Abstract
Muscle biopsies were obtained from the vastus lateralis before and after 84 days of bed-rest from six control (BR) and six resistance-exercised (BRE) men to examine slow- and fast-twitch muscle fibre contractile function. BR did not exercise during bed-rest and had a 17 and 40% decrease in whole muscle size and function, respectively. The BRE group performed four sets of seven maximal concentric and eccentric supine squats 2–3 days per week (every third day) that maintained whole muscle strength and size. Slow (MHC I) and fast (MHC IIa) muscle fibres were studied at 15°C for diameter, peak force (Po), contractile velocity (Vo) and force–power parameters. SDS-PAGE was performed on each single fibre after the functional experiments to determine MHC isoform composition. MHC I and IIa BR fibres were, respectively, 15 and 8% smaller, 46 and 25% weaker (Po), 21 and 6% slower (Vo), and 54 and 24% less powerful after bed-rest (P < 0.05). BR MHC I and IIa Po and power normalized to cell size were lower (P < 0.05). BRE MHC I fibres showed no change in size or Vo after bed-rest; however, Po was 19% lower (P < 0.05), resulting in 20 and 30% declines (P < 0.05) in normalized Po and power, respectively. BRE MHC IIa fibres showed no change in size, Po and power after bed-rest, while Vo was elevated 13% (P < 0.05). BRE MHC IIa normalized Po and power were 10 and 15% lower (P < 0.05), respectively. MHC isoform composition shifted away from MHC I fibres, resulting in an increase (P < 0.05) in MHC I/IIa (BR and BRE) and MHC IIa/IIx (BR only) fibres. These data show that the contractile function of the MHC I fibres was more affected by bed-rest and less influenced by the resistance exercise protocol than the MHC IIa fibres. Considering the large differences in power of human MHC I and IIa muscle fibres (5- to 6-fold), the maintenance of whole muscle function with the resistance exercise programme is probably explained by (1) the maintenance of MHC IIa power and (2) the shift from slow to fast (MHC I → MHC I/IIa) in single fibre MHC isoform composition.
Spaceflight is a unique environment that poses several physiological challenges to the human body. As the various space agencies around the world focus their attention on long duration stays aboard the International Space Station (ISS), implementation of effective exercise regimens will be essential for the health and well being of the crew members. In particular, maintenance of the musculoskeletal system will be necessary for successful long duration space travel (Baldwin et al. 1996; NASA, 2000). Previous studies have documented that skeletal muscle mass and strength are reduced with as little as 7 days of spaceflight (LeBlanc et al. 1995) and continue to decline with the length of exposure (cf. Adams et al. 2003). The reduction in muscle mass accounts for about two-thirds of the decrease in muscle strength. Thus, other physiological mechanisms, such as alterations in neural drive or intrinsic changes to the muscles fibres may be contributing to the deleterious changes in skeletal muscle function with unloading.
Data to support changes in the cross-bridge mechanics of human single muscle fibres have been shown with 17 days of spaceflight (Widrick et al. 1999), 17 days of bed-rest (Widrick et al. 1997), 37 days of bed-rest (Larsson et al. 1996) and 4 months of bed-rest (Yamashita-Goto et al. 2001). Taken together, these studies found that muscle fibres have a lower force per cross-sectional area and an elevated shortening velocity (Vo) following the unloading period. Thus, it appears that alterations in the contractile properties of human muscle do occur during periods of weightlessness and bed-rest. Furthermore, the changes observed between spaceflight and bed-rest closely mimicked each other, providing further evidence that bed-rest is a good ground-based analogue for studying changes in human muscle function (Widrick et al. 1999; Trappe et al. 2001c; Adams et al. 2003).
Resistance training is the most promising candidate for providing the proper stimulus to maintain muscle function while in space. However, the optimal exercise protocol has not been established (Baldwin et al. 1996; NASA, 2000; Adams et al. 2003). Given the time and energy requirements of crew members on board the ISS, the space agencies are interested in implementing effective countermeasure activities that minimize the exercise time of the crew members while at the same time maximizing the physiological benefits. Recently, our laboratory found that resistance training every third day during 21 days of unloading was sufficient to maintain muscle mass and strength (Schulze et al. 2002). Our study and others (Tesch et al. 1990; Dudley et al. 1991; Bamman et al. 1998) have concluded that to maximize resistance-training benefits, a programme must include concentric and eccentric muscle actions at a high intensity. While these initial studies are promising they are limited by the short duration of unloading. It is unknown if the same type of positive results would carry over to longer periods of unloading such as the current duration of ISS missions (range ∼90–180 days).
The intent of this investigation was to examine the alterations in the cellular mechanisms at the level of the single muscle fibre with long-duration (84 days) unloading in apparently healthy adults. In addition, high-intensity resistance training was employed in a separate group of volunteers to examine whether myocellular function could be maintained during a long-term period of unloading. We hypothesized that fibre diameter, force, force per cross-sectional area, power and normalized power would be reduced while shortening velocity would be elevated after bed-rest in subjects who did not perform any countermeasure activities. Conversely, we hypothesized that high-intensity resistance training every 2–3 days would be sufficient to maintain slow- and fast-twitch single fibre contractile characteristics with long-term bed-rest.
The unique aspects of this project were (1) the duration of bed-rest (84 days), (2) the use of a strict bed-rest (no countermeasures) control group for baseline measurements of muscle loss, (3) employment of resistance training every 2–3 days using a novel flywheel ergometer that has been designed for use in space (Berg & Tesch, 1994, 1998; Alkner et al. 2003; Tesch et al. 2003; Alkner & Tesch, 2004), and (4) measurement of contractile properties in individual slow- and fast-twitch muscle fibres from the vastus lateralis muscle.
Methods
Subjects
Twelve healthy men performed 90 days of 6 deg head-down tilt bed-rest. Subjects were divided into a bed-rest only (BR; n = 6) group and bed-rest with exercise (BRE; n = 6) group. Subjects in the BR group were 32 ± 2 years, 174 ± 2 cm, 70 ± 2 kg and had a BMI of 23 ± 1 kg m−2. Subjects in the BRE group were 32 ± 2 years, 174 ± 1 cm, 72 ± 2 kg and had a BMI of 24 ± 1 kg m−2. The bed-rest investigation was conducted in the facilities at the Institute for Space Medicine and Physiology (MEDES) Clinic in Toulouse, France.
Each volunteer was interviewed and had a general physical examination that included blood and urine chemistries. Trained personnel at the MEDES clinic performed all screening of the potential volunteers. Prior to the screening, all potential subjects were informed of all procedures and risks associated with the experimental testing. Informed consent was obtained from each volunteer. The study was conducted in accordance with the Declaration of Helsinki.
Whole muscle strength and size
To document muscle strength and size changes in response to the intervention protocols, isometric and dynamic muscle tests were performed before and after bed-rest in both groups. Magnetic resonance imaging (MRI) was conducted to determine muscle cross-sectional area (CSA) of the thigh muscles before and after bed-rest in both groups.
