Abstract
In the CA1 region of the hippocampus, LTP is thought to be initiated by a transient activation of NMDA receptors and is expressed as a persistent increase in synaptic transmission through AMPA receptors. To investigate the postsynaptic modifications of AMPA receptors involved in this enhanced synaptic transmission, the channel density and single-channel properties of extrasynaptic AMPA receptors located in synaptically active dendritic regions were examined following the induction of LTP. Following tetanic stimulation an outside-out patch was excised from the apical dendrite near the point of stimulation and saturating concentrations of glutamate were rapidly applied to the patch. AMPA current amplitude and duration were increased significantly in patches pulled from dendrites that expressed LTP. Non-stationary fluctuation analysis of AMPA currents indicated that AMPA channel number was nearly twofold larger than in controls, while single channel conductance and maximum open-probability were unchanged. Furthermore, while subtle changes in AMPA channel kinetics could also be observed, we did not find any evidence that receptor affinity or rectification properties were altered by LTP induction. Very similar results were found when CaMK-II activity was increased through the intracellular application of Ca/CaM. Together, we interpret our data to indicate that the stimuli used here produce an increased delivery of AMPA receptors to synaptically active regions of the apical dendrite without inducing any significant changes in their basic biophysical properties and that such delivery is a key element in this form of synaptic plasticity.
The most extensively studied form of activity-dependent synaptic plasticity is long-term potentiation (LTP) of glutamatergic synapses in CA1 pyramidal neurones of the hippocampus. One model of this process holds that an influx of Ca2+, mainly through NMDA receptors, initiates multiple mechanisms that result in an increase in transmission through AMPA-type glutamatergic receptors (Bliss & Collingridge, 1993). A postsynaptic mechanism thought to play an important role in this form of synaptic plasticity is the enhancement of AMPA receptor function through some type of receptor/channel modification (Malinow et al. 2000). There are several different modifications of AMPA receptors that might result in an increased synaptic efficacy and these include insertion of more receptors into the synaptic membrane (Malinow et al. 2000; Lu et al. 2001), increased channel open probability, increased single-channel conductance (Benke et al. 1998; Derkach et al. 1999), increased glutamate affinity and modification of channel kinetic properties.
There is evidence supporting an activity-dependent delivery of new, particularly GluR1-containing, AMPA receptors in various forms of long-term synaptic plasticity. Different types of immunocytochemical, over-expression approaches have suggested that an increased synaptic AMPA channel density follows the induction of a synaptic potentiation (Dudek & Bear, 1993; O'Brien et al. 1998; Heynen et al. 2000; Hayashi et al. 2000; Lu et al. 2001; Passafaro et al. 2001; Piccini & Malinow, 2002). There are still some questions, particularly in adult animals, however, revolving around whether most receptors are directly inserted into the postsynaptic density (PSD) or diffuse laterally into the synapse from an extrasynaptic compartment. Furthermore there is other evidence, although primarily in young animals and expression systems, that changes in AMPA channel phosphorylation state can increase single channel conductance (Benke et al. 1998; Poncer et al. 2002).
We investigated the changes in AMPA receptor function that accompany LTP by comparing the properties of AMPA receptors in outside-out patches pulled directly from synaptically active dendritic regions both in control conditions and following the induction of LTP and an experimentally induced increase in intracellular CaMK-II activity. The data suggest that, in the adult rats used here, an increase in channel number and not a CaMK-II-dependent change in channel properties is the primary mechanism of the postsynaptic changes in synaptic efficacy found following the induction of LTP. Furthermore, we have found that this increase does not occur throughout the activated neurone and is dependent upon the presence of active spines.
Methods
Hippocampal slices (400 μm) were prepared from 8- to 12-week-old Sprague-Dawley rats using standard procedures as described (Magee, 1998). According to methods approved by the LSUHSC Institutional Animal Care and Use Committee, rats were given a lethal dose of ketamine and xylazine, perfused through the ascending aorta with a 95% O2–5% CO2 bubbled solution just before death and then decapitated. Brains were rapidly removed and placed in cold oxygenated low-Ca2+/high-Mg2+ slicing solution. Experiments were conducted using an upright Zeiss Axioskop microscope fitted with differential interference contrast (DIC) optics using infrared illumination. All neurones had resting potentials between −60 and −75 mV. Patch pipettes were pulled from borosilicate glass tubing and had resistances of 5–8 MΩ when filled with internal solution. All currents were recorded with an Axopatch 200B (Axon Instruments, Union City, CA, USA). Data were acquired at 20 kHz and filtered at 2 kHz. The normal external solution contained 125 mm NaCl, 3.5 mm KCl, 1.25 mm NaH2PO4, 25 mm NaHCO3, 2 mm CaCl2, 1 mm MgCl2, 25 mm dextrose, bubbled with 95% O2 and 5% CO2 at 28–31°C (pH 7.4). The internal pipette solution consisted of 140 mm KMeSO4, 1 mm BAPTA, 10 mm Hepes, 4 mm NaCl, 0.28 mm CaCl2, 4.0 mm MgATP, 0.3 mm Tris2GTP, 14 mm phosphocreatine (pH 7.25 with KOH) and 50 μm spermine. The internal pipette solution for Ca/CaM experiments consisted of 140 mm KMeSO4, 0.5 or 5 mm EGTA, 10 mm Hepes, 4 mm NaCl, 1.5 or 6.2 mm CaCl2, 4.0 mm MgATP, 0.3 mm Tris2GTP, 2–10 μm calmodulin (CaM) and 14 mm phosphocreatine (pH 7.25 with KOH); 25 μm of calcineurin autoinhibitory peptide (CaN-AIP, Calbiochem) was added. The calculated (Patchers power tools by Francisco Mendez and Frank Würriehausen, http://www.mpibpc.gwdg.de/abteilungen/140/software/index.html) and measured (with MI-600 Ca2+-selective microelectrode from Microelectrode Inc., Bedford, NH, USA, using their standard protocol) free Ca2+ concentration was ∼48–60 μm (0.5 mm EGTA with 1.5 mm CaCl2 or 5 mm EGTA with 6.2 mm CaCl2). When we wanted to inhibit the Ca/CaM-dependent CaMK-II activity, 1 μm autoinhibitory peptide (AIP, Ishida et al. 1995; from Dr J. Haycock LSU, New Orleans, LA, USA) was added. The test stimulus was a short burst of three stimuli given at 100 Hz to the Schaffer collateral fibres via a Tungsten electrode positioned 20–30 μm from dendrite. A stimulus amplitude that was sufficient to initiate one or two action potentials (AP) was chosen. The average of three traces was used. One-second-long 100 Hz stimulation was given twice with a 30 s interval to potentiate the cell. A cell-attached recording electrode was used to measure synaptic efficacy before and after tetanus. The CA3 region was surgically removed and 20 μm bicuculline was added to reduce inhibitory input during the stimulation. APV (50 μm) and MK-801 (10 μm) were added in the ‘unpotentiated’ condition. Dendritic outside-out patches were excised at a distance of ∼200 μm distal to the soma. The fast application of agonist was performed as describe previously (Andrásfalvy & Magee, 2001). Double-barrelled application pipettes were fabricated from theta glass tubing and solutions were perfused through the control and agonist barrels at a rate of about 0.3 ml min−1 by means of a multi-lined peristaltic pump. The interface between the two solutions was moved across the membrane patch by means of a piezoelectric element, upon which the application pipette was mounted. The perfusion solutions contained 125 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 10 mm Hepes, 2.5 mm CaCl2 and 50 mm dextrose (pH 7.4). After each patch recording, the application speed was tested by breaking the patch and measuring the open tip current caused by a jump from 10% perfusion solution to normal perfusion solution. The 20–80% exchange times varied between 100 and 200 μs. Patches showing a current rise time greater than 1 ms were removed from analysis.
