Abstract
Ca2+ sensitivity of arterial contractility is governed by regulating myosin phosphatase activity in response to agonist stimuli. CPI-17, a myosin phosphatase inhibitor phosphoprotein, is phosphorylated concomitantly with agonist-induced contractile Ca2+ sensitization in mammalian artery. CPI-17 has not been detected in chicken artery, but is readily detectable in pigeon artery. To evaluate a role of CPI-17, we compared contractility of the arteries of ‘CPI-17-deficient’ chicken with those of CPI-17-rich rabbit and pigeon, and studied the effect of CPI-17-reconstitution in chicken artery. Other major regulatory/contractile proteins for Ca2+ sensitization are expressed in both chicken and rabbit arteries. Agonists, such as an α1-agonist and endothelin-1, produced significant contraction in arteries of all species under physiological Ca2+-containing conditions. Depletion of Ca2+ abolished these contractions in chicken but partially inhibited them in rabbit and pigeon arteries. Unlike CPI-17-rich tissues, chicken arteries exerted little Ca2+ sensitization in response to α1-agonist or endothelin-1. GTPγS produced a slight Ca2+ sensitizing effect in chicken artery, but this was significantly smaller compared with CPI-17-rich tissues. A PKC activator (PDBu) did not generate but rather reduced a contraction in both intact and α-toxin-permeabilized chicken artery in contrast to a large contraction in CPI-17-rich arteries. Myosin light chain phosphorylation was reduced by PDBu in chicken but elevated in rabbit artery. Addition of recombinant CPI-17 into β-escin-permeabilized chicken artery restored PDBu-induced and enhanced GTPγS-induced Ca2+ sensitization. Thus, CPI-17 is essential for G protein/PKC-mediated Ca2+ sensitization in smooth muscle.
The primary regulatory mechanism for smooth muscle contraction is the reversible phosphorylation of the regulatory myosin light chain (MLC20) at Ser19 by MLC20 kinase (MLCK) and MLC20 phosphatase (MLCP) (Hartshorne, 1987; Kamm & Stull, 1989). MLCK activity is Ca2+/calmodulin dependent, while MLCP functions independently of Ca2+ and is regulated by G protein-coupled signalling pathways (Somlyo & Somlyo, 2003). G protein activation leads to inhibition of MLCP and thus an increase in both MLC20 phosphorylation and contraction without change in Ca2+ (Kitazawa et al. 1991b; Kubota et al. 1992). This mode of regulation is termed Ca2+ sensitization and is an essential process for agonist-induced contraction of smooth muscle as well as for cytoskeletal reorganization and movement of non-muscle cells (Somlyo & Somlyo, 2003). At least two signalling pathways have been proposed for the inhibition of MLCP. First, MLCP activity can be inhibited through phosphorylation of the MLCP regulatory subunit, MYPT1, and this mechanism is thought to involve RhoA/Rho-kinase-dependent pathways (Kimura et al. 1996; Fukata et al. 2001; Somlyo & Somlyo, 2003). Although Thr695 of MYPT1 is likely to be a key residue for MLCP inhibition (Hartshorne et al. 1998; Feng et al. 1999), the exact signalling mechanisms surrounding this target are still controversial in smooth muscle tissues (Kitazawa et al. 2003; Niiro et al. 2003).
The second mechanism of MLCP inhibition is through phosphorylation of the smooth muscle-specific MLCP inhibitor protein, CPI-17 (PKC potentiated inhibitor protein-17 kDa; Eto et al. 1997; Kitazawa et al. 2000). When phosphorylated at Thr38 CPI-17 inhibits MLCP activity by 1000-fold (Eto et al. 1997; Senba et al. 1999). Selective depletion of CPI-17 by skinning of smooth muscle cells eliminates PKC-induced Ca2+ sensitization of artery, and the response can be reconstituted by addition of PKC and CPI-17 together but not by PKC alone (Kitazawa et al. 1999). Stimulation of arterial smooth muscle with not only PKC activators but also several agonists and the non-hydrolysable GTP analogue, GTPγS, induces phosphorylation of CPI-17 at Thr38 paralleling the contractile Ca2+ sensitization (Kitazawa et al. 2000, 2003; Niiro et al. 2003). This phosphorylation is suppressed by both GF-109203X (a PKC inhibitor) and Y-27632 (a Rho-kinase inhibitor). The expression level of CPI-17 among various types of smooth muscles correlates with the degree of PKC-mediated Ca2+ sensitization (Woodsome et al. 2001). In tonic artery, which expresses high CPI-17 and low MLCP, CPI-17 is thought to be a dominant player in MLCP inhibition and Ca2+ sensitization. These observations strongly suggest that CPI-17 mediates PKC-dependent Ca2+ sensitization in vascular smooth muscles.
To further elucidate the function of CPI-17, a crucial experiment would be to remove or down-regulate CPI-17 expression and study its effects on smooth muscle contractility and Ca2+ sensitization. Here, we report that CPI-17 is not detected in chicken smooth muscle tissues, such as artery, gizzard and small intestine. We investigated how PKC activation and G protein-coupled receptor activation affect contraction and MLC phosphorylation in the ‘CPI-17-deficient’ artery. Furthermore, we attempted the reconstitution of contractile Ca2+ sensitization in chicken artery with recombinant CPI-17. Our results strongly support the hypothesis that CPI-17 plays a key role in receptor-mediated Ca2+ sensitization in vascular smooth muscles. A part of these findings has been presented at the annual Biophysical Society Meeting (Li & Kitazawa, 1998).
Methods
Tissue preparation and force measurement
All animal procedures were approved by the Animal Care and Use Committee of Boston Biomedical Research Institute. Adult male New Zealand White rabbits, chickens (Charles River Laboratories, Wilmington, MA, USA) and homing pigeons (Double T Farm, Glenwood, IA, USA) were killed by halothane overdose. Smooth muscle strips (600 μm in width, 2–2.5 mm in length and natural wall thickness) were dissected from mesenteric arteries and mounted on a force transducer (AM801, SensoNor, Horten, Norway) set-up as previously described (Masuo et al. 1994). Force levels were monitored throughout the experiments.
Solutions
The compositions of external solutions for intact smooth muscle strips have been described previously (Woodsome et al. 2001). The standard relaxing solution used for resting states of the α-toxin- and β-escin-permeabilized strips contained the following (Masuo et al. 1994): 74.1 mm potassium methanesulphonate, 2 mm Mg2+, 4.5 mm MgATP, 1 mm EGTA, 10 mm creatine phosphate, 30 mm piperazine-N,N′-bis(2-ethanesulphonic acid), 1 mm dithiothreitol, pH 7.1 adjusted with KOH at 20°C. In the activating solution, 10 mm EGTA was used and a calculated amount of calcium methanesulphonate was added to give the final desired concentration of free Ca2+ ions. Ionic strength of 0.2 m was achieved by appropriately using more or less potassium methanesulphonate. For Triton X-100-skinned smooth muscle, the solution was modified to prevent the deterioration of contraction. The standard relaxing solution for the skinned smooth muscle had 0.13 m ionic strength, and contained 1 mm Mg2+, 3.5 mm MgATP, 0.5 μm calmodulin, 0.1% fatty acid-free BSA, 1 mm EGTA, 10 mm creatine phosphate, 30 mm piperazine-N,N′-bis(2-ethanesulphonic acid) and 1 mm dithiothreitol, pH 7.1 (adjusted with KOH). The experiments using intact smooth muscle were carried out at 30°C. All experiments for permeabilized smooth muscles were carried out at 20°C.
