Abstract
Tryptophan decarboxylase (TDC) is a cytosolic enzyme that catalyzes an early step of the terpenoid indole alkaloid biosynthetic pathway by decarboxylation of l-tryptophan to produce the protoalkaloid tryptamine. In the present study, recombinant TDC was targeted to the chloroplast, cytosol, and endoplasmic reticulum (ER) of tobacco (Nicotiana tabacum) plants to evaluate the effects of subcellular compartmentation on the accumulation of functional enzyme and its corresponding enzymatic product. TDC accumulation and in vivo function was significantly affected by the subcellular localization. Immunoblot analysis demonstrated that chloroplast-targeted TDC had improved accumulation and/or stability when compared with the cytosolic enzyme. Because ER-targeted TDC was not detectable by immunoblot analysis and tryptamine levels found in transient expression studies and in transgenic plants were low, it was concluded that the recombinant TDC was most likely unstable if ER retained. Targeting TDC to the chloroplast stroma resulted in the highest accumulation level of tryptamine so far reported in the literature for studies on heterologous TDC expression in tobacco. However, plants accumulating high levels of functional TDC in the chloroplast developed a lesion-mimic phenotype that was probably triggered by the relatively high accumulation of tryptamine in this compartment. We demonstrate that subcellular targeting may provide a useful strategy for enhancing accumulation and/or stability of enzymes involved in secondary metabolism and to divert metabolic flux toward desired end products. However, metabolic engineering of plants is a very demanding task because unexpected, and possibly unwanted, effects may be observed on plant metabolism and/or phenotype.
Plants produce large arrays of chemicals, many of which are referred to as secondary metabolites. Despite the minor role initially assigned to these molecules, secondary metabolites are now considered crucial for the interaction of plants with their environment (Verpoorte, 1998). Many secondary metabolites are also important therapeutic agents or pharmaceuticals, and the generally low abundance to which they accumulate has prompted extensive research into their biosynthetic pathways. To date, few plant secondary metabolic pathways have been fully characterized, and some have been partially characterized, although investigation into many is at an early stage. Nevertheless, it is clear that the biosynthesis of secondary metabolites is under strict developmental, temporal, and spatial control in plants (St. Pierre et al., 1999; De Luca and St. Pierre, 2000). As a consequence of the strictly regulated biosynthesis, natural products accumulate to only trace amounts in plants, and their extraction and purification is often difficult and cost intensive. Attempts to use plant cell cultures as an alternative source to natural products have also been problematic, often due to the lack of fully functional pathways required for the production of the target molecules. With the exception of the antibacterial, anti-inflammatory compound shikonin and the anticancer agent paclitaxel, no other secondary metabolites have so far been successfully produced on an industrial scale in plant cell cultures (Verpoorte et al., 2000).
Terpenoid indole alkaloids (TIAs) are a large group of secondary metabolites containing several therapeutically effective substances such as the anticancer agents vinblastine and vincristine produced by the Madagascar periwinkle (Catharanthus roseus). An early step in the biosynthesis of TIAs is catalyzed by Trp decarboxylase (TDC; EC 4.1.1.28), which, due to its position at the interface of primary and secondary metabolism, is one of the key enzymes that regulate TIA biosynthesis (Goddijn et al., 1993). TDC mediates the decarboxylation of l-Trp to produce tryptamine, a common precursor of several TIA species. As a result of research efforts from several laboratories, TDC is well characterized at the molecular and biochemical level, and cDNA and genomic clones are available, the latter allowing studies on the dissection of the cis-regulatory elements to be carried out (for review, see Facchini et al., 2000). TDC has been used in transgenic expression studies to create artificial metabolic sinks to determine the effects on the balance of metabolic flux down the branches from shikimate to aromatic amino acids other than Trp (Yao et al., 1995) or divert metabolic flux to promote the in vivo biosynthesis of novel substrates or products (Berlin et al., 1993; Chavadej et al., 1994). In addition, because the toxic compound 4-methyl-l-Trp is metabolized by TDC as an alternative substrate to l-Trp, TDC was proposed as a novel biochemical selectable marker (Goddijn et al., 1993).
With the continual elucidation of pathways, the cloning of genes encoding enzymes of secondary metabolism and, more recently, genes encoding transcriptional regulators of natural product biosynthesis, together with the development of appropriate transformation systems, different strategies are available for manipulating metabolic flux toward natural products of interest. In addition to the overexpression and antisense strategies, targeting enzymes to appropriate subcellular compartments may be envisaged as an alternative or complimentary strategy for increasing accumulation of specific products from proposed rate-limiting steps of a pathway by bringing together enzyme and substrate in the same compartment. The utility of such a strategy has been illustrated from reports studying amino acid biosynthesis in plants. For example, the biosynthesis of the essential amino acids Thr and Lys was significantly enhanced by targeting feedback-insensitive Asp kinase (Galili et al., 2000) or dihydropicolinic acid synthase (Falco et al., 1995) to the chloroplast, the site where the Asp family pathway is located and large amounts of precursors are available. Prompted by the successful results obtained in these studies, we investigated the effects of subcellular TDC localization on protein accumulation and enzyme activity toward determining whether targeting strategies may be useful when applied to enzymes of natural product biosynthesis. In particular, we wanted to examine the effects of localizing TDC to the chloroplast, the site of biosynthesis of the enzyme's natural substrate, l-Trp (Radwanski and Last, 1995). We also studied the effects of targeting TDC to the endoplasmic reticulum (ER) because it has been demonstrated that targeting proteins to the ER (Iturriaga et al., 1989; Wandelt et al., 1992; Boevink et al., 1996) significantly enhances accumulation of the respective recombinant protein in plant cells (Fiedler et al., 1997; Gomord et al., 1997; Fischer et al., 1999).
TDC is a cytosolic enzyme in TIA-producing plants such as C. roseus (De Luca and Cutler, 1987; Stevens et al., 1993), and ideally, studies on localization of TDC should be carried out using transgenic material from the plant expressing TDC as part of the pathway of interest; in our case, TIA biosynthesis in C. roseus. However, although transgenic undifferentiated C. roseus cell cultures can be recovered with relative ease, TIA biosynthesis is severely impeded in such cultures. Furthermore, a whole plant transformation system for C. roseus is not yet available. Thus, for the present studies, tobacco (Nicotiana tabacum) was chosen as the experimental system. As well as presenting a facile transformation system allowing the recovery of large numbers of transgenic plants, tobacco lacks the tdc gene and the downstream enzymes to further metabolize tryptamine. Therefore, accumulation of tryptamine in tobacco is a direct measure of TDC function in vivo within the target subcellular compartment.
