Abstract
The impact of chronic joint inflammation on articular vascular function in rats was investigated to address whether joint swelling and the associated vascular dysfunction are dependent upon a common prostanoid mechanism. Urinary nitrate/nitrite (NOx) and PGE2 excretion, knee joint diameter and body weight were measured following induction of adjuvant-induced arthritis (AIA). Ten days postinduction of AIA, joint vascular reactivity was assessed by measuring the perfusion response using a laser Doppler imager (LDI) to topical application of acetylcholine (ACh) and sodium nitroprusside (SNP). Four groups were compared: a non-inflamed control group and three AIA groups treated i.p. with vehicle, indomethacin or SC-236 (at equimolar doses). The selective cyclooxygenase-2 (COX-2) inhibitor (SC-236) was used to differentiate between COX-1 and -2-derived prostaglandins. Urinary NOx and PGE2 levels increased substantially during the early phase of AIA but decreased thereafter. Toxicity to indomethacin but not SC-236 was observed, as indicated by a marked decrease in body weight. Joint swelling was similarly attenuated by indomethacin and SC-236 (P = 0.0001 cf. vehicle-treated AIA; n = 5–6 per group), indicating that this is due to COX-2 and not COX-1 inhibition. The AIA-induced changes in urinary NOx and PGE2 were corrected by both COX inhibitors. While vascular reactivity to ACh and SNP was significantly attenuated by AIA (P < 0.002; n = 5–10 per group), the perfusion responses to these vasodilating agents were similar in all three AIA groups, demonstrating that the vascular dysfunction was not corrected by inhibition of either COX-1 or COX-2 enzymes. Furthermore, the attenuation of both ACh and SNP-induced responses in AIA suggest that vascular dysfunction was not exclusively endothelial in nature. In conclusion, the joint swelling and vascular dysfunction associated with AIA appear to be mediated, at least in part, by independent mechanisms. While COX-1/COX-2 inhibition reduced joint swelling, vascular dysfunction in AIA is independent of constitutive or inducible prostanoid mechanisms, and appears not to be solely endothelial-derived, but to involve other components such as the vascular smooth muscle.
Adjuvant-induced arthritis (AIA) is characterized by inflammation and aggressive pannus formation which leads to degradation of cartilage and bone (Verschure et al. 1989; Griffiths, 1992; Carpenter et al. 1994). AIA in the rat is an extensively studied model of inflammatory joint disease and it shares many features associated with rheumatoid arthritis (RA; Klareskog et al. 1989). Intra-articular hypoxia has been observed in animal models of joint inflammation (Najafipour & Ferrell, 1995) and is a feature of the rheumatoid joint (Richman et al. 1981). The relative intra-articular hypoxia and lactic acidosis in the arthritic joint suggests an insufficient blood flow (Falchuck et al. 1970; Wallis et al. 1985), which may be due to a combination of factors. This could include an inability of angiogenic processes to meet and support the growing demands of the proliferating pannus and synovial tissue, and/or the inflamed environment in the arthritic joint pre-disposing to vascular dysfunction (McDougall et al. 1995).
The production of prostaglandins (PGs), through the metabolism of arachidonic acid by cyclooxygenase (COX), is one of the key pathways involved in the pathogenesis of acute inflammation. There are two COX isoforms: COX-1 is constitutively expressed, performing housekeeping functions, and COX-2 is an inducible isoform rapidly up-regulated at inflammatory sites. COX-2 mRNA and protein are expressed in synovial tissues from rats with AIA (Anderson et al. 1996) as well as in synovium from patients with RA (Kang et al. 1996; Siegle et al. 1998). The functions of COX-1-derived prostaglandin include regulation of synovial vascular tone (Egan et al. 2001), but while prostaglandins are known to play an important role in acute joint inflammation (Egan et al. 2002), it is as yet unclear how their vascular role is affected during chronic arthritis. Furthermore, many current anti-inflammatory therapies target the prostanoid system, but their impact on synovial vascular function in chronic arthritis has not yet been established.
Non-steroidal anti-inflammatory drugs (NSAIDs) are used for the treatment of RA but can have adverse effects through their inhibition of COX-1. Selective inhibitors which target COX-2 have been developed in recent years to avoid such side-effects. Assessment of new anti-inflammatory therapies in pre-clinical studies are often limited to measurement of paw and joint swelling. However, longer-term consequences of established inflammatory processes include vascular dysfunction and this may contribute to inadequate perfusion of the arthritic joint. Previous studies have demonstrated that dilator responses to acetylcholine (ACh) were attenuated in the acutely inflamed joints of rabbits (Najafipour & Ferrell, 1993), and that the dilator response to substance P is reduced in chronically inflamed joints of rats at both 1 and 3 weeks post-induction of AIA (McDougall et al. 1995). Furthermore, ACh responses are attenuated in adjuvant arthritis (McDougall et al. 1998). These studies are indicative of vascular dysfunction, but do not address the location of such dysfunction (i.e. whether at the level of the endothelium or vascular smooth muscle) and whether this can be corrected by anti-inflammatory therapy.
It is well recognized that ACh can be used to stimulate the endothelial release of nitric oxide (NO) via muscarinic receptors on these cells. Sodium nitroprusside (SNP) is an NO donor that acts independently of the endothelium and is thus used as an experimental tool to assess the function of vascular smooth muscle. These vasoactive agents can be used to probe the nature and site of vascular dysfunction.