Cell permeabilization
After measuring the force of contractions induced by high K+ (154 mm) and by phenylephrine (30 μm) in freshly dissected strips, these strips were incubated in the standard relaxing solution for several minutes. For membrane permeabilization with α-toxin, the strips were treated for 30 min at 30°C with 20 μg ml−1 of purified Staphylococcus aureusα-toxin (List, Campbell, CA, USA) at pCa 6.7 buffered with 10 mm EGTA and then treated with 10 μm of the Ca2+ ionophore A23187 for 20 min at 25°C to deplete the SR of Ca2+ and maintain the cytoplasmic Ca2+ constant (Masuo et al. 1994). For membrane permeabilization with β-escin (Sigma), the strips were treated with 60 μmβ-escin in the relaxing solution for 45 min at 5°C and then for 20 min at 30°C together with 10 μm A23187 (Masuo et al. 1994). To make skinned preparations, we used 0.1% Triton X-100 (Sigma) in the relaxing solution for 30 min at 5°C and for 15 min at 30°C (Kitazawa et al. 1999). Experiments in intact smooth muscle preparations were carried out at 30°C. For all permeabilized preparations, experiments were carried out at 20°C to minimize the deterioration of contractility.
Measurement of MLC phosphorylation
Details of MLC phosphorylation measurements using two-dimensional gel electrophoresis have been previously described (Kitazawa et al. 1991a).
Expression and purification of recombinant CPI-17
Recombinant hexahistidine-tagged CPI-17 was expressed in E. coli and purified to homogeneity as previously described (Eto et al. 1997).
Antibodies
We used three different phosphorylation-independent and one phospho[Thr38]-dependent anti-CPI-17 antibodies. Chicken anti-CPI-17 IgY and rabbit anti-CPI-17 IgG were against hexahistidine-tagged porcine CPI-17 and affinity-purified (Senba et al. 1999; Kitazawa et al. 2000). Rabbit anti-CPI-17 peptide antibody was made against the residues 35–46 (100% identity between human and mouse) and affinity-purified. The rabbit anti-phospho[Thr38]-CPI-17 (anti-p[Thr38]) has been previously described (Kitazawa et al. 2000). These antibodies cross-react with pig, rabbit, rat, mouse and pigeon CPI-17 (Eto et al. 1999; Woodsome et al. 2001). The rabbit anti-phospho[Thr695]-MYPT1 (anti-p[Thr695]) has been previously described (Kitazawa et al. 2003). Rabbit polyclonal anti-PP1Cδ and PHI-1 antibodies were prepared and affinity-purified (Eto et al. 1999). Polyclonal anti-h-CaD and anti-h-CaP antibodies were provided by Dr K. Mabuchi (Boston Biomedical Research Institute). Monoclonal anti-MLC20, and polyclonal anti-actin, PKCα and PKCɛ were from Sigma. Monoclonal anti-RhoA, and polyclonal anti-G13α, anti-ERK1 and anti-p38MAPK were from SantaCruz Biotech (Santa Cruz, CA, USA). Monoclonal anti-Rho-kinase antibody was from Transduction Laboratories (Lexington, KY, USA), polyclonal anti-MYPT1 antibody from Babco (Richmond, CA, USA), and polyclonal anti-Raf1 antibody from Calbiochem. The dilutions of antibody used for Western blots were as follows: chicken anti-CPI-17 IgY antibody (× 10 000), rabbit anti-CPI-17 (× 2000), anti-phospho[Thr38]-CPI-17 (× 2000), anti-peptide(35–46)-CPI-17 (× 500), anti-PP1Cδ (× 10 000), anti-PHI-1 (× 2500), anti-h-CaD (× 10 000), anti-h-CaP (× 10 000), anti-MLC20 (× 2000), anti-actin (× 5000), anti-PKCα (× 10 000), anti-PKCɛ (× 7500), anti-RhoA (× 500), and anti-G13α (× 250), anti-ERK1 (× 1000) and anti-p38MAPK (× 1000), anti-Rho-kinase (× 1000), and anti-MYPT1 (× 5000).
Western blotting
Immuno-blotting experiments were performed as previously described in detail (Woodsome et al. 2001).
Reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was isolated from tissues using RNeasy Fibrous Tissue Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer's instructions. Purified RNA was eluted in 30 μl RNase-free dH2O, and the typical yield of RNA from 15 mg of wet smooth muscle tissue was ∼25 μg.
The reverse transcription and the subsequent PCR steps were carried out in a single-tube system using OneStep RT-PCR Kit (QIAGEN) according to the manufacturer's instructions. The primers were designed against the murine CPI-17 sequence as follows. For sense: 5′-GTC ACC GTC AAG TAC GAC CG-3′; and for anti-sense: 5′-GGT CCT GGC GGG GGC TCA GGC TG-3′ (Integrated DNA Technologies, Inc., IA, USA). Each reaction consisted of 50 μl total volume, containing 0.5–1 μg RNA, 0.6 μm each of sense and anti-sense primers, 8 U RNase inhibitor (Roche, Germany), 400 μm dNTP mixture, and the reverse transcriptase–Taq polymerase mixture. The reverse transcription reaction was carried out at 50°C for 30 min. The reaction mixture was directly subjected to the PCR step, which was run for 35 cycles of melting at 94°C for 45 s, annealing at 55°C for 45 s, extension at 72°C for 45 s, then further extended at 72°C for 10 min; 10 μl of the PCR product was analysed on a 1.3% agarose gel. DNA fragments were detected by ethidium bromide staining and the sequence was confirmed on both strands (Tufts Core Facility, Boston, MA, USA).
Statistics
Results are expressed as the means ± s.e.m. of n experiments. Statistical significance was evaluated with Student's two-tailed t test (P < 0.05).
Results
Expressions of CPI-17 and other proteins in chicken smooth muscle tissues
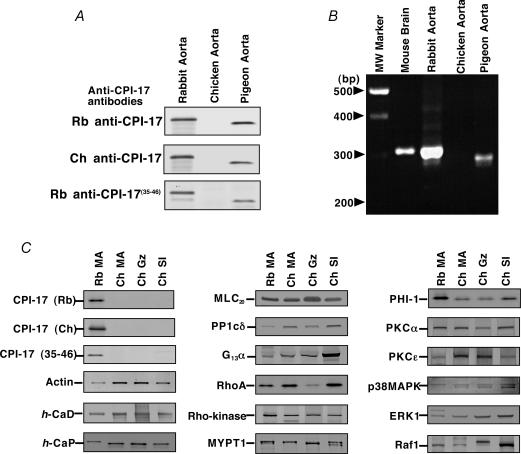
Three polyclonal anti-CPI-17 antibodies were used to detect CPI-17 in the SDS-extracts from arteries of rabbit (as a representative mammalian species known to express CPI-17 in the smooth muscle tissues; Woodsome et al. 2001), chicken and pigeon (as a control avian species). All immunoblots using these antibodies consistently showed no detectable expression of CPI-17 in chicken aorta (Fig. 1A) while high expression was found in rabbit and pigeon aortas. Moreover, RT-PCR was carried out from total RNA isolated from the aortas of all three species with mouse brain as positive control. Using a primer pair designed against regions of high homology among all known sequences of mammalian species (see Methods), a fragment of approximately 300 bp was generated from rabbit and pigeon aortas, but not from chicken aorta (Fig. 1B). In addition, CPI-17 protein was not detectable in chicken mesenteric artery (MA), gizzard (Gz) and small intestine (SI) from adult (Fig. 1C) and embryo (17-day) (not shown). PHI-1 (a member of the CPI-17 family; Eto et al. 1999), and the δ-isoform of type 1 protein phosphatase (a catalytic subunit of MLCP; Hartshorne et al. 1998) were expressed in chicken as well as rabbit smooth muscle tissues (Fig. 1C). Expressions of other signalling and contractile proteins such as actin, h-caldesmon (h-CaD), h-calponin (h-CaP), MLC20, G13α, RhoA, Rho-kinase (ROKα), MYPT1, PKCα and ɛ, p38MAPK, ERK1, and Raf-1 were more or less equivalent in rabbit and chicken tissues (Fig. 1C).
