Abstract
Recent studies indicate that cholecystokinin (CCK) and serotonin (5-hydroxytryptamine, 5-HT) act via vagal afferent fibres to mediate gastrointestinal functions. In the present study, we characterized the interaction between CCK and 5-HT in the vagal primary afferent neurones. Single neuronal discharges of vagal primary afferent neurones innervating the duodenum were recorded from rat nodose ganglia. Two groups of nodose ganglia neurones were identified: group A neurones responded to intra-arterial injection of low doses of cholecystokinin octapeptide (CCK-8; 10–60 pmol); group B neurones responded only to high doses of CCK-8 (120–240 pmol), and were also activated by duodenal distention. CCK-JMV-180, which acts as an agonist in high-affinity states and as an antagonist in low-affinity states, dose dependently stimulated group A neurones, but inhibited the effect of the high doses of CCK-8 on group B neurones. Duodenal perfusion of 5-HT evoked dose-dependent increases in nodose neuronal discharges. Some neurones that responded to 5-HT showed no response to either high or low doses of CCK-8. A separate group of nodose neurones that possessed high-affinity CCK type A (CCK-A) receptors also responded to luminal infusion of 5-HT. Further, a subthreshold dose of CCK-8 (i.e. 5 pmol) produced no measurable electrophysiological effects but it augmented the neuronal responses to 5-HT. This potentiation effect of CCK-8 was eliminated by CR 1409. From these results we concluded that the vagal nodose ganglion contains neurones that may possess only high- or low-affinity CCK-A receptors or 5-HT3 receptors. Some neurones that express high-affinity CCK-A receptors also express 5-HT3 receptors. Pre-exposure to luminal 5-HT may augment the subsequent response to a subthreshold dose of CCK.
The vagal nerve conveys primary afferent information from the intestinal mucosa to the brainstem. Activation of vagal afferent fibres results in inhibition of food intake, gastric emptying, and pancreatic secretion (Ritter et al. 1992; Li & Owyang, 1993, 1996; Schwartz & Moran, 1998). Electrophysiological studies indicate that exogenous cholecystokinin (CCK) stimulates vagal afferent fibres or the brainstem neurones (Davison & Clark, 1988; Richards et al. 1996). In a previous study using the nodose ganglia recording technique, we showed that CCK acted on both high- and low-affinity CCK-A receptors present on distinct vagal afferent fibres. The vagal CCK receptor field includes the regions innervated by gastric, coeliac and hepatic vagal branches (Li et al. 1999).
It has been demonstrated that intestinal perfusion of solutions with osmotic pressures ranging from 4 to 1100 mOsm, or perfusion of carbohydrates, increased vagal afferent firings (Mei & Garnier, 1986). Investigators have also reported that serotonin (5-hydroxytryptamine, 5-HT) increased the discharge of vagal afferent fibres from the stomach and proximal intestine of the ferret. We (Zhu et al. 2001), and others (Andrews & Davison, 1990; Blackshaw & Grundy, 1993), and have provided electrophysiological evidence that endogenously released 5-HT plays a major role in the signal transmission evoked by luminal factors to stimulate vagal nodose neurones.
Thus, endogenously released CCK and 5-HT are potent stimuli of vagal primary afferent neurones (Li et al. 1999; Zhu et al. 2001). Both responses are completely blocked by acute vagotomy and luminal anaesthetic, which suggests that both CCK and 5-HT may activate vagal afferent neurones via a paracrine or endocrine action after release from the intestinal mucosa. 5-HT and CCK are both implicated in pancreatic secretion, in the stimulation of gastrointestinal reflexes, and in the modulation of feeding behaviour. 5-HT is thought to play a role in visceral nociceptive mechanisms (Emeran & Gebhart, 1994; Bueno et al. 1997). In addition, 5-HT is involved in the regulation of motility and sensation in the gut (Grider et al. 1998; De Ponti & Tonini, 2001).
We have shown that, similar to CCK, non-CCK-mediated luminal stimuli such as osmolality, disaccharides, and mechanical stimulation, induce 5-HT release from intestinal enterochromaffin cells, which in turn activate 5-HT3 receptors on vagal afferent fibres to mediate luminal factor-stimulated pancreatic enzyme secretion (Li et al. 2000). In this manner, 5-HT acts as a paracrine substance to stimulate pancreatic secretion via a vagal cholinergic pathway (Li et al. 2001). Further, we have shown that infusion of a subthreshold dose of CCK potentiates vagal–vagal reflex-mediated pancreatic secretion stimulated by luminal 5-HT-dependent factors (Li & Owyang, 1996), which suggests that CCK and 5-HT may act synergistically to stimulate the vagal afferent pathway. Both CCK and 5-HT play an important role in the mediation of pancreatic enzyme secretion (Li & Owyang, 1993; Li et al. 2000, 2001). Together they account for most if not all postprandial pancreatic enzyme secretion. However, little is known about CCK and 5-HT interaction in vagal primary afferent neurones. We hypothesize that CCK and 5-HT may act separately on the nodose ganglion but they may also act synergistically to stimulate a specific population of vagal primary afferent neurones. In the current investigation, we performed electrophysiological studies in rats to determine the pattern of distribution of high- and low-affinity CCK-A and 5-HT3 receptors on vagal nodose neurones, and we examined the nature of the interaction between CCK and 5-HT in vagal primary afferent neurones.
Methods
Materials
5-HT, maltose and protease were purchased from Sigma-Aldrich (St Louis, MO). Cholecystokinin octapeptide (CCK-8, CR 1409 and L-365 260 were purchased from Research Biochemical International (Natick, MA), granisetron was purchased from GlaxoSmithKline Pharmaceuticals (Philadelphia, PA) and CCK-JMV-180 was purchased from Research Plus (Manasquan, NJ).
