Abstract
Animal studies have shown that cerebellar projections influence both excitatory and inhibitory neurones in the motor cortex but this connectivity has yet to be demonstrated in human subjects. In human subjects, magnetic or electrical stimulation of the cerebellum 5–7 ms before transcranial magnetic stimulation (TMS) of the motor cortex decreases the TMS-induced motor-evoked potential (MEP), indicating a cerebellar inhibition of the motor cortex (CBI). TMS also reveals inhibitory and excitatory circuits of the motor cortex, including a short-interval intracortical inhibition (SICI), long-interval intracortical inhibition (LICI) and intracortical facilitation (ICF). This study used magnetic cerebellar stimulation to investigate connections between the cerebellum and these cortical circuits. Three experiments were performed on 11 subjects. The first experiment showed that with increasing test stimulus intensities, LICI, CBI and ICF decreased, while SICI increased. The second experiment showed that the presence of CBI reduced SICI and increased ICF. The third experiment showed that the interaction between CBI and LICI reduced CBI. Collectively, these findings suggest that cerebellar stimulation results in changes to both inhibitory and excitatory neurones in the human motor cortex.
The cerebellum plays a major role in the planning, initiation and organization of movement (Allen & Tsukahara, 1974). These effects are mediated, in part, through its influence on the motor cortex and corticospinal outputs. Purkinje cells, the output neurones of the cerebellar cortex, have inhibitory connections with the deep cerebellar nuclei (DCN), which have a disynaptic excitatory pathway through the ventral thalamus to the motor cortex (Allen & Tsukahara, 1974). Inhibitory Purkinje cell output results in a reduction of excitatory output from DCN to the motor cortex that leads to modification of motor control. Abnormalities of these pathways may result in ataxia or a dysmetria of movement and schizophrenia that has been considered by some to be a dysmetria of thought (Andreasen et al. 1999). Consequently, the need to understand the cerebellar cortical connectivity is imperative if we are to understand the mechanisms that play a role in the pathophysiology of these complex disorders.
Activity in the cerebellothalamocortical pathway may be demonstrated non-invasively in humans. Electrical (Ugawa et al. 1991) or magnetic (Ugawa et al. 1995; Pinto & Chen, 2001) stimulation of the cerebellum 5–7 ms before magnetic stimulation of the motor cortex causes inhibition of the motor-evoked potential (MEP) produced by motor cortical stimulation. We will refer to this inhibition as cerebellar inhibition (CBI). Several lines of evidence suggest that CBI occurs at the level of the cerebral cortex (Granit & Phillips, 1957; Ito et al. 1970; Uno et al. 1970; Phillips & Porter, 1977; Ugawa et al. 1991; Ugawa et al. 1997).
TMS can also be used to examine at least two different cortico-cortical inhibitory processes: short-interval intracortical inhibition (SICI) and long-interval intracortical inhibition (LICI). In the SICI paradigm, a subthreshold conditioning stimulus (CS) followed by a suprathreshold test stimulus (TS) at short interstimulus intervals (ISIs; 1–5 ms) inhibits the MEP produced by the TS (Kujirai et al. 1993). LICI results in attenuation of the MEP when a suprathreshold CS is followed by a suprathreshold TS at long ISIs (50–200 ms; Valls-Sole et al. 1992; Wassermann et al. 1996). Several lines of evidence suggest that these forms of cortico-cortical inhibition are mediated by cortical inhibitory neuronal mechanisms (Fuhr et al. 1991; Inghilleri et al. 1993; Kujirai et al. 1993; Nakamura et al. 1997; Di Lazzaro et al. 1998; Chen et al. 1999). TMS can also be used to examine a cortico-cortical excitatory process known as intracortical facilitation (ICF). It has been suggested that ICF originates from excitatory postsynaptic potentials (EPSPs) transmitted by glutamate (Nakamura et al. 1997; Ziemann et al. 1998).
Animal studies have shown that cerebellar projections influence both excitatory and inhibitory neurones in the motor cortex. For example, Noda & Yamamoto (1984) reported that stimulation of the thalamic venterolateral nucleus or deep cerebellar nuclei activates pyramidal and non-pyramidal neurones in layers II and III in the cat motor cortex. Moreover, Holdefer et al. (2000) demonstrated a pattern facilitation and inhibition of neuronal activity in the primary motor cortex of the awake monkey following microstimulation of the cerebellar nuclei. Inhibition of motor cortex neurones was probably mediated through inhibitory interneurones within the motor cortex (Na et al. 1997) or by inhibition of thalamocortical projections in the motor thalamus (Ando et al. 1995). This type of cerebellothalamocortical connectivity has not, however, been demonstrated in humans.
The purpose of this study was to investigate the connectivity between the cerebellum and the motor cortex in humans by examining how CBI interacts with cortical inhibitory and excitatory circuits. Such connectivity can be explored with TMS in several ways. The first method examines the effects of controlled manipulation of TS intensities on CBI, SICI, LICI and ICF. If these phenomena share common mechanisms, their profiles of response under conditions of controlled manipulation should be similar. Second, connectivity can be explored by examining the impact of one inhibitory or excitatory phenomenon on the other. We anticipate that these findings will help to elucidate the mechanisms through which the cerebellum modulates the activity of the motor cortex.
Methods
Subjects
We studied 11 healthy, right-handed volunteers (mean age 41.7 years, s.d. 11.7 years, range 27–58 years; 7 males and 4 females). Handedness was confirmed using the Oldfield Handedness Inventory (Oldfield, 1971). Subjects were recruited through advertisements in the community and postings within the hospital. All subjects gave their written informed consent and the protocol was approved by the University Health Network Research Ethics Board in accordance with the declaration of Helsinki on the use of human subjects in experiments. Exclusion criteria included a self-reported comorbid medical illness or a history of drug or alcohol abuse.