The present study investigated the impact of chronic joint inflammation on articular vascular function and sought to address whether joint swelling and the associated vascular dysfunction are dependent upon a common prostanoid pathway. Given the importance of COX-1-derived prostaglandins in normal synovial vascular function (Egan et al. 2001), we compared the effect of a selective COX-2 inhibitor with a broad-spectrum COX inhibitor, indomethacin.
Methods
Experimental animals
Experiments were performed in male Wistar rats (300–400 g body weight; Charles River UK Ltd). Animals were housed in standard cages, had food and water available ad libitum and were maintained in a thermoneutral environment (23 ± 2°C). All procedures were performed in accordance with Home Office regulations.
Induction of arthritis
Adjuvant-induced monoarthritis has been previously described (McDougall et al. 1995). A total of 23 animals were used in the present investigation of which 18 underwent chronic, unilateral inflammation, the remaining five served as untreated (non-inflamed) control animals. Sham intra-articular injections were not administered to the control group in case this provoked a low-grade inflammatory response that would complicate interpretation of this study. Rats were deeply anaesthetized (2% halothane, 1 l min−1, O2, 1 l min−1, N2O, 1 l min−1; flexion withdrawal reflex absent), fur surrounding both knee joints shaved, 0.2 ml of Freund's complete adjuvant (FCA; Sigma, UK) injected into the right knee (0.1 ml into each of the anterior and posterior cavities) and the animals then allowed to recover. Preliminary work was performed in a group of rats (n = 6) where AIA was induced and allowed to develop for 28 days. Swelling developed and stabilized by day 10, remaining relatively constant thereafter. We therefore elected to focus on the first 10 days for the main experimental study.
Swelling, body weight, urine collection and drugs
Knee diameter measurements were made immediately prior to the induction of arthritis (day 0), then afterwards on days 1, 3, 6 and 10. Both ipsilateral (inflamed) and contralateral knee joint diameters were measured laterally across the joint line at the point of articulation using modified vernier callipers (Oditest, Kroeplin Gmbh, Germany). Measurements were compared with pre-injection diameter values. Body weight was monitored daily during the first week and at set intervals thereafter. Urine was also collected overnight in these animals via specialized metabolic cages at set days during the experiment. Four groups of animals were used for this study: AIA was induced in three groups of animals: vehicle (0.5% methylcellulose and 0.025% Tween-20; n = 6), SC-236 (n = 6) and indomethacin treated (n = 6). An untreated (non-inflamed) control group (n = 5) was also included. Intraperitoneal administration of drugs was given prophylactically, and then daily for the first 3 days and every second day thereafter. Both SC-236 and indomethacin were administered at equimolar concentrations of 5.6 and 5 mg kg−1, respectively. The indomethacin dose was supramaximal for inhibition of both COX isoforms (Wallace et al. 1999). The equimolar dose of SC-236 used was three orders of magnitude below the IC50 for COX-1 inhibition (Gierse et al. 1996). Osmolality measurements (Micro-Osmometer, 13/13 DR, Roebling, Germany) were taken as a measure of urine concentration. A standard solution of 300 mosmol kg−1 was used to calibrate the system prior to urine analysis.
Preparatory surgery
On day 10 of arthritis, terminal blood flow experiments were performed to assess vascular responsiveness in ipsilateral (inflamed) knees. Routine surgical procedures were performed to allow blood pressure measurement. Animals were deeply anaesthetized by injection of urethane (Sigma; 1.6 g kg−1, i.p.) and placed in dorsal recumbency. Temperature was monitored via a rectal probe and maintained at 37 ± 1°C (mean ±s.e.m.) over the course of the experiment by a thermal pad. Tracheostomy was performed with the animals breathing spontaneously throughout the experiments. Arterial blood pressure was monitored continuously via a cannula inserted into the left common carotid artery and linked to a pressure transducer (EM-751, Elcomatic, UK). Mean arterial pressure (MAP) was calculated by adding one-third of the pulse pressure to the diastolic pressure.
An ellipse of skin was removed from the medial aspect of the knee joint to reveal the underlying synovium. The overlying fascia and synovial fatty tissue were dissected clear to maximize exposure of superficial synovial blood vessels. The exposed knee was prevented from desiccation by dousing frequently with physiological saline (0.9% NaCl) warmed to the temperature of the exposed knee (31°C). An equilibration period of approximately 30 min was allowed prior to commencing vascular reactivity experiments, to ensure that blood pressure and synovial perfusion were stable.