Muscle strength measurements
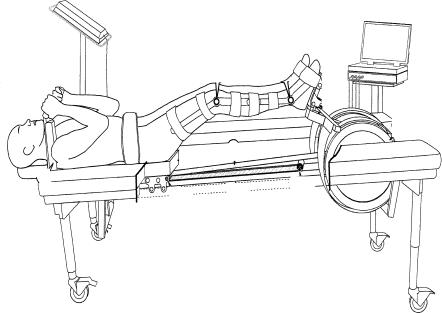
All muscle strength profiles were conducted on a specially designed flywheel ergometer (Berg & Tesch, 1994, 1998; Tesch et al. 2003) that was adapted for the 6 deg head-down tilt experiments (Alkner & Tesch, 2004). Measurements were obtained four times prior to bed-rest and on day 90 of bed-rest prior to reambulation. Maximal isometric squats with a knee angle of 90 deg and two sets of seven maximal concentric and eccentric actions were performed. Subjects were instructed to push with maximal force in the concentric phase, wait a brief moment just after the rotational turning point of the flywheel, then brake (i.e. eccentric phase) until reaching approximately 70 deg of knee angle. The ergometer was interfaced to a personal computer with customized software (MuscleLab, Ergotest AS, Langesund, Norway). Data were collected at 100 Hz. Dynamic test data consisted of concentric and eccentric peak force and concentric peak power. The data were averaged across repetitions for the set with the greatest average force.
MRI measurements
Muscle cross-sectional area (CSA) was obtained before bed-rest and on day 89 of bed-rest. Following 1 h of supine rest to control for the influence of postural-related fluid shifts on muscle size (Berg et al. 1993), MRI measurements were obtained for each subject. Subjects were supine and their heels were fixed on a non-metallic support to control joint and scan angle and to minimize compression of the legs against each other and the MRI gurney. Imaging was completed in a 1.0 T GE Siemens scanner (Somatam Impact, Erlangen, Germany) to determine the cross-sectional area (CSA) of the total quadriceps femoris, rectus femoris (RF), vastus lateralis (VL), vastus intermedius (VI), and vastus medialis (VM). Following the scout scans, interleaved transaxial images of 0.8 cm thick (TR/TE = 2000/20.0 ms, field of view 48 cm, 256 × 256 matrix) were taken from the top of the greater trochanter of the femur to the articular surface of the tibia.
MR images were transferred electronically from the scanner to a personal computer and analysed with Scion Image software (version 4.02) using manual planimetry. Analyses of the MR images began with the first proximal slice not containing gluteal muscle and continued distally to the last slice containing RF (Castro et al. 1999), because this region has been shown to represent the maximal CSA of the thigh (Narici et al. 1989). Muscle volume (MV; cm3) was taken as the summation of all the analysed slices for an individual muscle and determined for the RF, VL, VI, and VM and summed for the total quadriceps femoris.
Resistance training protocol
The BRE group trained on the flywheel ergometer every third day (2–3 days per week) beginning on day 5 of bed-rest. Each exercise session consisted of four sets of seven (4 × 7) maximal repetitions employing the supine (6 deg head-down tilt) squat exercise. Two minutes of rest was allowed between sets. Force, flywheel rotational velocity (work and power were calculated) and the knee joint angles were recorded during each training session. The device is described in detail by Alkner & Tesch (2004).
Muscle biopsy
A muscle biopsy (Bergstrom, 1962) from the vastus lateralis of the dominant leg was obtained from each subject prior to bed-rest and again on day 84 of bed-rest (prior to reambulation). The muscle biopsy was performed on day 84 to avoid interference with several other testing procedures that were being performed during the final week of bed-rest prior to reambulation of the subjects.
A portion of each muscle sample was sectioned longitudinally into several pieces, placed in cold skinning solution (see below) and stored at −20°C for later analysis of single muscle fibre physiology. Following a single muscle fibre experiment, each single fibre was analysed for myosin heavy chain (MHC) composition as described below. Following each muscle biopsy, all single fibre contractile measurements were completed within a 4 week period.
Skinning, relaxing and activating solutions
The skinning solution contained (mm): 125 potassium propionate, 2.0 EGTA, 4.0 ATP, 1.0 MgCl2, 20.0 imidazole (pH 7.0), and 50% (v/v) glycerol. The compositions of the relaxing and activating solutions were calculated using an iterative computer program described by Fabiato and Fabiato (1979). These solutions were adjusted for temperature, pH, and ionic strength using stability constants in the calculations (Godt & Lindley, 1982). Each solution contained (mm): 7.0 EGTA, 20.0 imidazole, 14.5 creatine phosphate, 1.0 free Mg2+, 4.0 free MgATP, KCl and KOH to produce and ionic strength of 180 mm and a pH of 7.0. The relaxing and activating solutions had a free [Ca2+] of pCa 9.0 and pCa 4.5, respectively (where pCa =−log Ca2+ concentration).
Single muscle fibre physiology experiments
On the day of an experiment, a 2–3 mm muscle fibre segment was isolated from a muscle bundle and transferred to an experimental chamber filled with relaxing solution where the ends were securely fastened between a force transducer (model 400A, Cambridge Technology, Watertown, MA, USA) and a DC torque motor (model 308B, Cambridge Technology) as described by Moss (1979). The instrumentation was arranged so that the muscle fibre could be rapidly transferred back and forth between experimental chambers filled with relaxing or activating solutions. The apparatus was mounted on a microscope (Olympus BH-2, Japan) so that the fibre could be viewed (× 800) during an experiment. Using an eyepiece micrometer, sarcomeres along the isolated muscle segment length were adjusted to 2.5 μm. All single muscle fibre experiments were performed at 15°C.
Unamplified force and length signals were sent to a digital oscilloscope (Nicolet 310, Madison, WI, USA) enabling muscle fibre performance to be monitored throughout data collection. Analog force and position signals were amplified (Positron Development, Dual Differential Amplifier, 300-DIF2, Ingelwood, CA, USA), converted to digital signals (National Instruments, Inc.) and transferred to a computer (Micron Electronics, Nampa, ID, USA) for analysis using customized software. Servo-motor arm and isotonic force clamps were controlled using a computer interfaced force (position controller (Positron Development, Force Controller, 300-FC1).
For each single muscle fibre experiment, a fibre with a compliance (calculated as fibre length divided by y-intercept) greater than 10%, and/or a decrease in peak force of more than 10% was discarded and not used for analysis. The within-fibre test/re-test of a single muscle fibre in our laboratory for the measurements of size, force–power relationships, peak force and contractile velocity were less than 1%. The coefficients of variation for the force transducer and servo-mechanical lever mechanism during the 1 year period we examined single muscle cell function from the volunteers as part of this investigation were 0.4 and 0.6%, respectively.
Single muscle fibre analysis
Individual muscle fibres were analysed for diameter, peak force (Po), maximal unloaded shortening velocity (Vo), and force–power characteristics. Detailed descriptions and illustrations of these procedures have been presented in our previous work (Trappe et al. 2003).