EMG recording
Surface EMG was recorded from the right and left first dorsal interosseous (FDI) muscles with disposable disc electrodes placed in a tendon-belly arrangement over the bulk of the FDI muscle and the first metacarpo-phalangeal joint. The subject maintained relaxation throughout the experiment and the EMG was monitored on a computer screen and via speakers at high gain. The signal was amplified (Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada), filtered (bandpass 2 Hz to 2.5 kHz), digitized at 5 kHz (Micro 1401, Cambridge Electronics Design, Cambridge, UK) and stored in a laboratory computer for offline analysis.
TMS procedure
This study involved three experiments. The first experiment examined the effects of test motor-evoked potential (MEP) size on SICI, LICI, ICF and CBI. The second experiment examined the effects of CBI on SICI and ICF. The third experiment examined the interactions between LICI and CBI.
TMS of the left motor cortex was performed with a 7 cm figure-of-eight coil and four Magstim 200 stimulators (The Magstim Company, Whitland, UK) connected via three Bistim modules in a ‘pyramid’ set-up. The output of each of the two pairs of Magstim 200 stimulators was connected to one Bistim module. The output from the two Bistim modules was directed to a third Bistim module that was connected to the TMS coil. This set-up allowed us to deliver up to four pulses of different stimulus intensities through the same coil at very short interstimulus intervals. The power attenuation of the pyramid system is about 15%, similar to a single Bistim system. The coil was placed at the optimal position for eliciting MEPs from the right FDI muscle. The optimal position was marked on the scalp with a felt pen to ensure identical placement of the coil throughout the experiment. The handle of the coil pointed backward and was perpendicular to the presumed direction of the central sulcus, about 45 deg to the midsagittal line. The direction of the induced current was from posterior to anterior and was optimal to activate the motor cortex transsynaptically (Werhahn et al. 1994; Kaneko et al. 1996).
TMS of the cerebellum was performed with a double-cone coil (110 mm mean diameter). The double-cone coil was centred over the right cerebellar hemisphere 3 cm lateral to the inion on the line joining the inion and the external auditory meatus. The current in the coil was directed downward, which induced upward current in the cerebellar cortex. This coil position and current direction was found to be optimal for suppressing the contralateral motor cortex (Ugawa et al. 1995; Werhahn et al. 1996). The active motor threshold for pyramidal tract activation was determined with the coil centred over the inion (Ugawa et al. 1994). The EMG was passed through a leaky integrator, and the EMG level was displayed on an oscilloscope. With visual and auditory feedback, the subjects maintained a constant background contraction of 10% of the maximum integrated EMG. Five trials were averaged, and the active motor threshold was the minimum intensity required to elicit MEPs of more than 50 μV above the background EMG. The threshold was determined to the nearest 5% of the stimulator output. The intensity for cerebellar stimulation was set at 5% of the stimulator output below the active motor threshold (Ugawa et al. 1995; Pinto & Chen, 2001).
This paragraph explains the various parameters used in the experiments. The motor threshold (MT) is expressed as a percentage of maximum stimulator output and was defined as the lowest intensity that produced MEPs of >50 μV in at least five out of 10 trials with the muscles relaxed. SICI and ICF were tested using paired TMS with a subthreshold CS preceding a suprathreshold TS. CS2 denotes a conditioning stimulus that occurred 2 ms before a TS and CS10 denotes a conditioning stimulus that occurred 10 ms before a TS. LICI was tested with the suprathreshold CS and TS (Valls-Sole et al. 1992). The CS precedes the TS by 100 ms and is termed CS100. CBI was tested with a CS delivered to the right cerebellar cortex followed by a suprathreshold TS delivered to the left motor cortex 5 ms later. This CS will be referred to as CCS5 (cerebellar conditioning stimulus). CCS5 was chosen because it consistently leads to CBI and the effects are probably related to cerebellar stimulation rather than stimulation of peripheral nerves or muscles (Ugawa et al. 1995; Werhahn et al. 1996; Pinto & Chen, 2001).
In all experiments the intensities of the TS were often adjusted to produce a target MEP size. An intensity of ‘TS 1mV’ indicates a stimulator setting (determined to the nearest 1% of the maximum stimulator output) that produces a peak-to-peak MEP amplitude of ≥1 mV in at least five out of 10 trials. Similarly, ‘TS 0.2 mV’ and ‘TS 4 mV’ indicate settings that produce peak-to-peak MEP amplitudes of ≥0.2 mV and ≥4 mV, respectively, in at least five out of 10 trials.
In Experiments 2 and 3, we compared the combined effects two inhibitory mechanisms versus that of one inhibitory mechanism alone. If we used the same test intensity throughout, the first inhibitory mechanism would decrease the test MEP amplitude upon which the second mechanism could operate. Therefore, in order to match for test MEP amplitude across trials we increased the test stimulus intensity to give a 0.5 mV test MEP in the presence of the first inhibitory mechanism. We then compared the effects of the second inhibitory mechanism on this 0.5 mV MEP to a 0.5 mV MEP that was elicited by a weaker single test pulse. Since both test MEP amplitude and test pulse intensity may be important in determining the degree of inhibition, but cannot be matched at the same time, we designed our protocols to match for test MEP amplitude and test pulse intensity on different trials.
