Abstract
We report here evidence for endogenous NO signalling in long-term (> 1 h) synaptic depression at the neuromuscular junction induced by 20 min of 1 Hz nerve stimulation. Synaptic depression was characterized by a 46% reduction in the end-plate potential (EPP) amplitude and a 21% decrease in miniature EPP (MEPP) frequency, but no change to MEPP amplitude, indicating a reduction in evoked quantal release. Both the membrane-impermeant NO scavenger cPTIO and the NOS inhibitor+ release from the sarcoplasmic reticulum and muscle contraction were blocked with dantrolene. These data suggest that the depression depends on transmission, but not muscle contraction. The calcineurin inhibitors cyclosporin A and FK506, as well as ODQ, an inhibitor of NO-sensitive soluble guanylyl cyclase, Rp-8-pCPT-cGMPS, an inhibitor of cGMP-dependent protein kinase, and the calmodulin antagonist phenoxybenzamine also blocked depression. We propose that low frequency synaptic transmission leads to production of NO at the synapse and depression of transmitter release via a cGMP-dependent mechanism. The NO could be generated either directly from the muscle, or possibly from the Schwann cell in response to an unidentified muscle-derived messenger. We showed that the long-lasting depression of transmitter release was due to sustained activity of the NO signalling pathway, and suggest dephosphorylation of NOS by calcineurin as the basis for continued NO production.
Nitric oxide (NO) has emerged as an important modulator of neurotransmitter release in both the CNS and PNS (Schuman & Madison, 1994; Garthwaite & Boulton, 1995; Prast & Philippu, 2001; Esplugues, 2002), potentiating and/or depressing transmission depending on the synaptic type and the history of synaptic activity (Schuman & Madison, 1994). The molecule is highly labile and therefore the primary means for controlling the biological action of NO is by regulation of nitric oxide synthase (NOS), the NO producing enzyme. The activity of most forms of the enzyme is tightly regulated by Ca2+–calmodulin (Ca2+–CaM; Bredt & Snyder, 1990) and hence Ca2+ transients associated with synaptic activity provide a mechanism for coupling neurotransmitter release with NO production.
A role for nitric oxide in modulation of transmission at the neuromuscular junction (NMJ) was first proposed from the observation that exogenous NO depresses transmitter release in both developing (Wang et al. 1995) and mature (Lindgren & Laird, 1994) NMJs. More recently, it has been demonstrated that endogenous nitric oxide modulates transmission at the mature NMJ (Ribera et al. 1998; Aonuma et al. 2000; Thomas & Robitaille, 2001).
There are several potential sources of NO at the NMJ, derived from NOS isoforms expressed in nerve terminals (Ribera et al. 1998), perisynaptic Schwann cells (Descarries et al. 1998) and postsynaptic muscle fibres (Nakane et al. 1993; Kobzik et al. 1994; Yang et al. 1997). Release of NO from perisynaptic Schwann cells can depress transmitter release at high frequencies of stimulation, and a damping down of transmission by tonic release of NO from muscle cells in the resting NMJ has also been demonstrated (Thomas & Robitaille, 2001). It has been proposed that activation of nNOS by a local increase in cytosolic Ca2+ may lead to an activity-dependent increase in NO production by skeletal muscle fibres (Kusner & Kaminski, 1996).
We tested for the involvement of NO signalling in a form of synaptic depression induced at the amphibian neuromuscular junction by a train of low frequency (1 Hz) stimulation. Endogenous NO appears to be involved in low frequency stimulation-induced depression in invertebrates (Aonuma et al. 2000); however, the source of the NO is unknown and it remains unclear whether a similar NO signalling pathway is active in vertebrates. It is also not clear from the work with invertebrates whether or not the action of NO in depression induced by low frequency stimulation is dependent on the soluble guanylyl cyclase (sGC)–cGMP pathway. Both cGMP-dependent and -independent NO pathways have been shown to modulate transmitter release at the amphibian neuromuscular junction, depending on the stimulus conditions (Thomas & Robitaille, 2001).
Here we demonstrate that 20 min of 1 Hz nerve stimulation induced a long-lasting depression of transmitter release at the NMJ, and that this form of synaptic plasticity is mediated by a nitric oxide pathway; to our knowledge, this is the first demonstration of the involvement of NO signalling in low frequency stimulation-induced depression at the mature vertebrate neuromuscular junction. We have identified a role for the muscle cell in depressing transmission by triggering a retrograde signalling pathway that decreases quantal release from the terminal. Our results are consistent with speculation in the literature that muscle-derived NO could potentially modulate transmission in response to synaptic activity (Kusner & Kaminski, 1996; Thomas & Robitaille, 2001). Depression was blocked by an inhibitor of NO-sensitive sGC and by an inhibitor of cGMP-dependent protein kinase, suggesting that the action of NO to depress transmitter release involves the sGC–cGMP pathway. We propose that the long lasting nature of the depression, after cessation of the 1 Hz stimulation routine, is due to dephosphorylation of NOS by calcineurin and sustained NO production.
Methods
Cane toads (Bufo marinus) were obtained from the wild in Queensland, Australia, and then maintained for up to 6 months in a large humidified tank with a 12 h light–dark cycle at between 22 and 25°C. Toads were fed twice weekly on a mixture of crushed rat pellets and lean minced beef. Animals were killed by double pithing according to procedures approved by the Animal Ethics Committee of The University of Western Australia. Iliofibularis muscles were isolated with sciatic nerves and ventral roots attached. Connective tissue was carefully cut away from the surface of muscles to facilitate impalement of muscle fibres. The preparations were maintained in a modified aerated amphibian Ringer solution (NaCl, 114 mm; KCl, 2 mm; glucose 5 mm; Mops, 10 mm; and CaCl2, 1.5 mm; adjusted to pH 7.4 with NaOH). Experiments were performed at room temperature (22–24°C).
