Abstract
We have identified the tracheal and laryngeal afferent nerves regulating cough in anaesthetized guinea-pigs. Cough was evoked by electrical or mechanical stimulation of the tracheal or laryngeal mucosa, or by citric acid applied topically to the trachea or larynx. By contrast, neither capsaicin nor bradykinin challenges to the trachea or larynx evoked cough. Bradykinin and histamine administered intravenously also failed to evoke cough. Electrophysiological studies revealed that the majority of capsaicin-sensitive afferent neurones (both Aδ- and C-fibres) innervating the rostral trachea and larynx have their cell bodies in the jugular ganglia and project to the airways via the superior laryngeal nerves. Capsaicin-insensitive afferent neurones with cell bodies in the nodose ganglia projected to the rostral trachea and larynx via the recurrent laryngeal nerves. Severing the recurrent nerves abolished coughing evoked from the trachea and larynx whereas severing the superior laryngeal nerves was without effect on coughing. The data indicate that the tracheal and laryngeal afferent neurones regulating cough are polymodal Aδ-fibres that arise from the nodose ganglia. These afferent neurones are activated by punctate mechanical stimulation and acid but are unresponsive to capsaicin, bradykinin, smooth muscle contraction, longitudinal or transverse stretching of the airways, or distension. Comparing these physiological properties with those of intrapulmonary mechanoreceptors indicates that the afferent neurones mediating cough are quite distinct from the well-defined rapidly and slowly adapting stretch receptors innervating the airways and lungs. We propose that these airway afferent neurones represent a distinct subtype and that their primary function is regulation of the cough reflex.
The cough reflex is initiated in animals and in human subjects by a variety of stimuli including capsaicin, citric acid, hypertonic saline, water, mechanical stimuli delivered to the large airways, cigarette smoke, autacoids such as bradykinin and prostaglandins, and to a lesser extent, neurotransmitters such as muscarinic receptor agonists, substance P and neurokinin A. It is clear that afferents arising bilaterally in the vagus nerves play an essential role in regulating the cough reflex, but it remains unclear precisely which afferent nerves are responsible for initiating cough and the mechanisms by which this defensive reflex is induced (Widdicombe, 1998; Karlsson & Fuller, 1999; Canning, 2002).
Evidence both for and against the hypotheses that C-fibres and rapidly adapting receptors (RARs) regulate coughing has been presented. C-fibre selective stimulants such as bradykinin and capsaicin are effective at evoking cough (Karlsson & Fuller, 1999). However, in anaesthetized animals, these stimuli have consistently failed to produce coughing. On the contrary, pulmonary C-fibre activation may inhibit coughing in anaesthetized animals (Tatar et al. 1988; Tatar et al. 1994). RARs are also activated by many stimuli that evoke coughing, and vagal cooling experiments are most consistent with the notion that RARs and not C-fibres are responsible for the cough reflex (Widdicombe, 1954a,b,c, 2003; Tatar et al. 1988). But stimuli such as thromboxane, histamine, neurokinins, leukotriene C4 and D4 (LTC4 and LTD4) and methacholine readily activate RARs and yet they are ineffective or only modestly effective at producing cough (Barnes et al. 1984; Joos et al. 1987; Fujimura et al. 1992; Sano et al. 1992; Takahama et al. 1993; Shinagawa et al. 2000; Widdicombe, 2003). These and other data indicate that coactivation of C-fibres and RARs, a unique mode of activation of C-fibres and/or RARs, or perhaps recruitment of a previously unrecognized afferent nerve subtype (‘cough receptors’; Widdicombe, 1954c) is required to precipitate coughing.
Coughing is evoked most readily from the larynx, trachea, extrapulmonary bronchi and large intrapulmonary bronchi (Widdicombe, 1954a,c; 1998). Vagal afferent neurones innervating the larynx, trachea and bronchi of guinea-pigs have been distinguished based on their ganglionic origin, conduction velocity, neurochemistry, sites of termination in the airways, and responsiveness to chemical and mechanical stimuli (Fox et al. 1993; Riccio et al. 1996; Hunter & Undem, 1999; McAlexander et al. 1999). In the present study we have used this pre-existing knowledge to identify the afferent neurones regulating cough in anaesthetized guinea-pigs. The data indicate that a subpopulation of myelinated, capsaicin-insensitive polymodal afferent neurones that are not readily classified as either rapidly or slowly adapting receptors are primarily responsible for regulating cough evoked from the trachea or larynx. We reintroduce the term ‘cough receptors’ for these nerves and speculate that their primary function is to regulate this important defensive reflex.
Methods
All experiments were carried out using male Hartley guinea-pigs (100–400 g, Hilltop, Scottdale, PA, USA) and were first approved by the Johns Hopkins University animal care and use committee. In some experiments, animals were anaesthetized with urethane (1 g kg−1i.p.). This dose of urethane provides a deep, stable anaesthesia persisting (up to 9 h) far longer than the duration of any experiments described. Throughout these experiments (which rarely lasted more than 2 h), the depth of anaesthesia was monitored by assessing paw withdrawal in response to a sharp pinch of the hindlimb and responses evoked by manipulating the exposed tissues in the neck (see below). Although not required by any animals used in this study, additional anaesthetic would have been provided if responses to these noxious stimuli had been noted.
At the end of the experiments carried out in vivo or for tissue harvest, animals were killed by asphyxiation in a vessel filled with 100% CO2 followed by exsanguination. The American Veterinary Medical Association recommends this method of killing for guinea-pigs.
Cough evoked in anaesthetized guinea-pigs
Guinea-pigs were anaesthetized and placed supine on a Plexiglas restraint resting on a warming pad. A midline incision in the neck exposed the trachea. The caudal-most segment of the trachea was cannulated with a bent, 15 gauge leur stub adaptor. Great care was taken not to disrupt the extrinsic innervation of the trachea or its blood supply when tightly tying the cannula in place. The cannula was then attached to a small length of tubing that terminated inside a water-jacketed organ bath continuously filled with fresh air. Moistened gauze was placed in the space surrounding the tubing to humidify the inspired air. (The tubing and organ bath were designed to approximate the warming and humidifying functions and the dimensions and resistance of the nose.) A pressure transducer was attached to a side port in the tracheal cannula to monitor respiratory efforts. All physiological parameters were recorded digitally using a Biopac data acquisition system (Biopac, Santa Barbara, CA, USA).
Once the trachea was cannulated, the remaining rostral segment of the extrathoracic trachea was opened lengthwise with an incision along its ventral-most aspect, through the cartilage rings. The incision extended from one to two cartilage rings rostral to the tracheal cannula through the cartilage ring adjacent to the larynx. A short length of polyethylene (PE) tubing was threaded through the larynx and nasal cavity and out of a nostril. Warmed, oxygenated Krebs buffer (composition (mm): NaCl (118), KCl (5.4), NaHPO4 (1), MgSO4 (1.2), CaCl2 (1.9), NaHCO3 (25) and dextrose (11.1)) was superfused (3 ml min−1) over the tracheal mucosa. The buffer was introduced into the tracheal lumen from the caudal-most exposed segment of the trachea, and removed at the rostral end of the trachea by attaching the PE tubing threaded through the upper airways to a gentle suction source. Throughout the dissection, cough reflexes evoked by any of the surgical manipulations were noted. Animals (<10%) that failed to cough during the dissection and failed to cough to any subsequent stimuli were excluded from all subsequent analyses. A schematic of the preparation of the trachea is provided in Fig. 1.
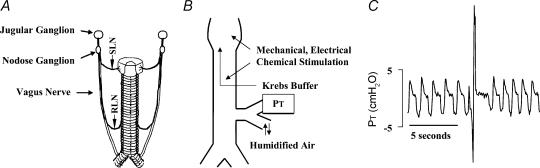
Figure 1. Schematic diagrams of the tracheal innervation, the preparation used to study cough, and a representative trace of a cough evoked in an anaesthetized guinea-pig.
A, diagram of the extrinsic innervation of the trachea, including the recurrent (RLN) and superior (SLN) laryngeal nerves. B, schematic diagram of the preparation used to study cough. Pressure changes at the tracheal cannula (PT) are used to monitor respiration and cough. C, representative trace of coughing initiated by mechanically probing the tracheal mucosa. Cough is defined visually by the experimenter and based on the timing (< 1 s for the entire manoeuvre) and magnitude of the inspiratory (appearing as a downward deflection in the pressure trace) and expiratory (>500% of expiratory pressure during tidal breathing) efforts.