Experiment 1: effects of test stimulus intensity on SICI, ICF, LICI and CBI
In this experiment we examined the effects of different TS intensities on SICI, ICF, LICI and CBI. For SICI (CS2) and ICF (CS10), the intensity of the CS was set to 80% of the MT (0.8MT). For LICI the intensity of the suprathreshold CS100 was adjusted to produce a peak-to-peak MEP amplitude of about 1 mV and for CBI the CCS5 was set at 5% of the stimulator output below the active motor threshold as described above. Each run consisted of 10 trials of TS alone and 10 trials within each of the four different conditioning stimulus conditions (CS2, CS10, CS100 and CCS5). The conditioning stimuli preceded the test stimuli in random order. The time between trials was 5 s. Three TS intensities (TS 0.2 mV, TS 1 mV and TS 4 mV) were studied in separate runs.
Experiment 2: effects of CBI on SICI and ICF
In this experiment, we investigated whether SICI and ICF are altered by CBI. Ten conditions were tested and are listed in Table 1 as 2A to 2J. Each run consisted of 10 trials of each of the 10 conditions delivered in a random order (i.e. total of 100 trials). Conditions 2A to 2D were used to determine SICI, ICF and CBI for a 0.5 mV test MEP. Since CBI inhibits the test response, and SICI and ICF may be altered by an attenuated test MEP, for conditions 2E to 2J the strength of the test stimulus was adjusted to produce 0.5 mV MEPs in the presence of an earlier CCS5 pulse. This test stimulus is referred to as ‘TS 0.5 mVCCS5’. This allowed us to match MEP amplitudes to produce a similar degree of corticospinal activation with and without a preceding CCS5. SICI and ICF in the presence of CBI were studied using three pulses in conditions 2I and 2J. We also measured SICI and ICF with the increased TS strength (TS 0.5 mVCCS5) in conditions 2F and 2G. Therefore, we designed this experiment to compare SICI and ICF in the presence of CBI (2I/2H and 2J/2H) to SICI and ICF in the absence of CBI matched for test MEP amplitude (0.5 mV; 2B/2A and 2C/2A) and TS intensity (TS 0.5 mVCCS5; 2F/2E and 2G/2E).
Table 1. Stimulus conditions used in Experiments 2 and 3.
| Condition | Stimulus intensity | ||||
|---|---|---|---|---|---|
| CS100 | CCS5 | CS10 | CS2 | TS | |
| 2 A | — | — | — | — | 0.5 mV |
| 2B | — | — | — | 0.8MT | 0.5 mV |
| 2C | — | — | 0.8MT | — | 0.5 mV |
| 2D | — | AMT–5% | — | — | 0.5 mV |
| 2E | — | — | — | — | 0.5 mVCCS5 |
| 2F | — | — | — | 0.8MT | 0.5 mVCCS5 |
| 2G | — | — | 0.8MT | — | 0.5 mVCCS5 |
| 2H | — | AMT–5% | — | — | 0.5 mVCCS5 |
| 2I | — | AMT–5% | — | 0.8MT | 0.5 mVCCS5 |
| 2J | — | AMT–5% | 0.8MT | — | 0.5 mVCCS5 |
| 3A | — | — | — | — | 0.5 mV |
| 3B | — | AMT–5% | — | — | 0.5 mV |
| 3C | 1 mV | — | — | — | 0.5 mV |
| 3D | — | — | — | — | 0.5 mVCS100 |
| 3E | — | AMT–5% | — | — | 0.5 mVCS100 |
| 3F | 1 mV | — | — | — | 0.5 mVCS100 |
| 3G | 1 mV | AMT–5% | — | — | 0.5 mVCS100 |
CS100 represents a conditioning stimulus delivered 100 ms before TS (Experiment 3), CCS5 is a contralateral conditioning stimulus delivered 5 ms before test stimulus and an intensity at 5% of the stimulator output below the active motor threshold (AMT–5%), CS10 is a conditioning stimulus delivered 10 ms before TS (Experiment 2), CS2 is a conditioning stimulus delivered 2 ms before TS (Experiment 2), MT represents the resting motor threshold and TS is the test stimulus. See Methods for definition of TS intensity.
Experiment 3: effects of LICI on CBI
In this experiment we investigated the effects of LICI on CBI. Seven conditions were tested and are listed in Table 1 as 3A to 3G. Each run consisted of 10 trials of each of the 7 conditions delivered in a random order (i.e. total of 70 trials). LICI and CBI for a 0.5 mV test MEP were determined from conditions 3B and 3C. Since CBI may be affected by test MEP amplitude and CS100 inhibits the test MEP, the strength of the test stimuli was adjusted to produce 0.5 mV MEPs in the presence of the CS100 pulse in conditions 3D to 3G. This test pulse is referred to as ‘TS 0.5 mVCS100’. The interactions between CBI and LICI were studied using three pulses in condition 3G. Therefore, we designed this experiment to compare CBI in the presence of LICI (3G/3F) to CBI in the absence of LICI matched for test MEP amplitude (0.5 mV) (3B/3A) and test stimulus intensity (TS 0.5 mVCS100; 3E/3D).
Data analysis
The peak-to-peak MEP amplitude for each trial was measured offline. Inhibition or facilitation was expressed as a ratio of the conditioned to mean unconditioned MEP amplitude for each subject. Ratios less than one indicate inhibition and ratios greater than one indicate facilitation. Values are expressed as mean ± s.d.