We report here a clear effect of compartmentation on TDC stability and in vivo function. Accumulation levels of TDC and its product, tryptamine, were significantly dependent on the target cell compartment, and the highest enzyme activity and product levels were achieved by targeting TDC to the chloroplast. However, transgenic tobacco plants expressing recombinant TDC in the chloroplast developed necrotic leaves closely resembling a lesion-mimic phenotype. The aberrant phenotype is likely to be a direct effect of the very high levels of tryptamine accumulated in plants expressing TDC in the chloroplast and we discuss this observation in the light of metabolic engineering.
RESULTS
Transient Expression of Targeted TDC in Tobacco Leaves
A transient expression assay of vacuum infiltrated leaves was initially carried out to evaluate the performance of the different TDC constructs. Recombinant Agrobacteria carrying the expression cassettes for targeting TDC to the chloroplast, the cytosol, or the ER (Fig. 1) were independently infiltrated into tobacco leaves.
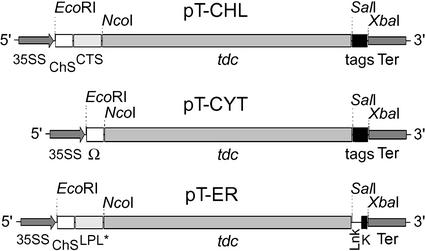
Figure 1.
Plant expression cassettes for targeting TDC to the chloroplast (pT-CHL), cytosol (pT-CYT), and ER (pT-ER). ChS, 5′-Untranslated region (UTR) of chalcone synthase; CTS, chloroplast-targeting signal of the potato (Solanum tuberosum) granule-bound starch synthase; Ω, 5′-UTR Ω sequence of tobacco mosaic virus; tags, c-myc/His6 tags; LPL*, plant codon optimized light chain leader peptide of the murine antibody 24 (Voss et al., 1995); Lnk, linker; K, KDEL sequence; 35SS, double enhanced cauliflower mosaic virus (CaMV) 35S promoter; Ter, CaMV termination sequence.
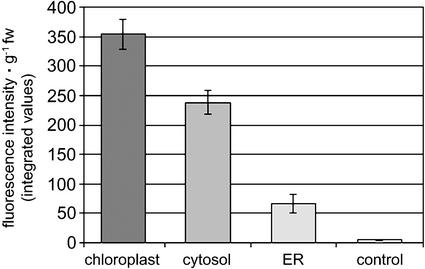
Because endogenous TDC activity was not detected in the present study or in previous studies in tobacco, the biosynthesis of tryptamine in infiltrated leaves transiently expressing targeted TDC was used as a direct evidence of in vivo enzyme function. Tryptamine levels in infiltrated leaves were measured using a fluorometric assay (Sangwan et al., 1998), and the results (fluorescence intensity per gram of fresh weight leaf material) are shown in Figure 2. Leaves transiently expressing chloroplast-targeted TDC showed a fluorescence intensity of tryptamine approximately 1.5-, 5-, and 72-fold higher than those expressing TDC in the cytosol, in the ER, or the background levels of control leaves infiltrated with nonrecombinant Agrobacteria, respectively (Fig. 2). Nevertheless, tryptamine fluorescence in leaves transiently expressing cytosolic or ER-targeted TDC was approximately 48.5- or 13.5-fold higher than the background signal of control leaves, respectively (Fig. 2).
Figure 2.
Fluorometric detection of tryptamine in the crude extract of tobacco SR1 leaves transiently expressing recombinant TDC targeted to different subcellular compartments. Bars represent the average value of fluorescence intensity g−1 fresh weight (fw) of leaf material calculated from the total integral of the 300- to 400-nm emission scan area of four infiltrated leaves per plant expression cassette. Control leaves were infiltrated with nonrecombinant agrobacteria. ses are shown.
Stable Expression and in Vivo TDC Function in Different Subcellular Compartments of Tobacco Plants
Because the transient expression studies provided evidence that the subcellular location of TDC affected its accumulation and/or activity, the effect of compartmentation on TDC was further studied in stably transformed tobacco plants. The levels of TDC protein were compared with in vivo enzymatic activities using immunoblot analysis and fluorometric assays in leaves harvested from at least 25 independent primary transgenic plants that harbored each construct.
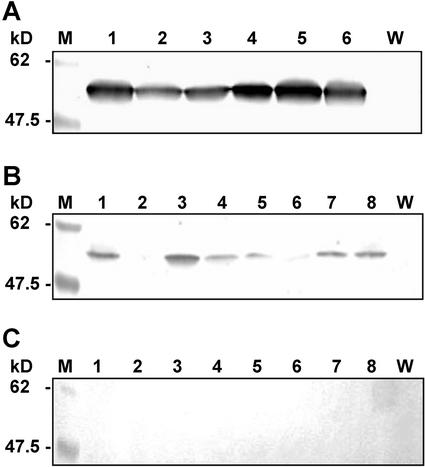
TDC is a homodimeric enzyme consisting of two monomers of approximately 49 to 55 kD (Pennings et al., 1989; Fernandez et al., 1989). Immunoblot analysis of protein extracts prepared from the leaves of T0 (and later T1) tobacco plants expressing chloroplast- or cytosol-targeted TDC identified a mass of 52 to 54 kD (Fig. 3, A and B), whereas no protein signal was detected in extracts prepared from leaves of T0 plants expressing ER-targeted TDC (Fig. 3C). These results indicated that the amount of ER-targeted TDC was below the detection limit of the immunoblot analysis. Therefore, subsequent analyses of T1 plants harboring the ER-targeting construct were not carried out.
Figure 3.
Immunoblot analysis of the crude TSP extract of independent transgenic tobacco plants expressing targeted TDC to the chloroplast (A), cytosol (B), and ER (C). The 9E10 anti-c-myc antibody (A and B) or the MA-GRP78 anti-KDEL antibody (C) was used to detect the recombinant TDC. M, Prestained protein marker. Lanes 1 through 6A and lanes 1 through 8, B and C, Leaf crude extract of transgenic T1 (A and B) and T0 plants (C). W, TSP of wild-type tobacco SR1 plants.
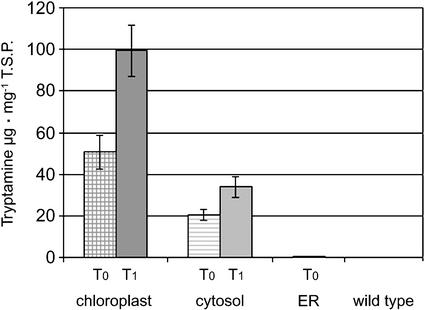
T0 plants were also subjected to quantitative analysis of tryptamine to study in vivo TDC function within the different subcellular compartments. The average level of tryptamine in the crude extract of leaves, harvested from the minimum of 25 independent T0 plants per targeting cassette, were 50.6 ± 8.1, 20.4 ± 2.5, and 0.6 ± 0.13 μg mg−1 total soluble protein (TSP) in plants expressing chloroplast-, cytosol-, or ER-targeted TDC, respectively (Fig. 4; Table I).
