Abstract
As there are wide interspecies variations in the molecular nature of the O2-sensitive Kv channels in arterial chemoreceptors, we have characterized the expression of these channels and their hypoxic sensitivity in the mouse carotid body (CB). CB chemoreceptor cells were obtained from a transgenic mouse expressing green fluorescent protein (GFP) under the control of tyrosine hydroxylase (TH) promoter. Immunocytochemical identification of TH in CB cell cultures reveals a good match with GFP-positive cells. Furthermore, these cells show an increase in [Ca2+]i in response to low PO2, demonstrating their ability to engender a physiological response. Whole-cell experiments demonstrated slow-inactivating K+ currents with activation threshold around −30 mV and a bi-exponential kinetic of deactivation (τ of 6.24 ± 0.52 and 32.85 ± 4.14 ms). TEA sensitivity of the currents identified also two different components (IC50 of 17.8 ± 2.8 and 940.0 ± 14.7 μm). Current amplitude decreased reversibly in response to hypoxia, which selectively affected the fast deactivating component. Hypoxic inhibition was also abolished in the presence of low (10–50 μm) concentrations of TEA, suggesting that O2 interacts with the component of the current most sensitive to TEA. The kinetic and pharmacological profile of the currents suggested the presence of Kv2 and Kv3 channels as their molecular correlates, and we have identified several members of these two subfamilies by single-cell PCR and immunocytochemistry. This report represents the first functional and molecular characterization of Kv channels in mouse CB chemoreceptor cells, and strongly suggests that O2-sensitive Kv channels in this preparation belong to the Kv3 subfamily.
Carotid body (CB) chemoreceptor activity underlies the hyperventilatory reflex under hypoxic conditions. Although there is still some controversy regarding the steps of the chemotransduction cascade in this organ and the molecular identity of the oxygen sensor, at the present it is firmly established that membrane depolarization of chemoreceptor cells is one of the initial steps in oxygen chemotransduction. Inhibition of potassium channels plays a pivotal role in achieving depolarization. The first oxygen modulated K+ channel was described in rabbit carotid body chemoreceptor cells (López-Barneo et al. 1988), and since then many other K+ channels have been shown to be oxygen sensitive in different chemosensory tissues from different species as well as in heterologous expression systems (Patel & Honore, 2001). Considerable tissue-specific differences in the O2-transduction process and in the transduction components (from O2 sensor to K+ channels) have been reported, yet there is a unifying end point in all cases, namely K+ channel inhibition. For this reason, the determination of the molecular constituents of the O2-sensitive K+ currents appears as a relevant issue in order to understand the molecular mechanisms of oxygen detection in hypoxia-sensitive tissues. In the past years, significant contributions to the molecular nature of the O2-sensitive K+ currents have been made in several chemosensitive tissues, including pulmonary arteries (Patel et al. 1997; Osipenko et al. 2000; Mandegar & Yuan, 2002), carotid bodies (Peers & Kemp, 2001; Sanchez et al. 2002), neuroepithelial bodies (Wang et al. 1996), and the clonal cell lines PC12, derived from pheochromocytoma cells (Conforti et al. 2000) and H-146, derived from neuroepithelial bodies (Hartness et al. 2001).
In the case of the CB, and due to the small size of the organ together with the cellular heterogeneity, the molecular identification of the channels has remained elusive for a long time. An additional difficulty comes from the observation that O2-sensitive K+ currents seem to be species specific: in the rat CB, both a large conductance, Ca2+-dependent K+ current and a background, leak K+ current have been reported to be specifically inhibited by hypoxia (Wyatt & Peers, 1995; Buckler, 1995; López-López et al. 1997), whereas in the rabbit CB hypoxia modulates a voltage-dependent, transient outward K+ current (Lopez-Lopez et al. 1993; Sanchez et al. 2002), and in the cat a non-inactivating K+ current (Chou & Shirahata, 1996). In the mouse CB, a decrease induced by sodium dithionite in voltage-dependent K+ currents has been described (He et al. 2002), although no further characterization of the currents was performed.
Numerous strains of transgenic knockout mice that might be valuable for the study of the oxygen chemotransduction process have been produced, including mice lacking a variety of potassium channels (Matsukawa et al. 2003). Despite the obvious advantages of studying the role of the different elements of the hypoxic transduction cascade with this experimental approach, studies in mouse CB are uncommon; obviously, the difficulties in obtaining and identifying chemoreceptor cells for a detailed morphological and functional characterization are a clear limitation of the mouse CB preparation. In this paper we provide the first characterization of the biophysical, pharmacological and molecular properties of the mouse CB Kv currents. We have used a transgenic mouse strain expressing green fluorescent protein (GFP) under the control of the rat tyrosine hydroxylase gene promoter (Sawamoto et al. 2001). In primary cultures obtained from CBs, GFP expression in TH-positive cells allows the positive identification of chemoreceptor cells. We demonstrate the expression of Kv2.2, Kv3.1, Kv3.2 and Kv3.3 subunits at the mRNA and for all but Kv2.2 also at protein level within isolated chemoreceptor cells. The pharmacological profile of the hypoxia-sensitive component of Kv current makes Kv3 subunits prime candidates for the molecular correlate of the native O2-sensitive voltage-dependent K+ current.
Methods
Dissociation and culture of CB cells
TH–GFP mice were maintained as heterozygotes by crossbreeding with wild-type C57BL/6J mice. Transgenic mice were identified by PCR using tail DNA and primers specific for the GFP sequences (Sawamoto et al. 2001). Adult heterozygote TH–GFP mice (2–4 months old) were killed by decapitation after diethyl ether anaesthesia. The protocols were approved by the Institutional Care and Use Committee of the University of Valladolid. Carotid artery bifurcations were dissected out and the CBs, together with a small patch of artery, were enzymatically dispersed with a modification of the previously described method (Pérez-García et al. 1992). Briefly, they were incubated at 37°C for 15 min in 2 ml of a collagenase solution (nominally Ca2+- and Mg2+-free Tyrode solution containing 2.5 mg ml−1 of collagenase and 6 mg ml−1 of albumin), washed, and additionally incubated for 15 min in 2 ml of a trypsin solution (1 mg ml−1 of trypsin and 6 mg ml−1 of albumin in nominally Ca2+- and Mg2+-free Tyrode solution). At the end of the second incubation 2 ml of growth medium (Dulbecco's modified Eagle's medium (DMEM)–Ham's nutrient mixture F12 with 5% fetal bovine serum (FBS)) were added, and the CBs were disrupted by repeatedly passing them through the tip of a fire-polished Pasteur pipette. The medium containing isolated cells was centrifuged and the pellet resuspended in growth medium. The dispersed cells were plated onto poly-l-lysine-coated coverslips with 2 ml of growth medium, and maintained in culture at 37°C for up to 72 h.