Induction of synaptic depression
Both iliofibularis muscle–nerve preparations from a single animal were tied at each tendon with silk and set up for stimulation in 3 ml organ baths. Muscles were stimulated via the nerve using a platinum–iridium suction electrode and the stimulation voltage and resting tension on each muscle were adjusted to produce a maximum twitch. Twitch amplitudes were recorded with Grass Instruments FT03 force transducers and experiments were aborted if the difference in maximum twitch amplitude between the two muscles was greater than 30%. Both muscles were pre-incubated in the relevant solution for each experiment (see Results) and then one muscle was stimulated continuously via the nerve at 1 Hz (1 ms square pulses at optimum voltage, usually 4.5–5.5 V) for 20 min, while the other muscle was left unstimulated.
Electrophysiological recordings
Electrophysiological recordings were performed using borosilicate glass microelectrodes (R = 7–20 MΩ) filled with 3 m KCl. All recordings were made from muscle fibres with membrane potentials more negative than −70 mV, and results from any one cell were discarded if the membrane potential depolarized by more than 10% before five synaptic potentials had been recorded. Impalements of cells from both muscles (control and stimulated) were performed in parallel, alternating between muscles. Experiments were discarded if less than five cells were sampled from a muscle. In experiments where spontaneous miniature end-plate potentials (MEPPs) were recorded, impalements were obtained within 1 h after termination of the 1 Hz stimulation routine. The 1 h window was selected because the nerve-evoked muscle twitch remained profoundly depressed at least 1 h after termination of the stimulation (see Results). To determine MEPP frequency, continuous recordings of no less than 3 min were obtained from each cell.
Muscles for EPP recordings were incubated in d-tubocurarine chloride (dTC, 0.6–1.6 μm, Sigma) to block muscle contractions. The concentration of dTC was varied throughout the year to compensate for seasonal variations in release probability (Bennett & Lavidis, 1991) and therefore to maintain the median amplitude of EPPs in control muscles within a workable range (1.4–4.0 mV). Muscles were normally exposed to the dTC immediately following the 1 Hz stimulation routine; impalements began 30 min later and stopped after 30 min of recording. EPPs were evoked at a frequency of 0.2 Hz by delivering supramaximal stimuli to the nerve via a suction electrode.
Synaptic potentials were recorded with an A-M Systems pre-amplifier (10 × gain) connected to a Powerlab 2/20 (ADInstruments). Scope software (version 3.6.11, ADInstruments) was used for recording amplitudes of synaptic potentials, while Chart software (version 4.2, ADInstruments) was used for recording MEPP frequency. Analysis of synaptic potential parameters was performed using the Peak Parameters extension for Chart.
Drugs
Stock solutions at 20 mm of 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yloxy-3-oxide potassium salt (cPTIO, Sigma), dantrolene sodium (Calbiochem), cyclosporin A (CsA, Calbiochem) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ, Sigma) and a 10 mm stock solution of FK506 (Calbiochem) were made up in DMSO. Working solutions, prepared immediately before use by dilution in Ringer solution, were at the following concentrations: cPTIO, 200 μm; dantrolene, 50 μm; CsA, 10 μm; FK506, 10 μm; and ODQ, 10 μm. Stock solutions of d-tubocurarine chloride hydrate (dTC, Sigma), hexamethonium bromide (Sigma) and 8-(4-chlorophenylthio)guanosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-pCPT-cGMPS, Biolog) were made up in Ca2+-free amphibian Ringer solution at 1, 40 and 2 mm and diluted in normal amphibian Ringer solution immediately before use to final concentrations of 0.6–1.6, 200 and 20 μm, respectively. A 50 mm stock solution of phenoxybenzamine hydrochloride (Calbiochem) was prepared in ethanol and then diluted in normal amphibian Ringer solution to a working concentration of 20 μm. One millimolar working solutions of Nω-nitro-l-arginine methyl ester (l-NAME, Sigma) were prepared in normal amphibian Ringer solution on the day of the experiment and kept on ice until used.
Statistical analysis
The mean amplitude and/or frequency of at least five synaptic potentials was determined for each fibre impaled. The median values of the fibre means for each muscle were then compared for statistical significance. Medians were considered to be preferable to means as estimates of the EPP and MEPP parameters because the small number of fibres sampled from each muscle (5–9) and the large variability in the parameters between fibres meant that means were often distorted by a large or small value from a single fibre. Hence, electrophysiological results show the mean ± s.e.m. of the median parameters for each muscle. Comparisons were always made between control and stimulated muscles from one animal, and therefore paired two-tailed Student's t tests were used to analyse all results.
Results
Synaptic depression due to low frequency stimulation
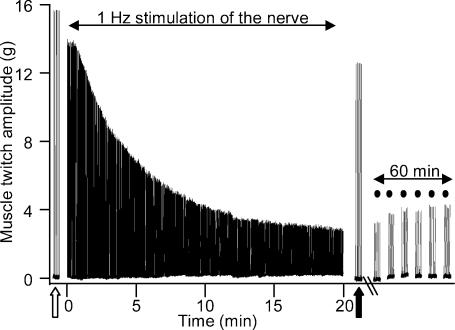
Low frequency (1 Hz) continuous stimulation of the nerve to the toad iliofibularis muscle resulted in a gradual decline in twitch amplitude, typically by 80% after 20 min of stimulation (Fig. 1). Only a minor component of the decline in force was due to changes directly associated with the muscle, such as fatigue, because the twitch amplitude in response to several stimuli delivered directly to the muscle belly was only 10–15% smaller after the 1 Hz nerve stimulation routine (filled arrow, Fig. 1) compared with before (open arrow). Moreover, depression in the nerve-evoked twitch was long lasting, as demonstrated by the depressed contractile response of the muscle to stimuli delivered via the nerve, at 10 min intervals, for 60 min after the 1 Hz nerve stimulation (dots, Fig. 1).
Figure 1. Low frequency nerve stimulation produces long-lasting depression of nerve-evoked muscle twitch at the amphibian neuromuscular junction.
