Abstract
This study examined the effects of short- and long-term aerobic training on the stable up-regulation of pyruvate dehydrogenase (PDH) and PDH kinase (PDK) in human skeletal muscle. We hypothesized that 8 weeks, but not 1 week, of aerobic training would increase total PDH (PDHt) and PDK activities compared to pretraining, and this would be detectable at the level of gene transcription (mRNA) and/or gene translation (protein). Resting muscle biopsies were taken before and after 1 and 8 weeks of aerobic cycle exercise training. PDHt and PDK activities, and their respective protein and mRNA expression, did not differ after 1 week of aerobic training. PDHt activity increased 31% after 8 weeks and this may be partially due to a 1.3-fold increase in PDH-E1α protein expression. PDK activity approximately doubled after 8 weeks of aerobic training and this was attributed to a 1.3-fold increase in PDK2 isoform protein expression. Similar to 1 week, no changes were observed at the mRNA level after 8 weeks of training. These findings suggest that aerobically trained human skeletal muscle has an increased maximal capacity to utilize carbohydrates, evident by increased PDHt, but increased metabolic control sensitivity to pyruvate through increased contribution of PDK2 to total PDK activity.
During exercise at maximal oxygen uptake, carbohydrates are the dominant fuel utilized in skeletal muscle, mainly in the form of muscle glycogen (see Hultman, 1995 for review). Repeated bouts of aerobic exercise have been shown to increase maximal oxygen uptake and muscle glycogen storage (see Henriksson, 1995 for review). In addition, training has also been shown to increase glycogen utilization during exercise at workloads eliciting maximal oxygen uptake (see Henriksson, 1995 for review). Central to this adaptation may be pyruvate dehydrogenase (PDH), which modulates carbohydrate metabolism by regulating the entrance of carbohydrate-derived acetyl units into the tricarboxylic acid cycle. To date, only two studies have examined the effects of training on human skeletal muscle total PDH (PDHt) activity, demonstrating no change after 7 days of aerobic exercise (Putman et al. 1998) and 5 weeks of strength exercise (Ward et al. 1986). However, a recent study reported an increase in PDHt activity with long-term aerobic exercise in mouse hindlimb (Houle-Leroy et al. 2000), suggesting prolonged aerobic training may alter skeletal muscle PDHt activity in humans.
PDH is a multienzyme complex, composed of pyruvate dehydrogenase (E1), dihydrolipoyl transacetylase (E2), and dihydrolipoyl dehydrogenase (E3; Denton et al. 1975; Wieland, 1983), along with a tightly associated protein (E3bp), anchoring E3 subunits to the E2 core (Harris et al. 1997), and two regulatory enzymes, PDH kinase (PDK) and PDH phosphatase (PDP; Denton et al. 1975; Wieland, 1983). To date, few studies have examined the relative transcriptional and translational responses of each PDH subunit during adaptive changes in PDHt, reporting changes exclusively in non-muscle tissues (Da Silva et al. 1993; Maury et al. 1995; Amessou et al. 1998).
Another skeletal muscle adaptive response to prolonged aerobic training, during submaximal exercise, is a greater reliance on fat for ATP synthesis (Henriksson, 1977; Hurley et al. 1986; Martin et al. 1993; Phillips et al. 1996b), resulting in a net glycogen sparing effect. In a recent study, we demonstrated an attenuated rise in PDH activation during the same absolute submaximal exercise after 7 weeks of prolonged aerobic exercise (LeBlanc et al. 2003). Thus, the control of PDH appears to play a central role in the training-induced shift from carbohydrate to fat metabolism in skeletal muscle during exercise; however, the mechanism is currently unknown. The activation of PDH is covalently regulated by PDK, which phosphorylates and inactivates, and PDP, which dephosphorylates and activates PDH. The activation of PDH is a dynamic system and alterations in PDK and/or PDP activity will affect PDH activity. With prolonged aerobic training, an attenuated activation of PDH may be due to a stable increased or decreased protein, and thus intrinsic activity, of PDK and PDP, respectively. Regulation of PDK may be central to control of the activation state of PDH in prolonged metabolic perturbations, including aerobic training, since skeletal muscle PDK activity responds to a number of physiological and pathological perturbations in rats (Fuller & Randle, 1984; Nakai et al. 1999; Vary & Hazen, 1999; Wu et al. 1999; Holness et al. 2000; Sugden et al. 2000; Peters et al. 2001a) and in humans (Peters et al. 1998, 2001b), whereas skeletal muscle PDP activity is unresponsive (Fuller & Randle, 1984).
The complexity of PDH control by PDK is enhanced by the presence of four isoforms (PDK1–4; Bowker-Kinley et al. 1998), with PDK2 and 4 being the most abundant isoforms represented in human skeletal muscle (Gudi et al. 1995). Each isoform has differing concentrations, specific activities, and kinetic properties, resulting in unique responses to certain metabolic demands. PDK4 responds to acute alterations in lipid availability and has been termed the ‘lipid-status’ responsive isoform (Sugden et al. 2001) whereas PDK2 demonstrates a higher sensitivity to the energy status of the cell (Popov, 1997; Bowker-Kinley et al. 1998) and is highly sensitive to pyruvate (Gudi et al. 1995; Popov, 1997; Bowker-Kinley et al. 1998), suggesting the importance of PDK2 in a training-induced attenuation of PDH activation during exercise post training.
Currently, little information is available regarding the adaptive changes in PDHt and/or PDK in human skeletal muscle with short- or long-term aerobic exercise, and whether these adaptations can be detected at the level of gene and/or protein expression. Thus, the purposes of the study were to determine if 1 and 8 weeks of aerobic exercise would (1) increase PDHt activity, with a concomitant increase in PDH subunit mRNA and protein, and (2) increase PDK activity, with a concomitant increase in PDK isoform mRNA and protein. We hypothesized that there will be no change in PDHt and PDK activity after 1 week of aerobic exercise, along with no change in mRNA or protein. In contrast, 8 weeks of aerobic exercise will increase both PDHt and PDK activities, with a concomitant increase in transcriptional and translational products, thus allowing for greater potential carbohydrate flux through PDH yet greater metabolic control of carbohydrate utilization through inactivation of PDH by PDK. Measurements of citrate synthase (CS) and cytochrome oxidase (COX) maximal activities and protein expression were used to confirm training-induced alterations to skeletal muscle gene expression.
Methods
Subjects
Eight healthy, active men were recruited to participate in the study (age 22 ± 1 years; height 181.6 ± 2.2 cm; weight 83.3 ± 2.6 kg). Individuals were asked to consume similar diets and refrain from caffeine, alcohol and exercise for 48 h before each trial. Oral and written explanation of the experimental protocol and its attendant risks were provided and informed written consent was obtained from each subject. The study was approved by the McMaster University Ethics Committee and was in accordance with the Declaration of Helsinki.
