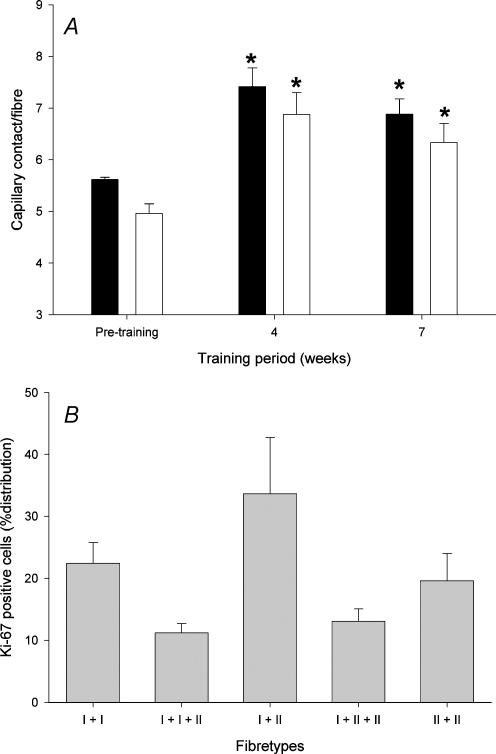
The number of capillaries in contact with muscle fibre type I increased from 5.79 ± 0.19 before training to 7.42 ± 0.35 and 6.88 ± 0.29 after 4 and 6.8 weeks of training, respectively (P < 0.05) (Fig. 3A). The number of capillaries in contact with muscle fibre type II increased from 5.22 ± 0.19 to 6.88 ± 0.42 and 6.33 ± 0.36 after 4 and 6.8 weeks of training, respectively (P < 0.05). No difference in the training-induced increase in capillaries in contact with muscle fibres was observed between type I and type II fibres (P > 0.05). The muscle fibre type ratio (type I: type II) did not change during the training period (1.64 ± 0.16 before the training period, 1.36 ± 0.18 after 4 weeks and 1.18 ± 0.11 after the training period) (P > 0.05).
Figure 3. Effect of high intensity (150% of V˙O2max) intermittent training on capillary distribution in relation to fibre types.
A, capillaries in contact with fibre type I (filled columns) or II (open columns) before, during and after the training period. *P < 0.05versus pretraining, n = 6. B, proliferating capillaries after 4 weeks of training in contact with two type I fibres (I + I), two type I and one type II fibres (I + I + II), one type I and one type II fibre (I + II), one type I and two type II fibres (I + II + II) or two type II fibres (II + II). n = 6.
Training resulted in a significant increase in Ki-67-positive cells per fibre after 4 weeks (from 0.01 ± 0.00–0.18 ± 0.05) (P < 0.05), whereas values after 2 and 6.8 weeks (0.12 ± 0.04 and 0.09 ± 0.02, respectively) were similar to pretraining levels (P > 0.05) (Fig. 2C). In the control leg the number of Ki-67-positive cells remained unaltered throughout the training period (P > 0.05).
The majority of the endothelial-related Ki-67-positive cells were shared between two fibres, of which 33.6 ± 9.0% were located between a type I and a type II fibre, and to a lesser extent between three fibres. Ki-67-positive cells associated with capillaries were localized equally to type I and type II fibres (Fig. 3B).
Study 2.
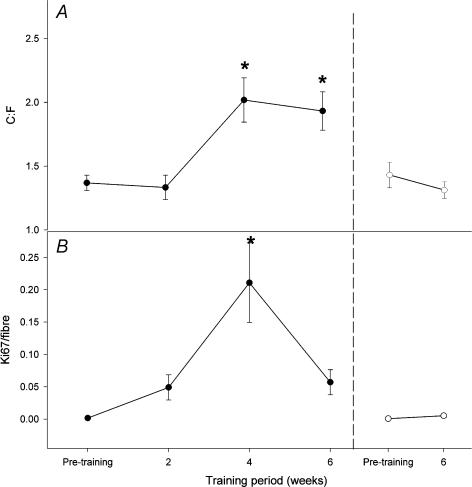
The C : F ratio increased from 1.36 ± 0.06 before training to 2.02 ± 0.17 and 1.93 ± 0.15 after 4 and 6 weeks of training, respectively (P < 0.05) (Fig. 4A). No change in C: F ratio was observed in the control leg (P > 0.05).