Sample Chart recording of an experiment showing the contractile response of the iliofibularis muscle to indirect stimulation via the sciatic nerve and to direct stimulation of the muscle itself using supramaximal stimuli delivered by a ring electrode around the muscle belly. Continuous 1 Hz stimulation of the sciatic nerve for 20 min produced a decrease in the amplitude of the nerve-evoked muscle twitch by approximately 80%. The nerve-evoked twitch remained small for at least 60 min after termination of the 1 Hz stimulation routine (filled circles). Also shown is the contractile response of the muscle to a small number of stimuli delivered directly to the muscle belly before (open arrow) and after (filled arrow) the 20 min of 1 Hz stimulation delivered via the nerve.
EPP amplitudes from low frequency stimulated muscles, recorded in the period between 30 and 60 min post-stimulation (see Fig. 2A for experimental protocol), were depressed by an average of 46% when compared with EPPs recorded in the control muscle from the same animal (Fig. 2B and C). MEPP amplitudes were identical in depressed and control muscles, averaging 0.62 mV (Fig. 2D), while the MEPP frequency was 21% lower in the stimulated preparations (Fig. 2E). These findings suggest that the 80% reduction in nerve-evoked muscle twitch amplitude observed as a result of continuous 1 Hz stimulation of the nerve is predominantly due to reduced transmitter release from the neuromuscular nerve terminal.
Figure 2. Low frequency nerve stimulation decreases transmitter release at the amphibian neuromuscular junction.
A, time course of experiments for recording of EPPs. Two nerve–muscle preparations were isolated from an animal, mounted in organ baths, and optimum tension and voltage were established (zero on timeline). One muscle was left unstimulated for 80 min before EPP recording (control), while the other was stimulated for 20 min via the nerve during this period (stimulated). All recordings of end-plate potentials were obtained within the time window indicated by the chequered pattern. Both muscles were exposed to dTC for 30 min before, and also during, EPP recording to partially block nicotinic AChRs and prevent muscle contraction. In subsequent figures only the experimental timelines of stimulated muscles are shown because in all cases the control experiment was the same as the stimulated except for the period of 1 Hz stimulation. B, average amplitude of EPPs recorded from control (open bar) and low frequency stimulated (filled bar) muscles in normal Ringer solution. Stimulation significantly reduced EPP amplitude (*P < 0.05, n = 5 pairs of muscles, Student's two-tailed paired t test). C, representative traces of EPPs recorded from iliofibularis muscles under control conditions and after 20 min of low frequency stimulation. There was no significant difference in MEPP amplitude (D) between control (open bar) and low frequency stimulated (filled bar) conditions over 5 experiments in normal Ringer solution; however, MEPP frequency (E) was significantly lower (*P < 0.05, n = 11 pairs of muscles, Student's two-tailed paired t test) in stimulated preparations (filled bar) compared with controls (open bar).
Signal for depression is triggered by transmission
When synaptic transmission to the muscles was partially blocked with the nicotinic nAChR antagonist dTC prior to the 1 Hz stimulation routine, preventing action potential firing in the majority of fibres (data not shown), we did not observe any depression of the EPP amplitude (Fig. 3A). Two main subtypes of nAChRs are expressed at the mature neuromuscular junction: muscle-type receptors that predominate postsynaptically and mediate synaptic transmission (Salpeter & Loring, 1985), and neuronal-type nAChRs, which are expressed both pre- and post-junctionally (Kimura et al. 1994; Tsuneki et al. 1995). Although the primary action of dTC at the neuromuscular junction is as an antagonist of muscle-type AChRs, pre-junctional effects of the compound have been documented (Prior et al. 1995) and presynaptic neuronal-type nAChRs have previously been implicated in low frequency stimulation-induced neuromuscular synaptic depression (Prior & Singh, 2000). To determine whether the observed effects of dTC were due to its action on muscle-type nAChRs, or to non-specific effects of the antagonist on presynaptic nAChRs, we replicated the above experiment using hexamethonium instead of dTC.
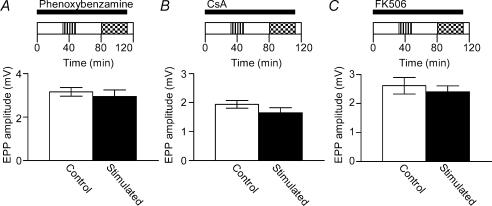
Figure 3. Low frequency nerve stimulation-induced depression is dependent on transmission, but not on muscle contraction.
Depression was not observed in response to low frequency nerve stimulation if muscles were pre-incubated with the muscle-type nAChR antagonist dTC (A, n = 6 pairs of muscles). Pre-incubation with dTC for 30 min typically reduced the nerve-evoked muscle twitch by approximately 90% (data not shown). In contrast, low frequency-induced depression was still observed (B) after incubation with the neuronal-type nAChR antagonist hexamethonium (200 μm); the average EPP amplitude in stimulated muscles was significantly lower than in controls (*P < 0.01, n = 7 pairs of muscles, Student's two-tailed paired t test). Significant depression of the EPP amplitude after low frequency stimulation was also observed when muscles were incubated with 50 μm dantrolene (*P < 0.05, n = 5 pairs of muscles, Student's two-tailed paired t test), a drug that blocks release of Ca2+ from the sarcoplasmic reticulum. In A, B and C the horizontal bar represents the period of exposure to the relevant drug (see legend to Fig. 2A for explanation of experimental design schematic).
Hexamethonium is a neuronal-type nAChR channel blocker that has only minimal effects on muscle-type nAChRs (Tian et al. 1997). Incubation with 200 μm hexamethonium, a concentration that has previously been shown to block neuronal nAChR-associated depression at the neuromuscular junction (Prior & Singh, 2000), did not block low frequency-induced depression (Fig. 3B), suggesting ACh is not acting back on the nerve terminal. Instead, these results using nAChR antagonists support the view that the induction of this form of depression is dependent on neuromuscular transmission, in particular the activity of muscle-type nAChRs.