Animal preparation
Experiments were performed on adult male Sprague-Dawley rats weighing 270–350 g that had been given free access to food and water. The animals were anaesthetized with a mixture of xylazine and ketamine (13 and 87 mg (kg body weight)−1, respectively). Supplemental doses of the anaesthetic agents were administered as needed to maintain a deep level of anaesthesia and muscle relaxation (supplemental doses of one-quarter of the initial dose were given every hour throughout the experiment or when a 20% increase in the rat's heart rate (50–60 beats min−1) above the level following the initial dose of anesthetic was observed). To prevent any undesirable movements, neuromuscular block was induced with pancuronium bromide (initial dose, 0.1 mg kg−1, i.v.; supplementary dose, 0.05 mg kg−1 h−1). The animals were ventilated with a respirator – a tracheal tube permitted artificial ventilation with room air (75–85 strokes min−1, 3.5–4.0 cm3 tidal volumes). A midline abdominal incision exposed the abdominal vagus, the stomach and the duodenum. Stimulation of the subdiaphragmatic vagus nerve was accomplished by placing a pair of Teflon-coated, pure gold wire electrodes (outside diameter, 76 μm) around the anterior and posterior trunks, about 2–3 cm above the gastroesophageal junction and above the accessory and coeliac branches of the vagus nerve. These stimulating electrodes were loosely sutured to the oesophagus to limit displacement. At the end of each experiment, an overdose of anaesthetic was administered to kill the animals. All experimental procedures were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Recording of single nodose neuronal activity
Rats were placed in a small animal stereotaxic frame (Kopf). Body temperature was maintained with a special heating pad. The right nodose ganglion was exposed by a short dorsal approach. Using an operating microscope, the ganglion sheath was removed and separated from the adjacent cervical sympathetic trunk and carotid artery. Protease (type XIV, 0.3 mg ml−1) was applied to the ganglion for 15 min. The recording microelectrodes were pulled from glass capillaries (A-M Systems, Everett,WA) using a micropipette puller and microelectrode beveller to obtain tips between 0.08 and 0.1 μm in diameter with a resistance of 50–70 MΩ. The bevelled glass micropipette filled with 1.0 m KCl was lowered into the nodose ganglion. Once a nodose ganglion neurone activated by the electrical vagal stimulation was identified, the response of that neurone to intra-arterial injection of CCK-8 and intraduodenal perfusion of test solutions was measured. A reference electrode was placed on a skin incision near the recording electrode. Recordings were only from gastrointestinal C-fibres, which were identified according to the following parameters measured in response to electrical stimulation of the abdominal vagus nerve: latency (60–80 ms); conduction distance between the stimulating electrode and the nodose ganglion (0.06 m); and conduction velocity (< 1.0 m s−1). Neuronal discharges recorded extracellularly were amplified by an A-M System high input-impedance pre-amplifier, monitored with an oscilloscope and audio monitor, displayed and stored on an IBM-compatible computer using Axon tape software. The basal discharge was monitored for 2 min to confirm the stability of the basal firing frequency. Consistent monitoring of each neurone was ensured by careful study of the firing pattern produced by each neurone and also by studying the amplitude and waveform of each spike.
Experimental design
Vagal nodose neuronal responses to CCK-8, CCK-JMV-180 and duodenal distention
CCK-8 and CCK-JMV-180 studies
The superior pancreaticoduodenal artery, which mainly supplies the duodenum (Hebel & Stromberg, 1976), was cannulated. The common hepatic artery was exposed and temporarily ligated. The gastroduodenal artery was punctured at its junction with the common hepatic artery. The catheter was inserted and threaded into the superior pancreaticoduodenal artery about 0.5 cm past the gastroepiploic artery (Cox, 1998). The catheter was fixed in place with a suture, and the puncture hole was sealed with cyanoacrylate glue. Once a stable single nodose neuronal recording was established, 1-min basal spontaneous firings were recorded. The neuronal responses to bolus injections of various doses of CCK (10, 30, 60, 120, and 240 pmol) into the superior pancreaticoduodenal artery were examined.
CCK-JMV-180 has been shown to interact with both classes of pancreatic CCK receptors, acting as an agonist in high-affinity states and as an antagonist in low-affinity states (Sato et al. 1989; Stark et al. 1989). We have previously shown that intravenous (i.v.) infusion of CCK-JMV-180 produces a dose-dependent increase in pancreatic protein secretion via a capsaicin-sensitive vagal afferent pathway (Li et al. 1997). In contrast, CCK-JMV-180 has also been shown to block the suppression of food intake by CCK; this is mediated by vagal low-affinity CCK-A receptors in rats (Weatherford et al. 1993). To identify the vagal CCK-A receptor affinity state that mediates CCK-evoked vagal afferent activities, we performed CCK-JMV-180 dose–response studies. CCK-JMV-180 (dissolved in dimethyl sulfoxide, Tween 80 and sterile 0.15 m NaCl; 8:1:1, v:v:v) was injected intra-arterially at doses of 1.0 and 4.0 μg. A maximum of five different doses of CCK-8 and CCK-JMV-180 or vehicle were delivered randomly in each neurone study. Recording was continued for 3 min, leaving 5 min between each dose. The neurones that did not respond to CCK-8 were discarded. A different neurone was identified after electrical stimulation of the subdiaphragmatic vagus nerve, and the experimental procedure was repeated. Responses to CCK were examined before and after i.v. administration of the CCK-A receptor antagonist CR 1409 and the CCK-B receptor antagonist L-365 260.
CCK receptor antagonist studies
Previous studies have shown that both CCK-A and CCK-B binding sites are transported towards the periphery of the rat vagus nerve (Julian & Lawrence, 1992). To determine whether the vagal afferent neuronal response evoked by intra-arterial injection of CCK-8 and CCK-JMV-180 involved either the CCK-A receptor or the CCK-B receptor, we examined the effects of the CCK-A receptor antagonist CR 1409 (10 mg kg−1 i.v. bolus injection, dissolved in 0.005 M NaOH) and the CCK-B receptor antagonist L-365 260 (3.5 μmol kg−1 h1, dissolved DMSO, Tween 80 and NaCl as above). It has been shown in the anaesthetized rat that the peptide antagonist CR 1409 prevented cerulein-stimulated pancreatic secretion in a dose-dependent manner. At a dose of 10 mg kg−1, CR 1409 abolished the pancreatic response to a near-maximum dose of cerulein (Niederau et al. 1989). Previously, we have shown that administration of CR 1409 but not the CCK-B antagonist L-365 260 completely abolished the nodose neuronal responses to endogenous CCK stimulation (Li et al. 1999). In addition, the effects of the CCK receptor antagonist L-364 718 (0.5 mg kg−1 i.v. bolus) or vehicle (4% DMSO/polysorbate 80) were examined in the current studies (Li & Owyang, 1996; Li et al. 1997).
In the anaesthetized rat, L-365 260 dose-dependently inhibited pentagastrin-induced stimulation of gastric acid secretion, with an ID50 of 2.5 μmol kg−1 h−1 (Attoub et al. 1998). Vagal nodose neuronal responses to CCK were monitored as previously described. The CCK-A and CCK-B receptor antagonists or vehicles were administered 10 min before CCK-8 administration.
Duodenal distention studies
Nodose ganglion neurones that respond to distention probably innervate the muscle layer, whereas those that respond to intestinal chemostimulation innervate the mucosa. To characterize the nodose neurones that were activated by CCK in the present study, we examined the responses of CCK-sensitive neurones to duodenal distention. Distention of the closed intestinal loop was achieved by closing the distal cannula and infusing 3 ml isotonic saline. Constant pressure distention was regulated by a distention control device. Intraduodenal pressure was monitored by connecting the distal catheter to a pressure transducer via a three-way stopcock. Animals with a cannula inserted into the duodenum but not distended (0 mmHg) served as control. Under our experimental conditions, infusion of 3 ml saline into a 20-cm isolated segment of intestine increased luminal pressure to 6–8 mmHg producing a non-noxious stimulation (Ness, 1988; Renehan et al. 1995).
