Abstract
We tested the hypothesis that inhibition of synthesis of either nitric oxide (NO) or vasodilating prostaglandins (PGs) would not alter exercise hyperaemia significantly, but combined inhibition would synergistically reduce the hyperaemia. Fourteen subjects performed 20 min of moderate rhythmic forearm exercise (10% maximal voluntary contraction). Forearm blood flow (FBF) was measured by Doppler ultrasound. Saline or study drugs were infused (2 ml min−1) into the forearm via a brachial artery catheter to locally inhibit synthesis of NO and PGs during steady state exercise (NG-nitro-l-arginine methyl ester (l-NAME), 25 mg over 5 min to inhibit NO synthase (NOS); and ketorolac, 3 mg over 5 min to inhibit cyclooxygenase (COX)). After achieving steady state exercise over 5 min (control), l-NAME was infused for 5 min, followed by 2 min saline, then by a 5 min infusion of ketorolac, and finally by 3 min of saline (n = 7). Drug order was reversed in seven additional subjects, such that single inhibition of NOS or COX was followed by combined inhibition. FBF during exercise decreased to 83 ± 2% of control exercise (100%) with NOS inhibition, followed by a transient decrease to 68 ± 2% of control during COX inhibition. However, FBF returned to levels similar to those achieved during NOS inhibition within 2 min (80 ± 3% of control) and remained stable through the final 3 min of exercise. When COX inhibition was performed first, FBF decreased transiently to 88 ± 4% of control (P < 0.01), and returned to control saline levels by the end of ketorolac infusion. Addition of l-NAME reduced FBF to 83 ± 3% of control, and it remained stable through to the end of exercise. Regardless of drug order, FBF was ∼80% of steady state control exercise (P < 0.01) during the last 30 s of exercise. We conclude that (1) NO provides a significant, consistent contribution to hyperaemia, (2) PGs contribute modestly and transiently, suggesting a redundant signal compensates for the loss of vasodilating PGs, and (3) NO and PG signals appear to contribute independently to forearm exercise hyperaemia.
The vasodilating ‘factors’ responsible for exercise hyperaemia and how they interact remain uncertain (Shepherd, 1983; Rowell et al. 1996; Joyner & Proctor, 1999a). Although the list of potential candidate dilators is extensive, few seem to account for more than a fraction of exercise hyperaemia when their synthesis or actions are inhibited (Radegran & Saltin, 1999; Bian et al. 2001; Frandsen et al. 2001; Boushel et al. 2002; Hillig et al. 2003; Merkus et al. 2003). In healthy humans there is evidence to suggest that both nitric oxide (NO) and prostaglandins (PGs) can contribute to exercise hyperaemia (Kapoor & Wilson, 1993; Endo et al. 1994; Gilligan et al. 1994; Dyke et al. 1995; Hickner et al. 1997; Maxwell et al. 1998; Duffy et al. 1999; Frandsen et al. 2001; Boushel et al. 2002; Hillig et al. 2003), while other studies have shown no role for these substances (Endo et al. 1994; Shoemaker et al. 1996, 1997; Lang et al. 1997; Radegran & Saltin, 1999).
The magnitude of the reported role of NO or PGs is variable and may depend on a variety of factors including the method used to measure blood flow (Doppler ultrasound versus plethysmography), the route of drug administration (local versus systemic), and the timing of drug administration (at rest versus during contractions). For example, plethysmography may tend to overestimate the contribution of either NO or PGs to the dilatation (Kapoor & Wilson, 1993; Wilson & Kapoor, 1993b; Gilligan et al. 1994; Dyke et al. 1995; Engelke et al. 1996; Duffy et al. 1999) as it does not necessarily reflect ‘active’ muscle blood flow regulation. Additionally, systemic infusion of NOS inhibitors increases blood pressure and probably evokes potentially confounding cardiovascular reflexes (Radegran & Saltin, 1999; Sheriff et al. 2000; Frandsen et al. 2001; Boushel et al. 2002). It is also unclear whether drugs given before exercise reach the blood vessels likely to be perfused during contractions, and previous studies using this approach suggest that NO is mainly important in regulating blood flow at rest but not during exercise (Radegran & Saltin, 1999; Frandsen et al. 2001). Finally, only a limited number of studies in humans have investigated, with mixed findings, how NO and PGs might interact to promote exercise hyperaemia (Duffy et al. 1999; Boushel et al. 2002).
With this information as background, we sought to determine whether local inhibition of NO and PG synthesis during rhythmic handgrip exercise would reduce exercise hyperaemia. Our main hypothesis was that inhibition of either NO or PGs would not alter exercise hyperaemia significantly, but combined inhibition would synergistically reduce the hyperaemia, suggesting that these dilator mechanisms are part of a larger ‘redundant’ control mechanism.
Methods
Subjects
All protocols and procedures were approved by the Institutional Review Board at Mayo Clinic and met the requirements for human studies outlined in the Declaration of Helsinki. Each subject provided his or her written informed consent prior to participation in this study.
Fourteen healthy volunteers participated in this study. Subjects were normotensive, non-smoking and non-obese, and were not taking any medications other than oral contraceptives. A blood sample was obtained from females (n = 7) less than 24 h prior to the study to ensure none were pregnant. All females were tested during the placebo phase of oral contraception, or in the luteal phase of their menstrual cycle to minimize possible cardiovascular effects of sex-specific hormones. All subjects fasted overnight or for at least 3 h when studied in the afternoon, and refrained from caffeine, alcohol and exercise for 24 h prior to the study.
Instrumentation, hemodynamic measurements, and drug administration
The brachial artery was catheterized under aseptic conditions after infiltration of the area with 1–2 ml of 1% lidocaine. A standard 5 cm 20-gauge Teflon catheter was inserted into the non-dominant arm, and continuously flushed with heparinized saline (2 units ml−1, 3 ml h−1). Heart rate was measured via three-lead ECG. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure.