Depression does not depend on muscle contraction
While our results suggest that neuromuscular transmission is important for the induction of depression, there is no requirement for the muscle to contract. We showed this by applying dantrolene to the muscles to block release of Ca2+ from the sarcoplasmic reticulum. Both control and stimulated preparations were incubated for 1.5 h in 50 μm dantrolene, after which time muscle contraction in response to nerve stimulation was typically decreased by approximately 85% (data not shown). In the presence of dantrolene, EPPs in muscles subjected to the 1 Hz stimulation routine were 55% smaller than those recorded from control muscles (Fig. 3C).
NO scavenger blocks induction of depression
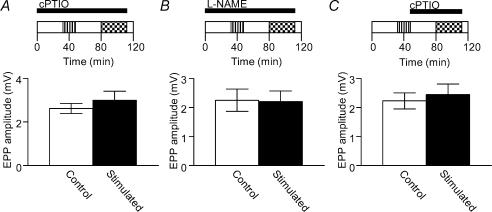
Evidence for NO signalling in the depression was found when 200 μm cPTIO, a NO scavenger, was added to the muscle-bathing medium throughout the time course of the experiments; controls had identical exposure to cPTIO, but were not stimulated. In the presence of cPTIO we did not observe any significant depression of EPP amplitude in response to repetitive low frequency stimulation (Fig. 4A). Pre-incubation with the NOS inhibitor L-NAME (1 mm) also blocked the induction of depression (Fig. 4B).
Figure 4. Endogenous NO mediates induction and maintenance of low frequency nerve stimulation-induced depression.
Exposure of muscles to the NO scavenger cPTIO throughout the experiments completely blocked the induction of low frequency stimulation-induced depression (A, n = 5 pairs of muscles, Student's two-tailed paired t test), as did exposure to the nitric oxide synthase inhibitor l-NAME (B, n = 5 pairs of muscles, Student's two-tailed paired t test). There was also no significant difference in EPP amplitudes between control (open bar) and stimulated muscles (filled bar) when cPTIO was added to the bathing medium after, but not during, the low frequency stimulation routine (C, n = 5 pairs of muscles, Student's two-tailed paired t test). In A, B and C the horizontal bars represent the period of exposure to the relevant drug (see legend to Fig. 2A for explanation of experimental design schematic).
These results, in light of the well-established capacity of exogenous NO to depress neuromuscular transmission (Lindgren & Laird, 1994; Wang et al. 1995; Aonuma et al. 2000; Thomas & Robitaille, 2001), indicate that NO signalling is critical to induction of the depression.
cPTIO is a cell membrane-impermeant NO scavenger (Ko & Kelly, 1999), and therefore the blockade of depression by cPTIO suggests that the NO produced in response to stimulation passes through the extracellular space before acting on the nerve terminal. Accordingly, NO must originate from a source outside the terminal, such as the muscle fibres and/or perisynaptic glial cells (Kusner & Kaminski, 1996; Yang et al. 1997; Descarries et al. 1998; Thomas & Robitaille, 2001). We would argue, based on our results using nAChR antagonists, that skeletal muscle fibres are the most likely source of NO depressing transmitter release from nerve terminals; the AChRs expressed on Schwann cells are predominantly, if not solely, of the muscarinic type (Robitaille et al. 1997) and therefore we would not expect the nicotinic AChR antagonist dTC to directly affect NO production by these cells. We cannot dismiss, however, the involvement of Schwann cells or indeed other surrounding cells given the possibility that the muscle may produce an unidentified transmissible factor that in turn activates NOS in these cells.
Sustained NO production is required for maintenance of synaptic depression
Synaptic depression was maintained for at least 60 min after termination of the 20-min low frequency stimulation routine (Fig 1 and 2B). We therefore investigated whether NO production is maintained throughout the period of the depression, or alternatively, whether NO production is only important for the induction of depression, with depression being maintained by a process downstream of NO. This was tested by applying the NO scavenger cPTIO to the preparation immediately after, but not during, the low frequency stimulation routine. If NO were involved in the induction but not in the maintenance of depression, we would expect that depression would still be observed if the NO scavenger were applied after termination of the depression routine. However, EPPs recorded from stimulated muscles were not depressed relative to controls with post-stimulation application of the NO scavenger (Fig. 4C). As NO is a very short-lived molecule, this result indicates that the prolonged depression of synaptic transmission is dependent on maintained activity of nitric oxide synthase (NOS) above baseline levels.
Synaptic depression is blocked by an inhibitor of calcineurin activity
The activation of nNOS by Ca2+–CaM is acute and therefore would not be expected to produce the long-term elevation of NO production that is implied by the result above. Another major determinant of the activity of nNOS is its phosphorylation state, with phosphorylation of the synthase leading to decreased catalytic activity (Bredt et al. 1992). Several members of the serine/threonine family of protein phosphatases have been implicated in dephosphorylating NOS and increasing its activity, including protein phosphatases 1, 2A and 2B (Komeima & Watanabe, 2001; Rameau et al. 2003).
Protein phosphatase 2B, also known as calcineurin, is of particular interest in this context because its activity is dependent on Ca2+–CaM binding (Stewart et al. 1982). Furthermore, it has been implicated in several forms of long-term depression in the CNS (Mulkey et al. 1994; Torii et al. 1995; Li et al. 2002). The induction of depression by low frequency stimulation in these experiments was blocked by the calmodulin inhibitor phenoxybenzamine (20 μm, Fig. 5A), implicating a Ca2+–CaM-dependent pathway in the depression. Our results suggest that calcineurin is also involved in long-term depression at the neuromuscular junction; low frequency stimulation did not produce significant depression of EPP amplitudes after pre-incubation of the muscle preparation with the calcineurin inhibitors CsA (10 μm, Fig. 5B) or FK506 (10 μm, Fig. 5C). We propose that the role of calcineurin in this system is to dephosporylate nNOS, increasing its activity and resulting in prolonged production of NO.
Figure 5. Induction of low frequency stimulation-induced depression is dependent on calmodulin and calcineurin activity.