Pre-experimental protocol
Individuals completed an initial incremental maximal exercise test on an electromagnetically braked cycle ergometer (Lode Excalibur, Quinton Instruments, Seattle, WA, USA) to determine maximal work capacity and maximal oxygen uptake (V˙O2 max) using a metabolic measurement system (Quinton Q-Plex 2, Quinton Instruments, Seattle, WA, USA).
Exercise training protocol
Individuals were aerobically trained on a cycle ergometer at a power output that reflected 75% of their maximal O2 uptake for 1 h per day, five days a week for a total of 8 weeks. For the first week, the 1 h of training was broken up into four 15 min intervals with 5 min rest in between. For the second and third weeks, the 1 h of training was broken up into three 20 min intervals with 5 and 2.5 min rest in between, respectively. For the fourth to eighth week, the 1 h of training was broken up into three 20 min intervals with 1 min rest in between. On the fourth and eighth week, individual subjects completed an incremental maximal exercise test to determine changes in maximal work capacity and O2 uptake so adjustments could be made to the workload to reflect 75% of their maximal O2 uptake, and to determine the adaptive response in maximal O2 uptake, respectively.
Muscle sampling
Two muscle biopsies (∼80–120 mg each) were obtained at rest before and after 1 and 8 weeks of aerobic exercise. Studies were performed at the same time of day. Individuals served as their own control. One thigh was prepared for needle biopsies of the vastus lateralis muscle as described by Bergström (1975). Incisions were made through the skin to the deep fascia under local anaesthesia (2% lidocaine without adrenaline). Resting muscle biopsies were taken 48 h after the last exercise bout, to avoid the transient effects on transcriptional rate postulated to occur up to 24 h after the last exercise bout (Neufer et al. 1998; Pilegaard et al. 2000). The first biopsy was immediately processed for mitochondrial isolation. The second biopsy was immediately frozen by plunging the needle into liquid nitrogen.
Enzyme activities
Intact mitochondria (Makinen & Lee, 1968; Peters et al. 1998) were extracted from 45 to 110 mg fresh muscle and an aliquot was used to determine PDK and PDHt activities (Fatinia et al. 1986; Peters et al. 1998) with the exception that PDK activities were determined at 37°C. The final mitochondrial suspension required an incubation time of 20 min at 30°C with 10 μm carbonyl cyanide m-chlorophenyl-hydrazone, which decreased ATP concentration to zero and resulted in complete conversion of PDH to the active form (Peters et al. 1998). As such, during the PDK activity assay, the time point that represents ‘zero time’ also represents ‘total PDH activity’ (Peters et al. 1998). Fractional recovery of intact mitochondria (25 ± 2%) was used to convert mitochondrial activities of PDHt to mmol min−1 (kg wet wt)−1 (Peters et al. 1998). CS and COX maximal enzyme activities were measured on 17–40 mg frozen muscle (Carter et al. 2001).
Western blotting
Mitochondria were diluted to a final protein concentration of 1 μg μl−1 as previously described (Peters et al. 2001b). Standard SDS–PAGE electrophoresis was performed as previously described (Peters et al. 2001b) with the exception of a 12% separating gel and 10 μg of mitochondrial protein loaded per lane. Electrophoretically separated proteins were transferred onto Protran nitrocellulose membranes (0.45 μm pore size, Schleicher & Schuell, NH, USA) using the Trans-blot semidry electrophoretic transfer cell (Bio-Rad, CA, USA) with a transfer buffer containing 34.8 mm Tris base, 31.2 mm glycine, 0.03% (w/v) SDS, and 20% (v/v) absolute ethanol. Membranes were incubated in TBST buffer (20 mm Tris base, 137 mm NaCl, 0.1% (v/v) Tween 20, pH 7.5) with 5% (w/v) non-fat dry milk for 1 h to block all non-specific binding sites. Membranes were then incubated for 1 h in 5% milk–TBST containing monoclonal antibodies against PDH subunits (E1α, E2, and E3bp; Molecular Probes, OR, USA), CS (Chemicon, CA, USA), or COX-II and -IV subunits (Molecular Probes, OR, USA), or polyclonal antibodies against PDK isoforms (PDK1–4; Santa Cruz Biotechnology, CA, USA). The membranes were washed and then incubated for 1 h in 5% milk–TBST containing either goat antimouse IgG (peroxidase conjugated, Sigma, Ontario, Canada) or bovine antigoat IgG (peroxidase conjugated, Santa Cruz Biotechnology, CA, USA). Membranes were again washed and antibody–antigen complexes were visualized on autoradiography film (Hyperfilm ECL, Amersham Biosciences, NJ, USA) after addition of chemiluminescent substrate (ECL, Amersham Biosciences). Relative densities were quantified using Scion Image (Scion Corporation, MD, USA). Blots were washed, stained with DB-71 (Hong et al. 2000) and total protein per lane was used to normalize loading between lanes on each blot. To correct for differences in blotting efficiency between gels, a rat standard was included in every gel. PDK antibodies were tested for cross-reactivity against purified PDK isoform proteins and did not cross-react with each other under the loading and detection conditions used for Western-blot analysis (data not shown).
Total RNA isolation and reverse transcription
Total RNA from 6–27 mg of frozen muscle was isolated using the FastRNA Kit-Green (Q-BIOgene, CA, USA) protocol and reagents. Total RNA concentrations were determined spectrophotometrically at 260 nm and purity at 280 nm. First strand cDNA was generated from 0.5 μg RNA using AMV Reverse Transcriptase (Promega, WI, USA) as previously described by Wadley et al. (2001). The cDNA was stored at −80°C for subsequent analysis.
Real-time PCR analysis
Primers were designed using Primer Express™ software package version 1.0 (Applied Biosystems, CA, USA) from gene sequences obtained from GenBank (CS, AF047042; PDH-E1α, NM_000284; PDH-E2, NM_001931; PDH-E3bp, NM_003477; PDK1, NM_002610; PDK2, NM_002611; PDK3, NM_005391; PDK4, NM_002612). The primer sequences were validated using BLAST (Altschul et al. 1990) to ensure homology between primer and desired mRNA of human skeletal muscle. Primer sequences are shown in Table 1. Quantification of mRNA expression was performed (in triplicate) by real-time PCR using the GeneAmp® 5700 sequence detection system (Applied Biosystems) as previously described (Wadley et al. 2001; Tunstall et al. 2002). To compensate for variations in input RNA amounts, and efficiency of reverse transcription, β-actin (GenBank, NM_001101) mRNA was quantified, and results were normalized to these values as previously described (Wadley et al. 2001; Tunstall et al. 2002). β-Actin mRNA levels did not change in response to 1 or 8 weeks of training (results not shown), similar to previous aerobic training studies in rat (Murakami et al. 1994) and human skeletal muscle (Wadley et al. 2001).