For Experiment 1, the effects of TS intensity on SICI, LICI, ICF and CBI were evaluated by repeated measures analysis of variance (ANOVA). If the effect of TS intensity was significant, Fisher's protected least significant difference (PLSD) post hoc test was used to detect differences among different TS intensities. Correlations between SICI, LICI, ICF and CBI were tested by Pearson product-moment correlation coefficients. In addition, it was found that the distribution for CBI values violated the assumptions of normality and homogeneity of variance and therefore was log transformed. For Experiment 2, SICI and ICF alone at different test stimulus intensities (TS 0.5 mV and TS 0.5 mVCCS5) and in the presence of CBI were compared using repeated-measures ANOVA. For Experiment 3, CBI alone at different test stimulus intensities (TS 1 mV and TS 0.5 mVCS100) and in the presence of LICI was compared using repeated-measures ANOVA. In some cases, we examined whether a mediational relationship exists between independent and dependent variables. That is, we explored the possibility that a mediating variable carries the relationship between two variables, thus helping to establish a causal relationship. This was conducted according to the statistical methods described by Baron & Kenny (1986). This analysis requires estimation of the following three regression equations: first, regressing the mediator variable (residualized change scores of SICI in the presence of CBI) on the independent variable (CBI); second, regressing the dependent variable (residualized change scores of ICF in the presence of CBI) on the independent variable (CBI); and third, regressing the dependent variable (residualized change scores of ICF in the presence of CBI) on both the independent variable (CBI) and the mediator variable (residualized change scores of SICI in the presence of CBI) simultaneously. In this third step, the relationship between the independent variable and dependent variable should be significantly reduced after controlling for the mediator. A significant reduction is determined by the Sobel test. The threshold for significance across all analyses was set at P < 0.05.
Results
Experiment 1: effects of test stimulus intensity on SICI, ICF, LICI and CBI
The MEP amplitude for TS alone was 0.23 ± 0.07 mV for TS 0.2 mV, 1.06 ± 0.29 mV for TS 1 mV and 2.91 ± 1.24 mV for TS 4 mV. The results are shown in Fig. 1. Separate within-group repeated-measures ANOVA demonstrated that increasing the TS intensity, from 0.2 to 4 mV, resulted in a significant decrease in CBI (F= 13.15, d.f. = 2,20, P= 0.0002) and LICI (F= 24.29, d.f. = 2,20, P < 0.0001) and a significant increase in SICI (F= 14.23, d.f. = 2,20, P= 0.0001). Increasing the TS intensity also resulted in a significant decrease in ICF (F= 6.74, d.f. = 2,20, P= 0.006). Post hoc testing showed that both CBI and LICI were significantly greater at 0.2 and 1 mV than at 4 mV TS intensity. In contrast, SICI was slightly facilitated at 0.2 mV but markedly inhibited at 1 and 4 mV. There was also a significant decrease in ICF at 1 and 4 compared to 0.2 mV.
Figure 1. Effects of increasing TS intensity on cortical inhibition and facilitation.
Data are from 11 subjects. Each measure is expressed as a ratio (mean ± s.e.m.) of the conditioned MEP amplitude to the unconditioned MEP amplitude. Values below 1 indicate inhibition and those greater than 1 indicate facilitation. With increasing TS intensity SICI increased, whereas LICI, CBI and ICF decreased.
Experiment 2: effects of CBI on SICI and ICF
The MEP amplitude for TS 0.5 mV was 0.74 ± 0.58 mV (Table 1: condition 2A) and for TS 0.5 mVCCS5 was 1.13 ± 1.20 mV (condition 2E). When a TS 0.5 mVCCS5 was preceded by CCS5 (condition 2H), the test MEP amplitude was 0.54 ± 0.44 mV. Thus, the test MEP amplitude for conditions 2A and 2H were closely matched. Figure 2 demonstrates the effects of combining CCS5 with CS2 in one representative subject and data for all the subjects are shown in Fig. 3. Compared to TS alone (Fig. 2A), a preceding CS2 (Fig. 2B) or CCS5 (Fig. 2C) inhibited the test response. However, when CCS5 and CS2 were applied together (Fig. 2D) there was no further inhibition compared to CCS5 or CS2 applied alone. The nature of the test MEP (TS 0.5 mV, TS 0.5 mVCCS5, CCS5–TS 0.5 mVCCS5; columns A, B and C in Fig. 3) had a significant effect on SICI (F= 4.91, d.f. = 2,20, P= 0.02; Fig. 3). Post hoc tests revealed a significant reduction in SICI in the presence of CBI compared to SICI alone when matched for test pulse intensity (i.e. TS 0.5 mVCCS5; Fig. 3: B versus C, P= 0.04) and a trend towards significance when matched for test MEP amplitude (i.e. TS 0.5 mV; Fig. 3: A versus C, P= 0.08), whereas SICI alone for the two TS intensities were not significantly different (Fig. 3: A versus B). Moreover, the change in SICI in the presence of CBI (calculated as a ratio of SICI in the presence of CBI to SICI alone) was greater in subjects with a stronger CBI and the correlation was significant (r =−0.77, P= 0.006; Fig. 4A) but it was not related to the strength of SICI (r =−0.22, P= 0.51; Fig. 4B).
Figure 2. Effects of CBI on SICI in a single subject.
These traces represent the average of 10 trials from a single subject. In all traces the TS intensity was adjusted to produce 0.5 mV MEPs when preceded by a CCS5 (i.e. TS 0.5 mVCCS5). A, response to TS 0.5 mVCCS5 alone (condition 2E). B, SICI alone. The conditioning stimulus (CS2) inhibited the test MEP (condition 2F) compared to A. C, CBI alone. The cerebellar conditioning stimulus (CCS5) also inhibited the test response (condition 2H) compared to A. D, combined CBI and SICI. When the CCS5 preceded the CS2 (condition 2I), CS2 led to facilitation rather than inhibition of the test MEP compared to C.
Figure 3. Effects of CBI on SICI and ICF.
