Abstract
Neonatal hypoxia alters the development of the hypoxic ventilatory response in rats and other mammals. Here we demonstrate that neonatal hypoxia impairs the hypoxic ventilatory response in adult male, but not adult female, rats. Rats were raised in 10% O2 for the first postnatal week, beginning within 12 h after birth. Subsequently, ventilatory responses were assessed in 7- to 9-week-old unanaesthetized rats via whole-body plethysmography. In response to 12% O2, male rats exposed to neonatal hypoxia increased ventilation less than untreated control rats (mean ±s.e.m. 35.2 ± 7.7%versus 67.4 ± 9.1%, respectively; P = 0.01). In contrast, neonatal hypoxia had no lasting effect on hypoxic ventilatory responses in female rats (67.9 ± 12.6%versus 61.2 ± 11.7% increase in hypoxia-treated and control rats, respectively; P > 0.05). Normoxic ventilation was unaffected by neonatal hypoxia in either sex at 7–9 weeks of age (P > 0.05). Since we hypothesized that neonatal hypoxia alters the hypoxic ventilatory response at the level of peripheral chemoreceptors or the central neural integration of chemoafferent activity, integrated phrenic responses to isocapnic hypoxia were investigated in urethane-anaesthetized, paralysed and ventilated rats. Phrenic responses were unaffected by neonatal hypoxia in rats of either sex (P > 0.05), suggesting that neonatal hypoxia-induced plasticity occurs between the phrenic nerve and the generation of airflow (e.g. neuromuscular junction, respiratory muscles or respiratory mechanics) and is not due to persistent changes in hypoxic chemosensitivity or central neural integration. The basis of sex differences in this developmental plasticity is unknown.
Humans born and raised at high altitude exhibit blunted ventilatory responses to hypoxia compared to sea level natives (Sørensen & Severinghaus, 1968a; Weil et al. 1971; Lahiri et al. 1976; Lahiri, 1981; Moore, 2000; Gamboa et al. 2003). Genetic factors are likely to contribute to this blunting (Moore, 2000), but several studies have identified a progressive decrease in hypoxic ventilatory sensitivity with increasing time spent at altitude in both sea level and high altitude natives (Weil et al. 1971; Byrne-Quinn et al. 1972; Lahiri et al. 1976; Lahiri, 1981). Thus, blunted hypoxic ventilatory responses may be acquired through prolonged exposure (years) to hypoxia, particularly when hypoxia occurs during infancy or childhood (Sørensen & Severinghaus, 1968a; Lahiri et al. 1976; Lahiri, 1981). Human infants with diseases or injuries causing postnatal hypoxia (e.g. congenital cyanotic heart disease, bronchopulmonary dysplasia) also develop low hypoxic ventilatory responses (Sørensen & Severinghaus, 1968b; Edelman et al. 1970; Blesa et al. 1977; Calder et al. 1994; Katz-Salamon et al. 1995, 1996). Together, these observations suggest that the hypoxic ventilatory response exhibits developmental plasticity in response to chronic hypoxia.
Developmental plasticity of the hypoxic ventilatory response has been confirmed using animal models of neonatal hypoxia (reviewed in Carroll, 2003; Fuller et al. in press). Chronic neonatal hypoxia from birth delays the maturation of carotid body hypoxic chemosensitivity and attenuates hypoxic ventilatory responses in mammals (Eden & Hanson, 1987; Hanson et al. 1989a, b; Sladek et al. 1993; Landauer et al. 1995; Wyatt et al. 1995; Sterni et al. 1999). The persistence of these effects has been studied in sheep and rats, with both species exhibiting prolonged blunting of the hypoxic ventilatory response that may last into adulthood (Sladek et al. 1993; Okubo & Mortola, 1990). Long-lasting plasticity after only 1–2 weeks of hypoxia is specific to development (Okubo & Mortola, 1990). Indeed, similar periods of hypoxia in adult mammals augment hypoxic chemoresponsiveness, an effect that reverses shortly after return to normoxia (Bisgard & Neubauer, 1995; Powell et al. 1998).
The mechanisms underlying persistent blunting of the hypoxic ventilatory response following neonatal hypoxia are unknown. Non-specific neural or physical impairment seems unlikely since rats exposed to neonatal hypoxia exhibit normal hypercapnic ventilatory responses (Okubo & Mortola, 1990). Since neonatal hypoxia diminishes carotid body function measured shortly after return to normoxia (Hanson et al. 1989b; Landauer et al. 1995; Wyatt et al. 1995; Sterni et al. 1999), continued carotid body impairment could be involved. However, this hypothesis has never been tested directly and there is some evidence for spontaneous recovery of carotid body chemosensitivity in continued hypoxia (Eden & Hanson, 1987) or shortly after return to normoxia (Sterni et al. 1999). Other changes could influence the hypoxic ventilatory response as well. For example, neonatal hypoxia alters lung morphology, respiratory mechanics and resting ventilation in rats (Okubo & Mortola, 1988, 1989, 1990), and may induce plasticity in respiratory muscles or efferent pathways (Kass & Bazzy, 2001). Further, previous studies reporting blunted hypoxic ventilatory responses in rats and sheep lack complete data on metabolic rate and/or blood gas measurements needed to verify that hypoxia-treated and control animals experienced the same hypoxic stimuli during assessment of the hypoxic ventilatory response (Okubo & Mortola, 1990; Sladek et al. 1993). Therefore, one purpose of the current study was to determine the site of respiratory plasticity after neonatal hypoxia. Sex influences the hypoxic ventilatory response (Tatsumi et al. 1995; Mortola & Saiki, 1996) and the expression of respiratory plasticity (Bavis & Kilgore, 2001; Behan et al. 2002; Genest et al. 2003). Since sex was not considered in previous studies of neonatal hypoxia (Okubo & Mortola, 1988, 1989, 1990; Sladek et al. 1993), we also investigated the influence of sex on the effects of neonatal hypoxia. We present evidence that neonatal hypoxia blunts adult hypoxic ventilatory responses in male rats only, and that this blunting does not involve persistent changes in peripheral chemoreceptors or central neural integration of chemoreceptor inputs.
Methods
All experimental procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison.
Experimental groups
We studied 7- to 10-week-old, male and female Sprague–Dawley rats (colony 236b, Harlan Sprague–Dawley, Inc., Madison, WI, USA) from two groups: (1) rats exposed to 10% O2 for the first postnatal week (‘neonatal hypoxia’; 15 litters), or (2) age-matched control rats born and raised in normoxia (‘control’; 15 litters). No more than two rats of each sex from each litter were used in each study (i.e. ventilation or phrenic nerve measurements); individual rats were studied once only.
Neonatal hypoxia
Fifteen litters (with their mothers) were placed in a chamber maintained at 10% O2 within 12 h after birth; preliminary studies indicated that placing litters into hypoxic conditions prior to, or immediately after, birth increased mortality. During the hypoxic exposure, chamber temperature and humidity were similar to those experienced by control rats; CO2 was maintained below 0.4% by regulating airflow through the chamber. Litters were exposed to hypoxia for 7 days, and then raised in room air under standard conditions.
Control
Control rats were born and raised in-house under normoxic conditions (21% O2). Twelve litters were born and raised in parallel to the neonatal hypoxia rats, but outside the chamber. Three additional litters were later raised for the first postnatal week in the chamber while the chamber was flushed with air. There were no differences between these groups of control rats, so data for control rats were pooled.
Oestrus cycle in female rats
The oestrus cycle was not standardized among female rats in these experiments. However, the stage of oestrus (pro-oestrus, oestrus, metoestrus or dioestrus) was determined for each rat at the time of study through light microscopy of vaginal smears prepared via vaginal lavage (Hebel & Stromberg, 1986).
Ventilatory and metabolic responses to hypoxia in awake rats
Ventilatory and metabolic responses to hypoxia were assessed in unanaesthetized control (n = 11 male; 10 female) and neonatal hypoxia (n = 11 male; 6 female) rats using a whole-body, flow-through plethysmograph (Olson, 1994). Rats were 7–9 weeks of age at the time of study (mean age: 57 ± 1 days).
Surgical preparation
Rats were implanted with indwelling arterial catheters and abdominal temperature telemetry transmitters (Mini-Mitter Co., Sunriver, OR, USA) at least 1 week prior to ventilation and metabolism experiments. Rats were administered buprenorphene (0.0025–0.005 µg (100 g)−1, s.c.) and then anaesthetized with pentobarbital (4 mg (100 g)−1, i.p.); local lidocaine (lignocaine) was applied to the exposed femoral artery. Adequacy of anaesthesia was determined throughout the surgery by the absence of a withdrawal reflex to tail and toe pinch. Catheters were advanced into the descending aorta via the left femoral artery and externalized through a small incision at the back of the neck. A body temperature transmitter was placed into the peritoneal cavity of each rat through a small, ventral abdominal incision.