Table 1.
Gene primer sequences
| Gene | Sense primer (5′–3′) | Antisense primer (5′–3′) |
|---|---|---|
| β-Actin | GAC AGG ATG CAG AAG GAG ATT ACT | TGA TCC ACA TCT GCT GGA AGG T |
| CS | GTG CCC ATA CCA GCC ACT TG | CTG CCA GCC CGT TCA TG |
| PDH-E1α | TGT GGA AGA ACT AAA GGA AAT TGA TGT | TTC CAA AGG TGG CTC AGG AT |
| PDH-E2 | CTC CCA CAG GTC CTG GAA TG | GTC CAA TAA CCC GCA GAA TGT |
| PDH-E3bp | CTG AGG ATG AAG AGG GAA ATG C | TCA TCA ACC ACT CGA CTC TCA CT |
| PDK1 | CCG CTC TCC ATG AAG CAG TT | TTG CCG CAG AAA CAT AAA TGA G |
| PDK2 | CCG CTG TCC ATG AAG CAG TT | TGC CTG AGG AAG GTG AAG GA |
| PDK3 | CAA GCA GAT CGA GCG CTA CTC | CGA AGT CCA GGA ATT GTT TGA TG |
| PDK4 | CCC GAG AGG TGG AGC ATT T | GCA TTT TCT GAA CCA AAG TCC AGT A |
Statistical analysis
All data are presented as means ± s.e.m. with n = 8. A two-way analysis of variance (ANOVA) with repeated measures was used to establish differences between condition and time. Tukey's post hoc test was used to determine significance. Assumptions for normality and independence were verified by generating appropriate residual plots. Data transformations (log, square root, and inverse square root) were used when appropriate to meet the above assumptions.
Results
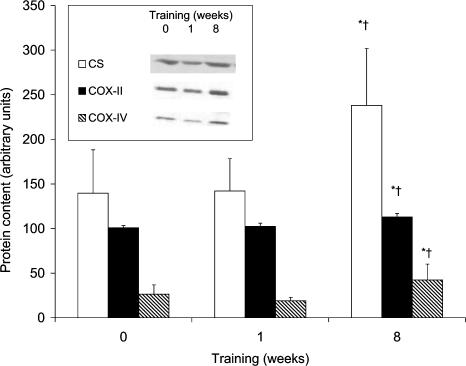
The effects of the exercise training programme are evident in a significantly increased maximal oxygen uptake after 8 weeks, increasing from 3.51 ± 0.15 to 4.05 ± 0.15 l min−1 (P < 0.05). This was accompanied by a 40 and 41% increase in CS and COX maximal activity, respectively, which was not seen after 1 week (Table 2). Proteins of CS and both COX subunits did not change after 1 week of aerobic training (Fig. 1). CS protein increased 54% after 8 weeks of training, as did COX-II and -IV protein, demonstrating a 12 and 62% increase, respectively. CS mRNAs were unchanged after 1 (1.11 ± 0.20-fold) and 8 (0.98 ± 0.15-fold) weeks of training compared to pretraining.
Table 2.
Maximal enzyme activities before and after 1 and 8 weeks of aerobic training
| 0 | 1 week | 8 weeks | |
|---|---|---|---|
| CS | 20.2 ± 1.6 | 23.3 ± 1.6 | 28.2 ± 1.7*† |
| COX | 12.4 ± 2.0 | 8.4 ± 1.1 | 17.5 ± 1.4*† |
| PDHt | 3.75 ± 0.24 | 3.65 ± 0.35 | 4.93 ± 0.38*† |
| PDK | 0.09 ± 0.01 | 0.11 ± 0.02 | 0.18 ± 0.03*† |
Values are means ± s.e.m. with n = 8. Enzyme activity expressed in mmol min−1 (kg wet wt)−1 except for PDK which is expressed min−1. CS, citrate synthase; COX, cytochrome c oxidase; PDHt, total pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase.
Significant difference from pretraining
significant difference from 1 week.
Figure 1. Mitochondrial protein content of CS, COX-II, and COX-IV before and after 1 and 8 weeks of aerobic training.
Inset, protein blots for a representative subject. Values are means ± s.e.m. with n = 8. *Significant difference from rest; †significant difference from 1 week.
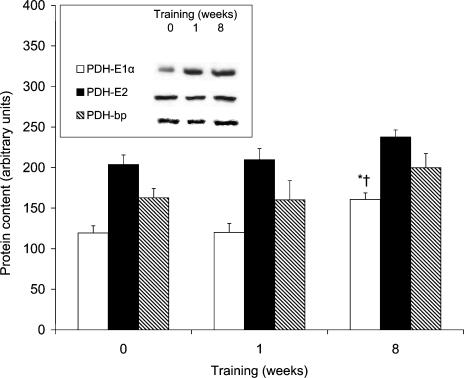
PDHt did not differ after 1 week of exercise but increased by 31% after 8 weeks (Table 2). There was no change in the protein expression of PDH subunits after 1 week of exercise and only PDH-E1α increased 1.3-fold after 8 weeks (Fig. 2). Both PDH-E1α and PDH-E3bp mRNA were unaffected by exercise training and PDH-E2 was lower after 8 weeks of exercise compared to 1 week (Table 3).
Figure 2. Mitochondrial protein content of PDH-E1a, PDH-E2, and PDH-E3bp before and after 1 and 8 weeks of aerobic training.
Inset, protein blots for a representative subject. Values are means ± s.e.m. with n = 8. *Significant difference from pretraining; †significant difference from 1 week.
Table 3.
Skeletal muscle mRNA content of PDH-E1a, PDH-E2, PDH-E3bp and PDK1-4 before and after 1 and 8 weeks of aerobic training
| 0 | 1 week | 8 weeks | |
|---|---|---|---|
| PDH-E1a | 1.296 ± 0.146 | 1.547 ± 0.209 | 1.277 ± 0.119 |
| PDH-E2 | 0.092 ± 0.009 | 0.119 ± 0.020 | 0.076 ± 0.009† |
| PDH-E3bp | 0.076 ± 0.008 | 0.102 ± 0.016 | 0.073 ± 0.009 |
| PDK1 | 0.004 ± 0.001 | 0.005 ± 0.001 | 0.003 ± 0.001 |
| PDK2 | 0.207 ± 0.038 | 0.269 ± 0.057 | 0.150 ± 0.017 |
| PDK3 | 0.002 ± 0.0002 | 0.002 ± 0.0002 | 0.002 ± 0.0001 |
| PDK4 | 0.005 ± 0.002 | 0.005 ± 0.002 | 0.005 ± 0.001 |
Values are means ± s.e.m. with n = 8. mRNA content expressed in arbitrary untis. PDH, pyruvate dehydrogenase; PDK, pyruvate dehydrogenase kinase.
