Abstract
Long-term potentiation (LTP) in the hippocampal CA1 region requires the activation of NMDA receptors (NMDARs). NMDAR activation in turn requires membrane depolarization as well as the binding of glutamate and its coagonist glycine. Previous pharmacological studies suggest that the glycine transporter type 1 (GlyT1) maintains subsaturating concentrations of glycine at synaptic NMDARs. Antagonists of GlyT1 increase levels of glycine in the synaptic cleft and, like direct glycine site agonists, can augment NMDAR currents and NMDAR-mediated functions such as LTP. In addition, stimulation of the glycine site initiates signalling through the NMDAR complex, priming the receptors for clathrin-dependent endocytosis. We have used a new potent GlyT1 antagonist, CP-802,079, with whole-cell patch-clamp recordings in acute rat hippocampal slices to determine the effect of GlyT1 blockade on LTP. Reverse microdialysis experiments in the hippocampus of awake, freely moving rats, showed that this drug elevated only the extracellular concentration of glycine. We found that CP-802,079, sarcosine and glycine significantly increased the amplitude of the NMDAR currents and LTP. In contrast, application of higher concentrations of CP-802,079 and glycine slightly reduced NMDAR currents and did not increase LTP. Overall, these data suggest that the level of glycine present in the synaptic cleft tightly regulates the NMDAR activity. This level is kept below the ‘set point’ of the NMDAR internalization priming mechanism by the presence of GlyT1-dependent uptake.
The N-methyl-d-aspartate receptor (NMDAR) plays a pivotal role in neural development, learning, memory, and synaptic plasticity (Bliss & Collingridge, 1993; Malenka & Nicoll, 1999). Long-term potentiation (LTP) of the Schaffer collateral synapses in the CA1 region of the hippocampus is the primary model system for the study of the associative synaptic modification thought to underline learning and memory (Bliss & Collingridge, 1993). This form of LTP requires presynaptic activity and postsynaptic depolarization (Brown et al. 1990). The postsynaptic depolarization is necessary due to the properties of the NMDAR, which require the relief of the Mg2+ block to open (Nowak et al. 1984). NMDAR activation also requires the binding of glutamate and the occupancy of the strychnine-insensitive glycine site (Johnson & Ascher, 1987). Once NMDARs are open, Ca2+ influx triggers synaptic plasticity (Bliss & Collingridge, 1993).
Glycine acts as a necessary coagonist at the NMDAR (Johnson & Ascher, 1987) and is considered to have a modulatory function because of its constant level (Kemp & Leeson, 1993). Glycine concentration in cerebrospinal fluid has been estimated to be in the low micromolar range (Westergren et al. 1994), concentrations sufficient to saturate the glycine site of the NMDAR under most physiological conditions. Indeed, the affinity of glycine for strychnine-insensitive glycine sites varies from 0.1 to 3 μm depending on the NR2 subunit make-up of the NMDAR complex (Danysz & Parsons, 1998). However, the concentration of glycine in the synaptic cleft could be reduced to well below 1 μm (150 nm) (Attwell et al. 1993; Roux & Supplisson, 2000) by glycine transporters (GlyT) strategically placed around the synapse (Smith et al. 1992; Zafra et al. 1995). A high-affinity glycine transporter type 1 (GlyT1) has been described in glia cells and its expression closely corresponds to the expression pattern of NMDARs (Smith et al. 1992).
Application of exogenous agonists for the glycine site (glycine or d-serine) or antagonists for GlyT1 has been demonstrated to enhance the amplitude of NMDAR currents in in vitro experiments (Wilcox et al. 1996; Bergeron et al. 1998; Chen et al. 2003; Martina et al. 2003). Bergeron et al. (1998) reported that, in rat hippocampal slices, application of 100 nm NFPS (N[3-(4-fluorophenil)-3-(4′-phenilphenoxy)]propylsarcosine), a highly potent and selective antagonist of GlyT1, caused a 50% enhancement in NMDAR currents in CA1 pyramidal neurones. These results confirm that the glycine site is not saturated at the synapse and that GlyT1 buffers the concentration of glycine in the synaptic cleft. In addition, it has been reported very recently that NFPS significantly enhanced LTP in the hippocampal dentate gyrus in vivo (Kinney et al. 2003), extending previous reports in vitro, in which glycine enhanced LTP (Tauck & Ashbeck, 1990). Overall, these data suggest that GlyT1 may be the main mechanism for regulation of glycine concentration at synapses.
The purpose of the present study is to examine the effect of the blockade of GlyT1 on LTP in rat hippocampal CA1 pyramidal cells.
Methods
Reverse microdialysis of CP-802,079 in rat hippocampus
Male Sprague-Dawley rats weighing 250–290 g were anaesthetized with ketamine (120 mg kg−1) and xylamine (20 mg kg−1) solution and placed in a stereotaxic frame. Surgery was performed in a designated surgery suite and took 30 min. Guide cannula were mounted over the hippocampus according to the atlas of Paxinos and Watson (AP-5.2, L-4.8, V-3.0) and affixed to the skull using dental cement and small machine screws. The animals recovered completely from the anaesthesia in 2–3 h. Following surgery and recovery, rats were individually housed in clean cages with fresh bedding. The animals were allowed to recover for 3 days under a 12 h light–dark cycle with free access to food and water. An analgesic (burenorphine 0.03 mg ml−1 was administered to the animals 15 min prior to anaesthetic recovery. If required, the dose of analgesic was repeated every 8–12 hours. On the day of the experiment, 4 mm tip microdialysis probes (Bioanalytical Systems) were inserted into the guide cannulas under 4% isoflurane anaesthesia in medical grade O2 (flowing at 2 l min−1) and perfused with ACSF containing (mm): 147 NaCl, 2.7 KCl, 1.3 CaCl2 and 2 MgCl2 at a flow rate of 2 μl min−1. The animals were anaesthetized for approximately 2–3 min prior to inserting the probes. The animals recovered in about 20–30 s. Under the conditions used, surgery suite and individual house, we have never had any incidence of postsurgery infection. Amino acid levels were allowed to stabilize for approximately 2 h prior to the initiation of each experiment. Samples were collected every 20 min in refrigerated fraction collector. Amino acid content in the dialysis samples was determined by gradient HPLC using fluorescence detection after derivitization with WATERS AccQ-Fluor reagents kits. CP-802,079 was dissolved in 100% DMSO at 10 mm prior to dilution to the final concentrations in ACSF. Dialysate concentrations of the amino acids are expressed as a percentage of the mean of the three fractions prior to infusion of CP-802,079.