Ventilation and metabolism measurements
Ventilation and metabolism measurements were made on unanaesthetized and unrestrained rats resting quietly in a 2 l, flow-through plethysmograph chamber. Air, saturated with water vapour, was forced into the chamber at 2 l min−1 through a high impedance orifice and exited the chamber through a variable impedance valve to a vacuum. Pressure fluctuations within the rat chamber, relative to a 2 l reference chamber, were detected with a differential pressure transducer (PM15E, Statham Instruments, Hato Rey, PR, USA) and used to calculate respiratory variables with customized computer software. The plethysmograph was calibrated before each experiment by rapid injection of 0.2 ml air into the chamber. The calibration signal was used with measurements of respiratory-related pressure fluctuations, body temperature (obtained via telemetry; Mini-Mitter Co., Sunriver, OR, USA) and chamber temperature to calculate tidal volume (Drorbaugh & Fenn, 1955); barometric pressure was measured at the start of each experiment. In five cases where the abdominal temperature transmitter malfunctioned at the time of study, tidal volume was calculated using the mean body temperature for preceding rats from the same sex and treatment group at the same inspired O2 fraction (FIO2). The computer software provided breath-by-breath analysis of ventilatory variables (frequency, tidal volume and minute ventilation) while rejecting pressure fluctuations caused by gross body movements. Inflow and outflow gases were monitored by O2 (FCX-MV, Fujikura, Ltd, Tokyo) and CO2 (LB-2, Beckman Instruments, Schiller Park, IL, USA) gas analysers and used to calculate metabolic rates at 10 min intervals.
Experimental protocol
All ventilatory and metabolic measurements were made during the light portion of the light:dark cycle. Rats were weighed and placed into the plethysmograph chamber flushed with air (21% O2) and given approximately 60 min to adjust to the chamber. Ventilatory and metabolic variables were recorded continuously for 30 min at 21% O2 (baseline), followed by 20 min periods at 12% O2, 21% O2 (recovery) and 10% O2; balance N2 in each case. Arterial blood samples (0.2–0.3 ml) were collected at the end of the first air exposure and at the end of each hypoxic exposure. Arterial PO2 and PCO2 (PaO2 and PaCO2, corrected to abdominal body temperature) were determined immediately with a blood analysis system (ABL-505, Radiometer, Copenhagen, Denmark).
Phrenic nerve responses to hypoxia in anaesthetized rats
Phrenic nerve responses to hypoxia were assessed in anaesthetized, paralysed, vagotomized and pump-ventilated control (n = 11 male, 5 female) and neonatal hypoxia (n = 11 male, 4 female) rats. Rats were 7–9 weeks of age at the time of study (mean age: 54 ± 1 days). To determine whether vagotomy influenced our results in male rats, additional control (n = 3) and neonatal hypoxia (n = 3) rats were studied before and after vagotomy. These rats were 9–10 weeks of age at the time of study (mean age: 67 ± 1 days).
Surgical preparation and phrenic nerve recording
Rats were weighed and placed into a sealed box containing isoflurane for rapid induction of anaesthesia, then maintained with 2.5% isoflurane (FIO2= 0.5, balance N2). Once anaesthetized, rats were pump-ventilated (Rodent Respirator, model 683, Harvard Apparatus, Holliston, MA, USA) through a tracheal cannula. Most rats were bilaterally vagotomized in the midcervical region (to prevent entrainment of phrenic neurograms to the ventilator). In additional rats, a piece of silk suture was placed loosely around each isolated but intact vagus nerve; these sutures were used to acutely vagotomize the rats at a later time point (see below). A catheter was placed into the right femoral vein, and rats were gradually converted to urethane anaesthesia (1.6–1.9 g kg−1, i.v.) over a 30 min period. Adequacy of anaesthesia was determined throughout the experiment by the absence of a withdrawal reflex and/or the absence of increases in blood pressure and phrenic nerve activity in response to a toe pinch; supplemental urethane (0.3 g kg−1, i.v.) was given as needed. A catheter was placed into the right femoral artery to monitor blood pressure (P23ID pressure transducer and P122 amplifier, Gould, Valley View, OH, USA) and to collect blood samples (0.2–0.3 ml). Arterial blood samples were analysed for PO2 and PCO2 with a blood analysis system (ABL-500, Radiometer, Copenhagen, Denmark) and corrected to rectal temperature, which was maintained at 37–38°C with a heated table. Once converted to urethane, rats were injected with the neuromuscular blocking agent pancuronium bromide (3.25–3.5 mg kg−1, i.v.) to prevent spontaneous breathing movements. Rats were continuously infused with sodium bicarbonate (5%)–lactated Ringer solution (1: 11 v/v) to maintain fluid and acid–base balance (2–2.5 ml h−1, i.v.). End-tidal CO2 partial pressure (PETCO2) was monitored throughout the experiment with a flow-through CO2 analyser (Capnogard, Novametrix, Wallingford, CT, USA) with sufficient response time to measure end-tidal gases in rats.
The left phrenic nerve was isolated using a dorsal approach, cut distally, desheathed, submerged in mineral oil, and placed on a bipolar silver wire electrode. PETCO2 was regulated near 40 Torr until starting the experiment (FIO2= 0.50). Electrical activity from the phrenic nerve was amplified (× 10 000), band-pass filtered (100 Hz to 10 kHz; Model 1800, A-M Systems, Carlsborg, WA, USA), and integrated (time constant = 50 ms; Moving Averager, Model MA-821RSP, CWE Inc., Ardmore, PA, USA). Integrated signals were recorded and analysed by computer using commercial software (WINDAQ version 2.18, Dataq Instruments, Akron, OH, USA).
Experimental protocol
Sixty minutes following surgery, the CO2 apnoeic threshold was determined by increasing ventilator rate and decreasing inspired CO2 until phrenic nerve activity stopped, then slowly raising PETCO2 until fictive breathing resumed (i.e. the apnoeic threshold). Baseline neural activity was standardized among preparations by maintaining PETCO2 3 Torr above the apnoeic threshold (FIO2= 0.50). After establishing baseline phrenic activity (20–30 min), an arterial blood sample was drawn. In vagotomized rats, isocapnic hypoxic responses were assessed at three levels of hypoxia (PaO2= 60, 50 and 40 Torr). Each hypoxic episode lasted 5 min, and arterial blood samples were collected during the final 30 s of each episode. Trials were accepted if PaO2 was ± 3 Torr of the target PaO2, and PaCO2 was ± 2 Torr of baseline. Rats were returned to FIO2= 0.50 for 5–10 min between bouts of hypoxia. After the final hypoxic challenge, the ‘maximal’ phrenic response to hypercapnia was measured by increasing PETCO2 to 85–90 Torr. Rats were then killed by an overdose of urethane.
A similar protocol was used for vagally intact rats. However, isocapnic hypoxic responses were assessed at only one level of hypoxia (PaO2= 50 Torr) followed by hypercapnia. After completing the hypoxic and hypercapnic trials, these rats were acutely vagotomized by pulling on sutures previously placed around the vagi; this procedure enabled us to transect the vagi without repositioning the rat. At least 15 min later, the apnoeic threshold was determined and baseline conditions re-established as described above. Measurements of the phrenic nerve responses to PaO2= 50 Torr and hypercapnia were then repeated. Rats were subsequently killed by an overdose of urethane and examined to confirm bilateral vagotomy.
Data analysis
Ventilatory and metabolic variables were analysed in 10 min bins throughout each experiment. Measurements included tidal volume (VT), respiratory frequency (fR), minute ventilation (V˙E), metabolic CO2 production (V˙CO2), CO2 convection requirement (V˙E/V˙CO2), and body temperature (Tb).
Phrenic activity was averaged in 30 s bins immediately preceding blood sampling during baseline and the fifth minute of hypoxia, and at the end of the hypercapnic challenge. A blinded design was used to conceal the identity of rats from the investigator during data collection and analysis. Variables included peak amplitude of integrated phrenic activity, phrenic burst frequency, and their product, minute phrenic activity. Changes from baseline in burst amplitude and minute activity were normalized as a percentage of baseline phrenic activity (% baseline) and as a percentage of phrenic activity during hypercapnia (% maximum). Since conclusions were not affected by the normalization method, only data expressed as a percentage of baseline are reported.