Significant difference from 1 week.
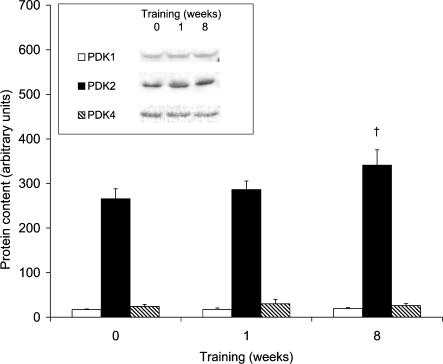
PDK activity did not differ after 1 week of exercise but approximately doubled after 8 weeks (Table 2). There was no change in the protein expression of PDK isoforms after 1 week of exercise and only PDK2 increased 1.3-fold after 8 weeks (Fig. 3). PDK3 was undetectable in the present study. PDK isoform mRNA levels were unaffected by exercise training (Table 3).
Figure 3. Mitochondrial protein content of PDK1, PDK2, and PDK4 before and after 1 and 8 weeks of aerobic training.
Inset, protein blots for a representative subject. Values are means ± s.e.m. with n = 8. †Significant difference from pretraining.
Discussion
The highly adaptive nature of skeletal muscle with repeated bouts of exercise allows for metabolic remodelling, leading to enhanced functional capacity. To our knowledge, this is the first study to examine the effects of short- and long-term aerobic training on enzyme activity and gene and protein expression of both PDH and PDK in human skeletal muscle. The major novel findings from the present study were that 8 weeks, but not 1 week, of aerobic training in human skeletal muscle resulted in (1) a 31% increase in PDHt activity, thus increasing the maximum potential capacity to utilize carbohydrates, and (2) a 2-fold increase in PDK activity, thus potentially attenuating the activation of PDH during submaximal exercise. Increased PDHt activity was accompanied by increased PDH-E1α protein expression whereas increased PDK activity was accompanied by increased PDK2 protein expression. Despite changes in activity and protein expression of PDHt and PDK, mRNA levels did not differ with training.
Mitochondrial oxidative capacity
An important by-product of repeated bouts of aerobic exercise is mitochondrial biogenesis (Hood, 2001), defined by increases in mitochondrial content following training. Training also increases the mitochondrial capacity to produce ATP, resulting in tightly coupled oxidative phosphorylation and a higher level of respiratory control. Depending on the training protocol and the pre-exercise trained state of subjects, mitochondrial adaptations may require 1–6 weeks before reaching a higher steady-state level (Hood, 2001). Thus the maximal activity and gene expression of representatives of the TCA cycle (CS) and electron transport chain (COX) will give insight into the adaptation to the training programme.
In the present study, 1 week of exercise did not alter the maximal activity or gene expression of CS and COX. This is consistent with the majority of previous studies, reporting that 5–12 days of aerobic exercise in humans did not change maximal activities of trained skeletal muscle mitochondrial enzymes (Green et al. 1991, 1992; Phillips et al. 1995a, 1996a; Putman et al. 1998). However, a couple of studies have demonstrated an increased CS maximal activity after 7–10 days of aerobic training (Chesley et al. 1996; Spina et al. 1996). The metabolic adaptations to short-term aerobic training remain controversial and will not be discussed in this paper. In contrast, after 8 weeks of exercise, the present study demonstrated an approximate 40% increase in maximal activities of CS and COX. This is consistent with another human study using a similar training programme (Carter et al. 2001). There was also a concomitant increase in gene expression of these two enzymes at the level of translation. The increases seen in CS and COX-II and -IV proteins are similar to previously reported values in exercise trained skeletal muscle of rats (Booth, 1991; Samelman et al. 2000) and humans (Dubouchaud et al. 2000; Bengtsson et al. 2001). Therefore, the present study demonstrates a clear adaptation in skeletal muscle typically seen with long-term aerobic exercise.
Short-term training – PDHt and PDK
Five days of aerobic exercise did not alter PDHt activity or PDH subunit expression in human skeletal muscle. This is consistent with a recent study which demonstrated that PDHt activity was unresponsive to 7 days of aerobic exercise in humans (60% V˙O2max for 2 h daily; Putman et al. 1998); however, PDH subunit expression was not measured.
As hypothesized, 5 days of aerobic exercise was not enough to elicit changes in PDK activity or the expression of any of the PDK isoforms. This is similar to a previous study, despite no measurements of PDK activity or isoform protein expression, which reported no change in PDK4 transcriptional rate or mRNA content in human skeletal muscle after 5 days of aerobic exercise (Pilegaard et al. 2000). Previous studies of short-term aerobic exercise in humans (5–10 days) have shown that metabolic adaptations precede changes to mitochondrial capacity, demonstrating reductions in lactate production and phosphocreatine and glycogen utilization (Green et al. 1991, 1992, 1995; Phillips et al. 1995b, 1996a; Putman et al. 1998). It was hypothesized that these adaptations were possibly mediated through allosteric modulation of PDH and glycogen phosphorylase (Putman et al. 1998). Thus any regulation of PDH with short-term aerobic exercise would be through acute regulators acting on PDK and PDP rather than changes to PDK or PDP maximal activities.
Long-term training – PDHt
The effect of long-term aerobic exercise on skeletal muscle carbohydrate utilization is an intricate balance between the control of carbohydrate utilization, through the activation of PDH, and the maximum potential capacity to utilize carbohydrates. In addition to increases in muscle mitochondrial volume, prolonged aerobic exercise training enhances the maximum potential carbohydrate flux in exercise trained compared to untrained skeletal muscle (see Goodyear & Kahn, 1998; Holloszy et al. 1998; Wojtaszewski & Richter, 1998; Henriksen, 2002 for review). This increased maximum potential for carbohydrate utilization may be at the level of PDH, through stable increases in PDHt activity. This is the first study to report an increased PDHt activity (31%) in human skeletal muscle after long-term aerobic exercise. In contrast, the only other study to examine the effects of repeated bouts of exercise on PDHt in humans reported no change in activity in triceps muscle after 5 weeks of strength exercise (Ward et al. 1986). Due to differences in muscle adaptation with strength and aerobic exercise, it is difficult to compare these studies.