Single fibre diameter
A video camera (Sony CCD-IRIS, DXC-107 A, Japan) connected to the microscope and interfaced to a computer allowed viewing on a computer monitor and storage of the digitized images of the muscle fibres. Fibre diameter was determined from a captured computer image taken with the fibre briefly suspended in air (<5 s). Fibre width (diameter) was determined at three points along the segment length of the captured computer image using public domain software (NIH Image v1.61). Fibre cross-sectional area was calculated from the mean width with the assumption that the fibre forms a cylindrical cross-section when suspended in air.
Single fibre Po
The outputs of the force and position transducers were amplified and sent to a microcomputer via a Laboratory-PC +12-bit data acquisition board (National Instruments, Inc.). Resting force was monitored and then the fibre was maximally activated in pCa 4.5 solution. Peak active force (Po) was determined in each fibre by computer subtraction of the force baseline from the peak force in the pCa 4.5 solution.
Single fibre Vo
Fibre Vo was measured by the slack test technique as previously described (Edman, 1979). The fibre was fully activated in pCa 4.5 and then rapidly released to a shorter length, such that force fell to baseline. The fibre shortened, taking up the slack, after which force began to redevelop. The fibre was then placed in relaxing solution and returned to its original length. The duration of unloaded shortening, or time between onset of slack and redevelopment of force, was determined by computer analysis. Four to six different activation and length steps (each ≤15% of fibre length (FL)) were used for each fibre, with the slack distance plotted as a function of the duration of unloaded shortening. Fibre Vo (FL s−1) was calculated by dividing the slope of the fitted line by the segment length and the data were normalized to a sarcomere length of 2.5 μm.
Single fibre power
Submaximal isotonic load clamps were performed on each fibre for determination of force–power parameters. Each fibre segment was fully activated in a pCa 4.5 solution and then subjected to a series of isotonic load steps. This procedure was performed at various loads so that each fibre was subjected to a total of 15–18 isotonic contractions.
For the force–velocity relationships, load was expressed as P/Po, where P is the force during load clamping, and Po is the peak isometric force developed prior to the submaximal load clamps. Force and shortening velocity data points derived from the isotonic contractions were fitted using the hyperbolic Hill equation (Hill, 1938). Only individual experiments in which r2 was greater than or equal to 0.98 were included for analysis.
Fibre power was calculated from the fitted force–velocity parameters (Po, Vmax and a/Po, where a is a constant). Absolute power (μN FL s−1) was defined as the product of force (μN) and shortening velocity (FL s−1). Normalized power (W l−1) was defined as the product of normalized force (i.e. fibre force per cross-sectional area) and shortening velocity.
MHC determination
Following the single muscle fibre physiology measurements, each fibre was solubilized in 80 μl of 10% SDS sample buffer and stored at −20°C until assayed (Giulian et al. 1983; Williamson et al. 2001). In order to determine the myosin heavy chain (MHC) composition, fibres were run on a Hoefer se 600 gel electrophoresis system that consisted of a 3.5% (w/v) acrylamide stacking gel with a 5% separating gel at 4°C. Following the gel electrophoresis, the gels were silver-stained as described by Giulian et al. (1983).
Statistical analysis and calculations
A two-way (group × time) ANOVA with repeated measures was used to determine if there were differences within and between groups for each of the following variables: whole muscle strength and size, single fibre diameter, Po, Po/CSA, Vo, Vmax, peak power, normalized peak power and a/Po. The number of studied fibres for an individual was averaged to represent a mean for MHC I and MHC IIa fibres. Significance was set at P < 0.05 and a Bonferroni post hoc test was used when significance was noted. All data are presented as means ±s.e.m.
Due to the low number of hybrid fibres studied before the bed-rest period, statistics were not performed on these fibre populations. However, these data are presented since these fibres do constitute a significant portion of the muscle profile following bed-rest.
Single fibre composite values
Due to the high proportion of hybrid fibres (which have a relative difference in their functional profile compared to fibres containing only one MHC isoform) after the bed-rest period, we implemented a weighted single fibre value system based upon the composition of the fibres studied from each subject. The resulting single fibre value for a given variable represents a ‘composite’ value that theoretically reflects the average for the entire muscle (in this case the vastus lateralis). For an example see Table 1. Combining all fibre types together, a pre-bed-rest composite Vo value would be 2.71 FL s−1 for this subject. Thus, the value 2.71 FL s−1 represents an average contractile speed for all the muscle fibres.
Table 1.
Example calculations for the composite contractile velocity (Vo) variable from a bed-rest subject (BR group) after the 84 day intervention period
| Variable | MHC I | MHC I/IIa | MHC IIa | MHC IIa/IIx | MHC I/IIa/IIx | Totals |
|---|---|---|---|---|---|---|
| Fibre number | 6 | 5 | 4 | 7 | 3 | 25 |
| Average Vo (FL s−1) | 1.04 | 2.04 | 3.67 | 4.50 | 1.73 | — |
| Total fibre Vo* | 6.24 | 10.20 | 14.68 | 31.50 | 5.19 | 67.81 |
| Composite Vo (FL s−1) ** | — | — | — | — | — | 2.71 |
Total fibre Vo calculated by multiplying fibre number by average Vo.
Composite Vo calculated by dividing total fibre Vo (67.81) by the total number of fibres (25).
We recognize that these calculations are theoretical and make the assumption that the MHC profile of the fibres we randomly studied from the vastus lateralis of our subjects is representative of the whole thigh (quadriceps femoris). In support of this assumption, we did perform a more comprehensive MHC analysis from these same muscle biopsies (Harber et al. 2003), which is in close agreement with the MHC profile presented here. Using these composite estimates, we were able to include the large increase in hybrid fibres in the bed-rest analysis in an attempt to make a link between the myocelluar functional results and the whole muscle results in this investigation. This procedure was conducted for the variables of diameter, Po, Po/CSA, Vo, power and normalized power for each individual and then averaged to represent the BR and BRE groups.
Results
Whole muscle size and function
Whole muscle size and functional data for the BR and BRE groups are shown in Table 2. Whole muscle size was reduced by 17%(P < 0.05) in the BR group and maintained in the BRE group. Isometric and isotonic measures of muscle function were reduced, on average, by 40% or more in the BR group. All indices of muscle function in the BR group showed significant losses compared to pre-bed-rest values. Conversely, the BRE group showed no significant decline in any of the isometric or isotonic muscle measurements.
Table 2.
Whole muscle quadriceps size and function from BR and BRE subjects before (Pre) and after (Post) 84 days of bed-rest
| BR group | BRE group | |||||
|---|---|---|---|---|---|---|
| Variable | Pre | Post | %Δ | Pre | Post | %Δ |
| MV (cm3) | 928 ± 47 | 770 ± 40* | −17 | 1111 ± 60 | 1105 ± 60 | 0 |
| MVC (N) | 1564 ± 191 | 876 ± 116* | −43 | 1388 ± 67 | 1216 ± 95 | −11 |
| CPF (N) | 1628 ± 57 | 969 ± 59* | −41 | 1427 ± 121 | 1442 ± 167 | +4 |
| EPF (N) | 1468 ± 49 | 937 ± 58* | −36 | 1433 ± 152 | 1299 ± 145 | −7 |
| PP (W) | 704 ± 90 | 365 ± 51* | −47 | 530 ± 42 | 568 ± 67 | +9 |
%Δ, percentage change.