Figure 4. Presence of capillaries and proliferating endothelial cells in human skeletal muscle before and after intense (90% of V˙O2max) intermittent training.
Capillary-to-fibre ratio (C : F; A) and endothelial cell associated proliferating cells (B) before, during and after a 6 week training period in the trained (•) and the control (○) leg. *P < 0.05versus pre-training, n = 7.
Four weeks of training induced an increase in Ki-67 immunopositive cells associated with capillaries (from 0.00 ± 0.00 to 0.21 ± 0.06) that was significantly different from 0, 2 and 6 weeks of training (Fig. 4B). The muscle fibre area after the training period was not different from that measured before the training period in the trained (4283.5 ± 137.6 μm2 before; 4545.2 ± 275.6 μm2 after) or the untrained (4245.7 ± 127.1 μm2 before; 4272.3 ± 211.5 μm2 after) legs (P > 0.05).
When comparing changes in C: F and Ki-67/fibre over time between Study 1 and Study 2 no differences were found (P > 0.05).
Endothelial cell proliferation with microdialysate
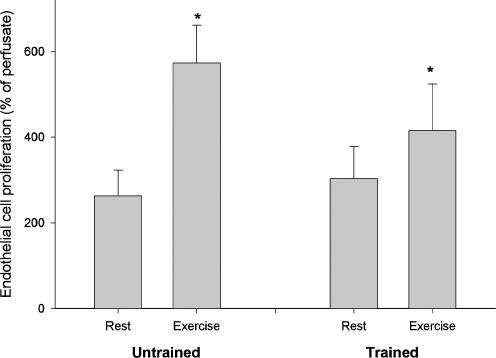
Addition of microdialysate from resting muscle to endothelial cells resulted in a higher level of proliferation (P < 0.05) than addition of perfusate (control), and the proliferation levels with resting dialysate were similar (P > 0.05) for the untrained (262 ± 60% of perfusate) and trained (303 ± 75% of perfusate) legs (Fig. 5). The proliferative effect of the dialysate obtained after exercise was greater than that at rest in both legs but not significantly different between the untrained (573 ± 87% of perfusate) and the trained (415 ± 108% of perfusate) legs.
Figure 5. Effect of intense (150% of V˙O2max) training on the endothelial cell proliferative effect of skeletal muscle microdialysate.
Proliferation of endothelial cells, as assessed by incorporation of bromodeoxyuridine (BrdU), after addition of skeletal muscle microdialysate from the trained and untrained leg obtained at rest (Rest) and during knee extensor exercise at 30 W (Exercise). *P < 0.05 versus rest, n = 6.
VEGF and bFGF localization
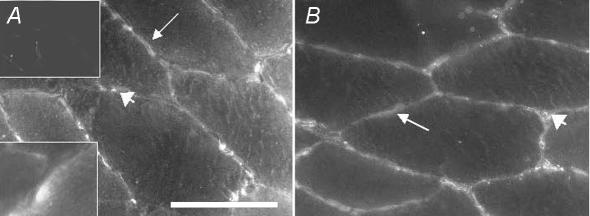
In skeletal muscle cells, weak positive VEGF staining was present in the cytosol whereas more distinct scattered staining was observed in the sarcolemma (Fig. 6A). The VEGF staining was more apparent in the cytosol and sarcolemma of type I muscle fibres than type II fibres. Positive VEGF staining was, moreover, observed between muscle cells and often in co-localization with endothelial cells. In a few skeletal muscle cells (approximately one out of 30) VEGF staining was concentrated in a pool in the cytosol in the periphery of the cells (inset in Fig. 6A). This peripheral pool was often localized adjacent to endothelial cells and sometimes to smooth muscle cells. bFGF was expressed in the cytosol, sarcolemma and between muscle cells (Fig. 6B). The staining between muscle cells was seldom co-localized with endothelial cells. Training did not lead to any changes in the distribution of VEGF or bFGF or in the number of VEGF-positive capillaries in either Study 1 or Study 2. VEGF and bFGF were rarely co-localized with smooth muscle cells.
Figure 6. Representative micrographs showing immunohistochemical staining of VEGF and bFGF in human skeletal muscle.