Electrophysiological methods
Ionic currents were recorded at room temperature (20–25°C) using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981). Whole-cell current recordings and data acquisition from CB chemoreceptor cells were made as previously described (López-López et al. 1997). The coverslips with the attached cells were placed at the bottom of a small recording chamber (0.2 ml) on the stage of an inverted microscope and perfused by gravity with the bath solution. This solution was connected to ground via a 3 m KCl agar bridge and an Ag–AgCl electrode. Patch pipettes were made from borosilicate glass (1.5 mm o.d., Clark Electromedical Instruments), double pulled (Narishige PP-83) and heat-polished (Narishige MF-83) to resistances ranging from 1.5 to 3 MΩ when filled with the internal solution. The composition of the bath solution was (mm): 141 NaCl, 4.7 KCl, 1.2 MgCl2, 1.8 CaCl2, 10 glucose, 10 Hepes (pH 7.4 with NaOH) and the pipette was filled with a solution containing (mm): 125 KCl, 4 MgCl2, 10 Hepes, 10 EGTA, 5 MgATP (pH 7.2 with KOH).
Whole-cell currents were recorded using an Axopatch 200 patch-clamp amplifier (Axon Instruments, Union city, CA, USA), filtered at 2 kHz (–3dB, 4-pole Bessel filter), and sampled at 10 kHz. When leak-subtraction was performed, an online P/4 protocol was used. Recordings were digitized with a Digidata 1200 A/D interface, driven by Clampex 8 software (Axon Instruments) on a Pentium computer.
Activation curves were obtained from families of 100–200 ms depolarizing steps from −60 to +80 mV followed by a 100 ms repolarizing pulse to −40 mV. The curves were constructed by plotting the maximal amplitude of the current during the repolarizing step to −40 mV (or the computed conductance, I/(V − Vrev)) against the voltage of the pulse. These curves were then fitted to a Boltzmann function to calculate the maximal amplitude (or conductance) and the midpoint of activation (V½). Deactivation kinetics was characterized by fitting the tail currents to a double exponential function.
Hypoxia was achieved by bubbling the reservoir that fed the perfusion chamber with 100% N2, obtaining a final PO2 level in the perfusion chamber below 10 mmHg. Oxygen levels where measured with small needle PO2 electrodes (Diamond General Development Corp., MI, USA) placed in the vicinity of the cells.
Sensitivity to tetraethyl ammonium ions (TEA) was analysed by studying the decrease in the peak current amplitude in depolarizing pulses to +40 mV upon application of increasing concentrations of TEA to the bath solution. TEA block was expressed as a percentage of inhibition of control current (mean of the current amplitude before and after TEA application). TEA dose–response curves were fitted to a one or several binding sites model with the function:
 |
The data were always best fitted with a two-binding-site model, obtaining two values of Bmax (B1 and B2) and two different Kd (K1 and K2) representing the amplitudes and the TEA affinities of the two components, respectively.
Electrophysiological data analyses were performed with the Clampfit subroutine of the pCLAMP software and with Origin 7.0 software (OriginLab Corp., Northampton, MA, USA). Pooled data are expressed as means ± standard error of the mean (s.e.m.). Statistical comparisons between groups of data were carried out with Student's two-tailed t test for paired or unpaired data, and values of P < 0.05 were considered statistically significant.
Ca2+ imaging
Chemoreceptor cells were incubated with 10 μm fura-2 AM (Molecular Probes, Eugene, OR, USA) at room temperature. After 60 min the culture coverslips were mounted in a perfusion chamber placed on the stage of a Nikon Diaphot 300 inverted microscope, and the cells were superfused with a solution containing (mm): 116 NaCl, 5 KCl, 1.1 MgCl2, 2 CaCl2, 25 NaHCO3, 10 glucose, 10 Hepes (pH 7.4 bubbling with 5% CO2–20% O2–75% N2). Temperature was kept at 37°C. Dual-wavelength measurements of fura-2 fluorescence were performed, using the two-way wavelength illumination system DX-1000 (Solamere Technology Group). A 75 W Xe lamp was used as light source (Optiquip). Light was focused and collected through a Nikon Fluor 40/1.30 objective. The wavelength for dye excitation was alternated between 340 and 380 nm, and fluorescence emission at 540 nm was collected with a SensiCam digital Camera (PCO CCD imaging). A 4 × 4 binning was applied to get images of 320 × 256 pixels (12 bits per pixel) at 0.5 Hz for both wavelengths. The illumination system and the camera were driven by Axon Imaging Workbench 4.0 (Axon Instruments) running on a Pentium computer. Calcium concentration was computed offline through the ratio images obtained from the background subtracted F340 and F380 images and calibration parameters measured in selected experiments (after perfusing the cells for 30 min with 0 Ca2+ plus 10 mm EGTA, or with 2 mm Ca2+-containing solutions in the presence of 50 μm ionomycin).
RT-PCR methods
Total RNA was extracted from mice cortex using Trizol (Gibco-BRL). Reverse transcription was carried out using MuLV reverse transcriptase (Applied Biosystems) at 42°C for 60 min. PCR experiments were performed in a thermal cycler (GeneAmp 9700, Perkin Elmer) using thin walled plastic tubes (PE Biosystems).
Unique PCR primers used for amplification of fragments of mouse Kv2.1, 2.2, 3.1, 3.2 and 3.3 were designed from GenBank sequences of these genes, using the Primer 3 website (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). In all cases, primers were designed in intron-spanning regions to overcome potential DNA contamination of the samples. Primer sequences were: mouse Kv2.1 (GenBank accession no. BC031776): AAGGAGCAGATGAACGAGGA (forward) and CGAGGAAGAGGATGAGCAAG (reverse); mouse Kv2.2 (GenBank accession nos XM_136479 and AK036239): AGGAAATGTGTGCGCTCTCT (forward) and AGCCTCTACGTGTGCCAACT (reverse); mouse Kv3.1 (GenBank accession no. NM_008421): GAGGACGAGCTGGAGATGAC (forward) and CAGGGCCAGGAAGATGATAA (reverse); mouse Kv3.2 (GenBank accession no. AK045425): ACCCTGGTGATGATGAGGAC (forward) and AGCACCCTCAGACCTACGAA (reverse); mouse Kv3.3 (GenBank accession no. NM_008422): for the first amplification: GCACGGACGAGTTCTTCTTC (forward) and ACCGTCTTGTTGCTGATGTG (reverse), and for the nested amplification: TGAGGAGGCACTGGACTCTT (forward) and ACCGTCTTGTTGCTGATGTG (reverse). We also designed control primers to detect a housekeeping gene (mouse β-actin, GenBank accession no. NM007393) and the GFP insert of TH–GFP transgenic mouse (green pelican GFP transformation vector, GenBank accession no. AF242362). Primers for β-actin were: ATGCCCACTGCCGCATCCTCTTCC (forward) and CACGATGGAGGGGCCGGACTCATC (reverse), and primers for GFP were: AAGTTCATCTGCACCACCG (forward) and TGCTCAGGTAGTGGTTGTCG (reverse).