Saline or study drugs were administered via the brachial artery catheter using a three-port connector system that permitted simultaneous measurements of arterial pressure during drug infusions. Saline and study drugs were infused at 2 ml min−1, and saline infusion at these rates did not alter basal blood flow. NG-Nitro-l-arginine methyl ester (l-NAME, 2.5 mg ml−1, Aerbio/Clinalfa, Darmstadt, Germany) and ketorolac (300 μg ml−1, trade name Toradol, Abbott Laboratories, Abbott Park, IL, USA) were diluted in saline immediately before use. Ketorolac was chosen to inhibit COX instead of indomethacin because of a United States restriction on parenteral indomethacin use solely for medical treatment. All subjects received the same total dose of drugs during exercise, such that the largest forearm (forearm volume was determined by water displacement) would receive a local delivery of drugs equivalent to 2–4 times higher than systemic doses used in previous studies. The dose of l-NAME was selected based on adjustments to whole-body intravenous infusions of l-NAME in previous work (Frandsen et al. 2001) and experience in our laboratory with l-NAME (Dinenno & Joyner, 2003). The dose of ketorolac was chosen as 10–20% of the systemic dose (15–30 mg). l-NAME was infused at 5 mg min−1 for 5 min (total dose = 25 mg, individual dose range: ∼17–35 mg l−1 forearm volume) to inhibit NOS. Ketorolac was infused at 600 μg min−1 for 5 min (total dose = 3 mg, individual dose range: ∼2–4 mg l−1 forearm volume) to inhibit COX.
Forearm blood flow and vascular conductance
Beat-to-beat FBF was measured as previously described (Shoemaker et al. 1997; Dinenno & Joyner, 2003; Rosenmeier et al. 2003). Briefly, a 4 MHz pulsed Doppler probe (Model 500 V, Multigon Industries, Mt Vernon, NY, USA) measured brachial artery mean blood velocity (MBV) proximal to the catheter insertion site. The probe insonation angle was 60 deg. A linear 7.0 MHz echo Doppler ultrasound probe (Acuson 128XP, Mountain View, CA, USA) was placed immediately proximal to the velocity probe to measure brachial artery diameter. Forearm blood flow was calculated as:
where FBF is in millilitres per minute, MBV is in centimetres per second, brachial diameter is in centimetres, and 60 is used to convert from millilitres per second to millilitres per minute. Forearm vascular conductance (FVC) was calculated as (FBF/mean arterial pressure) × 100, and expressed in millilitres per minute per 100 mmHg.
Rhythmic handgrip exercise
Rhythmic forearm handgrip exercise was performed using either a 2.8 kg weight for females, or a 4.1 kg weight for males, corresponding to ∼10% of maximal voluntary contraction (MVC). The weight was lifted 4–5 cm over a pulley at a duty cycle of 1 s contraction–2 s relaxation (20 contractions min−1) using audio and visual signals to ensure correct timing. We chose a low workload to minimize chances of fatigue, which could lead to movement artifacts that influence acquisition of the Doppler signal, as well as potential increases in muscle sympathetic nerve activity (MSNA) with fatigue. We did not measure each subject's MVC, and thus we based our estimates on laboratory experience that these workloads correspond to ∼10% MVC. Regardless, using forearm volume as a surrogate measure of muscle mass, both males and females exercised with remarkably similar relative workloads (males: 3.5 ± 0.1 g ml−1 forearm volume versus females: 3.3 ± 0.1 g ml−1 forearm volume).
Experimental protocol
Sequential drug infusion protocols
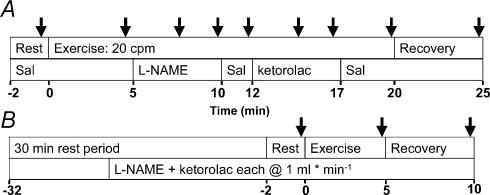
An experimental timeline is shown in Fig. 1. After 2 min of baseline recording with saline infusion (Rest), subjects began 20 min of forearm exercise at 20 contractions min−1. Saline was infused during the first 5 min (control exercise), at which point saline was replaced with l-NAME (n = 7) for 5 min. At 10 min exercise, l-NAME was changed to saline for 2 min, followed by a 5 min ketorolac infusion, and saline for the final 3 min of exercise, followed by 5 min of recovery. In the remaining seven subjects, the order of drug infusion was reversed; ketorolac was infused first, followed by l-NAME. Because inhibitors of NOS (like l-NAME) and COX (like ketorolac) demonstrate prolonged effects (hours), the protocol was designed such that after ‘single blockade’ with l-NAME or ketorolac, addition of the second drug resulted in a ‘double blockade’ condition during exercise.
Figure 1. Experiment timeline.
A, 30 min after catheterization, baseline measurements were taken for 2 min with saline infusion. Subjects performed rhythmic forearm handgrip exercise for 20 min, with either saline or drug continuously infused (2 ml min−1). At 5 min of exercise, l-NAME was infused for 5 min, followed by 2 min saline, then 5 min ketorolac, then 3 min saline, and finally 5 min recovery with saline. The drug order was reversed in 7 of 14 subjects. Arrows indicate approximate times that 30 s average data were collected for analysis, or the approximate time that nadir FBF were taken within l-NAME or ketorolac infusion; the timing of nadir varied between subjects (see Results). B, in 8 of the 14 subjects, l-NAME (2.5 mg ml−1) and ketorolac (300 μg ml−1) were infused at 1 ml min−1 each for 20 min after the first 20 min exercise bout. After this 30 min rest period, subjects performed 5 min forearm exercise at the same workload with concurrent infusion of l-NAME and ketorolac (both 1 ml min−1). FBF was measured for 2 min at rest, during exercise, and at 5 min recovery.
Double blockade experiment (combined NOS and COX inhibition prior to exercise onset)
We wanted to test the idea that the effectiveness of NO and PG inhibition on exercise hyperaemia depended on whether infusion of drugs occurred during exercise versus when exercise begins from a resting state with pharmacological inhibitors present. Eight of the 14 subjects (5 received l-NAME first, 3 received ketorolac first in the 20 min exercise bout) performed a second exercise bout 30 min after the end of the first bout to compare the hyperaemic responses to previous work in this area of study (Fig. 1B). During the last 20 min of the 30 min rest period and throughout the 5 min exercise bout, l-NAME (2.5 mg ml−1) and ketorolac (300 μg ml−1) were each infused at 1 ml min−1 to ensure complete and lasting inhibition of NOS and COX, respectively. Thus, we were able to compare data from the 20 min exercise bout (the 5 min control steady state exercise time point) to a 5 min exercise bout that started with combined NOS and COX inhibition. In these eight subjects total brachial artery infusions of l-NAME and ketorolac for the second exercise bout were 105 mg and 12.6 mg, respectively.
Time controls
Control experiments were performed in a separate group of five subjects to study the effects of time without drugs. These subjects performed the 20 min exercise protocol without catheterization or drug infusion, to ensure that steady state blood flow responses were stable over 20 min, and that any changes measured during drug infusions were not merely an artifact of exercise duration. Blood pressure was measured by an automated oscillometric approach (Cardiocap/5, Datex-Ohmeda, Louisville, CO, USA). Three of the five subjects also performed a second, 5 min exercise bout approximately 30 min after the end of the first 20 min bout.
