Abstract
To explore the electrophysiological properties of the interstitial cells of Cajal (ICCs) and fibroblast-like cells (FLCs), we developed a new preparation by treating the murine small intestine with collagenase. This thin muscle layer preparation contained at least two types of interstitial cells around the enteric nerve bundles, and the cluster of smooth muscle cells displayed a rhythmic contraction. We morphologically identified ICCs and FLCs and conducted patch clamp experiments on each type of cell. The c-kit-positive CD34-negative ICCs showed spontaneous and rhythmic potential fluctuations, and a large transient inward current was evoked by depolarization under voltage clamp conditions. Once the inward current was triggered, it took a regenerative time course and lasted approximately 500 ms. The current was inactivated by continuous depolarization, and by removal of external Ca2+. The application of acetylcholine (ACh) prolonged the duration of spontaneous depolarization as well as the depolarization-induced inward current. This inward current showed a reversal potential of around +3 mV and was considered to be due to non-selective cation channels. The c-kit-negative CD34-positive FLCs showed irregular or regular potential fluctuations, and spontaneous outward current was observed under voltage clamp conditions. This outward current showed a reversal potential of around −80 mV and might be classified as a potassium current. We failed to observe major time- and voltage-dependent currents except the above two currents in the interstitial cells.
The spontaneous electrical and mechanical activities of gastrointestinal smooth muscle are thought to arise from a group of cells specialized for pacemaker function, known as the interstitial cells of Cajal (ICCs; Huizinga et al. 1995; Hirst & Ward, 2003). Thus, clarification of the pacemaker mechanism in ICCs is a prerequisite for a deeper understanding of gastrointestinal movement. However, systematic electrophysiological analysis of the spontaneous and rhythmic depolarization is not available in freshly dissociated ICCs. This is because the identification of ICCs is difficult after the preparation of tissue for cell isolation. Thus, most experiments have been done after isolated ICCs have recovered their distinctive morphology during several days' culture. Although the electrophysiological properties might be changed in the artificial culture medium, the ICCs display spontaneous and rhythmic depolarizations. In these cells, the existence of several ionic currents has been demonstrated, such as non-selective cation currents (Thomsen et al. 1998; Koh et al. 2002), the Cl− currents (Tokutomi et al. 1995; Huizinga et al. 2002), and voltage-dependent inward currents (Kim et al. 2002).
To achieve a more systematic study of the electrical activity, we developed a new preparation of interstitial cells. Instead of completely dissociating individual cells, we treated the murine small intestine moderately with collagenase and dissected out a transparent sheet of tissue from the intestinal wall. When stretched in the recording chamber, the tissue had the smooth muscle cells and the interstitial cells in situ, around the myenteric plexus, and clusters of smooth muscle cells displayed spontaneous and rhythmic contractions. We could apply a patch electrode to interstitial cells, and recorded spontaneous depolarizations. Under voltage clamp conditions, two types of membrane current were observed. In one group of interstitial cells, a depolarizing pulse evoked a regenerative inward current, which lasted for several hundred milliseconds. The reversal potential was around +3 mV, suggesting an involvement of a non-selective cation current. In the other group, the whole-cell current showed spontaneous fluctuations with a more or less similar pattern to the potential fluctuations under current clamp conditions. The reversal potential of these current fluctuations was between −70 and −90 mV, suggesting spontaneous fluctuations of membrane K+ conductance. These electrophysiological findings were compared with the histological identification of cell types, and the pacemaker mechanisms of the murine small intestine will be discussed.
Methods
Use and treatment of animals were approved by the institutional animal use and care committee at the graduate school of medicine of Kyoto University.
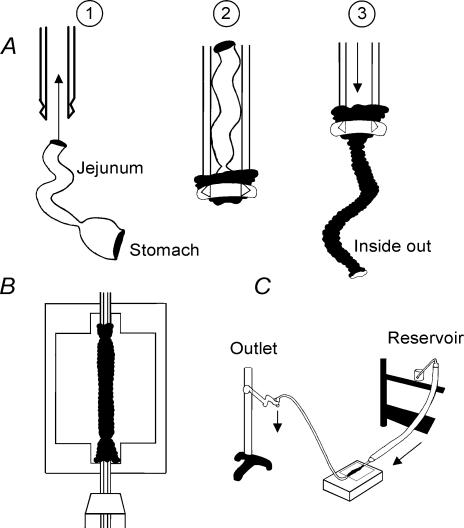
STD-ddY mice aged 7 weeks were deeply anaesthetized with diethylether, and killed by cervical dislocation. After exsanguinating by cutting the cervical artery, the epigastric area was opened and the small intestine including the stomach was dissected out. To apply enzyme solution on the serosal surface, the intestine was inverted by the technique shown in Fig. 1A. The jejunum was sucked from the anal end into a pipette of 5 mm in diameter, and the gastric wall was inverted around the pipette tip and fixed by ligation. The application of positive pressure into the pipette pushed out the intestine, resulting in an inside-out intestine. An intestinal segment of about 40 mm in length was cut and cannulated on both ends and fixed in the tissue chamber containing the nominally Ca2+-free solution as shown in Fig. 1B. The serosal cavity of the inverted intestine was perfused with the nominally Ca2+-free solution for about 5 min. The height of the perfusate reservoir was kept at 30–40 cm and the end of the outlet tubing was lifted, adjusting the flow rate to about 5 ml min−1 (Fig. 1C). Thus, a hydrostatic pressure of about 20–30 cmH2O was continuously applied to inflate the intestinal cavity. This inflation of the intestinal cavity was useful to achieve an effective enzymatic treatment of the intestinal wall.
Figure 1. Collagenase treatment of murine small intestine.
Schematic drawings of preparing the inverted intestinal segment (A), mounting the intestinal segment in the perfusion chamber (B) and the whole set up of the enzyme treatment (C). Mucosa and serosa are shown by black and white, respectively. See text for more detail.