Statistical comparisons between treatment groups (control versus neonatal hypoxia) were made separately for males and females. Body mass was compared between treatment groups by ANCOVA, with age included as a covariate, or by independent sample t tests. Baseline ventilation, metabolism and phrenic nerve activity, changes in ventilation at a single level of hypoxia, and CO2 apnoeic thresholds were compared between treatment groups by independent sample t tests. Changes in ventilation, metabolism, phrenic activity and blood gases at multiple levels of hypoxia, or before and after vagotomy, were compared between treatment groups using two-way repeated measures ANOVA and Student–Newman–Keuls post hoc tests. Statistical tests were run using SigmaStat version 2.03 or SPSS version 10.1 (SPSS Inc., Chicago, IL, USA). Differences were considered significant at P = 0.05.
Results
Body mass
At the time of study (i.e. ventilation or phrenic nerve measurements at 7–10 weeks of age), neonatal hypoxia rats tended to weigh less than control rats (Table 1), although this small difference was only statistically significant for female rats from the phrenic nerve studies (153 ± 6 g versus 175 ± 5 g, respectively; P = 0.02). The effect of neonatal hypoxia on body mass is more evident when rats from both studies are pooled. To eliminate variation caused by prior surgery in rats from the ventilation studies, mass at the time of catheter and temperature transmitter implantation (6–7 weeks of age) was used for these rats. Across this age range (i.e. 6–10 weeks of age), male neonatal hypoxia rats weighed 5–17% less than controls (P < 0.01) and female neonatal hypoxia rats weighed 14–15% less than controls (P < 0.001), after accounting for age-related variation.
Table 1.
Normoxic ventilation and metabolism for unanaesthetized control and neonatal hypoxia rats
| Male | Female | |||
|---|---|---|---|---|
| Control | Neonatal hypoxia | Control | Neonatal hypoxia | |
| n | 11 | 11 | 10 | 6 |
| Age (days) | 55 ± 1 | 56 ± 1 | 60 ± 0 | 60 ± 0 |
| Mass (g) | 210 ± 10 | 197 ± 7 | 189 ± 13 | 163 ± 5 |
| fR (breaths min−1) | 110 ± 7 | 110 ± 6 | 102 ± 8 | 96 ± 9 |
| VT (ml (100 g)−1) | 0.58 ± 0.03 | 0.67 ± 0.04 | 0.70 ± 0.05 | 0.69 ± 0.05 |
| V˙E (ml min−1 (100 g)−1) | 63.5 ± 4.4 | 73.7 ± 7.3 | 70.3 ± 5.7 | 65.1 ± 5.1 |
| V˙CO2 (ml min−1 (100 g)−1) | 1.85 ± 0.10 | 2.00 ± 0.16 | 1.90 ± 0.16 | 1.74 ± 0.12 |
| V˙E/V˙CO2 | 35.0 ± 2.4 | 36.6 ± 1.6 | 37.5 ± 2.2 | 38.1 ± 3.1 |
Values are mean ±s.e.m. No significant differences were detected between control and neonatal hypoxia rats (all P > 0.05).
Ventilatory and metabolic responses to hypoxia in awake rats
Baseline (normoxia)
Normoxic ventilation and metabolism were not significantly different between control and neonatal hypoxia rats, regardless of sex (Table 1; all P > 0.05). VT and V˙CO2 tended to be higher in male neonatal hypoxia rats versus control males, but these effects were not significant. Normoxic PaO2 levels were 8 Torr lower in male neonatal hypoxia rats (P = 0.04versus control), and a similar but non-significant trend was observed for females (P > 0.05) (Table 2). Normoxic PaCO2 levels were not affected by neonatal hypoxia (Table 2).
2.
Arterial blood gas values for unanaesthetized control and neonatal hypoxia rats breathing air (21% O2) and two levels of poikilocapnic hypoxia (12% and 10% O2)
| Level of hypoxia | |||
|---|---|---|---|
| Group | 21% O2 | 12% O2 | 10% O2 |
| PaO2 (Torr) | |||
| Male | |||
| Control | 107 ± 6 | 42 ± 2 | 32 ± 2 |
| Neonatal hypoxia | 98 ± 2* | 42 ± 1 | 34 ± 1 |
| Female | |||
| Control | 106 ± 7 | 45 ± 2 | 37 ± 2 |
| Neonatal hypoxia | 98 ± 1 | 43 ± 1 | 34 ± 0 |
| PaCO2 (Torr) | |||
| Male | |||
| Control | 37.0 ± 0.9 | 25.6 ± 0.6 | 20.3 ± 0.5 |
| Neonatal hypoxia | 36.6 ± 0.9 | 25.9 ± 0.7 | 20.9 ± 0.4 |
| Female | |||
| Control | 35.9 ± 1.5 | 25.3 ± 1.3 | 20.3 ± 1.0 |
| Neonatal hypoxia | 37.0 ± 2.1 | 26.7 ± 0.5 | 20.6 ± 0.3 |
Values are means ±s.e.m. for control (n = 10 male, 6 female) and neonatal hypoxia (n = 11 male, 4 female) rats.
P < 0.05versus control at the same FIO2.
Hypoxia
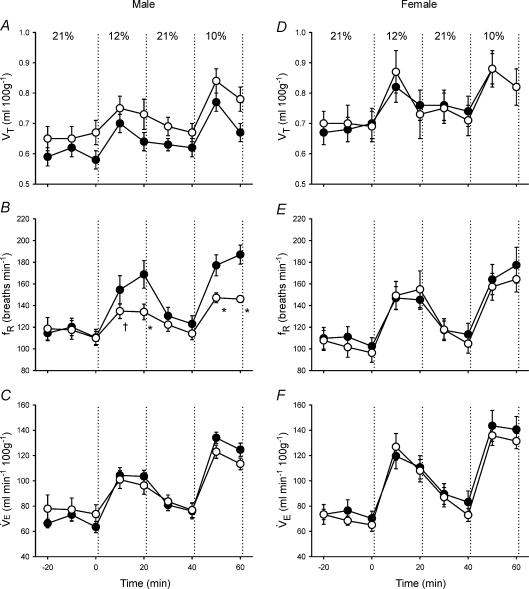
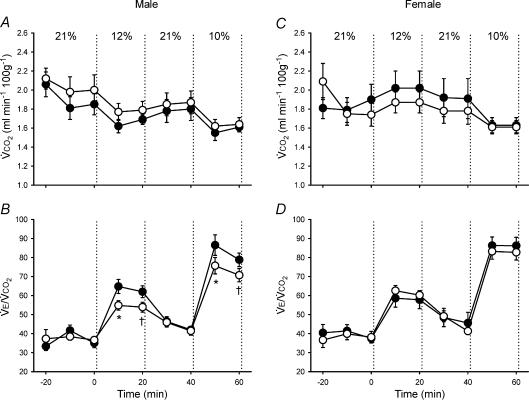
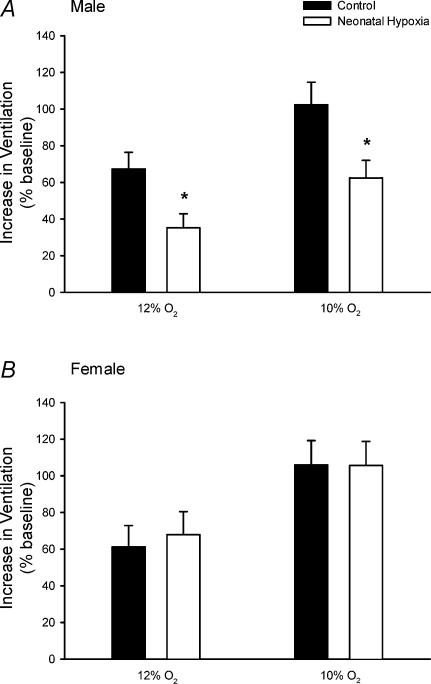
Control and neonatal hypoxia rats exhibited similar PaO2 and PaCO2 during acute exposure to 12% and 10% O2 regardless of sex (Table 2; all P > 0.05), thus enabling comparison of hypoxic ventilatory and metabolic responses at similar levels of arterial hypoxaemia. The effect of neonatal hypoxia on the acute ventilatory response differed between males and females (Figs 1–3). In males, neonatal hypoxia rats exhibited a smaller increase in fR during hypoxia than controls (treatment × time, P < 0.001), with no detectable change in their VT response (treatment and treatment × time, P > 0.05) (Fig. 1). As a result, changes in V˙E were also reduced in neonatal hypoxia rats (treatment × time, P < 0.01; Fig. 1). Since neonatal hypoxia rats tended to have higher normoxic V˙E, post hoc analysis failed to detect a difference between absolute values of V˙E during hypoxia (Fig. 1). However, the change in V˙E was significantly smaller in neonatal hypoxia rats (22.6 ± 4.1 versus 40.0 ± 4.3 ml min−1 (100 g)−1 in controls at 12% O2 and 39.7 ± 5.8 versus 61.1 ± 5.4 ml min−1 (100 g)−1 at 10% O2; both P < 0.02). Thus, the hypoxic ventilatory response (normalized to baseline V˙E) of neonatal hypoxia rats was only 52% and 61% of the response in control rats at 12% O2 and 10% O2, respectively (Fig. 2; both P < 0.02). The reduced hypoxic ventilatory response is also evident when V˙E is normalized to V˙CO2 (i.e. V˙E/V˙CO2) (treatment × time, P < 0.01; Fig. 3), particularly during the first 10 min of hypoxia.