When using isolated chemoreceptor cells as the source of RNA, 5–20 GFP-labelled cells were aspirated into the electrode without recording. The holder was either baked for 1 h at 200°C or treated with RNAse ZAP solution (Ambion, Inc., Austin, TX, USA). The electrode was filled with 7 μl of nominally RNAse-free pipette solution and the capillary glass used was baked overnight at 200°C. Sterile gloves were worn during the procedure to minimize RNAse contamination. After aspiration of the cells, the electrode was removed from the holder and its content was ejected into a 0.2 ml Eppendorf tube containing 1 μl of RNAsin (20 u μl−1, Applied Biosystems, Foster City, CA, USA) and kept at −80°C until the RT was performed. For the RT reaction, the contents of each tube were transferred to another 0.2 ml Eppendorf tube containing 1 μl of Random Hexamers (50 μm) and when necessary, diethylpyrocarbonate-treated water (DEPC water) was added to keep the final volume to 9 μl. The tubes were heated to 70°C for 5 min in a thermal cycler (Applied Biosystems) and incubated at 20°C for 10 min. During this time, a master mix containing 2 μl of 10 × PCR buffer, 4 μl of 25 mm MgCl2, 4 μl of mixed dNTPs (10 mm) and 1 μl of MulVRT (5000 μ ml−1) was added to each tube and single-strand cDNA was synthesized in a 1 h incubation at 42°C. The reaction was terminated by heating the mixture at 70°C for 10 min and then icing it. All reagents were obtained from Applied Biosystems. Aliquots (4 μl) of the cDNA obtained in the RT reaction were subjected to one or two rounds of amplification with conventional PCR to detect the expression of the various mRNAs. PCR conditions were: 95°C for 15 min then, 40 cycles of 95°C for 15 s anealing temperature for 20 s and 72°C for 20 s followed by 72°C for 10 min.
Immunofluorescence in CB cells
CB cells plated onto glass coverslips were fixed with 4% paraformaldehyde (PF) in phosphate buffer, pH 7.5, for 15 min at 20°C, washed in PBTx (phosphate-buffered saline (PBS), 0.1% Triton X-100), and blocked with PBTx-10 mg ml−1 BSA-2% normal goat serum for 10 min. Primary antibodies anti-Kv subunits, and other control antibodies, were diluted in blocking solution and incubated with the cells for 30–60 min at room temperature. After washes in PBTx, cells were incubated with secondary antibodies for 30 min. The fluorescently labelled secondary antibodies used were: Alexa 488/597-conjugated goat antirabbit/mouse secondary antibodies (Molecular Probes), and fluorescein isothiocyanate (FITC)-conjugated goat antimouse (Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA), and all of them were used at a final dilution of 1 : 1000. After washes in PBS, the coverslips were mounted with Vectashield H-1000 (Vector Laboratories Inc., Burlingame, CA, USA), and the cells were examined with the appropriate filters for immunofluorescence. The preparations were examined either in a Zeiss Axioscop microscope or in a Bio-Rad confocal microscope (Radiance 2100). When the confocal was not used, images were digitized with a CoolSnap CCD camera (Photometrics, CA, USA) and processed with PaintShop Pro (Jasc Software Inc.)
The anti-Kv antibodies were incubated in control experiments with the corresponding Kv peptide (10 μg ml−1) for 2 h before being added to the cells. Also, control labelling of secondary antibodies were included to discard non-specific labelling.
Rabbit polyclonal anti-Kv antibodies were obtained from Alomone and used at a 1 : 100 dilution and mouse monoclonal anti-TH antibody was purchased from Abcam (Cambridge, UK) and used at a 1: 2000 dilution.
Results
Characterization of mouse CB chemoreceptor cell cultures
As the expression of GFP in peripheral nervous system in TH–GFP mouse has not been previously characterized (Sawamoto et al. 2001), we first explored whether we could see GFP labelled cells in primary cultures of mouse CBs, and the degree of colocalization of TH and GFP (Fig. 1A). In most cases we found GFP labelled cells with morphological features similar to CB chemoreceptor cells from other species, being round, birefringent and often grouped in pairs or in glomeruli-like structures. However, cells with these morphological features were not always GFP positive and conversely, cells with fibroblast-like shapes were also labelled with GFP. Immunofluorescent labelling with TH revealed that all GFP-positive cells expressed TH, while there was in addition a smaller percentage (around 20%) of the cells that were TH positive but did not express GFP. Therefore, GFP labelled cells could be considered with a high degree of confidence as TH-expressing, chemoreceptor cells.
Figure 1. Characterization of CB chemoreceptor cell cultures from TH–GFP mouse.
A, GFP expression, anti-TH antibody labelling and brightfield images of two different CB chemoreceptor cell cultures. All GFP-positive cells in the primary cultures were labelled with the anti-TH antibody, but TH-immunostaining also detected a small number of cells not expressing GFP (arrow in left panel). B, changes in intracellular [Ca2+] in cells loaded with fura-2 are plotted against time. During the intervals indicated with the bottom bars, a N2-equilibrated solution was applied to the cells. GFP-expressing chemoreceptor cells (cells 1–3) showed a reversible increase of [Ca2+] during hypoxia, while this stimulus was without effect in GFP-negative cells (cells 4–6).
The functionality of the mouse CB cultures was assessed by investigating an integrated response, such as the increase of [Ca2+]i in response to hypoxic stimulation. We measured [Ca2+]i as an indicator of cell activation (Fig. 1B). When CB dispersed cells were stimulated with a N2-equilibrated solution, two clearly different patterns of responses were seen correlating with the presence of GFP. As expected, GFP-positive cells were sensitive to hypoxia, while GFP-negative cells were not. To exclude potential artefacts on the fluorometric ratio due to the expression of GFP, the same experiments were performed in wild-type mouse and, after the [Ca2+]i measurement experiments, TH-positive cells were identified by TH immunostaining. The results obtained were identical (data not shown).
Electrophysiological characterization of Kv currents in mouse chemoreceptor cells
Voltage-dependent K+ currents were studied in isolated, GFP-positive mouse CB cells. After establishing the whole-cell configuration, current–voltage (I–V) relationships for K+ currents were obtained every 2 min, applying groups of 200 ms depolarizing steps from −60 mV to +80 mV in 10 mV steps. The holding potential was −80 mV, and the depolarizing pulses were followed by a 100 ms repolarizing step to −40 mV. Figure 2A shows current records obtained in a typical chemoreceptor cell and the corresponding I–V relationship is depicted in Fig. 2B. Depolarization elicited outward currents with an apparent threshold for activation around −30 mV and very little inactivation during the 200 ms depolarizing pulse. The voltage dependence of activation was estimated by plotting the maximal amplitude of the tail currents immediately after repolarization against the potential of the preceding pulse (Fig. 2C, squares). However, when the kinetics of the tail currents was further characterized by fitting their time course to an exponential function, two different components were revealed (see inset in Fig. 2A), with time constants of 7.6 and 26.7 ms, and amplitudes that exhibit a similar voltage dependence (Fig. 2C, circles), although the fast component was clearly predominant, representing 75% of the total current. This analysis suggests the contribution of at least two different populations of channels to the macroscopic outward currents. The deactivation time course was consistently fitted to a bi-exponential function (n = 16), with time constants of 6.24 ± 0.52 ms (τfast) and 32.85 ± 4.14 (τslow). The fast component represented on average a 70 ± 3.4% of the amplitude of the tail currents.