Figure 4.
In vivo TDC function in transgenic tobacco plants. Bars represent the average amount of tryptamine in the leaf crude extract of 25 T0 and 15 T1 tobacco plants accumulating TDC in different subcellular compartments. Tryptamine amounts are in micrograms per milligram of TSP. ses are shown.
Table I.
Level of tryptamine in transgenic T0 and T1 tobacco plants
| pT-CTS
|
pT-CYT
|
pT-ER
|
|||
|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | |
| Meana | 50.584 (258.966) | 99.359 (660.784) | 20.366 (159.244) | 34.019 (227.981) | 0.591 (4.016) |
| se | 8.097 (39.154) | 12.255 (110.704) | 2.464 (25.103) | 4.949 (23.318) | 0.132 (0.766) |
| Minimum | 6.061 (61.92) | 32.757 (321.367) | 10.407 (61.881) | 14.953 (76.025) | 0.041 (0.759) |
| Maximum | 98.949 (570.124) | 196.966b (1,277.465) | 37.327 (346.991) | 62.604 (353.936) | 1.380 (10.701) |
Data represent statistical analysis of the fluorimetric quantitation of tryptamine synthesized in vivo in transgenic plants harboring cassettes for targeting TDC to different subcellular compartments. Levels of tryptamine are in microgram per milligram of TSP or microgram per gram fresh wt of leaf material (in parentheses).
The highest amount of tryptamine detected in fully expanded leaves of a transgenic T1 plant expressing recombinant TDC in the chloroplast is underlined.
Three to five T0 plants expressing TDC targeted to the chloroplast and to the cytosol that accumulated high TDC as well as high tryptamine levels (in the range of 61–98 μg mg−1 TSP and 32–37 μg mg−1 TSP, respectively) were selected to generate at least 15 T1 plants per construct. These plants were analyzed for levels of TDC and tryptamine. The average tryptamine levels were 99.36 ± 12.2 and 34.01 ± 4.9 μg mg−1 TSP (Table I), respectively, for plants expressing chloroplast- or cytosol-targeted TDC. Thus, the average and the maximum tryptamine levels in the two groups of T1 plants was about 2-fold higher than the levels detected in the corresponding T0 population (Table I).
Phenotypic Effects of TDC Expression
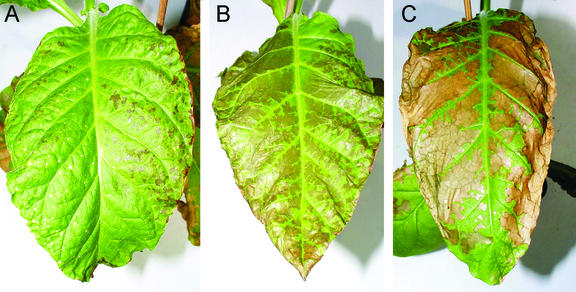
T0 and T1 tobacco plants accumulating TDC in the chloroplast showed a striking phenotype. One or 2 weeks before the flower buds developed, small necrotic areas appeared on the surface of older leaves (Fig. 5A). With the onset of the opening of fully developed flower buds, the lesions increased in number and size in the lower and middle leaves, whereas younger leaves showed only small necrotic areas at the leaf edges. At the end of the flowering, most of the leaf surface was necrotic, and strong deformation was apparent in the lower and middle leaves (Fig. 5, B and C). These symptoms were observed only in plants expressing chloroplast-targeted TDC and not in plants expressing TDC in the cytosol or ER. In addition, within the group of plants expressing chloroplast-targeted TDC, only those shown to accumulate high levels of TDC and tryptamine displayed symptoms, indicating a correlation between symptom severity and the level of enzyme and product.
Figure 5.
Leaf necrotic areas in transgenic plants expressing TDC in the chloroplast. Necrosis started to appear in the form of small HR-like necrotic lesions (A), and they increased in number and size as soon as the plants approached flowering when diffused necrosis with strong deformation of the leaf surface developed (B and C). The fertility of the plants was not affected, and seeds from T0 plants were used to cultivate T1 plants that showed the same phenotype with no reduction of fertility.
T0 plants with necrotic leaves were fully fertile and produced seeds that were used to generate T1 plants that showed the same phenotype displayed by T0 plants and no significant reduction of fertility.
Analysis of Chloroplast Preparations and Enzymatic Assays
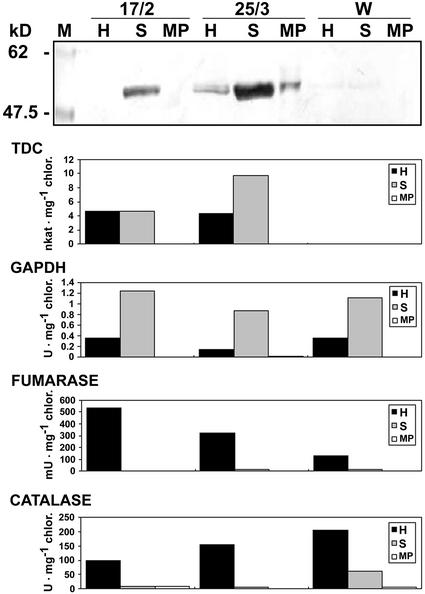
To confirm correct TDC targeting, chloroplasts were isolated from leaves of two T1 transgenic plants (17/2 and 25/3) harboring the chloroplast-targeting cassette and showing relatively high accumulation of TDC protein. More than 70% of the purified chloroplasts were intact as estimated by phase-contrast microscopy analysis. The homogenate (H), stroma (S), and membrane pellet (MP) of the purified chloroplasts were subjected to immunoblot analysis to study distribution of TDC in the different fractions. Furthermore, in vitro assays of marker enzymes were carried out to demonstrate that mitochondria (fumarase assay) and peroxisomes (catalase assay) did not contaminate chloroplasts. Glyceraldehyde-3-P dehydrogenase (GAPDH) was used as S-associated chloroplast marker and was assayed in the reductive direction (Wolosiuk and Buchanan, 1976). As shown in Figure 6, GAPDH activity peaked in the S fraction, whereas no activity was detected in the MP. No or very little fumarase and catalase activity was detected in the S and MP fractions, indicating that no or very little contamination by mitochondria or peroxisomes was present in the chloroplast preparations. Immunoblot analysis showed that the majority of TDC accumulated in the S (Fig. 6) and accordingly, the enzyme activity peaked or was fully recovered in this fraction. This result confirmed that recombinant TDC was correctly targeted to the chloroplast and was associated with the S. TDC was not detected in chloroplasts isolated from wild-type tobacco SR1 plants.