Measuring synaptic efficacy using cell-attached patch recordings
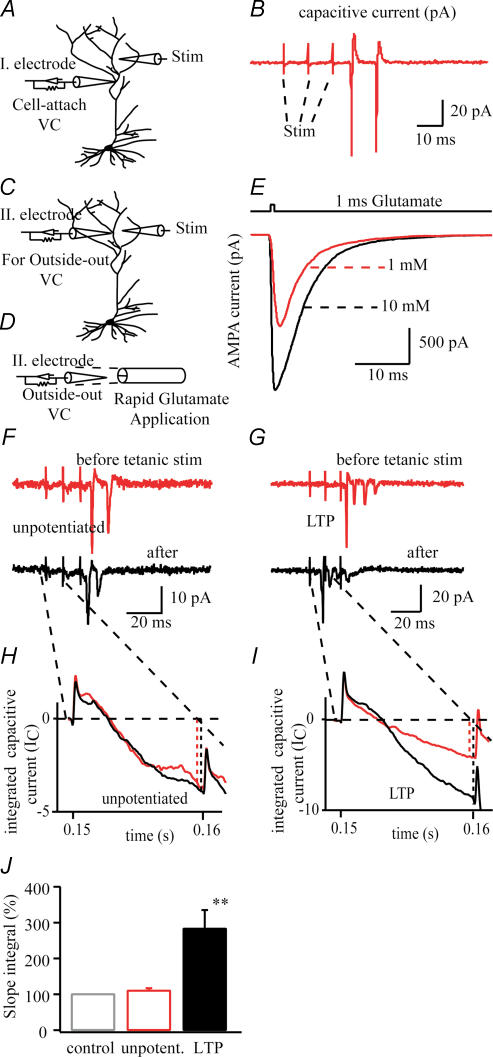
So as to not disturb the local intracellular environment, we monitored the electrical activity with a cell-attached patch recording for 10–20 min before and 20–30 min after tetanus stimulation (Frick et al. 2004). Every 20 s we gave three test stimuli (100 Hz), which were large enough to evoke one or two action potentials (APs). The recorded capacitive currents were analysed by two separate criteria to evaluate the change in synaptic efficacy. First, we measured the first spike latency from the beginning of the recorded trace, based on the notion that the increased synaptic efficacy increases the depolarization of the neurone, so it reaches the threshold earlier in time (Fig. 1). Second, we used the capacitive current traces as a measure of the evoked excitatory postsynaptic potantial (EPSP). The underlying idea is that during the potentiation the evoked EPSP increased and the elevated rate of voltage change (dV/dt) causes an elevated capacitive current (Ic = Cm × dV/dt, where Cm is patch capacitance). Indeed following potentiation the deflection increased after tetanus (274 ± 48%; n = 11, Fig. 1), while the deflection remained unchanged if the potentiation failed or was intentionally blocked by NMDA channel inhibition 110 ± 6%; n = 12, Fig. 1.
Figure 1.
A, diagram of a dendritic cell-attached patch recording from ∼150 μm distal from the soma. B, representative trace of capacitive currents evoked by three test stimuli using stimulating electrode ∼50 μm far from the recording electrode. Test stimuli were given every 20 s, for 10 min before and 20 min after 100 Hz tetanic stimulation to measure the synaptic efficacy (F–J). C, diagram of second recording electrode ∼20 μm from stimulating electrode and ∼200 μm from soma to excise an outside-out patch for fast glutamate application (D). E, representative traces of AMPA receptor-mediated glutamate current (Vh = –80 mV) induced by 1 ms, 1 mm or 10 mm glutamate. Glutamate at 1 mm was used to measure kinetic properties (Fig. 4) and current–voltage relationship of AMPA receptors (Fig. 5). F and G, cell-attached patch recordings from dendrites before and after tetanic stimulation without (G) and with (F) NMDA receptor blockers evoked by three test stimuli. H and I, the integration of these capacitive currents (IC; caused by evoked EPSPs) using the average of three traces to measure the synaptic efficacy change caused by tetanic stimulation. J, the change of synaptic efficacy measuring peak deflection after the first test stimulus in time before and after tetanic stimulation (see text, **P < 0.01).
Kinetic analysis
To measure the rise and the deactivation kinetics of the AMPA current, a 1 ms pulse of 1 mm glutamate was applied, while a 100 ms pulse was delivered for the measurement of desensitization kinetics. The rise time constants were determined from an exponential fit of the AMPA current rising phase while deactivation time constants were determined from an exponential fit of the decay phase of the same currents. The desensitization time constants were determined from a double exponential fit of the decay phase of the AMPA currents produced by the 100 ms glutamate application. The relative contribution of the separate components was expressed as the ratio of the fast to slow desensitization component amplitudes.
Non-stationary fluctuation analysis
Microscopic properties of AMPA channels were examined using non-stationary fluctuation analysis (Sigworth, 1980). AMPA currents were evoked every 2–3 s by a 1 ms pulse of 10 mm glutamate in the presence of 1 mm Mg2+. Between 20 and 100 traces per patch were obtained for analysis. The mean variance (σ2), determined from all responses, was plotted against the mean current for all responses. The plot was fitted with the function:
where I is the total current and i is the single-channel current, N is the number of available channels in the patch and σb2 is the variance of the background noise.