Measurement of vascular reactivity by laser Doppler imaging (LDI)
A detailed description of LDI measurement of perfusion in the exposed rat joint capsule has been previously published (Lam & Ferrell, 1993; McDougall et al. 1995, 1998, 2001). Briefly, a laser Doppler perfusion imager (Lisca, Linkoping, Sweden) was used to monitor relative changes in blood flow over the medial aspect of the exposed capsule. Drugs (ACh and SNP, both in the dose range 10−9–10−7 mol; Sigma) were applied topically in a 0.1 ml bolus per knee prior to LDI scans. These agents elicit vascular responses that are endothelial-dependent (ACh) or -independent (SNP) allowing the site of vascular dysfunction to be investigated. After initial control measurements using a saline vehicle to check stability, LDI scans (30 s duration) were performed immediately before and after drug application. The peak response was then compared to the vehicle response. The joints were allowed to recover for 10 min between drug administrations. Scans were performed in a darkened room using a red laser beam (He–Ne, 635 nm). The laser was scanned back and forth in a raster fashion over the joint, producing a colour-coded image. These images were later analysed by dedicated software (Moor Instruments Ltd, UK) to obtain a median flux value over the knee joint region. The biological zero values were measured as previously described (Najafipour & Ferrell, 1995) and subtracted from the perfusion values. Vascular conductance (inverse of vascular resistance) was calculated by dividing flux by the MAP value, with changes being expressed as a percentage compared to saline control.
Urinary PG determination
After centrifuging the urine samples, Bicyclo PGE2 (a stable metabolite of prostaglandin E2) was quantified using competitive binding enzyme-linked immunosorbent assay (ELISA; Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer's instructions. The microtitre plate was read using a spectrophotometer (Dynatech MR5000, Dynatech Laboratories Inc., Chantilly, VA, USA) set to 410 nm. Optical density was used to determine the percentage of bound labelled ligand. The sensitivity of the bicyclo PGE2 ELISA was 2 pg ml−1 (at 24°C). Sample values were corrected for urine concentration.
Urinary nitrite/nitrate determination
In view of the known involvement of nitric oxide (NO) in joint inflammation (Grabowski et al. 1996; Stichtenoth & Frölich, 1998; reviewed by Jang & Murrell, 1998) and the cross-talk between nitrergic and prostanoid pathways (Swierkosz et al. 1995), urinary nitrate/nitrite (NOx) excretion was measured as a marker of NO generation. In brief, urine (1.5 ml) was centrifuged at 10 000 g for 15 min, the supernatant removed and diluted 5-fold using deionized water. Nitrate standards were also reduced with vanadium to assess the percentage conversion efficiency of nitrate to nitrite. Each 50 µl sample (including nitrate and nitrite standards) was dispensed into a 96-well plate followed by 50 µl of freshly prepared vanadium chloride solution (200 mg in 25 ml 1 m HCl) and immediately followed by 50 µl of Griess reagent (2% sulphanilamide in 1.47 m hydrochloric acid, and 0.1%N-(1-naphthyl)ethylenediamine dihydrochloride in deionized water; Sigma; Miranda et al. 2001). After 30 min of colour development at 37°C, absorbance was measured on a microplate spectrophotometer (Dynatech MR5000) at 570 nm with 630 nm reference filter. Each sample was assayed in triplicate. NOx values were corrected for variations in urine concentration by dividing by the corresponding urinary osmolality values.
Statistical analysis
After image and data analysis, subsequent statistical analyses were performed by one- or two-way ANOVA as indicated in the text using Instat software (GraphPad, USA). Bonferroni post hoc tests were used for comparisons between treatment groups. All quoted P values are two-tailed; n values refer to the number of animals examined. Data are presented as mean ± standard error of the mean (s.e.m.).
Results
Joint swelling
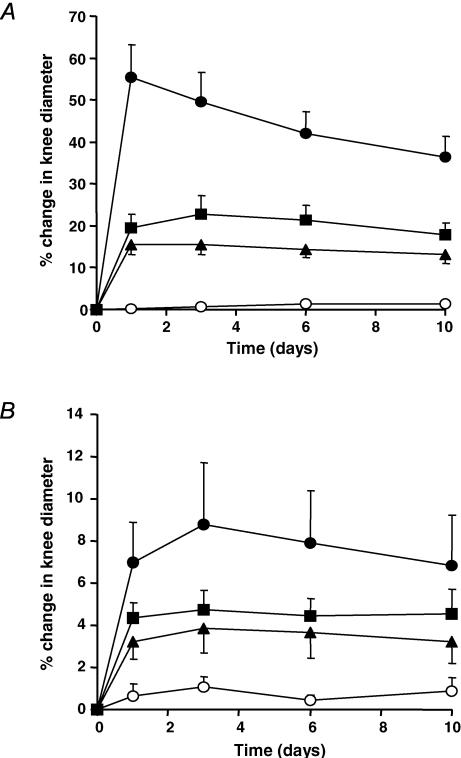
The vehicle-treated AIA group demonstrated a significant increase in knee joint diameter, peaking at day 1 (∼55%) and levelling by day 10 (Fig. 1A). Both SC-236 and indomethacin substantially attenuated the increase in joint diameter observed in vehicle-treated AIA animals.
Figure 1. Ipsilateral and contralateral knee joint diameters.
A, ipsilateral knee joint diameter in AIA. Diameter increased significantly in the vehicle-treated group (•) (P < 0.0001, one-way ANOVA). Both SC-236 (▴) and indomethacin (▪) significantly decreased swelling compared to vehicle-treated animals (P = 0.0001, two-way ANOVA). Knee joint diameter in control non-inflamed animals (○) did not significantly change with time (P = 0.138; n = 5–6; one-way ANOVA). B, contralateral knee joint diameter in AIA. Diameter increased significantly in the vehicle-treated AIA group (•) compared to the control non-inflamed group (P = 0.0001, two-way ANOVA). Both SC-236 (▴) and indomethacin (▪) decreased knee joint diameter by a magnitude proportionate to that of the ipsilateral knee (both P = 0.0001, two-way ANOVA, compared to untreated group). Control non-inflamed values (○) did not significantly change with time (P = 0.5; n = 5; one-way ANOVA).