Preparation of hippocampal slices
Coronal brain slices containing the hippocampus were obtained from Sprague-Dawley rats (21–28 days old). Prior to decapitation, the animals were anaesthetized using an isofluorane vaporizer (Stoelting, Wood Dale, IL, USA) in agreement with the guidelines of the Canadian Council of Animal Care. The concentration of isofluorane was 2–5%, the O2 flow rate was 1 l min−1. The brain was removed and placed in an oxygenated (95% O2–5% CO2) physiological solution, artificial cerebrospinal fluid (ACSF) at 4°C, containing (mm): 126 NaCl, 2.5 KCl, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2 and 10 glucose. The osmolarity of the ACSF was adjusted to 300 mosmol l−1 and the pH to 7.2. A block containing the region of interest was prepared, and sections (300 μm) were obtained with a vibrating microtome (Leica VT 1000S, Germany). The slices were stored for 1 h in an oxygenated chamber at room temperature before they were used for the experiments. The CA3 region of each slice was removed by a surgical cut.
Data recording and analysis
The LTP voltage-clamp experiments were performed with a solution containing (mm): 130 caesium methanesulphonate, to further minimize current attenuation, 10 Hepes, 10 KCl, 2 MgCl2, 0.2 EGTA, 2 ATP-Mg and 0.2 GTP-tris(hydroxy-methil) amino-methane. To record NMDAR currents, lidocaine N-ethyl bromide (QX-314, 5 mm) was added to the intracellular solution and caesium-BAPTA (10 mm) was used instead of EGTA. The pH of the intracellular solutions was adjusted to 7.2 and the osmolarity to 280–290 mosmol l−1. The pipettes had a resistance of 3–7 MΩ when filled with these solutions. In some experiments the synthetic peptide derived from the proline-rich domain of dynamin I (QVPSRPNRAP, dynaminPRD; Grabs et al. 1997; Wang & Linden, 2000) was added at 100 μg ml−1 to the intracellular solution.
Voltage-clamp recordings were obtained with a Multi-clamp 700A amplifier (Axon Instruments, Foster City, CA, USA) under visual control using differential interference contrast and infrared video microscopy (IR-DIC; Leica DMLFSA, Germany). The recordings were performed at room temperature from individual pyramidal cells of the CA1 region of the hippocampus voltage-clamped at −65 mV.
LTP experiments were performed in normal ACSF (normal extracellular Mg2+ concentration) while pharmacologically isolated NMDA current experiments were performed in an ACSF containing a low concentration of Mg2+ (see below).
Postsynaptic currents were evoked by electrical stimulation of the Schaffer collaterals with a bipolar microelectrode positioned in the stratum radiatum. The stimulation intensity consisted of 100 μs current pulses (10–200 μA) and was adjusted to evoke an EPSC amplitude in the range of 40–80 pA at a membrane potential (Vm) of −65 mV. Stimuli were delivered every 6 or 30 s when indicated. Bridge balance was monitored every 6 s (or 30 s) during the recordings. Recordings with series resistance higher than 25 MΩ were discarded. The recordings for the experiments using the pairing protocol to induce LTP were obtained in ACSF in the presence of picrotoxin (50 μm). The pairing protocol used to induce LTP was composed of three brief high frequency tetani (50 pulses at 100 Hz, 4 s intervals) given at the end of a long depolarization (3 min at 0 mV) (Chen et al. 1999). The pairing protocol was induced after 10–12 min of baseline in the absence or presence of drugs. It has been reported that the Ca2+ levels decay during the first minute (Chen et al. 1999). This protocol induced an increase of the synaptic responses lasting for more than 40 min.
To isolate the NMDAR-mediated component of evoked responses, we used ACSF containing a low concentration of MgCl2 (0.1 mm) with osmolarity maintained by CaCl2, and the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulphonamide (NBQX, 20 μm), the GABAa receptor antagonist picrotoxin (50 μm), the GABAb receptor antagonist 3-[[(3,4-dichlorophenyl)methyl]amino]propyl] diethoxymethyl) phosphinic acid (CGP 52432, 10 μm) and the glycine receptor antagonist strychnine (0.5 μm). NBQX is highly selective for AMPAR and does not act at the glycine site of the NMDAR (Yu & Miller, 1995).
Data were collected using pCLAMP 9 software (Axon Instrument, Foster City, CA). Analyses were performed off-line with the software IGOR (WaveMetrics Inc., Lake Oswego, OR, USA). The average of the responses during a 10 min period before LTP induction was taken as the baseline, and all the values were normalized to the baseline. The level of LTP was calculated from this normalized data as the average of all the responses recorded after the LTP induction. Each point shown in the graphs of Figs 3, 4 and 5 represents the average of the responses recorded in 60 s. Statistical significance of the results was determined with paired t tests (two-tailed). All values are expressed as means ±s.e.m.
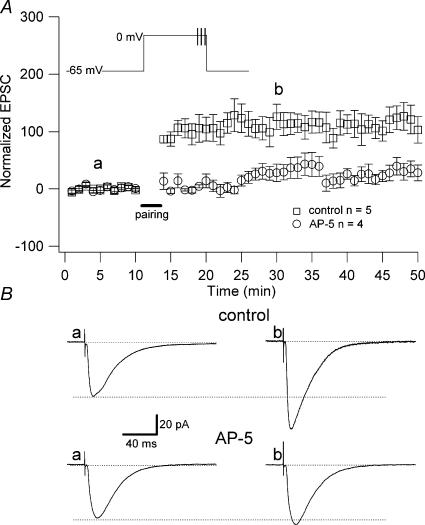
Figure 3. NMDAR-dependent LTP.