Figure 1. Expression of CPI-17 and other proteins in rabbit, pigeon and chicken smooth muscle tissues.
A, CPI-17 protein expression in rabbit, chicken and pigeon aortas. Top, a representative immunoblot probed using rabbit (Rb) anti-CPI-17 IgG antibody; middle, using chicken (Ch) anti-CPI-17 IgY; and bottom, using rabbit anti-CPI-17-peptide(35–46) IgG. Equal amounts of total protein (20 μg per lane) were added. The experiments were repeated three time using different animals of each species. B, CPI-17 mRNA expression. RT-PCR was performed from aortas of rabbit, chicken and pigeon. The mouse brain RNA was used as control. C, expression of other contractile and signalling proteins in Rb and Ch mesenteric artery (MA), chicken gizzard (Gz) and small intestine (SI). Per lane, 0.5 μg of total protein for actin and CaP, 2 μg for CaD and MLC20, and 20 μg for all others were applied. The experiments were repeated 4–6 times.
Agonist- and GTPγS-induced contraction and Ca2+ sensitization
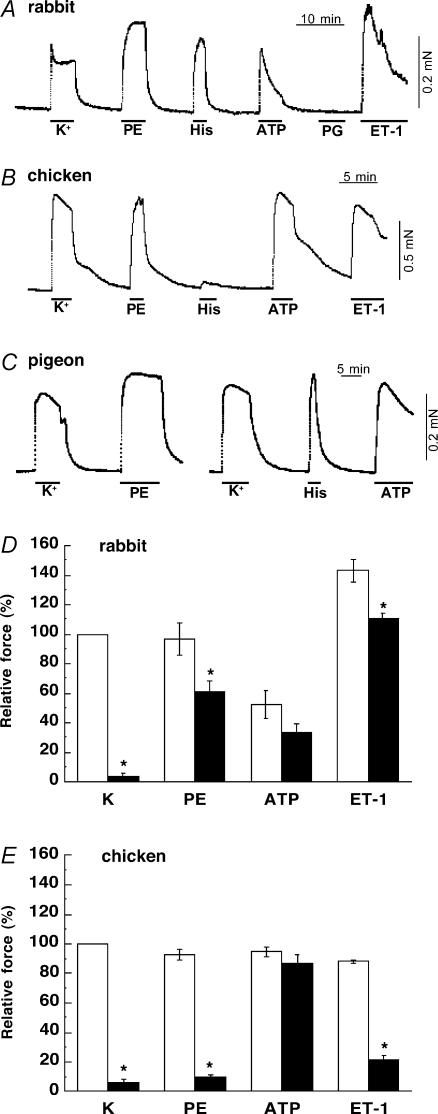
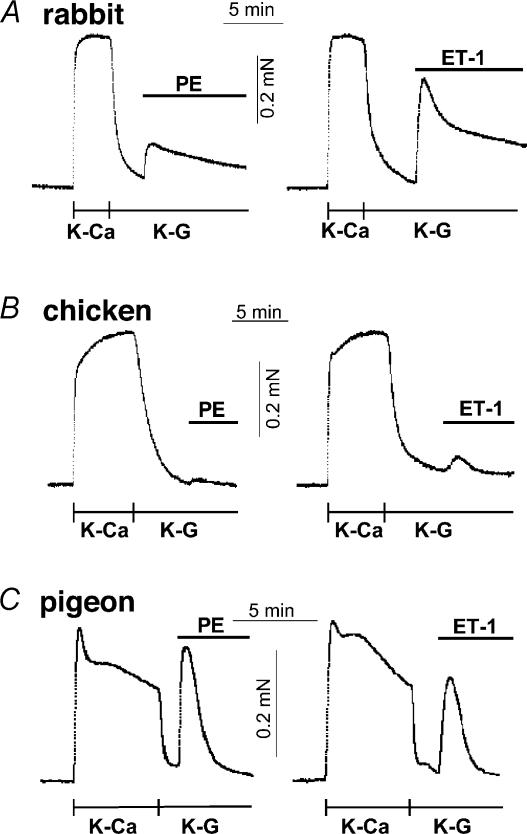
We used two types of excitatory agonists: the first class (α1-the agonist phenylephrine (PE), endothelin-1 (ET) and histamine) is linked to G protein-coupled Ca2+ sensitization (Somlyo & Somlyo, 2003), and the second (ATP) is coupled to ligand-gated non-selective cation channels (Abbracchio & Burnstock, 1998), and thus used as a negative control. Most of agonists except histamine evoked a large contraction to a level similar to that of high K+ (membrane depolarization) under the normal Ca2+-containing conditions in rabbit, pigeon and chicken mesenteric arteries (Fig. 2). It is known that, in rabbit artery, most of agonists can produce a large contraction even in the absence of Ca2+ influx (Himpens et al. 1990). Figure 2D and E summarizes the agonist-induced contractions and effect of a dihydropyridine Ca2+-channel blocker, nicardipine, in rabbit and chicken arteries, respectively. Nicardipine (0.2 μm) markedly diminished the development of high K+-induced contraction in both rabbit and chicken arteries. The blocker also markedly reduced PE- and ET-1-induced contractions in chicken artery (Fig. 2E), suggesting a large dependence on Ca2+ influx via dihydropyridine-sensitive Ca2+ channel. In rabbit artery, in contrast, agonist-induced contractions were only partially inhibited (Fig. 2D). ATP produced significant contraction in the presence of the blocker in both rabbit and chicken arteries. Removal of extracellular Ca2+ and addition of 2 mm EGTA under depolarized conditions almost abolished agonist-induced contractions in intact chicken artery (Fig. 3B). On the average, PE and ET-1 in the absence of Ca2+ produced only 3 ± 1% and 9 ± 2% (n = 5), respectively, of control high K+-induced contraction. In the CPI-17-rich arteries, however, even after removal of extracellular Ca2+ and further treatment with ryanodine to deplete the SR of Ca2+, significant contractions were induced by agonists without Ca2+ influx and Ca2+ release. In rabbit artery (Fig. 3A), PE and ET-1 evoked 21 ± 3% and 60 ± 7% of high K+-induced contraction (n = 5), respectively, and in pigeon artery (Fig. 3C), 75 ± 6% (n = 6) and 52 ± 6% (n = 3), respectively. These indicate significant contractile Ca2+ sensitization in rabbit and pigeon arteries. ATP-induced contraction was almost totally abolished under the Ca2+-free conditions in arteries of all species as predicted (not shown). These results together suggest little or no Ca2+ sensitization by agonists in intact chicken artery.
Figure 2. Effect of agonists in intact mesenteric arteries.