Single-channel conductance (γ) was determined as the chord conductance:
where Vh is the holding potential and Vrev is assumed to be 0 mV. The open probability (Po) was determined by the equation:
Dendritic region receiving stimulation
Optical recordings have previously shown that small subthreshold electrical stimulation of Schaffer collateral axons (using an electrode with a ∼5 μm tip located ∼30 μm from the dendrite) can produce synaptic input that arrives onto a CA1 pyramidal dendrite at least 50 μm distant to a point on the apical trunk that is perpendicular to the stimulating electrode (Magee et al. 1995; Magee & Johnston, 1997). Given these observations it is highly likely that a greater than 100 μm length of the apical dendrite would receive tetanized synaptic input during the large amplitude suprathreshold electrical stimulation.
Statistics
Numeric values are given as means ± standard error of the mean (s.e.m.). Error bars in the figures indicate standard error of the mean and are plotted only if they exceed the size of the symbol. Statistical significance was examined using one-way ANOVA followed by Tukey's post hoc test at the 5% and 1% level of confidence.
Results
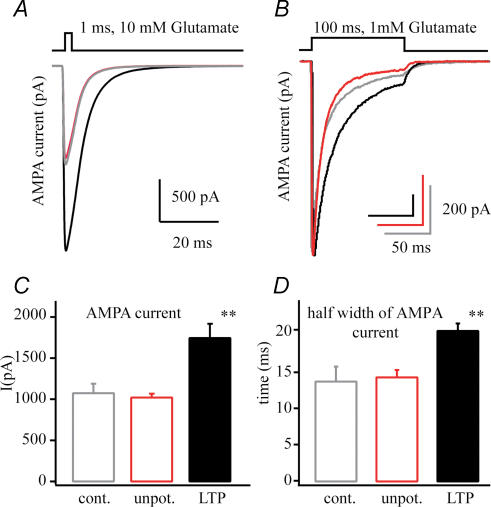
In our experiments we have investigated the impact of LTP induction on the properties of AMPA receptors on CA1 pyramidal neurones in the acute adult rat hippocampal slice. We excised outside-out patches from a region of the distal apical dendrite (∼200 μm distal from soma) that presumably received incoming synaptic activity 20–30 min following a 100 Hz, 1 s tetanic stimulation (Bannister & Larkman, 1995; Magee & Johnston, 1997; Megias et al. 2001). Glutamate (1 and 10 mm) was then rapidly applied to the outside-out patches and AMPA receptor properties (single-channel conductance, open probability, receptor-channel kinetics, transmitter affinity and receptor numbers) were quantified. These data are compared with patch data acquired in an ‘unpotentiated’ condition where NMDA receptor blockers were present to prevent synapse potentiation and with a control condition where no tetanic stimulation was given. We have found in patches pulled from potentiated cells that the AMPA receptor-mediated glutamate current was increased in amplitude by nearly 75% (1019 ± 47 pA versus 1742 ± 173 pA; n = 12, n = 11; P < 0.01) and that the current decay was slightly slowed compared to patches from the unpotentiated neurones (current half-width: control 13.8 ± 2.0 ms, n = 7; unpotentiated 14.6 ± 1.4 ms, n = 12; LTP 20.8 ± 1.0 ms; n = 11, P < 0.01, Fig. 2). A more detailed analysis follows.
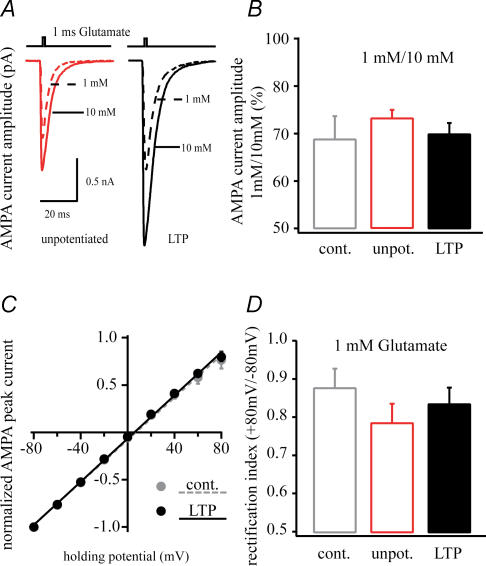
Figure 2. AMPA current changes following LTP.
A, representative average AMPA currents from patches pulled from a control (grey), an unpotentiated neurone (50 μm APV and 10 μm MK-801, red) and a potentiated neurone (100 Hz tetanus, black) showing the increase in current amplitude that selectively occurs following LTP induction. B, average AMPA currents evoked by 100 ms 1 mm glutamate application showing the increased current duration following potentiation. C, numerical representation of the AMPA current amplitude of patches excised from control (n = 7), unpotentiated (n = 12) and potentiated (n = 11) neurones. D, half-width of AMPA currents quantifying the significant increase of AMPA current duration followed by LTP (**P < 0.01).