Atropine and hexamethonium studies
High doses of CCK-8 may act on the vagal CCK-A receptors located on the muscle layer. Therefore, the vagal nodose neurones activated by CCK-8 may be stimulated by intestinal muscle contraction. To rule the possibility out that the nodose neuronal response to CCK was a result of an increase in muscle tone, secondary to the action of CCK on muscle itself, we performed an atropine study. Atropine (100 μg kg−1 h−1) was infused 10 min before injection of CCK. Similar studies were performed with hexamethonium (15 mg kg−1 bolus plus 7.5 mg kg−1 h−1 continuous infusion) to examine the role of pre-synaptic cholinergic neurones in the mediation of CCK-stimulated nodose neuronal firing.
Vagal nodose neuronal responses to CCK-8 plus CCK-JMV 180
In a separate study, we examined the effects of CCK-JMV-180, on the vagal neuronal firings induced by CCK-8. CCK-8 (various doses) and CCK-JMV-180 (4 μg) were infused simultaneously; and nodose recording was repeated as described above.
Vagal nodose neuronal responses to duodenal perfusion of 5-HT and intra-arterial injection of CCK-8
A 20-cm segment of small intestine, including the entire duodenum and the proximal jejunum, was isolated between two cannulas positioned at 4 cm (PE 60; i.d., 0.76 mm; o.d., 1.22 mm) and 24 cm (PE 190; i.d., 1.19 mm; o.d., 1.7 mm) from the pylorus. The cannulae were fixed in the intestine with sutures. After 1-min recordings at basal level, 5-HT (10−5 and 10−3 m, 1.5 ml) was administered intraduodenally at separate intervals. The distal cannula was kept open to permit drainage and to avoid an increase in intraluminal pressure. The two test solutions were delivered randomly over 1 min and then washed out using a syringe filled with isotonic NaCl. The recording of the nodose neurone continued for 3 min with a 10-min resting period between experiments. If a positive response to luminal stimuli was observed, that same neurone was used for CCK-8 dose–response studies as described above. The 5-HT3 antagonist granisetron (0.5 mg kg−1, i.v.) and the CCK-A antagonist CR 1409 were administered to a separate group of rats.
Interaction between CCK and luminal 5-HT
Because a group of neurones possessing high-affinity CCK-A receptors also responded to luminal infusion of 5-HT, we examined the nature of the interaction between CCK and 5-HT in vagal primary afferent neurones. Electrophysiological recordings of nodose neurones in response to intraduodenal perfusion of 5-HT and intra-arterial injection of CCK-8 were performed. After 1-min basal spontaneous firings were recorded, the neuronal responses to bolus injections of CCK-8 at doses of 5, 10 and 30 pmol were examined. When CCK-stimulated nodose neuronal responses were observed, those particular neurones were tested for their responsiveness to intraduodenal perfusion of 10−5 m 5-HT as described above. After rinsing the intestinal lumen with buffer solution, the discharge frequency always decreased. Neurones that exhibited an increased basal discharge frequency (> 4 impulses (20 s)−1) were not tested further. After a 5-min resting period, an intra-arterial injection of CCK-8 (5, 10 or 30 pmol) was administered as the duodenum was re-perfused with 5-HT. The effects of the CCK-A receptor antagonist CR-1409 were examined.
Data analysis
The single neuronal responses were examined using the Datapac software system 2000 (Run Technologies, Laguna Hills, CA) (Mei & Garnier, 1986). The pre-stimulus discharge frequency was assessed for 1 min to quantify the resting discharge. The discharge frequency after administration of CCK-8 or CCK-JMV-180 and after duodenal luminal perfusion of 5-HT was measured for 3 min. Time histograms were constructed for the 1-min period before the intestinal infusion of 5-HT and for the 2-min period after stimulation. The mean and standard deviation of nodose neuronal firing during the 30-s control period was compared with the maximal activity after the administration of the stimulus. A positive response was defined as an increase of 2 s.d.s from the mean in the maximal activity after the infusion of CCK-8, CCK-JMV-180 or luminal 5-HT, compared to the mean firing rate in the control period. The results were compared with those obtained after the pharmacological interventions. Results were expressed as means ± s.e.m. Significance tests were carried out using the appropriate Student paired or unpaired t test with the Newman-Keuls tests (InStat Biostatistics 2.01. Graphpad Software, Inc.) when multiple comparisons were made. P < 0.01 was considered statistically significant.
Results
Effects of CCK-8 and CCK-JMV-180 on nodose neuronal activity
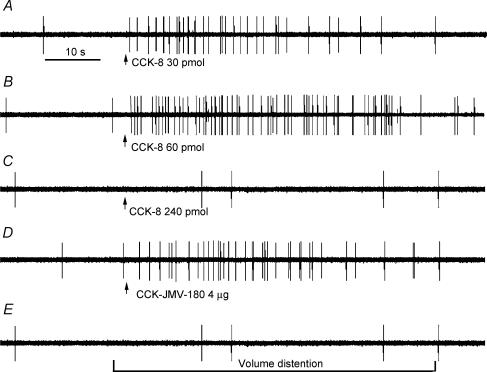
Data were collected from 141 recordings of single nodose ganglia neurones in 39 rats. All 141 neurones activated by electrical stimulation of the subdiaphragmatic vagus nerve were tested with intra-superior pancreaticoduodenal artery injection of CCK (5, 10, 30, 60, 120 and 240 pmol), and CCK-JMV-180 (1.0 and 4.0 μg). At most, five different doses of CCK-8 and two doses of CCK-JMV-180 were tested in each neurone. A total of 24 neurones (17%) responded to intra-arterial injections of CCK and CCK-JMV-180. All neurones displayed either silent or very low spontaneous activity (0–1.5 impulses (20 s)−1) before infusion of CCK-8 or CCK-JMV-180. Injection of CCK at dose of 5 pmol produced no measurable electrophysiological effect. In all cases, the threshold dose of CCK was greater than 5 pmol. The short latency of the CCK response (i.e. 1.0 ± 0.3 s) was probably the result of intra-arterial administration; CCK reached the intestinal mucosa soon after injection. Of the 24 neurones, two groups of vagal afferent neurones could be identified. Group A neurones (17 neurones) responded to low doses of CCK-8 (10, 30 and 60 pmol). The magnitude of the response was dependent on dose; the firing rate increased from 0 ± 0.5 to 6 ± 1, 15 ± 3 and 34 ± 6 impulses (20 s)−1, respectively (Fig. 1A and B). The response reached a plateau at 60 pmol CCK-8. The mean duration of the responses was 45.7 ± 2.7 s. Injection of CCK-8 at a dose of 120 pmol evoked a lesser response (4.5 impulses (20 s)−1) and at 240 pmol, this group of neurones failed to respond. An example of the original action potential record is presented in Fig. 1. In contrast, group B neurones (seven neurones) responded to high doses of CCK (120 and 240 pmol); the firing rate increased from basal the basal level (0 ± 1) to 21 ± 3 and 33 ± 5 impulses (20 s)−1, respectively. Low doses of CCK-8 had no effect on group B neurones. Only one neurone responded to both low and high doses of CCK. This particular neurone was also activated by duodenal distention.