A, the pairing protocol used to induce LTP was composed of three brief high frequency tetani given at the end of the long depolarization. Inset, diagram of the protocol. This protocol induced a significant and long lasting increase in the amplitude of the synaptic responses (control, □; 94 ± 16.6% above baseline, n = 5, P < 0.005). The LTP induced by this protocol was prevented by the application of AP-5 (○; 19 ± 6.82% above baseline; n = 4; P > 0.05). B, examples of EPSCs (each trace is an average of 50 traces) recorded in control (absence of drug) and in presence of AP-5, as indicated in A (a and b).
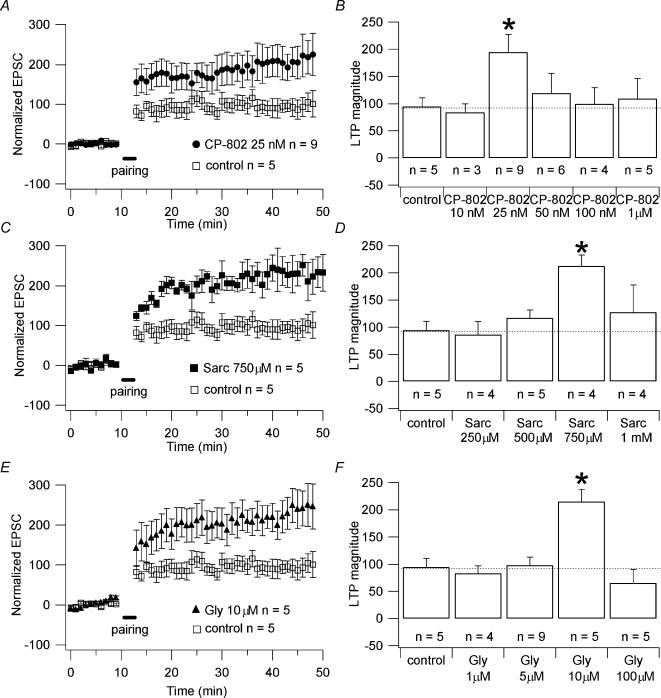
Figure 4. Effect of CP-802,079, sarcosine and glycine on NMDAR-dependent LTP.
A, C and E, pooled data of the effect of 25 nm CP-802,079 (n = 9; •), 750 μm sarcosine (n = 5; ▪) and 10 μm glycine (n = 5; ▵) on the magnitude of the LTP compared to the magnitude of LTP in absence of the drugs (control; n = 5; □). The cells recorded for the LTP experiments were obtained from different slices. Only one cell per slice was recorded. B, D and F, histograms showing the normalized increase in percentage of the amplitude of the EPSCs after the induction of LTP (indicated on the graphs as LTP magnitude) for different concentrations of CP-802,079, sarcosine and glycine, respectively. * Significant differences (P < 0.05) between the magnitude of LTP obtained in presence of the drug and the magnitude of LTP obtained in absence of the drugs (control). Each point on the graphs A, C and E is the average of the amplitude of the responses collected in 60 s (one stimulation every 6 s).
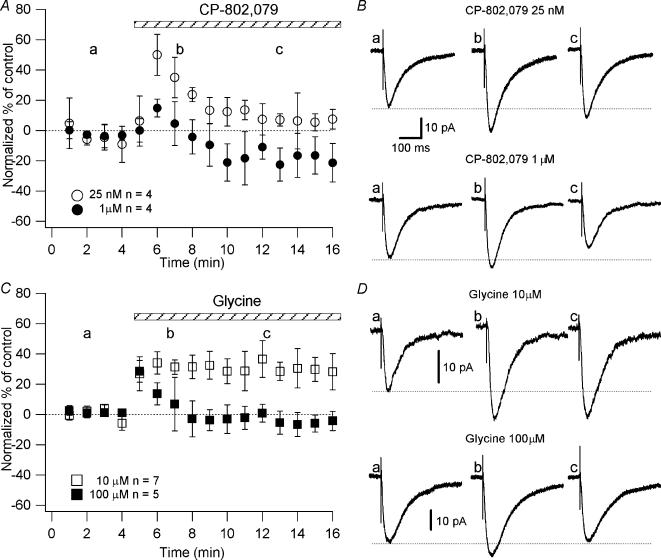
Figure 5. Effect of CP-802,079 and glycine on the NMDAR currents as a function of time.
Neurones were recorded in a low-Mg2+ ACSF in the presence of NBQX (20 μm), picrotoxin (50 μm), CGP 52432 (10 μm) and strychnine (0.5 μm). A and C, graphs plotting the normalized NMDAR currents amplitude as a function of time before and during the application of CP-802,079 (25 nm, n = 4; ○ and 1 μm, n = 4; •) and glycine (10 μm, n = 7; □ and 100 μm, n = 5; ▪), respectively. B and D, examples of NMDAR currents (each trace is an average of 20 traces) evoked by bipolar electrical stimuli at Vm = −70 mV, as indicated in A and C (a,b,c), respectively. Each point on the graphs is the average of the amplitude of the responses collected in 60 s (one stimulation every 6 s).
Drugs
Drugs tested with the LTP pairing protocol and NMDAR currents included sarcosine (250, 500, 750 and 1000 μm), glycine (5, 10, 25, 100 μm) and ({3-(4-chloro-phenyl)-3-[4-(thiazole-2-carbonyl)-phenoxy]-propyl}-methyl-amino)-acetic acid (CP-802,079; 10, 25, 50, 100, 1000 nm). The drugs were present throughout the LTP experiments. All drugs were obtained from RBI (Natick, MA, USA), with the exception of CGP 52432 (Tocris, Bristol, UK), CP-802,079 and dynaminPRD. CP-802,079 was synthesized at the Pfizer Global Research and Development facilities (Groton, CT, USA). DynaminPRD (QVPSRPNRAP) was synthesized by SIGMA genosys (Cambridgeshire, UK). Sarcosine is an N-methyl derivate of glycine and inhibits GlyT1, but not GlyT2, by competing with glycine as a substrate for the transporter.