Upon completion of the dissection, animals were allowed to breathe spontaneously and without any further manipulations for 10 min. Thereafter, we attempted to evoke cough by mechanical, electrical and chemical means. Cough was evoked mechanically by probing the tracheal or laryngeal mucosa with a von Frey filament (tip diameter: 0.8 mm) that delivers a force (4.7 mN) far exceeding the mechanical threshold described for all identified tracheal and laryngeal afferent nerve subtypes (Riccio et al. 1996). The mucosa was repeatedly probed for 10 s or until the animal coughed. Cough was evoked electrically using a custom-made bipolar (the distance between the poles is 1 mm) platinum electrode placed on the tracheal or laryngeal mucosa that delivered electrical stimuli (16 Hz, 12 V, 1 ms pulse duration, 10 s train) initiated from a Grass stimulator (Model S88). Optimal stimulation intensities were determined in preliminary experiments. We tried to evoke cough chemically by adding capsaicin (10 μm) or bradykinin (3 μm) directly to the tracheal perfusate, or with citric acid (0.01–1 m) applied topically in 100 μl aliquots. Cough was defined based on visual confirmation of a cough-like respiratory effort, and based on a change in tracheal pressure that produced a ≥500% increase in peak expiratory pressure preceded by an enhanced inspiratory effort (see Fig. 1).
Several interventions were performed prior to the equilibration period in an attempt to prevent coughing evoked from the trachea and larynx. The recurrent (RLN) or superior (SLN) laryngeal nerves were cut bilaterally in some experiments. In other experiments, the vagi were cut (either unilaterally or bilaterally) caudal to the SLNs. If cough was prevented by any of these manipulations, we attempted to evoke cough by mechanically probing the carina with PE10 threaded through the tracheal cannula or by acetone vapour inhalation (administered by holding a cotton swab saturated with acetone over the tracheal cannula). Cough evoked in this manner was monitored only visually, as the pressure transducer was necessarily removed to expose the tracheal cannula (Bergren & Sampson, 1982; Ravi et al. 1995; Takahama et al. 1997).
Respiratory reflexes evoked by intravenously administered histamine (1–10 μg kg−1) or bradykinin (0.2–0.5 nmol kg−1) or inhaled bradykinin (10 mg ml−1) were also studied. To administer drugs intravenously, the abdominal aorta and adjacent vena cava were cannulated as previously described (Mazzone & Canning, 2002b). The animals were then placed in a box continuously filled with room air. Pressure changes within the box were measured to monitor respiratory efforts. Blood pressure was monitored with a transducer attached to the arterial cannula. Intact animals were challenged with bradykinin by nebulizing the peptide into the box using an ultrasonic nebulizer (∼5 μm particle size; Mystique, Airsep, Buffalo, NY, USA) connected in series with the air pump. We also compared bradykinin-induced reflexes in conscious animals with those evoked following anaesthesia. Also, in some animals, bradykinin was administered by nebulization into the air warmed and humidified prior to inspiration through the tracheal cannula. Cough was defined visually and by its characteristic effects on the pressure trace.
Electrophysiology
Extracellular recording of action potentials induced by mechanical, electrical, or chemical activation of neuronal receptive fields in the larynx, trachea or mainstem bronchi were obtained as previously described (Riccio et al. 1996). The larynx, trachea and right bronchus were isolated with the right vagus nerve (including the nodose and jugular ganglion) intact. The preparation was pinned out in a Sylgard-coated Plexiglas chamber with the rostral aspect of the vagus nerve (and ganglia) threaded through a small hole and pinned to the bottom of an adjoining chamber. The hole connecting the chamber compartments was sealed with Vaseline to prevent mixing of the fluids in the chambers. The chambers were separately superfused (6 ml min−1) with Krebs buffer. Single fibre activity was obtained by moving a stimulating electrode along the surface of the airway, while a recording electrode was manipulated into and out of different locations in the ganglia until single unit activity was discriminated. After identifying the receptive field, characterizing the afferent nerve subtype and its conduction velocity (see below), the extrinsic pathways of the extrapulmonary afferent nerve fibres were determined by cutting the superior (SLN) or recurrent (RLN) laryngeal nerve, which in all cases terminated recorded action potentials initiated from the rostral tracheal or laryngeal receptive fields.
We also studied the electrophysiological properties of afferent neurones innervating the intrapulmonary airways and lungs in vitro. The lungs were freed of most of the blood by in situ perfusion with 20–30 ml of Krebs buffer (20–25°C containing 3 μm indomethacin) delivered through a feeding needle inserted into the pulmonary artery. The lungs were simultaneously insufflated with buffer (5–10 ml, 2–3 times) using a cannula (PE100) wedged into the trachea. The trachea and lungs with intact right-side vagal innervation were then dissected free and pinned to the bottom of a small Sylgard-lined Perspex chamber perfused with buffer (pH 7.4, 37°C, 4 ml min−1) just as described above. A piece of PE60 tubing was inserted into the pulmonary artery and connected to a Venoset Microdrip for continuous infusion of buffer (37°C, 4 ml min−1). Using an infusion pump (model 944, Harvard Apparatus, Holliston, MA), warmed, oxygenated buffer was also continuously infused (2 ml min−1) into the airways via the tracheal cannula. The lungs were pierced with a 27 gauge needle (1–2 mm deep, 2–8 times per lobe) to allow perfusate to exit the tissue. A P23AA pressure transducer (Statham, Hata Rey, PR, USA) attached to the chart recorder measured tracheal perfusion pressure.
Mechanically sensitive receptive fields in the airways and lungs were identified when mechanical stimuli (von Frey hair, 1800–3000 mN) bluntly applied to the airway mucosa or to the outer surface of a lung lobe evoked action potentials. Once these receptive fields were identified, a small concentric stimulating electrode was positioned over their discrete location in the airway or lung to determine axon conduction velocity (calculated by dividing the distance along the nerve pathway by the time between the shock artifact and the action potential evoked by electrical stimulation of the mechanically sensitive receptive field). Fibres were classified as C-fibres if they conducted action potentials at less than 1.3 m s−1 and Aδ-fibres if they conducted action potentials above 2.0 m s−1. In most experiments, one fibre/preparation was studied. Occasionally, two consecutive fibres were studied in the same preparation. In these instances, caution was used to avoid desensitization due to repeated agonist administration.
We studied the effects of histamine, methacholine, ATP receptor agonists and bradykinin on identified afferent neurones. Agonists were dissolved in 1 ml of Krebs buffer (37°C) and applied directly to the receptive field in the trachea/larynx preparations, or infused (50 μl s−1) into the lung via the pulmonary artery. Responses to lung distension were studied by increasing tracheal perfusion rate. Doubling the perfusion rate produced distending pressures that activated most intrapulmonary mechanoreceptors. To determine the adaptation index of the neurones, perfusion rates were again doubled, and held for 5–10 s. An adaptation index > 90% over the initial 5 s of the stimulus was used as a criterion for identifying rapidly adapting receptors.
In other preparations, we assessed the effects of altering tracheal luminal pressure on afferent nerve responsiveness in vitro and on respiration in vivo. The trachea was cannulated on its rostral and caudal ends and filled with Krebs buffer. One end of the trachea was connected to a syringe filled with Krebs buffer; the other cannula was connected to a pressure transducer. The effects of altering the luminal pressure on afferent neuronal discharge and on respiration were recorded as described above. Comparable studies were carried out in vitro, where the trachea was stretched either longitudinally or transversely. The trachea was tethered via cartilage rings to a manipulator that allowed varying degrees of transverse or longitudinal stretch. The effects of stretching on identified tracheal afferent neurones were recorded as described above.
Extracellular recordings from airway afferent neuronal cell bodies were performed using an aluminosilicate glass microelectrode as described elsewhere (Undem et al. 2004). Recordings were amplified (Microelectrode AC amplifier 1800, A-M Systems) and filtered (low cut off, 0·3 kHz; high cut off, 1 kHz) and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and a model TA240 chart recorder (Gould, Valley View, OH, USA). The data were stored and analysed on a Macintosh computer using the software TheNerveOfIt (sampling frequency 33 kHz, PHOCIS, Baltimore, MD, USA).