P < 0.05 from Pre. MV, muscle volume; MVC, maximal voluntary contraction; CPF, concentric peak force; EPF, eccentric peak force; PP, peak power.
Single muscle fibre MHC composition
The MHC profile is shown in Table 3. A total of 528 fibres were used for analysis in this investigation. Prior to the bed-rest period, both the BR and BRE groups had minimal hybrid fibres (13–14%) identified from the analysis. After the bed-rest period, the number of hybrid fibres (MHC I/IIa, IIa/IIx, or I/IIa/IIx) in the BR group increased (P < 0.05) to 49%. For the BR group, this appeared to be a fairly even distribution among the different hybrid types, with each type (I/IIa, IIa/IIx, I/IIa/IIx) contributing 15–17% to the total fibre population. In the BRE group, the total hybrid fibre population also increased (P < 0.05), to 31%, which was less (P < 0.05) than the increase in the BR group. The increase in hybrid fibres in the BRE group was most prominent in the MHC I/IIa fibre type, with no MHC I/IIa/IIx fibres identified in the pre- or post-bed-rest samples of this group. In the BR and BRE groups the increases in hybrid fibres resulted in decreases (P < 0.05) of 29 and 19% in MHC I fibres, respectively.
Table 3.
Single fibre myosin heavy chain (MHC) composition of vastus lateralis fibres used in physiological experiments from BR and BRE subjects before and after 84 days of bed-rest
| Group | MHC I | MHC I/IIa | MHC IIa | MHC IIa/IIx | MHC I/IIa/IIx | Total n | |
|---|---|---|---|---|---|---|---|
| BR | Pre | 73 (64%) | 8 (7%) | 26 (23%) | 7 (6%) | 0 (0%) | 114 |
| Post | 51 (35%) | 23 (15%) | 25 (17%) | 26 (17%) | 23 (16%) | 148 | |
| BRE | Pre | 58 (54%) | 2 (2%) | 34 (31%) | 14 (13%) | 0 (0%) | 108 |
| Post | 53 (35%) | 32 (21%) | 51 (34%) | 15 (10%) | 0 (0%) | 151 |
For each MHC type the number of fibres studied is shown with the percentage breakdown in parentheses.
Single muscle fibre diameter
Pre- and post-bed-rest fibre diameters are shown in Table 4. MHC I and IIa fibres were 15 and 8% smaller, respectivley, (P < 0.05) after the bed-rest period in the BR group. The BRE group did not show any significant changes in MHC I and IIa fibre diameters following bed-rest. After the bed-rest period, the MHC IIa fibres from the BR group were smaller (P < 0.05) compared to the BRE group.
Table 4.
Single muscle fibre diameter, peak force (Po) and specific force (Po/CSA) for all vastus lateralis myosin heavy chain (MHC) types from BR and BRE subjects before (Pre) and after (Post) 84 days of bed-rest
| MHC I | MHC I/IIa | MHC IIa | MHC IIa/IIx | MHC I/IIa/IIx | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Diameter (μm) | ||||||||||
| BR | 91 ± 3 | 77 ± 5* | 93 ± 5 | 80 ± 4 | 95 ± 5 | 87 ± 5† | 86 ± 6 | 86 ± 4 | N/A | 75 ± 2 |
| BRE | 87 ± 5 | 91 ± 5 | 102 ± 8 | 87 ± 4 | 97 ± 4 | 105 ± 8 | 88 ± 7 | 108 ± 9 | N/A | N/A |
| Po (mN) | ||||||||||
| BR | 0.65 ± 0.04 | 0.34 ± 0.05* | 0.69 ± 0.09 | 0.42 ± 0.05 | 0.88 ± 0.08 | 0.66 ± 0.07* | 0.77 ± 0.07 | 0.61 ± 0.07 | N/A | 0.41 ± 0.08 |
| BRE | 0.63 ± 0.06 | 0.51 ± 0.04 | 0.91 ± 0.16 | 0.63 ± 0.04 | 0.93 ± 0.10 | 0.97 ± 0.10 | 0.85 ± 0.11 | 0.84 ± 0.16 | N/A | N/A |
| Po/CSA (kN m−2) | ||||||||||
| BR | 101 ± 12 | 73 ± 9* | 103 ± 14 | 85 ± 12 | 124 ± 12 | 108 ± 9 | 158 ± 16 | 105 ± 10 | N/A | 89 ± 16 |
| BRE | 104 ± 5 | 83 ± 11 | 116 ± 37 | 106 ± 14 | 129 ± 14 | 117 ± 15 | 141 ± 10 | 90 ± 7 | N/A | N/A |
P < 0.05 from Pre.
P < 0.05 between groups.
Single muscle fibre Po and Po/CSA
Peak force and peak force per cross-sectional are shown in Table 4. As a result of the bed-rest, the BR groups' MHC I fibres showed a 47% decrease (P < 0.05) in Po, while the MHC IIa fibres showed a 25% decrease (P < 0.05) in Po. When corrected for cell size, the MHC I fibres still showed a decline (P < 0.05), suggesting that changes in fibre size could not explain all of the loss in peak force in these fibres. In contrast, MHC I and IIa fibres from the BRE group did not have any statistical reductions in Po or Po/CSA. While not significant, the MHC I fibres of the BRE group showed a trend for Po(P = 0.08) and Po/CSA (P = 0.10) to be lower.
Single muscle fibre shortening velocity
Contractile velocity of the muscle fibres was assessed using the slack test procedure (Vo) and the force–velocity procedure (Vmax) (see Methods). Both of these measurements provided a measure of shortening velocity and are shown in Table 5. The MHC I fibres had 21 and 22% drops (P < 0.05) in fibre Vo and Vmax, respectively, in the BR group. No change in MHC IIa fibre contractile velocity was found in the BR group. Likewise, no change in contractile velocity of the MHC I or IIa fibres was observed in the BRE group after bed-rest.
Table 5.
Single muscle fibre shortening velocity using the slack test procedure (Vo) and force–velocity procedure (Vmax) for all vastus lateralis myosin heavy chain (MHC) types from BR and BRE subjects before (Pre) and after (Post) 84 days of bed-rest
| MHC I | MHC I/IIa | MHC IIa | MHC IIa/IIx | MHC I/IIa/IIx | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Vo (FL s−1) | ||||||||||
| BR | 1.18 ± 0.08 | 0.93 ± 0.09* | 2.43 ± 0.61 | 1.86 ± 0.27 | 3.80 ± 0.44 | 3.58 ± 0.35 | 4.71 ± 0.62 | 4.24 ± 0.20 | N/A | 1.99 ± 0.39 |
| BRE | 1.30 ± 0.09 | 1.29 ± 0.06 | 2.98 ± 1.44 | 2.41 ± 0.50 | 3.75 ± 0.42 | 4.23 ± 0.33 | 4.78 ± 0.44 | 4.38 ± 0.17 | N/A | N/A |
| Vmax (FL s−1) | ||||||||||
| BR | 0.72 ± 0.11 | 0.56 ± 0.06* | 1.92 ± 0.62 | 0.90 ± 0.20 | 3.31 ± 0.36 | 2.23 ± 0.28 | 4.22 ± 0.71 | 3.56 ± 0.18 | N/A | 1.55 ± 0.54 |
| BRE | 0.67 ± 0.10 | 0.71 ± 0.10 | 1.85 ± 0.94 | 1.57 ± 0.31 | 2.92 ± 0.48 | 3.13 ± 0.22 | 4.22 ± 0.17 | 3.88 ± 0.27 | N/A | N/A |
P < 0.05 from Pre.