AMPA channel number
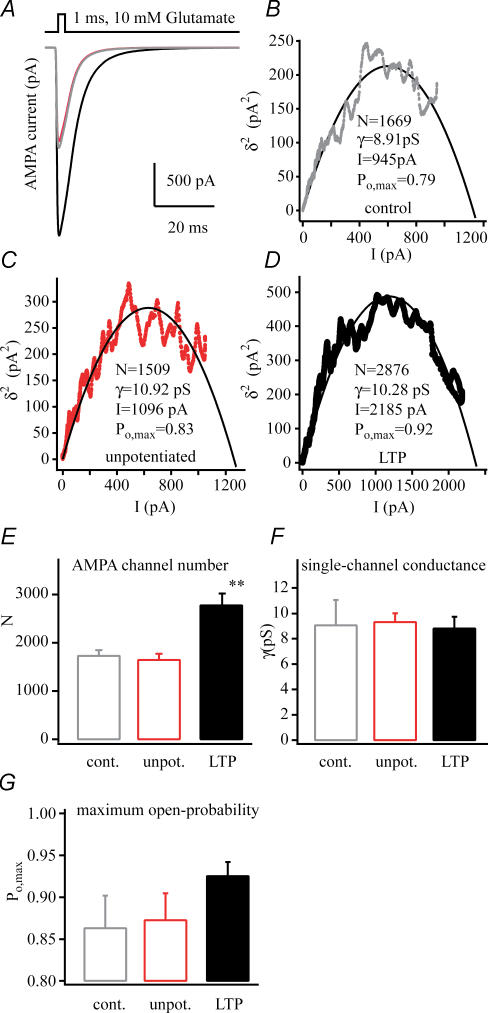
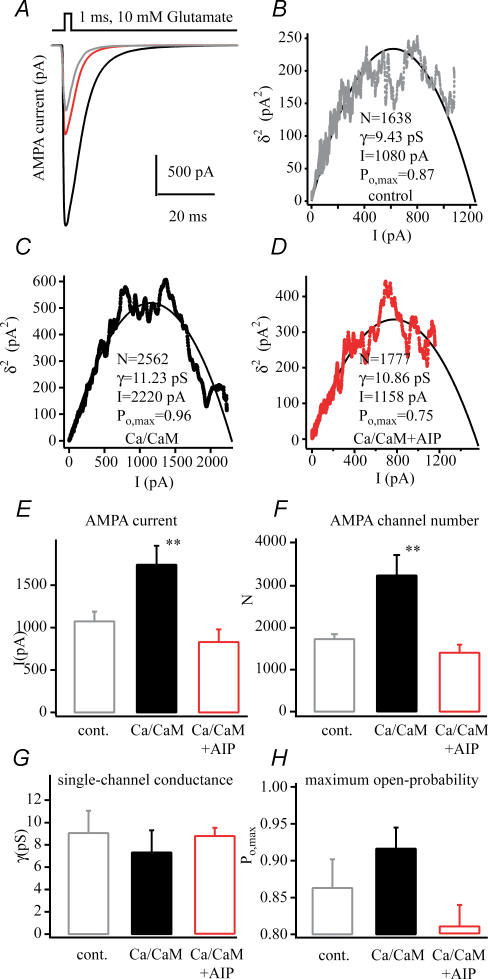
Sequential patch currents were submitted to a non-stationary fluctuation analysis (NSFA) to determine if the increased amplitude (Fig. 2) of the AMPA currents observed following LTP was due to either an increase in channel number or a change in single channel conductance (γ) or maximum probability of opening (Po,max). The NSFA of currents from potentiated cells (Fig. 3) showed that the number of AMPA receptors present in these patches was increased by 70% (1642 ± 126 versus 2771 ± 249; n = 12, n = 11; P < 0.01), while the single-channel conductance (9.3 ± 0.7 pS versus 8.8 ± 0.9 pS), and channel maximum open probability (0.87 ± 0.03 versus 0.92 ± 0.01) were insignificantly increased. These data indicate that the larger amplitude currents present in potentiated patches are likely to be almost exclusively due to an increase in channel numbers and not to any changes in γor Po,max.
Figure 3. AMPA channel biophysical properties following LTP.
A, representative average AMPA currents from patches pulled from a control (grey), an unpotentiated neurone (50 μm APV and 10 μm MK-801, red), and a potentiated neurone (100 Hz tetanus, black). B–D, plots of mean AMPA current versus variance that were used in non-stationary fluctuation analyses of the currents shown above. The number of channels in the patch (N), single channel conductance (γ), mean peak current amplitude (I) and maximum channel open probability (Po,max) calculated from the fit of the plots by a parabolic function are shown (see methods). Pooled data of AMPA channel numbers (E) of dendritic excised patches from control (n = 6), unpotentiated (n = 12) and potentiated cells (n = 11, **P < 0.01). F, single-channel conductance (γ) appears equal in every condition. G, the maximum open probability (Po,max) shows a slight but statistically insignificant increase in the potentiated cells. The results of non-stationary fluctuation analysis suggest the channel number changes instead of the channel properties during potentiation. All dendritic outside-out patches were excised ∼200 μm distal to the soma, 25–35 min after 100 Hz tetanic stimulation. Glutamate (1 ms, 10 mm) was used for maximal saturation of AMPA receptors for non-stationary fluctuation analysis.
Other channel properties
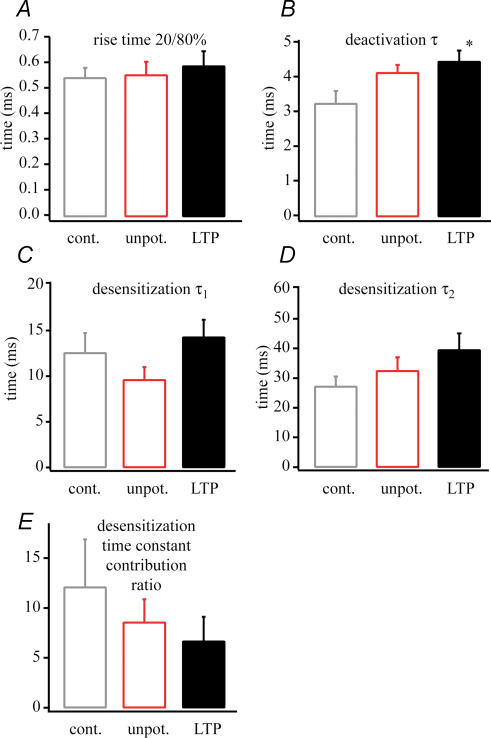
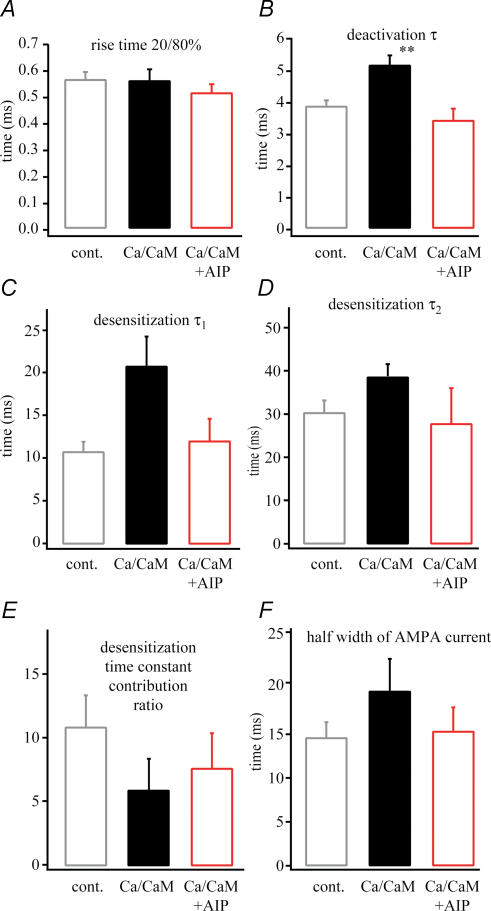
A more detailed analysis of the time course of AMPA receptor-mediated glutamate currents revealed subtle changes in decay kinetics following potentiation that may underlie the observed current prolongation. To measure the rise and the deactivation kinetics of the AMPA current a 1 ms pulse of 1 mm glutamate was applied, while a 100 ms pulse was delivered for the measurement of desensitization kinetics. The rise time constants were determined from an exponential fit of the AMPA current rising phase while deactivation time constants were determined from an exponential fit of the decay phase of the same currents. The desensitization time constants were determined from a double exponential fit of the decay phase of the AMPA currents produced by the 100 ms glutamate application. Current rise-times after potentiation (0.58 ± 0.05; n = 11) were identical with control (0.54 ± 0.04 ms; n = 7) and unpotentiated (0.55 ± 0.05 ms; n = 12) results. On the other hand, the time constant of channel deactivation was slightly increased following LTP induction (control 3.2 ± 0.3 ms, n = 7; unpotentiated 4.1 ± 0.2 ms, n = 10; potentiated 4.4 ± 0.3 ms, n = 11, P < 0.05 versus control). The fast (τ1) and slow (τ2) desensitization time constants were not significantly increased after LTP (τ1: control 12.5 ± 2.2 ms, n = 7; unpotentiated 9.5 ± 1.4 ms, n = 10; potentiated 14.2 ± 1.9 ms, n = 11) (τ2: control 27.1 ± 3.4 ms, n = 7; unpotentiated 32.4 ± 4.6 ms, n = 10; potentiated 39.4 ± 5.6 ms, n = 11). Also, the ratio of fast to slow desensitization components was not significantly decreased following LTP induction (control 12.0 ± 4.8, n = 7; unpotentiated 8.5 ± 2.3, n = 10; potentiated 6.6 ± 2.4, n = 11) (Fig. 4). We interpret these data to indicate that, although most of the changes in the individual kinetic parameters were in themselves statistically insignificant, the slight slowing observed in all of the measures of channel decay combine to produce a more prolonged AMPA current duration (half-width) following LTP induction.