Statistical analyses
Unless otherwise stated, data are presented as means ±s.e.m. of n experiments, where n is the number of separate animals studied. Analysis of variance was used to compare group means (respiratory rate, total coughs, conduction velocity, total action potentials). Post hoc comparisons for group means were made using Sheffe's F-test for unplanned comparisons. The ability of the various nerve cuts performed to prevent cough were compared using Chi-square analysis. A P-value of 0.05 was considered significant.
Reagents
AstraZeneca (Wilmington, DE, USA) and GlaxoSmithKline (King of Prussia, PA, USA) provided bradykinin and LTC4, respectively. ATP, α,β-methylene ATP, capsaicin, citric acid, histamine, indomethacin, isoproterenol, methacholine and urethane were purchased from Sigma (St Louis, MO, USA). The P2X receptor antagonist pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS) was purchased from Tocris (Ellisville, MO, USA). Stock solutions of these compounds were dissolved in water except for bradykinin (saline), capsaicin and indomethacin (ethanol) and LTC4 (methanol). Drugs were diluted further in Krebs buffer for in vitro studies, and in saline for administration in vivo.
Results
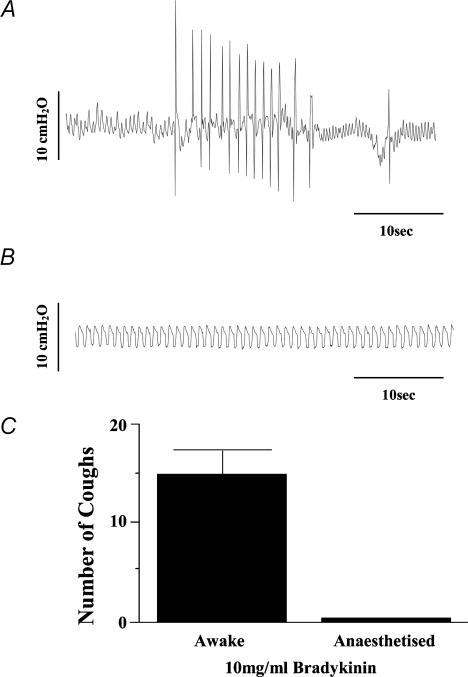
Baseline respiratory rate in the anaesthetized guinea-pigs averaged 52 ± 2 breaths min−1 (n = 74). All animals coughed during cannula placement in the trachea or larynx. Cough was also evoked in control animals by mechanical or electrical stimulation of the tracheal or laryngeal mucosa, mechanical probing of the carina, acetone vapour inhalation, or electrical stimulation of the central cut end of a vagus nerve. Unlike in conscious guinea-pigs, however, bradykinin inhalation (10 mg ml−1) failed to evoke coughing in the anaesthetized guinea-pigs (Table 1; Fig. 2). Bradykinin administered intravenously (0.2–0.5 nmol kg−1; n = 3) and aerosolized hypertonic saline (4%; n = 5) also failed to evoke coughing in the anaesthetized animals (data not shown).
Table 1.
Respiratory rate and coughing responses following cuts of the extrinsic innervation of the trachea and larynx in anaesthetized guinea-pigs
| Vagotomy | |||||
|---|---|---|---|---|---|
| Control | RLNs severed | SLNs severed | Unilateral | Bilateral | |
| Respiratory rate (breaths min−1) | 52 ± 2 (74) | 50 ± 5 (14) | 51 ± 3 (13) | 54 ± 4 (28) | 27 ± 4 (6) |
| Cough to mechanical stimulation | |||||
| Trachea | 11/16 | 0/12 | 6/10 | 2/3 | NA |
| Larynx | 13/14 | 1/12 | 9/10 | 21/25 | 0/6 |
| Carina | 3/3 | 2/2 | NA | NA | NA |
| Cough to Electrical Stimulation | |||||
| Trachea | 11/13 | 0/3 | 4/4 | NA | NA |
| Larynx | 7/13 | 0/4 | 7/7 | NA | NA |
| Rostral vagus nerves | NA | NA | NA | 22/28 | 6/6 |
| Cough response to acetone vapour | 15/25 | 7/9 | 12/13 | NA | NA |
See text for details of experimental design and methods for evoking and measuring cough. Unless otherwise stated, nerves were cut bilaterally. Coughing responses to the various stimuli are presented as the number of animals coughing/the number of animals tested. In experiments with unilateral vagotomy, either the right (n = 9) or left (n = 19) vagus nerve was cut, producing comparable results (not shown). NA: not assessed. RLN: recurrent laryngeal nerve. SLN: superior laryngeal nerve.
Figure 2. The effects of anaesthesia on bradykinin-evoked coughing in guinea-pigs.
Conscious (A, n = 12) or anaesthetized (B, n = 5) guinea-pigs were challenged for 10 min in a flow through chamber with nebulized bradykinin (10 mg ml−1). Pressure changes within the chamber were used to monitor respiration and coughing. Coughs evoked by bradykinin were counted and the average number of coughs is presented in C.
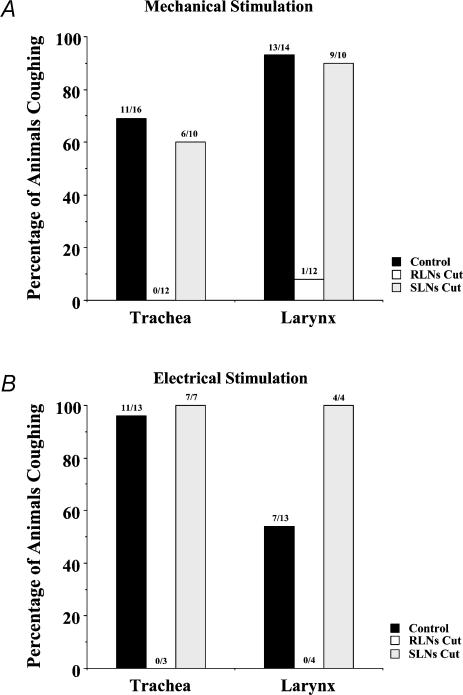
Cutting the superior (SLNs) or recurrent (RLNs) laryngeal nerves was without effect on respiratory rate. Cutting the SLNs was also without effect on the ability to evoke coughing. By contrast, cutting the RLNs prevented cough induced by electrical or mechanical stimulation of the tracheal or laryngeal mucosa (Table 1; Fig. 3). Bilateral RLN cuts did not prevent cough evoked by acetone vapour inhalation, however, nor cough evoked by mechanically probing the carina with PE10. As expected, bilateral vagotomy decreased respiratory rate and prevented cough evoked by mechanical stimulation of the larynx. Electrical stimulation of the central cut end of a vagus nerve following bilateral vagotomy did, however, evoke coughing. A unilateral cut of the right or left vagus nerve had little or no effect on respiratory rate and did not prevent coughing evoked by mechanical stimulation of the tracheal or laryngeal mucosa (Table 1).
Figure 3. The effect of cutting the superior (SLNs) or recurrent (RLNs) laryngeal nerves on coughing evoked by mechanically probing or electrically stimulating the tracheal or laryngeal mucosa of anaesthetized guinea-pigs.
Results are graphed as the percentage of animals coughing in response to mechanical (von Frey filament (4.7 mN), A) and electrical (16 Hz, 10 s, 12 V, 1 ms pulse duration, B) stimuli. The numbers above each bar indicate the number of animals that coughed/the number of animals that were challenged.
Identification of the afferent neurones mediating cough
The RLNs and SLNs carry the axons of all vagal afferent neurones innervating the larynx and extrathoracic trachea, with roughly equal numbers of these neurones projecting from the jugular and nodose ganglia (Kummer et al. 1992; S. B. Mazzone & B. J. Canning, unpublished observations). In the present study, an analysis of the projections of 281 afferent neurones was performed to determine the route of projection for the various vagal afferent nerve subtypes innervating the rostral trachea and larynx (characteristics of these subtypes have been described elsewhere; Riccio et al. 1996; Hunter & Undem, 1999; McAlexander et al. 1999; also see Fig. 4). We found that 88% (57/65 neurones studied) of tracheal and laryngeal afferent neurones with cell bodies in the nodose ganglia projected to the airways via the RLNs. The SLNs carried 84% (181/216 neurones studied) of the jugular ganglia neurones projecting to the larynx and rostral trachea (the first 5 cartilage rings adjacent to the larynx). Physiological differences in the neurones of each subtype that projected to the trachea and larynx via different extrinsic nerves were not apparent. Subclassification of the jugular ganglia neurones projecting to the larynx and rostral trachea revealed a preponderance of C-fibres (86%; 185/216 jugular ganglia neurones studied). This contrasted with the caudal trachea and bronchi, where the receptive fields of C-fibres arising from the jugular ganglia were less prevalent (91/198 or 46%) than the receptive fields of Aδ-fibres arising from the jugular ganglia (Table 2).