The contralateral knee joint in the vehicle-treated AIA group also increased significantly to a maximum (∼8%) by day 3 (Fig. 1B). Both SC-236 and indomethacin significantly decreased joint swelling by a magnitude proportionate to that of the ipsilateral knee. Joint diameters did not significantly change with time in untreated control animals, indicating the reproducibility of these measurements.
Body weight
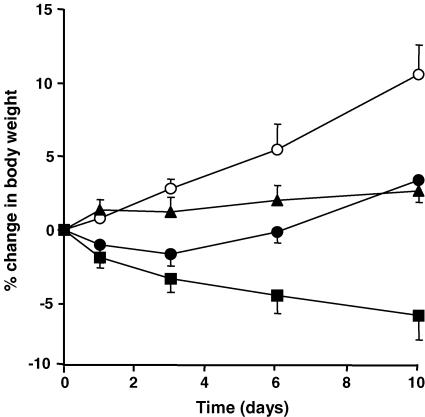
Body weight in control non-inflamed animals increased in a time-dependent fashion, differing significantly from vehicle-, SC-236- and indomethacin-treated groups. Body weight in vehicle- and SC-236-treated animals did not differ significantly from pre-induction values, whereas body weight decreased progressively with time in the indomethacin-treated group, indicating the toxic nature of this drug (Fig. 2). Animals treated with SC-236 and vehicle differed significantly from those treated with indomethacin.
Figure 2. Body weight.
Weights in control non-inflamed animals (○) increased in a time-dependent fashion (P < 0.0001, one-way ANOVA), differing significantly from vehicle-treated AIA (•), SC-236 (▴) and indomethacin (▪) groups (P < 0.0001, 0.0004 and 0.0001, respectively, two-way ANOVA). Animal weights in both vehicle- and SC-236-treated groups did not significantly differ with time, but decreased in the indomethacin-treated group (P < 0.029, one-way ANOVA). Body weights of SC-236 and vehicle groups differed significantly from those of the indomethacin group (P < 0.0001, respectively, two-way ANOVA). Data presented as mean ±s.e.m. (n = 5–6).
Urinary PG and NOx determination
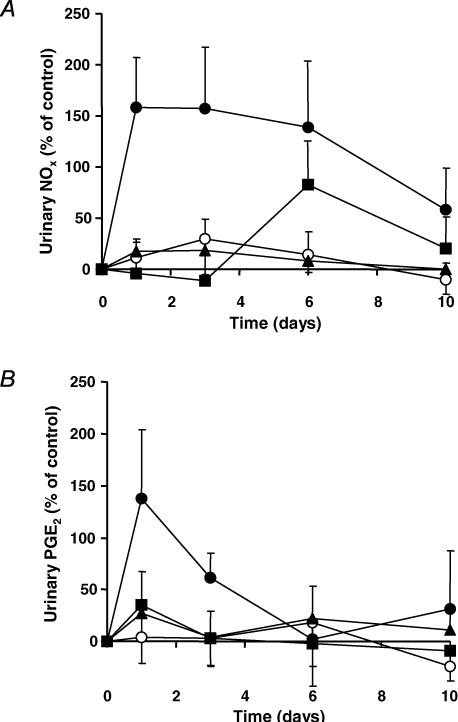
Urinary NOx increased significantly immediately after induction of inflammation, decreasing after day 6 towards baseline (Fig. 3A). This magnitude and profile in the vehicle group was significantly reduced by SC-236 and indomethacin. Importantly, both SC-236 and indomethacin groups decreased urinary NOx levels to near baseline control levels and did not significantly differ either from each other, or from the non-inflamed control group. The control group did not significantly change with time. There was an increase at day 6 in the indomethacin-treated group compared to SC-236-treated and control groups, possibly indicating an up-regulation of inducible nitric oxide synthase (iNOS) due to the overwhelming suppression of COX.
Figure 3. Urinary NOx and PGE2 changes.
A, urinary NOx changes in AIA. The vehicle-treated AIA group (•) was significantly different from SC-236 (▴), indomethacin (▪) and non-inflamed control (○) groups (P < 0.01, P < 0.005 and P < 0.01, respectively, two-way ANOVA). Both SC-236 and indomethacin reduced NOx levels to control levels (no significant difference from non-inflamed controls; two-way ANOVA). The non-inflamed controls did not significantly change with time (one-way ANOVA). Data presented as mean ± s.e.m. (n = 5–6). B, urinary PGE2 changes in AIA. There was an acute increase in PGE2 levels in the vehicle-treated AIA group (•) after day 1 (just failing to reach significance with time compared to SC-236 (▴), indomethacin (▪) and non-inflamed control (○) groups). The response over the first 3 days was significantly different from non-inflamed controls (P < 0.05, two-way ANOVA). The non-inflamed control group did not significantly change with time (one-way ANOVA) and did not differ significantly from either indomethacin or SC-236 (two-way ANOVA). Data presented as mean ±s.e.m. (n = 4).