Figure 6.
Immunoblot analysis and in vitro assay of TDC and marker enzymes in the H, S, and MP of chloroplast preparations from two T1 plants (17/2 and 25/3) accumulating TDC in the chloroplast and a wild-type tobacco plant (W). Bars represent the average value of the specific enzymatic activity expressed in units (catalase and GAPDH), milliunits (fumarase), or nanokatals (TDC) per milligram of chlorophyll.
DISCUSSION
To assess the potential of a targeting approach to the engineering of plant secondary metabolism, we investigated the effect of subcellular targeting on accumulation of TDC and its product tryptamine, in tobacco plants.
A transient assay, using vacuum infiltration of tobacco leaves, was performed to acquire preliminary information on the expression and in vivo function of recombinant TDC targeted to different subcellular compartments. Vacuum infiltration of leaves with suspensions of recombinant Agrobacteria has been described as a rapid and reliable tool for testing transient expression of recombinant proteins in young and intact leaves before approaching stable transformation of plants (Kapila et al., 1997). As tryptamine was detected in extracts of all but the infiltrated leaves of the negative control, it was concluded that TDC was transiently expressed and functional in vivo in different subcellular compartments. Moreover, leaves expressing different targeting cassettes displayed significantly different extractable tryptamine fluorescence with the highest signals detected in leaves transiently expressing chloroplast-targeted TDC. As we assumed that the transient expression of the different constructs resulted in efficient subcellular targeting of the corresponding recombinant enzyme, the results obtained in these studies anticipated the effect of subcellular TDC targeting on enzyme accumulation/stability and/or in vivo function. To our knowledge, this is the first report on transient expression and in vivo function of a recombinant enzyme of the secondary metabolism in intact tobacco leaves. A comparison of data from the transient assay and subsequent analysis of tryptamine levels in transgenic plants revealed similar relative levels of tryptamine for the three constructs used, demonstrating that the transient expression assay in intact leaves may represent a fast and reliable tool for studies of subcellular targeting and metabolic engineering.
The effect of compartmentation on accumulation and in vivo TDC function was clearly demonstrated in transgenic T0 and T1 plants by immunoblot analysis and fluorometric detection of tryptamine. The chloroplast-targeted and cytosolic TDC accumulated to detectable levels, whereas the ER-targeted TDC was below the detection limit of the immunoblot analysis. In addition, the chloroplast and cytosolic TDC showed bands of similar mass on the immunoblots, suggesting that the transit peptide of the chloroplast-targeted TDC was correctly processed. Immunoblot and biochemical analysis of chloroplasts purified from transgenic T1 plants demonstrated that TDC localized in the stroma.
In the majority of plants expressing a chloroplast-targeted TDC, the intensity of the protein signals on the immunoblots differed significantly from that displayed by plants expressing cytosolic TDC. Because this result was observed in detached leaves from T0 and T1 plants, we conclude that protein accumulation and stability were improved for the chloroplast-targeted TDC compared with the cytosolic counterpart. As reported by Bogorad (2000), a photosynthetic cell contains an average of 50 to 60 active chloroplasts, and the chloroplast resident proteases differ from those found in the cytosol. Thus, high accumulation of the chloroplast-targeted TDC may be accounted for by the large number of chloroplasts per cell and the higher protein stability resulting from the less effective proteolytic activity of the chloroplast resident proteases toward a naturally occurring cytosolic protein, such as TDC.
We speculate that the ER-targeted TDC was susceptible to degradation. The fluorescence intensity of tryptamine in infiltrated leaves transiently expressing ER-targeted TDC was about 13.5-fold higher than the background signal of the control, whereas in detached leaves of T0 plants expressing the ER-targeted TDC, the average level of tryptamine was approximately 600 ng mg−1 TSP with a maximum value of approximately 1.4 μg mg−1 TSP (Table I). This result showed that only a small protein fraction was functional in planta. In addition, in vitro TDC assays (Pennings et al., 1987), carried out by feeding l-Trp to the crude leaf protein extract of transgenic plants expressing the ER-targeted TDC, showed only a very low activity (data not shown). These data and the results of the immunoblot analysis corroborate our hypothesis of protein degradation, and it was concluded that the ER is not a suitable subcellular compartment for accumulation of TDC due to protein degradation and correspondingly low in vivo activity.
Additional evidence of compartmentation effects on the in vivo TDC activity was obtained through quantitative fluorometric analysis of tryptamine in the leaf crude extract of transgenic tobacco plants. The fluorometric detection described by Sangwan et al. (1998) proved reliable and reproducible for analysis of a large number of samples. However, some modification to this method was necessary because the amount of tryptamine in the leaf crude extracts of transgenic plants exceeded the extraction capacity of the volume of organic solvent suggested by Sangwan et al. (1998). Therefore, extraction of tryptamine was carried out with a larger volume of ethyl acetate, and, to acquire meaningful quantitative data, standard curves of tryptamine were constructed for each measurement. Other secondary metabolites coextracted in the organic phase gave no interference in the wavelength range used in the assay as showed by the analysis of the crude extract of wild-type leaves.
Aqueous solutions of tryptamine and l-Trp show a characteristic emission spectrum with a peak at 350 nm when excited at 280 nm. In our experiments, the maximum emission intensity of the extracted tryptamine was shifted to 340 nm. It is well known that organic solvents, due to their low polarity, can alter fluorescence of fluorophores, inducing the so-called Stokes' shift (Lakowicz, 1983). To acquire more information about the nature of the substance detected in the leaf crude extracts, we decided to compare the emission scan of standard solutions of tryptamine in tryptamine assay buffer (TRAB) and in ethyl acetate (EtOAc; data not shown). The emission peaks of the EtOAc solutions showed a 10-nm shift with maximum emission intensity at 340 nm. Therefore, the 340-nm emission peaks detected in the crude extract of leaves transiently or stably expressing TDC were the in vivo-synthesized tryptamine, and the observed shift was due to the organic solvent.
T0 and T1 plants expressing chloroplast-targeted TDC showed significantly higher tryptamine levels than those detected in leaves harvested from plants expressing cytosolic or ER-targeted TDC (Fig. 4). In particular, one T1 plant accumulated 196.96 μg mg−1 TSP or 1,277.47 μg g−1 fresh weight of leaves (Table I), which is the highest amount of tryptamine so far reported in the literature from studies expressing TDC in tobacco plants. This represents a 1.6-, 8.6-, and about 1.2-fold improvement on the maximum amount of tryptamine observed in mature transgenic T1 tobacco plants accumulating TDC in the cytosol, as reported by Leech et al. (1998), Poulsen et al. (1994), and Songstad et al. (1990), respectively. Levels of tryptamine in plants accumulating TDC in the cytosol were comparable with those obtained by Poulsen et al. (1994), but were lower than those reported by Leech et al. (1998) and Songstad et al. (1990).