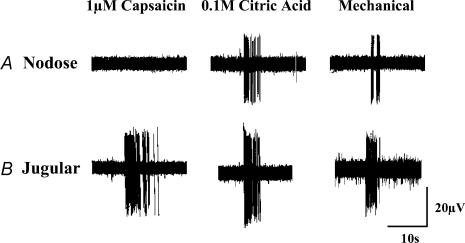
Figure 4. Representative recordings from tracheal afferent neurones originating in the nodose (A) and jugular (B) ganglia of guinea-pigs.
Three subtypes of vagal afferent neurones innervate the trachea, larynx and mainstem bronchi of guinea-pigs: jugular ganglia neurones (both Aδ- and C-fibres) that are activated by acid, punctate mechanical stimuli, capsaicin and bradykinin, and nodose ganglia neurones conducting action potentials in the Aδ range that are activated by acid and punctate mechanical stimuli. Neither capsaicin nor bradykinin activates or sensitizes nodose ganglia neurones for activation. See text and Tables 2 and 3 for further details.
Table 2.
Vagal afferent nerve subtypes innervating the rostral trachea and larynx of guinea pigs: ganglionic origin and extrinsic pathways
| Nodose Ganglia | Jugular Ganglia | |||
|---|---|---|---|---|
| Aδ-fibres | C-fibres | Aδ-fibres | C-fibres | |
| Laryngeal afferent nerves | ||||
| Percentage of fibres originating from ganglia | 100% (30/30) | 0% (0/30) | 18% (29/162) | 82% (133/162) |
| Percentage of subtype carried by the SLN | 23% (7/30) | — | 97% (28/29) | 89% (118/133) |
| Percentage of subtype carried by the RLN | 77% (23/30) | — | 3% (1/29) | 11% (15/133) |
| Rostral tracheal afferent nerves | ||||
| Percentage of fibres originating from ganglia | 100%(35/35) | 0% (0/35) | 3% (2/54) | 97% (52/54) |
| Percentage of subtype carried by the SLN | 3% (1/35) | — | 100% (2/2) | 63% (33/52) |
| Percentage of subtype carried by the RLN | 97% (34/35) | — | 0% (0/2) | 37% (19/52) |
See text for details. The rostral trachea is defined in this study as the first 5 tracheal rings caudal (and adjacent) to the larynx.
Tracheal and laryngeal afferent neurones carried by the superior laryngeal nerves (SLNs) were never found to project beyond the 5th tracheal ring caudal to the larynx. Thus, all vagal afferents (originating in both the jugular and nodose ganglia) studied that innervated the midcervical trachea from the 5th tracheal cartilage ring to the tracheal cartilage rings adjacent to the origin of the RLNs projected to the airways via the RLNs.
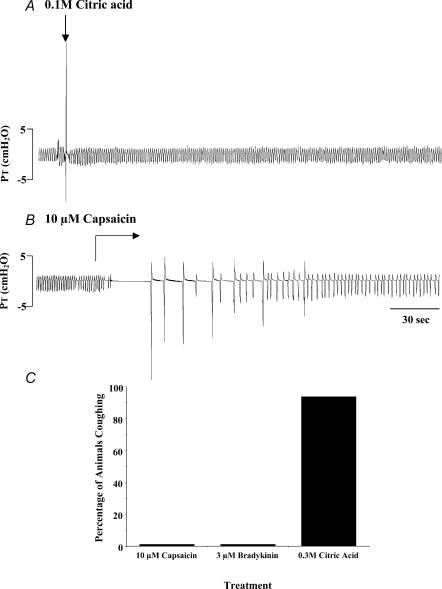
The afferent nerve subtypes regulating cough were further characterized by studying the effects of capsaicin, bradykinin and citric acid on cough. Both capsaicin and bradykinin, which selectively activate jugular ganglia neurones innervating the trachea and larynx (Fig. 4; Table 3; also see Riccio et al. 1996; Kajekar et al. 1999), were consistently ineffective at evoking cough in the anaesthetized guinea-pigs. Rather, both capsaicin and bradykinin evoked transient decreases in respiration, which resolved within 3–4 min and occasionally precipitated a delayed but sustained increase in respiratory rate. By contrast, tracheal challenge with citric acid, which activates both jugular and nodose ganglia afferent neurones innervating the trachea and larynx (Fig. 4; Table 3; also see Kollarik & Undem, 2002), evoked coughing in anaesthetized guinea-pigs and induced only a transient decrease in respiratory rate (Fig. 5).
Table 3.
Characteristics of vagal afferent neuronal subtypes innervating the airways and lungs of guinea-pigs
| RARs | Cough receptors | Neural crest C-fibres1 | Placodal C-fbres1 | |
|---|---|---|---|---|
| Ganglionic origin | nodose | nodose | jugular | nodose |
| Intrapulmonary terminations | Yes | Few | Yes | Yes |
| Extrapulmonary terminations | No | Yes | Yes | Few |
| Conduction velocity | ∼16 m s−1 | ∼5 m s−1 | < 1 m s−1 | < 1 m s−1 |
| Responsive to punctate mechanical stimuli | Yes | Yes | Yes | Yes |
| Responsive to stretch2 | Yes | No | No | No |
| Responsive to histamine | Yes3 | No | NA | NA |
| Responsive to methacholine | Yes3 | No | NA | NA |
| Responsive to bradykinin | NA | No | Yes | Yes |
| Responsive to ATP | Yes | No | No | Yes |
| Responsive to capsaicin | Yes3 | No | Yes | Yes |
| Responsive to acid | NA | Yes | Yes | NA |
| Responsive to 4% hypertonic saline | NA | No | Yes | NA |
Data are taken from the present study and from previous studies, all of which were carried out in vitro (Riccio et al. 1996; Kollarik & Undem, 2002; Undem et al. 2004). For clarity, the properties of other afferent neuronal subtypes identified in guinea pigs have been omitted.
C-fibre subtypes have been described in detail elsewhere (Undem et al. 2004).
Stretch refers to a mechanical stretch in either the longitudinal or circumferential axis of the airway, or to a distending pressure (20–100 cmH2O).
Activation of RARs by histamine, methacholine or capsaicin is prevented when the resulting airway smooth muscle contraction and/or bronchospasm is prevented. ‘Responsive’ implies that the neurones form action potentials upon stimulation. NA: not assessed. See text for further details.
Figure 5. Reflex responses initiated by citric acid, capsaicin and bradykinin applied topically to the tracheal mucosa of anaesthetized guinea-pigs.
A, representative trace of coughing evoked by 0.1 m citric acid applied to the tracheal mucosa. Respiration and coughing were monitored by recording tracheal pressure (PT) through a side port in the tracheal cannula (see Fig. 1). Citric acid (0.01–2 m) evoked cough when applied in 100 μl aliquots. At low concentrations, cough was not accompanied by marked changes in respiratory rate. At high concentrations of citric acid (0.3–2 m), however, prolonged (2–3 min) decreases in respiratory rate or apnoea occurred (not shown). B, unlike citric acid, capsaicin applied in 100 μl aliquots (3–10 μm) or continuously superfused as shown failed to evoke cough in anaesthetized guinea-pigs. Rather, capsaicin acutely and profoundly slowed or stopped respiration entirely, an effect that gradually reversed. At low concentrations of capsaicin (0.1–1 μm), after an initial slowing of rate, respiration eventually increased to above baseline rates. Note gasping initiated at the end of the sustained apnoeas. Tracheal challenge with bradykinin induced responses comparable to those induced by capsaicin. C, percentage of animals coughing in response to challenge with capsaicin (n = 15), bradykinin (n = 14) or citric acid (n = 19).