Discontinuous VEGF staining was observed in sarcolemma (arrow) and staining was weak in the cytosol of the muscle cells. VEGF staining was also observed in the extracellular space predominantly in association with endothelial cells (arrowhead). A, the insertion in the lower left corner shows VEGF staining in a cluster close to the periphery of the cell. In the upper left corner there is a negative control for which no primary antibody has been used. B, bFGF expression is detectable in cytosol and sarcolemma (arrow) of muscle cells and between muscle cells (arrowhead). Scale bar = 50 μm and applies to all panels.
Discussion
The present study demonstrates that high intensity intermittent endurance training induces endothelial cell proliferation and an enhanced capillarization within 4 weeks of training, with no further increase in capillarization at 7 weeks of training. The capillary growth-stimulating effect of the training appeared to be transient, as at 7 weeks of training the number of endothelial cell associated proliferating cells was back to pretraining levels. The increase in proliferating endothelial cells and in total capillaries were equally distributed around type I and type II muscle fibres. Furthermore, immunohistochemical analysis revealed that VEGF and bFGF were localized within the muscle cell as well as in the extracellular space, where VEGF, but not bFGF, was present mainly in endothelial cells. No changes in VEGF and bFGF protein distribution were observed with training.
Capillarization
In the current study the effect of two training protocols, both involving intermittent one-legged knee extensor exercise, using different intensities (150% and 90% of leg V˙O2max), was compared. The effect of training on capillarization and proliferating endothelial cells was similar in the two studies. In both studies an increase in capillarization was observed, which is in contrast to findings in studies using a lower exercise intensity (45% of V˙O2max) (Schantz et al. 1983). Thus, it appears that intermittent training of high intensity is effective in inducing angiogenesis, but there is little difference in angiogenic effect between exercise performed at 90% and 150% of leg V˙O2max.
In the present study no increase in capillarization was observed after 2 weeks of training, which is in contrast to findings in animal models where an increase in capillarization has already been observed after 4 days of electrical stimulation (Hudlicka, 1998). Nevertheless, after 4 weeks of training in our study, capillarization, as well as the number of proliferating cells co-localized with endothelial cells, which indicates newly developed capillaries, were found to be elevated. Only a few studies have examined the effect of training on the time course of angiogenesis in human skeletal muscle. However, in accordance with our observation, Andersen & Henriksson (1977) found that skeletal muscle capillarization was increased after 5 but not after 3 weeks of moderate intensity training. In the present study capillary supply did not increase further from 4 to 7 weeks of training and at 7 weeks the number of Ki-67-positive endothelial cells was not significantly different from pretraining. In addition, microdialysate obtained from all subjects showed a lower exercise-induced proliferating effect after training and, although there was no significant difference between the legs, the mean change in proliferative effect from rest to exercise was 175 ± 69% in the untrained and 32 ± 9% in the trained leg. In combination, these findings indicate that angiogenesis is important in the initial phase of adaptation to training but, as endothelial cells have a relatively long half-life (Bachetti & Morbidelli, 2000), the need for capillary growth may be reduced when an adequate capillary supply has been reached. Thus, a negative feed-back mechanism related to the level of capillarization appears to exist.
Our observation that microdialysate obtained from resting and exercising leg induced proliferation of endothelial cells is in agreement with findings obtained earlier by our group (Hoffner et al. 2003) and suggests the presence of angiogenic compounds. Since the microdialysis probes had a molecular mass cut-off of only 20 kDa, microdialysates were not likely to contain either VEGF (45 kDa) or high concentrations of any other high molecular weight compounds such as TGF-β (25 kDa). bFGF (18 kDa) could potentially have been present, even though the diffusion was probably limited due to its molecular size and it could be responsible for some of the proliferative effect observed. Nevertheless, our data indicate that the muscle interstitial fluid appears to contain one or several proliferative compound(s) of relatively low molecular weight. Furthermore, as the proliferative effect of the microdialysate obtained during exercise was greater than that obtained at rest, such proliferative compounds are seemingly increased in response to exercise. The present study did not unveil the identity of these compounds but several substances such as NO (RayChaudhury et al. 1996), endothelin-1 (ET-1) (Salani et al. 2000) and adenosine (Ethier et al. 1993) are regulators of endothelial cell proliferation and have been shown to be present in the human muscle interstitium at rest and to increase in concentration in response to exercise. Thus, one or several of these compounds are potential contributors to the proliferative effect of the microdialysate.