Data and statistical analysis
Data were collected and stored on a computer at 200 Hz and analysed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH, USA). Mean arterial pressure (MAP) was determined from the arterial pressure waveform and HR was derived from the ECG. The brachial artery MBV signal was divided manually into 10 s intervals, or about three contraction–relaxation cycles, to reduce the contraction-to-contraction variability of FBF. During analysis, it became clear that the time course of drug responses varied between subjects, making it impossible to pool the FBF responses to drug infusion at all time points during exercise. Therefore, raw data from three intervals (30 consecutive seconds) were averaged at the following time points: (1) the last 30 s of steady state exercise + saline, (2) the 30 s of the lowest FBF during l-NAME infusion, (3) the last 30 s of l-NAME infusion, (4) the last 30 s of saline prior to ketorolac, (5) the 30 s of the lowest FBF during ketorolac infusion, (6) the last 30 s of ketorolac infusion, (7) the last 30 s of exercise (plus saline), and (8) the last 30 s of 5 min of recovery (see arrows in Fig. 1). The order of these selected time points depended on the order of drug administration.
Since the absolute FBF values varied widely between males and females, data were expressed in the following manner: (1) absolute FBF or FVC; (2) normalized FBF responses (or FVC), where no blood flow was defined as 0%, and the 5 min steady state FBF level was defined as 100%. We feel the latter approach most accurately reflects the contributions of NO and PGs, as it reduces the variability of hyperaemic responses between males and females, and the interindividual variability due to the fact that absolute workloads were used for each sex (not a percentage of MVC). The absolute FBF and FVC data, as well as alternative expressions of FBF and FVC are displayed in Table 2, and select absolute and normalized data are summarized in the figures. We also expressed the data as the change in FBF above baseline FBF (or FVC) and the percentage change above baseline FBF (or FVC), where baseline FBF was defined as 0%, and the 5 min steady state FBF was defined as 100%. Despite the multiple manipulations of data, the pattern of changes in FBF and FVC were quite similar. Thus, select graphs and tables are presented for clarity.
Table 2.
Haemodynamic values for forearm exercise under sequential and double blockade conditions
| Variable | Condition | Baseline | 5 min exercise | Drug 1 | Saline | Drug 2 | End exercise | Recovery |
|---|---|---|---|---|---|---|---|---|
| HR (beats min−1) | LK | 59 ± 3 | 60 ± 3 | 59 ± 4 | 60 ± 2 | 55 ± 3 | 54 ± 3 | 52 ± 3* |
| KL | 57 ± 3 | 59 ± 3 | 59 ± 2 | 59 ± 2 | 58 ± 2 | 55 ± 2 | 53 ± 3 | |
| DB | 54 ± 2 | 54 ± 3 | — | — | — | — | 52 ± 2 | |
| MAP (mmHg) | LK | 91 ± 2 | 92 ± 2 | 93 ± 1 | 94 ± 1 | 96 ± 2† | 99 ± 2† | 96 ± 2† |
| KL | 97 ± 4 | 98 ± 4 | 98 ± 3 | 98 ± 3 | 99 ± 3 | 102 ± 3† | 104 ± 3† | |
| DB | 103 ± 2 | 105 ± 2 | 105 ± 3 | |||||
| Brachial artery | LK | 0.40 ± 0.02 | 0.40 ± 0.02 | — | — | — | — | 0.40 ± 0.02 |
| diameter | KL | 0.42 ± 0.03 | 0.43 ± 0.03 | — | — | — | — | 0.42 ± 0.03 |
| (cm) | DB | 0.37 ± 0.02 | 0.37 ± 0.02 | — | — | — | — | 0.37 ± 0.02 |
| FBF | LK | 44 ± 8 | 129 ± 15 | 107 ± 14* | 102 ± 11* | 88 ± 11* | 102 ± 14* | 27 ± 4‡ |
| (ml min−1) | KL | 38 ± 6 | 118 ± 11 | 105 ± 11 | 122 ± 11 | 96 ± 7* | 94 ± 10* | 33 ± 3 |
| DB | 22 ± 3 | 118 ± 11 | 25 ± 2 | |||||
| Delta FBF | LK | — | 85 ± 12 | 64 ± 10* | 58 ± 6* | 44 ± 8* | 59 ± 10* | −16 ± 6 |
| (ml min−1 above baseline) | KL | — | 80 ± 7 | 67 ± 8 | 84 ± 7 | 58 ± 6* | 56 ± 8* | −5 ± 5 |
| DB | — | 97 ± 10 | 4 ± 2 | |||||
| Normalized FBF | LK | 33 ± 5 | 100 | 83 ± 2* | 80 ± 4* | 68 ± 2*# | 79 ± 4* | 21 ± 2 |
| (% control exercise) | KL | 32 ± 2 | 100 | 88 ± 4* | 104 ± 4* | 83 ± 3* | 80 ± 5* | 28 ± 2 |
| Normalized FBF | LK | 0 | 100 | 76 ± 4* | 72 ± 6* | 52 ± 5* | 70 ± 6* | — |
| (% hyperaemic response) | KL | 0 | 100 | 82 ± 6 | 107 ± 5 | 74 ± 6* | 70 ± 7* | — |
| FVC | LK | 52 ± 10 | 140 ± 16 | 115 ± 14* | 108 ± 11* | 90 ± 11* | 104 ± 15* | 29 ± 5‡ |
| (ml min−1 100 mmHg−1) | KL | 39 ± 4 | 120 ± 9 | 106 ± 9 | 125 ± 9 | 98 ± 6* | 93 ± 9* | 31 ± 3 |
| DB | 23 ± 2 | 113 ± 14 | 25 ± 2 | |||||
| Delta FVC | LK | — | 88 ± 14 | 63 ± 13* | 56 ± 9* | 38 ± 11* | 52 ± 12* | −23 ± 8 |
| (ml min−1 100 mmHg−1 | KL | — | 81 ± 7 | 67 ± 7 | 86 ± 6 | 59 ± 6* | 54 ± 8* | −8 ± 4 |
| above baseline) | DB | — | 90 ± 12 | 2 ± 1 | ||||
| Normalized FVC | LK | 37 ± 6 | 100 | 81 ± 2* | 78 ± 4* | 64 ± 2*# | 73 ± 4* | 20 ± 2 |
| (% control exercise) | KL | 32 ± 2 | 100 | 88 ± 3* | 105 ± 4* | 82 ± 3* | 77 ± 5* | 26 ± 2 |
| Normalized FVC | LK | 0 | 100 | 68 ± 5* | 64 ± 9* | 39 ± 9* | 57 ± 5* | — |
| (% hyperaemic response) | KL | 0 | 100 | 82 ± 6 | 107 ± 6 | 73 ± 6* | 66 ± 7* | — |
Data summarized from same time points as 30-s average FBF data. Heart rate (HR) and brachial artery diameter were similar before and during exercise. Blood pressure did not change at the onset of exercise, but increased significantly by 20 min, regardless of drug order. The nadir values are presented for ketorolac. Data are expressed as means ±s.e.m. LK, l-NAME followed by ketorolac; KL, ketorolac followed by l-NAME; DB, double blockade with l-NAME + ketorolac for the 5-min exercise bout (n = 8). Various expressions of blood flow and vascular conductance are also displayed for comparison (explained in Methods).