Low frequency nerve stimulation did not induce depression of EPP amplitude in the presence of the calmodulin antagonist phenoxybenzamine (20 μm, A, n = 6 pairs of muscles, Student's two-tailed paired t test). There was also no significant difference in the amplitude of EPPs recorded from control (open bar) and 1 Hz stimulated (filled bar) muscles after incubation with 10 μm CsA (B, n = 5 pairs of muscles, Student's two-tailed paired t test) or 10 μm FK506 (C, n = 5 pairs of muscles, Student's two-tailed paired t test), inhibitors of the protein phosphatase calcineurin. In A, B and C the horizontal bar represents the period of exposure to phenoxybenzamine, CsA and FK506, respectively (see legend to Fig. 2A for explanation of experimental design schematic).
Synaptic depression involves a cGMP-dependent pathway
The proposed actions of NO to modulate synaptic function are diverse (for a review see Schuman & Madison, 1994); however, the primary pathway for action of NO in nerve terminals is activation of soluble guanylyl cyclase (sGC; Knowles et al. 1989), resulting in the production of cGMP, which can act by a variety of means to alter intracellular functions (Garthwaite & Boulton, 1995). The sGC–cGMP pathway has been implicated in tonic depression of ACh release by muscle-derived NO at the amphibian neuromuscular junction (Thomas & Robitaille, 2001).
To investigate the contribution of this pathway to the depression observed here we performed experiments using ODQ, a selective inhibitor of NO-sensitive sGC (Garthwaite et al. 1995). We observed that depression was completely blocked by incubation with 10 μm ODQ (Fig. 6A), which is consistent with NO acting through a cGMP-mediated pathway to depress transmitter release. However, ODQ has been shown to inhibit the activity of NOS, at least at concentrations greater than 30 μm (Feelisch et al. 1999). If ODQ were having a significant affect on NOS activity in our experiments, the outcome would be the same as the one shown in Fig. 6A, i.e. depression would be blocked. Therefore we investigated the effect of Rp-8-pCPT-cGMPS, an inhibitor of cGMP-dependent protein kinase, on depression and observed that low frequency stimulation did not depress EPP amplitudes in the presence of the compound (Fig. 6B). Together these results support a role for a sGC-cGMP pathway in the depression.
Figure 6. Activation of a soluble guanylyl cyclase–cGMP pathway is involved in low frequency nerve stimulation-induced depression.
Incubation of the muscles with 10 μm ODQ, an inhibitor of NO-sensitive guanylyl cyclase, completely blocked the induction of low frequency stimulation-induced depression (A, n = 5 pairs of muscles, Student's two-tailed paired t test). Depression was also blocked by incubation of the muscle with 10 μm Rp-8-pCPT-cGMPS, an inhibitor of cGMP-dependent protein kinase (B, n = 5 pairs of muscles, Student's two-tailed paired t test). The horizontal bars in A and B represent the period of exposure to ODQ and Rp-8-pCPT-cGMPS, respectively (see legend to Fig. 2A for explanation of experimental design schematic).
Discussion
Low frequency stimulation-induced depression is dependent on synaptic transmission
Depolarization of the postsynaptic cell is necessary for the induction of several forms of synaptic depression observed in the PNS (Cash et al. 1996) and CNS (for review see Linden & Connor, 1992). The same appears to be the case in our experiments; EPP amplitudes recorded in muscles stimulated continuously via the nerve at 1 Hz were not significantly different from controls in the presence of the muscle-type nAChR antagonist dTC. We confirmed that this effect of dTC was due to its activity on muscle-type nAChRs by showing that the onset of depression was unaffected by the neuronal-type nAChR antagonist hexamethonium. These results led us to conclude that the reduction in the level of transmitter secreted from the nerve terminals was dependent on activity of muscle-type nAChRs, and therefore implicated signalling from the postsynaptic muscle cell to the presynaptic neurone in the depression of the EPP amplitude.
Induction of depression is dependent on NO
Thomas & Robitaille (2001) have previously reported evidence for NO signalling in neuromuscular synaptic depression, specifically in high frequency- and adenosine- induced depression. The authors put forward a model in which transmission is modulated by muscle-derived NO at low levels of synaptic activity, and by NO derived from perisynaptic Schwann cells with high levels of nerve stimulation. The experimental protocol used by Thomas & Robitaille (2001), specifically the blockade of muscle activity by postsynaptic receptor antagonists, meant that they could not test directly whether transmission at low levels of nerve stimulation results in NO production and depression of release. Here, we confirmed that NO signalling is critical for the induction of low frequency transmission-dependent depression by blocking the depression with both the NO scavenger cPTIO and the nitric oxide synthase (NOS) inhibitor L-NAME.
NO is generated from l-arginine by the action of the NOS enzyme, which is expressed in several cell types at the NMJ, including perisynaptic Schwann cells (PSCs), skeletal muscle fibres and nerve terminals. The blockade of depression by cPTIO, a cell membrane-impermeant NO scavenger, is inconsistent with NO being produced by the nerve terminal itself, since NO produced by the nerve could act to decrease transmitter release without entering the extracellular space. Instead, this result, in combination with our observation that depression is blocked in the presence of the nicotinic AChR antagonist dTC, suggests that the most likely source of NO is the muscle cells; dTC would not be expected to affect PSCs, since AChRs expressed on these cells are muscarinic (Robitaille et al. 1997). Our results do not allow us to exclude the possibility that activation of nAChRs in the muscle indirectly activates NOS in the PSCs via an unknown muscle-derived transmissible factor. If such a transmissible factor exists, some components of the pathway outlined here could reside in the Schwann cell. This is a rather intriguing possibility, because it implies an integral relationship between three major cell types at the neuromuscular junction in determining the functional plasticity of the synapse. However, since activation of nNOS in the muscle cells is the most direct biochemical pathway to explain our results, and given that we have no evidence for muscle–glia signalling, our results will be discussed in terms of the more likely postsynaptic production of NO.