Figure 1. Response of a nodose ganglia neurone to intra-superior pancreaticoduodenal artery infusions of CCK-8 and CCK-JMV-180 and to intestinal volume distention.
Intra-arterial infusion of CCK-8 at doses of 30 pmol (A) and 60 pmol (B) produced a dose-dependent increase in nodose neuronal discharge frequency. C, administration of CCK-8 at 240 pmol evoked only a slight increase in nodose neuronal firing. D, administration of CCK-JMV-180 produced a marked increase in vagal afferent discharge in the same neurone as in A, which indicates the presence high-affinity CCK-A receptors on a subgroup of vagal primary afferent neurones. However, this same neurone failed to respond to intestinal distention (E).
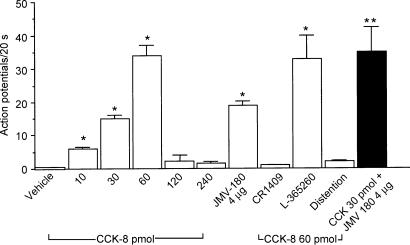
Administration of CCK-JMV-180 (1.0 and 4.0 μg) stimulated 13 of 24 neurones; nodose neuronal firings increased to 5.5 ± 0.2 and 19 ± 2 impulses (20 s)−1, respectively. All 13 neurones were activated by low doses of CCK. None of the neurones activated by high doses of CCK-8 responded to CCK-JMV-180. The mean discharge frequency of nodose ganglia neurones in response to various doses of CCK-8 is presented in Figs 2 and 3.
Figure 2. Discharges of group A nodose ganglia neurones in response to intra-arterial injections of CCK-8 and CCK-JMV-180 and to intestinal volume distention.
Of 141 neurones activated by electrical vagal stimulation, 24 neurones responded to intra-arterial injection of CCK-8 and CCK-JMV-180. Of these 24 neurones, two groups of neurones were identified. The discharge of group A neurones, which responded to low doses of CCK-8, were used for the analysis shown in this figure. Administration of CCK-8 at doses of 10, 30 and 60 pmol dose-dependently increased neuronal discharges. This group of neurones did not respond to high doses of CCK-8 (i.e. 120 and 240 pmol), and they failed to respond to intestinal volume distention. Twenty of 24 group A neurones were activated by intra-arterial injection of CCK-JMV-180. Administration of the CCK-A receptor antagonist CR 1409, but not the CCK-B receptor antagonist L-365,260, abolished nodose neuronal responses evoked by CCK-8 and CCK-JMV-180. Administration of CCK-JMV-180 in combination with CCK-8 (30 pmol) produced a further increase of neuronal firings compared with administration of CCK-8 alone. The filled bar represents the mean value of the neuronal discharges in response to CCK-8 (30 pmol) plus CCK-JMV-180. Values are means ± s.e.m. *P < 0.01 compared with vehicle; **P < 0.01 compared with CCK-8 at 30 pmol.
Figure 3. Discharges of group B nodose ganglia neurones in response to intra-arterial injections of CCK-8 and CCK-JMV-180 and to intestinal volume distention.
A, discharges of the group B neurones that responded to high doses of CCK-8 (i.e. 120 and 240 pmol). Neither administration of CCK at low doses nor administration of CCK-JMV-180 stimulated this group of neurones. In contrast to group A neurones, intestinal distention markedly increased the neuronal discharge frequency. Administration of atropine or hexamethonium however, had no effect on neuronal discharge stimulated by CCK-8. B, in a separate study of the 12 neurones, five were activated by high doses of CCK-8. This group of neurones did not respond to CCK-JMV-180. Administration of CCK-JMV-180 produced an 80% inhibition of neuronal responses stimulated by the 240 pmol dose of CCK-8. The filled bar represents the mean values of the neuronal discharges in response to CCK-8 (240 pmol) plus CCK-JMV-180. *P < 0.01 compared with vehicle; **P < 0.01 compared with CCK-8 at 240 pmol.
Of the 24 neurones activated by intra-arterial injection of CCK or CCK-JMV-180, five neurones were tested to determine whether stimulated response could be blocked by administration of the CCK-A receptor antagonist CR 1409. Indeed, CR 1409 abolished the responses to CCK-8 and CCK-JMV-180 in all five neurones. Four neurones were tested after administration of the CCK-B receptor antagonist L-365 260. L-365 260 had no effect on nodose neuronal firings stimulated by CCK-8 and CCK-JMV-180. The mean discharge frequency of the nodose ganglia neurones in response to various stimuli is presented in Figs 3 and 4. Administration of the CCK-A receptor antagonist L-364 718 completely abolished nodose neuronal responses to CCK-8 and CCK-JMV-180 in another three neurones tested.
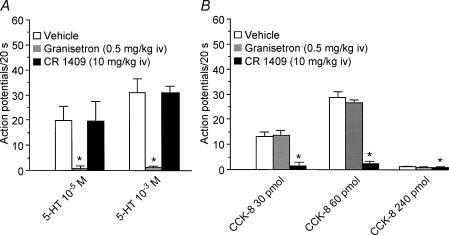
Figure 4. Responses of nodose ganglia neurones activated by both intraluminal perfusion of 5-HT and intra-arterial injection of CCK-8.
Of 32 neurones activated by electrical vagal stimulation, luminal perfusion of 5-HT (10−5 and 10−3 m) dose-dependently stimulated nodose neuronal firing in nine neurones A, administration of the 5-HT3 receptor antagonist granisetron but not the CCK-A receptor antagonist CR 1409, eliminated nodose neuronal responses evoked by luminal 5-HT stimulation. B, of the nine neurones that responded to luminal perfusion of 5-HT, five were activated by intra-arterial injection of low doses (i.e. 30 and 60 pmol) but not a high dose (240 pmol) of CCK-8. Administration of the CCK-A receptor antagonist CR 1409, but not the 5-HT3 receptor antagonist granisetron eliminated the neuronal responses evoked by CCK-8. Values are means ± s.e.m.