Figure 1. Changes in ventilation (tidal volume (VT), respiratory frequency (fR), minute ventilation V˙E)) during hypoxia in unanaesthetized male (A–C) and female (D–F) rats.
Values (mean ±s.e.m.) are given for 10 min bins throughout the experimental protocol (21% O2–12% O2–21% O2–10% O2); inspired O2 was switched from baseline (21% O2) to hypoxia at 0 min. Data are from adult rats raised in hypoxia for the first postnatal week (neonatal hypoxia (○): n = 11 males, 6 females) or in room air (control (•): n = 11 males, 10 females). *P < 0.05 versus control at the same level of hypoxia, †0.05 < P < 0.10 versus control at the same level of hypoxia. Although hypoxic V˙E did not differ significantly between treatment groups, the change in ventilation in response to hypoxia was significantly reduced in male neonatal hypoxia rats (treatment × time, P < 0.01; see also Fig. 2).
Figure 3. Changes in metabolism (metabolic CO2 production (V˙CO2)) and ventilation-to-metabolism ratio (V˙E/V˙CO2)during hypoxia in unanaesthetized male (A–B) and female (C–D) rats.
Values (mean ±s.e.m.) are given for 10 min bins throughout the experimental protocol (21% O2–12% O2–21% O2–10% O2); inspired O2 was switched from baseline (21% O2) to hypoxia at 0 min. Data are from adult rats raised in hypoxia for the first postnatal week (neonatal hypoxia (○): n = 11 males, 6 females) or in room air (control (•): n = 11 males, 10 females). *P < 0.05versus control at the same level of hypoxia, †0.05 < P < 0.10versus control at the same level of hypoxia.
Figure 2. Hypoxic ventilatory responses in unanaesthetized male (A) and female (B) rats.
The increase in V˙E (normalized to baseline; mean ±s.e.m.) in response to 12% O2 and 10% O2 is shown for adult rats raised in hypoxia for the first postnatal week (neonatal hypoxia (open columns): n = 11 males, 6 females) or in room air (control (filled columns): n = 11 males, 10 females). *P < 0.05versus control at the same level of hypoxia. Similar results are obtained when hypoxic responses are calculated as absolute changes in ventilation (ml min−1 (100 g)−1), rather than normalized as a percentage of baseline (see text).
In contrast to males, adult ventilatory responses to hypoxia were not affected by neonatal hypoxia in female rats (Figs 1–3; all P > 0.05). Most female rats from the neonatal hypoxia group were in either metoestrus or dioestrus at the time of study (5 of 6 rats), whereas control females were more evenly distributed among oestrus stages (6 of 10 rats in metoestrus or dioestrus). To determine whether the stage of oestrus cycle biased the results, ventilatory responses were re-analysed for female rats after restricting the sample to rats in metoestrus or dioestrus; these stages are similar with respect to serum hormone levels in the rat and are not consistently differentiated by researchers (Knobil & Neill, 1994). This restricted analysis yielded nearly identical results, with no evidence for blunted hypoxic ventilatory responses in female rats following neonatal hypoxia (P > 0.05; data not shown).
V˙CO2 (Fig. 3) and Tb responses to hypoxia were not affected by neonatal hypoxia in either sex (all P > 0.05).
Phrenic nerve responses to hypoxia in anaesthetized rats
Baseline (hyperoxia)
In male control and neonatal hypoxia rats, apnoeic thresholds (PETCO2) were similar (40 ± 2 versus 37 ± 3 Torr, respectively; P > 0.05), resulting in similar baseline PaCO2 levels (Table 3, P > 0.05). However, average baseline PaO2 levels were 25 Torr lower in neonatal hypoxia rats under baseline conditions (Table 3, P = 0.01). Baseline phrenic activity was similar between male control and neonatal hypoxia rats (burst frequency: 50 ± 1 versus 46 ± 2 bursts min−1, burst amplitude: 40 ± 2 versus 37 ± 3% of maximum, respectively; both P > 0.05). In female rats, no differences were detected between control and neonatal hypoxia rats in apnoeic threshold (39 ± 2 versus 41 ± 1 Torr), baseline blood gases (Table 3), or baseline phrenic activity (burst frequency: 37 ± 2 versus 36 ± 6 bursts min−1, burst amplitude: 40 ± 3 versus 31 ± 5% of maximum) (all P > 0.05).
Table 3.
Arterial blood gas values for anaesthetized, vagotomized control and neonatal hypoxia rats under baseline conditions (FIO2= 0.50) and during the three levels of hypoxia at which phrenic activity was measured
| Level of hypoxia | ||||
|---|---|---|---|---|
| Group | Baseline | 60 Torr | 50 Torr | 40 Torr |
| PaO2 (Torr) | ||||
| Male | ||||
| Control | 260 ± 6 | 61 ± 1 | 50 ± 1 | 40 ± 0 |
| Neonatal hypoxia | 235 ± 7* | 61 ± 1 | 50 ± 1 | 40 ± 1 |
| Female | ||||
| Control | 241 ± 9 | 60 ± 0 | 48 ± 1 | 41 ± 1 |
| Neonatal hypoxia | 232 ± 7 | 61 ± 1 | 50 ± 1 | 41 ± 0 |
| PaCO2 (Torr) | ||||
| Male | ||||
| Control | 45.9 ± 0.8 | 45.9 ± 0.7 | 45.7 ± 0.8 | 46.0 ± 1.1 |
| Neonatal hypoxia | 46.2 ± 0.9 | 45.7 ± 0.8 | 45.6 ± 0.8 | 46.0 ± 1.0 |
| Female | ||||
| Control | 46.2 ± 1.7 | 46.2 ± 1.3 | 45.2 ± 1.6 | 46.1 ± 1.9 |
| Neonatal hypoxia | 46.6 ± 1.2 | 46.4 ± 1.5 | 45.4 ± 1.2 | 45.8 ± 1.1 |
Values are means ±s.e.m. for control (n = 11 male, 5 female) and neonatal hypoxia (n = 11 male, 4 female) rats.
P < 0.05 versus control at the same FIO2.
Hypoxia
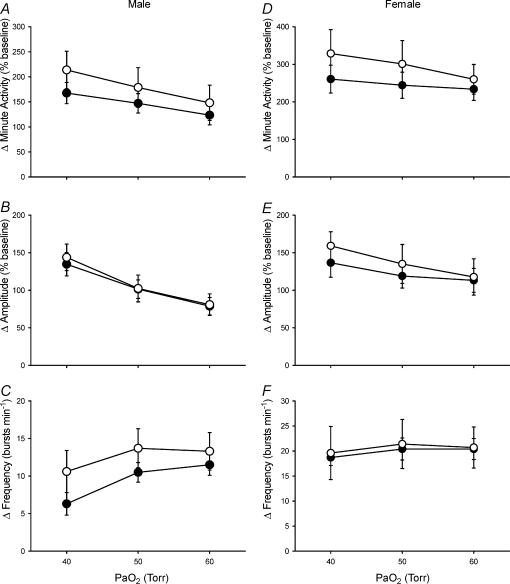
PaO2 and PaCO2 levels were similar between control and neonatal hypoxia rats during hypoxia and PaCO2 levels were isocapnic relative to baseline (mean ΔPaCO2 < 1 Torr) (Table 3; all P > 0.05). As expected, minute phrenic activity increased in all rat groups during hypoxia (Fig. 4; P < 0.001). However, there was no evidence for reduced phrenic responses to hypoxia, or changes in the pattern of these responses, in either male or female rats following neonatal hypoxia (Fig. 4; treatment and treatment ×PaO2, all P > 0.05). Indeed, there was a non-significant trend for minute phrenic activity to increase more in neonatal hypoxia rats than in controls. Sample sizes were insufficient to test for the effects of oestrus cycle on hypoxic phrenic responses in female rats, but (1) rats were distributed evenly in terms of stage of oestrus cycle within and among treatments and (2) phrenic responses did not change according to the stage of the oestrus cycle in young rats in a previous study from our laboratory (Zabka et al. 2001).