Results
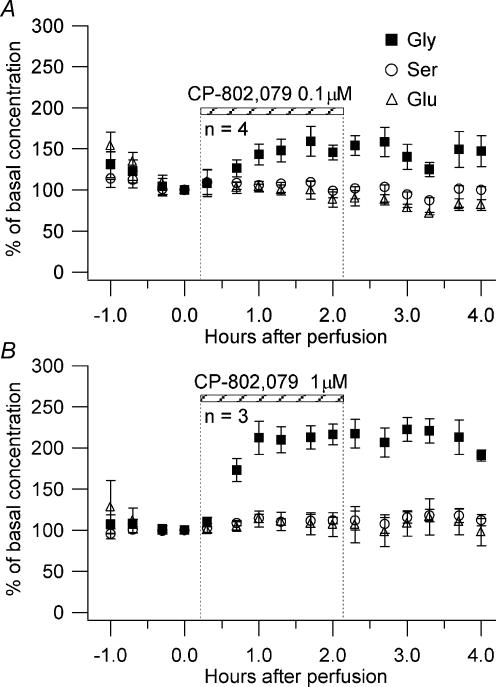
The structure of CP-802,079 is provided in Fig. 1. CP-802,079 is a potent antagonist for GlyT1 with an IC50 for inhibition of glycine uptake (3[H]glycine) of 16.1 nm in rat brain synaptosomes. The specificity of CP-802,079 has been evaluated based on its affinity for a diverse set of neurotransmitter sites including various dopaminergic, noradrenergic and serotonergic receptors using in vitro ligand binding (Table 1). In all cases, affinities for these sites were greater than 500 nm. Broad panel screening of related analogues of CP-802,079 has demonstrated this chemical series to be devoid of significant affinity for a wide range of ion channels and amino acid receptors including the glycine and glutamate sites of the NMDAR. To demonstrate the effect of GlyT1 inhibition on the extracellular concentration of glycine, we infused CP-802,079 into the hippocampus of awake, freely moving rats using reverse dialysis. Concentrations of 0.1 and 1 μm were selected to provide drug concentrations outside the microdialysis probe near the IC50 for inhibition of synaptosomal glycine uptake and at a 10-fold excess, assuming 10–20% efficiency in crossing the dialysis membrane. As shown in Fig. 2, infusion of CP-802,079 for 2 h resulted in a concentration-dependent elevation in dialysate levels of glycine. At the concentration estimated to approach its IC50, CP-802,079 produced a 59 ± 18% elevation in extracellular glycine (Fig. 2A) while the higher, presumably saturating concentration of the antagonist essentially doubled the extracellular concentration of glycine (peak concentration of 117 ± 13% above baseline) following drug infusion (Fig. 2B). Dialysate levels of serine and glutamate were unaffected by CP-802,079, consistent with CP-802,079 not affecting other amino acid transporters such as system ASC (alanine, serine and cyteine).
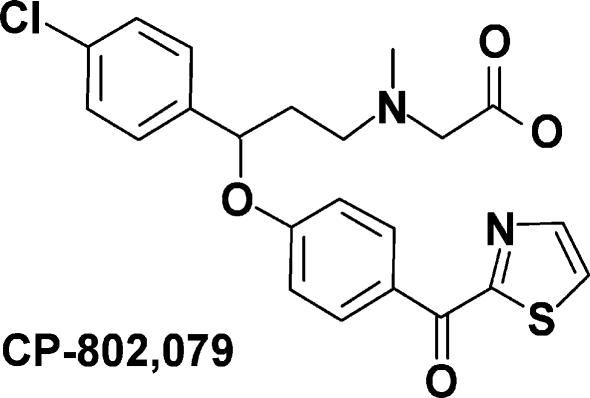
Figure 1. Structure of the GlyT1 inhibitor CP-802,079.
({3-(4-Chloro-phenyl)-3-[4-(thiazole-2-carbonyl)-phenoxy]-propyl}-methyl-amino)-acetic acid
Table 1. Receptor profile of CP-802,079.
| Receptor type (species) | Ki (nM) | Assay |
|---|---|---|
| 5-HT1a (cloned human) | >2000 | 3[H]8OH-DPAT binding |
| 5-HT2a (cloned rat) | >1200 | 3[H]ketanserin binding |
| Dopamine D2 (cloned human) | >900 | 3[H]spiperone binding |
| α1a adrenergic (cloned rat) | >1500 | 3[H]prazosin binding |
| α2a adrenergic (cloned human) | >1500 | 3[H]-RS-79948–197 binding |
| Histamine H1 (cloned human) | >1500 | 3[H]mepyramine binding |
| M1 muscarinic (cloned human) | >1200 | 3[H]N-methylscopolamine binding |
| GlyT2 (cloned rat) | >10000 | IC50 for 3[H]glycine uptake |
Figure 2. Hippocampal infusion of CP-802,079.
CP-802,079 at 0.1 μm (A) and 1 μm (B) was infused for 2 h into the rat hippocampus of 3 and 4 animals, respectively, and the levels of glycine (▪), serine (○) and glutamate (▵) measured in the extracellular space.
Effect of GlyT1 blockade on LTP
To study the effect of GlyT1 antagonism on synaptic plasticity, we recorded pyramidal cells from the CA1 region of rat hippocampus in acute slices and induced LTP with a pairing protocol based on Chen et al. (1999). Cells were held at −65 mV and stimuli given every 6 s (see Methods). We recorded 10 min of stable baseline of synaptic responses, followed by the pairing (see Methods). This protocol induced a 94 ± 16.6% (n = 5) increase above baseline of the synaptic responses lasting for more than 40 min (Fig. 3). This LTP was NMDAR dependent, since it was prevented by application of dl-2-amino-5-phosphonovaleric acid (AP-5, 50 μm; 19 ± 6.82% above baseline; n = 4; P > 0.05; Fig. 3). This protocol allows us to study the effect of the blockade of the GlyT1 on NMDAR-dependent LTP.
We first tested the application of CP-802,079 and the competitive GlyT1 substrate sarcosine on the magnitude of LTP. We observed that 25 nm CP-802,079 and 750 μm sarcosine caused increases in the amplitude of the responses after the induction of LTP of 195 ± 32% (n = 9; Fig. 4A) and 213 ± 20% (n = 4; Fig. 4C) above baseline, respectively. A similar effect was mimicked by 10 μm glycine (215 ± 22% above baseline; n = 5; Fig. 4E). These values were significantly greater than that measured in the absence of the drugs (control, 94 ± 16.6% above baseline; n = 5; P < 0.05 for all the drugs), while the difference between the augmentations due to the application of CP-802,079 (25 nm), sarcosine (750 μm) and glycine (10 μm) were not significantly different (P > 0.5).