The intestine was firstly treated by perfusing with 0.02% trypsin solution for 5 min, and then 0.04% collagenase (Wako collagenase) solution for 10 min. After the enzyme treatment, the intestine was moved to the enzyme- and Ca2+-free solution in a Petri dish, opened by cutting along the longitudinal axis, and washed several times. Small pieces (∼5 × 5 mm) of intestinal wall were dissected and fixed with the serosal side up on a surface of silicon rubber, and the smooth muscle layer was gently separated from the rest of the tissue using forceps and scissors. The thin muscle layer was stretched in the recording chamber under a dissection microscope and mounted on an inverted microscope. The recording chamber was perfused with control external solution.
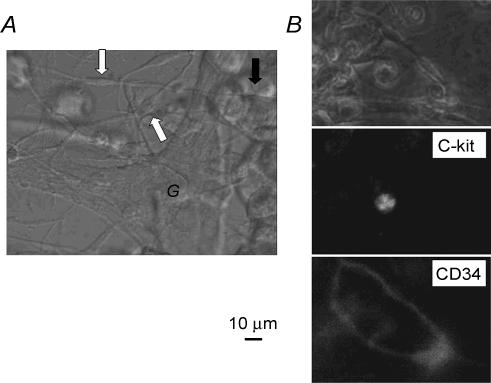
A representative tissue preparation is shown in Fig. 2A. The cells indicated by the white arrows are bipolar cells, extending processes to other cells. The size of the cell body is between 5 and 15 μm in width. These cells are located near the ganglia (G). The bipolar cells are considered as fibroblast-like cells (FLCs; Komuro, 1989; Horiguchi & Komuro, 2000) because of their peculiar shape and arrangement. In this picture, the much larger round cells are smooth muscle cells (black arrow), which were mostly rounded during rhythmic spontaneous contractions in the Ca2+-containing external solution. The spontaneous contractions continued in clusters of rounded smooth muscle cells.
Figure 2. Phase contrast and fluorescence micrographs of interstitial cells.
A, the myenteric plexus preparation obtained by treating the murine jejunum with collagenase. Around the plexus, the bipolar cells (indicated by white arrows) and the rounded smooth muscle cells (black arrow) are observed. Most of the smooth muscle cells were rounded, and removed by mechanical agitation during stretching and fixing the preparation onto the bottom of the recording chamber. Remaining clusters of the smooth muscle cells usually showed rhythmic contractions. B, double immunostaining with c-kit monoclonal antibody and CD34 monoclonal antibody. The top panel is the conventional phase contrast image, the middle the fluorescence image of c-kit, and the bottom the image of CD34 in the same preparation. The c-kit-positive cell was a round cell in the middle panel and the CD34-positive fusiform cells formed a network in the bottom panel. The calibration bar of 10 μm is common for all panels.
Direct immunofluorescence without fixation
The thin muscle layer preparation, removed from the intestinal wall after the enzyme treatment, was incubated for 20 min with anti-c-kit antibody (phycoerythrin (PE)-conjugated rat anti-mouse c-kit monoclonal antibody; eBioscience, San Diego, CA, USA) at a dilution of 1: 50. After washing twice with the control external solution, the same preparation was incubated for an additional 20 min with anti-CD34 antibody (fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD34 monoclonal antibody; BD Biosciences Pharmingen, San Diego, CA (USA) at a dilution of 1: 50, and washed twice with the control external solution. After the double immunostaining, the preparation was fixed on a glass plate filled with the control external solution, and observed using an ECLIPSE TE2000-U inverted microscope (Nikon, Kawasaki, Kanagawa, Japan) with fluorescence and phase-contrast microscopy (Fig. 2B). The two excitation beams of a xenon lamp (465–95 and 510–60 nm) and emission filters (515–55 and 590 nm) were sequentially used for selective detection of the red (PE) and blue (FITC) fluorochromes, respectively. Pictures were exported on a Pro2500 computer (EPSON, Matsumoto, Nagano, Japan) running AQUACOSMOS software (HAMAMATSU, Hamamatsu, Shizuoka, Japan). As shown in Fig. 2, the c-kit-positive cell was a small round cell and the CD34-positive cells were of fusiform shape and formed a network. Both cells were usually found close to each other. In the electrophysiological experiments, we could identify these cells by their characteristic shape and arrangement without the immunofluorescence. For convenience, we call the c-kit-positive cell type A and the CD34-positive cell type B in this study.
Electrophysiological measurements
The membrane potential and current were recorded with a perforated-patch whole-cell technique using an amplifier (EPC-7, Heka Electronik, Lambrecht, Pfalz, Germany, or Axopatch-1D amplifier, Axon Instruments, Union City, CA, USA). The output signal of the patch-clamp amplifier was sampled onto the computer memory via an A/D converter at 1–5 kHz for later data analysis. The electrode resistance, filled with the pipette solution, was around 10 MΩ. When series resistance compensation was employed to a level just below ringing, changes in the magnitude and time course of current on voltage jump were not marked in comparison to those before the compensation. Single cells, which were spatially separated from surrounding cells as examined under the microscope, were mainly used for voltage clamp experiments to avoid electrical coupling with other cells.
Solutions and drugs
The control external solution contained (mm) 140 NaCl, 5.4 KCl, 0.33 Na2HPO4, 1.8 CaCl2, 0.5 MgCl2, 5.5 glucose, 5 Na-pyruvate, and 5 Hepes, and the pH was adjusted to 7.4 with NaOH. The Ca2+-free solution was prepared by omitting CaCl2 from the external solution. The pipette solution in the whole-cell experiments contained (mm), 110 K-aspartate, 20 KCl, 1 MgCl2, 2 KH2PO4, 5 K2-ATP, 0.2 EGTA and 5 Hepes, and the pH was adjusted to 7.2 by adding KOH. To perform perforated-patch experiments, a pipette solution containing amphotericin-B (Sigma) was used. A stock solution of amphotericin-B was prepared by adding 6 mg solid to 100 μl dimethylsulphoxide. Prior to each experiment, 20 μl of stock solution was added to 2 ml of the pipette solution to give a final amphotericine-B concentration of 600 μg ml−1. As long as this pipette solution was kept in the refrigerator, it could be used for 6–8 h.