Sustained capsaicin challenges were used to desensitize jugular ganglia afferents to subsequent stimulation. In our in vitro electrophysiological studies, we found that continuous superfusion with 1 μm capsaicin initiated an immediate and sustained activation of jugular ganglia afferent neurones innervating the trachea, larynx or bronchi that eventually slowed and ceased 10–20 min into the challenge. Thereafter, these afferent neurones were rendered unresponsive to subsequent activation through chemical stimulation with 10 μm bradykinin, mechanical stimulation with a blunt probe, or even supramaximal electrical stimulation with a concentric ring electrode (n = 5). Nevertheless, a comparable desensitization (10 μm capsaicin for 30 min) of the jugular ganglia afferent neurones innervating the trachea and larynx in vivo did not prevent subsequently evoked coughing induced by mechanically probing the laryngeal mucosa (n = 4) or topical challenges with citric acid (100 μl of 0.01–0.3 m; n = 4).
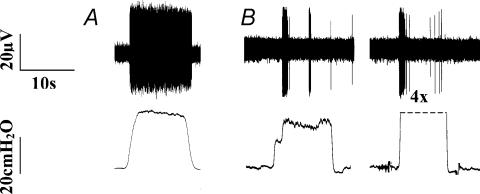
In subsequent studies we compared and contrasted the properties of the tracheal and laryngeal afferent neurones mediating cough with those of intrapulmonary stretch receptors. Throughout the remainder of the text, we will refer to the tracheal and laryngeal afferent neurones mediating cough as ‘cough receptors’. Intrapulmonary mechanoreceptors were activated in isolated perfused lungs by a distending pressure (evoked by increasing the tracheal perfusion rate; Fig. 6). Using this experimental design, threshold for activation of these afferent neurones was approximately 15 cmH2O. When a distending pressure roughly twice threshold was applied continuously for 5–10 s, 14 out of 24 identified neurones responded with a brief burst of action potentials and then adapted. The adaptation index of these fibres exceeded 90%, indicating that they were rapidly adapting recpetors (RARs). The remaining 10 fibres did not adapt or adapted only modestly to the distending pressure (adaptation index <20%), indicating that they were slowly adapting receptors (SARs).
Figure 6. Representative traces of the responses of intrapulmonary stretch receptors to sustained increases in distending pressures in vitro.
The upper traces in each panel are extracellular recordings from the cell bodies of vagal afferent neurones innervating the intrapulmonary airways. The lower traces in each panel are measurements of tracheal perfusion pressure (see text for further details of the experimental design). As expected, two subtypes of intrapulmonary stretch receptors were identified, slowly adapting (A), with a low threshold for activation by stretch (10–20 cmH2O) and an adaptation index of <20%, and rapidly adapting (B), also with a generally low threshold for activation by stretch (10–30 cmH2O) but an adaptation index of >80%. The rapidly adapting receptors remained rapidly adapting even when distending pressures were increased to 4 times (4X) threshold.
All intrapulmonary vagal afferent neurones responding to lung distension conducted action potentials at velocities ≥ 8 m s−1 (suggesting that their axons were myelinated) and had their cell bodies in the nodose ganglia. Mechanical probing of the lungs revealed that the majority of these intrapulmonary mechanoreceptors with cell bodies in the right nodose ganglia innervated the right middle lobe of the lungs. The precise location of their receptive fields (e.g. smooth muscle, epithelium) was not defined in this study.
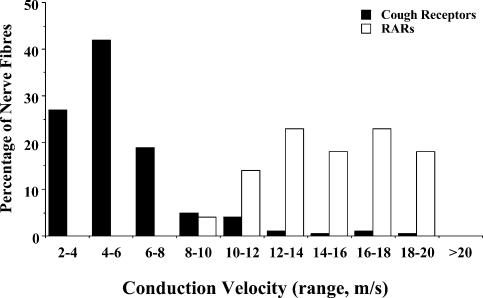
Conduction velocities of intrapulmonary RARs and the putative cough receptors
The conduction velocities of the 14 identifed RARs innervating the intrapulmonary airways exceeded the conduction velocities of the putative cough receptors (Fig. 7). Intrapulmonary RAR conduction velocities averaged 16 ± 1 m s−1 (range: 9–25 m s−1), roughly 3 times faster than the average conduction velocity of the extrapulmonary cough receptors (5.1 ± 0.2 m s−1; range: 2–8 m s−1; n = 136). Consistent with previous studies in both rats and guinea-pigs (Bergren & Sampson, 1982; Ho et al. 2001), the conduction velocities of the RARs approximated the conduction velocities of the SARs, which ranged between 10 and 25 m s−1.
Figure 7. Conduction velocity of airway and lung mechanoreceptors.
Graphic presentation of the distribution of conduction velocities of putative cough receptors innervating the trachea, larynx and bronchi (n = 136), and rapidly adapting stretch receptors (RARs) innervating the intrapulmonary airways and lungs (n = 14).
Mechanical and chemical sensitivity of intrapulmonary RARs and the putative cough receptors
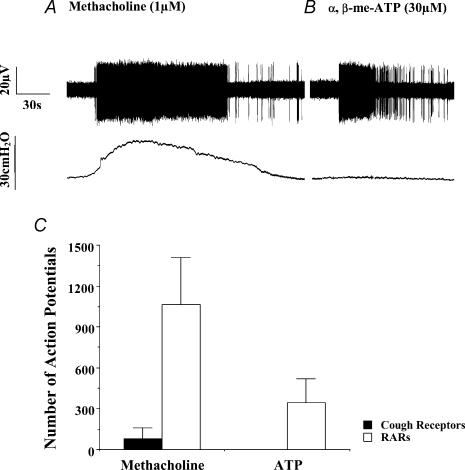
Administering a 1 ml solution of 1 μm methacholine via the pulmonary artery increased tracheal perfusion pressure (peak increases averaged 12 ± 2 cmH2O) and vigorously activated both RARs and SARs innervating the intrapulmonary airways in vitro (Fig. 8). As reported elsewhere (Bergren & Sampson, 1982; Sano et al. 1992; Bergren, 1997), putative RARs did not readily adapt to the bronchospasm. The responsiveness of putative SARs and RARs was not different in either peak action potential frequencies produced or in duration of response (data not shown). In 11 experiments, the intrapulmonary mechanoreceptors produced 1066 ± 344 action potentials in response to the 1 ml infusion of 1 μm methacholine, with peak frequencies of discharge averaging 40 ± 7 Hz. These responses to methacholine were functionally antagonized by 100 μm isoproterenol (administered 5 min prior to challenge). Thus, in four experiments on two RARs and two SARs, action potential discharge evoked by methacholine decreased from 1200, 313, 680 and 690 to 43, 0, 0 and 49, respectively (>97% reduction). Comparable results were obtained using a 1 ml pulmonary artery infusion of 10 μm histamine, which evoked 1195 ± 300 action potentials at a peak frequency of 47 ± 6 Hz (n = 5). In two RARs studied, 100 μm isoproterenol decreased the histamine-induced afferent nerve discharge from 658 and 1526 to 60 and 93 action potentials, respectively (> 90% reduction). Isoproterenol completely abolished the increases in tracheal perfusion pressure evoked by either histamine or methacholine.
Figure 8. Activation of intrapulmonary rapidly adapting receptors (RARs) by methacholine and by ATP receptor agonists in vitro.
A, coincident with the increases in tracheal perfusion pressure (depicted in the lower traces of each panel) evoked by pulmonary arterial administration of methacholine, RARs are activated robustly and in a non-adapting fashion (the upper traces in A and B are extracellular recordings from intrapulmonary RARs). This effect of methacholine can be mimicked by histamine and prevented by isoproterenol, indicating that airway smooth muscle contraction is the likely cause of this activation. B, in contrast to methacholine and histamine, ATP and the non-hydrolysable form of ATP, α,β-methylene ATP, have no effect on tracheal perfusion pressure but robustly activate airway stretch receptors. This effect of the purinergic receptor agonists is prevented by the P2X receptor antagonist PPADS. RARs and SARs responded identically to methacholine, histamine and to the ATP receptor agonists (not shown). C, mean ±s.e.m. action potentials initiated in intrapulmonary RARs and the putative cough receptors innervating the larynx, trachea and bronchi of guinea-pigs by challenges with methacholine (1–10 μm) and ATP (10–100 μm). See text for further details. Each bar is the mean ±s.e.m. of 4–8 separate experiments.