Figure 4. AMPA current kinetic changes following LTP.
The kinetic properties of the AMPA receptor currents. A, rise time. B, deactivation (measured at 1 ms, 1 mm glutamate application) time constant (τ). C, fast desensitization time constant (τ1). D, slow desensitization (measured at 100 ms, 1 mm glutamate application) time constant (τ2). E, the desensitization time constant contribution ratio. A slowing in kinetics can be seen during potentiation (n = 11, LTP versus control, *P < 0.05).
AMPA channel rectification properties were also compared under control conditions and following LTP induction. A plot of the AMPA receptor-mediated glutamate current amplitude versus holding potential (I–V) (from −80 mV to +80 mV, Fig. 5) shows that LTP induction did not produce any changes in the rectification properties of the AMPA receptors in our patches. Finally, the ability of potentiation induction to produce a change in the glutamate affinity of the AMPA receptors in our patches was examined. To do so the ratio of 1 mm to 10 mm glutamate peak current amplitudes were compared between the three groups and was found to be unaltered. These data suggest that the glutamate affinity of these receptors did not change for this range of concentrations (Fig. 5). Together the data presented thus far suggest that the increase in AMPA receptor-mediated current in patches from neurones showing synaptic potentiation is the result of an increase in AMPA receptor density in these synaptically active regions. It also appears that, other than a slight slowing of channel decay rates, there are no changes in the single-channel properties of the AMPA receptors responsible for the increase in patch current.
Figure 5. Potentiation does not change receptor affinity or channel rectification properties.
A, representative traces of 1 mm and 10 mm glutamate- (1 ms pulse) induced AMPA receptor-mediated currents of excised dendritic patch from control (n = 6), unpotentiated (n = 12) and potentiated (n = 11) cells. The ratio of 1 mm and 10 mm glutamate current (B) are similar in all cases suggesting that the glutamate affinity of AMPA receptors did not change during potentiation. C, the current–voltage relationship of control (filled grey circle) and LTP (filled black circle). The unpotentiated cells were similar (data not shown). D, the rectification index (peak current at +80/–80 mV) does not show any differences between these states.
CaMK-II activation increases AMPA currents
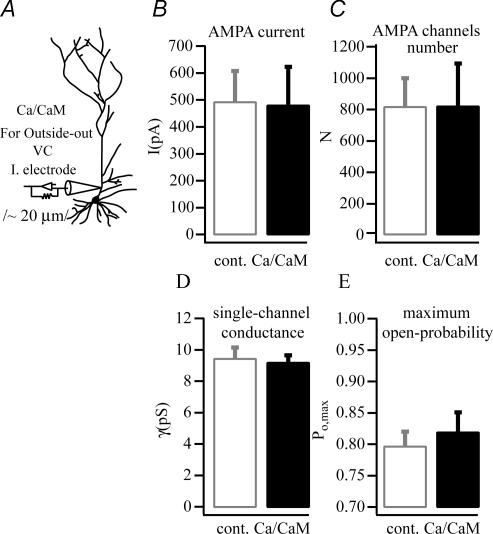
Earlier studies have suggested that calcium-activated calmodulin kinase II (CaMK-II) mimics electrically evoked potentiation and that many of the changes in synaptic efficacy, including AMPA receptor single channel conductance and receptor cycling associated with LTP, are the result of increased CaMK-II activation (Wang & Kelly, 1995; Hayashi et al. 2000). We thus filled CA1 dendrites with high free Ca2+ and calmodulin (CaM) (4: 1 ratio; Tao-Cheng et al. 2001) plus calcineurin autophosphorylation inhibitory peptide (CaN-AIP) (see Wang & Kelly, 1997) for 5–15 min to increase CaMK-II activity. Following this time, patches were excised, glutamate was rapidly applied as above and the AMPA receptor-mediated currents were analysed as in the previous experiments. As with electrical stimulation these patch currents were larger in amplitude (1039 ± 43 pA versus 1765 ± 124 pA; n = 7, n = 10) because the number of AMPA receptors in the patches was increased (1726 ± 120 versus 3246 ± 454; n = 7, n = 10) while γ(9.1 ± 0.7 pS versus 7.2 ± 0.4 pS) and Po,max (0.86 ± 0.03 versus 0.91 ± 0.03) were unaltered (see Fig. 6).
Figure 6. AMPA channel biophysical property changes following CaMK-II activation.
A, representative average AMPA currents from patches pulled from a control (grey), a CaMK-II-activated neurone (20 min Ca2+/CaM, black) and AIP-blocked CaMK-II-activated neurone (20 min Ca2+/CaM + AIP, red). B–D, plots of mean AMPA current versus variance that were used in non-stationary fluctuation analyses of the currents shown above. The number of channels in the patch (N), single channel conductance (γ), mean peak current amplitude (I) and maximum channel open probability (Po,max) calculated from the fit of the plots by a parabolic function are shown (see methods). E and F, pooled data of increased AMPA currents (E) and AMPA channel numbers (F) of dendritic excised patches from control (n = 7), CaMK-II (n = 10), CaMK-II + AIP (n = 6). G, single-channel conductance (γ) appears equal in every condition. H, the maximum open probability (Po,max) shows an insignificant increase in the CaMK-II-activated cells. The results of non-stationary fluctuation analysis suggest the channel number changes instead of the channel properties during potentiation. All dendritic outside-out patches were excised ∼200 μm distal to the soma, 20–30 min Ca2+/CaM intracellular application. Glutamate (1 ms, 10 mm) was used for maximal saturation of AMPA receptors for non-stationary fluctuation analysis (**P < 0.01).