Data are from 11 subjects. Both inhibition and facilitation are expressed as a ratio (mean ± s.e.m.) of the conditioned MEP amplitude to the unconditioned MEP amplitude. Values greater than 1 represent facilitation and those less than 1 represent inhibition. Points above A represent SICI and ICF using a TS that evokes a 0.5 mV MEP (i.e. TS 0.5 mV; conditions 2B/2A and 2C/2A) and points above B represent SICI and ICF with a TS that evokes a 0.5 mV MEP if preceded by a CCS5 stimulus (i.e. TS 0.5 mVCCS5; conditions 2F/2E and 2G/2E). Points above C demonstrate the triple stimulus approach, in which a CS2 or CS10 is preceded by CCS5 (conditions 2I/2H and 2J/2H). Here the test stimulus was TS 0.5 mVCCS5 (condition 2H). There was significantly less SICI and more ICF in the presence of CBI (C) compared to SICI and ICF in the absence of CBI (A and B).
Figure 4. Effects of the strengths of CBI and SICI on CBI—SICI interaction.
Data are from 11 subjects and each point represents 1 subject. A, the relationship between CBI and the change in SICI in the presence of CBI. CBI is expressed as a ratio of the conditioned MEP amplitude to the unconditioned MEP amplitude (2H/2E). The y-axis represents a ratio of SICI in the presence of CBI (2I/2H) to SICI alone (2F/2E). Change in SICI was significantly correlated with the strength of CBI. When the outlier with strong CBI was removed, the correlation remained significant (r = 0.74, P = 0.02). B, the relationship between SICI and the change in SICI in the presence of CBI. SICI is expressed as a ratio of the conditioned MEP amplitude to the unconditioned MEP amplitude (2F/2E). The y-axis represents a ratio of the SICI in the presence of CBI (2I/2H) to SICI alone (2F/2E). There was no correlation.
The nature of the test MEP (TS 0.5 mV, TS 0.5 mVCCS5, CCS5–TS 0.5 mVCCS5; columns A, B and C in Fig. 3) also had a significant effect on ICF (F= 6.78, d.f. = 2,20, P= 0.006; Fig. 3). Post hoc tests (PLSD) revealed a significant increase in ICF in the presence of CBI compared to ICF alone when matched for test pulse intensity (TS 0.5 mVCCS5; Fig. 3: B versus C, P= 0.02) and test MEP amplitude (TS 0.5 mV; Fig. 3: A versus C, P= 0.02) whereas ICF for the two TS intensities were not significantly different (Fig. 3: A versus B). Moreover, the change in ICF in the presence of CBI (calculated as a ratio of ICF in the presence of CBI to ICF alone) was correlated with the strength of CBI (r =−0.90, P < 0.01; Fig. 5A) but not with the strength of ICF (r =−0.42, P = 0.20; Fig. 5B).
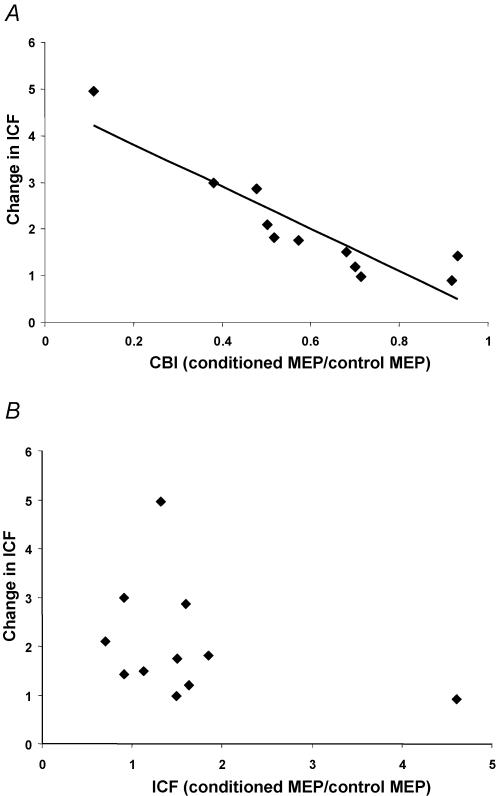
Figure 5. Effects of the strengths of CBI and ICF on CBI—ICF interaction.
Data are from 11 subjects and each point represents 1 subject. A, the relationship between CBI and the change in ICF in the presence of CBI. CBI is expressed as a ratio of the conditioned MEP amplitude to the unconditioned MEP amplitude (2H/2E). The y-axis represents a ratio of ICF in the presence of CBI (2J/2H) to ICF alone (2G/2E). Change in ICF was significantly correlated with the strength of CBI. B, the relationship between ICF and the change in ICF in the presence of CBI. ICF is expressed as a ratio of the conditioned MEP amplitude to the unconditioned MEP amplitude (2G/2E). The y-axis represents a ratio of the ICF in the presence of CBI (2J/2H) to ICF alone (2G/2E). There was no correlation.
We further examined the possibility that the CBI-induced change in ICF was mediated by the CBI-induced change in SICI, since there was also a significant correlation between the decrease in SICI when preceded by a CCS5 and the increase in ICF when preceded by a CCS5 (r = 0.74, P = 0.01). This was performed using a multiple regression analysis according to the approach described by Baron & Kenny (1986). We found that the CBI-induced change in ICF was mediated by the CBI-induced change in SICI (Sobel test =−2.13, P = 0.03).