It was hypothesized that the increased skeletal muscle PDHt activity after 8 weeks of exercise would be paralleled by increased PDH subunit protein expression. This was true only for PDH-E1α, which increased 1.3-fold after 8 weeks of exercise. This is consistent with previous studies examining tissues other than skeletal muscle. Maury et al. (1995) demonstrated an increased PDHt activity and PDH-E1α protein content in rat adipose tissue during the suckling–weaning transition, in which milk, a high-fat low-carbohydrate diet, is progressively replaced at weaning by an adult low-fat high-carbohydrate diet. In addition, livers of genetically obese (Amessou et al. 1998) and fat-free fed (Da Silva et al. 1993) rats demonstrate increased PDHt activity and PDH-E1α gene expression, with little or no change in the other subunits measured. This is of importance because the PDH-E1 tetramer (α2β2) carries out the non-reversible decarboxylation of pyruvate and the E1α subunit has three phosphorylation sites which, when phosphorylated, inactivate the PDH complex (Patel & Korotchkina, 2001). Although not measured in the present study, it would be expected that PDH-E1β would increase in parallel to PDH-E1α. In addition, a lack of significant increases in the other PDH subunits measured suggests that the amount of the E1α subunit, and possibly the E1 heterotetramer, is rate-limiting for the formation of new PDH complexes, as previously suggested for other tissues (Maury et al. 1995). Thus, it appears that an increased expression of the E1 subunit accommodates an increased PDHt activity in human skeletal muscle with long-term aerobic exercise.
Despite the increased PDHt activity and PDH-E1α with long-term aerobic exercise, no differences were detected in PDH subunit mRNA. Altered mRNA content is a product of the rate of mRNA production (gene transcription), degradation (mRNA stability), and subsequent protein production (translation). It is known that a single bout of exercise results in a transient increase in transcriptional rate of genes in skeletal muscle during the recovery period, believed to return to pre-exercise levels after 24 h (Neufer et al. 1998; Pilegaard et al. 2000). In addition, studies on cytochrome oxidase gene expression in rats have reported that increased mRNA in skeletal muscle with chronic electrical stimulation is a result of time-dependent increased mRNA stability (Freyssenet et al. 1999). There also appears to be translational and/or post-translational regulation of gene expression that occurs with exercise training in skeletal muscle of rats (Booth, 1991). The adaptation to repeat bouts of exercise may be due to the cumulative effects on any of these factors that contribute to gene expression, thus altering the level of skeletal muscle mRNA. As a result, it becomes difficult to interpret the mRNA results from the present study until further studies are done to determine what effect aerobic exercise training has on skeletal muscle mRNA stability and translational rates.
Long-term training – PDK
Long-term aerobic training results in a greater reliance on fat for ATP synthesis during submaximal exercise (Henriksson, 1977; Hurley et al. 1986; Martin et al. 1993; Phillips et al. 1996b), resulting in less reliance on carbohydrates and a net glycogen sparing effect. Activities of PDK and PDP, and resultant activation state of PDH, play an important role in this adaptation to training. In the current study, 8 weeks of aerobic exercise increased PDK activity approximately 2-fold. It is important to note that this increased PDK activity persisted after a rigorous mitochondrial preparation, and thus was not due to altered mitochondrial effectors known to increase PDK activity. The increased PDK post training reported in the present study is consistent with aerobic training in rats, demonstrating an increased PDK after 8 weeks of voluntary wheel running (Nakai et al. 1999). Increased PDK activity with long-term aerobic training would result in greater phosphorylation, and resulting inactivation, of the aforementioned 31% increased PDHt.
The long-term aerobic training-induced adaptations in skeletal muscle metabolism are not limited to the carbohydrate side. Previous studies have demonstrated greater adaptive increases in fat metabolism relative to carbohydrate metabolism in aerobically trained skeletal muscle (Davies et al. 1981; Wibom et al. 1992; Bizeau et al. 1998). Thus although aerobic training increases the maximal capacity to oxidize carbohydrates, there is a greater relative increase in enzymes that suppress carbohydrate flux (e.g. PDK) and enhance fat metabolism during submaximal exercise post training.
The increased PDK activity reported in the current study may be attributed to a 1.3-fold increase in PDK2 protein, with no change detected in protein of the other isoforms measured. To our knowledge, this is the first study to examine the effects of prolonged aerobic exercise training on the expression of PDK isoforms. Previous studies, focusing on dietary manipulation and acute disease states, reported an increased PDK4 protein in skeletal muscle during high fat diet (Holness et al. 2000), fasting (Wu et al. 1999; Sugden et al. 2000; Peters et al. 2001a), and insulin resistance (Wu et al. 1999) in rats and high fat diet in humans (Peters et al. 2001b). It appears that the adaptive response of skeletal muscle PDK4 is to acute alterations in fat availability, termed the ‘lipid status’-responsive PDK isoform (Sugden et al. 2001). In contrast, previous studies examining the less active but more abundant isoform, PDK2, report no change to acute physiological (Wu et al. 1999; Sugden et al. 2000; Peters et al. 2001a) or pathological (Wu et al. 1999) perturbations. PDK2 is important because it has a strong synergistic inhibition by ADP and dichloroacetate (DCA; Gudi et al. 1995; Bowker-Kinley et al. 1998), a unique response to high NADH/NAD ratio plus acetyl-CoA, increasing its activity to more than 300% of the control (Bowker-Kinley et al. 1998), and appears to respond to chronic perturbations, such as non-insulin-dependent diabetes mellitus (Majer et al. 1998). This evidence, along with the finding of the present study, suggests that chronic alterations to energy metabolism and energy status of the cell favour an up-regulation of PDK2, possibly making this the ‘energy status’-responsive PDK isoform.
A recent study conducted in our laboratory demonstrated a significantly lower activation of PDH during steady state submaximal exercise in individuals after 7 weeks of aerobic exercise (LeBlanc et al. 2003). We concluded that the altered flux through glycogen phosphorylase, influenced by cellular energy status, attenuated the exercise-induced increase in muscle [pyruvate], thus releasing inhibition on PDK. In the present study, the training-induced adaptive increase in skeletal muscle PDK2, which is highly sensitive to pyruvate, may be important in regulating PDH activation during submaximal exercise post training.