While PG levels tended to increase acutely in the vehicle-treated AIA group (just failing to reach significance with time compared to SC-236, indomethacin and controls), these returned to baseline by day 6 (Fig. 3B) and showed no further change, obviating the need for additional measurements. As with urinary NOx levels, SC-236 and indomethacin treatment reduced PG to baseline levels. PG levels in the control group did not significantly change with time and did not differ significantly from those of the indomethacin or SC-236 groups.
Vascular reactivity
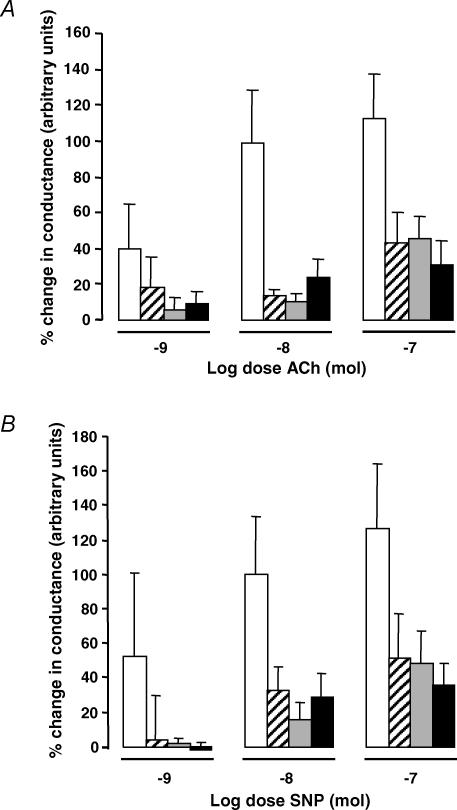
Exogenous application of ACh induced a dose-dependent synovial vasodilatation (Fig. 4A). The magnitude of this response in each of the AIA groups was significantly attenuated compared to the non-inflamed control group, but similar between SC-236-, indomethacin- and vehicle-treated AIA groups.
Figure 4. Vascular reactivity to ACh and SNP.
A, vascular reactivity to ACh in AIA rats. ACh induced a dose-dependent synovial vasodilatation (P = 0.009, two-way ANOVA), which was significantly attenuated in the vehicle (hatched columns; P < 0.002, Bonferroni post hoc), indomethacin (grey columns; P < 0.0001, Bonferroni post hoc) or SC-236-treated (filled columns; P < 0.0001, Bonferroni post hoc) AIA groups compared with the non-inflamed controls (open columns). There was no significant difference between the three AIA-treated groups. Data presented as mean ± s.e.m. (n = 5–10). B, vascular reactivity to SNP in AIA rats. SNP induced a dose-dependent synovial vasodilatation (P = 0.013, two-way ANOVA) which was significantly attenuated in the vehicle (hatched columns; P = 0.013, Bonferroni post hoc), indomethacin (grey columns; P = 0.0045, Bonferroni post hoc) or SC-236-treated (filled columns; P = 0.0002; Bonferroni post hoc) AIA groups compared with the non-inflamed controls (open columns). There was no significant difference between the three AIA-treated groups. Data presented as mean ±s.e.m. (n = 5–6).
Similarly, SNP application in all groups resulted in a dose-dependent synovial vasodilatation (Fig. 4B). The magnitude of vasodilator response was again substantially attenuated in the AIA groups compared to non-inflamed controls, and this reduced vascular responsiveness was not corrected by treatment with SC-236 or indomethacin.
Although there was no significance difference (P = 0.061; one-way ANOVA) between the basal vascular resistance of the synovium of non-inflamed and inflamed joints, resistance values tended to be lower in the inflamed groups, suggestive of inflammatory hyperaemia.
Discussion
This study has been the first to investigate the possible mechanisms underlying vascular dysfunction in a model of chronic arthritis. While many studies of joint inflammation have used swelling as their primary index, few have investigated vascular responsiveness. This is a neglected but important area for study as blood vessels play a key role in the development and maintenance of any inflammatory process. In the present study we explored possible mechanisms underlying synovial vascular dysfunction by measurement of vascular reactivity together with both prostanoid and nitrergic metabolites during the development of chronic joint inflammation.
Reduced responses to ACh have previously been described in acute (Lockhart et al. 1997) and chronic (McDougall et al. 1998) joint inflammation, possibly indicative of altered endothelial function. However, the effect of an NO donor such as SNP was not investigated in these early studies and thus dysfunction at the level of vascular smooth muscle could not be discounted. In the present study we observed that the vasodilator effect of both ACh and SNP in inflamed knees 10 days after the induction of AIA was similarly attenuated compared to healthy knees. This provides evidence that vascular dysfunction is not restricted to the endothelium, but may reside at the level of vascular smooth muscle. Precisely how this is mediated remains to be established and was beyond the scope of the present study. We can discount the possibility that altered vascular responsiveness was due to the administration of indomethacin or SC-236 since the vehicle-treated group showed very similar responses.
Interestingly, inhibition of either COX-2 (SC-236) or both COX isoforms (indomethacin) decreased the development of joint swelling (both ipsilateral and contralateral knees, Fig. 1A and B) by approximately 50%. The residual joint inflammation appears to be independent of prostanoid or nitrergic systems since the urinary PG and NOx levels in these treated animals were restored to baseline, suggesting the involvement of other pro-inflammatory systems.