Experiment 3: effects of LICI on CBI
The mean MEP amplitude for TS 0.5 mV alone was 0.62 ± 0.23 mV (condition 3A) and for the TS 0.5 mVCS100 test pulse was 1.57 ± 0.78 mV. With a TS 0.5 mVCS100 preceded by CS100 (condition 3F), the mean MEP amplitude was 0.57 ± 0.24 mV, similar to TS 0.5 mV alone (condition 3A). Consistent with the results of Experiment 1, LICI was greater with TS 0.5 mV (0.16 ± 0.17) than with TS 0.5 mVCS100 (0.46 ± 0.31; P = 0.0002, Student's paired t test). CBI, however, was not significantly different with TS 0.5 mV (0.85 ± 0.33) compared to TS 1mVCS100 (0.82 ± 0.21; P = 0.80, Student's paired t test). Figure 6 demonstrates the effects of combining a CS100 pulse with a CCS5 pulse in one representative subject. Compared to TS alone (Fig. 6A), a preceding CCS5 (Fig. 6B) inhibited the test response. In the presence of LICI, the CCS5 pulse no longer caused any inhibition (Fig. 6D compared to C). Data for the entire sample are shown in Fig. 7. The nature of the test MEP (TS 0.5 mV, TS 0.5 mVCS100, CCS5–TS 0.5 mVCS100; columns A, B and C in Fig. 7) had a significant effect on CBI (F = 4.11, d.f. = 2,20, P = 0.03). Post hoc tests (PLSD) revealed a significant reduction in CBI in the presence of LICI compared to CBI alone when matched for test pulse intensity (TS 0.5 mVCS100; Fig. 7: B versus C; P = 0.04) and test MEP amplitude (TS 0.5 mV; Fig. 7: A versus C; P = 0.05). The change in CBI in the presence of LICI (calculated as a ratio of CBI in the presence of LICI to CBI alone) did not correlate with the strength of LICI or CBI. Moreover, the MEP amplitude evoked by the conditioning pulse for LICI (CS100) did not correlate with the degree of LICI or the change in CBI in the presence of LICI.
Figure 6. Effects of LICI on CBI in a single subject.
Traces represent the averaged waveform for a single subject. A, response to TS 0.5 mV alone (condition 3A). B, CBI alone. A cerebellar conditioning stimulus (CCS5) inhibited the test response (condition 3B) compared to A. The TS was the same as in A. C, LICI alone. A conditioning stimulus (CS100) using a TS that evokes a 0.5 mV MEP if preceded by a CS100 stimulus (i.e. TS 0.5 mVCS100; condition 3F). The test MEP amplitude here is matched with that in A. D, combined LICI and CBI (condition 3G). Using both CS100 and CCS5 conditioning stimuli caused MEP facilitation compared to that shown in B and C.
Figure 7. Effects of LICI on CBI.
Data are from 11 subjects. Inhibition is expressed as a ratio of the conditioned MEP amplitude to the unconditioned MEP amplitude (mean ± s.e.m.). Values less than 1 represent inhibition. A, CBI using a TS that evokes a 0.5 mV MEP (i.e. TS 0.5 mV; condition 3B/3A). B, CBI using a TS that evokes a 0.5 mV MEP if preceded by a CS100 stimulus (i.e. TS 0.5 mVCS100; condition 3E/3D). C, the triple stimulus approach, in which a CCS5 is preceded by a CS100 conditioning stimulus (condition 3G/3F). Here the test stimulus was TS 0.5 mVCS100 (3F). CBI was less for the TS 0.5 mVCS100 (B) than the lower TS 0.5 mV (A). In the presence of LICI, CBI was significantly reduced when matched for test MEP amplitude (A versus C) and when matched for test stimulus intensity (B versus C).
Discussion
This study investigated the connectivity between the cerebellum with inhibitory and excitatory neurones of the human motor cortex. In Experiment 1, increasing TS intensities resulted in less LICI, CBI and ICF but greater SICI. In Experiment 2, SICI was reduced and ICF was increased in the presence of CBI. These changes were more pronounced in subjects with stronger CBI. In Experiment 3, it appears that the interaction between CBI and LICI resulted in a significant reduction in CBI.
Effect of CBI on SICI
Previous animal work suggests that cerebellar projections to the motor cortex terminate on both excitatory and inhibitory neurones (Noda & Yamamoto, 1984; Ando et al. 1995; Holdefer et al. 2000). If TMS of the cerebellum activates the inhibitory Purkinje cells, the excitatory drive from the DCN (dentate and interpositus nuclei) to the motor cortex via the ventrolateral nucleus of the thalamus would be reduced. However, if the cerebellothalamocortical pathway terminates on inhibitory neurones, as has been previously suggested (Na et al. 1997), it is anticipated that cerebellar stimulation would reduce local inhibitory mechanisms in motor cortex (Fig. 8). This is consistent with the results of Experiment 2, which showed that cerebellar stimulation inhibited SICI. Moreover, in subjects with greater cerebellar inhibition, there was more prominent reduction in SICI, lending further support to the hypothesis that cerebellar stimulation results in reduced local inhibitory activity.
Figure 8. A possible model that may explain our experimental findings.
Each diamond schematically represents a population of neurons mediating either inhibitory or facilitatory processes (i.e. SICI, LICI and ICF) or an anatomic location (i.e. DCN, VLN, IOP and PC). The diamond labelled ‘I’ represents cells leading to descending I-waves and ‘O’ represents corticospinal output neurones. LICI is shown to inhibit SICI based on the result of a previous study (Sanger et al. 2001). Jagged arrows represent the presumed site of TMS stimulation. The question marks indicate pathways that may explain some of our experimental findings, but whether they are involved remain speculative. Thick lines represent connections confirmed by these experimental findings. Our finding of reduced SICI in the presence of CBI can be explained by activation of the cerebellar Purkinje cell (PC) leading to suppression of excitatory output from deep cerebellar nuclei (DCN). This results in suppression of excitatory output from the ventrolateral nucleus of the thalamus (VL), leading to decreased excitatory drive to output neurones (causing decreased MEP amplitude) as well as inhibitory (SICI) interneurones (thick line). TMS-induced activation of corticospinal output neurones by the conditioning pulse for LICI may activate thalamic inhibitory neurones (TIN) or reticular nuclei neurones (RNN) that, in turn, inhibit thalamocortical neurones; this may account for the finding of decreased CBI in the presence of LICI. Alternatively, activation of the mossy fibres that come from the pontine nuclei (PN) via the pontocerebellar pathway may inhibit Purkinje cells through activation of inhibitory Golgi cells (GC) and basket cells (BC). Another possibility is that cortical projection activates the inferior olive (IO) and the collaterals of the climbing fibres also innervate the inhibitory GC and BC that may also lead to decreased PC output. It is important to note that while these pathways exist, their involvement in these experimental paradigms remains speculative.