Figure 2. Kv currents in TH–GFP cells from CB cultures.
A, the outward K+ currents elicited in one typical chemoreceptor cell with the voltage protocol depicted in the bottom of the figure. The region marked by the square was enlarged to show the fit of several tail current traces (from test pulses to −10, 0, +10 and +60 mV) to a bi-exponential function. Voltage pulses were applied every 5 s. B, plot of the current–voltage relationship obtained in this cell by measuring the peak current amplitude at each pulse (•). C, plot of the activation curve (▪) and the rapidly (○) and slowly (•) deactivating tail current amplitudes as a function of the voltage of the test pulse for the same cell shown in A. Activation curve was constructed from the peak current amplitude obtained at the beginning of the repolarizing pulse to −40 mV. The lines show the Boltzmann fits of the experimental data. According to the fit, the maximal amplitude of the activation curve in this cell was 206 pA and the midpoint of activation (V½) was 1.6 mV. The time constants of the two components are shown in the figure.
TEA sensitivity of the currents
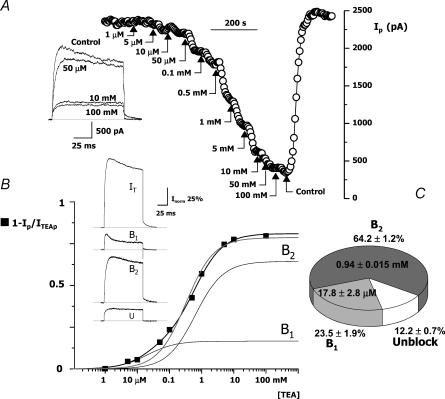
The electrophysiological properties of mouse CB chemoreceptor cells' Kv currents resemble those of Kv2 or some members of the Kv3 subfamily of channels, as described both in heterologous expression systems (Coetzee et al. 1999) and in native tissues (Baranauskas et al. 1999). A key pharmacological feature that allows discrimination between Kv2 and Kv3 currents is their sensitivity to block by external TEA. While Kv3 channels have been reported to be sensitive to TEA in the micromolar range, Kv2 subunits have a much lower affinity for TEA, with IC50 in the millimolar range (Coetzee et al. 1999). We tested the effect of TEA on the native CB chemoreceptor cells by analysing the reduction in the peak current amplitude during depolarizing pulses to +40 mV in the presence of increasing doses of TEA in the bath solution. A typical experiment is represented in Fig. 3A. TEA inhibition of the amplitude of the currents could be seen with concentrations as low as 5 μm, although increasing TEA up to 100 mm did not block completely outward currents (Fig. 3A). When TEA block was normalized to values between 0 (no block) and 1 (complete block of the currents), the resulting data was best fitted assuming two binding sites for TEA, with affinity constants of 20 μm (B1) and 0.63 mm (B2). The relative amplitude of these two components in the example shown was 17% and 64%, respectively. In addition to these two, there is a non-inactivating component that was unaffected by high concentrations of TEA, representing in this cell 19% of the total current. In spite of the difference in the affinity constants, a complete dissection of these components from the whole current can only be approximated. The subtracted traces depicted in Fig. 3B were computed from averaged traces obtained in control, under 50 μm and 100 mm TEA. However, it should be pointed out that in this cell 50 μm TEA should only block ∼70% of B1 and should also block ∼8% of B2.
Figure 3. Effect of TEA on the outward K+ currents of CB chemoreceptor cells.
A, peak current amplitude obtained in depolarizing pulses to +40 mV applied every 5 s in a cell while increasing concentrations of TEA were applied in the bath solution as indicated. The inset shows four representative traces obtained at the beginning of the experiment (control) and in the presence of 50 μm, 10 mm and 100 mm TEA. The figure also illustrates the full recovery of the current amplitude after TEA removal. B, TEA dose–response curve obtained with data from the same cell (▪) were fitted to a one-binding-site model (grey line) or a two-binding-site model (black line). The data were best fitted with the two-binding-site model. The functions representing the two components are also shown in the figure (B1 and B2). The inset shows the traces obtained in control conditions (IT) and the subtracted traces corresponding to the high (B1 = IT − ITEA50 μM) and low (B2 = ITEA50 μM−ITEA100 mM) TEA sensitivity and the unblock (U = ITEA100mM) fraction of the currents. C, the pie chart shows the average normalized distribution of the three components with the mean IC50 obtained from seven cells.
Figure 3C shows pooled data obtained from seven cells in which the full dose–response curve to TEA was studied. In all the cases, the best fit to the data was obtained with the two-binding-site model, with affinity constants that averaged 17.81 ± 2.8 and 940 ± 14.7 μm. The low-affinity component was the most abundant, representing 64.25 ± 1.2%, while the high-affinity component was 23.48 ± 1.9% of the current. The TEA-resistant fraction averaged 12.2 ± 0.7%. This pharmacological profile provided further support for our initial hypothesis regarding the expression of different Kv channel populations in mouse CB chemoreceptors.
Effect of hypoxia on Kv currents
As the presence in CB chemoreceptors of O2-modulated K+ currents is well establish in other species (see Introduction), we have tested the effect of hypoxia on the Kv currents of mouse chemoreceptor cells. We found that superfusion of the cells with a N2-equilibrated solution produced a reversible decrease in the amplitude of the currents at all voltages above the activation threshold. Representative traces elicited at four different test potentials in control conditions (C), during hypoxia (H), and after returning to normoxia (R), as well as the full I–V curve in these three conditions, are shown in Fig. 4. Hypoxic-sensitive currents, calculated by subtracting C and H traces, are shown in grey (d). Hypoxic inhibition of the currents was observed in 21 out of 23 cell tested, and the magnitude of this inhibition at +40 mV was 17.07 ± 1.2% (P < 0.001; Fig. 4, lower panel). In 14 of these cells the effect of hypoxia was explored over the whole range of voltages and on the activation curve by applying the protocol described before. Figure 5A shows the average activation curves in normoxia and hypoxia, fitted to a Boltzmann function.
Figure 4. Effect of hypoxia on Kv currents from mouse CB chemoreceptor cells.