MAP is different from baseline and 5 min exercise value (P > 0.05)
different from baseline value (P < 0.05)
different from control exercise value (P < 0.05)
different from all other exercise values (P < 0.05).
It was of interest to explore the effect of time within each drug order, and thus data were analysed by one-way analysis of variance (ANOVA) with repeated measures for each drug treatment order. The normalized data allowed for appropriate comparison of drug interaction effects using a two-way ANOVA with repeated measures. Tukey's HSD test was used in comparisons where appropriate. All data are expressed as means ± standard error of the mean (s.e.m.). Significance for all comparisons is P < 0.05.
Results
Subjects
Subject characteristics are summarized in Table 1. The mean age, BMI and sex did not differ between drug treatment groups (P > 0.7), and thus the data are presented as a pooled set. Additionally, no sex differences were observed with respect to the effects of NOS and COX inhibition, and therefore all data from each drug treatment group were pooled.
Table 1.
Subject Characterstistics
| Variable | l-NAME + Ketorolac (n = 7) | Ketorolac + l-NAME (n = 7) | P | Total (n = 14) |
|---|---|---|---|---|
| Sex (F/M) | 3/4 | 4/3 | 1.00 | 7/7 |
| Age | 25.7 ± 2 | 27.1 ± 3 | 0.70 | 26 ± 2 |
| BMI | 25.1 ± 1 | 24.7 ± 1 | 0.78 | 25 ± 1 |
The age, body mass index (BMI), and sex distribution were similar in both drug treatment groups, so data were combined. Data were expressed as mean ±s.e.m.
Cardiovascular response to forearm exercise
Brachial artery diameter, HR, MAP and the absolute and normalized FBF and FVC data are summarized in Table 2. During 20 min of exercise, brachial artery diameter did not change significantly from rest to exercise (P > 0.49). Heart rate did not change during exercise and/or drug infusion (P > 0.1), but was lower than baseline in recovery (P < 0.05). MAP did not change after 5 min of exercise (P > 0.99), but MAP did increase significantly by 20 min of exercise (P < 0.01; from 91 ± 2 to 99 ± 2 mmHg in LK, from 97 ± 4 to 102 ± 3 in KL). In the 5 min exercise bout 30 min after the first bout, completed by eight of the 14 subjects, brachial artery diameter, HR and MAP did not change from rest to exercise to recovery (P all > 0.68). In these subjects, baseline MAP was higher and FBF and FVC were lower prior to the second versus the first exercise bout. In the time control protocol, no changes were noted in brachial arterial diameter, MAP, or HR (not shown). The FBF and FVC responses are described below.
Effects of NOS inhibition followed by COX inhibition
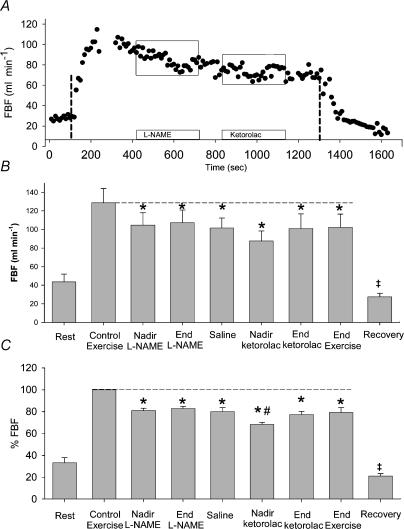
An individual FBF tracing is shown in Fig. 2A. Group means of absolute FBF from 30 s average time points for seven subjects are shown in Fig. 2B. After reaching steady state exercise over 5 min (129 ± 15 ml min−1), l-NAME reduced FBF to 104 ± 13 ml min−1 at the nadir (P < 0.05), which was similar to the end of l-NAME infusion (107 ± 14 ml min−1, P < 0.05 compared to control exercise), and remained stable through 2 min saline infusion (102 ± 11 ml min−1). Infusion of ketorolac further decreased FBF transiently to 88 ± 11 ml min−1 (P < 0.05 compared control exercise); it had returned to levels similar to infusion of l-NAME alone by the end of ketorolac (101 ± 16 ml min−1), and remained stable through to the end of exercise (102 ± 14 ml min−1).
Figure 2. Effects of NOS followed by COX inhibition.
A, an individual FBF tracing. Vertical lines indicate start and end of exercise. Open bars with boxes indicate infusion of l-NAME followed by ketorolac. B, mean absolute FBF data (+s.e.m.). l-NAME reduced FBF below control levels, although the subsequent decrease in FBF with ketorolac was not significantly lower than with l-NAME (P = 0.11). C, mean normalized FBF data (+s.e.m.). FBF data are normalized such that no FBF is defined as 0%, and steady state exercise FBF is defined as 100%. After reaching steady state exercise, l-NAME reduced FBF by ∼20%, and ketorolac transiently reduced FBF by 32%, returning to l-NAME levels by the end of ketorolac infusion. ‡Value is different from baseline (P < 0.05); *P < 0.05 compared to control exercise; #value is different than all other time points (P < 0.05).
Group means of normalized FBF values from 30 s average time points for seven subjects are shown in Fig. 2C. After reaching steady state exercise over 5 min (defined as 100%), l-NAME reduced FBF to 81 ± 2% of control levels at the nadir (P < 0.01), which was similar to the end of l-NAME infusion (83 ± 2% at P < 0.31), and remained stable through 2 min saline infusion (80 ± 4%). Infusion of ketorolac further decreased FBF transiently to 68 ± 2% of control (P < 0.043 compared to all other time points); it had returned to levels similar to end of infusion of l-NAME alone by the end of ketorolac (77 ± 3%, P = 0.31), and remained stable through to the end of exercise (79 ± 4%).