Single muscle fibre power
Absolute peak power and peak power normalized for muscle cell size of the MHC I and IIa fibres for the BR and BRE groups are shown in Table 6. The MHC I fibres from the BR group showed a 55% reduction (P < 0.05) in peak power after bed-rest. When adjusted for cell size, normalized peak power was still reduced (P < 0.05) by 41% in the MHC I fibres from the BR group. No significant reductions were observed in the MHC IIa fibres from the BR group as a result of the bed-rest. However, a trend (P = 0.10; −25%) towards lower peak power values was observed in the MHC IIa fibres from the BR group.
Table 6.
Single muscle fibre peak power (Absolute power) and peak power normalized to cell size (Norm power) for all vastus lateralis myosin heavy chain (MHC) types from BR and BRE subjects before (Pre) and after (Post) 84 days of bed-rest.
| MHC I | MHC I/IIa | MHC IIa | MHC IIa/IIx | MHC I/IIa/IIx | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Absolute power (μN FL s−1) | ||||||||||
| BR | 11.6 ± 1.2 | 5.2 ± 0.8* | 28.1 ± 8.2 | 11.5 ± 3.4 | 59.2 ± 8.2 | 44.7 ± 6.0 | 81.8 ± 11.2 | 44.9 ± 3.5 | N/A | 17.82 ± 8.41 |
| BRE | 12.8 ± 1.7 | 9.1 ± 0.8* | 50.0 ± 32.9 | 23.3 ± 4.8 | 68.2 ± 9.3 | 68.9 ± 7.1 | 86.2 ± 11.2 | 88.7 ± 17.3 | N/A | N/A |
| Norm power (W l−1) | ||||||||||
| BR | 1.89 ± 0.29 | 1.12 ± 0.13* | 3.84 ± 0.97 | 2.41 ± 0.76 | 8.28 ± 7.16 | 7.16 ± 0.51 | 16.84 ± 2.90 | 7.91 ± 0.72 | N/A | 4.07 ± 1.91 |
| BRE | 2.14 ± 0.17 | 1.47 ± 0.20* | 6.82 ± 5.03 | 3.97 ± 1.00 | 9.56 ± 1.29 | 8.26 ± 1.05 | 14.42 ± 1.29 | 9.39 ± 0.15 | N/A | N/A |
P < 0.05 from Pre.
The BRE group showed a 29% decline (P < 0.05) in MHC I fibre peak power. When adjusted for cell size, normalized power was still reduced (P < 0.05) in the MHC I fibres of the BRE group after bed-rest. No significant alterations were observed for the MHC IIa fibres of the BRE group following bed-rest.
Single muscle fibre composite data
Shown in Table 7 are the composite values for diameter, Po, Po/CSA, Vo, power and normalized power. The BR group had a reduction in all variables, with the exception of Vo. In contrast, the BRE maintained all parameters except for the normalized values of force and power.
Table 7.
Single muscle fibre vastus lasteralis composite data (see Methods for description) from BR and BRE subjects before (Pre) and after (Post) 84 days of bed-rest
| BR group | BRE group | |||||
|---|---|---|---|---|---|---|
| Variable | Pre | Post | %Δ | Pre | Post | %Δ |
| Diameter (μm) | 91 ± 3 | 82 ± 3* | −10 | 92 ± 4 | 97 ± 5 | +5 |
| Po (mN) | 0.71 ± 0.05 | 0.45 ± 0.03* | −36 | 0.69 ± 0.08 | 0.72 ± 0.05 | +4 |
| Po/CSA (kN m−2) | 109 ± 12 | 86 ± 6* | −21 | 116 ± 9 | 99 ± 11* | −15 |
| Vo (FL s−1) | 2.10 ± 0.13 | 2.34 ± 0.18 | +11 | 2.51 ± 0.18 | 2.80 ± 0.28 | +12 |
| Power (W l−1) | 27.9 ± 3.1 | 21.5 ± 1.8* | −23 | 39.3 ± 4.9 | 39.5 ± 4.6 | 0 |
| NPower (kN m−2 FL s−1) | 4.4 ± 0.6 | 3.9 ± 0.3 | −11 | 5.9 ± 0.7 | 5.1 ± 0.6 | −13 |
%Δ, percentage change.
P < 0.05 from Pre. Po, peak force; Po/CSA, force per cross-sectional area; Vo, unloaded shortening velocity; Power, absolute power; Npower, normalized power.
Discussion
One of the main goals of our research team over the past several years has been to document the loss in size and function of human skeletal muscle at the whole muscle and cellular level with unloading and to identify appropriate exercise countermeasures to offset this loss. Currently, an optimal exercise programme for protecting the skeletal muscles of humans during long-term unloading has not been elucidated. This project was a first attempt to examine the possibility that high-intensity, low-volume resistance exercise is sufficient to maintain skeletal muscle mass and function during long-duration unloading. The main finding from this investigation is that the resistance exercise programme used during bed-rest was effective for maintaining whole muscle size and function of the thigh muscles. However, muscle biopsies from the vastus lateralis showed that slow- and fast-twitch single muscle fibre function, along with the MHC profile of individual muscle fibres, was differentially affected by bed-rest. These data show that: (1) the MHC IIa fibres were protected by the resistance exercise programme, (2) the MHC I fibres were partially protected by the resistance exercise programme, and (3) both the BR and BRE groups showed a significant increase in hybrid fibres, with BRE showing less of an increase compared to BR.
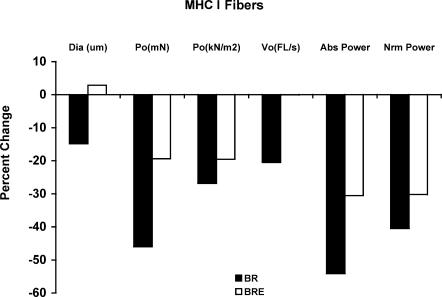
The single muscle fibre results are intriguing in that they appear to contrast the whole muscle findings. For the control group it was no surprise that both MHC I and IIa fibre functions were severely compromised as a result of the long-term bed-rest period (summarized in Figs 2 and 3). However, what was surprising was the reduction in some of the single cell functional parameters in the BRE group, despite the apparent maintenance of whole muscle size and function. The BRE MHC I fibres did maintain size and this, when coupled with the size preservation of the other fibre types in this group, provides an explanation of why whole muscle size was maintained. However, the BRE MHC I fibres showed a decline in the functional measures of strength, speed and power after bed-rest (Fig. 2). The BRE MHC IIa fibres maintained or increased cell size, peak force, contractile velocity, and power (Fig. 3). Although it appears that the exercise did have some positive benefit for the MHC I fibres, it was not sufficient to completely maintain single cell function compared to pre-bed-rest values.