The results of the immunoblot analysis and the quantitative analysis of tryptamine clearly indicated a correlation between levels of in vivo enzymatically active TDC and levels of product for plants harboring different targeting cassettes (Figs. 3 and 4; Table I) or plants expressing TDC in the same subcellular compartment (data not shown).
Leaves of transgenic T0 and T1 plants accumulating high levels of chloroplast-targeted TDC appeared to undergo uncontrolled cell death (CD) at specific developmental stages, whereas leaves of plants expressing cytosolic TDC never showed necrotic symptoms. To our knowledge, this is the first report of chloroplast TDC targeting where severe necrotic symptoms on leaves of tobacco plants expressing a recombinant TDC have been described, although Guillet et al. (2000) reported symptoms of root curling in tobacco seedlings coexpressing TDC and Tyr decarboxylase in the cytosol. The authors assigned this effect to perturbation in Trp levels required for the biosynthesis of auxins and normal root development. In transgenic tobacco plants constitutively expressing recombinant proteins, the development of early hypersensitive response (HR) symptoms and late diffused necrosis on the entire leaf surface similar to those we observed in the present study have been already described as a lesion-mimic phenotype (Abad et al., 1997) because the plant phenotype closely resembled that of so called lesion-mimic mutants that develop HR and uncontrolled CD in the absence of pathogen attack. In those studies, occurrence of symptoms correlated with high accumulation level of the recombinant proteins and were interpreted as the result of metabolic perturbation or as interference of the transgene expression with the disease resistance pathway (Dangl et al., 1996). Plants expressing chloroplast-targeted TDC clearly showed altered regulation of HR and of CD propagation, thereby reproducing a lesion-mimic phenotype. Moreover, because HR-like symptoms overlapped uncontrolled CD in old and young leaves, it was concluded that the lesion-mimic phenotype resembled the initiation and propagation classes of cell death mutants reported by Dangl et al. (1996).
Previous studies have shown that at physiological pH, tryptamine is an ionized molecule able to function as strong electron donor (Abu-Eittah and Abdou, 1996) and to complex cofactors such as FMN (Wilson, 1966) and adenine dinucleotide (Alivisatos et al., 1961). These chemical properties strongly indicate that tryptamine is a potential poison to chloroplast function. Because a good correlation between the severity of symptoms and the accumulation of TDC and tryptamine was observed, we speculate that the accumulation of TDC and the product tryptamine in the chloroplast of some transgenic plants exceeded a threshold of tolerance above which altered metabolism triggered a stress response. However, we cannot exclude the possibility that altered metabolism in plants expressing TDC in the chloroplast was a consequence of a severe depletion of l-Trp and the consequent reduction of flux into pathways stemming from the shikimate pathway located in the chloroplast. Thus, a more definitive conclusion of the nature of the necrotic symptoms we observed requires further investigation.
In conclusion, a recombinant enzyme of the plant secondary metabolism was targeted to subcellular compartment where it does not naturally occur. It was shown that some subcellular compartments are good candidates to enhance protein accumulation and in vivo enzyme function, whereas other compartments are not suitable for this purpose. Similar studies carried out in our group with strictosidine synthase, the enzyme catalyzing the step immediately downstream TDC in the biosynthesis of TIAs, provided further support to the notion that targeting enzymes of the secondary metabolism to different compartments of the plant cell may significantly influence protein accumulation and enzymatic activity (unpublished data).
At the onset of the present research, it was not possible to predict that transgenic plants would develop a lesion-mimic phenotype. However, our data are consistent with a growing awareness that the complex interactions of metabolic networks in plants may make it difficult to successfully engineer metabolic pathways, and in place of the desired outcome, unpredictable results and altered metabolic homeostasis may be generated. Thus, to improve the success of metabolic engineering, a better understanding of the complex interactions that regulate plant metabolism is required.
The results obtained show biotechnological relevance in that a substantial relative increase of a secondary metabolite was achieved by bringing together enzyme and substrate within the same subcellular compartment. This strategy might be particularly useful when applied to rate-limiting metabolic nodes in the production of desired end products of a secondary metabolic pathway. We demonstrated that TDC is functional in the chloroplast, and a transgenic T1 plant accumulated the highest tryptamine level so far reported. As shown in previous reports on improved amino acid biosynthesis, by targeting dihydropicolinic acid synthase and feedback-insensitive Asp kinase to the chloroplast (Falco et al., 1995; Galili et al., 2000), we here demonstrate that flux through amino acid biosynthetic pathways can be diverted in the chloroplast to enhance production of secondary metabolites such as tryptamine. However, because tobacco does not express enzymes to further metabolize tryptamine, a definitive statement on the value of the exploited strategy awaits the transfer of the present study to differentiated cell/organ cultures or whole plant systems containing functional chloroplasts and at least a segment of the TIA pathway downstream to tryptamine.
MATERIALS AND METHODS
Plasmid DNA, Bacteria, and Plants
The following plasmid DNA, bacteria, and plants were used in the present study: Plasmid DNA, pTSK (Leech et al., 1998), pUC18 (Messing, 1983), pGEM (Pharmacia, Freiburg, Germany), and pSS (Voss et al., 1995); bacteria, Escherichia coli SCS110 (Stratagene, Heidelberg) and Agrobacterium tumefaciens GV3101 (pMP90RK, GmR, KmR, and Rif R) (Koncz and Schell, 1986); plant, Nicotiana tabacum cv Petite Havana SR1. Plants were grown in a greenhouse under a 16-h photoperiod comprising natural daylight. The temperature was held at about 25°C during the day and about 22°C at night.
PCR Amplification of tdc
Tdc (GenBank accession no. M25151) was amplified by PCR using the TSK plasmid DNA (Leech et al., 1998) in combination with the primer tdc-forward (tfw) 5′-CGCGAGCTCCATGGGCAGCATTGATTCAAC-3′ and tdc-backward (tbw) 5′-CCCAAGCTTGTCGACGGCTTCTTTGAGCAAATCATC-3′.
SacI and NcoI as well as SalI and HindIII restriction sites were introduced, respectively, at the 5′ and 3′ end of tdc by amplification with the tfw and tbw primers. These restriction sites were used to clone tdc into cassettes for subcellular targeting. In addition, tbw was designed to delete the stop codon at the 3′ end of the tdc sequence to enable fusion of different 3′ tags or retention motifs.