In the left part of the figure, the traces obtained in one representative cells at the indicated voltages are shown in control conditions (C), during application of a N2-equilibrated solution (H), and after returning to the control, normoxic solution (R). The grey traces (d) show the subtraction of the hypoxia-inhibited currents. The current–voltage relationship obtained in this cell in the three situations, and the fraction of the current sensitive to hypoxia are also represented in the figure. The bar plot shows the mean effect of hypoxia as the percentage reduction in the peak current amplitude in depolarizing steps to +40 mV (black bar) and the current amplitude after washout (open bar). Control amplitude value in each individual cell was taken as 100%. The data are means ± s.e.m. of 21 cells. ***P < 0.001 as compared to control values.
Figure 5. Effect of hypoxia on Gmax and deactivation kinetics.
A, hypoxic inhibition of Kv currents is due to a decrease of Gmax. Average conductance curve obtained from pooled data of 14 cells in control (▪) and hypoxic conditions (□). The mean values of Gmax and V½ for control and hypoxia shown in the bar plots on the right were obtained from the individual fit of the conductance–voltage relationship of each cell to a Boltzmann function. ***P < 0.001 as compared to the control value. B, hypoxia affects selectively the fast deactivating component of the Kv currents. Representative tail currents obtained in repolarizing steps from +60 mV to −40 mV in control and during hypoxia application and their corresponding fits. Fit of control traces is shown by continuous line and fit of hypoxic traces by dashed lines. The bar plots on the right represent the average amplitude of the two components (Aslow and Afast) and the mean time constants (τslow and τfast) in the two conditions, control (filled bars) and hypoxia (empty bars). The data are means ± s.e.m. of 7 cells. **P < 0.01.
The mean values for maximal conductance (Gmax) and midpoint of activation (V½) from 14 CB cells studied with this protocol were 4.75 ± 0.47 nS and 4.2 ± 2.2 mV in control and 3.83 ± 0.44 nS and 6.66 ± 2.6 mV in hypoxic conditions. Low PO2 inhibition leads to a significant decrease in the maximal conductance without changing significantly the midpoint for activation. Kinetics of deactivation was also changed during the application of the hypoxic stimulus (Fig. 5B), and the analysis of the fast and slow components of the tail current revealed a differential sensitivity to hypoxia. As shown in the bar plots, hypoxia produced a 30% reduction in the amplitude of the fast component (P < 0.01) without changing the amplitude of the slow component. The time constants of these two components were not significantly modified by low PO2.
As these data pointed towards a differential effect of hypoxia in one population of channels, we further explored this possibility, studying the effect of hypoxia in the presence of low (μm) concentrations of TEA, enough to block the high-affinity component without affecting significantly the low-affinity one. The experimental protocol used is shown in Fig. 6. Whole-cell K+ currents were elicited by pulses to +40 mV applied every 5 s, and three hypoxic applications were performed, the second one in the presence of 10 μm TEA. Hypoxia alone produced a 20% decrease in the peak current amplitude in this cell. TEA application produced a larger inhibition (33%) and abolished the effect of hypoxia. The same lack of effect of hypoxia was observed in six similar experiments, in which concentrations of TEA between 10 and 50 μm were applied together with the N2-equilibrated solutions. The TEA concentration chosen in each experiment was previously found to inhibit most of the high-affinity component of the cell K+ current without modifying in a substantial way the low-sensitive component. These data indicate that hypoxia specifically inhibits the low-affinity TEA component of the K+ current.
Figure 6. Effect of hypoxia on Kv currents in the presence of TEA.
The graph shows the peak current amplitude in depolarizing pulses to +40 mV applied every 5 s. In this cell, hypoxic exposure was applied three times, during the indicated periods. The second hypoxic application was performed while the cell was superfused with a solution containing 10 μm TEA as indicated in the graph. Selected traces during the experiment (labelled with numbers 1–9), and the subtracted hypoxia-sensitive fractions (labelled d) are shown in the upper part of the figure. The right panel shows a bar plot with the average results obtained from six cells in which the same protocol was applied. In these cells, hypoxia alone produced a reduction in the peak current amplitude to an 86% of the initial value (white bar), but no effect of hypoxia was seen in the presence of TEA (grey bar), indicating that the hypoxia-sensitive portion of the current is blocked with this low TEA concentrations. **P < 0.01.
Molecular identification of Kv current constituents
The functional analysis of the Kv currents of mouse CB cells suggests the expression in these cells of Kv channels from the Kv2 and Kv3 subfamilies. We tested the presence of each of these Kv transcript in mouse CB chemoreceptor cells using RT-PCR and unique primers designed from mouse sequences. As all these transcripts have been found in CNS, we set the optimal PCR conditions for each pair of primers in RT-PCR reactions using mouse cortex as the RNA source.
Figure 7 shows the results of these amplifications. The amplification of a fragment of mouse β-actin was used as a control of the RT reaction from GFP cells and the positive control (C+) shows the amplification of each Kv channel from mouse cortex template. The rest of lanes in the ethidium bromide gels show the amplification of each Kv transcript from TH–GFP cells obtained from primary cultures. From these results, the transcription of Kv2.2, 3.1, 3.2 and 3.3 in mouse CB chemoreceptor cells is apparent. However, no Kv2.1 transcripts were detected in CB, although in the same samples we did obtain amplification of other transcripts (see M23 in Fig. 7).
Figure 7. Amplification products for Kv subunits obtained from GFP-positive cells' mRNA.
Transcript detection of the different Kv subunits was performed in RT products from pooled GFP-positive cells from mouse CB cultures. Positive control lanes (C+) show the expression of the corresponding Kv in mouse cortex. The M-number labels indicate the GFP-expressing cells' pool number used for each amplification. In the cases in which Kv transcripts were not detected in the cells (i.e. Kv2.1), control amplifications to demonstrate the detection of an ubiquitous transcript (β-actin) were performed. Amplifications were generally done with 3 mm Mg2+, except for Kv2.2, which was done with 1.5 mm Mg2+. The expected size of the PCR products was 595 bp (Kv2.1), 482 bp (β-actin), 359 bp (Kv2.2), 627 bp (Kv3.1), 575 bp (Kv3.2) and 378 bp (Kv3.3). The marks on the right of each gel correspond to molecular weights of 300, 500, 700 and 1000 bp as shown in the last image.