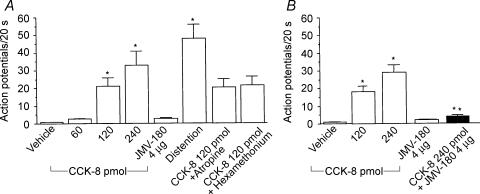
Sensitivity of CCK-sensitive neurones to duodenal distention
Twenty-four neurones were tested for their responsiveness to duodenal distention. Volume distention elicited a powerful increase in discharge frequency (39 ± 3 impulses (20 s)−1), which was maintained throughout the period of distention. The vagal afferent neurones that were activated by a low dose of CCK-8 failed to respond to volume distention (Fig. 1E). In contrast, five of seven neurones that were activated by high doses of CCK-8 responded to duodenal distention, but failed to respond to CCK-JMV-180, which suggests that those neurones expressing low-affinity CCK-A receptors also respond to duodenal distention.
Administration of atropine or hexamethonium
The effect of atropine on CCK-8-stimulated nodose neuronal firings was examined in six neurones. Of the six neurones, five neurones were activated both by high doses of CCK-8 and by duodenal volume distention, but CCK-JMV-180 evoked no response. The effect of hexamethonium was examined in nine neurones. Of these, four neurones were activated by both high doses of CCK-8 and duodenal distention, but failed to respond to CCK-JMV-180. We showed that administration of atropine or hexamethonium had no effect on vagal afferent neuronal firing in response to high doses of CCK-8 (Fig. 3), nor did this treatment affect the neuronal firing induced by duodenal distention.
Effects of CCK-JMV-180 on nodose neuronal activity stimulated by CCK-8
Data were collected from 21 recordings of single nodose ganglia neurones in 30 rats. All 21 units activated by electrical stimulation of the subdiaphragmatic vagus nerve were tested with intrasuperior pancreaticoduodenal artery injection of CCK-8 (30, 60, 120 and 240 pmol). Of the 21 neurones, 12 were activated by intra-arterial injection of various doses of CCK-8. Of these 12 neurones, seven were stimulated by low doses of CCK-8 (group A), and five were sensitive to high doses of CCK-8 (group B). To identify the vagal CCK-A receptor affinity state that mediates CCK-evoked vagal afferent neuronal firings, all 12 neurones were tested after administration of CCK-JMV-180 (4 μg). All of the group A neurones were stimulated by CCK-JMV-180; neuronal discharges increased to 19 ± 1.5 impulses (20 s)−1. In addition, CCK-8 at doses of 5 and 30 pmol were injected in combination with CCK-JMV-180. Administration of CCK-8 in combination with CCK-JMV-180 significantly augmented the nodose neuronal firings in four of seven neurones tested. The average discharge frequency is presented in Fig. 3. In group B, all five neurones were tested. Administration of CCK-JMV-180 (4 μg) produced an 80% inhibition of the nodose neuronal firings stimulated by high doses of CCK-8 (120 and 240 pmol). The average discharge frequency is presented in Fig. 3.
Effects of intraluminal infusions of 5-HT on nodose neuronal activity
Data were collected from 32 single neuronal recordings of nodose ganglia in 18 rats. All 32 units activated by electrical stimulation of the subdiaphragmatic vagus nerve were tested with intraduodenal perfusion of 5-HT (10−5 and 10−3 m). Nine of the 32 units responded to intraduodenal perfusion of 5-HT. Intestinal perfusion of 10−5 and 10−3 m 5-HT increased nodose neuronal discharges to 25 ± 6 and 47 ± 6 impulses (20 s)−1, respectively. The test solutions were delivered randomly over 1 min. After the intestinal lumen was rinsed with buffer solution, the discharge frequency decreased gradually.
We showed that i.v. administration of the 5-HT3 receptor antagonist granisetron abolished the response to luminal perfusion of 5-HT in four of four neurones tested. On the other hand, we found that administration of the 5-HT4 receptor antagonist GR125487 had no effect on the response to luminal 5-HT (data not shown). Of the nine neurones activated by intraduodenal perfusion of 5-HT, five responded to intra-arterial injection of CCK-8; four of nime neurones failed to respond to CCK-8 at any dose. Administration of granisetron had no effect on the nodose neuronal responses induced by CCK-8. In contrast, administration of the CCK-A receptor antagonist CR 1409 inhibited the responses evoked by intra-arterial CCK-8 injection, but had no effect on luminal 5-HT-induced nodose neuronal firings in another three of three neurones recorded. The average discharge frequency of nodose ganglia neurones in response to luminal 5-HT, and the effects of CR 1409 and granisetron are presented in Fig. 4.
Interaction between CCK-8 and luminal perfusion of 5-HT
Data were collected from 40 single neuronal recordings of nodose ganglia in 17 rats. All 40 neurones activated by electrical stimulation of the subdiaphragmatic vagus nerve were tested with intra-arterial injection of CCK-8. A subthreshold dose of CCK (i.e. 5 pmol) produced no measurable electrophysiological effect. Eleven of 40 neurones were activated by intra-arterial injection of CCK at a dose of 30 pmol; nodose firings increased from basal level (0 ± 0.5) to 16 ± 1.8 impulses (20 s)−1. Of the 11 neurones that responded to CCK-8, six were activated after luminal perfusion of 10−5 m 5-HT; firings increased from 1 ± 0.5 to 22.5 ± 3.5 impulses (20 s)−1. We showed that intra-arterial injection of a subthreshold dose of CCK (5 pmol) augmented the neuronal response to luminal 5-HT (Fig. 5). In addition, we showed that a 30 pmol dose of CCK further augmented neuronal firings when the luminal 5-HT perfusion was simultaneous.
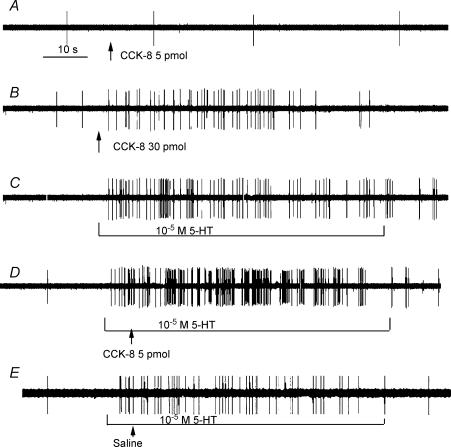
Figure 5. Effect of the interaction between CCK-8 and 5-HT on nodose neuronal firing.
Intra-arterial infusion CCK-8 at a dose of 30 pmol (B), but not 5 pmol (A) increased nodose neuronal firing. Intraduodenal infusion of 10−5 m 5-HT stimulated the same neurone (C). D, administration of the subthreshold dose of CCK-8 (i.e. 5 pmol) enhanced the nodose neuronal response to 5-HT (10−5 m). E, administration of saline had no effect on the nodose neuronal response to 5-HT (10−5 m).