With only an approximate 25% increase in PDK2 protein content in skeletal muscle, there appear to be other factors responsible for a twofold increase in PDK activity. One possible contributor may be the other PDK isoforms found in the muscle, but in lower quantities. Both PDK1 and 3 demonstrate 2–25 times higher specific activity compared to PDK2 and 4 (Bowker-Kinley et al. 1998). Thus, small changes in training-induced protein expression of PDK1 and 3 would result in large changes in relative contribution to total PDK activity. Other possible contributors other than increased protein synthesis may be the interaction between PDK and the PDH complex. PDK is known to exist in two states, intrinsic (bound) and free (unbound; Kerbey et al. 1984; Jones & Yeaman, 1991; Mistry et al. 1991). Intrinsic PDK binds to the innermost lipoyl domain of the E2 component of PDH (Ravindran et al. 1996) and represents only 15–30% of the total PDK activity (Kerbey et al. 1984; Mistry et al. 1991; Vary & Hazen, 1999). Shifts from free to intrinsic PDK may enhance the enzyme activity with little or no change in protein synthesis. In addition, intrinsic PDK activity is enhanced when associated with higher ratios of reduced or acetylated forms of the E2-lipoyl domain (Korotchkina & Patel, 2001). More specifically, the activity of PDK2 approximately doubled with reduced/acetylated E2 compared to the oxidized form (Korotchkina & Patel, 2001). To date, no study has examined the effects of long-term aerobic training on the expression of intrinsic and free PDK and the interaction between these two forms of PDK and the PDH-E2 lipoyl domain.
Similar to PDH mRNA, PDK isoform mRNA was unaltered with exercise training, which is consistent with a recent study that examined high-intensity training in human skeletal muscle (Nordsborg et al. 2003). Again, this may be due to the transient nature of gene expression of these proteins with exercise, which may not be detectable during the sampling time frame (48 h after the last exercise bout). Despite no change in PDK mRNA with aerobic exercise training, the reported increase in PDK2 protein would suggest an increased gene expression of this protein. It is possible that the cumulative effect of transient increases in PDK2 mRNA following each bout of exercise may have contributed to the stable increase in PDK2 protein after 8 weeks of exercise. Previous studies, possibly representing more short-term sustained perturbations, reported increased PDK4 mRNA with fasting (Wu et al. 1999; Peters et al. 2001a) and streptozotocin-induced diabetes (Wu et al. 1999) in rats and during recovery from a prolonged bout of exercise in 5 day trained humans (Pilegaard et al. 2000), with little or no change to PDK2 mRNA. It is believed that potential fat-dependent promoters, such as peroxisome proliferators-activated receptor α (see Sugden et al. 2001 for review), are responsible for the increased PDK4 transcription. To date, no study has examined which exercise-induced promoters may stimulate gene expression of PDK isoforms, more specifically PDK2. There are numerous mechanical, metabolic, neuronal and hormonal factors (see Fluck & Hoppeler, 2003 for review) that could contribute to an up-regulation of PDK2 expression with exercise training. Future experiments will have to determine which regulatory parameters are linked to altered PDK2 transcription and translation.
We have demonstrated in the present paper that prolonged aerobic training up-regulated the amount and activity of PDHt and one of its regulatory proteins, PDK. The other covalent regulator of PDH, PDP, was not measured in this study; however, it may be speculated that it also increased with exercise training to accommodate the overall increase in the enzyme complex. To date, there are two known isoforms of PDP in mammals; PDP1 and PDP2. PDP1 is Ca2+ sensitive and preferentially expressed in skeletal muscle, whereas PDP2 is relatively unaffected by the absence or presence of Ca2+, is insulin sensitive, and found more abundantly in liver and adipose tissue (Huang et al. 1998). Few studies have examined the effects of physiological or pathological perturbations on PDP, reporting mixed results. The only study to examine skeletal muscle reported no change with acute starvation and streptozotocin-induced diabetes in rats (Fuller & Randle, 1984). More work is needed to establish the relative contribution of PDP to skeletal muscle adaptations to more prolonged physiological (e.g. exercise training) or pathological (e.g. obesity, diabetes) conditions.
Summary and conclusion
The present study clearly demonstrates that long-term aerobic training influences carbohydrate utilization in human skeletal muscle. The alterations are evident at the level of PDH and its control. Eight weeks of aerobic exercise increased PDHt activity, thus increasing the maximal potential capacity to oxidize carbohydrates. This was mainly due to increased expression of PDH subunit genes, more specifically the E1α subunit. Also, aerobic exercise training increased PDK activity, thus increasing the level of control on PDH. This was in part due to increased expression of PDK2. As a result, exercise trained skeletal muscle would have an increased maximal capacity to utilize carbohydrates during maximal oxygen uptake, with an increased metabolic control sensitivity to pyruvate during submaximal exercise through increased PDK2.
Acknowledgments
We gratefully acknowledge Dr M. Hargreaves for supporting the mRNA measurement and analysis. We would like to thank Drs Mary Maj and Brian Robinson for kindly donating the purified PDK protein. We also would like to thank B. Easterbrook, for assistance during the training programme, and the subjects that participated in this study, for all their hard work and effort. This study was supported by an operating grant from the Canadian Institute of Health Research (GJFH) and Ontario Graduate Scholarship (PJL).