The organization of thalamocortical fibres in the cerebellothalamocortical system has been studied extensively in animals (for review see Shinoda et al. 1993). The thalamocortical projection to the motor cortex is primarily located in venterolateral (VL) nucleus of the thalamus. Electrophysiological recordings (Shinoda et al. 1982, 1985) have demonstrated that pyramidal tract neurones in areas 4 and 6 receive di- or trisynaptic inputs from both the dentate and interpositus nuclei of the cerebellum. Axons of these thalamocortical neurones terminate in cortical layers I, III, V and VI (Strick & Sterling, 1974; Shinoda & Kakei, 1989; Shinoda et al. 1993; Na et al. 1997). Cortical neurones in these layers, in turn, form divergent synaptic connections with pyramidal tract neurones, corticofugal neurones and interneurones that mediate cortical output (Shinoda et al. 1993). In the cortex, inhibitory interneurones producing fast IPSPs due to GABAA receptors are distributed throughout all cortical layers (Lund & Wu, 1997). Therefore, neurones that are likely to mediate SICI are anatomically located to form synaptic connections with thalamocortical afferents (Na et al. 1997). Thus, while reduction of test MEP from CBI may be related to reduced thalamocortical facilitation of neurones associated with cortical output, CBI may decrease SICI through reduced thalamocortical facilitation of cortical inhibitory interneurones mediating SICI. It has been speculated that this connectivity is important for the dynamic focusing of motor output (Shinoda et al. 1993).
Another possible explanation for decreased SICI in the presence of CBI is occlusion. Occlusion may occur if the same or overlapping populations of inhibitory interneurones mediate SICI and CBI. Therefore, in the presence of CBI, fewer inhibitory interneurones would be available to be activated by the SICI, leading to reduced SICI. Several observations, however, argue against this possibility. First, the difference in response of SICI and CBI to changes in TS intensities (Fig. 1) makes it unlikely that the same or overlapping neuronal population mediates SICI and CBI. Second, addition of a CCS5 pulse in the presence of SICI (Fig. 2D compared to B) resulted in MEP facilitation in three subjects. The occlusion model cannot explain this facilitation. It is likely that SICI at low test stimulus intensity represents effects of both SICI and short-latency intracortical facilitation (SICF, also known as facilitatory I wave interaction; Ilic et al. 2002; Roshan et al. 2003). In the presence of CBI, SICI is reduced, leaving SICF as the predominant system active at these short interstimulus intervals (Ilic et al. 2002). Third, the occlusion model predicts that subjects with greater CBI and SICI will have larger reduction of SICI in the presence of CBI. Although the change in SICI in the presence of CBI correlated with CBI (Fig. 4A), there was no correlation with SICI (Fig. 4B). Nevertheless, occlusion cannot be fully ruled out as a possible mechanism mediating decreased SICI in the presence of CBI.
Effect of CBI on ICF
In Experiment 2 we also found that ICF was increased in the presence of a cerebellar conditioning stimulus. Mediational statistics suggest that CBI-induced changes in ICF were mediated through changes in SICI. This finding suggests that ICF is not exclusively mediated by excitatory interneurones, but rather by a net balance between inhibition and excitation. In the presence of reduced inhibition (e.g. decreased SICI in the presence of CBI), excitatory circuits predominate, resulting in increased ICF.
Interaction between CBI and LICI
There are a number of similarities between CBI and LICI. Increasing TS intensities resulted in significant decrease in both LICI and CBI, suggesting that both inhibitory mechanisms predominantly target neurones activated at low intensities. Moreover, both CBI and LICI (Sanger et al. 2001) decrease SICI. However, these two inhibitory phenomena are probably mediated by different mechanisms because their durations of activation are different. LICI may last for 200 ms or longer, depending on the conditioning stimulus intensity (Valls-Sole et al. 1992), while CBI last up to 10 ms (Ugawa et al. 1995; Pinto & Chen, 2001). In addition, the effects of voluntary contraction are different for LICI and CBI. For LICI (Wassermann et al. 1996), the extent of inhibition is similar at rest and during voluntary muscle contraction, while CBI is reduced with voluntary contraction (Pinto & Chen, 2001), similar to SICI (Ridding et al. 1995).
Experiment 3 showed that CBI was reduced in the presence of LICI. One explanation is that CBI and LICI target the same population of cortical neurones and the interaction can be explained by a saturation or occlusion effect. However, this is unlikely because CBI did not change over the range of test stimulus intensities we used in Experiment 3 (Fig. 7), although occlusion cannot be entirely ruled out. Other possibilities include inhibition of CBI by LICI or inhibition of LICI by CBI at the cortical level. These are again unlikely, since we found no correlation between the degree of interaction between CBI and LICI and the strength of baseline LICI or the strength of baseline CBI. Since the degree of interaction also did not correlate with the MEP amplitude evoked by the CS100 pulse, it is probably not due to activation of the pyramidal tract.