As the identity of the proliferative compounds in the interstitium was unknown, it was not possible to obtain relative recovery for the compounds responsible for the proliferative effect. Nevertheless, in one of our previous studies an estimate of relative recovery was determined for several metabolites of different molecular size (albumin, adenosine and casein; Hoffner et al. 2003). The observed increases in relative recovery from rest to exercise for the metabolites examined (from 0 to 32%) were all less than the increases in proliferation rate (48–175%) observed with exercise in the present study. Thus, our findings of enhanced proliferation with exercise cannot be explained solely by an enhancement of probe recovery during exercise.
During the microdialysis experiment, training of both legs was performed at the same intensity (30 W) and thus the trained and untrained legs exercised at the same absolute but not relative intensities. After the training period the maximal oxygen uptake at 30 W represented 48 ± 3 and 43 ± 2% of leg V˙O2max of the untrained and trained legs, respectively. This minor difference is not likely to have affected the comparison between the untrained and the trained leg with regard to the exercise-induced proliferative effect of the muscle microdialysate.
Location of new capillaries in relation to muscle fibre types
In the present study the determination of endothelial cell associated proliferating cells allowed a sensitive examination of the location of capillary growth in relation to muscle fibre types. The analysis of muscle biopsies from the trained leg showed that the proliferating endothelial cells were equally distributed around type I and II muscle fibres. Accordingly, the number of capillaries per fibre as well as capillary density after training was not different for type I versus type II muscle fibres. The intense intermittent training employed in Study 1 required marked recruitment of type II fibres, as evidenced by analyses of glycogen depletion in individual fibres. The analysis showed that ∼70% of the type II fibres were depleted of glycogen whereas only 22% of the type I fibres were depleted (P. Krustrup, Y. Hellsten & J. Bangsbo, unpublished results). The present data showing a similar increase in capillarization around type I and type II fibre types therefore suggest that recruitment of a greater fraction of type II fibres than is recruited with exercise of moderate intensity does not lead to growth of capillaries specifically around type II fibres. This is somewhat in contrast to an earlier study by Daub et al. (1998) involving high intensity training, in which an increase in capillaries per fibre area was found for type I fibres, and not for type II fibres (Daub et al. 1982). It should be pointed out, however, that in this latter study by Daub et al. (1982) the training did not increase C: F ratio; in fact, a decrease in C : F ratio was observed for type II fibres. No single variable used to express capillarization will give a complete description of capillary supply. This is demonstrated in the study by Daub et al. (1982), where no changes were observed in C: F ratio for type I fibres, whereas an increase was present in capillaries per fibre area, primarily at the expense of a reduction in fibre size. Since no change in mean fibre area was found in the present study, the C : F values obtained must be considered reliable. The divergent findings of Daub et al. (1982) and the present study may be related to the initial training status of the subjects participating in the two studies. Whereas the present study used habitually active subjects, Daub and coworkers used ice-hockey players.
It may be noted that in the present study more than 90% of the proliferating cells were colocalized with endothelial cells, which is somewhat different from findings in rats which suggested that levels of capillary-linked BrdU-positive nuclei were only 1.5-fold higher than those of interstitial-linked BrdU-positive nuclei (Hudlicka et al. 2000). This discrepancy could be due to species differences or use of different methods to detect the proliferating cells. Ki-67 has been shown to detect proliferating cells more accurately than proliferating cell nuclear antigen (PCNA) (Aoyagi et al. 1995). The murine antibody of Ki-67 reacts with a human nuclear proliferation-associated antigen expressed in all active cell cycle phases (G1, S, G2, and mitosis), but not with G0 cells, which are consistently negative (Gerdes et al. 1984), and thus recognizes all proliferating cells and no quiescent cells. PCNA labelling occurs in the G1, S and G2 phases of the cell cycle and the PCNA antibody reacts most noticeably during the late G1 and early S phases. The half-life for PCNA (∼20 h) is longer than that for Ki-67 (< 2 h), which may be a cause of the observed differences in positive staining (Aoyagi et al. 1995).