Acetylcholine (ACh, Sigma) was dissolved in distilled water to give a concentration of 1 mm and diluted in the external solution to the appropriate final concentrations.
All experiments were performed at 35–36°C. The statistical data are given as mean ± s.d.
Results
Electrophysiological activities of type A cells
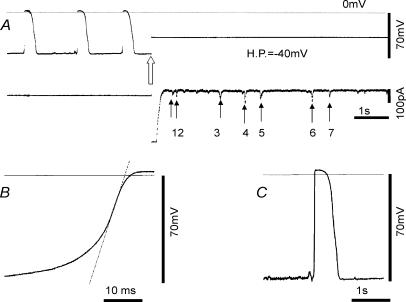
A representative record of spontaneous and repetitive depolarizations in a type A cell is shown in Fig. 3A. The resting potential was around −70 mV and depolarizations were observed with an average frequency of 16.2 min−1. The duration of depolarization at 50% repolarization level was 489.1 ± 32.8 ms (n = 5 events). The rising phase of the spontaneous depolarization was smooth as shown in Fig. 3B, and the maximum rate of rise was 7.1 V s−1. On average, it was 6.4 ± 0.6 V s−1 around −21.8 mV (n = 5 events). Note that the overshoot potential of the spontaneous depolarization was +3.1 mV. On switching to voltage clamp mode with a holding potential of −40 mV, the depolarizing step from the resting potential of −70 mV to −40 mV induced a large and transient inward current. The peak of the current was off the scale in this figure. Later, spontaneous transient inward currents with much shorter durations and smaller amplitudes were recorded. Figure 3C shows that the spontaneous depolarizations were preceded by much smaller transient depolarizations, which were probably induced by the spike-like inward currents observed at the holding potential in A.
Figure 3. Perforated patch recording from a type A cell.
A, the top trace is the potential recording, and the bottom is the membrane current. At the white arrow, the membrane potential was clamped at a holding potential (H.P.) of −40 mV. The dotted horizontal line at the top indicates the zero potential level. The amplitudes of the transient inward currents indicated by small arrows 1–7 were 36.9, 47.7, 75.4, 104.6, 70.8, 90.8, 52.3 pA respectively. B, the interrupted line was superimposed on the maximum rate of rise of the spontaneous depolarization, which was 7.1 V s−1 around −20.8 mV. C, the spontaneous depolarizations were preceded by much smaller transient depolarizations.
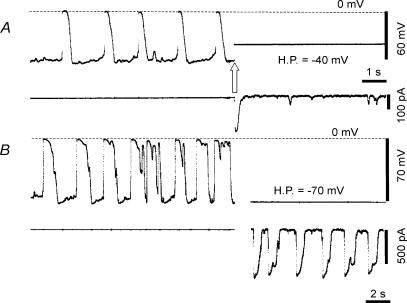
These electrical activities were variable between different experiments. The cell in Fig. 4A showed marked miniature fluctuations of the resting potential and full-blown spontaneous depolarization at a higher average frequency (45.2 min−1) than in Fig. 3. The maximum rate of rise was 3.6 ± 0.7 V s−1 around −22.2 mV (n = 5 events). The overshoot potential was +2.2 mV. The duration of the spontaneous depolarizations at 50% repolarization level was 269.5 ± 29.7 ms (n = 5 events). On clamping at a holding potential of −40 mV, a transient inward current was also evoked, but with smaller amplitude than in Fig. 3.
Figure 4. Perforated patch recordings from type A cells.
A, the top trace is the potential recording, and the bottom is the membrane current. At the white arrow the membrane potential was clamped at the holding potential (H.P.) of −40 mV. The dotted horizontal line at the top indicates the zero potential level. B, the same explanation as in A, except the holding potential was −70 mV. Note the slower time base for recording compared with A, and that the record was interrupted for about 1 min between the current clamp and the voltage clamp. The resting potential was −71.5 ± 2.3 mV and the positive peak of spontaneous depolarization (n = 5 events) was −2.1 ± 0.2 mV, the half duration 1160 ± 114 ms, the frequency 29.2 ± 8.6 min−1 and the maximum rate of rise 7.3 ± 0.3 V s −1at around −21.5 mV.
The cells in Figs 3 and 4A showed no repetitive activation of full-size inward current at a constant holding potential. Exceptionally, however, rhythmical inward currents as shown in Fig. 4B were observed in three experiments when cells were voltage clamped. In these cases, individual events of spontaneous depolarization showed multiple peaks as shown in Fig. 4B left part, and the duration of the compound was more than 1 s at 50% repolarization. Under voltage clamp, the duration of the spontaneous inward current was slightly shorter than the spontaneous depolarizations, but the multiple peaks were also obvious in some events. It should be noted that the duration of each peak within one event is of the same order as that observed in Fig. 3 or Fig. 4A (note the slower time base of record 4B than 4A). We speculate that these multiple-peak events might be recorded from cells that maintained electrical coupling with other spontaneously active type A cells. In fact, these cells extended processes to the neighbouring cells under the microscope. Therefore, we excluded these experiments from the detailed analysis described below. On average, in seven experiments, the resting potential was −72.5 ± 6.1 mV, the positive peak of depolarization −1.6 ± 3.8 mV, the frequency 26.8 ± 6.2 min−1, the duration at 50% repolarization level 553 ± 174 ms, and the maximum rate of rise 6.7 ± 2.5 V s−1 at −26.3 ± 4.3 mV, respectively. The input capacitance of five cells was 25.2 ± 4.7 pF.