Our results suggest that the blockade of GlyT1, as well as the direct application of glycine, augments the amplitude of the NMDAR currents, increasing therefore the impact that presynaptic activity has on the induction of LTP.
Effect of high concentration of glycine on LTP
Nong et al. (2003) reported that glycine and glutamate site activation of NMDARs together (as during excitatory neurotransmission) is necessary for the receptor to undergo endocytosis. We tested the effect of high concentrations of CP-802,079, sarcosine and glycine on the magnitude of the LTP. We found that concentrations of CP-802,079, sarcosine and glycine higher than 25 nm, 750 μm and 10 μm, respectively, did not cause a significant increase in the amplitude of the responses after the induction of LTP compared to that measured in the absence of the drugs (control). Indeed, at concentrations of 50, 100 and 1000 nm, CP-802,079 induced increases in the amplitude of the responses after the induction of LTP of 120 ± 26% (n = 6), 95 ± 30% (n = 4) and 109 ± 37% (n = 5) above baseline, respectively; 1 mm sarcosine caused an augmentation of 127 ± 50% (n = 4) and 100 μm glycine an augmentation of 67 ± 25% (n = 5) above baseline. These values were not significantly different from that obtained in the absence of the drugs (control; 94 ± 16.6% above baseline; n = 5; P > 0.5; Figs 4B, D and F).
These data suggest that the blockade of GlyT1, as well as the direct application of glycine, produce an increase in synaptic glycine that exceeds the concentration necessary for the saturation of the glycine site on NMDARs. This could lead to all the NMDARs being activated, and a certain percentage primed by glycine and endocytosed during the stimulation. As a result, in the presence of high concentrations of CP-802,079, sarcosine or glycine, a lower number of NMDARs will be available to support LTP than in the presence of lower concentrations of the drugs.
Effect of high concentration of glycine on NMDAR currents
To demonstrate that the lack of increase in the magnitude of LTP at high concentrations of CP-802,079 (1 μm) and glycine (100 μm) was due to the internalization of the NMDARs and consequently to a reduced NMDAR activation during the induction of LTP, we studied the effect of the application of CP-802,079 and glycine at different concentrations on the NMDAR currents. We expected that in the presence of high concentrations of CP-802,079 (1 μm) and glycine (100 μm) the amplitude of the NMDAR currents would be reduced. We induced the pairing protocol 10–12 min after the application of the drugs and observed the amplitude of the NMDAR currents at this time. To evoke postsynaptic glutamatergic currents, the Schaffer collaterals were stimulated with a bipolar electrode while the postsynaptic CA1 pyramidal cells were held at Vm = −70 mV. We delivered stimulation every 6 s as for the LTP experiments. The NMDAR-mediated component of the PSCs was pharmacologically isolated in a low-Mg2+ ACSF (see Methods) containing NBQX (20 μm), picrotoxin (50 μm), CGP 52432 (10 μm) and strychnine (0.5 μm). At high concentrations of CP-802,079 (1 μm) or glycine (100 μm), we observed an increase in the amplitude of the NMDAR currents (CP-802,079: 14.84 ± 5.82%, n = 4; glycine: 28.53 ± 7.32%, n = 5), followed by a progressive reduction (CP-802,079: –21.09 ± 12.3%, n = 4; glycine: −3.73 ± 6.9%, n = 5; after 10–12 min) (Figs 5A and C). In contrast, application of 10 μm glycine produced a significant increase in the amplitude of NMDAR currents (34.14 ± 7.35%, n = 7; P < 0.05; Figs 5C and D) that lasted over time. Moreover, the concentration of 25 nm, CP-802,079 caused a significant increase of the NMDAR currents (49.98 ± 13.5%, n = 4; P < 0.005) during the first minutes of the perfusion, followed by a reduction. Although the NMDAR currents were reduced compared to the beginning of the perfusion, they were still significantly larger compared to the control (13.31 ± 3.58%, n = 4; P < 0.05; Figs 5A and B).
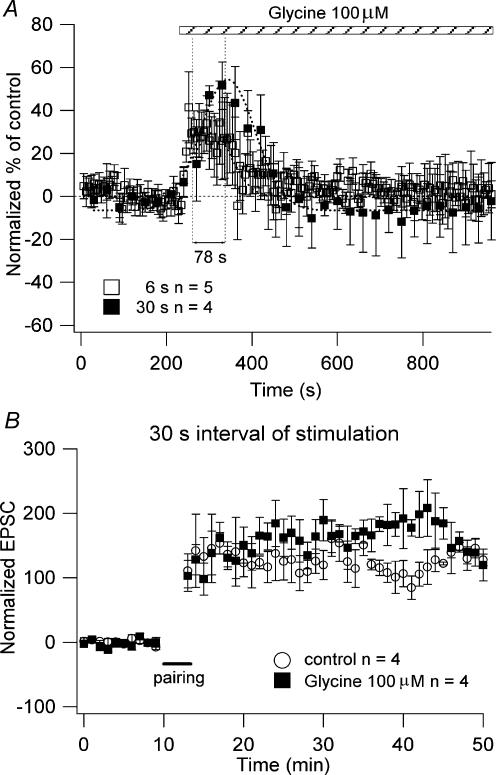
To verify if the lack of dose-dependent changes of glycine and CP-802,079 on NMDAR current amplitude and LTP magnitude was due to the frequency of stimulation, we performed experiments on NMDAR currents and LTP using a slower frequency of stimulation. We found that in the presence of 100 μm glycine the increase in the amplitude of the NMDAR currents using 30 s stimulation intervals was reached ∼78 s later than when 6 s stimulation intervals were used (Fig. 6A). We observed a reduction of the NMDAR current amplitude after a transient increase, as described in the experiments using 6 s interval of stimulation. There was no significant difference between both the increases (6 s: 34.34 ± 19.35%, n = 5; 30 s: 51.53 ± 11%, n = 4 at their peak of increase) and the decreases (6 s: 1 ± 6.33%, n = 4; 30 s: −2.2 ± 17.99%, n = 4; after 12 min in presence of 100 μm glycine) at either stimulation interval (P > 0.1; Fig. 6A). Note that the difference in the percentage NMDAR current amplitudes on the graphs shown in Figs 5 and 6 are due to the difference in the number of points averaged (10 and 1 for Figs 5 and 6, respectively).