Figure 2. A summary of the single muscle fibre parameters for the MHC IIa fibres for subjects in the bed-rest only (BR) group and bed-rest + resistance exercise (BRE) group.
For each variable the percentage change is shown.
With bed-rest, there was a large increase in the number of hybrid muscle fibres in both groups (Table 3). The BR group had an increase in hybrid fibres from 13 to 49%, while the BRE group had an increase in hybrid fibres from 14 to 31%. This shift in fibre composition was a directional shift from a slower contracting fibre to a faster contracting fibre. This slow-to-fast shift in myosin isoform composition is in agreement with previous unloading investigations in animals (Caiozzo et al. 1994, 1996) and humans (Zhou et al. 1995; Ohira et al. 1999). The MHC profile shift of the entire vastus lateralis would, in theory, lead to a faster contracting muscle. Or, in the case of this study, the shift in myosin isoform from slow to fast may have been sufficient to pre-serve whole muscle performance in the BRE group.
A unique finding from this study was the rather large number of MHC I/IIa/IIx fibres that were present in the control group muscles after bed-rest. Before bed-rest, neither the BR nor the BRE group had any identifiable MHC I/IIa/IIx. After bed-rest, however, a total of 23 fibres (16%) that had all three isoforms present were identified from the BR group. This is unique in that this fibre type is rarely observed in human skeletal muscle. Larrson et al. (1996) did not report any fibres containing all three isoforms from the human vastus lateralis muscle after 37 days of bed-rest. In our previous studies with short-term spaceflight (Widrick et al. 1999), ageing (Trappe et al. 2000, 2001b, 2003) and athletes (Trappe et al. 2001a), this MHC triple-hybrid isoform fibre has not been observed. This is in agreement with the work of others that has profiled the functional characteristics of normal active human skeletal muscle (Bottinelli et al. 1996, 1999, 2001). The functional profile of this novel MHC isoform falls in the spectrum between the MHC I/IIa hybrid and the pure MHC IIa isoform. The MHC IIx component of the MHC I/IIa/IIx fibre slightly increased the shortening velocity and power component compared to the MHC I/IIa fibres. The addition of the IIx isoform to the MHC I/IIa fibre may play a key role in increasing the contractile speed of fibres falling in the moderate range of functionality. The finding that long-term unloading resulted in one-sixth of the vastus lateralis muscle to be composed of the novel triple-hybrid isoform is reported here for the first time. The physiological role for this fibre type is presently unknown, as are the mechanisms that regulate selected muscle fibres to contain all three main isoforms in human skeletal muscle.
Most often single muscle fibre data are ‘compartmentalized’ into specific fibre types during analysis and when published. However, the vastus lateralis studied in this investigation was composed of a mixture of slow, fast, and a variety of hybrid muscle fibres, which comprise a continuum of functionality that contributes differentially to whole muscle performance. With this in mind, we wanted to provide an estimate of single muscle fibre function for all fibre types acting together (Table 7). This ‘composite’ value theoretically reflects the function of a given variable for multiple fibre types contracting together and provides some additional insight as to why whole muscle performance was unaffected in the BRE group. As can be seen in Table 7, the BR group had a reduction in single fibre size and all functional variables (except Vo), while the BRE group maintained most parameters. Of particular interest was single fibre power, since it is a product of strength and speed. The composite single muscle fibre power production was reduced by 23% in the BR group, but maintained in the BRE group, again providing additional information to help relate the single muscle fibre findings to whole muscle performance.
Another aspect worth noting was that the MHC I fibres appeared to be more affected in the control group compared to the MHC IIa fibres. This is in agreement with animal data showing that anti-gravity muscles that are composed primarily of slow-twitch fibres, such as the soleus, are more affected by unloading compared to muscles with a fast-twitch fibre component (Fitts et al. 2000). Following 5 weeks of bed-rest, LeBlanc et al. (1988) reported a 30% loss in human whole muscle peak torque of the calf and reported that whole muscle performance was less impacted at higher angular velocities. This also supports the idea that slow-twitch fibres appear to have a greater loss in function with unloading compared to fast-twitch fibres. From the current investigation, single muscle fibre power was reduced in the MHC I fibres by nearly 60%, and only about 25% in the MHC IIa fibres. This suggests that the lack of a 1 g load that is typically placed upon the muscles for normal daily activities (walking, simple tasks, posture, etc.) may play a bigger role in preserving these fibres than previously thought. As a result, resistance training alone may not be sufficient to preserve the contractile function of slow-twitch fibres during long-term unloading.
Single fibre data from vastus lateralis muscle have consistently shown that MHC IIa fibres produce 5- to 6-fold more power compared to MHC I fibres (Table 6; Trappe et al. 2000, 2003). In the current investigation, the MHC IIa fibres were ∼5-fold more powerful compared to the MHC I fibres for both the BR and BRE groups prior to bed-rest. Following bed-rest, the power relationship between the MHC I and IIa fibres was altered in both groups. For the BR group the MHC IIa fibres were 8–9 times more powerful compared to the MHC I fibres after bed-rest. This ratio was slightly altered in the BRE group after bed-rest, with the MHC IIa fibres having a ∼7-fold greater power output. This highlights the importance of the fast-twitch fibre types in their ability to generate power and their potential contribution to whole muscle power. Granted the MHC I fibres were more affected by bed-rest in both groups, but the decline in MHC I size, contractile speed and power would, in theory, have much less of an impact to a decline in whole muscle power output than if the MHC IIa fibres were not protected by exercise countermeasures. The maintenance of the MHC IIa power output in the BRE subjects of the current investigation would thus appear to be one of the major contributing factors to the similar whole muscle results pre- and post-bed-rest.
Single muscle fibre contractile velocity was reduced in the control group, which is both in contrast to (Widrick et al. 1997, 1999; Yamashita-Goto et al. 2001) and agreement with (Larsson et al. 1996; Widrick et al. 2002) previous human studies. With the exception of one investigation (Yamashita-Goto et al. 2001), studies with no exercise during the unloading period have all shown a decline in contractile speed at the single fibre level. Studies with designed countermeasures or mandated exercise programmes while in orbit have shown an elevation in single fibre contractile velocity (Widrick et al. 1997, 1999). Findings from the current investigation provide further evidence that during periods of unloading with no exercise single fibre contractile speed declines and when exercise countermeasure programmes are employed, single fibre contractile velocity is elevated. Thus, it does appear that muscle activity plays a significant role in modulating contractile velocity in each specific fibre type. As we (Trappe et al. 2000, 2001b) and others (Bottinelli, 2001) have reported, this alteration in contractile speed can occur without a change in MHC isoform. While the mechanism for this physiological alteration is unknown, several candidates such as undetected MHC isoforms (Bottinelli, 2001), alterations in myosin light chain composition (Bottinelli, 2001) or glycation of the myosin head (Ramamurthy et al. 2001) have been postulated.