The fact that NOx levels were reduced by COX inhibition suggests a strong interrelationship between nitrergic and prostanoid pathways. While low levels of NO are reported to activate COX (Stadler et al. 1991; Salvemini et al. 1993), this role appears to be reversed at high levels. The mechanism may involve NO binding to the haem moiety of COX at high concentrations, and converting it to the ferrous-inactive form (reviewed in Mitchell et al. 1995). Alternatively, nitrosylation of the cysteine groups on COX may undermine COX activity (Kennedy et al. 1994) and NO may also alter the availability of the transcription factors (e.g. NF-κB) required for COX-2 induction, thereby reducing its expression (Dela Torre et al. 1997). Thus a close interaction between NOS and COX pathways is clearly evident. It should be noted that the higher levels of urinary nitrate excreted in the vehicle-treated AIA animals was not due to increased food intake, since body weight was significantly lower in these rats compared to the untreated control group. Thus the increased urinary NOx levels are likely to have arisen from the inflammatory response itself.
While body weight increased with time in the untreated control group, it remained unchanged in the vehicle (and SC-236) -treated AIA groups, suggesting this aggressive inflammatory response impacts on food and possibly water intake. In the indomethacin-treated animals there was evidence of gastrointestinal haemorrhage, suggested by the presence of dark colouration of the faeces (observed after day 5). These findings may explain the marked loss in body weight in this group, which was significantly lower than SC-236- or vehicle-treated rats. This finding is consistent with previous studies which have found that slightly higher doses of indomethacin resulted in elevated mortality (Naciazek-Wieniawska & Krus, 1975). It is widely accepted that long-term inhibition of both COX isoforms through the use of conventional NSAIDs may produce ulceration of the stomach lining and gastric mucosa (Roth, 1996). This has led to the development of selective COX-2 inhibitors to avoid such detrimental side-effects (Laine et al. 1999), and this appears to be borne out in the present study.
In addition to the swelling of the inflamed knee, a smaller (approximately 7-fold less) but consistent response was also present in the contralateral knee (Fig. 1B). We have previously reported (McDougall et al. 1995) selective swelling of the knee joint in response to intra-articular injection of adjuvant, with no associated change in ankle diameter. This confirms the absence of a systemic response in the adjuvant model of arthritis, and demonstrates that bilateral responses in the present study were specific to the region of the insult in the ipsilateral knee. It is thought that this contralateral effect is neurogenically mediated through descending pathways operating via the spinal cord (Kidd et al. 1989). Evidence for this phenomenon comes from the fact that following induction of adjuvant monoarthritis, there is a bilateral elevation of substance P and calcitonin gene-related peptide levels in the dorsal root ganglia (Mapp et al. 1993) and in the synovial fluids of both treated and untreated joints (Bileviciute et al. 1993). The fact that COX inhibition decreased contralateral knee joint diameter by approximately 50% suggests that PG and/or NO may be partly responsible.
The most striking observation of the present study was that, while joint swelling was substantially alleviated by both indomethacin and SC-236, the vasodilator responses to ACh and SNP in drug-treated groups did not differ from those of vehicle-treated animals. The extent of this vascular dysfunction persisted despite the fact that both COX inhibitors also prevented the AIA-induced rise in urinary PG and NO levels. Thus, while the joint swelling data demonstrates that the response is partly COX dependent, the associated vascular dysfunction appears to be independent of the prostanoid and nitrergic systems. This importantly suggests that joint swelling and vascular dysfunction appear to be mediated, at least in part, by independent mechanisms. Mechanical factors such as raised intra-articular pressure cannot explain this vascular dysfunction since there was no significant difference between basal vascular resistance in untreated compared with inflamed joints. Indeed, this also discounts increased sympathetic vasoconstrictor tone as a causative factor in the vascular dysfunction of the chronically inflamed joint. Furthermore, while the joint effusion arising as part of the inflammatory response would be expected to increase intra-articular pressure, a previous study by our group demonstrated that this dissipates with time due to the viscoelastic creep of the joint capsule (Wood & Ferrell, 1984).
The design of the present study allows us to go one step further. By comparing both a broad-spectrum and a selective COX inhibitor we can differentiate between COX-1- and COX-2-mediated effects. This was important to establish since we have previously reported that basal perfusion of the normal joint is dependent on COX-1, but not COX-2 (Egan et al. 2001). The fact that indomethacin and SC-236 similarly failed to restore vascular function demonstrates that neither COX isoform mediates such dysfunction. The observation that urinary PG levels had returned to baseline by day 10 in the vehicle-treated group also argues that vascular dysfunction is unrelated to the prostanoid pathway. Thus, it would appear that there are other pro-inflammatory mediators working independently of the prostanoid pathway. As ACh and SNP evoke vascular responses via the nitrergic pathway, it could be argued that this may be a possible site for the occurrence of vascular dysfunction, and indeed there is evidence for NO being a pro-inflammatory mediator (Moncada et al. 1991). However, although urinary NOx levels remained elevated in the vehicle-treated group right through to 10 days postinduction, SC-236 treatment reduced this to baseline (Fig. 3A) and yet vascular dysfunction persisted (Fig. 4). These results do not therefore support vascular dysfunction associated with chronic inflammation arising through nitrergic mechanisms. Although outwith the scope of the present focused study, this could be confirmed by administration of NOS inhibitors. Other candidate pathways may include cytokines (e.g. interleukin-1 and tumour necrosis factor-α) and possibly protease-activated receptors (PARs). Recent work has demonstrated an important role for PAR-2 in chronic joint inflammation (Ferrell et al. 2003). Future studies could attempt to address the mechanism underlying inflammation-induced vascular dysfunction, which may include inhibition of cytokines or PAR-2 activation.