Contractions induced by high K+ and agonists in intact rabbit (A), chicken (B) and pigeon arteries (C) under the normal Ca2+ (2 mm)-containing conditions (n = 6). Before the experiments were started, strips were repeatedly stimulated with a cycle of high K+ for 3 min and rest for 10 min until the steady state contraction was obtained. PE, 30 μm phenylephrine; His, 30 μm histamine; ATP, 200 μm; PG, 1 μm prostaglandin F2α; ET-1, 1 μm endothelin-1. D and E show a summary of high K+- and agonist-induced contractions under the normal conditions (open column) and in the presence of 0.2 μm nicardipine (filled column) in rabbit and chicken MA, respectively (n = 4). *Significant difference from respective control contraction in the absence of nicardipine.
Figure 3. Agonist-induced contractions under the Ca2+-free, depolarized conditions in intact rabbit (A), chicken (B) and pigeon arteries (C).
High K+-induced contraction was abolished with removal of Ca2+ and addition of 2 mm EGTA. Then, 30 μm PE or 1 μm ET-1 was added. K-Ca, high-K+ solution containing 2 mm Ca2+. K-G, Ca2+-free, high-K+ solution containing 2 mm EGTA. Rabbit and pigeon arteries were pre-treated with 1 μm ryanodine in the K-G solution containing 30 mm caffeine for 30 min to deplete the SR of Ca2+ (Himpens et al. 1990). (n = 3–6.)
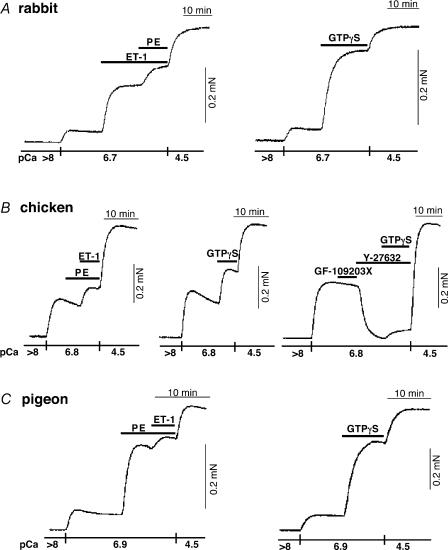
Figure 4 displays representative force traces showing effects of excitatory agonists and G protein activator on the contractile response of α-toxin-permeabilized and A23187-treated rabbit, chicken and pigeon vascular smooth muscles, in which intracellular Ca2+ stores were depleted and cytoplasmic Ca2+ concentration was maintained with 10 mm EGTA (Kitazawa et al. 1991a). In rabbit mesenteric artery at constant pCa 6.7, a single dose of a saturated concentration of PE and ET-1 produced significant contractions from 8 ± 1 to 52 ± 3 and 44 ± 6% (n = 3) of the maximum induced at pCa 4.5, respectively. In the pigeon artery at pCa 6.9, PE and ET-1 similarly potentiated from 19 ± 3 to 54 ± 7 (n = 6) and 56 ± 8%(n = 4), respectively. These potentiated contractions by different agonists are additive (Kitazawa et al. 1991) and the mixture of the agonists produced a larger contraction than a single agonist alone, but the amplitude was limited by the maximum force capacity (Fig. 4A and C). The non-selective G protein activator, GTPγS, markedly enhanced a submaximal contraction from 13 ± 3 to 77 ± 3% (n = 5) in rabbit artery (Fig. 4A) and from 16 ± 3 to 78 ± 3% (n = 5) for pigeon (Fig. 4C), indicating very significant G protein-mediated Ca2+ sensitization. In chicken artery, however, PE did not produce any significant contraction at constant Ca2+ (n = 7; Fig. 4B). ET-1 and GTPγS did significantly enhance a submaximal contraction at pCa 6.8 from 27 ± 3 to 44 ± 2% (n = 3) and from 32 ± 2 to 58 ± 2% (n = 9), respectively (Fig. 4B). This enhancement was significantly smaller as compared to those of rabbit and pigeon artery.
Figure 4. Effects of agonists and GTPγS on contractile force at clamped Ca2+ in α-toxin-permeabilized and A23187-treated mesenteric arteries.
A, left, a representative trace of effect of cumulative application of ET-1 (1 μm) and PE (30 μm) on submaximal contraction at pCa 6.7 in α-toxin-permeabilized rabbit arteries (n = 3). Right, GTPγS (30 μm)-induced contraction at pCa 6.7 (n = 5). B, left, PE did not increase a submaximal contraction at pCa 6.8 in α-toxin-permeabilized chicken artery (n = 7). ET-1 produced a significant contraction at pCa 6.8 (n = 5). Middle, GTPγS-induced contraction at pCa 6.8 (n = 9). Right, effect of 3 μm GF-109203X and 10 μm Y-27632 on pCa 6.8- and 30 μm GTPγS-induced contraction in chicken artery (n = 6). C, left, PE- and ET-1-induced contraction at pCa 6.9 in α-toxin-permeabilized pigeon arteries (n = 3). Right, GTPγS-induced contraction at pCa 6.9 (n = 5).
The Rho-kinase inhibitor, 10 μm Y-27632, more effectively inhibited basal and GTPγS-induced contractions in α-toxin-permeabilized chicken artery (Fig. 4B) than those of rabbit (Uehata et al. 1997; Kitazawa et al. 2000). In chicken artery, the pCa 6.8-induced contraction was insensitive to 3 μm GF-109203X (Kitazawa et al. 2000), a PKC inhibitor (n = 3), but markedly decreased by Y-27632 from 51 ± 4 to 1 ± 1% of maximum (n = 6). The basal Ca2+ sensitivity was significantly decreased but maximal contraction at pCa 4.5 was not altered (n = 3). In rabbit artery, the basal contraction at pCa 6.5 was significantly reduced from 14 ± 2 to 7 ± 3% (n = 3). In the presence of Y-27632, GTPγS only minimally increased the reduced contraction to 6 ± 4% in chicken artery (n = 6; Fig. 4B) while still significantly increased to 46 ± 5% in rabbit artery (n = 3). In contrast, a G protein inhibitor, GDPβS (0.3 mm), did not significantly inhibit the basal Ca2+-activated contraction in chicken artery (54 ± 9 versus 53 ± 7% at pCa 6.7; n = 3).