Figure 6. Effect of glycine on the NMDAR currents and LTP as a function of the frequency of stimulation.
A, the neurones were recorded in a low-Mg2+ ACSF in the presence of NBQX (20 μm), picrotoxin (50 μm), CGP 52432 (10 μm) and strychnine (0.5 μm). The graph plots the normalized NMDAR currents amplitude as a function of time during the application of 100 μm glycine (□, 6 s interval of stimulation, n = 5; ▪, 30 s interval of stimulation, n = 4). Each point on the graphs is the amplitude normalized respect to the baseline of the responses collected every 6 or 30 s. B, the LTP experiments were performed delivering stimuli every 30 s. The graph shows the pooled data of the effect of 100 μm glycine (▪; n = 4) on the magnitude of the LTP compared to the magnitude of the LTP in absence of the drugs (○, control; n = 4). The cells recorded for the LTP experiments were obtained from different slices. Only one cell per slice was recorded.
Since the experiments on NMDAR currents using stimuli delivered every 30 s in presence of 100 μm glycine showed no difference in the amplitude of the NMDAR currents after 12 min of glycine perfusion compared to the NMDAR currents recorded using stimuli delivered every 6 s, we expected the magnitude of the LTP to be the same as the magnitude of the LTP observed in absence of glycine (control). The LTP experiments performed in the presence of 100 μm glycine using 30 s stimuli intervals (160 ± 20% above baseline, n = 4) showed no significant difference from the control (absence of drugs: 123 ± 19% above baseline, n = 4; P > 0.5; Fig. 6B), as was the case for the LTP experiments using 6 s of stimuli intervals (Figs 4E and F). These data suggest that the lack of effect of high concentrations of glycine on the NMDAR currents amplitude and on the LTP magnitude was not due to the frequency of stimulation.
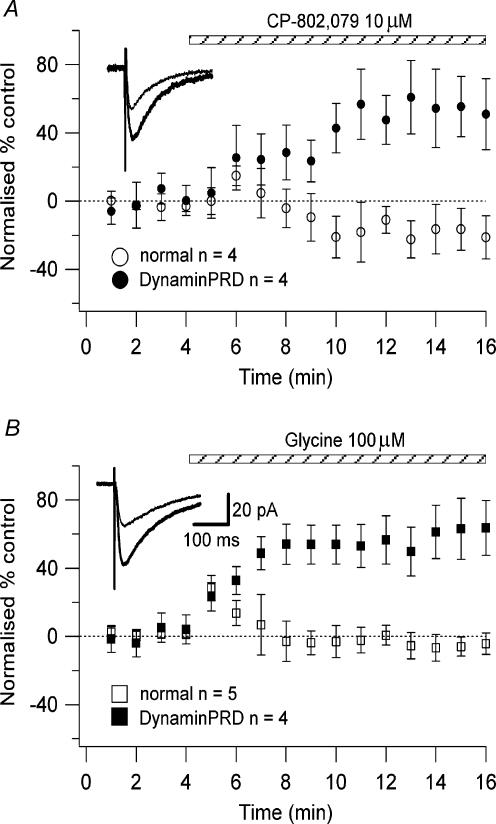
To determine whether the lack of effect of high concentrations of glycine and CP-802,079 on the LTP magnitude and on the NMDAR current amplitude was due to glycine priming the receptors for clathrin-dependent endocytosis, we dialysed the cells with the synthetic peptide derived from the proline-rich domain of dynamin I (dynaminPRD; Grabs et al. 1997). This peptide has been shown to impair clathrin-dependent endocytosis in the lamprey reticulospinal synapse (Shupliakov et al. 1997), in rat brain synaptic vescicle preparations (Marks & McMahon, 1998), and in cultured Purkinje neurones (Wang & Linden, 2000). We recorded NMDAR currents, as described above, with 100 μg ml−1 dynaminPRD in the intracellular solution during whole-cell recordings. In the presence of 10 μm CP-802,079 or 100 μm glycine we observed a significant increase in the amplitude of the NMDAR currents (CP-802,079: 55.27 ± 17.7%, n = 4; glycine: 63.93 ± 10.92%, n = 4; P < 0.05; Figs 7A and B), greater than that recorded with the normal intracellular solution (CP-802,079: 14.84 ± 5.82%, n = 4; glycine: 28.53 ± 7.32%, n = 5). This increase was not followed by a progressive reduction (Fig. 7).
Figure 7. Effect of CP-802,079 and glycine on the NMDAR currents blocking the clathrin-dependent endocytosis.
The neurones were recorded in a low-Mg2+ ACSF in the presence of NBQX (20 μm), picrotoxin (50 μm), CGP 52432 (10 μm) and strychnine (0.5 μm) using a normal intracellular solution and an intracellular solution with the addition of 100 μg ml−1 dynaminPRD. The stimuli to evoke the NMDAR currents were given every 6 s. The graphs plot the normalized NMDAR currents amplitude as a function of time during the application of 10 μm CP-802,079 (A: ○, normal intracellular solution, n = 4; •, dynaminPRD 100 μg ml−1, n = 4) and 100 μm glycine (B: □, normal intracellular solution, n = 5; ▪, dynaminPRD 100 μg ml−1, n = 4). Each point on the graphs is the amplitude normalized with respect to the baseline of the responses collected in 1 min. Insets, examples of NMDAR currents (each trace is an average of 20 traces) recorded in absence (thin line) and presence of CP-802,079 or glycine (thick line).
To further verify that the effect of CP-802,079 was due to the increase in the ambient glycine, as previously reported for NFPS, we performed the experiments described above with 300 nm NFPS. The highest concentrations used for both CP802,079 (10 μm) and NFPS (300 nm) enhanced the NMDA current in the presence of internal dynaminPRD (data not shown), suggesting that these two selective GlyT1 antagonists induced similar effects.
These data strongly suggest that the lack of effect of high concentrations of glycine on the LTP magnitude and NMDAR current amplitude was due to the glycine priming the receptors for clathrin-dependent endocytosis, as shown by Nong et al. (2003).