Activation of an autonomous inward current by depolarization
Figure 5 shows a conventional protocol of voltage clamp used to examine the nature of membrane currents. The holding potential was −80 mV. During the test pulse of 20 mV hyperpolarization, or 10 or 20 mV depolarizations (upper panels), no time-dependent change in the membrane currents was observed except the spike-like capacitive transients at the pulse onset and offset. The resting input resistance of the whole cell was 0.7 GΩ and a slight inward rectification was obvious from the larger current jump during the 20 mV hyperpolarization than that during the corresponding depolarization.
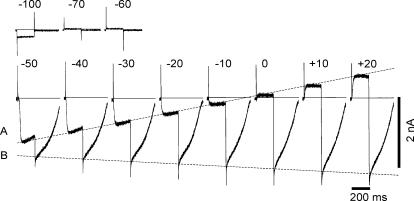
Figure 5. Depolarization-induced inward currents (AI current) in the type A cell.
The current records in response to test pulses of 200 ms in duration to the potentials indicated above each record. The holding potential was −80 mV. The zero current level is indicated by the horizontal lines. The line B was superimposed at the peaks of the inward tail currents recorded at the holding potential. The broken line A was superimposed at the peaks of evoked currents recorded at the test potential.
With the 30 mV depolarization, a large inward current was evoked after a delay of ∼20 ms. Surprisingly, this inward current showed an autonomous time course once it was evoked by depolarizing clamp pulses, and lasted for about 500 ms irrespective of the duration of the depolarizing pulse (see Fig. 8A). We call this inward current autonomous inward current, ‘AI current’ in this study. With larger depolarizations, the delay for the current activation was shortened and the rising phase of AI current became faster. At the onset of the test pulse to 0 mV, the transient peak immediately after the capacitive current was only slightly inward, suggesting the presence of a very small inward current system if any. The line B superimposed at the peaks of AI current (when panels are aligned with a constant interval) indicates that slightly larger activation was evoked with larger depolarization. The interrupted line A indicates that the conductance of AI current is almost linear, and the slope gave a membrane resistance of ∼35 MΩ with a reversal potential around 0 mV. This whole-cell input resistance is about 50 times smaller than the resting input resistance of 0.76 ± 0.41 GΩ in three experiments, indicating that both the duration and the overshoot potential of spontaneous depolarizations are determined almost solely by the AI current.
Figure 8. Reversal potential of the AI current.
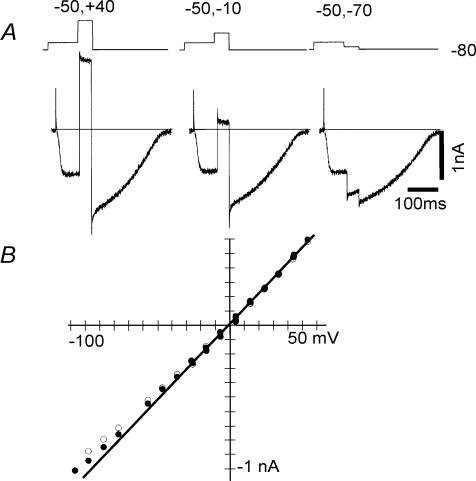
Measurements of the I–V relationship of the AI current using square pulses (A). In A, the AI current was evoked by depolarization to −50 mV (100 ms in duration) from a holding potential of −80 mV; 50 ms test pulses to various levels were applied during the maximum activation of the AI current and repolarized to −80 mV. The current magnitude at the beginning of the 50 ms test pulses (•) and at the end of test pulse (○) were plotted against the membrane potential in B.
Apparent inactivation of AI current by the membrane depolarization
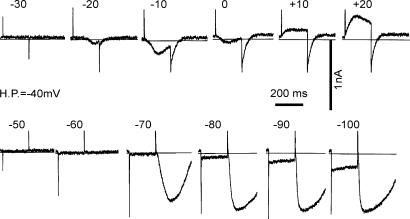
When the holding potential was shifted from −80 mV to −40 mV in the same cell as used in Fig. 5, depolarization to −30 mV failed to activate AI current (Fig. 6). A depolarizing pulse to −20 mV induced a small AI current with a delay of ∼100 ms. With a depolarizing pulse to −10 mV, the amplitude of the current was enlarged and the delay became shorter. However, AI current during depolarization to 0 mV was smaller than at −10 mV, suggesting that the test potential is close to the reversal potential as described later. At +10 and +20 mV, the evoked current was outward. It should be noted that the peak time even at +20 mV was 65 ms, which is much more delayed than that observed with a holding potential of −80 mV. On switching off the depolarizing pulses, a transient AI current was recorded, but the duration was much shorter than that observed with a holding potential of −80 mV.
Figure 6. Inactivation of the AI current by membrane depolarization.
The same experiment as shown in Fig. 5. The upper panels show the membrane currents induced by depolarizing pulses for 200 ms from the holding potential (H.P.) of −40 mV, and the lower panels show those in response to hyperpolarizing pulses to −50∼–100 mV as indicated above the current recordings. The horizontal line indicates the zero current level. The AI current system was activated during the depolarization to more than −20 mV, or after the hyperpolarizing pulses to −70, −80, −90 and −100 mV, but the currents were smaller in magnitude and shorter in duration than those obtained with a holding potential of −80 mV.
With hyperpolarizing pulses, no obvious time-dependent current was observed during the test pulse. However, after clamping back to −40 mV from −70 mV, a slow increase in the inward current (AI current) was triggered. The peak of the inward current was 710 pA, and the half duration was 181.5 ms. With larger hyperpolarizations of −80, −90 and −100 mV, the rising phase of AI current became faster, and the peak time became shorter. The duration of AI current was prolonged from −80 to −90 mV and almost saturated with the test pulse to −100 mV. It should be noted that this dependency of AI current on the preconditioning potential is totally different from the Marcovian behaviour of the usual ionic channels, indicating that the AI current is dependent on intracellular mechanisms as described in the previous literatures (van Helden et al. 2000; Hirst & Ward, 2003).