Distribution of VEGF and bFGF
VEGF protein distribution has mainly been examined in rabbit and rat muscles (Annex et al. 1998; Cherwek et al. 2000; Amaral et al. 2001; Hudlicka et al. 2002). Such studies have shown that VEGF is localized to the matrix between muscle cells and to some extent to endothelial cells and other non-muscle cells, but is not present within the skeletal muscle cells. In the present study VEGF protein was localized to endothelial cells as well as to the cytosol and sarcolemma of skeletal muscle cells, with the strongest staining observed in type I muscle fibres. This suggests a species difference, with a higher amount of VEGF present in the muscle cells of human muscle compared to rat and rabbit skeletal muscle. In support of this proposition, VEGF mRNA has been reported to be present in the cytoplasma of muscle cells in human skeletal muscle (Gustafsson et al. 2002). However, Rissanen et al. (2002) did not observe the presence of VEGF protein in non-atrophic ischaemic human skeletal muscle. The reason for these divergent findings is unclear but may be due to the muscle being ischaemic. In the present study VEGF was, moreover, rarely localized to smooth muscle cells. This observation is in conflict with findings that isolated human vascular smooth muscle cells express VEGF mRNA (Brogi et al. 1994). However, the present study is the first to detect VEGF protein in normal human muscle and it might be that VEGF and bFGF are not localized to vascular smooth muscle cells in human skeletal muscle.
In the present study we noted occasional pools of VEGF staining mainly localized in the vicinity of endothelial and smooth muscle cells. A similar observation has been made in patients with chronic ischaemia in which VEGF pools were present in several regenerating skeletal muscle cells (Rissanen et al. 2002). It may be speculated that the presence of these pools of VEGF in the skeletal muscle is due to normal maintenance processes in the muscle cells and blood vessels.
Assessment of VEGF protein after exercise has, for the most part, been derived from whole extracts of muscle and it has therefore not been clear how the distribution of VEGF is influenced by exercise training. In animal studies, immunohistochemical analysis of VEGF protein in matrix has shown an up-regulation after chronic stimulation of rat and rabbit skeletal muscle (Annex et al. 1998; Amaral et al. 2001; Hudlicka et al. 2002), which was partly due to an increase in VEGF localized to capillaries (Hudlicka et al. 2002). In the present study there were no changes in the number of capillaries staining positive for VEGF and the immunohistochemical technique was not sufficiently sensitive to detect possible small increases in the amount of VEGF protein. This discrepancy between the observations in animal studies and our findings might be due to the use of electrical stimulation versus exercise training, time of assessment after training/stimulation and species differences. In accordance with our data showing a similar distribution of capillaries in relation to muscle fibre types there was no apparent change in VEGF distribution in relation to fibre type throughout the training period.
In the present study, no differences in the amount or distribution of bFGF was observed. This is in agreement with training studies in humans showing that an exercise bout or exercise training does not alter bFGF mRNA levels (Gustafsson et al. 1999; Richardson et al. 2000). bFGF has, as in the present study, been localized to the sarcolemma of muscle cells and to the matrix between muscle cells, but only to a minor extend to smooth muscle cells. Bovine endothelial cells have been reported to express bFGF (Schweigerer et al. 1987), but in the present study only sparse positive staining was found in the vicinity of capillaries.
Conclusion
In summary, this study demonstrates that intense intermittent endurance training induces an increase in endothelial cell proliferation and an increased capillarization that is equally distributed around type I and type II fibres in human skeletal muscle. The capillary growth occurs within 4 weeks and appears to be transient, as evidenced by a reduction in proliferating endothelial cells and no further capillarization after 7 weeks of training. Proliferative compounds other than VEGF are released in response to exercise and are likely to be important for training-induced angiogenesis. VEGF and bFGF were localized in skeletal muscle cells, with VEGF also present in the majority of capillaries, but the distribution of these growth factors was not found to be altered during the training period.
Acknowledgments
This study was supported by grants from the Danish National Research Foundation (504-14) and the Danish Ministry of Culture.