The effects of histamine and methacholine described above and elsewhere and their sensitivity to isoproterenol indicate that intrapulmonary mechanoreceptors, either RARs or SARs, are activated indirectly by spasmogens, secondary to effects on perfusion pressure and/or secondary to the smooth muscle contraction itself. Tracheal distension, however, even to pressures exceeding 200 cmH2O, consistently failed to evoke cough in vivo (n = 6) and did not initiate action potentials in the cough receptors when studied in vitro (n = 5). The cough receptors were also unresponsive to transverse (n = 4) or longitudinal stretch (only 1/12 fibres responded to stretching the trachea from its passive length to a length exceeding that of the trachea in situ), and when studied in the open tracheal preparation, were unresponsive to smooth muscle constriction (80–100% of the maximum contraction) evoked by 10 μm histamine (n = 4), 0.1 μm LTC4 (n = 4), or 10 μm methacholine (n = 4). Only one fibre responded to the spasmogens, discharging action potentials in response to both histamine and methacholine. In the closed tracheal tube preparation, 10 μm methacholine failed to evoke action potential discharge in an additional four nodose ganglia neurones innervating the trachea. These neurones were nevertheless highly sensitive to punctate mechanical stimuli, which as mentioned above (Table 1), evoked coughing in the anaesthetized guinea-pigs.
Further evidence that the afferent nerves mediating cough are insensitive to airway smooth muscle contraction comes from in vivo studies of cough. Thus, electrical stimulation (32 Hz, 6–8 V, 20 s) of the caudal cut end of a vagus nerve (the contralateral vagus nerve was intact), which evokes marked airways obstruction and tracheal smooth muscle contraction (Mazzone & Canning, 2002b), did not evoke cough and had little if any effect on respiratory rate (4 ± 4% increase; n = 7). Methacholine, 30 μm, added to the tracheal perfusate also failed to evoke cough (n = 3), while histamine given intravenously evoked tachypnoea (respiratory rate increased 193 ± 31% during the 60 s following an i.v. infusion of 10 μg kg−1 histamine; n = 3) but no coughing. Nevertheless, subsequent mechanical stimulation of the larynx or 0.1 m citric acid applied topically to the trachea evoked coughing in all of these animals.
The cough receptors were not activated by capsaicin (1–10 μm; also see Fig. 4) or bradykinin (1–3 μm; n = 9) and as shown in Fig. 5, consistently failed to evoke coughing when applied to the tracheal mucosa in vivo in anaesthetized guinea-pigs. We also found that bradykinin does not directly sensitize the cough receptors to activation. Using a concentric ring electrode placed over their defined receptive fields, electrical activation thresholds for five cough receptors averaged 5.1 ± 0.9 V before and 5.2 ± 0.8 V after 3 μm bradykinin challenge. This contrasts with the effects of bradykinin on bronchopulmonary C-fibres, which are readily activated by this inflammatory peptide. Like the cough receptors, however, intrapulmonary C-fibres (arising from both the jugular and nodose ganglia) were unresponsive to lung distending pressures ≤ 40 cmH2O (data not shown; n = 5–12; also see Table 3).
ATP (10–100 μm) had no effect on extrapulmonary cough receptors (n = 13) whereas the purine (1 ml of 30 μm administered via the pulmonary artery) activated intrapulmonary mechanoreceptors (343 ± 177 action potentials, peak frequency: 17 ± 5 Hz; n = 5; Fig. 8). This effect of ATP on the intrapulmonary afferents appeared to be direct and receptor dependent, inasmuch as it was not accompanied by a change in tracheal perfusion pressure, and was mimicked by the non-hydrolysable form of ATP, α,β-methylene ATP (1 ml of 30 μm), which also failed to increase tracheal perfusion pressure. Amongst nine intrapulmonary afferent neurones studied (4 RARs, 4 SARs, 1 not characterized), all responded to the non-hydrolysable agonist (874 ± 193 action potentials, peak frequency: 37 ± 3 Hz). This effect of α,β-methylene ATP was abolished by the P2X receptor antagonist PPADS (100 μm administered in a 1 ml bolus 15 min prior to challenge with α,β-methylene ATP (1 ml of 30 μm)). In three experiments, RARs produced 1186 ± 325 action potentials before and 0 ± 0 action potentials after PPADS pretreatment (Table 3). Despite its ability to activate intrapulmonary RARs, however, aerosolized α,β-methylene ATP (1 μm to 1 mm; n = 5) did not evoke coughing in conscious guinea-pigs, all of which coughed frequently upon subsequent challenge with citric acid.
Discussion
The data presented in this study indicate that afferent neurones with cell bodies in the nodose ganglia are primarily responsible for regulating the cough reflex initiated from the trachea and larynx of guinea-pigs. These afferent neurones, the putative cough receptors, possess attributes that are quite unique to this subset of afferent neurones and are thus not readily classified as RARs, SARs or C-fibres. The data presented also indicate that cough initiated following activation of bronchopulmonary C-fibres is distinct from the cough reflex initiated by stimulation of the cough receptors. Finally, we speculate that afferent nerve subtypes may interact both in the periphery and in the central nervous system to regulate the cough reflex.
Identification of the cough receptors innervating the extrapulmonary airways
In vitro electrophysiological analyses have identified three subtypes of vagal afferent neurones innervating the larynx, trachea and mainstem bronchi of guinea-pigs: capsaicin-sensitive afferent neurones (both Aδ- and C-fibres) that originate in the jugular ganglia and are relatively insensitive to punctate mechanical stimuli but are activated by capsaicin, bradykinin, hypertonic saline and acid, and afferent neurones conducting action potentials in the Aδ range that originate in the nodose ganglia and are insensitive to capsaicin and bradykinin, relatively insensitive to hypertonic saline, but highly sensitive to punctate mechanical stimuli and acid (Riccio et al. 1996; Kajekar et al. 1999; Kollarik & Undem, 2002). In vivo, cough was elicited by mechanical, electrical or acidic stimulation of the tracheal or laryngeal mucosa of anaesthetized guinea-pigs, whereas tracheal application of capsaicin or bradykinin did not evoke cough. We also observed that cutting the recurrent nerves abolished coughing induced by stimulating the tracheal or laryngeal mucosa whereas cutting the superior laryngeal nerves did not prevent coughing. Subsequent electrophysiological analyses of the projections of tracheal and laryngeal afferent nerves revealed that 88% of nodose ganglia neurones project to the rostral trachea and larynx via the recurrent nerves. We conclude from these studies that capsaicin-insensitive nodose ganglia neurones play a primary role in regulating cough evoked from the extrapulmonary airways.
Selectively preserving tracheal and laryngeal afferent innervation carried by the superior laryngeal nerves, which is comprosed almost exclusively of capsaicin-sensitive C-fibres, did not sustain the cough reflex evoked by mechanical or electrical stimulation of the trachea or larynx and neither capsaicin nor bradykinin applied to the trachea or larynx evoked cough. These results are consistent with previous studies (Tatar et al. 1988, 1994). That anaesthesia may prevent capsaicin-sensitive afferent nerve activation seems highly unlikely (Coleridge & Coleridge, 1994; Lee & Pisarri, 2001; Canning et al. 2001; Mazzone & Canning, 2002a). Clearly, then, in anaesthetized animals, capsaicin-sensitive nerve activation is not sufficient for initiating cough from the trachea or larynx. We would assert, however, that capsaicin-sensitive nerve activation is also not necessary for initiating cough. Thus, while the stimuli that evoked cough from the trachea and larynx (mechanical or electrical stimulation, citric acid) may activate both nodose and jugular ganglia neurones, capsaicin did not by itself evoke cough and prior capsaicin desensitization did not inhibit subsequently evoked cough.
Unique physiological properties of the cough receptors
We propose that the afferent neurones mediating cough identified here are not adequately described as either rapidly or slowly adapting receptors and comprise an anatomically and physiologically distinct subset of airway afferent nerves. We base this assertion on the following arguments. (1) Unlike RARs or SARs (Sano et al. 1992; Schelegle & Green, 2001; Widdicombe, 2003), the cough receptors are unresponsive to smooth muscle contraction and/or lung distension (present study; Fox et al. 1993). (2) Cough receptor conduction velocity is much slower than the conduction velocity of RARs or SARs (present study; Bergren & Sampson, 1982; Ho et al. 2001; Undem et al. 2004), and much faster than that of C-fibres (Riccio et al. 1996; Ho et al. 2001). (3) The cough receptors are primarily localized to the extrapulmonary airways whereas RARs and SARs, at least in guinea-pigs (Bergren & Sampson, 1982; Keller et al. 1989; Riccio et al. 1996), are localized to the intrapulmonary airways and lungs. (4) Histamine and ATP receptor agonists activate RARs and yet the cough receptors are unresponsive to histamine and purines which, incidently, also fail to evoke coughing. And (5) lung collapse and/or modestly negative airway pressures activates RARs (Widdicombe, 1954b; Ho et al. 2001) whereas pronounced negative pressures (−100 cmH2O) produced in the lumen of the trachea failed to evoke cough or any reflexes in vivo in guinea-pigs (authors' unpublished observations).