Additionally, the increase in CaMK-II activity produced a similar AMPA current kinetic profile as electrical stimulation. Here, we again observed a slight but significant change in channel deactivation, but only insignificant slowing of the various desensitization parameters (see Fig. 7). Finally, inclusion of 1 μm autophosphorylation inhibiting protein (AIP, n = 6; Ishida et al. 1995) in the pipette prevented all of the alterations produced by CaMK-II activation (see Figs 6 and 7). These data indicate that, as in other studies, CaMK-II activation can mimic the effects of electrically evoked synaptic potentiation. They furthermore, suggest that native AMPA receptors in hippocampal CA1 pyramidal neurones do not respond to increased CaMK-II activity with an increase in γbut instead with a increase in channel density.
Figure 7. AMPA current kinetic changes following CaMKII activation.
The kinetic properties of the AMPA receptor current. A, rise time. B, deactivation (measured at 1 ms, 1 mm glutamate application) time constant (τ). C, fast desensitization time constant (τ1). D, slow desensitization time constant (τ2). E, the desensitization (measured at 100 ms, 1 mm glutamate application) time constant contribution ratio of dendritic excised patches from control (n = 7), CaMK-II (n = 10), CaMK-II + AIP (n = 6). A slowing in kinetics can be seen during Ca/CaM treatment. AIP application with Ca/CaM shows the same values as in control. F, half-width of AMPA currents demonstrate the increase of AMPA current decay followed by CaMK-II activation (**P < 0.01).
Dendritic region showing AMPA current potentiation
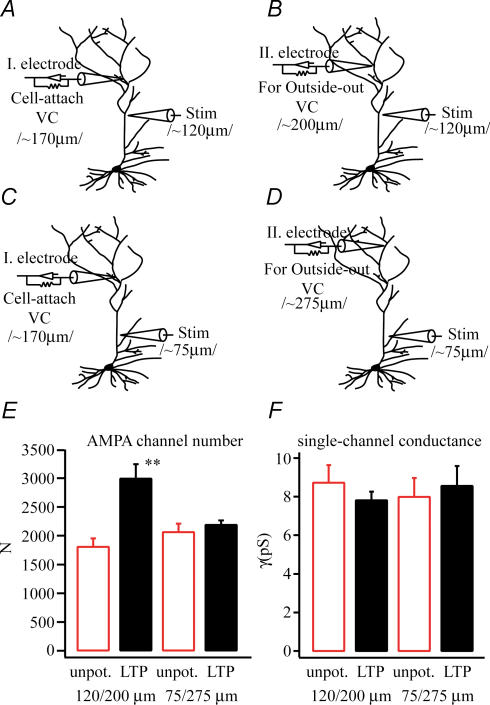
Next we wanted to monitor the spatial extent of the increase in patch AMPA currents in the apical dendrite following the strong electrical stimulation we were using. To do so we excised outside-out patches at different distances from the stimulation and recording sites (see Fig. 8). In all cases we produced a level of synaptic activation that was similar (number of action potentials evoked: proximal stimulation 3.3 ± 0.8 versus distal stimulation 3.5 ± 1.6) and equally capable of inducing LTP in the activated synapses (shift in spike latency: proximal stimulation before tetanus 25.0 ± 0.6 ms, after tetanus 14.2 ± 2.0 ms; distal stimulation before tetanus 22.5 ± 4.3 ms, after tetanus 15.2 ± 0.4 ms; n = 3, n = 6). Spike latency is the measured time between the first test stimulus and the first evoked spike. Following tetanic stimulation we could detect a large increase in AMPA channel numbers (1804 ± 146 versus 2988 ± 252; n = 4; P < 0.01) when the patch was pulled from a region of the apical dendrite that was up to 80 μm away from the stimulation site (patch site: 200 ± 20 μm from soma; stimulation site: 120 ± 20 μm from soma). We did not, however, observe any such changes (2060 ± 148 versus 2185 ± 80; n = 3; P > 0.05) when the patch was pulled from greater distances from the stimulation site (patch site: 275 ± 20 μm; stimulation site: 75 ± 20 μm). These data suggest that while the potentiation did affect a large part of the dendrite (∼150 μm), it did not affect all of the arborization (Fig. 8). In fact we suspect that the dendritic region affected by the stimulation is not much larger than the region receiving synaptic stimulation (see methods), especially considering the less than precise localization of LTP induction (Engert & Bonhoeffer, 1997).
Figure 8. Regionalization of AMPA receptor changes.
A and B, the experimental configuration for pulling the outside-out patch near the site of the LTP induction (80–100 μm distal to the stimulation site). C and D, the experimental configuration for pulling patches at a site that was distant to the stimulation site (∼200 μm distant). Pooled data (LTP n = 4, unpotentiated n = 3) show that AMPA channel number (E) is increased when the patch was pulled near the site of stimulation but not when it was far away. There were no changes in single-channel conductance under any conditions.
Are synapses and or dendritic spines required for the LTP-associated increases in AMPA receptor currents? To investigate this question we activated CaMK-II in the proximal dendritic region (only ∼20 μm away from soma) where there are virtually no excitatory synapses (Bannister & Larkman, 1995; Megias et al. 2001). After 5–15 min filling this region of the dendrite with our high Ca/CaM-containing solution, outside-out patches were excised and the standard AMPA current analyses produced. In contrast to what was observed above at synaptically dense regions of the dendrite, we did not see any increases in AMPA current in the patches pulled from this region of the dendrite (491 ± 116 pA versus 479 ± 144 pA; n = 9 and 7, respectively). Furthermore, NSFA showed that there was no increase in AMPA channel number or any alteration in single channel properties (Fig. 9). These observations strongly suggest that the underlying machinery of increasing AMPA channel number in the sub- and/or extrasynaptic membrane must be related somehow to the special synaptic or spine structures.
Figure 9. Synaptic structures are required for the increase in AMPA channel numbers caused by CaMK-II activation.
A, intracellular application of Ca/CaM (n = 7) at the proximal part of the apical dendrites (∼20 μm away from soma) did not have an impact on (control n = 9) any properties of excised AMPA receptors, B–E), suggesting the localization dependence of phosphorylation governs receptor insertion or lateral transportation.