Time dependency of AI current activation and removal of inactivation
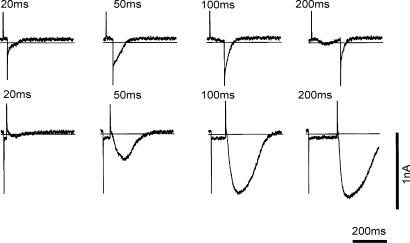
To examine the time course of AI current activation by depolarization, the duration of the depolarizing pulse was varied as shown in the upper panels of Fig. 7. The amplitude of the tail current became larger as the depolarizing pulse duration was increased from 20 to 100 ms. This fact may indicate that the current activation is in fact time dependent. With the 200 ms pulse, AI current is partially inactivated within the depolarizing pulse, and the tail current at the end of the pulse was smaller than that obtained with the 100 ms pulse.
Figure 7. Time course of activation by depolarization and removal of inactivation by hyperpolarization.
The holding potential was −40 mV and depolarizing (0 mV) or hyperpolarizing (−80 mV) pulses of various durations were applied in the upper and lower panels, respectively. The zero current level is indicated by the horizontal lines.
When the duration of the hyperpolarizing pulse to −80 mV was prolonged from 20 to 100 ms, the amplitude of AI current was increased, and nearly saturated with a 200 ms duration. Note the duration of AI current was prolonged with longer hyperpolarizations. It is concluded that both the amplitude and the duration of AI current are decreased via inactivation of unknown underlying mechanisms.
Reversal potential of AI current
In Fig. 8, the I–V relationship of the AI current was measured. As indicated in A, the 50 ms test pulses were applied to variable levels during the maximum activation of the AI current. It is evident that AI current shows no obvious time-dependent changes during a test pulse. The current levels immediately after the capacitive current were plotted against the membrane potential of the test pulse in B, with filled circles (open circles are at the end of the test pulse). The reversal potential determined from the superimposed line was +3 mV. The average of reversal potentials was +3.3 ± 2.5 mV (n = 3).
Effects of removing Ca2+ from the external solution
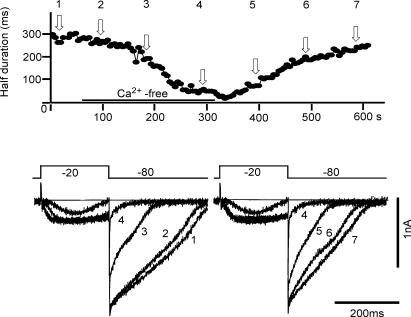
Previous studies (Ward et al. 2000; Yamazawa & Iino, 2002) suggested an involvement of Ca2+ release from intracellular Ca2+ stores in generating the spontaneous depolarizations in ICCs. To explore this mechanism, we perfused the preparation with a Ca2+-free external solution. The upper graph of Fig. 9 indicates the time course of changes in the duration of the tail current, and the lower panel shows the original current recordings obtained with a depolarizing pulse to −20 mV. The four current recordings, obtained at different experimental times were superimposed in the lower panel; those in the left panel were obtained after switching the external solution to the nominally Ca2+-free solution, and those in the right panel after reapplying Ca2+. The amplitude of inward current during the test pulse gradually decreased with time after removing Ca2+. On clamping back to −80 mV, the inward tail current progressively decreased in both amplitude and duration in the Ca2+-free solution. The reverse sequence of changes was observed when the control Ca2+-containing solution was applied (right panel). These findings support the hypothesis that the intracellular Ca2+ store determines both the time course and the amplitude of AI current.
Figure 9. Effects of removing [Ca2+]o on the AI current.
The upper graph indicates the time course of changes in the duration of the tail current, which was measured from the pulse offset to the half-decay time of the tail current. The lower panel shows the original current recordings. The pulse protocol is indicated above the current recordings. The four current recordings obtained at the different experimental times of the corresponding number in the graph were superimposed in the lower panel.
Effects of applying 1 μm ACh
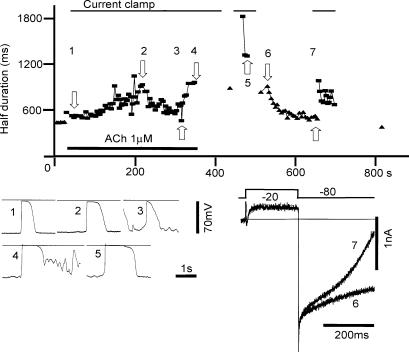
The spontaneous depolarization is enhanced by applying ACh to the muscle layer or ICCs (Keef et al. 1992; Kim et al. 2003). To further confirm the responsibility of AI current for the pacemaker potential, the perfusate of the recording chamber was switched from the control external solution to the same solution containing 1 μm ACh. The application of ACh gradually prolonged the duration of the spontaneous depolarization to 200 s (original records 1 and 2 in Fig. 10). From 200 s to 300 s, the resting potential was slightly depolarized by 3–4 mV, and small fluctuations were observed, and the duration of the spontaneous depolarization was slightly decreased as indicated by the original record 3. After switching back to the control perfusate, the membrane fluctuations decreased and the prolongation of the spontaneous depolarization was marked as observed in records 4 and 5 (Fig. 10).
Figure 10. Effects of applying 1 μm ACh.