References
- Amaral SL, Linderman JR, Morse MM, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation. 2001;8:57–67. [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol. 1998;274:H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- Aoyagi M, Yamamoto M, Wakimoto H, Azuma H, Hirakawa K, Yamamoto K. Immunohistochemical detection of Ki-67 in replicative smooth muscle cells of rabbit carotid arteries after balloon denudation. Stroke. 1995;26:2328–2331. doi: 10.1161/01.str.26.12.2328. [DOI] [PubMed] [Google Scholar]
- Bachetti T, Morbidelli L. Endothelial cells in culture: a model for studying vascular functions. Pharmacol Res. 2000;42:9–19. doi: 10.1006/phrs.1999.0655. [DOI] [PubMed] [Google Scholar]
- Bangsbo J. Physiology of intermittent exercise. In: Garret WE, Kirkendall D, editors. Exercise, Basic and Applied Science. Philadelphia, USA: Lippincott, Williams & Wilkins; 1999. pp. 53–66. [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90:649–652. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- Cherwek DH, Hopkins MB, Thompson MJ, Annex BH, Taylor DA. Fiber type-specific differential expression of angiogenic factors in response to chronic hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2000;279:H932–H938. doi: 10.1152/ajpheart.2000.279.3.H932. [DOI] [PubMed] [Google Scholar]
- Daub WD, Green HJ, Houston ME, Thomson JA, Fraser IG, Ranney DA. Cross-adaptive responses to different forms of leg training: skeletal muscle biochemistry and histochemistry. Can J Physiol Pharmacol. 1982;60:628–633. doi: 10.1139/y82-085. [DOI] [PubMed] [Google Scholar]
- Denis C, Chatard JC, Dormois D, Linossier MT, Geyssant A, Lacour JR. Effects of endurance training on capillary supply of human skeletal muscle on two age groups (20 and 60 years) J Physiol (Paris) 1986;81:379–383. [PubMed] [Google Scholar]
- Ethier MF, Chander V, Dobson JG., Jr Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol. 1993;265:H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Gustafsson T, Knutsson A, Puntschart A, Kaijser L, Nordqvist AC, Sundberg CJ, Jansson E. Increased expression of vascular endothelial growth factor in human skeletal muscle in response to short-term one-legged exercise training. Pflugers Arch. 2002;444:752–759. doi: 10.1007/s00424-002-0845-6. [DOI] [PubMed] [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- Hoffner L, Nielsen JJ, Langberg H, Hellsten Y. Exercise but not prostanoids enhance levels of vascular endothelial growth factor and other proliferative agents in human skeletal muscle interstitium. J Physiol. 2003;550:217–225. doi: 10.1113/jphysiol.2002.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlicka O. Is physiological angiogenesis in skeletal muscle regulated by changes in microcirculation? Microcirculation. 1998;5:5–23. [PubMed] [Google Scholar]
- Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown MD, Silgram H. Inhibition of capillary growth in chronically stimulated rat muscles by N(G)-nitro-1-arginine, nitric oxide synthase inhibitor. Microvasc Res. 2000;59:45–51. doi: 10.1006/mvre.1999.2193. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Milkiewicz M, Cotter MA, Brown MD. Hypoxia and expression of VEGF-A protein in relation to capillary growth in electrically stimulated rat and rabbit skeletal muscles. Exp Physiol. 2002;87:373–381. doi: 10.1113/eph8702285. [DOI] [PubMed] [Google Scholar]
- Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- Morrow NG, Kraus WE, Moore JW, Williams RS, Swain JL. Increased expression of fibroblast growth factors in a rabbit skeletal muscle model of exercise conditioning. J Clin Invest. 1990;85:1816–1820. doi: 10.1172/JCI114640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RayChaudhury A, Frischer H, Malik AB. Inhibition of endothelial cell proliferation and bFGF-induced phenotypic modulation by nitric oxide. J Cell Biochem. 1996;63:125–134. doi: 10.1002/(sici)1097-4644(19961101)63:2<125::aid-jcb1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA, Wagner PD. Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol. 1999;277:H2247–H2252. doi: 10.1152/ajpheart.1999.277.6.H2247. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am J Pathol. 2002;160:1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salani D, Taraboletti G, Di Rosano LCV, Borsotti P, Giavazzi R, Bagnato A. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization In vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Henriksson J, Nygaard E, Andersen P, Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann N Y Acad Sci. 1977;301:3–29. doi: 10.1111/j.1749-6632.1977.tb38182.x. [DOI] [PubMed] [Google Scholar]
- Schantz P, Henriksson J, Jansson E. Adaptation of human skeletal muscle to endurance training of long duration. Clin Physiol. 1983;3:141–151. doi: 10.1111/j.1475-097x.1983.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]