To further clarify the synergistic interaction between these two agents, an additional study was performed in 10 rats. Twelve of 35 neurones activated by electrical stimulation of the subdiphragmatic vagus nerve were stimulated with an intra-arterial injection of CCK-8 at a dose of 10 pmol. Nodose neuronal firings increased from 0 ± 0.5 to 5.8 ± 0.5 impulses (20 s)−1. Of these 12 neurones, seven were activated after luminal perfusion of 5-HT; firing increased from 1 ± 0.5 to 19.5 ± 2.5 impulses (20 s)−1. During intraduodenal perfusion of 5-HT, intra-arterial injection of CCK (10 pmol) evoked an increase in discharge frequency to 36 ± 3 compared with 20.5 ± 3.5 impulses (20 s)−1 after vehicle (saline) injection. The total increase in neuronal discharge was greater than the sum of neuronal discharges stimulated by CCK-8 and luminal 5-HT alone, suggesting a potentiation effect of these two agents on vagal nodose neuronal activities. Administration of the CCK-A receptor antagonist CR 1409 eliminated the augmented responses induced by CCK-8. The mean discharge frequency of the nodose neurones is shown in Fig. 6.
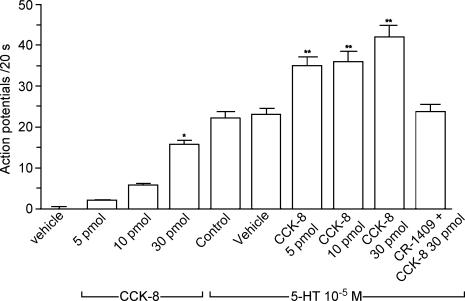
Figure 6. Discharge of nodose ganglia neurones in response to intra-arterial injection of CCK-8 and intraluminal perfusion of 5-HT, and the effect of the interaction between CCK-8 and 5-HT.
Of the 40 neurones activated by electrical stimulation of the vagus nerve, a subthreshold dose of CCK (i.e. 5 pmol) produced no measurable electrophysiological effect. Eleven of 40 neurones were activated by intra-arterial injection of CCK at a dose of 30 pmol. Of the 11 neurones that responded to CCK-8, six were activated after luminal perfusion of 5-HT. Intra-arterial injection of a subthreshold dose of CCK (5 pmol) enhanced the neuronal responses to luminal 5-HT. Furthermore, injection of CCK (30 pmol) against a background luminal perfusion of 5-HT produced a further increase of neuronal firing. Administration of the CCK-A receptor antagonist CR 1409 eliminated the enhanced responses induced by CCK-8. *P < 0.01 compared with vehicle; **P < 0.01 compared with 5-HT- control.
Discussion
In the present study, using the nodose ganglia recording technique in anaesthetized rats, we showed that the vagal nodose ganglion contains neurones that may possess high- or low-affinity CCK-A receptors or 5-HT3 receptors. Some neurones that express high-affinity CCK-A receptors also contain 5-HT3 receptors, so prior exposure to subthreshold doses of CCK may augment the subsequent response to luminal 5-HT.
Vagal afferent fibres represent a major target for CCK, mediating many digestive functions (Li & Owyang, 1993, 1996; Smith et al. 1981). CCK has been shown to stimulate gastric muscle mechanoreceptors in the rat (Davison & Clarke, 1988; Schwartz & Moran, 1998) although inhibition of gastric motor activity by CCK-8 has also been reported. However, electrophysiological studies suggest that mucosal afferents in the gastroduodenal region appear to be stimulated by CCK in the ferret and the rat (Li & Owyang, 1993; Richards et al. 1996). CCK dose–response studies using pancreatic acini typically reveal a biphasic dose–response relationship. Although clearly defined subtypes of the type A receptor have not been identified, CCK has been shown to interact with two different affinity states in pancreatic acinar cells (Sato et al. 1989; Stark et al. 1989). One site has high affinity and low capacity and is associated with stimulation of pancreatic enzyme release at CCK concentrations up to 100 pm. The other site has low affinity and high capacity and is thought to mediate the inhibition of CCK-stimulated pancreatic enzyme release. It is not known whether these two sites represent distinct proteins or different affinity states of the same receptor protein. The COOH-terminal heptapeptide CCK analogue CCK-JMV-180 provides a functional discrimination between these two states. CCK-JMV-180 has been shown to interact with both classes of pancreatic CCK receptors, acting as an agonist at the high-affinity state and an antagonist at the low-affinity state (Sato et al. 1989; Stark et al. 1989). Another study (Li et al. 1997) demonstrated that activation of gastric mechanosensitive vagal afferent fibres and suppression of food intake by CCK in the rat were mediated by vagal low-affinity CCK-A receptors. We have shown that, in contrast to its effect on satiety, i.v. infusion of CCK-JMV-180 stimulates pancreatic secretion to a similar degree to that evoked by a physiological dose of CCK-8. CCK-JMV-180 did not inhibit but rather augmented the pancreatic responses to both exogenous and endogenous CCK, which suggests that under physiological conditions, CCK acts through high-affinity CCK-A receptors to mediate pancreatic protein secretion (Weatherford et al. 1993). These responses were abolished by vagotomy, which suggests mediation by high-affinity CCK-A receptors. Further, electrophysiological recording of the nodose ganglion neurones, showed that administration of CCK-JMV-180 inhibited the neuronal response to endogenously released CCK during diversion of bile-pancreatic juice in some but not all neurones (Li et al. 1999). Therefore, both low- and high-affinity CCK receptors must coexist in the vagal nodose ganglion. Activation of these CCK receptors in different affinity states may mediate different digestive and metabolic functions.
In the current study, we examined the single vagal nodose neuronal responses to intra-arterial injection of CCK-8 at various doses and showed that CCK-8 clearly can act on both high- and low-affinity CCK-A receptors found on a distinct population of nodose neurones. The mechanisms underlying the phenomenon of low-dose CCK stimulation and high-dose CCK inhibition of a certain group or population of CCK-sensitive afferents are unclear; however, our previous investigations have demonstrated that protein kinase C (PKC) might be involved in this negative feedback regulation. When dispersed pancreatic acini were incubated with the high-affinity CCK-A receptor agonist CCK-JMV-180 and the PKC activator, an inhibition of enzyme secretion was observed (Tsunoda & Owyang, 1995; Lankisch et al. 2002). The activation of PKC may cause phosphorylation of serine or threonine on the CCK-A receptor, resulting in desensitization of the receptor (Ozcelbi & Miller, 1995). Therefore, it appears that PKC activated by high-dose CCK inhibits the activity of the high-affinity CCK-A receptor. A similar mechanism may operate within the vagal afferent fibres bearing low- and high-affinity CCK receptors.