A semantic argument could be made that the afferent neurones described here are merely a subset of rapidly adapting receptors that are unique only because they are localized to the extrapulmonary airways, and that their site of termination largely defines their physiological attributes (Widdicombe, 2003). Without question, the cough receptors are myelinated, mechanically sensitive and rapidly adapt to a sustained (punctate) mechanical stimulation. But these attributes alone do not adequately describe the physiological properties of the well-defined rapidly adapting receptors, nor does classifying the cough receptors as RARs adequately describe the physiology of this afferent nerve subtype. Their insensitivity to changes in transpulmonary pressures and stretch, their insensitivity to spasmogens and the different reflexes they initiate upon activation clearly differentiate the cough receptors from the intrapulmonary rapidly adapting receptors. It is noteworthy that bronchospasm evoked by NKA, histamine and methacholine, which stimulates RARs, is generally ineffective at evoking cough (instead producing a reflex bronchospasm and tachypnoea; present study; Joos et al. 1987; Sano et al. 1992; Fujimura et al. 1992; Takahama et al. 1993; Canning et al. 2001; Mazzone & Canning, 2002a). It is also important to note that rapid adaptation to a mechanical probe is in no way like rapid adaptation to a sustained distending pressure. Indeed, RARs adapt poorly to airway smooth muscle contraction (Bergren & Sampson, 1982; Bergren, 1997) or lung collapse (Ho et al. 2001), facts that highlight the importance of context when using the terms rapidly or slowly adapting. Finally, it would also seem inappropriate to use the term irritant receptors to describe the cough receptors, as these afferent nerves, at least in guinea-pigs, are essentially unresponsive to a variety of ‘irritants’ (e.g. capsaicin, bradykinin, 5-HT, prostanoids, hypertonic saline; Fox et al. 1993; Riccio et al. 1996; Kajekar et al. 1999).
How cough receptors innervating the extrapulmonary airways are activated in vivo under natural conditions is unclear. Aspirate and accumulated mucus would likely activate these afferents and it is tempting to speculate that aspirate and airways secretions are the primary natural triggers of coughing. Smooth muscle contraction is unlikely to be a primary mechanism for initiating cough (Fujimura et al. 1992; present study). It is also unclear if results obtained in guinea-pigs are generally applicable to all mammalian species that cough. In dogs and in cats, for example, both RARs and SARs that respond to distension and/or smooth muscle contraction can be localized to the trachea (Widdicombe, 1954b; Schelegle & Green, 2001; Widdicombe, 2003). This would appear to differ from guinea-pigs, where few if any stretch receptors have been localized to the extrapulmonary airways (Riccio et al. 1996; Keller et al. 1989). It should be restated, however, that bronchoconstriction is a poor stimulus for coughing in any species, and bronchodilators are not very effective at preventing cough (Fujimura et al. 1992). These observations suggest at least some similarities in the cough reflex across species.
We were unable to demonstrate a role for afferent neurones carried by the superior laryngeal nerves in coughing, an observation that seems to contradict the results of some previous studies carried out in guinea-pigs (Tsubone et al. 1991; Ishii et al. 1998). If anything, our results confirm those of other investigators suggesting an inhibitory role of superior laryngeal afferent nerves in coughing (Karlsson et al. 1991; Tatar et al. 1996). Although our assessment of the role of the superior laryngeal nerves in coughing was far more limited than those of Tsubone, Ishii and colleagues, it is interesting that the superior laryngeal nerves are also not essential to the cough reflex in humans (Stockwell et al. 1993).
The role of C-fibres in regulating the cough reflex.
We have presented further evidence that C-fibre activation does not initiate coughing in anaesthetized animals. It is unclear how these data can be reconciled with the evidence that C-fibre activation initiates coughing in conscious animals (Karlsson & Fuller, 1999; Canning, 2002; present study). Amongst the most effective tussive stimuli identified (e.g. capsaicin, bradykinin and citric acid), each activates C-fibres and initiates cough in conscious animals. In guinea-pigs, prior capsaicin desensitization prevents cough evoked by citric acid, while TRPV1 or neurokinin receptor antagonists attenuate or abolish coughing evoked by capsaicin, bradykinin and citric acid (Forsberg & Karlsson, 1986; Bolser et al. 1991; Lalloo et al. 1995; Advenier et al. 1997; Bolser et al. 1997; authors' unpublished observations). Neurokinin receptor antagonists also prevent bradykinin- and capsaicin-induced reflex bronchospasm (Canning et al. 2001; Mazzone & Canning, 2002a). Given that neurokinins appear to be localized exclusively to the peripheral and central nerve terminals of bronchopulmonary C-fibres in guinea-pigs (Kummer et al. 1992; Riccio et al. 1996; Hunter & Undem, 1999; Mazzone & Canning, 2002a; Undem et al. 2004), these data indicate that C-fibre activation in some way initiates coughing.
We speculate that C-fibre-mediated cough is distinct from coughing initiated by directly activating the cough receptors. The effects of general anaesthesia would suggest that consciousness plays an important role in C-fibre-dependent cough. Perhaps bronchopulmonary C-fibre activation produces the urge to cough and not the uncontrollable coughing initiated by activation of the cough receptors following aspiration of food or water, for example. Patients challenged with capsaicin and presented with a traffic light indicating that they should suppress their urge to cough (red light), or cough if they feel that they must (green light), respond very differently to the tussive stimuli. The red light can essentially abolish the coughing evoked by capsaicin challenge in these patients (Hutchings et al. 1993). It is difficult to imagine that a comparable suppressive effect could be exerted over the cough initiated by aspiration.
Finally, it seems likely that C-fibre-dependent cough requires coactivation of other airway afferent nerve subtypes for the full reflex to be initiated. Peripherally acting neurokinin receptor antagonists can effectively abolish citric acid- and capsaicin-induced cough in conscious guinea-pigs (Advenier et al. 1997; Hay et al. 2002; Canning, 2002). This would suggest that axon reflex-dependent effects of C-fibre activation play an important role in cough induced in guinea-pigs. This coughing may involve activation of the intrapulmonary RARs, since the cough receptors, as discussed above, are not activated by bronchospasm. Alternatively, CNS-dependent reflexes initiated by C-fibre activation such as mucus secretion may result in cough receptor activation. C-fibres may also mediate coughing through interactions at the level of the CNS (Bolser et al. 1997; Canning, 2002). We reported such an interaction between afferent nerve subtypes regulating bronchospasm (Mazzone & Canning, 2002a), and our recent studies would suggest that such interactions also regulate coughing (S. B. Mazzone & B. J. Canning, unpublished observations).
Acknowledgments
This research was funded by grants from the National Institutes of Health, Bethesda, Maryland. Stuart Mazzone is a National Health and Medical Research Council of Australia CJ Martin Fellow (007188).