Figure 4. Hypoxic phrenic responses in anaesthetized and vagotomized male (A–C) and female (D–F) rats.
The increases in phrenic minute activity, burst amplitude and burst frequency (normalized to baseline; mean ±s.e.m.) in response to three levels of isocapnic hypoxia (arterial PO2 (PaO2) = 40, 50 and 60 Torr) are shown for adult rats raised in hypoxia for the first postnatal week (neonatal hypoxia (○): n = 11 males, 4 females) or in room air (control (•): n = 11 males, 5 females). No significant differences were detected between neontatal hypoxia and control rats (all P > 0.05).
Vagotomy
Since vagotomy could alter hypoxic responses or obscure the effects of neonatal hypoxia by disrupting afferent pathways from some peripheral chemoreceptors (e.g. aortic or abdominal chemoreceptors) and stretch receptors, additional rats were studied before and after bilateral vagotomy. Prior to vagotomy, the presence of intact vagi was evident by entrainment of phrenic activity to the ventilator cycle (i.e. one phrenic burst per ventilator cycle) and by inhibition of phrenic activity upon hyperinflation of the lungs (i.e. Breuer-Hering reflex). Subsequent vagotomy was confirmed by an absence of these effects, and by post mortem inspection. Thus, acute vagotomy significantly decreased baseline phrenic burst frequency from ∼61 bursts min−1 (ventilator setting) to 47 ± 3 and 49 ± 4 bursts min−1 in control and neonatal hypoxia rats, respectively (P < 0.01); no differences were detected between treatment groups (treatment and treatment × vagotomy, P > 0.05). The drop in phrenic burst frequency following vagotomy was accompanied by a significant increase in baseline phrenic amplitude (control: 37 ± 5 to 51 ± 2% maximum, neonatal hypoxia: 27 ± 3 to 36 ± 4% maximum; overall effect of vagotomy, P = 0.001). The effect of vagotomy on phrenic burst amplitude did not differ between treatment groups (treatment × vagotomy, P > 0.05), but baseline amplitude was smaller in neonatal hypoxia rats in this small sample (P = 0.05). The PETCO2 corresponding to the apnoeic threshold also decreased following vagotomy (control: 46 ± 2 to 44 ± 3 Torr, neonatal hypoxia: 44 ± 4– to 36 ± 5 Torr; overall effect of vagotomy, P = 0.01), as previously reported by Boden et al. (1998).
Hypoxic phrenic responses before and after vagotomy, as well as the effects of vagotomy per se, were similar between control and neonatal hypoxia rats (treatment and treatment × vagotomy, all P > 0.05; Fig. 5). Overall, vagotomy influenced the pattern but not the magnitude of the hypoxic phrenic response (Fig. 5). Prior to vagotomy, rats responded to hypoxia (PaO2= 50 Torr) with an increase in phrenic burst amplitude while burst frequency remained constant (i.e. phrenic burst frequency remained entrained to the ventilator). After vagotomy, phrenic burst amplitude increased to a lesser extent during hypoxia (P = 0.02), but greater burst frequency increases (P = 0.02) maintained similar minute phrenic activity responses to hypoxia (P > 0.05).
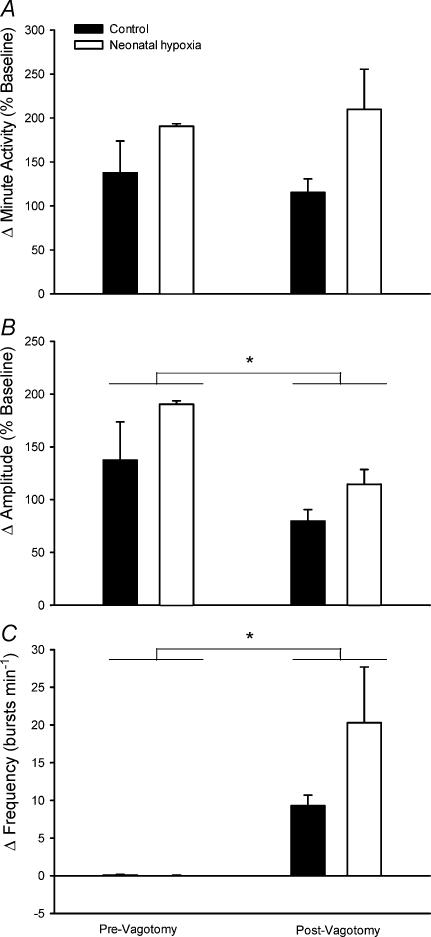
Figure 5. Influence of vagotomy on hypoxic phrenic responses in anaesthetized male rats.
The increases in phrenic minute activity (A), burst amplitude (B) and burst frequency (C) (normalized to baseline; mean ±s.e.m.) in response to isocapnic hypoxia (PaO2 = 50 Torr) before and after vagotomy for adult rats raised in hypoxia for the first postnatal week (neonatal hypoxia (open columns), n = 3) or in room air (control (filled columns), n = 3). *Significant difference between pre- and postvagotomy hypoxic responses (i.e. main effect for vagotomy, P < 0.05). No significant differences were detected between neonatal hypoxia and control rats (P > 0.05).
Discussion
Neonatal hypoxia attenuated the hypoxic ventilatory response in adult male rats in the present study, confirming earlier studies in rats (Okubo & Mortola, 1990), sheep (Sladek et al. 1993), and humans (Sørensen & Severinghaus, 1968a, b; Lahiri et al. 1976; Lahiri, 1981). However, this effect was not observed in female rats at 7–9 weeks of age, indicating that this form of developmental plasticity is sexually dimorphic. Although previous studies have not investigated the influences of sex on neonatal hypoxia-induced respiratory plasticity, other forms of respiratory plasticity differ between males and females (Bavis & Kilgore, 2001; Behan et al. 2002; Genest et al. 2003). Another new finding is that hypoxic phrenic responses are not reduced in adult rats following neonatal hypoxia, an observation that provides important insights concerning the site of plasticity (see ‘Potential sites of plasticity’ below).
Hypoxic ventilatory responses were assessed in 7- to 9-week-old rats, so the effects of neonatal hypoxia in male rats persisted at least 6–8 weeks post hypoxia. In contrast, similar periods of hypoxia do not elicit lasting changes in the hypoxic ventilatory response when delivered to mature rats (Okubo & Mortola, 1990). In fact, chronic hypoxia in prenatal or adult mammals generally enhances hypoxic ventilatory responses (Bisgard & Neubauer, 1995; Powell et al. 1998; Peyronnet et al. 2000; Fuller et al. (in press)). Thus, the inhibitory effects of neonatal hypoxia appear to be specific to postnatal development, although details of the critical period for this plasticity are not yet known. While the plasticity persists for at least weeks to months, we lack sufficient data to address its ultimate duration. It is possible, for example, that female rats exhibit similar plasticity, but that their ventilatory responses recover more quickly than in males. Thus, it is not yet known whether the sex differences identified in this study are qualitative (i.e. capacity to express plasticity) or quantitative (i.e. magnitude and duration of plasticity).
The effects of neontatal hypoxia on normoxic ventilation in rats differ somewhat between the present study and the earlier work of Okubo & Mortola (1988, 1990). Okubo & Mortola (1988, 1990) found that normoxic V˙E was greater in adult rats (∼50 days of age) following neonatal hypoxia, primarily due to a greater tidal volume. Hyperventilation was confirmed in their study by lower PaCO2 levels (Okubo & Mortola, 1988). There was little evidence for persistent hyperventilation following neonatal hypoxia in the present study despite similar experimental protocols. Although normoxic VT, and therefore V˙E, tended to be higher following neonatal hypoxia in male rats, this difference was not statistically significant and reflected a slightly higher metabolic rate. Indeed, normoxic V˙E/V˙CO2 and PaCO2 were unchanged by neonatal hypoxia in the present study. Our data are more similar to studies on sheep where normoxic ventilation and PaCO2 were normal following developmental hypoxia (Sladek et al. 1993). Nevertheless, despite differences in the effects on resting ventilation, attenuated hypoxic ventilatory responses were observed in each of these studies (Okubo & Mortola, 1990; Sladek et al. 1993; present study), suggesting that the blunted hypoxic ventilatory response is independent from altered resting ventilation.