FVC expressed as a percentage of control exercise levels (Table 2) was similar to the normalized FBF analysis, except that a statistically significant nadir was seen with l-NAME (78 ± 2% nadir versus 81 ± 2% at l-NAME end, P < 0.05). The pattern of blood flow (or FVC) changes in response to drug infusion during exercise was very similar irrespective of whether the data were expressed as absolute FBF, change from baseline FBF,%FBF, or percentage of hyperaemic response (Table 2).
The individual timing of drug response varied between subjects. l-NAME reduced FBF in all seven subjects, but the nadir occurred 234 ± 31 s into l-NAME infusion (range 50–300 s after l-NAME started). Addition of ketorolac to l-NAME reduced FBF transiently in all seven subjects. The nadir occurred 167 ± 27 s into ketorolac infusion (range 40–270 s after ketorolac started).
Effects of COX inhibition followed by NOS inhibition
An individual FBF tracing is shown in Fig. 3A. Group means of absolute FBF from 30 s average time points from seven subjects are shown in Fig. 3B. After reaching steady stated FBF by 5 min exercise (118 ± 11 ml min−1), ketorolac infusion caused a transient reduction in FBF to 105 ± 11 ml min−1 at the nadir, but increased to control levels by the end of ketorolac infusion (115 ± 12 ml min−1). Two minutes of saline did not alter FBF (122 ± 11 ml min−1). Addition of l-NAME decreased FBF to 92 ± 7 ml min−1 at the nadir (P < 0.05 compared to control exercise), which was similar (P > 0.37) to the end of l-NAME infusion (96 ± 7 ml min−1). Resumption of saline for 3 min had no further effect on FBF (94 ± 9 ml min−1) compared to the end of l-NAME infusion.
Figure 3. Effects of COX followed by NOS inhibition.
A, an individual FBF tracing. Vertical lines indicate start and end of exercise. Open bars with boxes indicate infusion of ketorolac followed by l-NAME. B, mean absolute FBF data (+s.e.m.). The decrease in FBF with ketorolac was not significantly lower than control with l-NAME (P > 0.05), but l-NAME reduced FBF by ∼20%. C, mean normalized FBF data (+s.e.m.) FBF data are normalized such that no FBF is defined as 0%, and steady state exercise FBF is defined as 100%. After reaching steady state exercise, ketorolac transiently reduced FBF by ∼12%, returning to control levels by the end of ketorolac infusion. l-NAME infusion reduced FBF by ∼20%, which remained stable through 20 min exercise. *Value is different (P < 0.05) from control exercise, ketorolac end and saline.
Group means of normalized FBF from 30 s average time points from seven subjects are shown in Fig. 3C. After reaching steady stated FBF by 5 min exercise, ketorolac infusion caused a transient reduction in FBF to 88 ± 4% of control levels (P < 0.02) at the nadir, but increased to control levels by the end of ketorolac infusion (97 ± 3%, P = 0.38). Two minutes of saline did not alter FBF. Addition of l-NAME decreased FBF to 79 ± 3% at the nadir (P < 0.01 compared to control exercise), which was similar (P > 0.37) to the end of l-NAME infusion (83 ± 3%), and was significantly different from all earlier time points except the ketorolac nadir (P = 0.08–0.22). Resumption of saline for 3 min had no further effect on FBF (80 ± 5% of control) compared to the end of l-NAME infusion (P > 0.7). Various expressions of FBF listed in Table 2 exhibited similar patterns of change.
Normalized FVC showed similar results (Table 2) except that the nadir of l-NAME was slightly lower than the end of l-NAME (79 ± 4 versus 83 ± 4%, P < 0.05). The pattern of blood flow (or FVC) changes in response to drug infusion during exercise were very similar whether the data were expressed in absolute FBF, change from baseline FBF,%FBF, or percentage of hyperaemic response. Various expressions of FBF listed in Table 2 exhibited similar patterns of change.
The nadir to ketorolac occurred 177 ± 39 s after ketorolac infusion started (range 42–300 s after ketorolac started). Addition of l-NAME reduced FBF, with a nadir occurring at 260 ± 12 s after l-NAME infusion started (range 210–300 s after l-NAME started).
Comparison of both drug treatments
Two-way repeated measures ANOVA of the normalized (steady state FBF equal to 100%) data indicated a significant effect of time (P < 0.0001), drug order (P < 0.004), and importantly, a significant drug order by time interaction effect (P < 0.02). In fact, after reaching steady state FBF by 5 min, FBF was different between drug orders at most time points except the end of the second drug infusion (after both l-NAME and ketorolac) and the end of exercise. Both groups displayed approximately 20% lower FBF after receiving both l-NAME and ketorolac (79 ± 4% of control when l-NAME was infused first, 80 ± 5% of control when ketorolac was infused first, P > 0.8 between drug orders). Similar results were seen when normalized FVC data were analysed (FVC was 75 ± 5% of control FVC at the end of exercise).
FBF was lower at 5 min post-exercise compared to baseline (46 ± 5 ml min−1versus 30 ± 3 ml min−1, P < 0.02) indicating that we used adequate doses of l-NAME and ketorolac, resulting in reduced baseline FBF.
Effects of combined NOS and COX inhibition prior to exercise onset on forearm hyperaemic responses
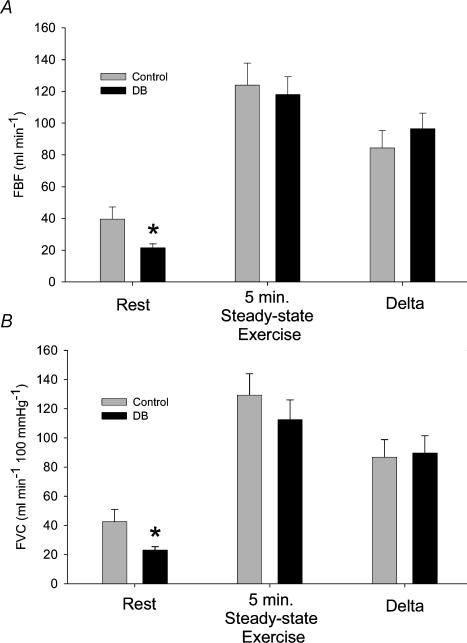
It was of interest to compare the hyperaemic responses to handgrip exercise under control conditions with the responses to exercise when resting FBF was decreased due to the presence of l-NAME plus ketorolac prior to the start of exercise. The results of this comparison are shown in Fig. 4. As expected, baseline FBF was lower after l-NAME + ketorolac (DB, double blockade). ANOVA indicated that the response to exercise was similar between control and DB (P > 0.2, Fig. 4A). A t test also confirmed that the exercise-induced increase in FBF (delta) in response to exercise was similar in control and DB conditions (P = 0.37). Since treatment with l-NAME + ketorolac led to an increase in MAP, we also expressed the FBF data as FVC, and the results are similar to the FBF results in that the FVC response to exercise was similar (P > 0.6) in control and DB conditions, as was the delta (P > 0.8; Fig. 4B).