There are three main isoforms of NOS (inducible, endothelial and neuronal), all of which are expressed in skeletal muscle (Stamler & Meissner, 2001; Wang et al. 2001). Although inducible NOS (iNOS) is observed in skeletal muscle (Punkt et al. 2002) it is constitutively active and therefore its activity, related to the level of protein expression, would not be expected to change over the time course of the depression observed here. Ca2+–CaM affects the activity of both endothelial and neuronal NOS (eNOS and nNOS, respectively) and consequently both are potential candidates for activity-dependent regulation of NO release. Expression of eNOS is predominantly associated with the skeletal muscle vasculature (Gath et al. 1996), and consequently it is unlikely to be involved in modulation of synaptic transmission. In contrast, nNOS is abundantly expressed in skeletal muscle and it is concentrated to the sarcolemma (Kobzik et al. 1994), particularly the neuromuscular endplate (Chao et al. 1996; Kusner & Kaminski, 1996; Yang et al. 1997), and is therefore ideally positioned for modulating synaptic function in response to transmission. Therefore, although we did not test directly which isoforms of NOS are involved in the depression, the expression and regulation of the various NOS isoforms in skeletal muscle indicate that nNOS activity is probably the dominant source of activity-dependent NO production.
Depression is not dependent on release of Ca2+ from the sarcoplasmic reticulum
Activation of nNOS by Ca2+–CaM may occur either through influx of Ca2+ from extracellular fluid, or by release of Ca2+ from intracellular stores, such as the sarcoplasmic reticulum in muscle fibres. A role for Ca2+ from intracellular stores in these experiments seems unlikely because the depression persisted in the presence of dantrolene, which blocks sarcoplasmic reticulum Ca2+ release.
Therefore, we favour extracellular Ca2+ entering through nAChRs (Vernino et al. 1994) and/or postsynaptic voltage-dependent Ca2+ channels (VDCCs, Vijayaraghavan et al. 1992; Rathouz & Berg, 1994) as the main activator of NOS, rather than a global change in intracellular [Ca2+] due to excitation–contraction coupling. We have suggested the possible involvement of VDCCs in the depression because it is not clear from our methods whether or not firing of a muscle action potential is critical for induction of the depression. There is evidence for modulation of transmission by a local postsynaptic Ca2+ signal in nerve–muscle cocultures (Cash et al. 1996); however, events occurring ‘downstream’ of Ca2+ entry into the muscle cells are still not well defined, and to our knowledge it has not been directly demonstrated that local postsynaptic Ca2+ transients can modulate transmission at the mature NMJ.
Maintenance of depression requires prolonged production of NO
Having established that NO production is necessary for the induction of low frequency depression, we also observed that sustained NO production is required for maintenance of the depression, as depression was not seen if NO was scavenged with cPTIO after the stimulation routine. Interestingly, because NO is so labile, this result implies that low frequency stimulation results in prolonged activation of NOS above baseline levels.
NOS activation by Ca2+–CaM is acute and therefore would be expected to return to baseline levels after termination of the 1 Hz stimulation protocol. Aside from Ca2+–CaM binding, an important mechanism for physiological regulation of nNOS activity is the phosphorylation state of the enzyme (Bredt et al. 1992). Tonic phosphorylation of nNOS is observed in many systems, producing a tonic dampening of the catalytic activity of nNOS, while dephosphorylation has the opposite effect, leading to an increase in NO production (Dawson et al. 1993). We tested for this in our experiments by blocking the action of calcineurin, a protein phosphatase that has previously been shown to dephosphorylate nNOS (Dawson et al. 1993), and did not observe depression in the EPP amplitude in response to the 1 Hz nerve stimulation.
There are several reasons to hypothesize that the prolonged production of NO observed here might be due to NOS dephosphorylation by calcineurin. Firstly, calcineurin requires binding of Ca2+ and Ca2+–CaM for activation (Stewart et al. 1982). Thus there is an established mechanism whereby Ca2+ transients associated with repetitive stimulation could lead to activation of calcineurin, and subsequently to dephosphorylation of nNOS and production of NO. Our observation that depression was blocked by the calmodulin antagonist phenoxybenzamine is consistent with the involvement of such a pathway. Secondly, Yasuda et al. (2003) have monitored the activity of calcineurin in rat cortical slices and observed that 15 min of 1 Hz stimulation, similar to the stimulation routine used here, produced a prolonged increase in calcineurin activity. They showed that the activity of the phosphatase was elevated for the whole observation period, up to 25 min after termination of the stimulation routine. If such prolonged calcineurin activity was induced by the stimulus routine used in our experiments, it could produce a prolonged elevation of NOS activity and a long-lasting depression. Thus it is reasonable to conclude that the depression observed in our experiments resulted from activation of NOS by Ca2+–CaM, both directly, leading to acute production of NO, and indirectly, through binding of Ca2+–CaM to calcineurin, leading to a longer lasting production of NO.
Induction of depression by low frequency stimulation is via a cGMP-dependent signalling pathway
The most common mode of action of NO is by activation of NO-sensitive soluble guanylyl cyclase resulting in the production of cGMP. It has been proposed that NO acts through both cGMP-dependent and -independent pathways at the NMJ, with the dominance of a particular pathway determined by the level of synaptic activity (Thomas & Robitaille, 2001). We observed that depression was completely blocked by ODQ, an inhibitor of NO-sensitive guanylyl cyclase, and also by Rp-8-pCPT-cGMPS, an inhibitor of cGMP-dependent protein kinase, implicating a cGMP-dependent pathway in the depression. We have shown that NO originates from a source outside the terminal, most likely the muscle cell, but that depression results from a presynaptic change in transmitter release. Therefore we have proposed that the NO-sensitive sGC implicated in these experiments is located in the presynaptic terminal, with NO diffusing across the synaptic cleft to act on the sGC and depress transmission. If we consider this finding in the context of the work by Thomas & Robitaille (2001), who observed that NO tonically produced by skeletal muscle cells decreases transmitter release via a cGMP-dependent mechanism, our results may reflect increased activity, under the appropriate stimulus conditions, of a pathway that is tonically active at resting neuromuscular junctions.