Given their lower V˙E/V˙CO2, it is surprising that hypoxic blood gases were not significantly altered in neonatal hypoxia rats compared to untreated controls. Indeed, previous studies in rats have observed or predicted changes in PaCO2 of 1–3 mmHg for somewhat smaller reductions in hypoxic ventilatory response (Mortola & Saiki, 1996; Bavis et al. 2003). There is no clear explanation for the current results, although we can suggest two possibilities. First, blood gases were drawn at the end of each 20 min hypoxic exposure, whereas the difference in V˙E/V˙CO2 tended to be greatest during the first 10 min of hypoxia and was not quite statistically significant (P = 0.06) during the second 10 min; blunted hypoxic responsiveness is also most pronounced during the initial moments of hypoxia in sheep treated with neonatal hypoxia (Sladek et al. 1993). Thus, our blood sampling protocol may have missed the effect of neonatal hypoxia on arterial PCO2. The second possibility is that blood gases are not altered by neonatal hypoxia in rats, consistent with the generally normal blood gases during hypoxia in neonatal hypoxia-treated sheep (Sladek et al. 1993). Thus, rats and sheep may be capable of adequate blood gas regulation after neonatal hypoxia despite apparent reductions in hypoxic ventilatory responsiveness. Since V˙E/V˙CO2 has been altered, the most likely explanation for this observation is that gas exchange has been enhanced by changes in physiological dead space, either by constriction of upper airways or a smaller increase in ventilation–perfusion heterogeneity during hypoxia, thereby compensating for hypoxic hypoventilation in neonatal hypoxia rats.
Potential sites of plasticity
Plasticity in the hypoxic ventilatory response could occur at many levels of the chemoreflex, including changes in gas exchange and metabolism (peripheral or central) chemoreceptors, central neural integration, efferent neural pathways, respiratory muscles, or respiratory mechanics/mechanoreceptors (see also Fuller et al. 2004). By combining measurements of ventilatory, metabolic and phrenic nerve activity, the present study excludes several of these sites as major, proximate contributors to plasticity in the hypoxic ventilatory response following neonatal hypoxia.
Respiratory environments experienced during development may impair pulmonary gas exchange. For example, adult rats exposed for the first month of life to chronic hyperoxia (60% O2) exhibit lower PaO2 levels, and an increased alveolar–arterial PO2 difference, relative to normoxia-reared rats while breathing the same hypoxic gas mixtures (Ling et al. 1996). Therefore, comparisons of hypoxic ventilatory responses at fixed levels of inspired O2 may be influenced by differences in blood gases rather than changes in the ventilatory control system. Similarly, given the important influence of metabolism on ventilation (Mortola, 1996), differences in metabolism and metabolic responses to hypoxia may lead to incorrect conclusions regarding hypoxic ventilatory responses. Neither blood gases nor metabolic rates were measured during hypoxia in the previous study on neonatal hypoxia in rats (Okubo & Mortola, 1990), although rats exposed to neonatal hypoxia exhibited morphological changes (e.g. larger lungs and alveoli) that could alter lung function (Okubo & Mortola, 1989). There was some evidence for impaired gas exchange following neonatal hypoxia in the present study. Specifically, male (and to a lesser extent female) rats had lower baseline PaO2 whether spontaneously breathing or artificially ventilated. However, hypoxic blood gases and metabolism were similar between rats treated with neonatal hypoxia and control rats. Thus, hypoxia-treated and control rats experienced the same respiratory stimuli, similar to an earlier study on sheep in which hypoxic ventilatory responses were assessed at similar PaO2 levels (Sladek et al. 1993). Accordingly, neither changes in gas exchange nor metabolism explain the blunted ventilatory response to hypoxia following neonatal hypoxia in male rats.
Although we did not measure the output of O2-sensitive chemoreceptors or the central neural integration of chemoafferent activity directly, our data suggest that blunted hypoxic ventilatory responses are not caused by persistent changes in these pathways following neonatal hypoxia. By measuring phrenic nerve responses to carefully regulated changes in blood gases, we were able to assess collective neural mechanisms including chemosensation and central neural integration without influences from neuromuscular transmission or the mechanical act of breathing. We found no evidence of net impairment of the hypoxic chemoreflex upstream of the phrenic nerve in either male or female rats. Previous studies in rats support aspects of this conclusion. Although hypoxic sensitivity of the carotid body, the primary O2 sensor in rats, is blunted or absent in newborn rats and other species when born and raised in hypoxia (Hanson et al. 1989a, b; Sladek et al. 1993; Landauer et al. 1995; Wyatt et al. 1995; Sterni et al. 1999), normal carotid body responses return after 5–10 weeks of hypoxia (Eden & Hanson, 1987). Thus, although perinatal hypoxia delays maturation of carotid body chemoreceptors, chemosensitivity eventually develops despite continued hypoxia (i.e. spontaneous recovery). Similarly, Sterni et al. (1999) observed spontaneous recovery of hypoxic sensitivity in carotid body type I cells isolated from rat pups after varying durations of neonatal hypoxia. The in vitro hypoxic chemosensitivity (assessed via increased intracellular calcium during acute hypoxia) was abolished at 3, 11 and 18 days of age. However, if the carotid body was harvested from 18-day-old pups that had been returned to normoxia 1 week earlier (i.e. at 11 days of age), substantial hypoxic chemosensitivity had returned. Based on these studies, long-lasting impairment of carotid body chemosensitivity seems unlikely following neonatal hypoxia, consistent with normal hypoxic phrenic responses in adult rats.
Although the carotid body is the principal source of hypoxic chemosensitivity in rats, additional O2-sensitive sites have been reported (Martin-Body et al. 1985; Bisgard & Neubauer, 1995; Forster, 2003). These alternate central and peripheral O2 sensors normally contribute little to the acute hypoxic ventilatory response (e.g. Martin-Body et al. 1985; see also Bavis & Mitchell, 2003) unless up-regulated by mechanisms of plasticity (Forster, 2003). However, it is possible that loss of some or all of these O2 sensors could diminish the hypoxic ventilatory response in rats (Martin-Body et al. 1985). Since the CNS remained intact while measuring hypoxic ventilatory and phrenic responses, it is unlikely that central O2-sensitive neurones are responsible for blunted ventilatory responses following neonatal hypoxia. On the other hand, vagotomy prevents assessment of peripheral chemoreceptors that send afferents to the CNS via the vagus nerve (Martin-Body et al. 1985). However, since hypoxic phrenic responses were similar in intact and vagotomized rats, and were not affected by neonatal hypoxia, there is little evidence for lasting effects on the O2 sensitivity of non-carotid body hypoxic chemoreceptors following neonatal hypoxia.
Hypoxic ventilatory measurements were made on spontaneously breathing animals under poikiolocapnic conditions in rats (Okubo & Mortola, 1990; present study) and sheep (Sladek et al. 1993). Thus, differences in CO2 sensitivity could, in principle, alter the magnitude of the hypoxic ventilatory response by influencing hypocapnia-induced ventilatory inhibition. Moreover, since phrenic responses were measured under isocapnic conditions, altered CO2 sensitivity could explain the discrepancy between ventilatory and phrenic responses to hypoxia. Hypercapnic ventilatory responses were not studied and only maximal phrenic responses to hypercapnia were measured in the present study. However, Okubo & Mortola (1990) reported that hypercapnic ventilatory responses were normal in adult rats after neonatal hypoxia. Thus, altered CO2 sensitivity is unlikely to explain the blunted hypoxic ventilatory responses of adult rats following neonatal hypoxia.
Normal hypoxic phrenic responses following neonatal hypoxia support the conclusion that peripheral chemoreceptor and CNS function are also normal in adult male rats. Thus, the mechanism of plasticity must occur at a site between phrenic nerve activity and the production of airflow, such as neuromuscular transmission, respiratory muscle function or respiratory mechanics (or related feedback control). Neonatal hypoxia appears to delay, but not prevent, maturation of the neuromuscular junction and reduces the ability of the diaphragm to generate force (Kass & Bazzy, 2001); however, the persistence of these effects is unknown. Even if these effects persist into adulthood, they may not explain the blunted hypoxic ventilatory response observed here since reduced contractile force would alter tidal volume versus respiratory frequency (Fig. 1). Moreover, since hypercapnic ventilatory responses are not affected by neonatal hypoxia (Okubo & Mortola, 1990), neonatal hypoxia does not cause persistent impairment in the capacity to respond to increased respiratory drives.