Although each specific fibre type showed a decline or a tendency for a decline in fibre Vo for the control group, the composite Vo data in the control group indicated an increase of 11%. This is in close agreement with the 12% increase in the composite single fibre contractile velocity for the exercise group. As stated earlier, both the BR and BRE groups had an increase in hybrid muscle fibres, resulting in a slow- to fast-fibre transition following the bed-rest period. When combining the fibre type shift information with the contractile velocity data it becomes apparent that each individual fibre type had a decrease in contractile velocity, but the overall contractile velocity profile of the vastus lateralis was faster due to the myosin isoform changes. While isolated individual muscle fibres provide important information about a specific fibre type, this information may be misleading from a more global or whole muscle perspective.
In animals using the hindlimb unloading model, in vitro preparations have consistently reported contractile velocity to be elevated (cf. Fitts et al. 2000). What is interesting about these data is that no exercise during the hindlimb unloading period still resulted in an increase in single fibre Vo, which is in contrast to human studies with no exercise during unloading. However, muscle activity, as measured by EMG activity, initially was reduced in these animals and then gradually increased with the period of unloading (Alford et al. 1987). This suggests that the muscle was not in a state of reduced contractile activity during unloading in animals, except for the first few days of hindlimb suspension, and may have contributed to the rise in Vo. In contrast, human EMG activity has been shown to be reduced by ∼35–40% after 90–180 days of unloading (Lambertz et al. 2001). These data provide some evidence that the electrical activity to the muscle may differ with unloading among animals and humans and therefore direct comparisons between species and models of unloading should be made with caution.
Previous bed-rest studies of 37 days (Larsson et al. 1996) and 4 months (Yamashita-Goto et al. 2001) have reported a decline in MHC I fibre peak force normalized to cell size (i.e. specific force) of ∼40%. Slow- and fast-twitch muscle fibres from the control subjects (BR group) in the current study showed declines in specific force of ∼25 and ∼14%, respectively. Shorter duration periods of unloading have shown no change in specific force with bed-rest (Widrick et al. 1997) or spaceflight (Widrick et al. 1999). Given that the latter two studies employed periodic testing during the unloading period, one might speculate that specific force may be maintained with unloading provided there is an exercise programme. However, the slow- and fast-twitch muscle fibres from the exercise group of the current investigation also showed a decline in specific force, indicating that unloading duration and not exercise per se may be a contributing factor in specific force alterations. Riley et al. have shown that the actin protein is preferentially lost compared with myosin with 17 days of spaceflight (Riley et al. 2002) and bed-rest (Riley et al. 1998), but this did not translate into a decline in specific force of fibres from the soleus (Widrick et al. 1997, 1999). Thus, some other aspect of the contraction process (i.e. alterations in the contractile or myofibrillar proteins, calcium kinetics, force per cross-bridge, etc.) must be occurring to influence force per unit area in single muscle fibres during periods of long-term unloading.
A deficit in the energy intake by humans during bed-rest or while in space poses an additional concern that has been linked to muscle wasting (Ferrando et al. 1996; Stein et al. 1999). The voluntary energy intake of crew members while in space has been reported to be reduced by ∼20% (Stein et al. 1999). In the current investigation, subjects were fed prepared meals by the staff that were nutritionally balanced. For both the BR and BRE groups, the amount of energy consumed along with the composition of carbohydrates, fats, and proteins was consistent before, during and after the bed-rest period (Table 8). Of particular importance was the protein intake, since this has been shown to be critical for the anabolic and catabolic aspects of muscle protein turnover (Ferrando et al. 2002). Subjects from the current investigation consumed, on average, 90–100 g of protein per day, which is equivalent to 1.2–1.4 g (kg body weight)−1. This level of protein intake is in excess of the current recommended daily allowance (RDA) in the United States (0.8 g (kg body weight)−1) and within the range recommended for space travellers (Stein, 1994). Thus, it does not appear that the muscle alterations observed in the current investigation can be directly attributed to poor nutrition.
Table 8.
Average nutritional daily intake information from the pre-bed-rest (15 day average), bed-rest (90 day average) and post-bed-rest (15 day average) phases for the BR and BRE groups
| Group | Energy intake (kcal) | Carbohydrate (g) | Fat (g) | Protein (g) | |
|---|---|---|---|---|---|
| BR | Pre | 2355 ± 87 | 318 ± 14 (55%) | 76 ± 3 (29%) | 95 ± 2 (16%) |
| Bed-rest | 2083 ± 87 | 255 ± 15 (50%) | 75 ± 3 (32%) | 92 ± 2 (18%) | |
| Post | 2222 ± 104 | 281 ± 21 (51%) | 78 ± 2 (32%) | 93 ± 2 (17%) | |
| BRE | Pre | 2504 ± 57 | 345 ± 9 (56%) | 79 ± 2 (28%) | 100 ± 1 (16%) |
| Bed-rest | 2215 ± 58 | 290 ± 8 (53%) | 74 ± 2 (30%) | 94 ± 3 (17%) | |
| Post | 2474 ± 89 | 337 ± 12 (55%) | 81 ± 4 (29%) | 97 ± 4 (16%) |
The numbers in parentheses represent the percentage contributions of each nutrient.
In summary, this investigation demonstrated that high-intensity, low-volume resistance exercise every 2–3 days was effective for maintaining whole muscle strength and size during long-term bed-rest. Physiological studies on single muscle fibres from the vastus lateralis showed that the contractile function was preserved in the fast-twitch fibres, but not in the slow-twitch fibres from subjects who performed the resistance exercise. This discrepancy between the in vivo (whole muscle) and in vitro (single muscle fibre) studies appears to be related, in part, to the increase in hybrid muscle fibres and the relative functional contribution of the different fibre types. For the BRE group, the shift away from the slower less powerful fibres to faster more powerful fibres (MHC I → MHC I/IIa) coupled with the 5-fold greater power output of the fast-twitch fibres compared to the slow-twitch fibres, was sufficient to maintain whole muscle function. In light of the inability of the resistance training to maintain the functional characteristics of the slow-twitch fibres, additional exercise interventions that target and provide adequate loading for this fibre type should be considered.
Figure 1. A summary of the single muscle fibre parameters for the MHC I fibres for subjects in the bed-rest only (BR) group and bed-rest + resistance exercise (BRE) group.
For each variable the percentage change is shown.
Acknowledgments
This investigation was supported by National Institutes of Health (NIH) grant AG18409 (S. Trappe), NIH grant AG00831 (T. Trappe), the Swedish National Space Board (P. Tesch) and the European Space Agency.