The thin horizontal line indicates perfusion of the control external solution containing 1 μm ACh. The duration of the spontaneous depolarization was measured at the 50% amplitude level and plotted against the experimental time (▪ in the upper graph). The voltage clamp recordings (holding potential = −80 mV, and the test pulse = −20 mV, 200 ms) were conducted before and after switching the ACh solution and the durations of the AI current (measured from the test pulse onset to the half-decay time of the inward tail current) were plotted (▴). In the lower left panel, the spontaneous depolarizations recorded at the experimental times 1–5 were demonstrated. In the lower right panel, two current recordings at experimental times 6 and 7 were superimposed
Although the prolongation of the spontaneous depolarization was not very consistent, probably due to the fluctuations of the resting membrane potential, the prolongation of the AI current was observed as evident by comparing the current obtained soon after washing out ACh. Along with the gradual removal of ACh, the duration of AI current decreased almost monotonically (upper graph and lower right panel in Fig. 10). Essentially the same findings were observed in an additional two experiments.
A different type of the membrane fluctuations
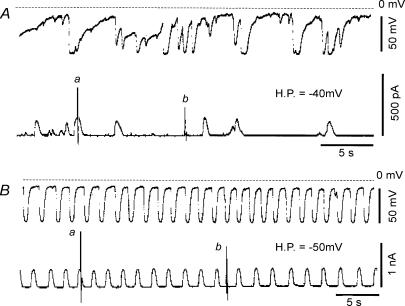
So far, the rhythmic depolarizations caused by the inward current system have been described. In addition to this type of fluctuation in the type A cell, we observed a different type of membrane fluctuation in the type B cell. It was characterized by rapid hyperpolarizations followed by slowly developing depolarizations. The hyperpolarization occurred irregularly in most cells as shown in Fig. 11A (upper panel). The positive and negative peaks of potential fluctuations were −17.3 mV and −77.9 mV, respectively. The maximum rate of fall was 5.81 ± 0.81 V s−1 (n = 5 events) around −58.8 mV. Although the duration and amplitude of individual events were variable, the large hyperpolarization, which was more than 50 mV in amplitude, lasted for more than 1 s. The average frequency of the large hyperpolarization was 5.2 ± 2.5 min−1 in 10 experiments.
Figure 11. Spontaneous rapid hyperpolarizations and outward currents in the type B cell.
The potential fluctuations (upper panels) and current fluctuations (lower panels) recorded from two different cells are demonstrated in A and B. The interrupted horizontal line indicates the zero potential level in the upper panels. The zero current level in the lower panels is nearly overlapped on the base line of the current recordings. In both A and B, the vertical deflections in current recordings a and b were evoked by applying the ramp pulse, and the peaks of the currents were out of scale in a of the lower panels. H.P., holding potential.
When the membrane potential was clamped at −40 mV, transient outward currents occurred spontaneously. The amplitude of the currents was less than 150 pA. The duration and amplitude of the currents were variable as shown in Fig. 11A (lower panel).
In a few cells, rhythmic potential fluctuations were observed as shown in Fig. 11B (upper panel). The positive and negative peak potentials were −10.6 ± 0.8 mV and −63.6 ± 0.5 mV, respectively (n = 5 events). The frequency and duration of hyperpolarizations at 50% level were 38.8 ± 3.2 min−1 and 538 ± 31 ms, respectively (n = 5 events). The maximum rate of fall was 0.87 ± 0.13 V s−1 around −39.5 mV (n = 5 events). In three experiments, the average negative peak potential was −66.1 ± 3.7 mV, the positive peak potential −8.6 ± 1.5 mV, the frequency 27.6 ± 11.4 min−1, the duration of hyperpolarization at 50% level 622 ± 180 ms, and the maximum rate of fall −0.95 ± 0.17 V s−1 around −33.7 mV. Note that the frequency of these potential fluctuations is within the range of that in the slow wave, 10–40 min−1 (Hirst & Ward, 2003). Although the contraction of smooth muscle was inhibited by 2 μm nicardipine in one experiment, there was no obvious change in the rhythm of potential and current fluctuations (not shown).
When the membrane potential was clamped at −50 mV, rhythmic outward currents occurred at similar frequency to the hyperpolarizations. In an average from five events immediately before and after the clamp, the frequencies were 35.1 ± 3.1 min−1 and 36.1 ± 3.7 min−1, respectively, and the durations at 50% level were 520.0 ± 35.8 ms and 460.0 ± 20.0 ms, respectively. These findings support the hypothesis that these rapid outward currents are responsible for the rapid hyperpolarizations. The amplitude was 300–350 pA at −50 mV and 60–70 pA at −70 mV in the same cell. Although the input capacitance was not measured in this cell, other type B cells had a mean input capacitance of 26.4 ± 1.9 pF (n = 4 cells).
These spontaneous outward currents observed at a constant membrane potential indicate the presence of voltage-independent and intracellular mechanisms underlying these outward currents. In all type B cells, no AI current was evoked by depolarizing pulse.
The reversal potential of the spontaneous current fluctuations
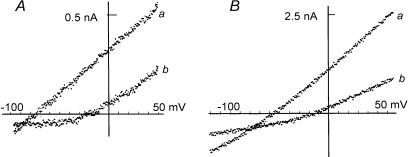
To determine the nature of the current system, which may underlie the spontaneous potential changes in the type B cells, the I–V curves were directly measured by applying ramp command pulses during the current fluctuations (Fig. 11, lower panels in A and B). The vertical linear deflections of the currents are due to the application of constant ramp pulses. When the ramp pulses were applied at the peaks of the outward currents (a), the membrane conductance was larger than that measured at base line (b) in both experiments. The I–V relations in Fig. 12 were determined from the descending limb of the ramp pulse (from +50 mV to −100 mV) as indicated by the corresponding letters in Fig. 11A and B. The reversal potentials in these two experiments were −83 mV and −81 mV, respectively, determined from the crossing points of the two I–V curves. In an average of irregular and regular fluctuations from three cells, the reversal potentials were −82.0 ± 7.5 mV and −77.7 ± 3.5 mV, respectively. It was suggested that the activation of the outward current was due to a K+ current system.