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amessou M, Fouque F, Soussi N, Desbuquois B, Hainaut I, Girard J, Benelli C. Longitudinal study of tissue- and subunit-specific obesity-induced regulation of the pyruvate dehydrogenase complex. Mol Cell Endocr. 1998;144:139–147. doi: 10.1016/s0303-7207(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Bengtsson J, Gustafsson T, Widegren U, Jansson E, Sundberg CJ. Mitochondrial transcription factor A and respiratory complex IV increase in response to exercise training in humans. Pflugers Arch. 2001;443:61–66. doi: 10.1007/s004240100628. [DOI] [PubMed] [Google Scholar]
- Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Laboratory Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Bizeau ME, Willis WT, Hazel J. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol. 1998;85:1279–1284. doi: 10.1152/jappl.1998.85.4.1279. [DOI] [PubMed] [Google Scholar]
- Booth FW. Cytochrome c protein synthesis rate in rat skeletal muscle. J Appl Physiol. 1991;71:1225–1230. doi: 10.1152/jappl.1991.71.4.1225. [DOI] [PubMed] [Google Scholar]
- Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329:191–196. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky MA. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharm. 2001;79:386–392. [PubMed] [Google Scholar]
- Chesley A, Heigenhauser GJF, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Physiol. 1996;270:E328–E335. doi: 10.1152/ajpendo.1996.270.2.E328. [DOI] [PubMed] [Google Scholar]
- Da Silva LA, De Marcucci OL, Kuhnle ZR. Dietary polyunsaturated fats suppress the high-sucrose-induced increase of rat liver pyruvate dehydrogenase levels. Biochim Biophys Acta. 1993;1169:126–134. doi: 10.1016/0005-2760(93)90197-h. [DOI] [PubMed] [Google Scholar]
- Davies KJA, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- Denton RM, Randle PJ, Bridges BJ, Cooper RH, Kerbey AL, Pask HT, Severson DL, Stansbie D, Whitehouse S. Regulation of mammalian pyruvate dehydrogenase. Mol Cell Biochem. 1975;9:27–53. doi: 10.1007/BF01731731. [DOI] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Fatinia HR, Vary TC, Randle PJ. Modulation of pyruvate dehydrogenase kinase activity in cultured hepatocytes by glucagon and n-octanoate. Biochem J. 1986;234:233–236. doi: 10.1042/bj2340233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity – from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- Freyssenet D, Connor MK, Takahashi M, Hood DA. Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle. Am J Physiol. 1999;277:E26–E32. doi: 10.1152/ajpendo.1999.277.1.E26. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, Randle PJ. Reversible phosphorylation of pyruvate dehydrogenase in rat skeletal-muscle mitochondria. Effects of starvation and diabetes. Biochem J. 1984;219:635–646. doi: 10.1042/bj2190635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Green HJ, Cadefau J, Cusso R, Ball-Burnett M, Jamieson G. Metabolic adaptations to short-term training are expressed early in submaximal exercise. Can J Physiol Pharm. 1995;73:474–482. doi: 10.1139/y95-060. [DOI] [PubMed] [Google Scholar]
- Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol. 1992;72:484–491. doi: 10.1152/jappl.1992.72.2.484. [DOI] [PubMed] [Google Scholar]
- Green HJ, Jones S, Ball-Burnett M, Smith D, Livesey J, Farrance B. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol. 1991;70:2032–2038. doi: 10.1152/jappl.1991.70.5.2032. [DOI] [PubMed] [Google Scholar]
- Gudi R, Bowker-Kinley MM, Kedishvili NY, Zhao Y, Popov KM. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J Biol Chem. 1995;270:28989–28994. doi: 10.1074/jbc.270.48.28989. [DOI] [PubMed] [Google Scholar]
- Harris RA, Bowker-Kinley MM, Wu P, Jeng J, Popov KM. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex. J Biol Chem. 1997;272:19746–19751. doi: 10.1074/jbc.272.32.19746. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ. Exercise effects of muscle insulin signaling and action. Invited review: effects of acute exercise and exercise training on insulin resistance. J Appl Physiol. 2002;93:788–796. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- Henriksson J. Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol. 1977;270:661–675. doi: 10.1113/jphysiol.1977.sp011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson J. Muscle fuel selection: effects of exercise and training. Proc Nutr Soc. 1995;54:125–138. doi: 10.1079/pns19950042. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci. 1998;3:1011–1027. doi: 10.2741/a342. [DOI] [PubMed] [Google Scholar]
- Holness MJ, Kraus A, Harris RA, Sugden MC. Target upregulation of pyruvate dehydrogenase kinase (PDK)-4 in slow-twitch skeletal muscle underlies the stable modification of the regulatory characteristics of PDK induced by high-fat feeding. Diabetes. 2000;49:775–781. doi: 10.2337/diabetes.49.5.775. [DOI] [PubMed] [Google Scholar]
- Hong H-Y, Yoo G-S, Choi J-K. Direct blue 71 staining of proteins bound to blotting membranes. Electrophoresis. 2000;21:841–845. doi: 10.1002/(SICI)1522-2683(20000301)21:5<841::AID-ELPS841>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hood DA. Plasticity in skeletal, cardiac, and smooth muscle invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Houle-Leroy P, Garland T, Jr, Swallow JG, Guderley H. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. J Appl Physiol. 2000;89:1608–1616. doi: 10.1152/jappl.2000.89.4.1608. [DOI] [PubMed] [Google Scholar]
- Huang B, Gudi R, Wu P, Harris RA, Hamilton J, Popov KM. Isoenzymes of pyruvate dehydrogenase phosphatase. DNA-derived amino acid sequences, expression, and regulation. J Biol Chem. 1998;273:17680–17688. doi: 10.1074/jbc.273.28.17680. [DOI] [PubMed] [Google Scholar]
- Hultman E. Fuel selection, muscle fibre. Proc Nutr Soc. 1995;54:107–121. doi: 10.1079/pns19950041. [DOI] [PubMed] [Google Scholar]
- Hurley BF, Nemeth PM, Martin WH, III, Hagberg JM, Dalsky GP, Holloszy JO. Muscle triglyceride utilization during exercise: effect of training. J Appl Physiol. 1986;60:562–567. doi: 10.1152/jappl.1986.60.2.562. [DOI] [PubMed] [Google Scholar]
- Jones BS, Yeaman SJ. Long-term regulation of pyruvate dehydrogenase complex. Evidence that kinase-activator protein (KAP) is free pyruvate dehydrogenase kinase. Biochem J. 1991;275:781–784. doi: 10.1042/bj2750781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbey AL, Richardson LJ, Randle PJ. The roles of intrinsic kinase and kinase/activator protein in the enhanced phosphorylation of pyruvate dehydrogenase complex in starvation. FEBS Lett. 1984;176:115–119. doi: 10.1016/0014-5793(84)80923-0. [DOI] [PubMed] [Google Scholar]
- Korotchkina LG, Patel MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem. 2001;276:37223–37229. doi: 10.1074/jbc.M103069200. [DOI] [PubMed] [Google Scholar]
- LeBlanc PJ, Howarth KR, Gibala MJ, Heigenhauser GJF. Effects of aerobic training on human skeletal muscle metabolism at rest and during submaximal exercise. Med Sci Sport Exerc. 2003;35:S211. [Google Scholar]
- Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol General Metab. 1998;65:181–186. doi: 10.1006/mgme.1998.2748. [DOI] [PubMed] [Google Scholar]