Discussion
Summary
In recent years many attempts have been made to clarify the mechanisms by which synaptic efficacy is increased following the induction of LTP. In this study we have characterized the changes in functional dendritic AMPA receptors in hippocampal CA1 pyramidal neurones from adult rats that are produced by, or at least coincide with, LTP induction. The main observations are as follows. (1) Tetanus-induced potentiation significantly increased the amplitude of AMPA receptor-mediated glutamate currents in outside-out patches pulled from the apical dendrites of adult rats. (2) This increase is due to an increase in the number of AMPA receptors in the patches. (3) AMPA currents from potentiated dendritic regions also decay more slowly, but there was no single kinetic parameter responsible for this slowing. (4) Other properties of AMPA receptors such as single-channel conductance, channel rectification and glutamate affinity do not show any alterations following potentiation. (5) Increases in intracellular CaMK-II activity mimic the changes in AMPA receptor properties that were observed following tetanus-induced potentiation. (6) The dendritic region affected by the synaptic stimulation is not much greater than the area receiving input and requires the presence of excitatory synapses. These data extend the observations of others groups that have previously reported an increase in postsynaptic glutamate responsivity following LTP induction or CaMKII activation (Shirke & Malinow, 1997; Montgomery et al. 2001). We interpret our data to indicate that the stimuli used here produce an increased delivery of AMPA receptors to synaptically active regions of the apical dendrite without inducing any significant changes in their basic biophysical properties. We suspect that the primarily extrasynaptic receptors sampled by our patches (see below) will ultimately arrive at activated PSDs via the regulated process of receptor cycling between the synaptic and extrasynaptic pools of AMPA receptors.
Origin of AMPA receptors
In our previous work (Andrásfalvy et al. 2003) we made an effort to determine the origin of the excised receptors on outside-out patches, using GluR1−/− mice. In these mice there is a nearly complete (∼97%) loss of AMPA receptor currents in outside-out patches from both spiny (synaptic) and non-spiny (non-synaptic) regions, while purely synaptic currents (mEPSCs) are reduced only ∼30% at proximal locations. That AMPA receptor patch currents are equally reduced in both spiny and non-spiny regions of GluR1−/− mice suggests that either the receptors in outside-out patches are from extrasynaptic regions or that synaptic and extrasynaptic currents were both equally reduced in these mice. However, the observation that synaptic currents (mEPSCs) are, in fact, much less reduced than outside-out patch currents (30% versus 97% reduction), leaves us to conclude that the AMPA receptors in our outside-out patches are mostly from extrasynaptic regions. These extrasynaptic regions could be either the dendrite trunk or the non-synaptic regions of spines (Andrásfalvy et al. 2003). A probable explanation for this result is that the very tight connection between the postsynaptic density (PSD) and the active zone (AZ) membrane at the synapse proper does not allow the receptors located there to be readily pulled into outside-out patches, while other receptors nearby the PSD are available. We thus conclude that LTP induction in CA1 pyramidal neurones leads to an increase in the extrasynaptic pool of AMPA receptors in dendritic regions near activated synapses.
AMPA receptor cycling and delivery during synaptic plasticity
Previous studies have demonstrated differently regulated incorporation of subunit-specific AMPA receptors into synapses as one underlying mechanism of various synaptic plasticity phenomena (Heynen et al. 2000; Shi et al. 2001; Kim et al. 2001; Lu et al. 2001; Passafaro et al. 2001; Piccini & Malinow, 2002; Grosshans et al. 2002). In these schemes, GluR2/3 receptors are thought to be delivered directly into the synapse and continuously recycled within the synapse through exo- and endocytosis (Shi et al. 2001; Kim et al. 2001; Meng et al. 2003). GluR1/2 receptors are thought to initially insert into the extrasynaptic membrane and then move laterally into the postsynaptic density depending on the activity pattern of the synapse (Zamanillo et al. 1999; Chen et al. 2000; Mack et al. 2001; Shi et al. 2001; Lu et al. 2001; Passafaro et al. 2001; Piccini & Malinow, 2002; Andrásfalvy et al. 2003; Meng et al. 2003; but see Hoffman et al. 2002). Thus, a baseline of synaptic efficacy is thought to be established by GluR2/3 receptors, with the regulated delivery of GluR1/2 receptors either directly or indirectly increasing synaptic weight depending upon the associative history of the synapse.
In this work, we have shown that the decay times of AMPA receptor-mediated glutamate currents have increased and that Po,max did not decrease following synaptic potentiation. Based on our previous observations (Andrásfalvy et al. 2003) that AMPA currents from GluR1-deficient mice (probably GluR2/3) decay faster and have a lower Po,max than AMPA currents from wild-type mice (probably GluR1/2), we suggest that the increased amount of AMPA receptors present in the potentiated patches are mediated by GluR1/2 AMPA receptors. This concept meshes nicely with the idea that GluR1 containing AMPA receptors are delivered to the synapses in an activity-dependent manner.
In addition to this, other groups have reported that LTP induction or CaMKII activation results in an increase in AMPA receptor single channel conductance (Benke et al. 1998; Derkach et al. 1999). Such an LTP-induced increase in γ as found for synaptic receptors in CA1 pyramidal cells of juvenile rats (mEPSCs) (Benke et al. 1998). Our observations that there is an increase in the density of functional AMPA receptors in the extrasynaptic regions following LTP induction do not preclude additional changes in AMPA receptor properties once these channels reach the PSD. On the other hand, the CaMK-II phosphorylation study (Derkach et al. 1999) was performed using GluR1 homomeric receptors expressed in a HEK cell-line. Our data indicate that CaMK-II-dependent phosphorylation of native adult AMPA receptors (probably GluR1/2 heteromeres) does not produce the same effect on single channel conductance. Obviously this disparity may be due to the expression of different receptors in different systems (Wenthold et al. 1996). Additionally, the work of Hayashi et al. (2000) showed that the phosphorylation of GluR1(S831) is not crucial during LTP, while the mutation of GluR1(T887) could inhibit LTP by inhibiting GluR1 binding to the PDZ domain, reducing the transportation of those receptors to the synapse.
Exocytosis or cluster formation and lateral drift of extrasynaptic receptors into synapses
NMDA receptor trafficking through lateral diffusion in and out of synapses has been shown (Tovar & Westbrook, 2002). Furthermore, Choquet and colleagues (Tardin et al. 2003) have reported that half of the GluR2-containing synaptic receptors are mobile and this mobility is affected by pharmacological treatments that modify receptor accumulation at synapses (O'Brien et al. 1998). Their results suggested a dynamic equilibrium between the extrasynaptic and synaptic receptor pools, supporting the idea that the regions around synapses form a reserve pool zone and transition area, providing available receptors for recruitment at synapses.