Figure 12. Measurement of the reversal potential.
The I–V curves in the left and right panels were obtained from experiments shown in Fig. 11A and B, respectively. The ramp pulses were applied at the peak of the outward current and at the resting level as shown in Fig. 11. The I–V curves a obtained at the peak of the outward current crossed at −83 and −81 mV with the curves b obtained at the resting condition.
Discussion
In the present study we developed a new preparation, in which we could identify two types of interstitial cells, and performed patch clamp experiments on these cells. The two types of cells in this preparation were often adjacent to, but distinct from each other in morphology and immunoreactivity. One type of cell was mostly rounded, but much smaller than smooth muscle cells, often located near nerve bundles, and was c-kit-positive and CD34-negative. Another type of cell was spindle-shaped, often forming a network, found close to nerve bundles, and was c-kit-negative and CD34-positive. It has been reported that c-kit-positive ICCs and CD34-positive FLCs were close to each other around both the myenteric plexus and the deep muscular plexus (Horiguchi & Komuro, 2000; Vanderwinden et al. 2000). In our preparation the deep muscular area was largely removed. Therefore, we thought that c-kit positive cells might correspond to ICC-MYs and CD34-positive cells might be FLCs. The electrophysiological properties of FLCs have not been described.
Kito & Suzuki (2003) recorded the pacemaker potential by penetrating conventional microelectrodes into ICC-MYs embedded in smooth muscle tissues of mouse small intestine. The resting potential of −72.5 mV in the type A cells is within the range recorded in situ by Kito & Suzuki (2003), which was −62.5 to −78.9 mV. The typical pacemaker potential in situ had an amplitude of 47.4–68.1 mV and the maximum rate of rise 1.51 V s−1. These values are lower than those obtained in the type A cells (70.9 mV and 6.7 V s−1, respectively) in the present study. However, it is expected that the electrical coupling of ICC-MYs with non-pacemaker cells including smooth muscle cells in situ should attenuate the pacemaker potential in ICC-MYs. Furthermore, both the relatively long half-width of pacemaker potential in situ (∼1.0 s) and the ‘noisy pattern of pacemaker potential’ are similar to the recorded events shown in Fig. 4B, which leads us to speculate that this type A cell might have electrical coupling with other pacemaker cells in our preparation. It should also be noted that the frequency (26.1 min−1) in situ is almost equal to that in the present study (26.8 min−1). Taken together, these findings support that the type A cells in the present study correspond to the ICC-MY revealed in situ. Kito & Suzuki (2003) separated the pacemaker potential into two components using pharmacological tools; the Ca2+-mediated primary component and the Cl−-mediated plateau component. However, the level of the entire current trace during the test pulse shifted almost linearly according to the test potential, with the reversal potential at around +3 mV in Fig. 5. This fact strongly suggests one current component might underly the pacemaker potential in the present study.
The detailed analysis of voltage clamp records depends on the completeness of the voltage control. The sharp spike-like capacitive current on voltage steps as evident in Figs 5–8 suggest feasibility of the voltage clamp in the present study. We recorded two types of potential fluctuation distinctive for each type of cell. The first type was the rhythmic depolarization attributable to the activation of the transient AI current in type A cells. The conductance of AI current was in the range of several tens of nanosiemens, which was approximately 20 times larger than the resting membrane conductance. No other major time- and voltage-dependent currents were observed. Thus, the overshoot potential of the rhythmic depolarization was almost equal to the reversal potential of AI current. Furthermore, the current magnitude underlying the maximum rate of rise in the spontaneous depolarization (6 V s−1) multiplied with the input membrane capacitance (25 pF) agreed well with the amplitude of the AI current (100–200 pA) at the corresponding membrane potential of −20 mV (Fig. 8B).
Ion channels may be classified according to their ion-selectivity and channel gating. The reversal potential of the AI current was around +3 mV, and the current–voltage relationship was almost linear. We consider that AI current is generated by non-selective cation channels. This is because the reversal potential of the AI current is different from the Cl− equilibrium potential in ICCs. The cell-attached single-channel recording of the Cl− current (Huizinga et al. 2002) revealed a reversal potential of −34 mV, which is consistent with the asymmetric distribution of Cl− across the cell membrane. We assume that the intracellular Cl− concentration was not much modified under the perforated patch experiment using amphotericin-B in the present study. A more definitive experiment would be to examine the reversal potential of the AI current at various [Na+]o. In preliminary experiments, however, the duration of the AI current was largely prolonged as in the case of increasing [Ca2+]o, when 90 mm Na+ was replaced by equimolar N-methyl-d-glucamine. The finding may suggest secondary changes in the intracellular ion concentrations. Alternatively, the ion selectivity of the AI current might be examined quantitatively by single-channel recording or by using a method of rapid solution change.
The second type of membrane depolarization was newly observed in the spindle-shaped cells, which may correspond to FLCs described by Horiguchi & Komuro (2000). This membrane depolarization occurred spontaneously and was most probably induced by the deactivation of K+ currents. The reversal potential of the membrane current fluctuations was more negative than −70 mV, and the current showed virtually no time-dependent change except the fluctuations induced by unknown cytosolic factors, such as variation in the Ca2+ concentration. This K+ conductance might be due to the SK3 channels, small conductance Ca2+-activated K+ channels, expressed in FLCs (Vanderwinden et al. 2002; Fujita et al. 2003). In addition, rhythmic potential fluctuations of type B cells were similar to slow waves in frequency, which might agree with the observations of Vanderwinden et al. (2002) and Fujita et al. (2003), that the SK3-immunopositive cells formed gap junctions with ICCs or smooth muscle cells.