PKC-induced contraction and Ca2+ sensitization
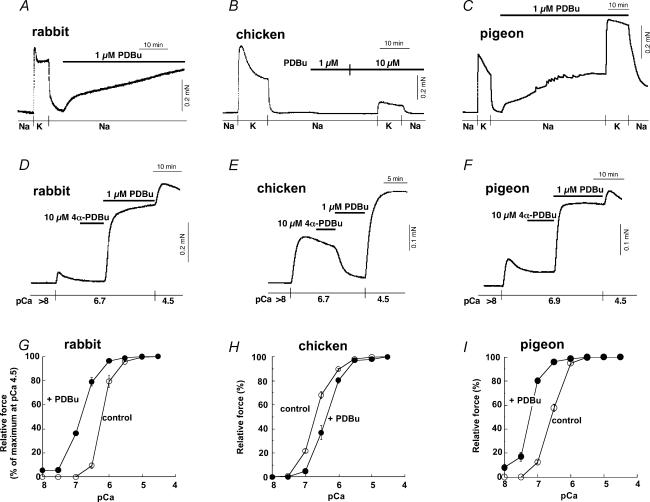
In rabbit and pigeon mesenteric arteries, the PKC activator, phorbol 12,13-dibutyrate (PDBu, 1 μm), generated a contraction at rest to a significant level (60 ± 10% of high K+-induced contraction in rabbit and 85 ± 9% for pigeon; n = 6) (Fig. 5A and C). In the chicken mesenteric artery, in contrast, even 10 μm PDBu evoked no measurable contraction at rest (n = 22)and, rather, inhibited high-K+-induced contraction (Fig. 5B). In α-toxin-permeabilized, A23187-treated rabbit and pigeon arteries, PDBu but not an inactive isomer, 4α-PDBu (Masuo et al. 1994), significantly enhanced a contraction (from 4 ± 1% at pCa 6.7 to 64 ± 6% of maximum contraction obtained at pCa 4.5 for rabbit and from 12 ± 3% at pCa 6.9 to 78 ± 4% for pigeon; n = 8) (Fig. 5D and F) and increased the contractile Ca2+ sensitivity (Fig. 5G and I). In the permeabilized chicken artery, in contrast, PDBu failed to increase and rather decreased a contraction (from 44 ± 2% at pCa 6.7 to 11 ± 2% of maximum; n = 13) (Fig. 5E) similar to that in intact preparations and reduced the Ca2+ sensitivity (Fig. 5H) whereas 4α-PDBu had no effect. A synthetic diacylglycerol (DAG), 1,2-sn-dihexanoylglycerol (Masuo et al. 1994), at 10 μm also enhanced a submaximal contraction in rabbit (from 11 ± 4% at pCa 6.7 to 68 ± 4%; n = 4) and pigeon arteries (from 18 ± 1% at pCa 6.9 to 71 ± 6%; n = 4) but reduced a submaximal contraction in chicken artery (from 39 ± 5% at pCa 6.7 to 6 ± 1%; n = 3) similar to the effect of phorbol ester. PDBu significantly increased MLC20 phosphorylation from 23 ± 3 to 49 ± 4% of total MLC20 (n = 5) at pCa 6.7 in rabbit artery, while PDBu significantly decreased MLC20 phosphorylation from 48 ± 2 to 35 ± 2% (n = 4) at pCa 6.7 in chicken artery, paralleling the effect on contraction. Notably, control Ca2+ sensitivity of both contraction and MLC phosphorylation in chicken mesenteric artery and contractile Ca2+ sensitivity in pigeon artery were higher than that of rabbit mesenteric artery (Fig. 5). The high Ca2+ sensitivity under control conditions and PDBu-induced Ca2+ desensitization were also observed in chicken carotid artery (not shown).
Figure 5. Effect of phorbol ester (PDBu) on contractile force in mesenteric arteries.
Time course of contractile effect of 1 μm PDBu following high K+-induced contraction in intact rabbit (A), chicken (B) and pigeon MA (C). Na, normal extracellular solution; K, high (154 mm)-K+ solution. Middle panel, effect of 1 μm PDBu on Ca2+-induced contraction in α-toxin-permeabilized rabbit (D), chicken (E) and pigeon MA (F). Height of pCa 4.5-induced contraction represents a maximal force in each arterial strip. Bottom panel (n = 4), control contractile Ca2+ sensitivity (○) and effect of 1 μm PDBu (•) in rabbit (G), chicken (H) and pigeon MA (I).
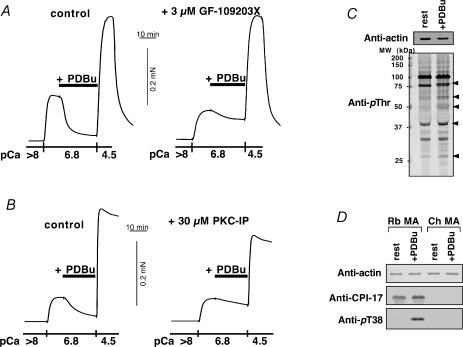
PKC inhibitors, GF-109203X (3 μm) and PKC pseudosubstrate inhibitor peptide(19–31) (PKC-IP; 30 μm) both, but not a conventional PKC isoform-specific inhibitor Go6976 (10 μm), significantly inhibited PDBu-induced relaxation in α-toxin- and β-escin-permeabilized chicken mesenteric artery, respectively (Fig. 6A and B). PDBu significantly increased phosphorylation levels of several proteins in intact chicken artery monitored by immunoblotting with anti-phosphothreonine antibody (Fig. 6C). Using phospho[Thr38]CPI-17-specific antibody, the immunoblots showing Thr38 phosphorylation of CPI-17 by PDBu was clearly identified in rabbit artery, but not found in chicken artery (Fig. 6D).
Figure 6. Effect of PKC inhibitors on PDBu-induced relaxation and immunoblots using anti-phosphoprotein antibodies in chicken mesenteric artery.
A, effect of 3 μm GF-109203X on 1 μm PDBu-induced relaxation in α-toxin-permeabilized chicken mesenteric artery (n = 3). Left trace, control PDBu-induced relaxation. Right trace, 3 μm GF-109203X was added 10 min before and present throughout the experiment. B, effect of 30 μm PKC pseudosubstrate inhibitor peptide(19–31) (PKC-IP; the peptide of 2000 Da cannot penetrate cells through α-toxin pores but can through β-escin pores; Kobayashi et al. 1989) on 1 μm PDBu-induced relaxation in β-escin-permeabilized chicken artery (n = 3). Left, control. Right, 30 μm PKC-IP was added 10 min before increase in Ca2+ and was present throughout the experiment. C, protein phosphorylation by PDBu in chicken artery (n = 3). Intact chicken artery strips were exposed to either the normal external solution (rest) or 1 μm PDBu-containing solution for 10 min and quickly frozen. The SDS-extracts were used to monitor phosphorylation levels by immunoblotting using anti-phosphothreonine (anti-pThr) antibody. For immunoblotting for actin, the extracts were diluted 30 times. Arrowheads show more than twice increase in blot density. D, phosphorylation of CPI-17 at Thr38 by 1 μm PDBu in intact rabbit (Rb) and chicken (Ch) mesenteric artery (MA) (n = 3). Top panel shows immunoblots using anti-actin, middle panel using anti-CPI-17, and lowest panel using anti-pThr38 CPI-17 antibodies. SDS-extract containing 20 μg of total protein was applied in each lane for anti-CPI-17 and anti-pT38 immunoblottings. For actin, the extracts were diluted 40 times. Phosphorylation level of CPI-17 at Thr38 was dramatically increased in the PDBu-treated rabbit MA (13, 16). Either polyclonal anti-CPI-17 or anti-pThr38 antibody did not show any bands in chicken MA extracts in the presence and absence of PDBu.
Reconstitution of Ca2+ sensitization in chicken artery
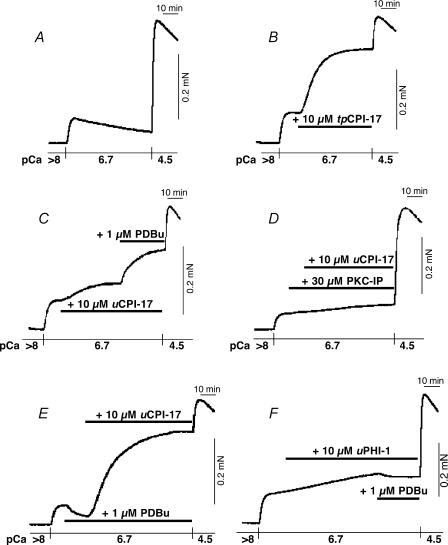
In Triton X-100-skinned smooth muscle in which endogenous CPI-17, if any, was depleted (Kitazawa et al. 1999), addition of 5 μm pre-thiophosphorylated recombinant CPI-17 (tpCPI-17) consistently enhanced a basal contraction to near maximum levels at pCa 6.7 in Triton X-100-skinned artery smooth muscles from all three species (for rabbit, from 4 ± 1 to 74 ± 4% of maximum at pCa 4.5; for pigeon, from 24 ± 2 to 96 ± 2%; for chicken, from 7 ± 2 to 77 ± 4%; n = 3). This result suggests that the activated CPI-17 if expressed is able to inhibit the in situ MLCP and induce a significant Ca2+ sensitization in chicken artery similar to rabbit and pigeon arteries.