Most of the neurones (5 of 7) activated by high doses of CCK-8 also responded to duodenal distention, which suggests that these afferent fibres are polymodal and their terminals are located in the intestinal muscle layer. These neurones possessed low-affinity CCK-A receptors as they did not respond to CCK-JMV-180.
We showed that administration of atropine or hexamethonium had no effect on vagal afferent neuronal firing in response to high doses of CCK-8, nor did this treatment affect the neuronal firing induced by duodenal distention. These observations suggest that the vagal nodose neuronal response evoked by intra-arterial injection of high doses of CCK-8 was not the result of an increase in muscle tone, secondary to the action of CCK on the intestinal muscle itself or on the enteric cholinergic pathway, which are known to stimulate intestinal contraction. CCK-8 also does not act via a pre-synaptic cholinergic pathway to stimulate nodose neuronal firing.
Enterochromaffin cells have been shown to secrete 5-HT spontaneously and in response to a wide variety of stimuli. Large numbers of 5-HT-containing cells are found in the proximal duodenum. These cells have a morphology that is consistent with a paracrine ‘sensory’ role. It is interesting that a CCK-A receptor antagonist has been reported to reduce the vagal afferent-mediated inhibitory effects on gastric emptying induced by intra-intestinal infusion of maltose (Raybould & Hölzer, 1992), leading to the suggestion that carbohydrates may stimulate CCK release, which in turn activates vagal afferent fibres. In a recent electrophysiological study, we showed that intraduodenal perfusions of maltose, glucose and hypertonic saline stimulated nodose ganglion in rats (Zhu et al. 2001). These vagal primary afferent neurones were also sensitive to exogenous luminally applied 5-HT at concentrations that mimicked physiological levels. Intravenous administration of a 5-HT3 antagonist blocked these responses, which suggests that nodose neuronal responses to luminal osmolarity and to the digestion products of carbohydrates are mediated by the release of endogenous 5-HT from the mucosal enterochromaffin cells. This released 5-HT acts on the 5-HT3 receptors on vagal afferent fibres.
Given the facts that endogenously released 5-HT and CCK both act on vagal afferent fibres innervating the intestinal mucosa to stimulate vagal afferent neurones, and, as we observed in a previous study, infusion of a subthreshold dose of CCK potentiated vagal–vagal reflex-mediated pancreatic secretion stimulated by luminal 5-HT-dependent factors (Li & Owyang, 1996), it is possible that 5-HT and CCK may activate the same population of nodose ganglia neurones. Inconsistency currently surrounds the interaction of 5-HT and CCK in the vagal afferent nerve. Previous research involving electrophysiological recording from filaments of afferent fibres of the cervical vagus in the ferret reported that a number of single units that responded to systemic administration of 5-HT were activated by CCK-8 (Blackshaw & Grundy, 1993). However, recording single unit firings extracted from mesenteric nerve bundles of the rat showed that individual units responded either to CCK or to 5-HT, but not to both (Hillsley & Grundy, 1998). In our current study, using single vagal afferent neuronal recording, we demonstrated that there were nodose neurones that responded to luminal perfusion of 5-HT, but not to different doses of CCK-8. However, five of nine nodose ganglion neurones that were activated by CCK-8 also responded to intraluminal perfusion of 5-HT. Similarly, six of 11 nodose neurones possessing high-affinity CCK-A receptors also responded to luminal perfusion of 5-HT. Interestingly, we showed that although a subthreshold dose of CCK-8 produced no measurable electrophysiological effects, it augmented the neuronal response to luminal 5-HT perfusion. The potentiation effect was eliminated by a CCK-A receptor antagonist.
The interaction between CCK and 5-HT at the level of the nodose ganglion may modulate afferent postprandial signals arising from the gastrointestinal tract and thus influence the central nervous system regulation of gastrointestinal functions. This positive interaction may explain the augmented response of pancreatic secretion to CCK after stimulation by 5-HT-dependent luminal stimuli (Li & Owyang, 1996) and it may provide an explanation of how a small increase in the plasma CCK level is sufficient to produce a robust postprandial pancreatic secretion. Previous research has shown that vagal afferent fibres respond to gastric load, duodenal load and close coeliac arterial administration of CCK (Schwartz et al. 1991, 1995). CCK administration potentiates subsequent responses to gastrointestinal perfusion of nutrients (Schwartz et al. 1991, 1995). It is well known that the satiety induced by CCK results from its interaction with other signals triggered by stimuli within the gastrointestinal tract. Previous studies have shown that duodenal glucose infusion, which activates vagal primary afferent neurones by the release of 5-HT (Zhu et al. 2001; Li et al. 2001) enhances suppression of sham feeding by CCK (Cox, 1994). The positive interaction between CCK and 5-HT on the vagal primary afferent neurones, as demonstrated in the current study, may contribute to maximizing the satiety action of CCK. In addition, the positive interaction between CCK and 5-HT at the level of vagal nodose ganglion may have other physiological and clinical implications. Alosetron has been reported to significantly reduce pain scores after colorectal distention (De Ponti & Tonini, 2001), and clinical trials have demonstrated that the drug is moderately effective in controlling symptoms in female patients with irritable bowel syndrome (Talley, 2003). Most patients with irritable bowel syndrome also show an exaggerated response to CCK in colonic motility and visceral pain (Roberts-Thomson et al. 1992). The present study showed that 5-HT and CCK-8 may act synergistically to stimulate vagal primary afferent neurones; an observation that may provide a functional basis to help explain why most patients with irritable bowel syndrome exhibit CCK hypersensitivity.
Acknowledgments
This investigation was supported by the National Institute of Diabetes and Digestive and Kidney Disease Grants R01-DK-51717 (Y.L.), R01-DKDK-48419 and P30-DK 34933 (C.O.), and the Michigan Life Science Corridor Grant 1635 (Y. L.).