References
- Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hind limb suspension. Exp Neurol. 1987;96:635–649. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- Alkner BA, Berg HE, Kozlovskaya I, Sayenko D, Tesch PA. Effects of strength training, using a gravity-independent exercise system, performed during 110 days of simulated space station confinement. Eur J Appl Physiol. 2003;90:44–49. doi: 10.1007/s00421-003-0850-2. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, White TP, Arnaud SB, Edgerton VR, Kraemer WJ, Kram R, Raab-Cullen D, Snow CM. Musculoskeletal adaptations to weightlessness and development of effective countermeasures. Med Sci Sports Exerc. 1996;28:1247–1253. doi: 10.1097/00005768-199610000-00007. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol. 1998;84:157–163. doi: 10.1152/jappl.1998.84.1.157. [DOI] [PubMed] [Google Scholar]
- Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid Volume after transition from standing to supine. Acta Physiol Scand. 1993;148:379–385. doi: 10.1111/j.1748-1716.1993.tb09573.x. [DOI] [PubMed] [Google Scholar]
- Berg HE, Tesch A. A gravity-independent ergometer to be used for resistance training in space. Aviat Space Environ Med. 1994;65:752–756. [PubMed] [Google Scholar]
- Berg HE, Tesch PA. Force and power characteristics of a resistive exercise device for use in space. Acta Astronaut. 1998;42:219–230. doi: 10.1016/s0094-5765(98)00119-2. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Muscle electrolytes in man. Scand J Clin Invest. 1962;14(suppl. 68):7–110. [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story. Pflugers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force–velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol. 1999;9:87–95. doi: 10.1016/s1050-6411(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol. 1994;76:1764–1773. doi: 10.1152/jappl.1994.76.4.1764. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Haddad F, Baker MJ, Herrick RE, Prietto N, Baldwin KM. Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J Appl Physiol. 1996;81:123–132. doi: 10.1152/jappl.1996.81.1.123. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Dudley GA, Tesch PA, Miller BJ, Buchanan P. Importance of eccentric actions in performance adaptations to resistance training. Aviat Space Environ Med. 1991;62:543–550. [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Paddon-Jones D, Wolfe RR. Alterations in protein metabolism during space flight and inactivity. Nutrition. 2002;18:837–841. doi: 10.1016/s0899-9007(02)00930-9. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol. 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983;129:277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J General Physiol. 1982;80:279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber M, Gallagher P, Creer A, Mazzetti S, Trappe T, Alkner B, Tesch PA. Human single muscle fiber morphology with 84-d bedrest and resistance exercise. Faseb J. 2003;17:A959. [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc B. 1938;126:136–195. [Google Scholar]
- Lambertz D, Perot C, Kaspranski R, Goubel F. Effects of long-term spaceflight on mechanical properties of muscles in humans. J Appl Physiol. 2001;90:179–188. doi: 10.1152/jappl.2001.90.1.179. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Berg HE, Frontera WR. Effects of removal of weight-bearing function on contractility and myosin isoform composition in single human skeletal muscle cells. Pflugers Arch. 1996;432:320–328. doi: 10.1007/s004240050139. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Gogia P, Schneider V, Krebs J, Schonfeld E, Evans H. Calf muscle area and strength changes after five weeks of horizontal bed rest. Am J Sports Med. 1988;16:624–629. doi: 10.1177/036354658801600612. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Rowe R, Schneider V, Evans H, Hedrick T. Regional muscle loss after short duration spaceflight. Aviat Space Environ Med. 1995;66:1151–1154. [PubMed] [Google Scholar]
- Moss RL. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J Physiol. 1979;292:177–192. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol. 1989;59:310–319. doi: 10.1007/BF02388334. [DOI] [PubMed] [Google Scholar]
- NASA. Critical Path Roadmap Baseline Document 000502. 2000 On-line http://criticalpath.jsc.nasa.gov. [Google Scholar]
- Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita-Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, Edgerton VR. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol. 1999;87:1776–1785. doi: 10.1152/jappl.1999.87.5.1776. [DOI] [PubMed] [Google Scholar]
- Ramamurthy B, Hook P, Jones AD, Larsson L. Changes in myosin structure and function in response to glycation. Faseb J. 2001;15:2415–2422. doi: 10.1096/fj.01-0183com. [DOI] [PubMed] [Google Scholar]
- Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Disproportionate loss of thin filaments in human soleus muscle after 17-day bed rest. Muscle Nerve. 1998;21:1280–1289. doi: 10.1002/(sici)1097-4598(199810)21:10<1280::aid-mus6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Thin filament diversity and physiological properties of fast and slow fiber types in astronaut leg muscles. J Appl Physiol. 2002;92:817–825. doi: 10.1152/japplphysiol.00717.2001. [DOI] [PubMed] [Google Scholar]
- Schulze K, Gallagher P, Trappe S. Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc. 2002;34:303–313. doi: 10.1097/00005768-200202000-00019. [DOI] [PubMed] [Google Scholar]
- Stein TP. Protein requirements for long term missions. Adv Space Res. 1994;14:157–166. doi: 10.1016/0273-1177(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Stein TP, Leskiw MJ, Schluter MD, Donaldson MR, Larina I. Protein kinetics during and after long-duration spaceflight on MIR. Am J Physiol. 1999;276:E1014–E1021. doi: 10.1152/ajpendo.1999.276.6.e1014. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Thorsson A, Colliander EB. Effects of eccentric and concentric resistance training on skeletal muscle substrates, enzyme activities and capillary supply. Acta Physiol Scand. 1990;140:575–580. doi: 10.1111/j.1748-1716.1990.tb09035.x. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Trieschmann JT, Ekberg A. Hypertrophy of chronically unloaded muscle subjected to resistance exercise. J Appl Physiol. 2003;46:1451–1458. doi: 10.1152/japplphysiol.01051.2003. [DOI] [PubMed] [Google Scholar]
- Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties. Med Sci Sports Exerc. 2001a;33:48–56. [PubMed] [Google Scholar]
- Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol. 2001b;281:C398–C406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- Trappe SW, Trappe TA, Lee GA, Widrick JJ, Costill DL, Fitts RH. Comparison of a space shuttle flight (STS-78) and bed rest on human muscle function. J Appl Physiol. 2001c;91:57–64. doi: 10.1152/jappl.2001.91.1.57. [DOI] [PubMed] [Google Scholar]
- Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol. 2000;89:143–152. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915–930. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrick JJ, Romatowski JG, Bain JL, Trappe SW, Trappe TA, Thompson JL, Costill DL, Riley DA, Fitts RH. Effect of 17 days of bed rest on peak isometric force and unloaded shortening velocity of human soleus fibers. Am J Physiol. 1997;273:C1690–C1699. doi: 10.1152/ajpcell.1997.273.5.c1690. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Trappe SW, Romatowski JG, Riley DA, Costill DL, Fitts RH. Unilateral lower limb suspension does not mimic bed rest or spaceflight effects on human muscle fiber function. J Appl Physiol. 2002;93:354–360. doi: 10.1152/japplphysiol.01245.2001. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol. 2001;91:1955–1961. doi: 10.1152/jappl.2001.91.5.1955. [DOI] [PubMed] [Google Scholar]
- Yamashita-Goto K, Okuyama R, Honda M, Kawasaki K, Fujita K, Yamada T, Nonaka I, Ohira Y, Yoshioka T. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol. 2001;91:417–424. doi: 10.1152/jappl.2001.91.1.417. [DOI] [PubMed] [Google Scholar]
- Zhou MY, Klitgaard H, Saltin B, Roy RR, Edgerton VR, Gollnick PD. Myosin heavy chain isoforms of human muscle after short-term spaceflight. J Appl Physiol. 1995;78:1740–1744. doi: 10.1152/jappl.1995.78.5.1740. [DOI] [PubMed] [Google Scholar]