Model of low frequency stimulation induced depression at the neuromuscular junction
A summary of the cellular mechanisms for long-lasting activity-dependent depression of transmitter release suggested by our experiments is illustrated in Fig. 7. The main feature of this scheme, which distinguishes it from previous models of NO signalling at the neuromuscular junction (Thomas & Robitaille, 2001; Esplugues, 2002), is the role played by the skeletal muscle cell in generating an activity-dependent retrograde signal to reduce quantal release from the terminal.
Figure 7. Model of depression induced at the neuromuscular junction by repetitive low frequency nerve stimulation.
According to the main pathway in the model, repetitive low frequency stimulation of the nerve results in activation of muscle-type nAChRs and a local increase in postsynaptic free Ca2+, due to entry of extracellular Ca2+ through nAChRs and/or voltage-dependent Ca2+ channels. This Ca2+ then binds to calmodulin in the muscle cell, and the Ca2+–CaM complex has both direct and indirect actions on nNOS activity. Ca2+–CaM binds to nNOS, resulting in an acute increase in NO production, as well as stimulating the protein phosphatase calcineurin, which in turn dephosphorylates nNOS and increases its activity. The activation of calcineurin by Ca2+–CaM is long-lasting, maintaining nNOS activation and NO production long after cessation of the stimulus routine. The NO produced by these mechanisms diffuses to the nerve terminal to decrease the quantal release via an unknown sGC–cGMP-dependent mechanism. Alternatively, signalling between the muscle cell and the terminal may occur indirectly via the Schwann cell. Such a pathway is indicated by the dashed line and involves the activity-dependent production of an unknown muscle-derived messenger, which in turn activates NOS in the Schwann cell.
The most direct cellular pathway to explain the results presented here involves Ca2+ entry through muscle-type nAChRs and/or voltage-dependent Ca2+ channels activating skeletal muscle nNOS and triggering the production of NO. In this scheme the NO itself is the retrograde messenger, acting back on the terminal via a cGMP-dependent mechanism to depress transmission. The model incorporates a role for calcineurin in the long term regulation of NOS activity and NO production.
Alternatively, it is possible that the retrograde signal is transmitted indirectly via the Schwann cell. Such a scheme would require the activity-dependent release of an unidentified muscle-derived messenger, which in turn activates NOS in the Schwann cell (dashed arrow Fig. 7). Both the direct and indirect pathways require muscle cell activation to produce the long-lasting change in synaptic efficacy.
To our knowledge, this is the first demonstration that NO signalling can produce long-term depression of transmission at the neuromuscular junction in response to synaptic activity. Our results demonstrating the transmission-dependent modulation of quantal release by NO, along with the findings of Thomas & Robitaille (2001), who identified a role for glial-derived NO in neuromuscular plasticity, provide a comprehensive framework for understanding the role of NO signalling in neuromuscular function.
Acknowledgments
This work was supported by an Australian Postgraduate Award and a Jean Rogerson Supplementary Postgraduate Scholarship awarded to S.J.E.
References
- Aonuma H, Nagayama T, Takahata M. Modulatory effects of nitric oxide on synaptic depression in the crayfish neuromuscular system. J Exp Biol. 2000;203:3595–3602. doi: 10.1242/jeb.203.23.3595. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lavidis NA. Probabilistic secretion of quanta from the release sites of nerve terminals in amphibian muscle modulated by seasonal changes. Neurosci Lett. 1991;134:79–82. doi: 10.1016/0304-3940(91)90513-s. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; Identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267:10976–10981. [PubMed] [Google Scholar]
- Bredt D, Snyder S. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Dan Y, Poo M-M, Zucker RS. Postsynaptic elevation of calcium induces persistent depression of neuromuscular synapses. Neuron. 1996;16:745–754. doi: 10.1016/s0896-6273(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Chao DS, Silvagno F, Xia H, Cornwell TL, Lincoln TM, Bredt DS. Nitric oxide synthase and cyclic GMP-dependent protein kinase concentrated at the neuromuscular endplate. Neuroscience. 1996;76:665–672. doi: 10.1016/s0306-4522(96)00367-3. [DOI] [PubMed] [Google Scholar]
- Dawson T, Steiner J, Dawson V, Dinerman J, Uhl G, Snyder S. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:9808–9812. [Google Scholar]
- Descarries LM, Cai S, Robitaille R. Localization and characterization of nitric oxide synthase at the frog neuromuscular junction. J Neurocytol. 1998;27:829–840. doi: 10.1023/a:1006907531778. [DOI] [PubMed] [Google Scholar]
- Esplugues JV. NO as a signalling molecule in the nervous system. Br J Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol. 1999;56:243–253. doi: 10.1124/mol.56.2.243. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Gath I, Closs EI, Godtel-Armbrust U, Schmitt S, Nakane M, Wessler I, Forstermann U. Inducible NO synthase II and neuronal NO synthase I are constitutively expressed in different structures of guinea pig skeletal muscle: implications for contractile function. FASEB J. 1996;10:1614–1620. doi: 10.1096/fasebj.10.14.9002553. [DOI] [PubMed] [Google Scholar]
- Kimura I, Tsuneki H, Dezaki K, Nojima H, Kimura M. Monoclonal antibody to β2 subunit of neuronal nicotinic receptor depresses the postjunctional non-contractile Ca2+ mobilization in the mouse diaphragm muscle. Neurosci Lett. 1994;180:101–104. doi: 10.1016/0304-3940(94)90497-9. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from 1-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Kelly PT. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:6784–6794. doi: 10.1523/JNEUROSCI.19-16-06784.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Komeima K, Watanabe Y. Dephosphorylation of nNOS at Ser847 by protein phosphatase 2A. FEBS Lett. 2001;497:65–66. doi: 10.1016/s0014-5793(01)02389-4. [DOI] [PubMed] [Google Scholar]