References
- Andrews PL, Davison JS. Activation of vagal afferent terminals by 5-HT is mediated by 5-HT3 receptors in the anaesthetized ferret. J Physiol. 1990;422:92P. [Google Scholar]
- Attoub S, Moizo L, Laignwau JP, Alchepo B, Lewin MJ, Bado A. YM022, a highly potent and selective CCKB antagonist inhibiting gastric acid secretion in the rat, the cat and isolated rabbit glands. Fundam Clin Pharmacol. 1998;12:256–262. doi: 10.1111/j.1472-8206.1998.tb00952.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. doi: 10.1016/s0016-5085(97)70056-8. [DOI] [PubMed] [Google Scholar]
- Cox JE. Duodenal sucrose and glucose infusions enhance suppression by cholecystokinin of sham feeding. Am J Physiol. 1994;266:R466–R471. doi: 10.1152/ajpregu.1994.266.2.R466. [DOI] [PubMed] [Google Scholar]
- Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol. 1998;274:R1390–R1396. doi: 10.1152/ajpregu.1998.274.5.R1390. [DOI] [PubMed] [Google Scholar]
- Davison JS, Clarke GD. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. Am J Physiol. 1988;255:G55–G61. doi: 10.1152/ajpgi.1988.255.1.G55. [DOI] [PubMed] [Google Scholar]
- De Ponti F, Tonini M. Irritable bowel syndrome: new agents targeting serotonin receptor subtypes. Drugs. 2001;61:317–332. doi: 10.2165/00003495-200161030-00001. [DOI] [PubMed] [Google Scholar]
- Emeran AE, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine 4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg RW. Anatomy of Laboratory Rat. Baltimore: Williams & Wilkins; 1976. [Google Scholar]
- Hillsley K, Grundy D. Serotonin and cholecystokinin activate different populations of rat mesenteric vagal afferents. Neurosci Lett. 1998;225:63–66. doi: 10.1016/s0304-3940(98)00690-9. [DOI] [PubMed] [Google Scholar]
- Julian GM, Lawrence CB. Selectivity of cholecystokinin (CCK) receptor antagonists, MK-329 and L-365,260, for axonally-transported CCK binding sites on the rat vagus nerve. Neurosci Lett. 1992;137:229–231. doi: 10.1016/0304-3940(92)90410-9. [DOI] [PubMed] [Google Scholar]
- Lankisch TO, Tsunoda Y, Lu Y, Owyang C. Characterization of CCKA receptor affinity states and Ca2+ signal transduction in vagal nodose ganglia. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1002–G1008. doi: 10.1152/ajpgi.00313.2001. [DOI] [PubMed] [Google Scholar]
- Li Y, Hao Y, Owyang C. High-affinity CCK-A receptors on the vagus nerve mediate CCK-stimulated pancreatic secretion in rats. Am J Physiol. 1997;273:G679–G685. doi: 10.1152/ajpgi.1997.273.3.G679. [DOI] [PubMed] [Google Scholar]
- Li Y, Hao YB, Zhu JX, Owyang C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-CCK-stimulated pancreatic secretion in rats. Gastroenterology. 2000;118:1197–1207. doi: 10.1016/s0016-5085(00)70373-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Owyang C. Vagal afferent pathways mediate physiological action of cholecystokinin on pancreatic secretion. J Clin Invest. 1993;92:418–424. doi: 10.1172/JCI116583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Owyang C. Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin-dependent duodenal stimuli via vagal afferent fibres in the rat. J Physiol. 1996;494:773–782. doi: 10.1113/jphysiol.1996.sp021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu XY, Owyang C. Intestinal serotonin acts as a paracrine substance to mediate pancreatic secretion stimulated by non-CCK-dependent luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281:G916–G923. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhu J, Owyang C. Electrical physiological evidence for high- and low-affinity CCK-A receptors. Am J Physiol. 1999;277:G469–G477. doi: 10.1152/ajpgi.1999.277.2.G469. [DOI] [PubMed] [Google Scholar]
- Mei N, Garnier L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst. 1986;16:159–170. doi: 10.1016/0165-1838(86)90022-6. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Niederau M, Niederau C, Strohmeyer G, Grendell JH. Comparative effects of CCK receptor antagonists on rat pancreatic secretion in vivo. Am J Physiol. 1989;256:G150–G157. doi: 10.1152/ajpgi.1989.256.1.G150. [DOI] [PubMed] [Google Scholar]
- Ozcelbi F, Miller LJ. Phosphopeptide mapping of cholecystokinin receptors on agonist-stimulated native pancreatic acinar cells. J Biol Chem. 1995;270:3435–3441. doi: 10.1074/jbc.270.7.3435. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Hölzer HH. Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by carbohydrate. Neurosci Lett. 1992;141:236–238. doi: 10.1016/0304-3940(92)90902-j. [DOI] [PubMed] [Google Scholar]
- Renehan WE, Zhang X, Beierwaltes WH, Fogel R. Neurons in the dorsal motor nucleus of the vagus may integrate vagal and spinal information from the GI tract. Am J Physiol. 1995;268:G780–G790. doi: 10.1152/ajpgi.1995.268.5.G780. [DOI] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J Physiol. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Brenner L, Yox DP. Participation of vagal sensory neurons in putative satiety signals from upper gastrointestinal tract. In: Ritter S, Ritter RC, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. Boca Raton, Ann Arbor Boston: CRC Press; 1992. pp. 221–248. [Google Scholar]
- Roberts-Thomson IC, Fettman MJ, Jonsson JR, Frewin DB. Responses to cholecystokinin octapeptide in patients with functional abdominal pain syndromes. J Gastroenterol Hepatol. 1992;7:293–297. doi: 10.1111/j.1440-1746.1992.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Sato S, Stark HA, Martines J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation, calcium mobilization and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 1989;257:G202–G209. doi: 10.1152/ajpgi.1989.257.2.G202. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol. 1991;261:R64–R69. doi: 10.1152/ajpregu.1991.261.1.R64. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol. 1998;274:R1236–R1242. doi: 10.1152/ajpregu.1998.274.5.R1236. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, Tougas G, Moran TH. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides. 1995;16:707–711. doi: 10.1016/0196-9781(95)00033-g. [DOI] [PubMed] [Google Scholar]
- Smith G, Jerome C, Bushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effects of cholecystokinin in the rat. Science. 1981;213:1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- Stark HA, Sharp CM, Sutliff VE, Martinez J, Jensen RT, Gardner JD. CCK-JMV-180: a protein that distinguishes high-affinity cholecystokinin receptors from low-affinity cholecystokinin receptors. Biochim Biophys Acta. 1989;1010:145–150. doi: 10.1016/0167-4889(89)90154-7. [DOI] [PubMed] [Google Scholar]
- Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:750. doi: 10.1111/j.1572-0241.2003.07306.x. [DOI] [PubMed] [Google Scholar]
- Tsunoda Y, Owyang C. High-affinity CCK receptors are coupled to phospholipase A2 pathways to mediate pancreatic amylase secretion. Am J Physiol. 1995;269:G435–G444. doi: 10.1152/ajpgi.1995.269.3.G435. [DOI] [PubMed] [Google Scholar]
- Weatherford SC, Laughton WB, Salabarria J, Danho W, Tilley JW, Netterville LA, Schwartz GJ, Moran TH. CCK satiety is differentially mediated by high- and low-affinity CCK receptors in mice and rats. Am J Physiol. 1993;264:R244–R249. doi: 10.1152/ajpregu.1993.264.2.R244. [DOI] [PubMed] [Google Scholar]
- Zhu JX, Wu XY, Owyang C, Ying L. Intestinal serotonin acts as a paracrine substance to mediate vagal transmission evoked by luminal factors in the rat. J Physiol. 2001;530:431–442. doi: 10.1111/j.1469-7793.2001.0431k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]