Construction of the Plant Expression Cassettes
The tdc cDNA (obtained by PCR amplification as above) was initially cloned as a SacI/HindIII fragment into pUC18 and was then subcloned, via NcoI/SalI, into pUC or pGEM derivatives containing different targeting and tag sequences. The following targeting cassettes were constructed: T-CHL (chloroplast) was generated by cloning tdc as a NcoI/SalI fragment between the 5′ cDNA sequence of the potato granule-bound starch synthase chloroplast-targeting signal (CTS; V. Hoppmann, S. Di Fiore, S. Zimmermann, N. Emans, T. Rademacher, R. Fischer, and S. Schillberg, unpublished data) and the 3′-c-myc/His6 tags; T-CYT (cytosol) was generated from the constructs scFv24CW (Zimmermann et al., 1998) and scFv24H (Spiegel et al., 1999) by cloning tdc as a NcoI/SalI fragment between the 5′ Ω-UTR of the tobacco mosaic virus (Schmitz et al., 1996) and the 3′-c-myc/His6 tags; and T-ER (endoplasmic reticulum) was generated from the construct biscFv2429-KDEL (Fischer et al., 1999) by cloning tdc as a NcoI/SalI fragment between the plant codon-optimized 5′-light chain leader peptide sequence of the murine antibody 24 (LPL*; Voss et al., 1995) and a 3′ sequence encoding the KDEL ER retention signal (Munro and Pelham, 1986).
The recombinant tdc cDNAs including the 5′ targeting sequences and the 3′ tags were subcloned as a EcoRI/XbaI fragment into the pSS plant expression vector (Voss et al., 1995) between the constitutive CaMV 35S double enhanced promoter and the CaMV terminator sequence, resulting in the plant expression vectors pT-CHL, pT-CYT, and pT-ER for targeting the TDC protein to the chloroplast, cytosol, and ER of plant cells, respectively (Fig. 1).
Transformation of Tobacco Plants
The plant expression vectors were introduced into A. tumefaciens GV3101 cells by electroporation using a Gene Pulser II electroporation system (Bio-Rad, Hercules, CA) according to the manufacturer's instructions.
In preliminary experiments, TDC expression and in vivo function was evaluated using a transient expression assay of vacuum-infiltrated tobacco leaves with recombinant Agrobacteria (Kapila et al., 1997). Four young leaves (approximately 6–12 cm in length) per targeting cassette were randomly selected from different plants, infiltrated, and incubated in sealed trays on wet paper (Whatman, Clifton, NJ) at 25°C with a 16-h photoperiod for 60 h. At the end of the incubation, the leaves were weighed, frozen in liquid nitrogen, and stored at −80°C until analyzed. Tobacco leaves infiltrated with nonrecombinant Agrobacteria were used as negative control.
Stably transformed transgenic tobacco plants were obtained by inoculating tobacco leaf discs with recombinant Agrobacteria, as described by Horsch et al. (1985).
Transgenic T1 plants were generated by germinating seeds harvested from selected T0 plants on Murashige and Skoog minimal organics medium (Sigma, Deisenhofen, Germany) supplemented with 2% (w/v) Suc, 0.4 μg mL−1 thiamin, 2 μg mL−1 Gly, 0.5 μg mL−1 nicotinic acid, 0.5 μg mL−1 pyridoxine, and 100 μg mL−1 kanamycin as selectable marker.
Protein Analysis
TSP was prepared from tobacco leaves transiently or stably expressing recombinant TDC as described by Leech et al. (1998) with the following plant extraction buffer (PEXB) in a 1:1.5 (w/v) ratio: 100 mm sodium phosphate, pH 7.5, 2 mm EDTA, 4 mm dithiothreitol, and 5% (w/v) of polyvinylpolypyrrolidone. From each T0 and T1 plant a single fully expanded leaf of similar age and size was collected and was used to prepare crude TSP extracts as above. TSP was subjected to SDS-PAGE followed by electroblotting onto nitrocellulose membranes (Hybond-C; Amersham Life Science, Braunschweig, Germany) and immunoblot analysis. The primary 9E10 anti-c-myc antibody (clone no. CRL-1729; American Type Culture Collection, Manassas, VA) was used to detect the chloroplast- and cytosol-targeted TDC, whereas the mouse anti-GRP78 anti-KDEL antibody (StressGen Biotechnologies, Victoria, BC) was used to detect the ER-targeted TDC. The goat anti-mouse IgG heavy + light chain-specific alkaline phosphatase-conjugated antibody (Jackson ImmunoResearch, West Grove, PA) was used as secondary antibody. Development of the blots was carried out with a solution of nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate (Pierce, Rockford, IL).
TSP content of the leaf crude extracts was determined in triplicate with the Bradford protein assay (Bio-Rad) using bovine serum albumin as internal standard.
Fluorometric Detection of Tryptamine
Tryptamine, accumulated in vivo in vacuum-infiltrated leaves or in leaves detached from transgenic T0 and T1 plants, was detected according to the method of Sangwan et al. (1998) with minor modifications. In brief, aliquots (10–100 μL) of leaf crude extracts prepared with PEXB as described above were mixed with TRAB (buffer system 2A as described by Sangwan et al., 1998) to a final volume of 1 mL and were alkalinized as described. Extraction of tryptamine was carried out with the addition of 5 mL of EtOAc, vortexing for 30 s to emulsify the solvent, and buffer phases followed by 5 min centrifugation at 1,500g in a swinging bucket rotor at room temperature to favor phase separation. The organic phase was subjected to fluorometric analysis by using an Aminco Bowman AB2 luminescence spectrometer (Spectronic Instruments, Rochester, NY). Tryptamine was detected at 280 nm excitation and 340 nm emission wavelengths with 4-nm slit width for excitation and emission light and with the photomultiplier voltage set to 550 V. Fluorescence intensity and integrated values of the tryptamine emission scans were recorded against a blank of PEXB in TRAB and against a negative control made of the crude extract of wild-type tobacco leaves in TRAB. For each T0 and T1 plant, detection of tryptamine was carried out with the same leaf crude extract used for immunoblot analysis of TDC expression. Two samples for each crude extract were analyzed in triplicate to obtain six data points per plant. The concentration of tryptamine in the crude extract of transgenic plants was expressed in micrograms per milligram TSP and was extrapolated from a calibration curve of standard solutions of tryptamine in the leaf crude extract (10- to 100-μL aliquots) of wild-type plants. The standards of tryptamine were extracted with the same volume (5 mL) of EtOAC.