In order to further demonstrate the presence of particular Kv subunits in chemoreceptor cells, we analysed the cellular distribution of these channel subunits at the protein level with immunocytochemistry. Commercially available antibodies (anti-Kv2.1 and anti-Kv3.1–3.4) were used. First, we tested cross-reaction of these antibodies in mouse tissues, by comparing the labelling in isolated cells from rat or mouse SCG, where their presence has been reported. Co-localization of Kv channels and TH within the same cells was observed both in rat SCG sympathetic neurones with double immunocytochemistry and in TH–GFP mouse SCG cells stained with each of the anti-Kv used in the study. Specificity of the labelling was confirmed by preincubation with the control peptide (data not shown). After these control experiments, we studied the expression of these channel proteins in CB chemoreceptor cell cultures obtained from either TH–GFP or wild-type mice. In this latter case, we performed double-immunocytochemistry with each of the anti-Kv antibodies, and the anti-TH antibody as a marker for chemoreceptor cells. Kv3.2 was predominately present in chemoreceptor cells and was also seen in a small number of TH-negative cells (Fig. 8A). The same pattern of distribution also holds true for Kv3.1, which was present in most chemoreceptor cells and was also expressed by TH-negative cells. On the contrary, and in agreement with our RT-PCR results from chemoreceptor cells, most Kv2.1 expression was detected in non-chemoreceptor cells, although occasionally we found coexpression of Kv2.1 and GFP (see arrow in Fig. 8A). Finally, the possibility that Kv3 channels can be heterotetramers composed of fast-inactivating (Kv3.3 and Kv3.4) and non-inactivating (Kv3.1 and Kv3.2) subfamily members led us to look for the expression of Kv3.3 and Kv3.4 in our cultures (Fig. 8B and C). We found that Kv3.4 was expressed exclusively in TH-negative cells. Although in short-term cultures many Kv3.4 labelled cells were morphologically indistinguishable from TH-expressing cells, with longer time in cultures Kv3.4-positive cells show the morphological aspect of smooth muscle cells. The merged panels in the figure show the absence of colocalization of GFP and Kv3.4. With respect to Kv3.3, we found expression in almost all the cells in our culture, and as shown in the merged panels in this case all chemoreceptor cells expressed Kv3.3 protein. In view of this ubiquitous labelling, we confirm the specificity of the anti-Kv3.3 by preincubation of the cells with the blocking peptide (Fig. 8C, lower panels).
Figure 8. Immunofluorescence labelling in mouse CB chemoreceptor cells.
A, the presence of Kv3.2 and Kv3.1b in TH-expressing cells was shown both by immunofluorescence labelling of TH–GFP cells and by double labelling with TH and the corresponding anti-Kv antibody. On the contrary, Kv2.1 labelling was mainly found in GFP-negative cells, although colocalization could occasionally be detected (see arrow in the lower panels). B, Kv3.4 is not present in GFP-positive cells; there is no overlap of green and red fluorochromes in the merged panels. The change in the morphology of the Kv3.4-positive cells is evidenced by comparing a 1-day culture (upper panel) and a 5-day culture (lower panel). C, immunofluorescence labelling of Kv3.3 in mouse CB chemoreceptor cells shows the expression of Kv3.3 in every GFP-positive cell (yellow in the merged panels). The specificity of the Kv3.3 ubiquitous labelling was confirmed by the loss of Kv3.3 tagging in cells preincubated with the control peptide (CP). In this case, there is no colour change in the merged panel compared to the TH/GFP panel.
Discussion
In this work we have successfully set up a preparation of mouse CB dispersed chemoreceptor cells suitable for electrophysiological and molecular characterization. The data presented here constitute the first electrophysiological and pharmacological characterization of Kv currents in mouse chemoreceptor cells, and point to Kv3 channels as the molecular correlate of the O2-sensitive voltage-dependent K+ currents in this preparation. Consistently with the functional studies, the use of RT-PCR and immunocytochemistry confirms the expression in chemoreceptor cells of several members of the Kv2 and Kv3 subfamilies.
The use of the TH–GFP transgenic mouse strain, which allows the identification of chemoreceptor cells, overcomes several problems associated with the scarcity of material in this preparation. The small size of the organ forced us to dissect out the CB together with a segment of carotid artery, so that, apart from the intrinsic cell heterogeneity of the CB, in our primary cultures there was a significant amount of endothelial and smooth muscle cells. As these vascular smooth cells are also excitable cells endowed with voltage-dependent channels, they would represent a significant source of contaminating results in the absence of a chemoreceptor cell marker. To avoid false-positive results in the identification of CB chemoreceptor cells, special care was taken during dissociation to separate and discard superior cervical ganglion (SCG), which would be a contaminating source of TH-expressing cells and hence of GFP-positive cells. However, SCG is a clearly delimited structure that was easily dissected out of the carotid artery bifurcation, and besides, when we obtained SCG cultures from TH–GFP mice, the GFP-positive cells had the morphology of sympathetic neurones, unmistakable both in shape and in size from chemoreceptor cells (data not shown). A similar approach (cell-specific GFP labelling) has been recently described for the electrophysiological characterization of voltage-dependent currents in defined taste cells of mice, by using a transgenic mouse expressing GFP under the control of the gusducin promoter (Medler et al. 2003). As an additional control, we have checked that the expression of GFP in TH-expressing cells did not modify the functional properties of the cultures (see above), by duplicating some of the experiments in wild-type mouse. For these latter experiments, we have chosen the same mouse strain as the TH–GFP mice (C57BL/6J) as differences in the size and morphology of the CB (and also in the ventilatory responses) have been described among different strains (Yamaguchi et al. 2003). In fact, we have observed important differences in shape and location of CBs between C57BL/6J and BALB/c strains, although no further studies have been carried out.
Electrophysiological analysis of mouse CB GFP-positive cells showed a relatively high density of voltage-gated outward currents (512 ± 47 pA pF−1 at +40 mV, n = 21). The characterization of the deactivation kinetics of these currents suggests the presence of at least two components in the outward K+ current (Figs 2 and 3), one with a faster deactivation, representing around 70% of the total tail amplitude and one slower that represents the remaining 30%. More clear evidence of the presence of more than one population of Kv channels can be obtained by studying TEA sensitivity of the voltage-dependent currents. It is evident from the data in Fig. 3 that there is a poor fit of the data to a single binding-site function; on the contrary, the dose–response curve to TEA is best fitted with a two-binding-site model. Altogether, the electrophysiological properties and TEA sensitivity profile of the currents are consonant with the contribution to the mouse CB K+ currents of two different populations of channels, probably belonging to the Kv2 and Kv3 subfamily members.
Kv3 genes expressed in heterologous expression systems give rise to K+ currents with some conspicuous features: the currents become apparent with membrane depolarization more positive than −20 mV, have a fast rate of deactivation upon repolarization and are blocked by TEA at micromolar concentrations (Coetzee et al. 1999; Rudy & McBain, 2001). The degree of inactivation depends on the specific subunit composition, ranging from non-inactivating channels (Kv3.1–Kv3.2 combinations) to fast inactivating ones (Kv3.4 homotetramers). The presence of a single fast inactivating subunit (Kv3.3 or Kv3.4) in a heteromultimeric channel is sufficient to impart mild inactivation properties to the channel complex (Rudy & McBain, 2001). Currents with properties remarkably similar to those mediated by Kv3 channels in heterologous systems have been recorded in neuronal populations that express Kv3 subunits (Baranauskas et al. 1999; Chow et al. 1999; Matsukawa et al. 2003). Our data in mouse CB cells makes very plausible the hypothesis that the molecular correlate of the high-TEA-sensitive current could be heterotetramers composed of Kv3.3 subunits with Kv3.1 and/or Kv3.2.