In β-escin-permeabilized chicken mesenteric artery strips, in which G protein-coupled signal transduction and PKC signalling were still retained (Kobayashi et al. 1989), 10 μmtpCPI-17 markedly increased a pCa 6.7-activated contraction from 17 ± 4% to 66 ± 5% of maximum contraction at pCa 4.5 with a half-time (t½) of 11 ± 1 min (n = 3; Fig. 7B compared to control Fig. 7A), comparable to those in β-escin-permeabilized rabbit femoral artery (Li et al. 1998) and also in Triton X-100-skinned chicken mesenteric artery. Unphosphorylated CPI-17 (uCPI-17) slowly (t½ of 17 ± 1 min) increased the basal contraction to a significantly lower level (from 19 ± 3 to 45 ± 5% of maximum; n = 4; Fig. 7C) than that of tpCPI-17 (Fig. 7B). PKC-IP (30 μm) inhibited the uCPI-17- (Fig. 7D) but not tpCPI-17-induced contraction (not shown), suggesting that spontaneous activity of endogenous PKC is responsible for the main fraction of uCPI-17-induced contraction. In the presence of uCPI-17, 1 μm PDBu significantly enhanced the contraction from 43 ± 5 to 66 ± 2% (n = 3; Fig. 6C). PDBu alone, however, reduced the basal pCa 6.7-induced contraction (Fig. 7E) similar to that in α-toxin-permeabilized artery (Fig. 5E). The uCPI-17, but not a mutant [T38A] CPI-17 (Kitazawa et al. 1999), enhanced the PDBu-reduced contraction (7 ± 0%) to higher level (71 ± 4%) with a shorter t½ of 13 ± 0 min (n = 3; Fig. 7E) than those in the absence of PDBu (Fig. 7C before addition of PDBu). The uCPI-17 in the presence of PDBu significantly reduced the speed of relaxation (t½ was changed from control 2.4 ± 0.1 min to 5.5 ± 0.2 min; n = 3) from pCa 4.5-induced contraction when MLCK activity was blocked by removal of Ca2+ and addition of 10 mm EGTA and 0.2 mm ML-9, a MLCK kinase inhibitor (Masuo et al. 1994), suggesting inhibition of MLCP by CPI-17. Addition of unphosphorylated recombinant PHI-1 (10 μm) caused very slow development (t½ > 40 min) of contraction at pCa 6.7 to a low level from 13 ±5 to 25 ± 5% of maximum at 80 min (Fig. 7F). Unlike in the presence of unphosphorylated CPI-17, PDBu did not potentiate the contraction in the presence of unphosphorylated PHI-1 (25 versus 24 ± 5%; n = 3).
Figure 7. Reconstitution of PKC-induced contractile Ca2+ sensitization in β-escin-permeabilized chicken mesenteric artery.
A, control force trace at pCa 6.7. Height of contraction at pCa 4.5 shows a maximum force. B, effect of 10 μm pre-thiophosphorylated recombinant CPI-17 (tpCPI-17) on submaximal contraction at pCa 6.7. C, effect of 10 μm unphosphorylated recombinant CPI-17 (uCPI-17) and 1 μm PDBu. D, effect of uCPI-17 in the presence of 30 μm PKC-IP.E, effect of uCPI-17 in the presence of 1 μm PDBu. F, effect of 10 μmuPHI-1 and PDBu. Each force trace is a representative of three to four similar experiments.
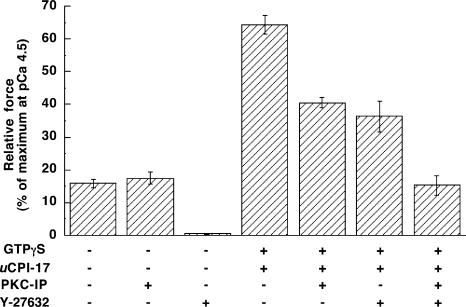
Figure 8 summarizes the effects of inhibitors on CPI-17 plus GTPγS-mediated Ca2+ sensitization of β-escin-permeabilized chicken artery at pCa 6.7. PKC-IP had no effect on the basal contraction even in the presence of GTPγS (n = 3) while either 10 μm Y-27632 or 3 μm H-1152 (a more selective Rho-kinase inhibitor; Sasaki et al. 2002) strongly diminished the basal pCa 6.7-activated contraction even in the presence of GTPγS to 1 ± 0%(n = 4) and 0 ± 0%(n = 3) similar to those in α-toxin-permeabilized preparations (Fig. 4B). GTPγS (30 μm) alone significantly but only partially potentiated the basal contraction at pCa 6.7 from 16 ± 1 to 27 ± 4% of maximum (n = 3), similar to that observed in α-toxin-permeabilized arterial smooth muscle (Fig. 4B). In the presence of GTPγS, uCPI-17 rapidly (t½ of 10 ± 1 min) enhanced the contraction to 64 ± 3% (Fig. 8) not significantly different from those of tpCPI-17 (Fig. 7B). The amplitude and speed of potentiation were significantly (P < 0.05 and 0.01, respectively) higher than those of uCPI-17 alone (Fig. 7C). Addition of PKC-IP partially eliminated the contractility induced by uCPI-17 in the presence of GTPγS to 41 ± 2%(n = 3). Y-27632 also partially reduced contraction induced by CPI-17 plus GTPγS at pCa 6.7–36 ± 5%. Addition of Y-27632 and PKC-IP together suppressed the contractility induced by uCPI-17 plus GTPγS to a level not significantly different from the basal level (P= 0.89; n = 5).
Figure 8. Effects of PKC-IP and Y-27632 on GTPγS-induced Ca2+ sensitization in the presence of uCPI-17 in β-escin-permeabilized chicken mesenteric artery.
Strips were pre-activated in pCa 6.7 solutions for 10 min and then 30 μm PKC-IP and/or 10 μm Y-27632 were applied. After a new steady state level of contraction was achieved in the presence of the inhibitors, 30 μm GTPγS and 10 μm uCPI-17 were added. n = 3–4.