Isolation of Chloroplasts
Young leaves from transgenic T1 plants expressing chloroplast-targeted TDC were weighed, cut into small pieces, and homogenized in ice-cold grinding buffer (GR; 50 mm HEPES-KOH, pH 7.5, 0.33 m sorbitol, 5 mm sodium ascorbate, 0.025% [w/v] bovine serum albumin, 2 mm EDTA, 1 mm MgCl2, and 1 mm MnCl2) with a 1:10 (w/v) ratio. Homogenization of the leaf material was carried out with a single 3-s pulse in a blender (Waring, New Hartford, CT) set at high speed. The crude H was filtered through Miracloth (Calbiochem, San Diego) and pulse centrifuged at 7,000 rpm and 4°C in a SS 34 rotor (Sorvall Products, Newtown, CT). The supernatant was decanted while the chloroplast-enriched pellet was resuspended in 2 mL of GR buffer, loaded onto a two-step density gradient (30% and 80% [w/v] Percoll solution in GR buffer), and centrifuged for 5 min at 7,000 rpm and 4°C in a HB6 swinging bucket rotor (Sorvall). Intact chloroplasts that sedimented at the interface of the 30% and 80% (w/v) Percoll solution were collected, 10-fold diluted in GR buffer, and pulse centrifuged at 7,000 rpm in a SS34 rotor (Sorvall) at 4°C. The chloroplast pellet was gently resuspended in 200 μL of suspension buffer (50 mm HEPES-KOH, pH 7.5, and 0.33 m sorbitol). Integrity of the chloroplasts was estimated using phase-contrast light microscopy. Aliquots (1 mL) of the H fraction were stored at −70°C for further analysis. The chloroplasts were freeze-thawed and a 100 μL aliquot was centrifuged at 106,000g for 1 h to separate the S from the MP of envelopes and thylakoid membranes. TSP of the H, S, and MP fraction was determined in triplicate as described. For each fraction, the same amount of TSP (approximately 4 μg) was loaded on a SDS-polyacrylamide gel and was subjected to electrophoresis and immunoblot analysis.
Enzymatic Assays
The activity of catalase, fumarase, GAPDH, and TDC was assayed in the H, S, and MP fraction of purified chloroplasts.
The in vitro assay for catalase was performed according to Luck (1965) by monitoring the decline in absorbance of H2O2 at 240 nm. Fumarase was assayed by monitoring absorbance of the in vitro-synthesized fumarate at 240 nm according to the method of Racker (1950) with the modification described by Hatch (1978). GAPDH was assayed in the reductive direction as described by Wolosiuk and Buchanan (1976). TDC was assayed according to Pennings et al. (1987) with the difference that the in vitro-synthesized tryptamine was detected fluorometrically as described above. All the assays were carried out in triplicate. Specific activity of the marker enzymes was expressed in units (micromoles per minute) or mU (nanomoles per minute) per milligram chlorophyll.
Chlorophyll content (milligram per milliliter) in the H and chloroplasts S + MP was determined in triplicate according to the method of Arnon (1949) as modified by Bruinsma (1961).
ACKNOWLEDGMENT
We thank Verena Hoppmann for providing the pGEM derivative for chloroplast targeting.
Footnotes
This work was supported in part by the European Commission (Training and Mobility of Researchers fellowship no. FAIR–CT98–5015 to S.D.F.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010889.
LITERATURE CITED
- Abad MS, Hakimi SM, Kaniewski WK, Rommens CM, Shulaev V, Lam E, Shah DM. Characterization of acquired resistance in lesion-mimic transgenic potato expressing bacterio-opsin. Mol Plant-Microbe Interact. 1997;10:635–645. doi: 10.1094/MPMI.1997.10.5.635. [DOI] [PubMed] [Google Scholar]
- Abu-Eittah RH, Abdou MM. Charge transfer interactions between some tryptamines and iodine. J Chim Phys. 1996;93:1958–1973. [Google Scholar]
- Alivisatos SGA, Ungar F, Jibril A, Mourkides GA. Non-enzymic reactions of indoles with pyridine coenzymes and related structures. Biochim Biophys Acta. 1961;51:361–372. doi: 10.1016/0006-3002(61)90178-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin J, Rugenhagen C, Dietze P, Fecker LF, Goddijn OJM, Hoge JHC. Increased production of serotonin by suspension and root cultures of Peganum harmala transformed with a tryptophan decarboxylase cDNA clone from Catharanthus roseus. Transgenic Res. 1993;2:336–344. [Google Scholar]
- Boevink P, Santa Cruz S, Hawes C, Harris N, Oparka KJ. Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J. 1996;10:935–941. [Google Scholar]
- Bogorad L. Engineering chloroplasts: an alternative site for foreign genes, proteins, reactions and products. Trends Biotechnol. 2000;18:257–263. doi: 10.1016/s0167-7799(00)01444-x. [DOI] [PubMed] [Google Scholar]
- Bruinsma J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Chavadej S, Brisson N, McNeil JN, De Luca V. Redirection of tryptophan leads to production of low indole glucosinolate canola. Proc Natl Acad Sci USA. 1994;91:2166–2170. doi: 10.1073/pnas.91.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Dietrich Robert A, Richberg Michael H. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Cutler AJ. Subcellular localization of enzymes involved in indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol. 1987;85:1099–1102. doi: 10.1104/pp.85.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, St. Pierre B. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 2000;5:168–173. doi: 10.1016/s1360-1385(00)01575-2. [DOI] [PubMed] [Google Scholar]
- Facchini PJ, Huber-Allanach KL, Tari LW. Plant aromatic l-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry. 2000;54:121–138. doi: 10.1016/s0031-9422(00)00050-9. [DOI] [PubMed] [Google Scholar]
- Falco SC, Guida T, Locke M, Mauvais J, Sanders C, Ward RT, Webber P. Transgenic canola and soybean seeds with increased lysine. Biotechnology. 1995;13:577–582. doi: 10.1038/nbt0695-577. [DOI] [PubMed] [Google Scholar]
- Fernandez JA, Owen TG, Kurz WGW, De Luca V. Immunological detection and quantitation of tryptophan decarboxylase in developing Catharanthus roseus seedlings. Plant Physiol. 1989;91:79–84. doi: 10.1104/pp.91.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler U, Phillips J, Artsaenko O, Conrad U. Optimization of scFv antibody production in transgenic plants. Immunotechnology. 1997;3:205–216. doi: 10.1016/s1380-2933(97)00014-6. [DOI] [PubMed] [Google Scholar]
- Fischer R, Schumann D, Zimmermann S, Drossard J, Sack M, Schillberg S. Expression and characterization of bispecific single-chain Fv fragments produced in transgenic plants. Eur J Biochem. 1999;262:810–816. doi: 10.1046/j.1432-1327.1999.00435.x. [DOI] [PubMed] [Google Scholar]