References
- Advenier C, Lagente V, Boichot E. The role of tachykinin receptor antagonists in the prevention of bronchial hyperresponsiveness, airway inflammation and cough. Eur Respir J. 1997;10:1892–1906. doi: 10.1183/09031936.97.10081892. [DOI] [PubMed] [Google Scholar]
- Barnes NC, Piper PJ, Costello JF. Comparative effects of inhaled leukotriene C4, leukotriene D4, and histamine in normal human subjects. Thorax. 1984;39:500–504. doi: 10.1136/thx.39.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergren DR. Sensory receptor activation by mediators of defense reflexes in guinea-pig lungs. Respir Physiol. 1997;108:195–204. doi: 10.1016/s0034-5687(97)00030-3. [DOI] [PubMed] [Google Scholar]
- Bergren DR, Sampson SR. Characterization of intrapulmonary, rapidly adapting receptors of guinea pigs. Respir Physiol. 1982;47:83–95. doi: 10.1016/0034-5687(82)90094-9. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Aziz SM, Chapman RW. Ruthenium red decreases capsaicin and citric acid-induced cough in guinea pigs. Neurosci Lett. 1991;126:131–133. doi: 10.1016/0304-3940(91)90536-3. [DOI] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC, O'Reilly S, McLeod RL, Hey JA. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968, in the guinea-pig and cat. Br J Pharmacol. 1997;121:165–170. doi: 10.1038/sj.bjp.0701111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ. Interactions between vagal afferent nerve subtypes mediating cough. Pulm Pharmacol Ther. 2002;15:187–192. doi: 10.1006/pupt.2002.0363. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Reynolds SM, Mazzone SB. Multiple mechanisms of reflex bronchospasm in guinea pigs. J Appl Physiol. 2001;91:2642–2653. doi: 10.1152/jappl.2001.91.6.2642. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Pulmonary reflexes: neural mechanisms of pulmonary defense. Annu Rev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- Forsberg K, Karlsson JA. Cough induced by stimulation of capsaicin-sensitive sensory neurons in conscious guinea-pigs. Acta Physiol Scand. 1986;128:319–320. doi: 10.1111/j.1748-1716.1986.tb07981.x. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Barnes PJ, Urban L, Dray A. An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J Physiol. 1993;469:21–35. doi: 10.1113/jphysiol.1993.sp019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effects of methacholine induced bronchoconstriction and procaterol induced bronchodilation on cough receptor sensitivity to inhaled capsaicin and tartaric acid. Thorax. 1992;47:441–445. doi: 10.1136/thx.47.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DW, Giardina GA, Griswold DE, Underwood DC, Kotzer CJ, Bush B, Potts W, Sandhu P, Lundberg D, Foley JJ, Schmidt DB, Martin LD, Kilian D, Legos JJ, Barone FC, Luttmann MA, Grugni M, Raveglia LF, Sarau HM. Nonpeptide tachykinin receptor antagonists. III. SB 235375, a low central nervous system-penetrant, potent and selective neurokinin-3 receptor antagonist, inhibits citric acid-induced cough and airways hyper-reactivity in guinea pigs. J Pharmacol Exp Ther. 2002;300:314–323. doi: 10.1124/jpet.300.1.314. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med. 1999;159:1943–1948. doi: 10.1164/ajrccm.159.6.9808078. [DOI] [PubMed] [Google Scholar]
- Hutchings HA, Morris S, Eccles R, Jawad MS. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993;87:379–382. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- Ishii R, Furuta M, Hashimoto M, Naruse T, Gallico L, Ceserani R. Effects of moguisteine on the cough reflex induced by afferent electrical stimulation of the superior laryngeal nerve in guinea pigs. Eur J Pharmacol. 1998;362:207–212. doi: 10.1016/s0014-2999(98)00760-2. [DOI] [PubMed] [Google Scholar]
- Joos GF, Pauwels RA, Van Der Straeten ME. Effect of inhaled substance P and neurokinin A on the airways of normal and asthmatic subjects. Thorax. 1987;42:779–783. doi: 10.1136/thx.42.10.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajekar R, Proud D, Myers AC, Meeker SN, Undem BJ. Characterization of vagal afferent subtypes stimulated by bradykinin in guinea pig trachea. J Pharmacol Exp Ther. 1999;289:682–687. [PubMed] [Google Scholar]
- Karlsson JA, Fuller RW. Pharmacological regulation of the cough reflex — from experimental models to antitussive effects in Man. Pulm Pharmacol Ther. 1999;12:215–228. doi: 10.1006/pupt.1999.0207. [DOI] [PubMed] [Google Scholar]
- Karlsson JA, Hansson L, Wollmer P, Dahlback M. Regional sensitivity of the respiratory tract to stimuli causing cough and reflex bronchoconstriction. Respir Med. 1991;85(Suppl. A):47–50. doi: 10.1016/s0954-6111(06)80254-4. [DOI] [PubMed] [Google Scholar]
- Keller E, Kohl J, Koller EA. Location of pulmonary stretch receptors in the guinea-pig. Respir Physiol. 1989;76:149–157. doi: 10.1016/0034-5687(89)90093-5. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Lalloo UG, Fox AJ, Belvisi MG, Chung KF, Barnes PJ. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J Appl Physiol. 1995;79:1082–1087. doi: 10.1152/jappl.1995.79.4.1082. [DOI] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2002a;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. An in vivo guinea pig preparation for studying the autonomic regulation of airway smooth muscle tone. Auton Neurosci. 2002b;99:91–101. doi: 10.1016/s1566-0702(02)00053-x. [DOI] [PubMed] [Google Scholar]
- McAlexander MA, Myers AC, Undem BJ. Adaptation of guinea-pig vagal airway afferent neurones to mechanical stimulation. J Physiol. 1999;521:239–247. doi: 10.1111/j.1469-7793.1999.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi K, Singh M, Julka DB. Properties of rapidly adapting receptors of the airways in monkeys. Macaca mulatta, Respir Physiol. 1995;99:51–62. doi: 10.1016/0034-5687(94)00072-8. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Tsubone H, Sugano S. Vagal afferent activities and respiratory reflexes during drug-induced bronchoconstriction in the guinea pig. J Vet Med Sci. 1992;54:989–998. doi: 10.1292/jvms.54.989. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol. 2001;125:17–31. doi: 10.1016/s0034-5687(00)00202-4. [DOI] [PubMed] [Google Scholar]
- Shinagawa K, Kojima M, Ichikawa K, Hiratochi M, Aoyagi S, Akahane M. Participation of thromboxane A2 in the cough response in guinea-pigs: antitussive effect of ozagrel. Br J Pharmacol. 2000;131:266–270. doi: 10.1038/sj.bjp.0703553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell M, Lang S, Yip R, Zintel T, White C, Gallagher CG. Lack of importance of the superior laryngeal nerves in citric acid cough in humans. J Appl Physiol. 1993;75:613–617. doi: 10.1152/jappl.1993.75.2.613. [DOI] [PubMed] [Google Scholar]
- Takahama K, Fuchikama T, Isohama Y, Kai H, Miyata T. Neurokinin A but not neurokinin B and substance P induces codeine-resistant coughs in awaked guinea-pigs. Regul Pept. 1993;42:236–237. doi: 10.1016/0167-0115(93)90045-a. [DOI] [PubMed] [Google Scholar]
- Takahama K, Wakuda I, Fukushima H, Isohama Y, Kai H, Miyata T. Differential effect of codeine on coughs caused by mechanical stimulation of two different sites in the airway of guinea pigs. Eur J Pharmacol. 1997;329:93–97. doi: 10.1016/s0014-2999(97)10110-8. [DOI] [PubMed] [Google Scholar]
- Tatar M, Karcolova D, Pecova R, Brozmanova M. The role of partial laryngeal denervation on the cough reflex in awake guinea-pigs, rats and rabbits. Pulm Pharmacol. 1996;9:371–372. doi: 10.1006/pulp.1996.0051. [DOI] [PubMed] [Google Scholar]
- Tatar M, Sant'Ambrogio G, Sant'Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol. 1994;76:2672–2679. doi: 10.1152/jappl.1994.76.6.2672. [DOI] [PubMed] [Google Scholar]
- Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol. 1988;402:411–420. doi: 10.1113/jphysiol.1988.sp017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubone H, Sant'Ambrogio G, Anderson JW, Orani GP. Laryngeal afferent activity and reflexes in the guinea pig. Respir Physiol. 1991;86:215–231. doi: 10.1016/0034-5687(91)90082-t. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee M-G, Weinreich D, Myers AC, Kollarik Two distinct phenotypes of vagal afferent C-fibers innervating the lungs. J Physiol. 2004 doi: 10.1113/jphysiol.2003.060079. in press DOI 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol. 1954a;123:55–70. doi: 10.1113/jphysiol.1954.sp005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol. 1954b;123:71–104. doi: 10.1113/jphysiol.1954.sp005034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Respiratory reflexes excited by inflation of the lungs. J Physiol. 1954c;123:105–115. doi: 10.1113/jphysiol.1954.sp005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol. 1998;114:5–15. doi: 10.1016/s0034-5687(98)00076-0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. Functional morphology and physiology of pulmonary rapidly adapting receptors (RARs) Anat Rec. 2003;270A:2–10. doi: 10.1002/ar.a.10003. [DOI] [PubMed] [Google Scholar]