The AI current was triggered by the depolarizing voltage step and inactivated by a continuous depolarization (Figs 6 and 7). This apparent voltage-dependent gating, however, might be caused secondarily to other molecular mechanisms. van Helden et al. (2000) proposed that the membrane depolarization activates phospholipase C (PLC) via an unknown process and leads to synthesis of IP3, and the resultant increase in IP3 triggers the release of Ca2+ through IP3 receptor-operated Ca2+ release channels in the sarcoplasmic reticulum (SR). Finally the increase of [Ca2+]i may activate the AI current. In fact, the measurement of intracellular [Ca2+] simultaneously in both smooth muscle cells and ICCs (Yamazawa & Iino, 2002) revealed that [Ca2+]i increased during the slow wave. It should be noted that three positive-feed-back mechanisms are involved in these processes. Firstly, the activation of the AI current further activates PLC via membrane depolarization, secondly the release of Ca2+ from SR further activates the Ca2+ release channel in SR, and lastly an influx of Ca2+ through the non-selective cation channel (AI current), if any, may further increase [Ca2+]i. Thus, once the AI current has been triggered, its time course is autonomously determined. The pivotal role of the first voltage-dependent mechanism in the repetitive pacemaker depolarizations under current clamp conditions is indicated by the experimental finding that spontaneous and repetitive activation of AI current was not observed after the voltage clamp was switched on. We speculate that the spontaneous activation of the AI current under voltage clamp (Fig. 4B) was due to incomplete electrical coupling of the recorded (voltage clamped) cell with adjacent pacemaking cells, as suggested in the voltage clamp experiments using the multiple-cell preparation of cultured ICC (Koh et al. 1998; Kim et al. 2002).
The apparent inactivation of the AI current might be attributed to a decrease of [Ca2+]i caused by single or multiple steps in the above cascade. The hypothesis is also in agreement with the prolongation of the AI current by the ACh stimulation. It was demonstrated by Ganitkevich & Isenberg (1993) that the activation of PLC by ACh enhances the positive feedback underlying the depolarization-induced increase in [Ca2+]i. Ward et al. (2000) suggested the involvement of mitochondria in the activation process of the non-selective cation current. In the present study, however, we did not examine this possibility.
Tokutomi et al. (1995) demonstrated that the spontaneous activity of the intestinal c-kit positive cells was inhibited by applying EGTA into the cell or by omitting Ca2+ from the extracellular medium. In the present study, the depletion of the extracellular [Ca2+] gradually decreased both the amplitude and duration of the AI current. This finding well supports van Helden's hypothesis, where the increase in [Ca2+]i takes the pivotal role in the generation of the slow wave. We consider that the Ca2+ store within the cell was gradually depleted when the extracellular [Ca2+] was decreased.
The activation of the AI current was triggered by small membrane depolarization. The present study revealed spontaneous inward current of less than 100 pA and less than several tens of milliseconds in duration under the voltage clamp condition. We hypothesize that the summation of these small inward currents triggers spontaneous depolarization via massive activation of the AI current. In the present study we did not systematically examine the nature of these small transient inward currents.
Here we compare the AI current observed in the present study with various currents reported in previous studies. The pioneering work by Tokutomi et al. (1995) suggested the involvement of a Cl− conductance to the pacemaker potential. However, the pacemaker current recorded by Thomsen et al. (1998) in the murine ICC was well defined as a non-selective cation current by determining the reversal potential, which was different from the Cl− equilibrium potential (−34 mV). The reversal potential ranged from +5 to +25 mV, which is in rough agreement with the reversal potential of AI current and supports a non-selective cation current. The current–voltage relationship determined from the spontaneous current recorded at different clamp potentials shows a linear relation, which is apparently similar to the ohmic conductance of the AI current in Fig. 8. However, the amplitude of the ‘pacemaker’ current (∼100 pA at −80 mV) was much smaller than the value of 2–3 nA for the AI current. This might be due to both the lower experimental temperature (room temperature versus 35°C), and the use of 10 mm EGTA in the patch pipette (ruptured patch versus perforated patch) in the experiment of Thomsen et al. (1998). We speculate that the pacemaker current in Thomsen et al. (1998) might correspond to the small transient currents observed in the present study.
Acknowledgments
We thank Dr H. Jo for helping to take pictures of the preparations, and for discussions during the course of study we thank especially Dr M. Takano at Kyoto University, Professor H. Suzuki at Nagoya city University and Dr S. Nakayama at Nagoya University. This study was supported by Grants in Aid for Scientific Research to A.N. from the Ministry of Education, Culture, Sports, Science and Technology (MECSST) of Japan. The study was also supported by the Leading Project for Biosimulation of MECSST.
References
- Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphae-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/WV mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. The W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterol. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- Keef KD, Ward SM, Stevens RJ, Frey BW, Sanders KM. Electrical and mechanical effects of acetylcholine and substance P in subregions of canine colon. Am J Physiol. 1992;262:G298–G307. doi: 10.1152/ajpgi.1992.262.2.G298. [DOI] [PubMed] [Google Scholar]
- Kim TW, Koh SD, Ordog T, Ward SM, Sanders KM. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J Physiol. 2003;546:415–425. doi: 10.1113/jphysiol.2002.028977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797–810. doi: 10.1113/jphysiol.2002.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T. Three-dimensional observation of the fibroblast-like cells associated with the rat myenteric plexus, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 1989;255:343–351. doi: 10.1007/BF00224117. [DOI] [PubMed] [Google Scholar]
- Thomsen L, Robinson TL, Lee JCF, Farraway LA, Hughes MJG, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, Nishikawa S, Nakao J, Imamura T, Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit positive cells. Pflugers Arch. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, De Kerchove d'Exaerde A, Jr, Gillard K, Panthier JJ, De Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–358. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, De Laet MH, Vanderhaeghen JJ, Schiffmann SN. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000;302:145–153. doi: 10.1007/s004410000264. [DOI] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, Von Der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]