Neonatal hypoxia may alter respiratory control through effects on respiratory mechanics and associated feedback control (i.e. mechanoreceptors and their CNS integration). Neonatal hypoxia increases lung and respiratory system compliance and reduces pulmonary resistance in adult rats (Okubo & Mortola, 1989). These changes could contribute to higher tidal volumes following neonatal hypoxia (Okubo & Mortola, 1988, 1990; present study). On the other hand, altered respiratory mechanics sufficient to constrain hypoxic ventilatory responses are difficult to reconcile with normal hypercapnic ventilatory responses in the same rats (Okubo & Mortola, 1990). The conditions used to assess mechanics may offer a solution to this paradox. Since mechanics were measured during normoxia (Okubo & Mortola, 1989), they may not accurately reflect physiological changes in respiratory mechanics that are unique to hypoxia. Indeed, hypoxia induces a complex suite of changes in airway resistance and the mechanical function of the lung (Pérez Fontán, 1996). If neonatal hypoxia alters airway and lung responses to acute hypoxia, it may also alter ventilatory responses in a hypoxia-specific manner. For example, if total resistance of the respiratory system is relatively greater during hypoxia in rats exposed to neonatal hypoxia, a smaller increase in respiratory frequency may result. The experimental preparation used in phrenic nerve studies bypasses changes in respiratory mechanics and may therefore be unaltered by neonatal hypoxia. Hypoxia-specific changes in respiratory mechanics (e.g. airway resistance) appear to be the most likely cause of blunted hypoxic ventilatory responses in male rats following neonatal hypoxia. However, this hypothesis warrants additional study.
Critique of methods
To localize the site of long-lasting plasticity following neonatal hypoxia, we studied ventilatory and phrenic responses to hypoxia in adult rats. Comparing experiments in anaesthetized and unanaesthetized animals is inherently difficult, but previous work in various laboratories, including our own, supports our approach. For example, developmental hyperoxia impairs carotid body function (Ling et al. 1997; Bisgard et al. 2003), causing reductions in both ventilatory and phrenic responses to hypoxia (Ling et al. 1996, 1997; Bavis et al. 2003). Similarly, enhanced phrenic motor output following intermittent hypoxia in anaesthetized rats (i.e. phrenic long-term facilitation), which occurs by a CNS mechanism, is also observed as increased ventilation in awake rats (Mitchell et al. 2001; McGuire et al. 2003; Fuller et al. in press). Thus, changes in phrenic activity in anaesthetized rats resulting from changes in peripheral chemoreceptor and CNS function generally predict changes in ventilation in unanaesthetized rats. However, the reverse is not necessarily true. Since phrenic nerve activity requires multiple steps before initiating gas flow (e.g. neuromuscular transmission) and involves additional regulation (e.g. mechanoreceptors), normal phrenic responses may fail to produce normal ventilatory responses. Of course, phrenic nerve activity reflects neural drive to the diaphragm only, whereas ventilation is produced by the coordinated effort of many respiratory muscles. Nevertheless, since the blunted ventilatory response following neonatal hypoxia resulted from reduced respiratory frequency, phrenic activity should be representative of all respiratory muscle groups. The ultimate plasticity must result from changes in respiratory rhythm generation or its afferent feedback associated with changes in respiratory mechanics.
Comparison of neonatal hypoxia and hyperoxia
Similar to neonatal hypoxia, neonatal hyperoxia (30–60% O2) for as little as 1 week attenuates respiratory responses to hypoxia in adult rats, provided that hyperoxia occurs during the first two postnatal weeks (Ling et al. 1996, 1997; Bavis et al. 2002, 2003). However, developmental hyperoxia impairs both ventilatory and phrenic responses to hypoxia by attenuating carotid body hypoxic responses (Ling et al. 1997; Bisgard et al. 2003). Thus, neonatal hypoxia and hyperoxia produce similar effects on the hypoxic ventilatory response through different mechanisms. Since hyperoxia impairs the development of carotid body function, it seems logical to suggest that hypoxia has the opposite effect, enhancing carotid body function and, perhaps, lessening the effects of altered mechanics. However, as previously noted, most data indicate that postnatal hypoxia delays the maturation of carotid body O2 sensitivity (Hanson et al. 1989a, b; Sladek et al. 1993; Landauer et al. 1995; Wyatt et al. 1995; Sterni et al. 1999). Nevertheless, the long-term consequences of neonatal hypoxia to carotid body function have not been studied directly. Interestingly, there was a non-significant trend for enhanced phrenic responses in intact and vagotomized adult rats following neonatal hypoxia (Figs 3 and 4). Although this observation is consistent with enhanced carotid body sensitivity, the data were variable and the effect was not statistically significant (even after combining all rats into a single analysis to increase sample size; P > 0.05, data not shown). Thus, we found no clear evidence that neonatal hypoxia and hyperoxia have opposite effects on adult carotid body function.
Implications
Humans may experience chronic hypoxia during postnatal development as a result of disease or residence at high altitudes, but the long-term consequences of neonatal hypoxia on the hypoxic ventilatory response have been debated (Edelman et al. 1970; Blesa et al. 1977; Vargas et al. 1998; Gamboa et al. 2003). Controlled studies with animal models indicate that the hypoxic ventilatory response may be diminished for long periods following relatively short exposures to neonatal hypoxia (Okubo & Mortola, 1990; Sladek et al. 1993; present study). Thus, chronic hypoxia during development may contribute to the reduced hypoxic ventilatory responses observed in high altitude populations (Moore, 2000), as well as in some animals exposed to hypoxia in burrows (Boggs, 1991). A unique finding of the present study is that males may be more susceptible to these effects than females, although the reason for this difference between the sexes requires further investigation.
The potential health consequences of blunted hypoxic ventilatory responses following neonatal hypoxia are not clear. Altered hypoxic responses have been implicated in a number of respiratory disorders and may be a risk factor for sudden infant death syndrome (e.g. Hunt, 1989; Gaultier, 2000, 2001). However, our data suggest that, at least in some cases, the body may compensate for changes in the hypoxic ventilatory responses and adequately defend arterial blood gases during acute hypoxic challenges. If verified, the time course for this compensation may delineate a period of heightened vulnerability to hypoxia.
Acknowledgments
We gratefully acknowledge the assistance of C. F. Thomas and A. G. Zabka in determining oestrus stage in female rats. This study was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-53319 and HL-65383. R. W. Bavis was supported by Training Grant HL-07654 and National Research Service Award HL-70506. D. D. Fuller was supported by a fellowship from the Francis Family Foundation.