Figure 4. Effect of blocking NOS and COX before exercise onset.
From the 20 min exercise bout, resting and 5 min steady state (saline, control exercise) data were compared to resting and 5 min steady state (DB, double blockade) data from the 5 min exercise bout. A, the mean FBF levels (+s.e.m.) at rest were less in DB, but the exercise FBF was similar (P > 0.2), as was the change from baseline (P > 0.37). B, as DB caused modest increases in blood pressure, the FVC data (+s.e.m.) are also shown. The FVC response to exercise was similar in control and DB conditions (P > 0.6), as was the increase in FVC above baseline (P > 0.8). *Value is different from control in Rest condition (P < 0.05).
Time controls
Five subjects performed non-invasive time controls. No changes were noted in brachial artery diameter, HR, or MAP from rest to exercise to recovery. FBF increased above baseline levels but remained similar across time, returning to baseline by 5 min post-exercise. Similar results were seen during the 5 min exercise bout that followed 30 min after the first 20 min bout. Brachial artery diameter, HR, MAP and FBF values were similar to the control FBF (at 5 min of exercise) in the 20 min exercise bout performed 30 min prior (P > 0.05). The increase in blood flow above baseline during the first 5 min of exercise was similar between the 20 min and 5 min exercise bouts (77 ± 12 ml min−1versus 78 ± 9 ml min−1, P > 0.25).
Discussion
There are four main observations from this study. First, local inhibition of NOS during exercise with l-NAME reduced exercise forearm blood flow by ∼20% regardless of drug treatment order. Second, local inhibition of PG synthesis during exercise with ketorolac caused a transient reduction in forearm blood flow, suggesting other vasodilating factors acted rapidly to restore the hyperaemic response. Third, the effects of NOS inhibition on exercise hyperaemia were not affected by PG synthesis inhibition indicating that NO was not the factor that restored the flow after PG synthesis inhibition. Fourth, when exercise was repeated after inhibition of synthesis of both NO and PGs, the rise in forearm blood flow above baseline was similar to control conditions, suggesting other signals restore the hyperaemic response and mask the normal contribution of NO and/or PGs to steady state exercise blood flow. These observations support the concept that NO and PGs both contribute significantly to exercise hyperaemia, that their effects appear independent, and that factors other than NO rapidly restore blood flow after PG inhibition.
Role of nitric oxide in exercise hyperaemia
The role of NO in exercise hyperaemia has received much attention in recent years, but discrepant findings have not allowed definitive conclusions as to the significance of NO in regulating blood flow during muscle contractions (Wilson & Kapoor, 1993a; Endo et al. 1994; Gilligan et al. 1994; Hirai et al. 1994; Shen et al. 1994; Dyke et al. 1995; Shoemaker et al. 1997; Duffy et al. 1999; Radegran & Saltin, 1999; Sheriff et al. 2000; Frandsen et al. 2001; Boushel et al. 2002). There are several possible reasons for these discrepant findings. First, the use of plethysmography for blood flow estimates requires brief pauses in contractions for accurate measurements and thus reflects post-exercise, not active muscle blood flow regulation (Wilson & Kapoor, 1993a; Shoemaker et al. 1997; Duffy et al. 1999; Joyner et al. 2001; Gordon et al. 2002). Second, systemic NOS inhibition results in a significant elevation in arterial blood pressure and evokes baroreflex-mediated reductions in sympathetic vasoconstrictor tone. This withdrawal of sympathetic outflow and subsequent vasodilatation could essentially ‘mask’ the physiological contribution of NO to exercise hyperaemia under these conditions (Radegran & Saltin, 1999; Sheriff et al. 2000; Frandsen et al. 2001; Boushel et al. 2002). Third, it is possible that the use of different arginine analogues to inhibit NOS might contribute to the equivocal findings. In this context, it appears that the metabolite of l-NAME, l-Nitroarginine (l-NA), is a much more potent inhibitor of NOS than NG-monomethyl-l-arginine (l-NMMA) (Vargas et al. 1991). Further, it has recently been suggested that l-NAME might inhibit nNOS to a greater extent than l-NMMA, and thus might be the more appropriate NOS inhibitor to use during muscle contractions because of the contraction-induced calcium release and stimulation of nNOS in skeletal muscles (Vargas et al. 1991; Thomas et al. 1998b).
In this study we demonstrated that blood flow measured continuously with Doppler ultrasound to contracting forearm muscles fell by about 20% when NOS was inhibited using relatively high doses of the arginine analogue l-NAME (Figs 2 and 3), and FBF remained reduced throughout the exercise bout. Given that we administered l-NAME via a brachial artery catheter and during contractions, we probably avoided the confounding cardiovascular reflexes associated with systemic doses of l-NAME. In fact, our findings are quite similar to those of Sheriff et al. (2000) who used ganglionic blockade to eliminate the confounding effects of systemic NOS inhibition and found that exercise hyperaemia was reduced ∼30% in the dog hindlimb. If the slight blood pressure elevations during forearm exercise in our study did evoke significant reductions in sympathetic outflow, then we might have underestimated the contribution of NO to exercise hyperaemia in the present study. Taken together, it appears that NO, possibly derived from nNOS (Kobzik et al. 1994; Thomas et al. 1998; Roberts et al. 1999; Lau et al. 2000; Grange et al. 2001) might normally account for about 20–30% of the blood flow response to exercise. However, it is clear that robust levels of exercise hyperaemia can occur in the absence of NO, meaning that NO is important normally, but not obligatory, to this response (Radegran & Saltin, 1999; Sheriff et al. 2000; Bian et al. 2001; Frandsen et al. 2001).
Role of prostaglandins in exercise hyperaemia
The contribution of vasodilating PGs to exercise hyperaemia is unclear (Kapoor & Wilson, 1993; Lang et al. 1997; Boushel et al. 2002). In this study we used acute brachial artery administration of the cyclooxygenase inhibitor ketorolac during handgrip exercise and showed a significant ∼12% reduction in FBF that recovered rapidly. Our observations are consistent with several previous studies in healthy humans that have relied on measurements of forearm blood flow using venous occlusion plethysmography, which in fact measures post-exercise blood flow. In these studies either local (i.e. brachial artery) or oral systemic administration of non-steroidal anti-inflammatory agents that inhibit COX can reduce blood flow to forearm muscles after contractions (Kilbom & Wennmalm, 1976; Wilson & Kapoor, 1993b). However, our results differ from studies demonstrating no role for PGs in regulating active muscle blood flow during steady state exercise. These studies differed from ours in that PG synthesis was inhibited prior to, and not during, exercise (Shoemaker et al. 1996; Lang et al. 1997; Merkus et al. 2003).