In conclusion, joint swelling and vascular dysfunction associated with AIA appear to be mediated, at least in part, by independent mechanisms. While COX-1/COX-2 inhibition reduced joint swelling, vascular dysfunction in AIA is independent of constitutive or inducible prostanoid mechanisms, and appears not to be solely endothelial-derived, but to involve components such as the vascular smooth muscle.
Acknowledgments
This work was supported by Searle (Monsanto Corporation, USA) and the University of Paisley Research funds. We gratefully acknowledge the expert technical assistance of Lynette Dunning and Marion Drew.
References
- Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bileviciute I, Lundeberg T, Ekblom A, Theodorsson E. Bilateral changes of substance P-, neurokinin A- and neuropeptides Y-like immunoreactivity in rat knee joint synovial fluid during acute monoarthritis. Neurosci Lett. 1993;153:37–40. doi: 10.1016/0304-3940(93)90071-r. [DOI] [PubMed] [Google Scholar]
- Carpenter MA, Everett LD, Hall MA. High-resolution magnetic resonance imaging of arthritic pathology in the rat knee. Skeletal Radiol. 1994;23:429–437. doi: 10.1007/BF00204603. [DOI] [PubMed] [Google Scholar]
- Dela Torre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B P50 binding kinetics by S-nitrosylation. Biochem Biophys Res Commun. 1997;238:703–706. doi: 10.1006/bbrc.1997.7279. [DOI] [PubMed] [Google Scholar]
- Egan CG, Lockhart JC, Ferrell WR, Day SM, McLean JS. Pathophysiological basis of acute inflammatory hyperaemia in the rat knee: roles of cyclo-oxygenase-1 and -2. J Physiol. 2002;539:579–587. doi: 10.1113/jphysiol.2001.013473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CG, Lockhart JC, McLean JS, Ferrell WR. Expression of constitutive but not inducible cyclooxygenase maintains articular perfusion in the rat knee. Exp Physiol. 2001;86:191–197. doi: 10.1113/eph8602129. [DOI] [PubMed] [Google Scholar]
- Falchuck KH, Goetzl E, Kulka JP. Respiratory gases of synovial fluid. Am J Med. 1970;49:223–231. doi: 10.1016/s0002-9343(70)80078-x. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K. A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem. 1996;271:15810–15814. doi: 10.1074/jbc.271.26.15810. [DOI] [PubMed] [Google Scholar]
- Grabowski PS, England AJ, Dykhuizen R, Copland M, Benjamin N, Reid DM, Ralston SH. Elevated nitric oxide production in rheumatoid arthritis. Detection using the fasting urinary nitrate: creatinine ratio. Arthritis Rheum. 1996;39:643–647. doi: 10.1002/art.1780390416. [DOI] [PubMed] [Google Scholar]
- Griffiths RJ. Characterisation and pharmacological sensitivity of antigen arthritis induced by methylated bovine serum albumin in the rat. Agents Actions. 1992;35:88–95. doi: 10.1007/BF01990957. [DOI] [PubMed] [Google Scholar]
- Jang D, Murrell GA. Nitric oxide in arthritis. Free Radic Biol Med. 1998;24:1511–1519. doi: 10.1016/s0891-5849(97)00459-0. [DOI] [PubMed] [Google Scholar]
- Kang RY, Freire-Moar J, Sigal E, Chu C-Q. Expression of cyclooxygenase-2 in human and an animal model of rheumatoid arthritis. Br J Rheumatol. 1996;35:711–718. doi: 10.1093/rheumatology/35.8.711. [DOI] [PubMed] [Google Scholar]
- Kennedy TA, Smith CJ, Marnett LJ. Investigations of the roles of cysteines in catalysis by prostaglandin endoperoxide synthase. J Biol Chem. 1994;269:27357–27364. [PubMed] [Google Scholar]
- Kidd BL, Mapp PI, Gibson SJ, Polak JM, O'Higgins F, Buckland-Wright JC, Blake DR. A neurogenic mechanism for symmetrical arthritis. Lancet. 1989;2:1128–1130. doi: 10.1016/s0140-6736(89)91491-8. [DOI] [PubMed] [Google Scholar]
- Klareskog L. What can we learn about arthritis from animal models? Springer Semin Immunopathol. 1989;11:315–333. doi: 10.1007/BF00197310. [DOI] [PubMed] [Google Scholar]
- Laine L, Harper S, Simon T, Bath R, Johanson J, Schwartz H, et al. A randomized trial comparing the effect of rofecoxib, a cyclooxygenase 2-specific inhibitor, with that of ibuprofen on the gastroduodenal mucosa of patients with osteoarthritis. Rofecoxib Osteoarthritis Endoscopy Study Group. Gastroenterol. 1999;117:776–783. doi: 10.1016/s0016-5085(99)70334-3. [DOI] [PubMed] [Google Scholar]
- Lam FY, Ferrell WR. Acute inflammation in the rat knee joint attenuates sympathetic vasoconstriction but enhances neuropeptide-mediated vasodilatation assessed by laser Doppler perfusion imaging. Neuroscience. 1993;52:443–449. doi: 10.1016/0306-4522(93)90170-k. [DOI] [PubMed] [Google Scholar]