- Kusner LL, Kaminski HJ. Nitric oxide synthase is concentrated at the skeletal muscle endplate. Brain Res. 1996;730:238–242. doi: 10.1016/0006-8993(96)00675-0. [DOI] [PubMed] [Google Scholar]
- Li S-T, Kato K, Tomizawa K, Matsushita M, Moriwaki A, Matsui H, Mikoshiba K. Calcineurin plays different roles in group II metabotropic glutamate receptor- and NMDA receptor-dependent long-term depression. J Neurosci. 2002;22:5034–5041. doi: 10.1523/JNEUROSCI.22-12-05034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Long-term depression of glutamate currents in cultured cerebellar purkinje neurons does not require nitric oxide signalling. Eur J Neurosci. 1992;4:10–15. doi: 10.1111/j.1460-9568.1992.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Lindgren CA, Laird MV. Nitroprusside inhibits neurotransmitter release at the frog neuromuscular junction. Neuroreport. 1994;5:2205–2208. doi: 10.1097/00001756-199410270-00054. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Nakane M, Schmidt HHHW, Pollock JS, Forstermann U, Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Prior C, Singh S. Factors influencing the low-frequency associated nicotinic ACh autoreceptor-mediated depression of ACh release from rat motor nerve terminals. Br J Pharmacol. 2000;129:1067–1074. doi: 10.1038/sj.bjp.0703161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior C, Tian L, Dempster J, Marshall IG. Prejunctional actions of muscle relaxants: Synaptic vesicles and transmitter mobilization as sites of action. General Pharmacol. 1995;26:659–666. doi: 10.1016/0306-3623(94)00246-j. [DOI] [PubMed] [Google Scholar]
- Punkt K, Naupert A, Wellner M, Asmussen G, Schmidt C, Buchwalow IB. Nitric oxide synthase II in rat skeletal muscles. Histochem Cell Biol. 2002;118:371–379. doi: 10.1007/s00418-002-0465-4. [DOI] [PubMed] [Google Scholar]
- Rameau GA, Chiu L-Y, Ziff EB. NMDA receptor regulation of nNOS phosphorylation and induction of neuron death. Neurobiol Aging. 2003;24:1123–1133. doi: 10.1016/j.neurobiolaging.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rathouz MM, Berg DK. Synaptic-type acetylcholine receptors raise intracellular calcium levels in neurons by two mechanisms. J Neurosci. 1994;14:6935–6945. doi: 10.1523/JNEUROSCI.14-11-06935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera J, Marsal J, Casanovas A, Hukkanen M, Tarabal O, Esquerda JE. Nitric oxide synthase in rat neuromuscular junctions and in nerve terminals of Torpedo electric organ: Its role as regulator of acetylcholine release. J Neurosci Res. 1998;51:90–102. doi: 10.1002/(SICI)1097-4547(19980101)51:1<90::AID-JNR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Jahromi B, Charlton M. Muscarinic Ca2+ responses resistant to muscarinic antagonists at perisynaptic Schwann cells of the frog neuromuscular junction. J Physiol. 1997;504:337–347. doi: 10.1111/j.1469-7793.1997.337be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter MM, Loring RH. Nicotinic acetylcholine receptors in vertebrate muscle: Properties, distribution and neural control. Prog Neurobiol. 1985;25:297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Stamler JA, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80) FEBS Lett. 1982;137:80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Thomas S, Robitaille R. Differential frequency-dependent regulation of transmitter release by endogenous nitric oxide at the amphibian neuromuscular junction. J Neurosci. 2001;21:1087–1095. doi: 10.1523/JNEUROSCI.21-04-01087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Prior C, Dempster J, Marshall I. Hexamethonium- and methyllycaconitine-induced changes in acetylcholine release from rat motor nerve terminals. Br J Pharmacol. 1997;122:1025–1034. doi: 10.1038/sj.bjp.0701481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii N, Kamishita T, Otsu Y, Tsumoto T. An inhibitor for calcineurin, FK506, blocks induction of long-term depression in rat visual cortex. Neurosci Lett. 1995;185:1–4. doi: 10.1016/0304-3940(94)11210-a. [DOI] [PubMed] [Google Scholar]
- Tsuneki H, Kimura I, Dezaki K, Kimura M, Sala C, Fumagalli G. Immunohistochemical localization of neuronal nicotinic receptor subtypes at the pre- and postjunctional sites in mouse diaphragm muscle. Neurosci Lett. 1995;196:13–16. doi: 10.1016/0304-3940(95)11824-g. [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe K, Dani J. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. J Neurosci. 1994;14:5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+ Neuron. 1992;8:353–362. doi: 10.1016/0896-6273(92)90301-s. [DOI] [PubMed] [Google Scholar]
- Wang M-X, Murrell DF, Szabo C, Warren RF, Sarris M, Murrell GAC. Nitric oxide in skeletal muscle: inhibition of nitric oxide synthase inhibits walking speed in rats. Nitric Oxide. 2001;5:219–232. doi: 10.1006/niox.2001.0348. [DOI] [PubMed] [Google Scholar]
- Wang T, Xie Z, Lu B. Nitric oxide mediates activity-dependent synaptic suppression at developing neuromuscular synapses. Nature. 1995;374:262–266. doi: 10.1038/374262a0. [DOI] [PubMed] [Google Scholar]
- Yang CC, Alvarez RB, Engel WK, Haun CK, Askanas V. Immunolocalization of nitric oxide synthases at the postsynaptic domain of human and rat neuromuscular junctions – light and electron microscopic studies. Exp Neurol. 1997;148:34–44. doi: 10.1006/exnr.1997.6663. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Higashi H, Kudo Y, Inoue T, Hata Y, Mikoshiba K, Tsumoto T. Imaging of calcineurin activated by long-term depression-inducing synaptic inputs in living neurons of rat visual cortex. Eur J Neurosci. 2003;17:287–297. doi: 10.1046/j.1460-9568.2003.02449.x. [DOI] [PubMed] [Google Scholar]