- Makinen MW, Lee C-P. Biochemical studies of skeletal muscle mitochondria. I. Microanalysis of cytochrome content, oxidative and phophorylative activities of mammalian skeletal muscle mitochondria. Arch Biochem Biophys. 1968;126:75–82. doi: 10.1016/0003-9861(68)90561-4. [DOI] [PubMed] [Google Scholar]
- Martin WH, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS, Holloszy JO. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol. 1993;265:E708–E714. doi: 10.1152/ajpendo.1993.265.5.E708. [DOI] [PubMed] [Google Scholar]
- Maury J, Kerbey AL, Priestman DA, Patel MS, Girard J, Ferre P. Pretranslational regulation of pyruvate dehydrogenase complex subunits in white adipose tissue during the suckling-weaning transition in the rat. Biochem J. 1995;311:531–535. doi: 10.1042/bj3110531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry SC, Priestman DA, Kerbey AL, Randle PJ. Evidence that rat liver pyruvate dehydrogenase kinase activator protein is a pyruvate dehydrogenase kinase. Biochem J. 1991;275:775–779. doi: 10.1042/bj2750775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Shimomura Y, Fujitsuka N, Nakai N, Sugiyama S, Ozawa T, Sokabe M, Horai S, Tokuyama K, Suzuki M. Enzymatic and genetic adaptation of soleus muscle mitochondria to physical training in rats. Am J Physiol. 1994;267:E388–E395. doi: 10.1152/ajpendo.1994.267.3.E388. [DOI] [PubMed] [Google Scholar]
- Nakai N, Sato Y, Oshida Y, Fujitsuka N, Yoshimura A, Shimomura Y. Insulin activation of pyruvate dehydrogenase complex is enhanced by exercise training. Metabolism. 1999;48:865–869. doi: 10.1016/s0026-0495(99)90220-2. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Ordway GA, Williams RS. Transient regulation of c-fos, αB-crystallin, and hsp70 in muscle during recovery from contractile activity. Am J Physiol. 1998;274:C341–C346. doi: 10.1152/ajpcell.1998.274.2.C341. [DOI] [PubMed] [Google Scholar]
- Nordsborg N, Bangsbo J, Pilegaard H. Effect of high-intensity training on exercise-induced gene expression specific to ion homeostasis and metabolism. J Appl Physiol. 2003;95:1201–1206. doi: 10.1152/japplphysiol.00257.2003. [DOI] [PubMed] [Google Scholar]
- Patel MS, Korotchkina LG. Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases. Exp Mol Med. 2001;33:191–197. doi: 10.1038/emm.2001.32. [DOI] [PubMed] [Google Scholar]
- Peters SJ, Harris RA, Heigenhauser GJF, Spriet LL. Muscle fiber type comparison of PDH kinase activity and isoform expression in fed and fasted rats. Am J Physiol. 2001a;280:R661–R668. doi: 10.1152/ajpregu.2001.280.3.R661. [DOI] [PubMed] [Google Scholar]
- Peters SJ, Harris RA, Wu P, Pehleman TL, Heigenhauser GJF, Spriet LL. Human skeletal muscle PDH kinase activity and isoform expression during a 3-day high-fat/low-carbohydrate diet. Am J Physiol. 2001b;281:E1151–E1158. doi: 10.1152/ajpendo.2001.281.6.E1151. [DOI] [PubMed] [Google Scholar]
- Peters SJ, St Amand TA, Howlett RA, Heigenhauser GJF, Spriet LL. Human skeletal muscle pyruvate dehydrogenase kinase activity increases after a low-carbohydrate diet. Am J Physiol. 1998;275:E980–E986. doi: 10.1152/ajpendo.1998.275.6.E980. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Grant SM. Decreased glucose turnover after short-term training is unaccompanied by changes in muscle oxidative potential. Am J Physiol. 1995a;269:E222–E230. doi: 10.1152/ajpendo.1995.269.2.E222. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Grant SM. Increased clearance of lactate after short-term training in men. J Appl Physiol. 1995b;79:1862–1869. doi: 10.1152/jappl.1995.79.6.1862. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJF, Grant SM. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol. 1996a;270:E265–E272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol. 1996b;81:2182–2191. doi: 10.1152/jappl.1996.81.5.2182. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Popov KM. Regulation of mammalian pyruvate dehydrogenase kinase. FEBS Lett. 1997;419:197–200. doi: 10.1016/s0014-5793(97)01453-1. [DOI] [PubMed] [Google Scholar]
- Putman CT, Jones NL, Hultman E, Hollidge-Horvat MG, Bonen A, McConachie DR, Heigenhauser GJF. Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am J Physiol. 1998;275:E132–E139. doi: 10.1152/ajpendo.1998.275.1.E132. [DOI] [PubMed] [Google Scholar]
- Ravindran S, Radke GA, Guest JR, Roche TE. Lipoyl domain-based mechanism for the integrated feedback control of the pyruvate dehydrogenase complex by enhancement of pyruvate dehydrogenase kinase activity. J Biol Chem. 1996;271:653–662. doi: 10.1074/jbc.271.2.653. [DOI] [PubMed] [Google Scholar]
- Samelman TR, Shiry LJ, Cameron DF. Endurance training increases the expression of mitochondrial and nuclear encoded cytochrome c oxidase subunits and heat shock proteins in rat skeletal muscle. Eur J Appl Physiol. 2000;83:22–27. doi: 10.1007/s004210000241. [DOI] [PubMed] [Google Scholar]
- Spina RJ, Chi MM-Y, Hopkins MJ, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7–10 days of cycle exercise. J Appl Physiol. 1996;80:2250–2254. doi: 10.1152/jappl.1996.80.6.2250. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Bulmer K, Holness MJ. Fuel-sensing mechanism intergrating lipid and carbohydrate utilization. Biochem Soc Trans. 2001;29:272–278. doi: 10.1042/0300-5127:0290272. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Kraus A, Harris RA, Holness MJ. Fibre-type specific modification of the activity and regulation of skeletal muscle pyruvate dehydrogenase kinase (PDK) by prolonged starvation and refeeding is associated with targeted regulation of PDK isoenzyme 4 expression. Biochem J. 2000;346:651–657. [PMC free article] [PubMed] [Google Scholar]
- Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol. 2002;283:E66–E72. doi: 10.1152/ajpendo.00475.2001. [DOI] [PubMed] [Google Scholar]
- Vary TC, Hazen S. Sepsis alters pyruvate dehydrogenase kinase activity in skeletal muscle. Mol Cell Biochem. 1999;198:113–118. doi: 10.1023/a:1006993910781. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Tunstall RJ, Sanigorski A, Collier GR, Hargreaves M, Cameron-Smith D. Differential effects of exercise on insulin-signaling gene expression in human skeletal muscle. J Appl Physiol. 2001;90:436–440. doi: 10.1152/jappl.2001.90.2.436. [DOI] [PubMed] [Google Scholar]
- Ward GR, MacDougall JD, Sutton JR, Toews CJ, Jones NL. Activation of human muscle pyruvate dehydrogenase with activity and immobilization. Clin Sci. 1986;70:207–210. doi: 10.1042/cs0700207. [DOI] [PubMed] [Google Scholar]
- Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol. 1992;73:2004–2010. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- Wieland OH. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JFP, Richter EA. Glucose utilization during exercise: influence of endurance training. Acta Physiol Scand. 1998;162:351–358. doi: 10.1046/j.1365-201X.1998.0322e.x. [DOI] [PubMed] [Google Scholar]
- Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999;48:1593–1599. doi: 10.2337/diabetes.48.8.1593. [DOI] [PubMed] [Google Scholar]