Discussion
CPI-17 was undetectable in chicken smooth muscles (aorta, mesenteric artery, gizzard and small intestine) by immunoblotting or RT-PCR, whereas it was expressed in mammalian smooth muscle tissues, such as rabbit femoral artery (at 7 μm) and rabbit vas deferens (at 0.8 μm) (Woodsome et al. 2001). The antigen of three antibodies and the primers used for RT-PCR in this study are derived from known mammalian CPI-17 sequences. The possibility therefore remains that CPI-17 was not detected in chicken smooth muscle tissues due to substantial sequence differences from known mammalian CPI-17 sequences at both the nucleotide and amino acid levels. Yet, the fact that CPI-17 was easily detected in another avian species (pigeon) together with the results of the functional studies strongly supports the idea that CPI-17 is deficient in chicken smooth muscle tissues examined. Interestingly, MYPT1 of MLCP is known to undergo phenotypic switch around the time of hatching from leucine zipper positive to negative isoforms in chicken gizzard smooth muscle (Khatri et al. 2001), and this coincided with the development of cGMP-resistant phenotype in the tissue. However, we did not observe changes in CPI-17 immuno-detectability between embryonic and adult chicken smooth muscle tissues. Therefore, the ‘deficiency’ of CPI-17 in chicken smooth muscle may not be developmentally regulated, and instead this might be a result of extensive inbreeding of cloned strains for agricultural purposes. Further genomic study is needed to clarify the nature of the mutation in chicken.
The absence of CPI-17 in chicken artery presented a useful model system for studying the role of CPI-17 in Ca2+ sensitization. In chicken and rabbit/pigeon arteries, agonists, such as PE, ET-1 and ATP, can produce a contraction to a level similar to that induced by high K+. Histamine, known to strongly promote phosphorylation of CPI-17 in rabbit femoral artery (Kitazawa et al. 2000), failed to induce contraction in chicken mesenteric artery, possibly due to lack of CPI-17, histamine receptor and/or the specific G-proteins coupled to the receptor. In rabbit and pigeon arteries, in the absence of extracellular Ca2+ in the ryanodine-treated intact muscles or at clamped Ca2+ concentration in the α-toxin-permeabilized, A23187-treated muscles, agonists still produced large contractions, showing significant Ca2+ sensitization (Himpens et al. 1990; Kitazawa et al. 1991a). In chicken artery, in contrast, PE and ET-1 evoked only minor contraction under the same conditions. These results suggest that agonist- as well as high K+-induced contractions in chicken artery are predominantly regulated by classical Ca2+-dependent mechanism such as MLC20 phosphorylation by Ca2+/calmodulin-activated MLCK (Hartshorne, 1987; Kamm & Stull, 1989). The absence of extracellular Ca2+ without ryanodine treatment or the presence of Ca2+ channel blocker also strongly reduced agonist-induced contractions in chicken artery, further suggesting a minor role for the SR Ca2+ release during agonist-induced contraction. The direct G protein activator, GTPγS, produced significantly less Ca2+ sensitization in chicken than in rabbit and pigeon arteries. The G protein activation with supplement of uCPI-17 in chicken artery was able to make a larger and faster Ca2+ sensitization similar to those of CPI-17-rich arteries of rabbit and pigeon. These results together suggest that CPI-17 is at least partially required for strong G-protein coupled receptor-mediated Ca2+ sensitization of smooth muscle.
PKC activation did not evoke significant contraction in either intact or permeabilized chicken artery, while the same PKC activators can elicit a large contraction in rabbit and pigeon arteries. A reasonable interpretation of our data is that the absence of CPI-17 is accountable for the lack of PKC-mediated Ca2+ sensitization in chicken smooth muscles. Several lines of evidence suggest that PKC itself is functional in chicken artery. First, active but not inactive form of PDBu and also DAG analogue affected (reduced) the Ca2+-activated contraction in chicken artery. Second, both Ca2+-dependent and Ca2+-independent isoforms of PKC and signalling proteins implicated in the PKC pathway (such as MAPKs, Raf-1, h-CaP, and h-CaD; Morgan & Gangopadhyay, 2001) were expressed in chicken smooth muscles as well as in rabbit artery. Third, PDBu significantly increased phosphorylation levels of several proteins in chicken artery detected by immunoblotting. Finally, PKC inhibitors reduced PDBu-induced relaxation in chicken artery. Therefore, that PKC activation was not capable of developing a contraction in chicken artery strongly suggests that this is due to the lack of CPI-17. Yet, chicken artery retains the contractile ability to respond to PKC activation, provided that CPI-17 is made available. This is demonstrated by the large Ca2+ sensitization induced by PKC activator and recombinant uCPI-17 together in chicken artery. It is consistent with our previous finding in rabbit smooth muscles (Woodsome et al. 2001), that the expression level of CPI-17 is a key factor in determining the contractile profile of PKC-mediated Ca2+ sensitization in various smooth muscle tissues. There are some reports, however, demonstrating that phorbol ester caused a relaxation of high K+- and/or agonist-induced contractions in some types of intact mammalian smooth muscle tissues that could express CPI-17 (Mitsui & Karaki, 1993; Tajima et al. 1997; Chakder et al. 2001). In those cases, the major relaxant action was correlated with a reduction in intracellular Ca2+ by phorbol ester but not Ca2+ desensitization similar to that observed in the CPI-17-deficient chicken artery (this study).
Interestingly, the closely related phosphatase inhibitor protein, PHI-1 (Eto et al. 1999), is expressed in chicken, suggesting that this protein is unable to couple PKC signalling to contraction despite their similarities. In fact, unlike CPI-17, 10 μm recombinant PHI-1 incorporated into chicken artery did not elicit PDBu-induced Ca2+ sensitization. This can be also supported by biochemical evidence that phosphorylation of PHI-1 by PKC was much slower (about one-fifth; M. Eto, unpublished result) and IC50 of phosphorylated PHI-1 for MLCP was about one order of magnitude higher than those of CPI-17 under similar conditions (Eto et al. 1999). These results suggest that PHI-1 is not responsible for PKC-mediated MLCP inhibition. The thin filament-associated proteins, h-CaD and h-CaP, are also expressed in chicken as well as rabbit smooth muscles. Even under Ca2+-clamped conditions, however, PKC activation caused a relaxation associated with a decrease in MLC20 phosphorylation in chicken artery, while in rabbit artery PDBu-induced contraction was developed together with an increase in MLC20 phosphorylation (Itoh et al. 1993; Masuo et al. 1994). Supplement of uCPI-17 into chicken artery switched PKC activator-induced relaxation to contraction. This is consistent with the fact that Ca2+ sensitization in Triton X-100-skinned rabbit artery (in which endogenous CPI-17 and PKC are depleted while h-CaD and h-CaP still remain as in intact preparations) is reconstituted by addition of PKC together with uCPI-17 but not PKC alone (Kitazawa et al. 1999). Since CPI-17 and PKC activator together markedly reduced the relaxation rate even when MLCK was blocked, it strongly supports the model that PKC-induced contraction is mediated through the CPI-17/MLCP pathway in smooth muscles.
PKC is also believed to at least partly mediate G protein-coupled receptor signalling in Ca2+ sensitization (Yoshida et al. 1994; Brozovich, 1995; Parsons et al. 1996; Buus et al. 1998; Kitazawa et al. 2000). How does GTPγS trigger the Ca2+ sensitization in the CPI-17-deficient artery in which PKC activation does not lead to contraction? The Rho-kinase inhibitor, Y-27632 or H-1152, effectively inhibited GTPγS-induced contractions in chicken artery similar to rabbit smooth muscles (Uehata et al. 1997; Sasaki et al. 2002), suggesting that the Ca2+ sensitizing pathway downstream of G protein requires Rho-kinase activation. However, consistent with the results from rabbit smooth muscles (Kitazawa et al. 2003; Niiro et al. 2003), phosphorylation of MYPT1 Thr695 (an inhibitory site in chicken sequence; Feng et al. 1999) was unchanged during GTPγS-induced contraction in chicken artery (not shown). Signalling pathways involved in Ca2+ sensitization mediated by RhoA/Rho-kinase in CPI-17-deficient chicken artery (this study) and CPI-17-minimal rabbit phasic visceral smooth muscles (Woodsome et al. 2001; Kitazawa et al. 2003) are unclear and remain to be determined. Interestingly Y-27632 and H-1152 also strongly suppressed Ca2+-dependent contractility of chicken artery similar to an appreciable degree of inhibition of the tonic component of high K+-induced contraction (Mita et al. 2002; Urban et al. 2003). This is consistent with the reports that RhoA and Rho-kinase in rabbit arteries are activated in response to elevation of cytosolic Ca2+ concentration (Urban et al. 2003; Sakurada et al. 2003). Y-27632 might be more sensitive in Ca2+-induced contraction of arterial smooth muscle, as compared to Ca2+ sensitization. The CPI-17-deficient chicken smooth muscle will be a useful model for studying not only the role of CPI-17 but also other potential mechanism(s) regulating smooth muscle contractility.