The findings from the present study demonstrate that under normal conditions PGs contribute to exercise hyperaemia, but that when their synthesis is inhibited ‘redundant control’ systems operate to rapidly restore the hyperaemic response to control levels. We believe that this observation is one of the first and clearest examples of redundant control operating during exercise, an idea that has received much discussion but limited support until now (Joyner & Proctor, 1999; Nishikawa et al. 2000; Halcox et al. 2001; Boushel et al. 2002; Hillig et al. 2003; Merkus et al. 2003). While we do not know exactly which PGs contribute to the responses we saw, it is generally accepted that prostacyclin is the predominant relaxing factor produced via COX (Taddei et al. 1995; Taddei et al. 1997; Dornyei et al. 1998).
Sequential inhibition of nitric oxide and prostaglandins during exercise
We initially proposed that inhibition of either PG or NO synthesis would have little impact on exercise hyperaemia, but that when production of either pathway was inhibited the contribution of the other would become more prominent as part of a redundant control scheme (Boushel et al. 2002; Hillig et al. 2003). For example, we expected to see the total contribution of NO (as demonstrated by the reduction in flow seen with l-NAME administration) to increase after inhibition of PG synthesis. Clearly this did not occur, suggesting that the dilating substance that rapidly restored blood flow following ketorolac was not NO. Likewise the decrement in flow seen with ketorolac after l-NAME did not increase suggesting that a compensatory increase in the local synthesis of dilating PGs to limit the reduction in forearm flow caused by the loss of NO did not occur. The question then becomes what dilating factor might have restored the transient fall in flow caused by inhibition of PGs?
One possible candidate dilator is endothelial derived hyperpolarizing factor (EDHF). Evidence from other sources suggests that production of this dilating compound is normally suppressed by NO and PGs (Nishikawa et al. 2000; Halcox et al. 2001). As both PGs and EDHF are arachidonic acid metabolites, blocking COX with ketorolac may ‘shunt’ arachidonic acid toward the P450 pathway acutely, resulting in increased synthesis of EDHF. Other possible dilating substances might include adenosine or related compounds that are thought to contribute significantly to exercise hyperaemia. However, the relationship between adenosine, NO and PGs during exercise has not been explored. Clearly, more studies are necessary to elucidate these complex interactions during muscle contractions in humans.
Combined NOS and COX inhibition prior to exercise onset
Our experimental design allowed reasonable comparison between the differing methods of pharmacological inhibition: during exercise versus inhibition in a resting state. When a second bout of exercise was performed following a 30 min rest period after both l-NAME and ketorolac had been given, resting forearm blood flow was reduced, but the rise in flow above baseline with handgrip exercise was similar (Fig. 4). This finding is comparable to the findings from Boushel et al. (2002), who demonstrated that (at a low workload) combined NOS and COX inhibition prior to knee extensor exercise did not significantly effect quadriceps hyperaemia. However, they did report a significant effect of combined NOS and COX inhibition as exercise intensity increased, similar to our findings when NO and PGs were inhibited during exercise. Thus, it is possible that the relative contributions of NO and PGs we observed may increase as exercise intensity increases, as suggested by recent work (Boushel et al. 2002). Collectively, these findings suggest that when NO and PGs are inhibited prior to exercise onset other redundant signals can compensate to produce a normal hyperaemic response during low intensity exercise. Thus, from an experimental design standpoint, we believe that administration of NOS and COX inhibitors during muscle contractions is a useful approach to test the importance of these vasodilator substances (or interactions between them) in regulating exercise hyperaemia.
Experimental considerations
It is important to consider potential limitations to this study. First, it is difficult to definitively demonstrate the efficacy of the inhibiting drugs. However, the local dose of ketorolac we gave was equal to or higher than either systemic or local doses of related compounds that have been used in previous studies that clearly either limited production of PGs (Kilbom & Wennmalm, 1976; Kapoor & Wilson, 1993), or had an obvious effect on blood flow responses (Kilbom & Wennmalm, 1976; Kapoor & Wilson, 1993). Additionally, the local dose of l-NAME we gave (25 mg total, or 17–35 mg l−1 forearm volume) was quitehigh by any standard; similar doses have caused marked reductions in the forearm vasodilator response to acetylcholine (Dinenno & Joyner, 2003), and lower relative doses inhibited skeletal muscle NOS activity by 67% (Frandsen et al. 2001). Thus, we feel confident that we gave adequate local doses of the drugs. Another possible limitation is our use of the isolated forearm model and the associated problems of extrapolating results to the locomotor muscles of the legs. It is possible that NO and PGs contribute differently to hyperaemia in the forearm than the thigh. While this is a concern, use of the forearm permitted us to conduct this study without the problem of engaging counter-regulatory cardiovascular reflexes that can cloud the interpretation of results in the leg. A third concern is our interpretation of the l-NAME results. It is possible that the main blood flow lowering actions of l-NAME might be restoring sympathetic vasoconstrictor control of blood flow to active muscles (Thomas, 1997; Thomas et al. 1998). However, we have conducted detailed studies on related topics and there is little support for NO in functional sympatholysis in the forearm exercise model, and hence this should not confound the interpretation of the present findings (Dinenno & Joyner, 2003; Rosenmeier et al. 2003). Fourth, as noted above, the contributions of NO or PGs might have differed markedly had we used a higher exercise intensity (Boushel et al. 2002). Finally, in any study investigating blood flow response there are multiple issues relating to how best to express the data. We analysed our data using multiple indices of vasodilatation and the pattern of results and conclusions is independent of the approach used.