The other component of the current is a delayed-rectifier, non-inactivating, K+ channel that is sensitive to TEA in the low millimolar range. These properties are compatible with those described for Kv2 channels and for some Kv1 subfamily members (i.e. Kv1.2, 1.3 or 1.6) in heterologous systems. However, while the threshold for activation of Kv2 channels (Von, −20 to −30 mV) and the midpoint of activation (V½∼10 mV) are similar to those observed in mouse CB cells, Kv1 current channels have a more negative onset, between −40 and −60 mV, and show more hyperpolarized V½ values (between −30 and −5 mV; Coetzee et al. 1999). Although we cannot exclude the contribution to the macroscopic currents of chemoreceptor cells of some Kv1 channels, whose kinetic properties could be modified by heteromultimerization and/or association with accessory subunits (Song, 2002), our functional studies strongly suggest the presence of Kv2 channels in our preparation, a point that was confirmed by the molecular characterization showing the expression of Kv2.2 in GFP-expressing CB cells. Delayed rectifier currents expressed by Kv2 channels are ubiquitously found in excitable cells (Brahmajothi et al. 1996; Baranauskas et al. 1999; Malin & Nerbonne, 2002), where they show properties that resemble those of recombinant channels, although some differences in biophysical properties were noticed.
The data from immunocytochemistry and PCR studies confirm the presence of several of the postulated channel subunits contributing to voltage-dependent K+ currents of mouse CB chemoreceptor cells, including three members of the Kv3 subfamily (Kv3.1, 3.2 and 3.3) and one member of the Kv2 subfamily, Kv2.2. Excluding Kv2.2, for which there is no antibody available, we have demonstrated the Kv expression both at the mRNA and protein levels. No Kv3.4 protein expression was found in chemoreceptor cells (Fig. 8), but this subunit was abundantly expressed in vascular myocytes in our cultures. Regarding Kv2.1, we did not detect mRNA expression in PCR performed from GFP-expressing cells, although antiKv2.1 antibody identified a small (20%) population of chemoreceptor cells. The PCR data were obtained from pooled GFP-positive cells, so that we cannot extract any conclusions regarding cellular distribution of the mRNAs studied. However, if we look at the protein expression pattern determined by the immunocytochemical data, with the exception of the Kv3.3 subunit, which seems to be present in every chemoreceptor cell, the Kv subunits do not show a homogeneous distribution: Kv3.1 was found in 83% and Kv3.2 was present in 85% of TH-positive cells. Also, none of the channels studied was exclusively found in chemoreceptor cells, although Kv3.2 subunits seemed to be the most specific marker (70% of the Kv3.2-labelled cells were GFP positive, while only 50% of the Kv3.1- and 35% of the Kv3.3-expressing cells were also GFP positive). This heterogeneity in the distribution of ion channels has been reported in many other preparations (Ribera, 1996; Schultz et al. 2001; Malin & Nerbonne, 2002), including CB from other species (Pérez-García et al. 1992; Sanchez et al. 2002) and may reflect a mechanism for regulating cellular excitability in a cell-type-specific manner.
Similarly to what happens in CB chemoreceptor cells from different species (López-Barneo et al. 1988, 1997; Wyatt & Peers, 1995), hypoxia was able to reversibly decrease the amplitude of voltage-dependent outward K+ currents in the mouse CB cells. The observed reduction in the maximal conductance of the current accounted for the hypoxic inhibition, and the lack of modification of the V½ excludes that the effect of hypoxia could be an artefact due to a rightward shift in the current–voltage relationship (Figs 4 and 5).
Analysis of the effect of hypoxia on the tail currents and in the presence of low concentrations of TEA suggests that hypoxia inhibits specifically the fast deactivating and high-affinity TEA components of the voltage-dependent K+ currents. As discussed above, those properties make highly plausible the hypothesis that those components could be mediated by the members of the Kv3 family and therefore it seems reasonable to speculate that Kv3 channels are the O2-sensitive, voltage-dependent K+ channels in the mice.
As mentioned in the introduction, a great number of Kv channels have been described as being modulated by hypoxia both in chemosensory tissues and in heterologous expression systems. Among these, Kv3 channels have been postulated as the O2-sensitive K+ channels in several studies. Wang and coworkers have shown evidence for the presence in mouse pulmonary neuroepithelial bodies of an O2 sensing mechanism consisting of an O2 sensor protein complex (NADPH oxidase) coupled to O2-sensitive K+ channel. By using in situ hybridization and Northern blot analysis they localize and identify mRNAs encoding the different components of NADPH oxidase and the H2O2-sensitive K+ channel Kv3.3 (Wang et al. 1996). More recently, other authors (Osipenko et al. 2000) showed that hypoxia inhibited Kv3.1b channels expressed in L929 cells, and also a K+ current in rabbit pulmonary artery smooth muscle (PASM) cells with functional properties resembling those of Kv3.1 channels. They demonstrated Kv3.1b expression in PASM cells and the lack of effect of hypoxia in the presence of TEA concentrations that abolish Kv3.1b current while preserving other delayed rectifier currents. However, a clear demonstration of the role of Kv3 channels in these preparations (and consequently of the outcome of its hypoxic inhibition) has not been provided, and the same considerations apply to the present work. Kv3 genes are mainly thought to enable rapid spiking in the central nervous system by limiting action potential duration and refractory period (Massengill et al. 1997; Martina et al. 1998; Rudy & McBain, 2001). However, Kv3 channels are also found in non-fast-spiking neurones and other cells types (Chow et al. 1999; Betancourt & Colom, 2000) suggesting that they perform functions other than sustain rapid firing, in spite of their perfect adaptation to this task. As their voltage threshold for activation is outside the range of membrane potentials encountered in resting excitable cells, inhibition of voltage-dependent K+ channels by hypoxia is unlikely to cause membrane depolarization; functional effects of hypoxia on these channels would become apparent only if cells were already depolarized. Nevertheless, the inhibition of Kv channel activity may enable depolarization to be maintained during hypoxia, amplifying the primary response. Further studies directed to answer this question by exploring the effect of hypoxia on resting membrane potential as well as the presence and possible O2 modulation of other families of K+ channels (and their accessory subunits) will allow the determination of the molecules involved in the hypoxic transduction cascade in this preparation. In addition, this characterization will benefit from the possibility of using transgenic mice with deletion of some of the identified channels subunits (Lau et al. 2000; Matsukawa et al. 2003) as a tool to understand the contribution of these channels to excitability of the cells.
Acknowledgments
We thank Esperanza Alonso for excellent technical assistance and Drs M. D. Ganfornina and D. Sanchez for advice with the maintenance and growth of the TH–GFP mice colony and for critical reading of the manuscript. We thank Dr Okano for the gift of TH–GFP strain. This work was supported by Instituto de Salud Carlos III Grant G03/045 and DGICYT Grants BFI2001-1691 and BFI2001- 1713 and by an FISS Grant to the Red Respira-SEPAR. O.C. is a fellow of the Spanish MCYT and E.M. is a postdoctoral fellow of the Spanish FISss.