- Galili S, Guenoune D, Wininger S, Hana B, Schupper A, Ben-Dor B, Kapulnik Y. Enhanced levels of free and protein-bound threonine in transgenic alfalfa (Medicago sativa L.) expressing a bacterial feedback-insensitive aspartate kinase gene. Transgenic Res. 2000;9:137–144. doi: 10.1023/a:1008991625001. [DOI] [PubMed] [Google Scholar]
- Goddijn OJM, Schouten PMV, Schilperoort RA, Hoge JHC. A chimaeric tryptophan decarboxylase gene as a novel selectable marker in plant cells. Plant Mol Biol. 1993;22:907–912. doi: 10.1007/BF00027376. [DOI] [PubMed] [Google Scholar]
- Gomord V, Denmat LA, Fitchette-Laine AC, Satiat-Jeunemaitre B, Hawes C, Faye L. The C-terminal HDEL sequence is sufficient for retention of secretory proteins in the endoplasmic reticulum (ER) but promotes vacuolar targeting of proteins that escape the ER. Plant J. 1997;11:313–325. doi: 10.1046/j.1365-313x.1997.11020313.x. [DOI] [PubMed] [Google Scholar]
- Guillet G, Poupart J, Basurco J, De Luca V. Expression of tryptophan decarboxylase and tyrosine decarboxylase genes in tobacco results in altered biochemical and physiological phenotypes. Plant Physiol. 2000;122:933–943. doi: 10.1104/pp.122.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch MD. A simple spectrophotometric assay for fumarate hydratase in crude tissue extracts. Anal Biochem. 1978;85:271–275. doi: 10.1016/0003-2697(78)90299-3. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Iturriaga G, Jefferson RA, Bevan MW. Endoplasmic reticulum targeting and glycosylation of hybrid proteins in transgenic tobacco. Plant Cell. 1989;1:381–390. doi: 10.1105/tpc.1.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapila J, De Rycke R, Van Montagu M, Angenon G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997;122:101–108. [Google Scholar]
- Koncz C, Schell J. The promoter of T-L DNA Gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Lakowicz JR. Principles of Fluorescence Spectroscopy. New York: Plenum Press; 1983. [Google Scholar]
- Leech MJ, May K, Hallard D, Verpoorte R, De Luca V, Christou P. Expression of two consecutive genes of a secondary metabolic pathway in transgenic tobacco: molecular diversity influences levels of expression and product accumulation. Plant Mol Biol. 1998;38:765–774. doi: 10.1023/a:1006000229229. [DOI] [PubMed] [Google Scholar]
- Luck H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1965. pp. 886–888. [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham HRB. An HSP-70-like protein in the endoplasmic reticulum: identity with the 78-kilodalton glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Pennings EJ, Hegger I, van der Heijden R, Duine JA, Verpoorte R. Assay of tryptophan decarboxylase from Catharanthus roseus plant cell cultures by high-performance liquid chromatography. Anal Biochem. 1987;165:133–136. doi: 10.1016/0003-2697(87)90210-7. [DOI] [PubMed] [Google Scholar]
- Pennings EJM, Verpoorte R, Goddijn OJM, Hoge JHC. Purification of tryptophan decarboxylase from a Catharanthus roseus cell suspension culture. J Chrom. 1989;483:311–318. [Google Scholar]
- Poulsen C, Goddijn OJM, Hoge JHC, Verpoorte R. Anthranilate synthase and chorismate mutase activities in transgenic tobacco plants overexpressing tryptophan decarboxylase from Catharanthus roseus. Transgenic Res. 1994;3:43–49. [Google Scholar]
- Racker E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-acotinic acids. Biochim Biophys Acta. 1950;4:211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Radwanski ER, Last RL. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan RS, Mishra S, Kumar S. Direct fluorometry of phase-extracted tryptamine-based fast quantitative assay of l-tryptophan decarboxylase from Catharanthus roseus leaf. Anal Biochem. 1998;255:39–46. doi: 10.1006/abio.1997.2377. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Prüfer D, Rohde W, Tacke E. Non-canonical translation mechanisms in plants: efficient in vitro and in planta initiation at AUU codons of the tobacco mosaic virus enhancer sequence. Nucleic Acids Res. 1996;24:257–263. doi: 10.1093/nar/24.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songstad DD, De Luca V, Brisson N, Kurz WGW, Nessier CL. High levels of tryptamine accumulation in transgenic tobacco expressing tryptophan decarboxylase. Plant Physiol. 1990;94:1410–1413. doi: 10.1104/pp.94.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel H, Schillberg S, Sack M, Holzem A, Naehring J, Monecke M, Liao Y-C, Fischer R. Accumulation of antibody fusion proteins in the cytoplasm and ER of plant cells. Plant Sci. 1999;149:63–71. [Google Scholar]
- Stevens LH, Blom TJM, Verpoorte R. Subcellular localization of tryptophan decarboxylase, strictosidine synthase and strictosidine glucosidase in suspension cultured cells of Catharanthus roseus and Tabernaemontana divaricata. Plant Cell Rep. 1993;12:573–576. doi: 10.1007/BF00233063. [DOI] [PubMed] [Google Scholar]
- St. Pierre B, Vazquez-Flota FA, De Luca V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell. 1999;11:887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpoorte R. Exploration of nature's chemodiversity: the role of secondary metabolites as leads in drug development. Drug Discovery Today. 1998;3:232–238. [Google Scholar]
- Verpoorte R, van der Heijden R, Memelink J. Engineering the plant cell factory for secondary metabolite production. Transgenic Res. 2000;9:323–343. doi: 10.1023/a:1008966404981. [DOI] [PubMed] [Google Scholar]
- Voss A, Niersbach M, Hain R, Hirsch HJ, Liao YC, Kreuzaler F, Fischer R. Reduced virus infectivity in N. tabacum secreting a TMV-specific full-size antibody. Mol Breed. 1995;1:39–50. [Google Scholar]
- Wandelt CI, Khan MR, Craig S, Schroeder HE, Spencer D, Higgins TJ. Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 1992;2:181–192. doi: 10.1046/j.1365-313x.1992.t01-41-00999.x. [DOI] [PubMed] [Google Scholar]
- Wilson JE. Studies on the electronic nature of flavin-indole and flavin-purine complexes. Biochemistry. 1966;5:1351–1359. doi: 10.1021/bi00868a031. [DOI] [PubMed] [Google Scholar]
- Wolosiuk RA, Buchanan BB. Studies on the regulation of chloroplast NADP-linked glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1976;251:6456–6461. [PubMed] [Google Scholar]
- Yao K, De Luca V, Brisson N. Creation of a metabolic sink for tryptophan alters the phenylpropanoid pathway and the susceptibility of potato to Phytophthora infestans. Plant Cell. 1995;7:1787–1799. doi: 10.1105/tpc.7.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Schillberg S, Liao Y-C, Fisher R. Intracellular expression of TMV-specific single-chain Fv fragments leads to improved virus resistance in Nicotiana tabacum. Mol Breed. 1998;4:369–379. [Google Scholar]