We must also consider an alternative explanation for our findings. We cannot exclude the possibility that in the 20 min exercise bout, l-NAME and ketorolac merely reduce blood flow to ‘resting’ muscle and NO and PGs are not obligatory for normal exercise hyperaemia. We find this unlikely for the following reasons. First, we infused the drugs during steady state hyperaemia, and thus l-NAME and ketorolac were most likely delivered to the vessels important for modulating hyperaemia. Second, the transient drop in FBF with ketorolac is inconsistent with the idea that PGs are only important to resting FBF. We presume l-NAME is influencing FBF in a similar manner as ketorolac, without the acute compensation. Finally, studies on the coronary blood flow response to exercise (infusing inhibitors only at rest) also support our results from the 5 min exercise bout indicating redundant (unknown) vasodilator signals may provide for a ‘normal’ hyperaemic response when resting flow is reduced. That we can achieve similar results to previous human studies in our ‘double blockade’ protocol (and similar results to the studies of coronary blood flow – all of which infuse inhibitors at rest (Bian et al. 2001; Merkus et al. 2003)), yet show striking effects with sequential inhibition during exercise highlights the fact that the timing of inhibition appears to heavily influence the interpretation of which signals are important for exercise hyperaemia. Thus the interpretation we favour is that NO and PGs are normally active in forearm exercise hyperaemia, but signals other than NO and PGs are able to compensate when these are blocked prior to exercise. Additional studies will be needed to confirm this interpretation.
Summary
In this study the role of vasodilating NO and PGs acting alone and in combination was explored and we demonstrated that both of these signals clearly contribute to exercise hyperaemia in humans. The studies with l-NAME suggest a significant (but not obligatory) role for NO in exercise hyperaemia. The transient blood flow reductions seen during inhibition of PG synthesis and subsequent effects of l-NAME suggest the rapid action of an NO-independent ‘redundant’ dilator system. Thus, inhibition of NO and PGs during exercise suggests that these two signals act in a parallel fashion in control of forearm exercise hyperaemia. Once NO and PGs are inhibited at rest, other factors are responsible for restoration of exercise hyperaemia. What these signals are and how they might interact with NO and PGs during exercise await further exploration.
Acknowledgments
The authors wish to thank Shelly K. Roberts, Pamela Engrav, Karen P. Krucker, Diane E. Wick, Branton G. Walker, Christopher P. Johnson, Dr Niki M. Dietz and Dr John H. Eisenach for assistance. We also thank the enthusiastic volunteers for participating. This study was supported by grants from the National Institutes of Health (WGS-HL69692, FAD-AG05912, MJJ-HL46493, and General Clinical Research Center-RR00585)
References
- Bian X, Tune JD, Downey HF. KATP+ channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol. 2001;281:H831–H837. [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory. J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornyei G, Kaley G, Koller A. Exercise training augments flow-dependent dilation in rat skeletal muscle arterioles. Role of endothelial nitric oxide and prostaglandins. Am J Physiol. 1998;275:H831–H836. doi: 10.1152/ajpheart.1998.275.3.H831. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and no to metabolic vasodilation in the human forearm. Am J Physiol. 1999;276:H663–H670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation. 1994;90:2886–2890. doi: 10.1161/01.cir.90.6.2886. [DOI] [PubMed] [Google Scholar]
- Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996;81:1807–1814. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-l-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- Gordon MB, Jain R, Beckman JA, Creager MA. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vascular Med. 2002;7:163–168. doi: 10.1191/1358863x02vm439oa. [DOI] [PubMed] [Google Scholar]
- Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Narayanan S, Cramer-Joyce L, Mincemoyer R, Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. Am J Physiol. 2001;280:H2470–H2477. doi: 10.1152/ajpheart.2001.280.6.H2470. [DOI] [PubMed] [Google Scholar]
- Hickner RC, Fisher JS, Ehsani AA, Kohrt WM. Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. Am J Physiol. 1997;273:H405–H410. doi: 10.1152/ajpheart.1997.273.1.H405. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B, Hellsten Y. Cytochrome p450 2c9 plays an important role in the regulation of exercise-induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol. 2003;546:307–314. doi: 10.1113/jphysiol.2002.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: The use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Proctor DN. Muscle blood flow during exercise: The limits of reductionism. Med Sci Sports Exerc. 1999;31:1036–1040. doi: 10.1097/00005768-199907000-00017. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Wilson JR. Contribution of prostaglandins to exercise-induced vasodilation in humans. J Appl Physiol. 1993;75:2740–2744. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]
- Kilbom A, Wennmalm A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol. 1976;257:109–121. doi: 10.1113/jphysiol.1976.sp011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Lang CC, Chomsky DB, Butler J, Kapoor S, Wilson JR. Prostaglandin production contributes to exercise-induced vasodilation in heart failure. J Appl Physiol. 1997;83:1933–1940. doi: 10.1152/jappl.1997.83.6.1933. [DOI] [PubMed] [Google Scholar]
- Lau KS, Grange RW, Isotani E, Sarelius IH, Kamm KE, Huang PL, Stull JT. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- Merkus D, Haitsma DB, Fung TY, Assen YJ, Verdouw PD, Duncker DJ. Coronary blood flow regulation in exercising swine involves parallel rather than redundant vasodilator pathways. Am J Physiol. 2003;285:H424–H433. doi: 10.1152/ajpheart.00916.2002. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol. 2000;279:H459–H465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276:H1951–H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Jasman A, Balon TW. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol. 1999;277:E390–E394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB, O'Leary DS, Kellogg DLJ. Integration of cardiovascular control systems in dynamic exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 772–838. [Google Scholar]
- Shen W, Xu X, Ochoa M, Zhao G, Wolin MS, Hintze TH. Role of nitric oxide in the regulation of oxygen consumption in conscious dogs. Circ Res. 1994;75:1086–1095. doi: 10.1161/01.res.75.6.1086. [DOI] [PubMed] [Google Scholar]
- Shepherd JT. Circulation to skeletal muscle. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, Peripheral Circulation and Organ Blood Flow. III. Bethesda: American Physiological Society; 1983. pp. 319–370. [Google Scholar]
- Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation. Am J Physiol. 2000;279:H726–H732. doi: 10.1152/ajpheart.2000.279.2.H726. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Naylor HL, Pozeg ZI, Hughson RL. Failure of prostaglandins to modulate the time course of blood flow during dynamic forearm exercise in humans. J Appl Physiol. 1996;81:1516–1521. doi: 10.1152/jappl.1996.81.4.1516. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Thomas GD. Nitric oxide mediates contraction-induced attenuation of sympthetic vasoconstriction in rat skeletal muscle. J Physiol. 1997;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha -adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas HM, Cuevas JM, Ignarro LJ, Chaudhuri G. Comparison of the inhibitory potencies of NG-methyl-, NG-nitro- and NG-amino-L-arginine on EDRF function in the rat: Evidence for continuous basal EDRF release. J Pharm Exper Therap. 1991;257:1208–1215. [PubMed] [Google Scholar]
- Wilson JR, Kapoor S. Contribution of endothelium-derived relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol. 1993a;75:2740–2744. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Kapoor SC. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am J Physiol. 1993b;265:H171–H175. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]