Overall, our experiments suggest that CP-802,079 causes augmentation or reduction of the NMDAR currents through regulation of the endogenous levels of glycine. This effect is mimicked by the direct application of glycine.
Discussion
Our results show that blockade of the transporters that regulate the levels of glycine, as well as direct application of glycine, increases the amplitude of the NMDAR currents and LTP when the concentration of glycine is brought up to the saturating level. We also found that blockade of GlyT1 or direct application of glycine at concentrations that exceed the level of saturation of the NMDAR glycine site resulted in slightly reduced NMDAR currents and no increase in LTP magnitude.
NMDARs are heteromultimeric ligand-gated channels composed of three different subunit families (NR1, NR2A–D, NR3A–B), as identified to date (Dingledine et al. 1999). The glycine-binding site is located on the NR1 subunit of the NMDAR, but it is the type of NR2 subunit coassembled with NR1 that controls the affinity of NMDAR for glycine (Kutsuwada et al. 1992; Priestley et al. 1995; Kew et al. 1998). Glycine has approximately a 10-fold higher affinity for receptors containing the NR2B, NR2C, or NR2D subunits than for those containing NR2A (Buller et al. 1994; Laurie & Seeburg, 1994; Priestley et al. 1995). The glycine concentration in the synaptic cleft is estimated to be well below the Kd value for glycine binding, 100–500 nm for high affinity receptors (Buller et al. 1994; Priestley et al. 1995; Kew et al. 1998) and ∼800 nm for low affinity receptors (Kew et al. 1998). We found that 10 μm glycine increased the amplitude of the response after the induction of LTP at a level comparable to that obtained after the application of 25 nm CP-802,079 and 750 μm sarcosine (Fig. 3). This concentration of glycine caused an increase in the amplitude of the NMDAR currents of ∼50%. This increase was responsible for enhanced LTP due, presumably, to augmented Ca2+ influx into the cell. A greater Ca2+-dependent insertion of AMPARs in the membrane compared to that in the absence of glycine has been shown to result in an increase in the magnitude of LTP (Shi et al. 1999; Hayashi et al. 2000; Lu et al. 2001).
It has been reported that stimulation of the glycine site initiates signalling through the NMDAR complex, priming the receptors for clathrin-dependent endocytosis (Nong et al. 2003). Nong et al. (2003) reported that activation of NMDARs at the glycine and glutamate sites together is necessary for the receptor to be endocytosed. Since the levels of glycine are tightly regulated in the synaptic cleft by the presence of a certain number of GlyT1s (Danysz & Parsons, 1998), the NMDARs may be protected from regulated internalization because the basal extracellular concentration of glycine is below the ‘set point’ of the internalization priming mechanism (Nong et al. 2003).
We observed that, when the concentration of CP-802,079 and glycine were elevated (1 and 100 μm), an initial short-lasting increase in the amplitude of the NMDAR currents was gradually followed by a progressive reduction of the currents, consistent with an internalization of the NMDARs. The intial short-lasting increase in the amplitude of the currents was probably due to the presence of receptors with different affinities for glycine. In presence of hypothetical glycine concentrations (300 nm to 1 μm; Supplisson & Bergman, 1997), the NMDARs with a relatively low affinity for glycine (Kd=∼800 nm; Kew et al. 1998) are only ∼20% to ∼65% occupied, whereas almost all high affinity receptors (Kd = 100–500 nm) (Buller et al. 1994; Laurie & Seeburg, 1994; Priestley et al. 1995) would be saturated. The addition of exogenous glycine could enhance the NMDAR responses by recruiting low affinity NMDARs and, in the presence of high glutamatergic activity, initiating the process of internalization. This suggests that the application of high concentrations of CP-802,079 (1 μm) or glycine (100 μm) also results in the activation of NMDARs not occupied at ambient glycine concentrations. Indeed, the temporary augmentation in the amplitude of the NMDAR currents shown in Fig. 4 could indicate the transient activation of a larger population of NMDARs. As the process of internalization takes place, the NMDAR currents will be reduced to levels not significantly different from the control (Fig. 4). The fact that we do not see a reduction in the magnitude of the LTP compared to control could be due to the fact that we induced the pairing protocol after 10–12 min of drugs application; consequently only a certain percentage of receptors will be internalized. Indeed, Nong et al. (2003) observed that after 20 min of 100 μm glycine application ∼60% of the receptors were internalized, while after 10 min only ∼30% of the receptors were internalized. This suggests that the remaining proportion of NMDARs is sufficient to increase the amplitude of the responses after the induction of LTP to levels similar to that in control. This hypothesis could also explain why 25 nm CP-802,079 caused a significant increase of the NMDAR currents followed by a reduction. Indeed, we suggest that if 25 nm CP-802,079 blocks sufficient GlyT1s to elevate the concentration of glycine close to the threshold for the internalization priming mechanism, a percentage of high affinity receptors could consequently be internalized. This hypothesis was also supported by the elimination of the reducing effect of high doses of glycine and CP-802,079 on NMDAR current in experiments performed with the peptide dynaminPRD in the intracellular solution. Indeed, this synthetic peptide, which is derived from the proline-rich domain of dynamin 1, is known to impair synaptic vesicle endocytosis (Grab et al. 1997; Shupliakov et al. 1997; Marks & McMahon, 1998; Wang & Linden, 2000). We also ruled out the possibility that high extracellular glycine concentrations enhance NMDAR desensitization because the reducing effects of high concentrations of glycine disappeared in the presence of dynaminPRD. It is also important to point out that any direct effect of CP-802,079 at the NMDAR, e.g. receptor blockade, would be unaffected by dynaminPRD. The ability of the peptide to prevent the gradual loss of the augmentation response to CP-802,079 is consistent with this effect being mediated exclusively by a CP-802,079-induced elevation of extracellular glycine.
We also observed that the lack of effect of a saturating concentration of exogenous glycine on the NMDAR current amplitude and LTP magnitude was not due to the frequency of synaptic activity.