In conclusion, the absence of CPI-17 strongly correlates with the lack of PKC-dependent and at least in part with the minimal agonist-induced Ca2+ sensitization of contraction in chicken arterial smooth muscle. Thus, CPI-17 is one of the key players in the G protein-coupled receptor-mediated Ca2+ sensitization in vascular smooth muscles.
Acknowledgments
We thank Dr Katsuhide Mabuchi for kind gift of anti-CaP and anti-CaD antibodies, and also Kazuyo Kitazawa and Mallappa Anitha for technical assistance. We thank Dr Lin Li for two-dimensional gel analysis of myosin light chain phosphorylation in the early stage of the present project. This work was supported by National Institute of Health grants R01HL51824 and HL70881 to T.K and a Scientist Development Grant from the AHA National Center to M.E.
References
- Abbracchio MP, Burnstock G. Purinergic signalling: Pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- Brozovich FV. PKC regulates agonist-induced force enhancement in single alpha-toxin-permeabilized vascular smooth muscle cells. Am J Physiol. 1995;37:C1202–C1206. doi: 10.1152/ajpcell.1995.268.5.C1202. [DOI] [PubMed] [Google Scholar]
- Buus CL, Aalkjaer C, Nilsson H, Juul B, Moller JV, Mulvany MJ. Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries. J Physiol. 1998;510:577–590. doi: 10.1111/j.1469-7793.1998.577bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakder S, Sarma DNK, Rattan S. Mechanism of internal anal sphincter smooth muscle relaxation by phorbol 12,13-dibutyrate. Am J Physiol. 2001;280:G1341–G1350. doi: 10.1152/ajpgi.2001.280.6.G1341. [DOI] [PubMed] [Google Scholar]
- Eto M, Karginov A, Brautigan DL. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: Its specific location in smooth muscle. FEBS Lett. 1997;410:356–360. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trend Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ. Biochemistry of the contractile process in smooth muscle. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 423–482. [Google Scholar]
- Hartshorne DJ, Ito M, Erdodi F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Himpens B, Kitazawa T, Somlyo AP. Agonist-dependent modulation of Ca2+-sensitivity in rabbit pulmonary artery smooth muscle. Pflugers Arch. 1990;417:21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- Itoh H, Shimomura A, Okubo S, Ichikawa K, Ito M, Konishi T, Nakano T. Inhibition of myosin light chain phosphatase during Ca2+-independent vasocontraction. Am J Physiol. 1993;265:C1319–C1324. doi: 10.1152/ajpcell.1993.265.5.C1319. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Regulation of smooth muscle contractile elements by second messengers. Ann Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem. 2001;276:37250–37257. doi: 10.1074/jbc.M105275200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng JH, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–889. doi: 10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP. G-protein-mediated Ca2+-sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991a;266:1708–1715. [PubMed] [Google Scholar]
- Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci U S A. 1991b;88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in Triton X-100-demembranated rabbit arterial smooth muscle. J Physiol. 1999;520:139–152. doi: 10.1111/j.1469-7793.1999.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Kitazawa T, Somlyo AV, Somlyo AP. Cytosolic heparin inhibits muscarinic and α-adrenergic Ca2+ release in smooth muscle. J Biol Chem. 1989;264:17997–18004. [PubMed] [Google Scholar]
- Kubota Y, Nomura M, Kamm KE, Mumby MC, Stull JT. GTPγS-dependent regulation of smooth muscle contractile elements. Am J Physiol. 1992;262:C405–C410. doi: 10.1152/ajpcell.1992.262.2.C405. [DOI] [PubMed] [Google Scholar]
- Li L, Eto M, Lee MR, Morita F, Yazawa M, Kitazawa T. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol. 1998;508:871–881. doi: 10.1111/j.1469-7793.1998.871bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kitazawa T. PKC activators fail to sensitize α-toxin-permeabilized chicken smooth muscle to Ca2+ Biophys J. 1998;74:A151. [Google Scholar]
- Masuo M, Reardon S, Ikebe M, Kitazawa T. A novel mechanism for the Ca2+-sensitizing effect of protein kinase C on vascular smooth muscle: Inhibition of myosin light chain phosphatase. J General Physiol. 1994;104:265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita M, Yanagihara H, Hishinuma S, Saito M, Walsh MP. Membrane depolarization-induced contraction of rat caudal arterial smooth muscle involves Rho-associated kinase. Biochem J. 2002;364:431–440. doi: 10.1042/BJ20020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui M, Karaki H. Contractile and relaxant effects of phorbol ester in the intestinal smooth muscle of guinea-pig taenia caeci. Br J Pharmacol. 1993;109:229–233. doi: 10.1111/j.1476-5381.1993.tb13558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan KG, Gangopadhyay SS. Signal transduction in smooth muscle: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol. 2001;91:953–962. doi: 10.1152/jappl.2001.91.2.953. [DOI] [PubMed] [Google Scholar]
- Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J. 2003;369:117–128. doi: 10.1042/BJ20021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SJ, Summer MJ, Garland CJ. Phospholipase A2 and protein kinase C contribute to myofilament sensitization to 5-HT in the rabbit mesenteric artery. J Physiol. 1996;491:447–453. doi: 10.1113/jphysiol.1996.sp021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation–induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline) sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Senba S, Eto M, Yazawa M. Identification of trimeric myosin phosphatase (PP1M) as a target for a novel PKC-potentiated protein phosphatase-1 inhibitory protein (CPI17) in porcine aorta smooth muscle. J Biochem Tokyo. 1999;125:354–362. doi: 10.1093/oxfordjournals.jbchem.a022294. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Tajima M, Hori M, Ozaki H, Karaki H. Effect of phorbol esters on cytosolic Ca2+ level, myosin phosphorylation and muscle tension in high K+-stimulated bovine tracheal smooth muscle. Jpn J Pharmacol. 1997;74:195–201. doi: 10.1254/jjp.74.195. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Urban NH, Berg KM, Ratz PH. K+ depolarization induces RhoA kinase translocation to caveolae and Ca2+ sensitization of arterial muscle. Am J Physiol. 2003;285:C1377–C1385. doi: 10.1152/ajpcell.00501.2002. [DOI] [PubMed] [Google Scholar]
- Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Suzuki A, Itoh T. Mechanisms of vasoconstriction induced by endothelin-1 in smooth muscle of rabbit mesenteric artery. J Physiol. 1994;477:253–265. doi: 10.1113/jphysiol.1994.sp020188. [DOI] [PMC free article] [PubMed] [Google Scholar]