LICI and CBI may interact at subcortical sites (Fig. 8). For example, the suprathreshold conditioning stimulus of LICI may disrupt the cerebellothalamocortical inhibitory pathway at the level of the motor thalamus. Animal studies have shown that cortical stimulation results in activation of reticular nuclei neurones and thalamic inhibitory neurones, which, in turn, inhibit the cerebellothalamocortical pathway (Ando et al. 1995; Zhang & Jones, 2004). Another possibility is that motor cortex stimulation from the CS100 pulse influences the cerebellar cortex through activation of the mossy fibres that come from the pontine nuclei via the corticopontocerebellar pathway (Kelly & Strick, 2003). Activation of this pathway may inhibit Purkinje cells through activation of inhibitory neurones (i.e. Golgi and basket cells) in the cerebellar cortex. Delivery of the cerebellar CS in the presence of inhibited Purkinje cells would produce the loss of CBI seen in this experiment. Yet another possibility is that motor cortex stimulation results in decreased Purkinje cell inhibitory output through activation of the inferior olive (Schwarz & Welsh, 2001). Here, the collaterals of climbing fibres from the inferior olive also innervate the Golgi (Schulman & Bloom, 1981) and basket cells (Lemkey-Johnston & Larramendi, 1968), leading to suppression of Purkinje cells.
In conclusion, these findings suggest that cerebellar stimulation results in changes to both inhibitory and excitatory neurones in the human motor cortex.
References
- Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974;54:957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Ando N, Izawa Y, Shinoda Y. Relative contributions of thalamic reticular nucleus neurons and intrinsic interneurons to inhibition of thalamic neurons projecting to the motor cortex. J Neurophysiol. 1995;73:2470–2485. doi: 10.1152/jn.1995.73.6.2470. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Granit R, Phillips CG. Effects on Purkinje cells of surface stimulation of the cerebellum. J Physiol. 1957;135:73–92. doi: 10.1113/jphysiol.1957.sp005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN, Miller LE, Chen LL, Houk JC. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol. 2000;84:585–590. doi: 10.1152/jn.2000.84.1.585. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10:64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemkey-Johnston N, Larramendi LM. Types and distribution of synapses upon basket and stellate cells of the mouse cerebellum: an electron microscopic study. J Comp Neurol. 1968;134:73–112. doi: 10.1002/cne.901340106. [DOI] [PubMed] [Google Scholar]
- Lund JS, Wu CQ. Local circuit neurons of macaque monkey striate cortex. IV. Neurons of laminae 1–3A. J Comp Neurol. 1997;384:109–126. doi: 10.1002/(sici)1096-9861(19970721)384:1<109::aid-cne7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Na J, Kakei S, Shinoda Y. Cerebellar input to corticothalamic neurons in layers V and VI in the motor cortex. Neurosci Res. 1997;28:77–91. doi: 10.1016/s0168-0102(97)00031-x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T, Yamamoto T. Response properties and morphological identification of neurons in the cat motor cortex. Brain Res. 1984;306:197–206. doi: 10.1016/0006-8993(84)90369-x. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. Corticospinal Neurons. Their Role in Movement. London: Academic Press; 1977. [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res. 2001;140:505–510. doi: 10.1007/s002210100862. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman JA, Bloom FE. Golgi cells of the cerebellum are inhibited by inferior olive activity. Brain Res. 1981;210:350–355. doi: 10.1016/0006-8993(81)90908-2. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Welsh JP. Dynamic modulation of mossy fiber system throughput by inferior olive synchrony: a multielectrode study of cerebellar cortex activated by motor cortex. J Neurophysiol. 2001;86:2489–2504. doi: 10.1152/jn.2001.86.5.2489. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Futami T, Kano M. Synaptic organization of the cerebello-thalamo-cerebral pathway in the cat. II. Input-output organization of single thalamocortical neurons in the ventrolateral thalamus. Neurosci Res. 1985;2:157–180. doi: 10.1016/0168-0102(85)90010-0. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Kakei S. Distribution of terminals of thalamocortical fibers originating from the ventrolateral nucleus of the cat thalamus. Neurosci Lett. 1989;96:163–167. doi: 10.1016/0304-3940(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Kakei S, Futami T, Wannier T. Thalamocortical organization in the cerebello-thalamo-cortical system. Cereb Cortex. 1993;3:421–429. doi: 10.1093/cercor/3.5.421. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yamazaki M, Futami T. Convergent inputs from the dentate and the interpositus nuclei to pyramidal tract neurons in the motor cortex. Neurosci Lett. 1982;34:111–115. doi: 10.1016/0304-3940(82)90161-6. [DOI] [PubMed] [Google Scholar]
- Strick PL, Sterling P. Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J Comp Neurol. 1974;153:77–106. doi: 10.1002/cne.901530107. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, Sakai K, Furubayashi T, Machii K, Kanazawa I. Magnetic stimulation over the cerebellum in patients with ataxia. Electroencephalogr Clin Neurophysiol. 1997;104:453–458. doi: 10.1016/s0168-5597(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation of corticospinal pathways at the foramen magnum level in humans. Ann Neurol. 1994;36:618–624. doi: 10.1002/ana.410360410. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- Uno M, Yoshida M, Hirota I. The mode of cerebello-thalamic relay transmission investigated with intracellular recording from cells of the ventrolateral nucleus of cat's thalamus. Exp Brain Res. 1970;10:121–139. doi: 10.1007/BF00234726. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Taylor J, Ridding M, Meyer BU, Rothwell JC. Effect of transcranial magnetic stimulation over the cerebellum on the excitability of human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:58–66. doi: 10.1016/0013-4694(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jones EG. Corticothalamic inhibition in the thalamic reticular nucleus. J Neurophysiol. 2004;91:759–766. doi: 10.1152/jn.00624.2003. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]