It has been reported that the NMDAR response elicited in the nominal absence of glycine is the result of contamination with low background concentrations of glycine ranging from 20 to 130 nm (Benveniste et al. 1990; Lerma et al. 1990; Kew et al. 2000). These levels are not enough to saturate the NMDAR glycine site and induce endocytosis, and consequently the glycine site should be saturated by glycine and/or d-serine release in the synaptic cleft. The mechanisms of glycine and d-serine release are still not well-known. However, in the vicinity of glutamatergic synapses, active release of glycine and d-serine from glial cells is present (Danysz & Parsons, 1998; Billups & Attwell, 2003). We hypothesize that, in presence of 25 nm or 1 μm CP-802,079, the blockage of the GlyT1s allows glycine released from the glial cells to accumulate in the synaptic cleft. In presence of 25 nm CP-802,079, the level of glycine increases but the presence of GlyT1s that have not been blocked by the CP-802,079 keep the level of glycine close to the threshold for the internalization priming mechanism, while in presence of 1 μm CP-802,079 the level of glycine can increase up to saturating levels because all GlyT1s are blocked.
Hypofunction of NMDAR might contribute to some symptoms of schizophrenia (Javitt & Zukin, 1991; Tsai & Coyle, 2002). Treatment with GlyT1 antagonists such as CP-802,079 may be an effective way to increase glycine site occupancy and may represent a new therapeutic approach to reversing NMDAR hypofunction in disorders such as schizophrenia. Our results suggest that blockade of GlyT1 causes an increase in the NMDAR function and LTP, provided the level of glycine does not exceed the saturating level at the NMDAR glycine site. Consequently, we hypothesize that the NMDAR activity is tightly regulated by the level of glycine present in the synaptic cleft. This level is kept below the ‘set point’ of the NMDAR internalization priming mechanism by the presence of a certain number of GlyT1s. Since LTP is a mechanism underlying memory formation, these results suggest that antagonists of GlyT1 could enhance learning and memory processes.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) and the National Alliance for Research on Depression and Schizophrenia. We thank C. Metivier for technical assistance. M. Martina is supported by a fellowship from CIHR.
References
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Benveniste M, Mienville JM, Sernagor E, Mayer ML. Concentration-jump experiments with NMDA antagonists in mouse cultured hippocampal neurons. J Neurophysiol. 1990;63:1373–1384. doi: 10.1152/jn.1990.63.6.1373. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc Natl Acad Sci USA. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups D, Attwell D. Active release of glycine or d-serine saturates the glycine site of NMDA receptors at the cerebellar mossy fibre to granule cell synapse. Eur J Neurosci. 2003;18:2975–2980. doi: 10.1111/j.1460-9568.2003.02996.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brown TH, Kairiss EW, Keenan CL. Hebbian synapses: biophysical mechanisms and algorithms. Annu Rev Neurosci. 1990;13:475–511. doi: 10.1146/annurev.ne.13.030190.002355. [DOI] [PubMed] [Google Scholar]
- Buller AL, Larson HC, Schneider BE, Beaton JA, Morrisett RA, Monaghan DT. The molecular basis of NMDA receptor subtypes: native receptor diversity is predicted by subunit composition. J Neurosci. 1994;14:5471–5484. doi: 10.1523/JNEUROSCI.14-09-05471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Muhlhauser M, Yang CR. Glycine transporter-1blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003;89:691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- Chen HX, Otmakhov N, Lisman J. Requirements for LTP induction by pairing in hippocampal CA1 pyramidal cells. J Neurophysiol. 1999;82:526–532. doi: 10.1152/jn.1999.82.2.526. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons AC. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem. 1997;272:13419–13425. doi: 10.1074/jbc.272.20.13419. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultered mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp JA, Leeson PD. The glycine site of the NMDA receptor — five years on. Trends Pharmacol Sci. 1993;14:20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Kew JN, Koester A, Moreau JL, Jenck F, Ouagazzal AM, Mutel V, Richards JG, Trube G, Fisher G, Montkowski A, Hundt W, Reinscheid RK, Pauly-Evers M, Kempt JA, Bluethmann H. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J Neurosci. 2000;20:4037–4049. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Development changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GG, Sur C, Burno M, Mallorga PJ, Williams JB, Figueroa DJ, Wittmann M, Lemaire W, Conn PJ. The glycine transporter type 1 inhibitor N-[3-(4′-fluorophenyl)-3-(4′-phenylphenoxy) propyl] sarcosine potentiates NMDA receptor-mediated resposes in vivo and produces an antipsychotic profile in rodent behavior. J Neurosci. 2003;23:7586–7591. doi: 10.1523/JNEUROSCI.23-20-07586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-D-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci USA. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WY, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation — a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Martina M, Krasteniakov NV, Bergeron R. D-Serine differently modulates NMDA receptor function in rat CA1 hippocampal pyramidal cells and interneurons. J Physiol. 2003;548:411–423. doi: 10.1113/jphysiol.2002.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdelles B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanantly transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman RS, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDAR activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin—SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL. Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron. 1992;8:927–935. doi: 10.1016/0896-6273(92)90207-t. [DOI] [PubMed] [Google Scholar]
- Supplisson S, Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauck DL, Ashbeck GA. Glycine synergistically potentiates the enhancement of LTP induced by a sulfhydryl reducing agent. Brain Res. 1990;519:129–132. doi: 10.1016/0006-8993(90)90070-r. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Westergren I, Nyström B, Hamberger A, Nordborg C, Johansson BB. Concentrations of amino acids in extracellular fluid after opening of the blood—brain barrier by intracarotid infusion of protamine sulfate. J Neurochem. 1994;62:159–165. doi: 10.1046/j.1471-4159.1994.62010159.x. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Fitzsimonds RM, Johnson B, Dichter MA. Glycine regulation of synaptic NMDA receptors in hippocampal neurons. J Neurophysiol. 1996;76:3415–3424. doi: 10.1152/jn.1996.76.5.3415. [DOI] [PubMed] [Google Scholar]
- Yu W, Miller RF. NBQX, an improved non-NMDA antagonist studied in retinal ganglion cells. Brain Res. 1995;692:190–194. doi: 10.1016/0006-8993(95)00665-d. [DOI] [PubMed] [Google Scholar]
- Zafra F, Aragón C, Oliveres L, Danbolt NC, Gimenez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]