References
- Baranauskas G, Tkatch T, Surmeier DJ. Delayed rectifier currents in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1/3.2 K+ channels. J Neurosci. 1999;19:6394–6404. doi: 10.1523/JNEUROSCI.19-15-06394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt L, Colom LV. Potassium (K+) channel expression in basal forebrain cholinergic neurons. J Neurosci Res. 2000;61:646–651. doi: 10.1002/1097-4547(20000915)61:6<646::AID-JNR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Brahmajothi MV, Morales MJ, Liu S, Rasmusson RL, Campbell DL, Strauss HC. In situ hybridization reveals extensive diversity of K+ channel mRNA in isolated ferret cardiac myocytes. Circ Res. 1996;78:1083–1089. doi: 10.1161/01.res.78.6.1083. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. Effects of hypoxia on resting (leak) potassium conductance in carotid body type-I cells of the neonatal rat. J Physiol. 1995;489P:56. [Google Scholar]
- Chou CL, Shirahata M. Two types of voltage-gated K channels in carotid body cells of adult cats. Brain Res. 1996;742:34–42. doi: 10.1016/s0006-8993(96)00987-0. [DOI] [PubMed] [Google Scholar]
- Chow A, Erisir A, Farb C, Nadal MS, Ozaita A, Lau D, Welker E, Rudy B. K+ channel expression distinguishes subpopulations of parvalbumin- and somatostatin-containing neocortical interneurons. J Neurosci. 1999;19:9332–9345. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz dM, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Conforti L, Bodi I, Nisbet JW, Millhorn DE. O2-sensitive K+ channels: role of the Kv1.2-subunit in mediating the hypoxic response. J Physiol. 2000;524:783–793. doi: 10.1111/j.1469-7793.2000.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high-resolution current recordings from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartness ME, Lewis A, Searle GJ, O'Kelly I, Peers C, Kemp PJ. Combined antisense and pharmacological approaches implicate hTASK as an airway O2 sensing K+ channel. J Biol Chem. 2001;276:26499–26508. doi: 10.1074/jbc.M010357200. [DOI] [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Sanders K, Sundar K, Hoidal J, Fidone S. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am J Physiol. 2002;282:C27–C33. doi: 10.1152/ajpcell.2002.282.1.C27. [DOI] [PubMed] [Google Scholar]
- Lau D, de Miera EV-S, Contreras D, Ozaita A, Harvey M, Chow A, Noebels JL, Paylor R, Morgan JI, Leonard CS, Rudy B. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–9085. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J, López-López JR, Ureña J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez JR, De Luis DA, Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J Physiol. 1993;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López JR, Gonzalez C, Pérez-García MT. Properties of ionic currents from isolated adult rat carotid body chemoreceptor cells: effect of hypoxia. J Physiol. 1997;499:429–441. doi: 10.1113/jphysiol.1997.sp021939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Delayed rectifier K+ currents, IK, are encoded by Kv2 alpha-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci. 2002;22:10094–10105. doi: 10.1523/JNEUROSCI.22-23-10094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandegar M, Yuan JX. Role of K+ channels in pulmonary hypertension. Vasc Pharmacol. 2002;38:25–33. doi: 10.1016/s1537-1891(02)00123-4. [DOI] [PubMed] [Google Scholar]
- Martina M, Schultz JH, Ehmke H, Monyer H, Jonas P. Functional and molecular differences between voltage-gated K+ channels of fast-spiking interneurons and pyramidal neurons of rat hippocampus. J Neurosci. 1998;18:8111–8125. doi: 10.1523/JNEUROSCI.18-20-08111.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massengill JL, Smith MA, Son DI, O'Dowd DK. Differential expression of K4-AP currents and Kv3.1 potassium channel transcripts in cortical neurons that develop distinct firing phenotypes. J Neurosci. 1997;17:3136–3147. doi: 10.1523/JNEUROSCI.17-09-03136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa H, Wolf AM, Matsushita S, Joho RH, Knopfel T. Motor dysfunction and altered synaptic transmission at the parallel fiber-Purkinje cell synapse in mice lacking potassium channels Kv3.1 and Kv3.3. J Neurosci. 2003;23:7677–7684. doi: 10.1523/JNEUROSCI.23-20-07677.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipenko ON, Tate RJ, Gurney AM. Potential role for kv3.1b channels as oxygen sensors. Circ Res. 2000;86:534–540. doi: 10.1161/01.res.86.5.534. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Molecular physiology of oxygen-sensitive potassium channels. Eur Respir J. 2001;18:221–227. doi: 10.1183/09031936.01.00204001. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honore E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J. 1997;16:6615–6625. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, Kemp P. Acute oxygen sensing: diverse but convergent mechanisms in airway and arterial chemoreceptors. Respres. 2001;2:145–149. doi: 10.1186/rr51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García MT, Obeso A, López-López JR, Herreros B, Gonzalez C. Characterization of cultured chemoreceptor cells dissociated from adult rabbit carotid body. Am J Physiol. 1992;263:C1152–C1159. doi: 10.1152/ajpcell.1992.263.6.C1152. [DOI] [PubMed] [Google Scholar]
- Ribera AB. Homogeneous development of electrical excitability via heterogeneous ion channel expression. J Neurosci. 1996;16:1123–1130. doi: 10.1523/JNEUROSCI.16-03-01123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Lopez-Lopez JR, Perez-Garcia MT, Sanz-Alfayate G, Obeso A, Ganfornina MD, Gonzalez C. Molecular identification of Kvalpha subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid body. J Physiol. 2002;542:369–382. doi: 10.1113/jphysiol.2002.018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JH, Volk T, Ehmke H. Heterogeneity of Kv2.1 mRNA expression and delayed rectifier current in single isolated myocytes from rat left ventricle. Circ Res. 2001;88:483–490. doi: 10.1161/01.res.88.5.483. [DOI] [PubMed] [Google Scholar]
- Song WJ. Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res. 2002;42:7–14. doi: 10.1016/s0168-0102(01)00305-4. [DOI] [PubMed] [Google Scholar]
- Wang D, Youngson C, Wong V, Yeger H, Dinauer MC, Vega-Saenz ME, Rudy B, Cutz E. NADPH-oxidase and a hydrogen peroxide-sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell lung carcinoma cell lines. Proc Natl Acad Sci U S A. 1996;93:13182–13187. doi: 10.1073/pnas.93.23.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995;483:559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Balbir A, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O'Donnell CP, Shirahata M. Structural and functional differences of the carotid body between DBA/2J and A/J strains of mice. J Appl Physiol. 2003;94:1536–1542. doi: 10.1152/japplphysiol.00739.2002. [DOI] [PubMed] [Google Scholar]