- Lockhart JC, McMurdo L, Ferrell WR. Vascular reactivity to acetylcholine is attenuated in the inflamed rabbit synovium. J Physiol. 1997;501:106P. [Google Scholar]
- McDougall JJ. Abrogation of α-adrenergic vasoactivity in chronically inflamed rat knee joints. Am J Physiol Regul Integr Comp Physiol. 2001;281:R821–R827. doi: 10.1152/ajpregu.2001.281.3.R821. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Elenko RD, Bray RC. Cholinergic vasoregulation in normal and adjuvant monoarthritic rat knee joints. J Auton Nerv Syst. 1998;72:55–60. doi: 10.1016/s0165-1838(98)00087-3. [DOI] [PubMed] [Google Scholar]
- McDougall JJ, Karimian SM, Ferrell WR. Prolonged alteration of vasoconstrictor and vasodilator responses in rat knee joints by adjuvant monoarthritis. Exp Physiol. 1995;80:349–357. doi: 10.1113/expphysiol.1995.sp003852. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Terenghi G, Walsh DA, Chen ST, Cruwys SC, Garrett N, et al. Monoarthritis in the knee induces bilateral and time-dependent changes in substance P and calcitonin gene-related peptide immunoreactivity in the spinal cord. Neurosci. 1993;57:1091–1096. doi: 10.1016/0306-4522(93)90051-g. [DOI] [PubMed] [Google Scholar]
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Larkin S, Williams TJ. Cyclooxygenase-2: regulation and pharmacology. Pharmacol Rev. 1995;43:109–142. doi: 10.1016/0006-2952(95)00212-x. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Naciazek-Wieniawska A, Krus S. Studies on the cumulation of the toxic effect of indomethacin. Pol Med Sci Hist Bull. 1975;15:35–39. [PubMed] [Google Scholar]
- Najafipour H, Ferrell WR. Sympathetic innervation and α-adrenoceptor profile of blood vessels in the posterior region of the rabbit knee joint. Br J Pharmacol. 1993;108:79–84. doi: 10.1111/j.1476-5381.1993.tb13443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafipour H, Ferrell WR. Comparison of synovial PO2 and sympathetic vasoconstrictor responses in normal and acutely inflamed rabbit knee joints. Exp Physiol. 1995;80:209–220. doi: 10.1113/expphysiol.1995.sp003841. [DOI] [PubMed] [Google Scholar]
- Richman AI, Su EY, Ho G., Jr Reciprocal relationship of synovial fluid volume and oxygen tension. Arthritis Rheum. 1981;24:701–705. doi: 10.1002/art.1780240512. [DOI] [PubMed] [Google Scholar]
- Roth SH. NSAID gastropathy. A new understanding. Arch Intern Med. 1996;156:1623–1628. [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Nat Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle I, Klein T, Backman JT, Saal JG, Nüsing RM, Fritz P. Expression of cyclooxygenase 1 and cyclooxygenase 2 in human synovial tissue: differential elevation of cyclooxygenase 2 in inflammatory joint diseases. Arthritis Rheum. 1998;41:122–129. doi: 10.1002/1529-0131(199801)41:1<122::AID-ART15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991;147:3915–3920. [PubMed] [Google Scholar]
- Stichtenoth DO, Frölich JC. Nitric oxide and inflammatory diseases. Br J Pharmacol. 1998;37:246–257. doi: 10.1093/rheumatology/37.3.246. [DOI] [PubMed] [Google Scholar]
- Swierkosz TA, Mitchell JA, Warner TD, Botting RM, Vane JR. Co-induction of nitric oxide synthase and cyclo-oxygenase: interactions between nitric oxide and prostanoids. Br J Pharmacol. 1995;114:1335–1342. doi: 10.1111/j.1476-5381.1995.tb13353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschure PJ, Van Noorden CFJ, Dijkstra CD. Macrophages and dendritic cells during the early phase of antigen-induced arthritis in rats. Immunochemical analysis of cryostat sections of whole rat knee joint. Scand J Immunol. 1989;29:371–381. doi: 10.1111/j.1365-3083.1989.tb01136.x. [DOI] [PubMed] [Google Scholar]
- Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch inflammation. Br J Pharmacol. 1999;126:1200–1204. doi: 10.1038/sj.bjp.0702420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JM, Simkin PA, Nelp WB. Low synovial clearance of iodine provides evidence of hypoperfusion in chronic rheumatoid synovitis. Arthritis Rheum. 1985;28:1096–1104. doi: 10.1002/art.1780281004. [DOI] [PubMed] [Google Scholar]
- Wood L, Ferrell WR. Response of slowly adapting articular mechanoreceptors in the cat knee joint to alterations in intra-articular volume. Ann Rheum Dis. 1984;43:327–332. doi: 10.1136/ard.43.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]