References
- Bavis RW, Kilgore DL., Jr Effects of embryonic CO2 exposure on the adult ventilatory response in quail: does gender matter. Respir Physiol. 2001;126:183–199. doi: 10.1016/s0034-5687(01)00206-7. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol. 2003;94:399–409. doi: 10.1152/japplphysiol.00374.2002. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Mitchell GS. Critical developmental period for hyperoxia-induced blunting of hypoxic phrenic responses in rats. J Appl Physiol. 2002;92:1013–1018. doi: 10.1152/japplphysiol.00859.2001. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Olson EB, Jr, Vidruk EH, Bisgard GE, Mitchell GS. Level and duration of developmental hyperoxia influence impairment of hypoxic phrenic responses in rats. J Appl Physiol. 2003;95:1550–1559. doi: 10.1152/japplphysiol.01043.2002. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol. 2002;131:65–77. doi: 10.1016/s1569-9048(02)00038-1. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 617–668. [Google Scholar]
- Bisgard GE, Olson EB, Jr, Wang Z-Y, Bavis RW, Fuller DD, Mitchell GS. Adult carotid chemoafferent responses to hypoxia after 1, 2 and 4 weeks of postnatal hyperoxia. J Appl Physiol. 2003;95:946–952. doi: 10.1152/japplphysiol.00985.2002. [DOI] [PubMed] [Google Scholar]
- Blesa MI, Lahiri S, Rashkind WJ, Fishman AP. Normalization of the blunted ventilatory response to acute hypoxia in congenital cyanotic heart disease. N Engl J Med. 1977;296:237–241. doi: 10.1056/NEJM197702032960501. [DOI] [PubMed] [Google Scholar]
- Boden AG, Harris MC, Parkes MJ. Apneic threshold for CO2 in the anesthetized rat: fundamental properties under steady-state conditions. J Appl Physiol. 1998;85:898–907. doi: 10.1152/jappl.1998.85.3.898. [DOI] [PubMed] [Google Scholar]
- Boggs DF. Comparative control of respiration. In: Parent RA, editor. Comparative Biology of the Normal Lung. Boca Raton: CRC Press; 1991. pp. 309–350. [Google Scholar]
- Byrne-Quinn E, Sodal IE, Weil JV. Hypoxic and hypercapnic ventilatory drives in children native to high altitude. J Appl Physiol. 1972;32:44–46. doi: 10.1152/jappl.1972.32.1.44. [DOI] [PubMed] [Google Scholar]
- Calder NA, Williams BA, Smyth J, Boon AW, Kumar P, Hanson MA. Absence of ventilatory response to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia. Implications for the risk of sudden infant death. Pediatr Res. 1994;35:677–681. doi: 10.1203/00006450-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol. 2003;94:375–389. doi: 10.1152/japplphysiol.00809.2002. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Edelman NH, Lahiri S, Braudo MD, Cherniack NS, Fishman AP. The blunted ventilatory response to hypoxia in cyanotic congenital heart disease. N Engl J Med. 1970;282:405–411. doi: 10.1056/NEJM197002192820801. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol. 1987;392:11–19. doi: 10.1113/jphysiol.1987.sp016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J Appl Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bavis RW, Mitchell GS. Pharmacology and Pathophysiology of the Control of Breathing. New York: Marcel Dekker; Respiratory neuroplasticity: respiratory gases, development, and spinal injury. in press. [Google Scholar]
- Gamboa A, Léon-Velarde F, Rivera ChM, Palacios J-A, Pragnell TR, O'Connor DF, Robbins PA. Acute and sustained ventilatory responses to hypoxia in high-altitude natives living at sea level. J Appl Physiol. 2003;94:1255–1262. doi: 10.1152/japplphysiol.00856.2002. [DOI] [PubMed] [Google Scholar]
- Gaultier C. Development of the control of breathing: implications for sleep-related breathing disorders in infants. Sleep. 2000;23(Suppl. 4):S136–S139. [PubMed] [Google Scholar]
- Gaultier C. Abnormalities of the chemical control of breathing: Clinical correlates in infants and children. Pediatr Pulmonol Suppl. 2001;23:114–117. [PubMed] [Google Scholar]
- Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation and sex-specific plasticity of the hypoxic ventilatory response in awake rats. J Physiol. 2003;554:543–557. doi: 10.1113/jphysiol.2003.052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Eden GJ, Nijhuis JG, Moore PJ. Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn. In: Lahiri S, Forster RE, Davies RO, Pack AI, editors. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. New York: Oxford University Press; 1989b. pp. 113–120. [Google Scholar]
- Hanson MA, Kumar P, Williams BA. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J Physiol. 1989a;411:563–574. doi: 10.1113/jphysiol.1989.sp017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW. Anatomy and Embryology of the Laboratory Rat. Germany: BioMed-Verlag, Wörthsee; 1986. [Google Scholar]
- Hunt CE. Impaired arousal from sleep: relationship to sudden infant death syndrome. J Perinatol. 1989;9:184–187. [PubMed] [Google Scholar]
- Kass LJ, Bazzy AR. Chronic hypoxia modulates diaphragm function in the developing rat. J Appl Physiol. 2001;90:2325–2329. doi: 10.1152/jappl.2001.90.6.2325. [DOI] [PubMed] [Google Scholar]
- Katz-Salamon M, Eriksson M, Jonsson B. Development of peripheral chemoreceptor function in infants with chronic lung disease and initially lacking hyperoxic response. Arch Dis Child. 1996;75:F4–F9. doi: 10.1136/fn.75.1.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Salamon M, Jonsson B, Lagercrantz H. Blunted peripheral chemoreceptor response to hyperoxia in a group of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1995;20:101–106. doi: 10.1002/ppul.1950200209. [DOI] [PubMed] [Google Scholar]
- Knobil E, Neill JD. The Physiology of Reproduction. New York: Raven Press; 1994. [Google Scholar]
- Lahiri S. Adaptive respiratory regulation – lessons from high altitudes. In: Hovarth SM, Yousef MK, editors. Environmental Physiology. Aging, Heat and Altitude. New York: Elsevier North Holland; 1981. pp. 341–350. [Google Scholar]
- Lahiri S, DeLaney RG, Brody JS, Simpser M, Velasquez T, Motoyama EK, Polgar C. Relative role of environmental and genetic factors in respiratory adaptation to high altitude. Nature. 1976;261:133–135. doi: 10.1038/261133a0. [DOI] [PubMed] [Google Scholar]
- Landauer RC, Pepper DR, Kumar P. Effect of chronic hypoxaemia from birth upon chemosensitivity in the adult rat carotid body in vitro. J Physiol. 1995;485:543–550. doi: 10.1113/jphysiol.1995.sp020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol. 1996;495:561–571. doi: 10.1113/jphysiol.1996.sp021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response. Respir Physiol. 1997;110:261–268. doi: 10.1016/s0034-5687(97)00091-1. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol. 2003;95:1499–1508. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD. Respiratory effects of sectioning the carotid sinus glossopharyngeal and abdominal vagal nerves in the awake rat. J Physiol. 1985;361:35–45. doi: 10.1113/jphysiol.1985.sp015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Moore LG. Comparative human ventilatory adaptation to high altitude. Respir Physiol. 2000;121:257–276. doi: 10.1016/s0034-5687(00)00133-x. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Ventilatory responses to hypoxia in mammals. In: Haddad GG, Lister G, editors. Tissue Oxygen Deprivation. From Molecular to Integrated Function. New York: Marcel Dekker; 1996. pp. 433–477. [Google Scholar]
- Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: gender differences. Respir Physiol. 1996;106:21–34. doi: 10.1016/0034-5687(96)00064-3. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP. Long-term respiratory effects of neonatal hypoxia in the rat. J Appl Physiol. 1988;64:952–958. doi: 10.1152/jappl.1988.64.3.952. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP. Respiratory mechanics in adult rats hypoxic in the neonatal period. J Appl Physiol. 1989;66:1772–1778. doi: 10.1152/jappl.1989.66.4.1772. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol. 1990;259:R836–R841. doi: 10.1152/ajpregu.1990.259.4.R836. [DOI] [PubMed] [Google Scholar]
- Olson EB., Jr Physiologic dead space increases during initial hours of chronic hypoxemia with or without hypocapnia. J Appl Physiol. 1994;77:1526–1531. doi: 10.1152/jappl.1994.77.3.1526. [DOI] [PubMed] [Google Scholar]
- Pérez Fontán JJ. Mechanical function of the lungs and airways during hypoxia. In: Haddad GG, Lister G, editors. Tissue Oxygen Deprivation. From Molecular to Integrated Function. New York: Marcel Dekker; 1996. pp. 335–350. [Google Scholar]
- Peyronnet J, Roux JC, Géloën A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol. 2000;524:525–537. doi: 10.1111/j.1469-7793.2000.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Sladek M, Parker RA, Grogaard JB, Sundell HW. Long-lasting effect of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr Res. 1993;34:821–828. doi: 10.1203/00006450-199312000-00025. [DOI] [PubMed] [Google Scholar]
- Sørensen SC, Severinghaus JW. Irreversible respiratory insensitivity to acute hypoxia in man born at high altitude. J Appl Physiol. 1968a;25:217–220. doi: 10.1152/jappl.1968.25.3.217. [DOI] [PubMed] [Google Scholar]
- Sørensen SC, Severinghaus JW. Respiratory insensitivity to acute hypoxia persisting after correction of tetralogy of Fallot. J Appl Physiol. 1968b;25:221–223. doi: 10.1152/jappl.1968.25.3.221. [DOI] [PubMed] [Google Scholar]
- Sterni LM, Bamford OS, Wasicko MJ, Carroll JL. Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol. 1999;277:L645–L652. doi: 10.1152/ajplung.1999.277.3.L645. [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Hannhart B, Moore LG. Influence of sex steroids on ventilation and ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Marcel Dekker; 1995. pp. 829–864. [Google Scholar]
- Vargas M, León-Velarde F, Monge C, Palacios J-A, Robbins PA. Similar hypoxic ventilatory responses in sea-level natives and high-altitude natives living at sea level. J Appl Physiol. 1998;84:1024–1029. doi: 10.1152/jappl.1998.84.3.1024. [DOI] [PubMed] [Google Scholar]
- Weil JV, Byrne-Quinn E, Sodal IE, Filley GF, Grover RF. Acquired attenuation of chemoreceptor function in chronically hypoxic man at high altitude. J Clin Invest. 1971;50:186–194. doi: 10.1172/JCI106472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci U S A. 1995;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS. Time-dependent hypoxic respiratory responses in female rats are influenced by age and the estrus cycle. J Appl Physiol. 2001;91:2831–2838. doi: 10.1152/jappl.2001.91.6.2831. [DOI] [PubMed] [Google Scholar]