Our previous work has also suggested that the number of AMPA receptors in excised outside-out patches (from mostly subsynaptic and extrasynaptic regions) is proportional to the synaptic receptor pool, lending support to the concept that any changes in the size of one pool will be manifested in the other (Andrásfalvy & Magee, 2001; Smith et al. 2003; Andrásfalvy et al. 2003). Therefore our data suggest that an increase in the number of functional AMPA receptors present in the reserve receptor pool (either through lateral diffusion or regulated exocytosis) is one important event that occurs following the induction of LTP in CA1 pyramidal neurones. We would also expect that these additional receptors would then be available to find their way, through interactions with transporting (stargazin, GRIP, etc.) and scaffolding/anchoring (actin, PSD, etc.) proteins (Lisman & Zhabotinsky, 2001; Lisman et al. 2002), to synapses that had been activated during the LTP inducing stimulation.
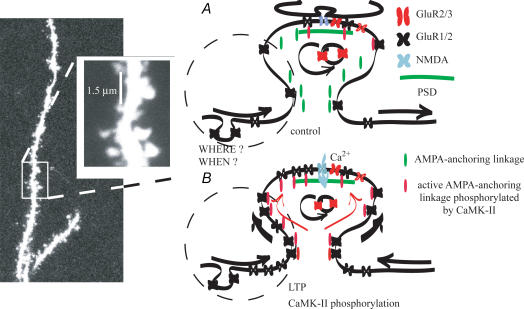
Demonstrating our conclusion we present a simple model here (Fig. 10). Under resting conditions the GluR2/3 AMPA receptors are continuously exo- and endocytosed directly into the PSD. Most of the GluR1/2 AMPA receptors are mobile in the extra- and subsynaptic area and continuously move in and out of the PSD by lateral drift, but are not anchored there. When Ca2+ flows through NMDA receptor channels during depolarization, many phosphorylation cascades (e.g. CaMK-II) that activate transporting and anchoring proteins are initiated (AMPA-anchoring linkage). AMPA-anchoring linkages that are set together during phosphorylation move the mobile GluR1/2 receptors towards the PSD where they are then anchored. Lateral drift of more extrasynaptic receptors makes more receptors available in the sub- or juxtasynaptic region. The number of GluR1/2 receptors in this region mirrors the increase in synaptic AMPA receptors.
Figure 10. Model of AMPA receptor redistribution or transportation into the subsynaptic and synaptic area during LTP induction in adult hippocampal neurones.
The picture shows a distal apical dentritic trunk and on the magnified insert the scale bar indicates the average tip aperture of our pipettes. A, the dashed circle indicates the putative outside-out patch membrane area containing extrasynaptic and sub- or juxtasynaptic AMPA receptors. Under resting conditions the GluR2/3 (red) AMPA receptors are continuously exo- and endocytosed directly into the PSD (green line). Most of the GluR1/2 AMPA receptors (black) are mobile in the extra- and subsynaptic area and continuously moving in and out of the PSD by lateral drift, but are not anchored there. B, when Ca2+ flows through NMDA ion channels (blue) during depolarization, many phosphorylation cascades (e.g. CaMK-II) are initiated, which activate transporting and anchoring proteins (AMPA-anchoring linkage). AMPA-anchoring linkages that are set together during phosphorylation move the mobile GluR1/2 receptors towards the PSD where they are then anchored. Lateral drift of more extrasynaptic receptors makes more receptors available in the sub- or juxtasynaptic region. The number of GluR1/2 receptors in this region mirrors the increase in synaptic AMPA receptors.
Acknowledgments
We are grateful to Dr J. Haycock for the AIP. This work was supported by National Institutes of Health grants NS35865 and NS39458.
References
- Andrásfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrásfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol. 2003;552:35–45. doi: 10.1113/jphysiol.2003.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister NJ, Larkman AU. Dendritic morphology of CA1 pyramidal neurons from the rat hippocampus. II. Spine distribution. J Comp Neurol. 1995;360:161–171. doi: 10.1002/cne.903600112. [DOI] [PubMed] [Google Scholar]
- Benke TA, Lüthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Synapse specificity of long-term potentiation breaks down at short distances. Nature. 1997;388:279–284. doi: 10.1038/40870. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee JC, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7:1–10. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28:527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Sprengel R, Sakman B. Molecular dissection of hippocampal theta-burst pairing potentiation. Proc Natl Acad Sci U S A. 2002;99:7740–7745. doi: 10.1073/pnas.092157999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lu W-Y, Man H-Y, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors includes membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Mack V, Burnashev N, Kaiser KMM, Rozov A, Jensen V, Hvalby Ø, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in GluR-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci. 1998;18:7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Christofi G, Christie BR, Johnston D. Subthreshold Ca2+ influx mediated through LVA calcium channels in the dendrites of CA1 pyramidal neurons. J Neurophysiol. 1995;74:1335–1342. doi: 10.1152/jn.1995.74.3.1335. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–223. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102:527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Pavlidis P, Madison DV. Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron. 2001;29:691–701. doi: 10.1016/s0896-6273(01)00244-6. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nature Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Piccini A, Malinow R. Critical postsynaptic density 95/disc large/zonula occludens-1 interactions by glutamate receptor 1 (GluR1) and GluR2 required at different subcellular sites. J Neurosci. 2002;22:5387–5392. doi: 10.1523/JNEUROSCI.22-13-05387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by alpha-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shirke AM, Malinow R. Mechanisms of potentiation by calcium-calmodulin kinase II of postsynaptic sensitivity in rat hippocampal CA1 neurons. J Neurophysiol. 1997;78:2682–2692. doi: 10.1152/jn.1997.78.5.2682. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. The variance of sodium current fluctuations at the node of ranvier. J Physiol. 1980;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GCR, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Vinade L, Pozzo-Miller LD, Reese TS, Dosemeci A. Calcium/calmodulin-dependent protein kinase II clusters in adult rat hippocampal slices. Neurosci. 2001;115:435–440. doi: 10.1016/s0306-4522(02)00451-7. [DOI] [PubMed] [Google Scholar]
- Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Postsynaptic injection of Ca2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron. 1995;15:443–452. doi: 10.1016/0896-6273(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Wang JH, Kelly PT. Postsynaptic calcineurin activity downregulates synaptic transmission by weakening intracellular Ca2+ signaling mechanisms in hippocampal CA1 neurons. J Neurosci. 1997;17:4600–4611. doi: 10.1523/JNEUROSCI.17-12-04600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos JII, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby Ø, Jensen V, Burnashev N, Rozov A, Kaiser KMM, Köster HJ, Borchardt T, Worley P, Lübke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]